Abstract
Executive functions of the prefrontal cortex (PFC) are sensitive to local dopamine (DA) levels. Although sex differences distinguish these functions and their dysfunction in disease, the basis for this is unknown. We asked whether sex differences might result from dimorphisms in the glutamatergic mechanisms that regulate PFC DA levels. Using antagonists selective for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors, we compared drug effects on in vivo microdialysis DA measurements in the PFC of adult male and female rats. We found that baseline DA levels were similar across sex, AMPA antagonism decreased PFC DA in both sexes, and NMDA antagonism increased DA in males but decreased DA in females. We also found that, at subseizure-producing drug levels, γ-aminobutyric acid (GABA)-A antagonism did not affect DA in either sex but that GABA-B antagonism transiently increased PFC DA in both sexes, albeit more so in females. Finally, when NMDA antagonism was coincident with GABA-B antagonism, PFC DA levels in males responded as if to GABA-B antagonism alone, whereas in females, DA effects mirrored those induced by NMDA antagonism. Taken together, these data suggest commonalities and fundamental differences in the intracortical amino acid transmitter mechanisms that regulate DA homeostasis in the male and female rat PFCs.
Keywords: hyperdopaminergia, hypodopaminergia, NMDA-R hypofunction, Schizophrenia
Introduction
The prefrontal cortices (PFCs) of humans and animals mediate higher-order executive functions including working memory, behavioral flexibility, and decision-making (Goldman-Rakic et al. 1990; Dalley et al. 2004; Tandon 2013). Prefrontal dysfunction is also implicated in the cognitive deficits seen in neurological disorders including Parkinson's disease (Dubois and Pillon 1997; Zgaljardic et al. 2003; Narayanan et al. 2013) and schizophrenia (Eisenberg and Berman 2010; Ortiz-Gil et al. 2011). The executive operations of the PFC are sensitive to intracortical dopamine (DA) levels and are affected when these levels deviate from optimal ranges (Murphy et al. 1996; Verma and Moghaddam 1996; Zahrt et al. 1997; Landau et al. 2009; Cools and D'Esposito 2011). Thus, factors such as disease processes, drug stimulation, and others that move PFC DA levels outside of these limits produce deficits in these and other PFC-dependent behaviors (Davis et al. 1991; Goldberg et al. 2003; Javitt 2007; Arnsten 2009; Scott and Aperia 2009; Seeman 2009). At the same time, it is also known that executive functions and the incidence and/or severity of executive dysfunctions in disease are often significantly different in males and females. For example, in humans (Overman et al. 1996; Woolley et al. 2010), non-human primates (Goldman et al. 1974; Overman et al. 1996; Lacreuse et al. 1999), and rodents (Roof et al. 1993; Faraji et al. 2010), males tend to outperform females on spatial cognitive and working memory tasks while in disorders including Parkinson's disease (Miller and Cronin-Golomb 2010) and schizophrenia (Leung and Chue 2000; Mendrek and Stip 2011), males are more susceptible to and more impaired by deficits in executive function than females.
The question arises as to whether sex differences in PFC-dependent behavioral operations might be related to sex differences in the functionally critical DA systems that underlie them. Recent tract tracing studies in rats showed that, for mesoprefrontal projections, there is a nearly 2-fold female over male difference in the proportions of DA to non-DA afferents that make up these pathways (Kritzer and Creutz 2008). However, PFC DA levels measured in tissue homogenates have been shown to be either similar across sex (Tanila et al. 1994; Duchesne et al. 2009) or higher in males (Dalla et al. 2008). These seemingly contradicting observations could be reconciled by sex differences in the regulatory mechanisms that set functional PFC DA levels. These include strategically placed, intracortical receptor subtype-specific glutamatergic (GLU) influences that tonically and flexibly regulate DA levels (Jedema and Moghddam 1996; Takahata and Moghaddam 1998; Del Arco and Mora 1999; Wu et al. 2002; Aubele and Kritzer 2012) and that have been recently shown to be highly sensitive to circulating gonadal hormone levels in adult male rats (Aubele and Kritzer 2012). Using in vivo microdialysis and reverse dialyses, drug and dual drug challenges we examined whether these receptor subtype-specific mechanisms of PFC DA regulation might also differ across sex.
Previous studies—carried out to date exclusively in the male brain—have shown that tonic regulation of PFC DA tone is achieved in part by a balance of offsetting receptor subtype-specific intracortical GLU influences (Jedema and Moghddam 1996; Takahata and Moghaddam 1998; Del Arco and Mora 1999; Wu et al. 2002; Balla et al. 2009; Aubele and Kritzer 2012). More specifically, studies combining techniques of in vivo microdialysis or electrophysiology with drug challenge have shown that N-methyl-d-aspartate (NMDA)-mediated GLU actions engage PFC interneurons to inhibit the PFC's descending drive over the ventral midbrain and tonically suppress PFC DA levels (Del Arco and Mora 2002; Jackson et al. 2004; Homayoun and Moghaddam 2007; Aubele and Kritzer 2012; Povysheva and Johnson 2012). In contrast, local α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-mediated systems excite PFC pyramidal neurons, including those projecting to the ventral tegmental area and tonically stimulate mesoprefrontal DA neurons and DA release back in the PFC (Jedema and Moghddam 1996; Takahata and Moghaddam 1998; Wu et al. 2002; Aubele and Kritzer 2012). Taken together, with what have largely been shown to be phasic, resetting actions of intracortical γ-aminobutyric acid (GABA)-A or GABA-B receptor-mediated influences (Yonezawa et al. 1998; Balla et al. 2009; Del Arco et al. 2011), these regulatory processes play important roles in modulating electrophysiological properties (Tong et al. 1996; Wang et al. 2010; Povysheva and Johnson 2012) and complex behaviors (Aultman and Moghaddam 2001; Feenstra et al. 2002; Fejgin et al. 2009) associated with the PFC. Likewise, they have also been repeatedly implicated in the hyper- and hypodopaminergia associated with PFC dysfunction in disease (Lewis et al. 1999, 2012; Cryan and Kaupmann 2005; Kehrer et al. 2008; Kantrowitz and Javitt 2010; Gonzalez-Burgos and Lewis 2012). Here, we tested hypotheses for sex differences in the organization and/or operations of these regulatory circuits. This was done by combining in vivo microdialysis with reverse dialysis administration of receptor subtype-selective GLU (NMDA and AMPA) and GABA (GABA-A and GABA-B) antagonists and HPLC with electrochemical detection to quantitatively compare drug effects, alone and in combination, on DA levels in the PFC of adult male and in female rats where estrous cycle stage was tracked.
Materials and Methods
Animal Subjects
A total of 60 adult male and 53 female Sprague-Dawley rats (Taconic Farms, Germantown, NY, USA) were used. Animals were housed in a specific pathogen-free environment in same sex pairs under a 12/12 h light/dark cycle (lights on at 0700) with food (Purina PMI Lab Diet: Prolab RMH 3000) and water available ad libitum. Animals were 8–10 weeks of age and weighed between 200 and 400 g at the time of the microdialysis studies. For the females, vaginal lavage was used to identify the stage of the estrus cycle subjects were in on the day of the microdialysis experiments. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Stony Brook University and were designed to minimize animal use and discomfort.
Stereotaxic Placement of Guide Cannulae
Craniotomies were performed 24 h before the microdialysis experiments under aseptic conditions and using intraperitoneal injections of ketamine (90 mg/kg) and xylazine (10 mg/kg) as anesthesia. Stereotaxic coordinates were used to place guide cannulae (14 mm, CMA Microdialysis, North Chelmsford, MA, USA) within the left pregenual medial PFC area corresponding to plate 8 of Paxinos and Watson (1998). Cannulae were secured to the skull with shallow anchor screws and dental cement. After surgeries, animals were given single doses of buprenorphine (0.03 mg/kg) and returned to home cages for recovery.
In Vivo Microdialysis, DA Detection, and DA Measurement
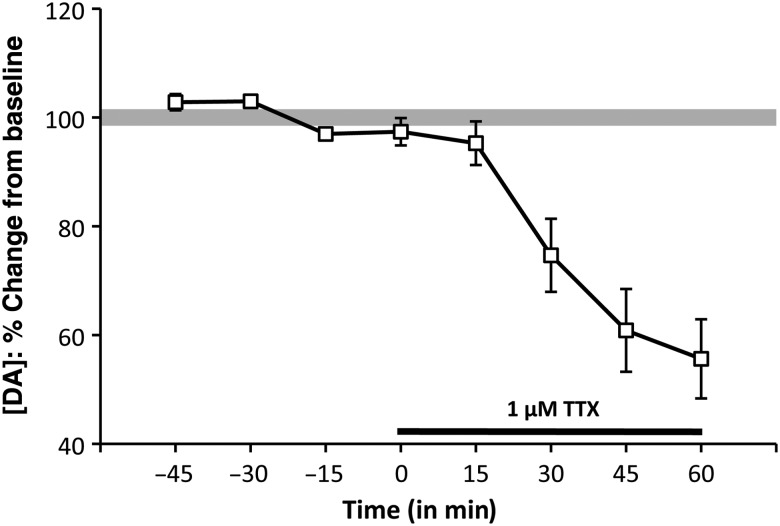
Microdialysis studies took place during animals' subjective nights. Animals were typically asleep for the duration of the experiment. Rats were placed in clear bowls (Raturn, BioAnalytical Systems) and allowed to acclimate for 10 min. Microdialysis probes (100 000 Da cutoff, 2 mm Polyethersylphone exposed membrane tip, CMA Microdialysis) were then gently inserted through guide cannulae and perfused with artificial cerebrospinal fluid (aCSF; 145 mM NaCl; 2.8 mM KCl; 1.2 mM MgCl2; 0.25 mM ascorbic acid; 5.4 mM d-glucose, 1.2 mM CaCl2, pH 6.8) at a flow rate of 2 μL/min for a 2-h equilibration period. Sensitivity of PFC DA levels to tetrodotoxin (TTX) after the equilibration period and for the duration of the subsequent drug application period was established in a subset of test subjects (n = 7) by reverse dialysis delivery of 1 μM TTX (Fig. 1). While the low dose of TTX used established patency of stimulated DA terminal release for the duration of the experimental timelines, it does not fully rule out the possibility for a small pool of TTX-insensitive DA that may lead to an underestimation of drug effects. For all other subjects, baseline dialysates were collected (10 µL) and directly injected into the HPLC every 15 min using an online autoinjector (Pollen-8, BAS). Because of the comparative design of all within- and across-subject assessments and the hours-long experimental timelines involved, uncorrected rather than no-net flux methods were used to measure basal DA levels. After obtaining 3 consecutive stable, uncorrected baseline measures (DA levels within 5% of each other), drug was added to the aCSF [5–50 μM bicuculline (Sigma-Aldrich Chemical Co., St. Louis, MO, USA), 30–60 µM CGP52432 (Tocris Bioscience), 100–150 µM 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) (Tocris Bioscience), 100–670 μM D(-)-2-amino-5-phophonopentanoic acid (APV) (Sigma-Aldrich Chemical Co.), or 50–200 µM picrotoxin (Sigma-Aldrich Chemical Co.)] and infused for 120 min while dialysates were collected (every 15 min); exceptions included the co-administration study where CGP52432 was administered for 45 min followed by a 90-min co-infusion of CGP52432 and APV and the dose–response studies in which each drug concentration of APV was administered for 75 min and each concentration of CGP52432 was administered for 45 min. With the exception of the dose response studies, after drug delivery, infusion of aCSF was resumed and dialysates were collected until DA levels returned to within 5% of pre-drug baseline values. It should be noted that a small number of subjects in the dose–response studies did not receive every drug concentration due to problems occasionally encountered over the long experimental timelines of these studies.
Figure 1.
Timeline/line graph showing the effects of reverse dialysis application of 1 μM TTX on extracellular prefrontal DA levels, expressed as mean percent changes from baseline (±standard error of the mean) in male rats. The solid black line beneath the line graph marks the drug-infusion period. Following 15 min of drug application, PFC DA levels began to decrease and continued to do so for the 60-min TTX application period.
Dialysate samples (10 μL) were directly injected into an HPLC system (PM 92-E pump, BAS, West Lafayette, IN, USA) and analyzed using a microbore column (UniJet, 1.0 mm inner diameter, 100 mm length, 3 μm Octadecylsilane particles; BAS) and a BioAnalytical Systems LC-Epsilon detector (BAS). The Eapp was +0.65 V versus the Ag/AgCl reference electrode. The mobile phase consisted of 14.5 mM NaH2PO4; 30 mM sodium citrate; 10 mM diethylamine HCl; 2.2 mM 1-octanesulfonic acid; 0.027 mM ethylenediaminetetraacetic acid; 7.2% acetonitrile (v/v); 1% tetrahydrofuran (v/v), pH 6.0 (with phosphoric acid). All chemicals used were purchased from Sigma-Aldrich Chemical Co.
DA peaks were isolated and quantified (ng/mL) in relation to a series of DA standards of known concentrations (2, 10 ng/mL) run before and after each microdialysis study. DA concentration (fmol/μL) was calculated using the ChromGraph Software (BAS) and measurements of “peak area.” Drug-induced changes in the DA level were also quantified as a percent of pre-drug baseline. Probe efficiency was determined to be 10–18%, and an overall detection limit of 8 fmol was achieved.
Estrous Stage Determination, Euthanasia, Histology, and Determination of Probe Placement
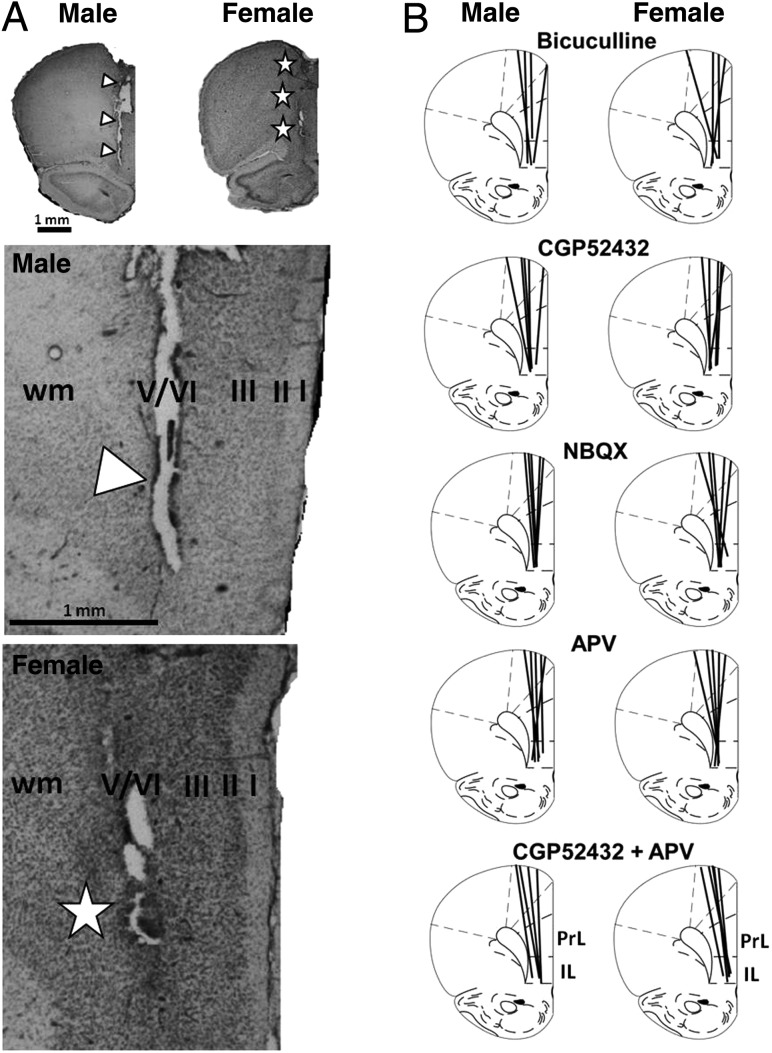
At the conclusion of microdialysis studies, female rats were vaginally lavaged and vaginal cytology was used to determine estrus cycle stage (Marcondes et al. 2002; Goldman et al. 2007). All rats were euthanized by rapid decapitation. Brains were removed and post-fixed for 2–4 days in a 10% buffered formaldehyde solution containing 30% sucrose for cryoprotection. Once fixed, brains were rapidly frozen in powdered dry ice and serially sectioned in a coronal plane on a freezing microtome (40 μm). A 1 of 4 series sections taken from the level of mid-olfactory bulb to the genu of the corpus callosum were slide mounted and counterstained with 0.5% cresyl violet (Fig. 2). Light microscopic evaluation was used to map probe tracks in relation to cortical cytoarchitecture. Only those cases where dialysis probes were confirmed to have spanned the deep layers of the left prelimbic and infralimbic medial PFC were included in the analysis (Fig. 2).
Figure 2.
Representative photomicrographs (left panels, A) and line drawings (right panels, B) showing the locations of microdialysis probe tracks in relation to cytoarchitectonic areas of the prefrontal cortex in male and female rats. The tissue sections shown (A) are counterstained with cresyl violet. Visible damage from the probe tracks is identified by white triangles (male) and white stars (female). Higher power photomicrographs of these probe tracks show their relation to cortical layers (I–VI) and white matter (wm). Line drawings (B) and their cytoarchitectonic boundaries are as per plate 8 of the Paxinos and Watson rat atlas; the locations of microdialysis probe tracks are depicted as black lines. The antagonist drug study is identified above line drawing pairs. Scale bar = 1 mm. IL, infralimbic cortex; PrL, prelimbic cortex; wm, white matter
Statistical Analysis
Uncorrected basal DA levels were compared across groups using a one-way analysis of variance (ANOVA). Drug effects on PFC DA levels were evaluated using two-way ANOVAs with repeated measures design, where sex served as the independent factor and the 15-min sample bins as the repeated measure. When significant sex, time, or sex by time interactions were found, post hoc Bonferroni analyses were used to identify when along study timelines drug effects on PFC DA levels significantly diverged between males and females. Additionally, within-sex one-way ANOVAs with repeated measures design, with the 15-min sample bins serving as the within-subjects factors, were run to determine at which time point during drug administration DA concentrations was significantly different from baseline. In all cases, a P-value of <0.05 was accepted as a significant and 0.05 < P ≤ 0.09 was designated as a near significant.
Results
Baseline Extracellular PFC DA Levels in Male and Female Rats Across the Estrous Cycle
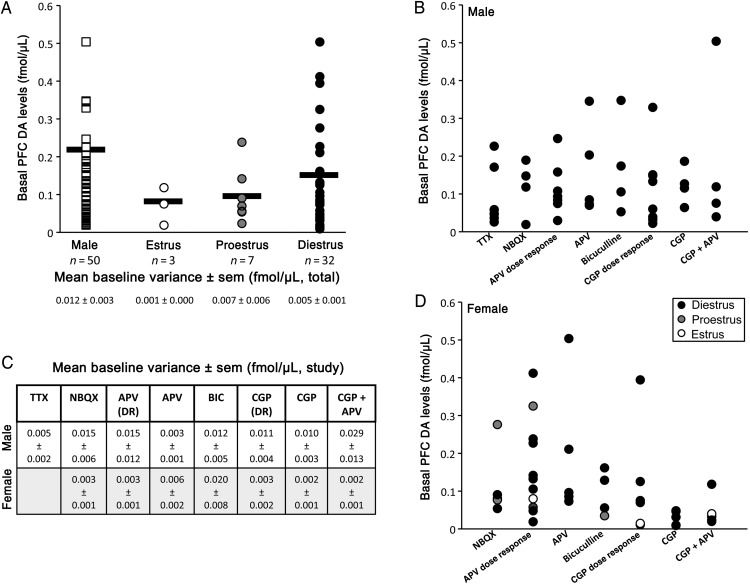
Uncorrected, basal extracellular PFC DA levels were measured prior to drug delivery in all animal subjects. In males rats, these values ranged from 0.02 to 0.50 fmol/μL and had a group average of 0.238 fmol/µL (±0.06 SEM, Fig. 3). A somewhat broader and slightly lower range of uncorrected basal DA concentrations was found among the female rats. Although there was substantial overlap, it was also noted that the highest DA levels mapped to females in diestrus (from 0.01 to 0.50 fmol/µL; group average 0.169 fmol/μL, ±0.04 SEM) that intermediate uncorrected basal DA levels were found for proestrus females (from 0.02 to 0.24 fmol/μL; group average 0.099 fmol/µL, ±0.03 SEM), and that the lowest values were associated with the estrus females (from 0.02 to 0.12 fmol/μL; group average 0.063 fmol/μL, ±0.03 SEM, Fig. 3). One-way ANOVA, carried out between males and all females and between males and females separated by estrous cycle stage, identified no significant main effects of group.
Figure 3.
Scatter plots showing mean basal dopamine level concentrations (fmol/μL) measured from all individual subjects included in this study (A). Data from males are shown in white squares; data from females are shown in circles and separated by estrous cycle stage—estrus (open circles), proestrus (grey circles), and diestrus (black circles, A). Group means are depicted by horizontal black bars (A). No significant differences were found in basal dopamine levels among any of these groups in comparisons in which males were compared with a single combined pool of all female subjects. Scatter plots showing mean basal dopamine level concentrations (fmol/µL) in individual male (B) and female (D) subjects separated by the drug study. Mean baseline variance, that is, the average difference between actual baseline measurements and mean basal dopamine level (±SEM, fmol/μL) for each animal, are also shown for total numbers of animal subjects (A) and separated by the drug study (C).
Comparisons of Glutamate Receptor Antagonism on Extracellular PFC DA Levels in Male and Female Rats
AMPA Receptor Antagonism
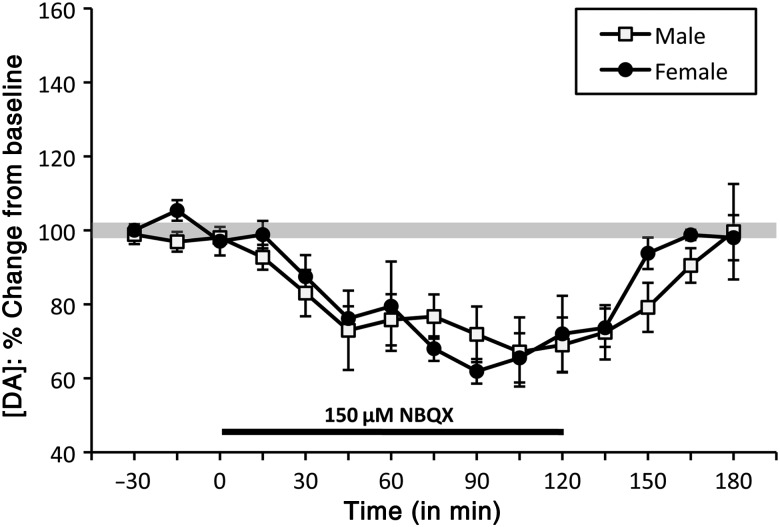
The effects of the AMPA antagonist NBQX on PFC DA levels were initially assessed across a range of concentrations in male (n = 8) and female [diestrus (n = 6) and proestrus (n = 2)] subjects. These studies showed that, at concentrations lower than 100 μM, NBQX had no discernable effects on PFC DA levels and at concentrations higher than 150 μM, drug precipitation interfered with the HPLC analysis. However, at 150 μM, infusion of the AMPA antagonist NBQX reliably and similarly decreased extracellular PFC DA in both sexes (Fig. 4). Thus, in males (n = 5) and in females [in diestrus (n = 3) or proestrus (n = 2)], uncorrected basal DA levels began to fall within 15 min of drug application, and dropped to maximally depressed extracellular DA concentrations that were roughly 30% below baseline within 45 min (Fig. 4). DA levels remained depressed for the remainder of the drug application period and upon drug removal returned to pre-drug concentrations within 45–60 min (Fig. 4). A two-way ANOVA (repeated measures) identified significant main effects of NBQX on PFC DA level (F14,112 = 11.62, P < 0.0001). However, no significant main effects of sex and no significant interactions between sex and drug treatment on PFC DA level. The main effects of drug were explored further in one-way ANOVA that revealed significant main effects of time/drug (F9,63 = 6.245–8.479, P < 0.0001) and post hoc testing that showed that PFC DA levels in males and females dropped to values that were significantly lower than baseline within 45 min of drug onset and remained significantly lower than baseline until the time of drug removal (P ≤ 0.001–0.048).
Figure 4.
Timeline/line graphs showing the effects of reverse dialysis application of NBQX on extracellular prefrontal DA levels expressed as mean percent change from baseline (±standard error of the mean) in male (white squares) and female (black circles) rats. The solid black line beneath the line graphs marks the drug infusion period. In both sexes, NBQX application decreased in prefrontal DA levels within 30 min.
NMDA Receptor Antagonism
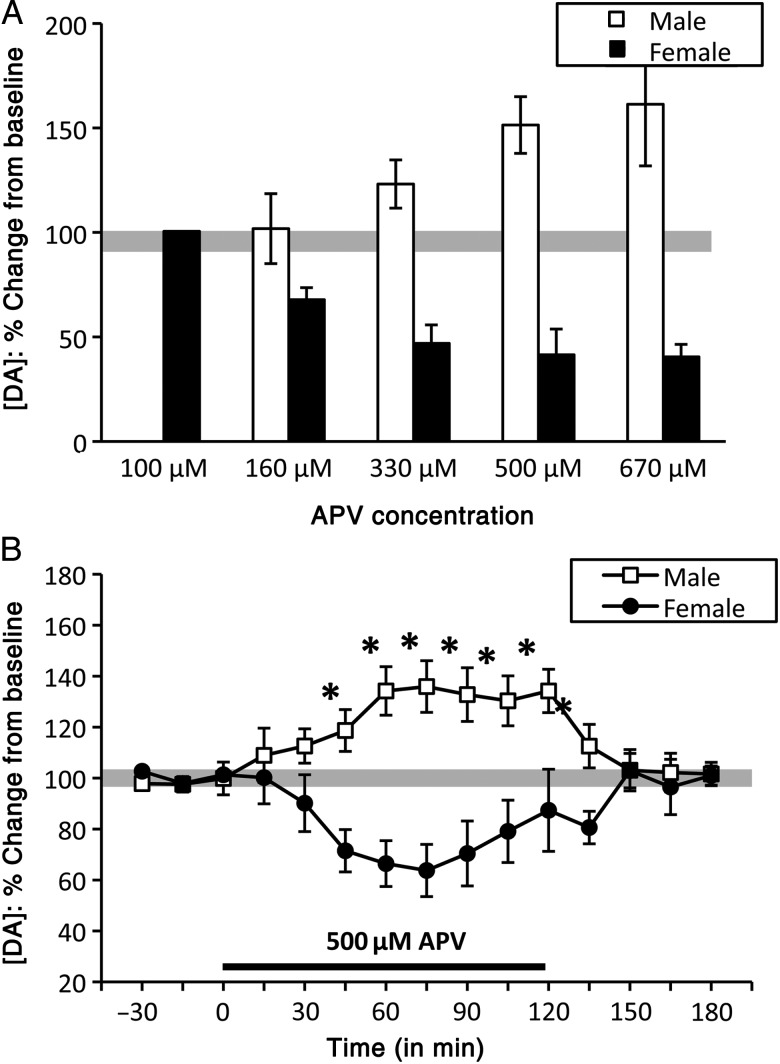
The effects of reverse dialysis intra-PFC infusion of the NMDA antagonist APV on local DA levels were initially assessed across 5 concentrations of ranging from 100 to 670 μM in males (n = 13) and females [diestrus (n = 13), proestrus (n = 2), and estrus (n = 1)]. These studies showed that drug concentrations of 100 and 160 μM in males and of 100 μM in females had no effect on PFC DA level (Fig. 5A). In male rats, intermediate doses of APV (330 and 500 μM) increased PFC DA levels by 30–50% in a concentration-dependent manner, and the effects of 500 and 670 μM APV were similar. In contrast, in the females, 160, 330, and 500 μM APV decreased PFC DA levels by 40–50% in a concentration-dependent manner, with depressive effects of 500 and 670 μM APV being maximal and similar (Fig. 5A). A 2-h drug-infusion protocol using 500 μM APV further showed that, in males (n = 5), PFC DA levels began to rise within 15 min of drug onset, reached peak concentrations of 30–40% above baseline roughly 60 min later, remained elevated until drug offset and returned to pre-drug levels within approximately 30 min (Fig. 5B). In females [diestrus (n = 5)], PFC DA levels were more sluggish in responding. However, 15–30 min after drug infusion, PFC DA concentrations dropped to levels of 30–40% below baseline, remained depressed until drug offset, and returned to baseline within 30 min (Fig. 5B). An initial two-way ANOVA (repeated measures) identified significant main effects of sex (F1,8 = 16.05, P = 0.0039), and significant interactions between sex and APV treatment (F14,112 = 7.184, P < 0.0001) on PFC DA levels. Within-sex, one-way ANOVAs further revealed significant main effects of time/drug on PFC DA levels in males and females (F9,79 = 3.381–6.387, P ≤ 0.0001–0.002). Finally, post hoc comparisons showed that, in males, APV stimulated DA levels that were significantly higher than baseline from 60 min of drug infusion until drug removal (P = 0.001–0.015), that in females, APV decreased DA to levels that were significantly to near significantly lower than baseline from 60 min of drug infusion to drug offset (P = 0.040–0.087), and that the effects of APV effects on PFC DA levels were significantly different in males versus females from 45 min after drug infusion until drug removal (P ≤ 0.0001–0.0032).
Figure 5.
Bar graphs (A) showing opposite, dose-dependent effects of APV on extracellular prefrontal DA in male (white bars) and female (black bars) rats across a range of drug concentrations. APV at 100 and 160 μM had no effects on prefrontal DA levels in females (black bars) or males (grey bars), respectively. At higher doses, APV progressively increased prefrontal DA levels in males, and decreased prefrontal DA levels in females. In both sexes, maximum drug effects on extracellular DA levels were achieved at 500 μM APV. (B) Timeline/line graphs showing the effects of 500 μM APV on extracellular prefrontal DA levels, expressed as mean percent change from baseline (±standard error of the mean) in male (white squares) and female (black circles) rats. The solid black line beneath the line graphs in B marks the drug-infusion period. Infusion of 500 μM APV rapidly increased DA levels in males, but decreased DA levels in females. Asterisks identify the time points where DA levels in males and females were significantly different from each other (P < 0.05).
Effects of GABA Blockade on NMDA Receptor Antagonism on Extracellular PFC DA Levels in Male and Female Rats
The striking dimorphisms noted in APV effects on PFC DA levels suggested that APV's DA-regulating actions engaged GABAergic interneurons in males (as expected), but not in females. This was tested in in vivo microdialysis studies using a reverse dialysis dual drug challenge in which NMDA- and GABA receptors were both antagonized. These were preceded by studies in which the effects of GABA-A and GABA-B receptor antagonism alone were characterized.
GABA-A Receptor Antagonism
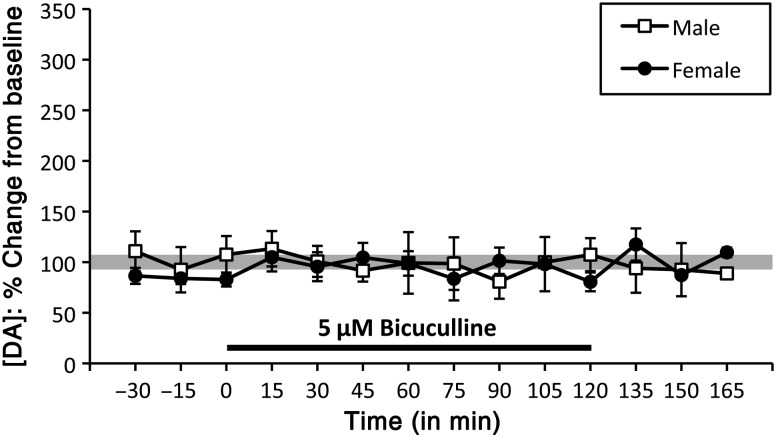
The effects of both the non-competitive antagonist picrotoxin and the competitive antagonist bicuculline were assessed across a range of doses (picrotoxin: 50–200 µM; bicuculline: 5–50 µM). Neither drug at even the highest concentrations had quantifiable effects on PFC DA levels. However, at all concentrations of picrotoxin tested and at all concentrations of bicuculline above 5 μM, piloerection or discernable seizure activity developed over the 2-h drug-infusion period in at least some of the animals. Only 5 μM bicuculline could be infused for the entirety of the drug-delivery period without producing obvious seizure activity in any of the subjects. Like the higher doses used, over the 2 h delivery, 5 μM bicuculline had no measureable effects on PFC DA levels in males (n = 4) or females [(diestrus (n = 3) and proestrus (n = 1)]. Rather, PFC DA levels remained within a few percent of pre-drug baselines throughout the drug application period and after drug offset (Fig. 6). A two-way ANOVA (repeated measures) of these data identified no significant main effects of sex or drug, and no significant interactions between them.
Figure 6.
Timeline/line graphs showing the effects of reverse dialysis application of 5 μM bicuculline on extracellular prefrontal DA levels, expressed as mean percent changes from baseline (±standard error of the mean) in male (white squares) and female (black circles) rats. The solid black line beneath the line graphs marks the drug-infusion period. Bicuculline did not have any significant effects on DA concentration in either males or females at the 5-μM concentration, the highest concentration to be administered without inducing seizure activity in any animal subjects.
GABA-B Receptor Antagonism
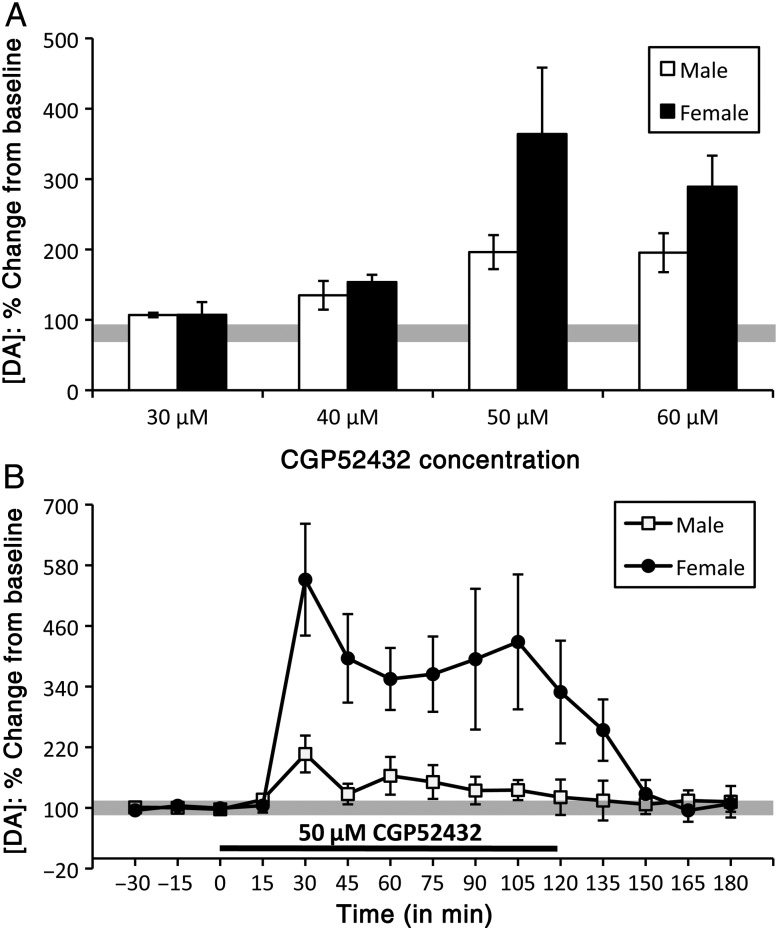
The effects of reverse dialysis infusion of the GABA-B antagonist CGP52432 were tested across a range of concentrations. Below 40 μM, infusion of CGP52432 had no effects on PFC DA level and at concentrations at and above 70 μM, while CGP52432 increased PFC DA level, piloerection, and/or motor seizures also developed in some of the males (n = 20) and females [diestrus (n = 15), proestrus (n = 1), and estrus (n = 1)] tested. However, when infused at either 50 μM or at 60 μM, CGP52432 had similar, albeit sex-specific effects on PFC DA levels, with no observable motor effects in any subjects (Fig. 7A). More specifically, in males (n = 5), roughly 30 min after drug onset (50 μM), DA concentrations transiently peaked at levels that were about 2 times higher than baseline. Over the next 15 min, DA levels dropped to concentrations of about 60% above baseline and continued to decline thereafter, returning to pre-drug baseline levels 15–30 min before drug offset (Fig. 7B). The effects of CGP52432 in female rats [diestrus (n = 5)] were similar but were larger overall (Fig. 7B). Thus, 30 min after drug application, DA levels showed a roughly 5-fold peak before quickly dropping to levels that were approximately 3 times higher than baseline and then undergoing a slow decrement for the remainder of the drug application period, and a return to baseline within 15–30 min of drug offset (Fig. 7B). An initial two-way ANOVA (repeated measures, data collected using 50 μM CGP52432) identified significant main effects of sex (F1,8 = 6.938, P = 0.03), significant main effects of drug treatment/time (F15,120 = 8.219, P < 0.0001), and significant interactions between the two (F15,120 = 4.424, P < 0.0001) on PFC DA level. Separate within-sex, one-way ANOVA (repeated measures) also revealed significant main effects of drug/time on PFC DA level in both sexes (F9,79 = 2.338–7.881, P ≤ 0.001–0.023). Finally, post hoc comparisons showed that, in males, peak DA concentrations were significantly higher than baseline (P = 0.008), that in females DA levels were significantly to near significantly higher than baseline from its peak until drug removal (P ≤ 0.001–0.079), and that the effects of CGP52432 on extracellular PFC DA levels were significantly to near significantly greater in females than in males for the majority of the drug application period (P = 0.0002–0.09, Fig. 7B).
Figure 7.
Bar graphs (A) showing the maximum effects of CGP52432 on extracellular prefrontal DA at different drug concentrations. Timeline/line graphs (B) showing the effects of reverse dialysis application effects of CGP52432 on extracellular prefrontal DA levels, expressed as mean percent change from baseline (±standard error of the mean) in male (white) and female (black) rats. The solid black line beneath the line graphs in B marks the drug-infusion period. (A) In both sexes, the lowest concentration of CGP52432 (30 μM) had no effect on extracellular DA levels and maximal, seizure-free effects were reached at the 50-μM concentration. (B) Within 30 min of application, CGP52432 (50 μM) increased in prefrontal DA levels in males (white squares) and more so in females (black circles).
Combined NMDA/GABA-B Receptor Antagonism
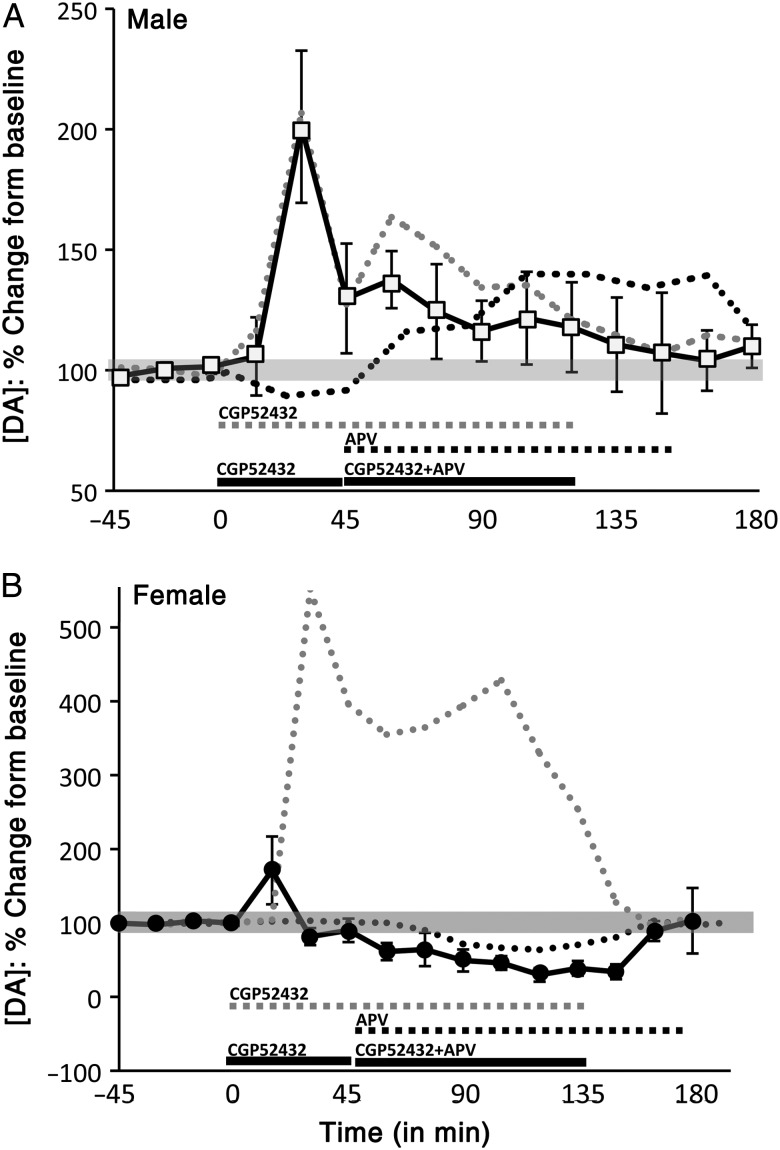
A dual drug reverse dialysis infusion challenge was carried out in which CGP52432 (50 μM) was introduced 45 min prior to adding 500 μM of APV to the dialysate and co-infusing both drugs for an additional 90 min (Fig. 8). In male rats (n = 5), the prior infusion of CGP52432 blocked the expected DA-stimulating actions of APV (Fig. 8A). Thus, 30 min after CGP52432 infusion, DA levels transiently peaked at levels that were about 2-fold higher than baseline but 15 min, these levels dropped back to within roughly 60% of baseline and continued to fall thereafter to near pre-drug baseline levels, despite the infusion of APV (Fig. 8A). In female rats [diestrus (n = 3), proestrus (n = 1), and estrus (n = 1)], the co-infusion protocol had no obvious effects on APV's suppression of PFC DA levels (Fig. 8B). Thus, while CGP52432 administration induced its expected peaks in DA level, when APV co-infusion commenced, PFC DA levels quickly dropped to levels that were 30–40% below baseline that were sustained until drug offset (Fig. 8B). An initial two-way ANOVA (repeated measures) identified significant main effects of sex (F1,10 = 12.609, P = 0.005), significant main effects of drug treatment (F15,150 = 3.114, P < 0.001), and significant interactions between sex and drug treatment (F15,150 = 3.853, P < 0.0001) on PFC DA levels. Within-sex, one-way ANOVA further revealed significant main effects of time/drug on PFC DA levels in both sexes (F10,95 = 3.509–4.737, P ≤ 0.001). Allowed post hoc analyses showed that, in males, only the DA peaks that followed CGP52432 application were significantly higher than baseline (P < 0.001). In females, the CGP52432-induced DA peaks were also significantly higher than baseline (P = 0.065), and following co-infusion of APV, DA levels dropped to concentrations that were nearly significantly lower than baseline (P = 0.077).
Figure 8.
Timelines/line graphs showing the effects of reverse dialysis infusion of CGP52432 followed by co-infusion of CGP52432 and APV (black line) on extracellular prefrontal DA levels, expressed as mean percent change from baseline (±standard error of the mean) in (A) male (white square) and (B) female (black circle) rats. For reference, the effects of infusion of CGP52432 (gray dashed line) and APV (black dashed line) alone are also graphed. (A) In males, initial infusion of CGP52432 alone increased extracellular DA levels as expected (gray dashed line), while the subsequent addition of APV to the infusate failed to increase DA levels as would be expected from APV infusion alone (medium gray dashed line). Rather, DA levels continued to decline as in conditions of CGP52432 infusion alone (gray dashed line). (B) In females, initial infusion of CGP52432 alone also produced expected increases in extracellular DA levels (light gray dashed line). However, the subsequent addition of APV to the infusate decreased DA to depressed levels that were similar to those evoked by APV alone (black dashed line), and that were markedly different from the elevated PFC DA levels associated with CGP52432 infusion (light gray dashed lines).
Discussion
The executive functions that are associated with the PFC are highly sensitive to local, extracellular DA concentration (Murphy et al. 1996; Verma and Moghaddam 1996; Zahrt et al. 1997; Landau et al. 2009; Cools and D'Esposito 2011). The DA levels of the PFC are governed by regulatory circuits and mechanisms that tonically set and phasically re-set PFC DA tone. Not surprisingly, factors that interfere with these processes and move PFC DA levels away from optimal operational settings, for example, stress, drug stimulation, disease (Davis et al. 1991; Deutch 1992; Murphy et al. 1996; Zahrt et al. 1997; Goldberg et al. 2003; Arnsten and Li 2005; Niwa et al. 2010; Dumontheil et al. 2011; Cervenka et al. 2012) negatively impact executive function. Stimulated in part by sex differences that characterize DA-sensitive executive functions and executive dysfunctions related to prefrontal hyper- or hypodopaminergia (Ott et al. 1996; Overman et al. 1996; Szymanski et al. 1996; Leung and Chue 2000; Goldstein et al. 2002; Petry et al. 2002; Canuso and Pandina 2007; Usall et al. 2007; Miller and Cronin-Golomb 2010; Lai et al. 2012; Feinstein and Kritzer 2013), this study asked whether there might be also be dimorphisms in the intracortical GLU mechanisms that regulate PFC DA levels. Recent in vivo microdialysis, reverse dialysis drug application studies showed that the intracortical AMPA and NMDA receptor-mediated GLU mechanisms that are well known to tonically regulate PFC DA levels in the male brain (Feenstra et al. 1995, 2002; Jedema and Moghddam 1996; Takahata and Moghaddam 1998; Aubele and Kritzer 2012) are sensitive to changes in circulating gonadal steroids in adult male rats (Aubele and Kritzer 2012). Here, similar microdialysis/drug challenge approaches were used for the first time to compare the effects of AMPA and NMDA receptor antagonists on extracellular PFC DA levels in male and female rats. These studies showed that the effects of AMPA antagonists on PFC DA levels were indistinguishable across sex. More specifically, AMPA antagonism, which has been repeatedly shown to depress extracellular PFC DA levels in males (Jedema and Moghddam 1996; Jin 1997; Takahata and Moghaddam 1998; Aubele and Kritzer 2012), was found to have qualitatively and quantitatively similar effects in females. In contrast, NMDA antagonism had markedly different effects on PFC DA baseline levels in males and females. Thus, although NMDA antagonism increased extracellular DA in males as expected (Feenstra et al. 1995, 2002; Jedema and Moghddam 1996; Takahata and Moghaddam 1998; Aubele and Kritzer 2012), its infusion in females significantly decreased PFC DA levels. These latter findings suggested that long-held views of intracortical NMDA-mediated influences as being levied on PFC interneurons (Yonezawa et al. 1998; Homayoun and Moghaddam 2007) describe DA regulation in the male, but not in the female PFC. Instead, our data suggest that NMDA antagonism in females acts independently of interneurons in regulating PFC DA levels. This was borne out in co-infusion studies where antagonism of intra-PFC GABAergic signaling blocked the DA-potentiating effects of the NMDA antagonist APV in males, but had no effect on APV's DA-depressing actions in females. In the sections below, these findings along with those from foundation studies examining the effects of intra-PFC infusion of GABA-A and GABA-B antagonists are considered further in relation to the extant literature describing amino acid transmitter regulation of PFC DA tone and are incorporated into sex-specific models of the seemingly disparate ways in which the male and the female PFCs regulate basal DA levels.
Glutamatergic Regulation of PFC DA Levels in the Male and Female Brain
Prefrontal DA-regulating GLU systems were compared across sex using reverse dialysis intra-PFC infusion of the AMPA antagonist NBQX and the NMDA antagonist APV. These along with other antagonists of AMPA and NMDA GLU receptors have been extensively used in prior in vivo microdialysis studies of PFC DA regulation in rats. Carried out exclusively in males, these studies along with data from male subjects of the present all show that, in this sex, local AMPA antagonism depresses extracellular PFC DA levels (Jedema and Moghddam 1996; Takahata and Moghaddam 1998; Del Arco and Mora 1999; Wu et al. 2002; Aubele and Kritzer 2012), and that local NMDA antagonism has opposite, PFC DA-potentiating effects (Feenstra et al. 1995; Jedema and Moghddam 1996; Takahata and Moghaddam 1998; Del Arco and Mora 1999; Aubele and Kritzer 2012). These data have been interpreted as evidence for a tonic AMPA receptor-mediated drive and a tonic NMDA receptor-mediated suppression over mesocortical DA systems and DA overflow in the PFC. Our studies suggest that this is only half correct for the female brain. Thus, while intra-PFC infusion of NBQX dose-dependently decreased extracellular PFC DA levels in both sexes, APV also dose-dependently decreased extracellular PFC DA levels in females—the opposite of its stimulatory actions in males. Thus, while in males intracortical NMDA and AMPA receptor-mediated actions exert opposing, functionally balancing influences over mesocortical DA systems, in females both receptor subtypes appear to tonically drive them.
The DA-depressing actions of APV observed here in females are similar to those previously seen in male rats that have undergone long-term gonadectomy (Aubele and Kritzer 2012). That the effects of gonadectomy (GDX) on APV were attenuated by supplementing GDX rats with testosterone propionate but not estradiol suggests that diminished androgen is responsible for the anomalous drug effects observed (Aubele and Kritzer 2012). It is thus tempting to speculate that the chronically low levels of androgen experienced by the female brain likewise contribute to their sex-unique mode of NMDA-mediated PFC DA homeostasis. Similar to what has been posited for GDX male rats (Aubele and Kritzer 2012), the impact of APV on PFC DA levels in female rats also suggests an NMDA-mediated influence that directly excites PFC pyramidal cells, including those projecting to the ventral midbrain. This is fundamentally different from all current, male-specific evidence of NMDA receptor-mediated effects on PFC DA homeostasis as being translated through GABAergic PFC interneurons (Yonezawa et al. 1998; Homayoun and Moghaddam 2007). These sex-specific circuit schemas are consistent with outcomes from the present CGP52432/APV co-infusion studies, wherein the effects of NMDA antagonism on PFC DA levels were found to be dependent on GABAergic signaling in males, but independent of GABAergic inhibition in females.
The list of activational hormone effects and/or sex differences among NMDA receptors and NMDA receptor-mediated actions in rat cerebral and hippocampal cortices is considerable and continues to grow. These include effects of estrogens and androgens on pyramidal cell spine densities (Gould et al. 1990; Woolley and McEwen 1993; Leranth et al. 2003, 2004; Hajszan et al. 2008), on NMDA receptor numbers, affinities, and/or subunit compositions (Smith and McMahon 2006; Taherianfard et al. 2012; Vedder et al. 2013), and on measures of NMDA-dependent toxicity and synaptic plasticity (Pozzo-Miller et al. 1999; Kajta et al. 2001; de Olmos et al. 2008; Smith et al. 2010). We hypothesize that the sex differences in NMDA-mediated PFC DA homeostatic mechanisms identified in this study have origins in a differential NMDA receptor-mediated activation of ventral tegmental area (VTA)-projecting PFC pyramidal cells. How this occurs is unknown but could be related to differences in genomic androgen actions as these corticofugal neurons are heavily invested with the requisite intracellular receptive machinery in both male and female rats (Aubele and Kritzer 2012). As previously argued for GDX male rats, targets of this genomic activation could include protein kinase C which is known to be activated by AR-dependent androgen signaling (Nguyen et al. 2009) and is capable of stimulating Ca-dependent NMDA receptor deactivation (Lu et al. 2000). However, other alternatives, including impact on NMDA receptor trafficking similar to that recently demonstrated for the neurosteroid pregnenolone (Kostakis et al. 2013), should also be considered.
GABAergic Regulation of PFC DA Levels in the Male and Female Brain
In setting up the dual drug-infusion paradigm, the impact of GABA-A and GABA-B antagonists on PFC DA levels was investigated in male and female rats using reverse dialysis infusion of picrotoxin, bicuculline, and CPG52432. Although sex differences and/or hormone effects have been described in susceptibility to ictal activity including that induced by bicuculline and picrotoxin (Pericic et al. 1996; Bujas et al. 1997; Frye 2008, 2010), we found that seizure-producing thresholds—defined by us as drug concentrations producing piloerection and/or motor twitches in at least some animals over the course of the 2-h drug application period—were similar in males and females for all 3 antagonists. For all 3 as well, these thresholds were substantially lower to half of the drug concentrations used in previous in vivo microdialysis/reverse dialysis studies focused on the male rat PFC, which also found no effects on basal DA levels or on GLU overflow in the ventral tegmentum (Santiago et al. 1993; Harte and O'Connor 2005; Fallon et al. 2007; Balla et al. 2009). Thus, although the range of drug concentrations tested in our studies is low, consensus cross-study findings that subsume a substantially larger range of drug doses at least provisionally suggest that the negative results reported here for both bicuculline and picrotoxin in the male and female PFC may not be due to insufficient receptor occupancy.
Previous studies using drugs including CGP52432 at concentrations of up to 2-fold higher than those used here have also found no effects of GABA-B antagonism on either PFC DA or ventral midbrain GLU levels in the male brain (Takahata and Moghaddam 1998; Harte and O'Connor 2005; Balla et al. 2009). However, our data suggest a tonic GABA-B-mediated suppression of PFC DA levels in both male and female rats. This discrepancy may be related to the fact that, in our hands, GABA-B blockade produced surges in PFC DA concentration that in males appeared and returned to near-baseline levels within about 30 min. Thus, it is possible that the 30-min sampling periods combined with the exclusive use of male subjects in each of the prior studies (Harte and O'Connor 2005; Fallon et al. 2007; Balla et al. 2009) averaged out and obscured these transient GABA-B responses. We also found that CGP52432 produced transient spikes in PFC DA levels in female rats that were significantly larger, and that took significantly longer to return to baseline than those produced in males. In both cases, it must be considered that these peaks are driven by undetected, drug-induced seizure activity in the PFC. However, an alternative explanation is that the surges in the PFC DA level induced by CGP52432 trigger a GABA-B-independent, rectifying response(s). An attractive candidate for this resetting mechanism may be DA activation of intracortical DA D2 receptors. Although DA affects PFC excitability in complex, concentration and receptor subtype-dependent ways (Seamans and Yang 2004; Tseng and O'Donnell 2004; Williams and Castner 2006), in the male brain DA D2 agonists, have been shown to activate fast-spiking PFC interneurons (Tseng and O'Donnell 2004, 2007a, b), to stimulate local GABA overflow (Retaux et al. 1991; Grobin and Deutch 1998), to mediate DA potentiation of GLU-stimulated GABA overflow (Del Arco and Mora 2000), and to inhibit PFC pyramidal cell activity in part via GABA-A sensitive means (Tseng and O'Donnell 2007). These and other D2-mediated actions are concentration-dependent and typically emerge only under conditions of elevated to supranormal DA levels (Zheng et al. 1999; Del Arco and Mora 2000). Moreover, DA D2 stimulation's net inhibitory actions have been shown, to date only in the male brain, to emerge after adolescence (O'Donnell 2010), suggesting intriguing links to circulating gonadal steroids. Given the importance of D2 signaling to PFC network operations (Seamans et al. 2001; Xu et al. 2009; Gruber et al. 2010) and to clinical therapeutics (Laruelle et al. 2005; Masana et al. 2012), it will be important to use indices of cortical excitability and/or additional drug challenge strategies to resolve the basis for the self-limiting spikes in DA level that we observed in males and in females following the intra-PFC infusion of CGP52432.
Conclusion
A large body of work sums to identify tonic intracortical AMPA-mediated stimulation of VTA-projecting PFC pyramidal cells that drives mesoprefrontal DA neurons and keeps PFC DA levels elevated and an opposing intracortical NMDA-mediated drive of GABAergic PFC interneurons that inhibits VTA-projecting pyramidal cells and holds PFC DA levels in check (Feenstra et al. 1995; Jedema and Moghddam 1996; Takahata and Moghaddam 1998; Del Arco and Mora 1999; Wu et al. 2002; Aubele and Kritzer 2012). Taken together, these mechanisms form bases for contemporary computational models of PFC function (Berridge and Robinson 1998; Durstewitz et al. 2000; Durstewitz and Seamans 2002; O'Donnell 2003; Seamans and Yang 2004) that are strongly influential in shaping etiologic thinking about mental illness and its treatment (Lewis and Moghaddam 2006; Seeman 2009; Javitt 2010; Moghaddam and Javitt 2012; Stan and Lewis 2012). However, the foundation studies all share limitations of only examining male subjects. The present comparative studies add new information about NMDA receptor-mediated DA homeostatic mechanisms that appear to be oppositely tuned in the female compared with male PFC, and about tonic, DA-suppressing GABA-B receptor-mediated effects that appear to be significantly larger in females compared with males. It may be important to consider these findings further in 2 contexts. The first is the sexually dimorphic mesoprefrontal projections, where DAergic cells of origin are roughly 2 times more numerous in female rats than in males (Swanson 1982; Deutch et al. 1991; Carr and Sesack 2000; Margolis et al. 2006; Kritzer and Creutz 2008). The second is basal PFC DA concentrations that are by most accounts similar across sex (Tanila et al. 1994; Duchesne et al. 2009). From these we hypothesize that, in females, potent intra-PFC, DA-facilitating GLU influences, uniquely conferred by both of its major classes of cortical ionotropic receptors, drive mesoprefrontal systems that are characterized by a doubling of constituent DA cells of origin relative to males. While this could potentiate DA levels in the female compared with male PFC, findings suggesting a significantly more powerful GABA-B-mediated DA inhibition in females could aid in maintaining basal DA concentrations that are more similar across sex. These sex-specific means of PFC DA homeostasis may also shape the sex differences that differentiate PFC functions in humans and animals and also shed new light on the neurobiology relevant to females' disproportionate vulnerability to PTSD and other anxiety disorders associated with PFC hyperdopaminergia, and their relative protection from cognitive deficits associated with PFC hypodopaminergia in disorders such as schizophrenia.
Funding
This work was supported by the National Institute of Neurological Disorder and Stroke at the National Institutes of Health (RO1-NS41966 to M.F.K.).
Notes
Conflict of Interest: None declared.
References
- Arnsten AF. 2009. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 23(Suppl 1):33–41. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. 2005. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 57:1377–1384. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF. 2012. Androgen influence on prefrontal dopamine systems in adult male rats: localization of cognate intracellular receptors in medial prefrontal projections to the ventral tegmental area and effects of gonadectomy and hormone replacement on glutamate-stimulated extracellular dopamine level. Cereb Cortex. 22:1799–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aultman JM, Moghaddam B. 2001. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology (Berl). 153:353–364. [DOI] [PubMed] [Google Scholar]
- Balla A, Nattini ME, Sershen H, Lajtha A, Dunlop DS, Javitt DC. 2009. GABAB/NMDA receptor interaction in the regulation of extracellular dopamine levels in rodent prefrontal cortex and striatum. Neuropharmacology. 56:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. 1998. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 28:309–369. [DOI] [PubMed] [Google Scholar]
- Bujas M, Pericic D, Jazvinscak M. 1997. Influence of gender and gonadectomy on bicuculline-induced convulsions and on GABAA receptors. Brain Res Bull. 43:411–416. [DOI] [PubMed] [Google Scholar]
- Canuso CM, Pandina G. 2007. Gender and schizophrenia. Psychopharmacol Bull. 40:178–190. [PubMed] [Google Scholar]
- Carr DB, Sesack SR. 2000. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 38:114–123. [DOI] [PubMed] [Google Scholar]
- Cervenka S, Hedman E, Ikoma Y, Djurfeldt DR, Ruck C, Halldin C, Lindefors N. 2012. Changes in dopamine D2-receptor binding are associated to symptom reduction after psychotherapy in social anxiety disorder. Transl Psychiatry. 2:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. 2011. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 69:e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K. 2005. Don't worry ‘B’ happy! A role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 26:36–43. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, Daskas S, Papadopoulou-Daifoti Z. 2008. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav. 93:595–605. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. 2004. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 28:771–784. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. 1991. Dopamine in schizophrenia—a review and reconceptualization. Am J Psychiatry. 148:1474–1486. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. 1999. Effects of endogenous glutamate on extracellular concentrations of GABA, dopamine, and dopamine metabolites in the prefrontal cortex of the freely moving rat: involvement of NMDA and AMPA/KA receptors. Neurochem Res. 24:1027–1035. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. 2000. Endogenous dopamine potentiates the effects of glutamate on extracellular GABA in the prefrontal cortex of the freely moving rat. Brain Res Bull. 53:339–345. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. 2002. NMDA and AMPA/kainate glutamatergic agonists increase the extracellular concentrations of GABA in the prefrontal cortex of the freely moving rat: modulation by endogenous dopamine. Brain Res Bull. 57:623–630. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Ronzoni G, Mora F. 2011. Prefrontal stimulation of GABAA receptors counteracts the corticolimbic hyperactivity produced by NMDA antagonists in the prefrontal cortex of the rat. Psychopharmacology (Berl). 214:525–536. [DOI] [PubMed] [Google Scholar]
- de Olmos S, Bueno A, Bender C, Lorenzo A, de Olmos J. 2008. Sex differences and influence of gonadal hormones on MK801-induced neuronal degeneration in the granular retrosplenial cortex of the rat. Brain Struct Funct. 213:229–238. [DOI] [PubMed] [Google Scholar]
- Deutch AY. 1992. The regulation of subcortical dopamine systems by the prefrontal cortex: interactions of central dopamine systems and the pathogenesis of schizophrenia. J Neural Transm Suppl. 36:61–89. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ. 1991. Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cereb Cortex. 1:273–292. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B. 1997. Cognitive deficits in Parkinson's disease. J Neurol. 244:2–8. [DOI] [PubMed] [Google Scholar]
- Duchesne A, Dufresne MM, Sullivan RM. 2009. Sex differences in corticolimbic dopamine and serotonin systems in the rat and the effect of postnatal handling. Prog Neuropsychopharmacol Biol Psychiatry. 33:251–261. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Roggeman C, Ziermans T, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T. 2011. Influence of the COMT genotype on working memory and brain activity changes during development. Biol Psychiatry. 70:222–229. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. 2002. The computational role of dopamine D1 receptors in working memory. Neural Netw. 15:561–572. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. 2000. Neurocomputational models of working memory. Nat Neurosci. 3(Suppl):1184–1191. [DOI] [PubMed] [Google Scholar]
- Eisenberg DP, Berman KF. 2010. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 35:258–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon S, Shearman E, Sershen H, Lajtha A. 2007. The effects of glutamate and GABA receptor antagonists on nicotine-induced neurotransmitter changes in cognitive areas. Neurochem Res. 32:535–553. [DOI] [PubMed] [Google Scholar]
- Faraji J, Metz GA, Sutherland RJ. 2010. Characterization of spatial performance in male and female Long-Evans rats by means of the Morris water task and the ziggurat task. Brain Res Bull. 81:164–172. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH, van Uum JF. 2002. Behavioral arousal and increased dopamine efflux after blockade of NMDA-receptors in the prefrontal cortex are dependent on activation of glutamatergic neurotransmission. Neuropharmacology. 42:752–763. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, van der Weij W, Botterblom MH. 1995. Concentration-dependent dual action of locally applied N-methyl-d-aspartate on extracellular dopamine in the rat prefrontal cortex in vivo. Neurosci Lett. 201:175–178. [DOI] [PubMed] [Google Scholar]
- Feinstein I, Kritzer MF. 2013. Acute N-methyl-d-aspartate receptor hypofunction induced by MK801 evokes sex-specific changes in behaviors observed in open-field testing in adult male and proestrus female rats. Neuroscience. 228:200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejgin K, Palsson E, Wass C, Finnerty N, Lowry J, Klamer D. 2009. Prefrontal GABA(B) receptor activation attenuates phencyclidine-induced impairments of prepulse inhibition: involvement of nitric oxide. Neuropsychopharmacology. 34:1673–1684. [DOI] [PubMed] [Google Scholar]
- Frye CA. 2010. Effects and mechanisms of progestogens and androgens in ictal activity. Epilepsia. 51(Suppl 3):135–140. [DOI] [PubMed] [Google Scholar]
- Frye CA. 2008. Hormonal influences on seizures: basic neurobiology. Int Rev Neurobiol. 83:27–77. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. 2003. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 60:889–896. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. 2007. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 80:84–97. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Crawford HT, Stokes LP, Galkin TW, Rosvold HE. 1974. Sex-dependent behavioral effects of cerebral cortical lesions in the developing rhesus monkey. Science. 186:540–542. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Funahashi S, Bruce CJ. 1990. Neocortical memory circuits. Cold Spring Harb Symp Quant Biol. 55:1025–1038. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Cohen LS, Horton NJ, Lee H, Andersen S, Tohen M, Crawford A, Tollefson G. 2002. Sex differences in clinical response to olanzapine compared with haloperidol. Psychiatry Res. 110:27–37. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. 2012. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 38:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. 1990. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 10:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin AC, Deutch AY. 1998. Dopaminergic regulation of extracellular gamma-aminobutyric acid levels in the prefrontal cortex of the rat. J Pharmacol Exp Ther. 285:350–357. [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O'Donnell P. 2010. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 30:17102–17110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. 2008. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm Behav. 53:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte M, O'Connor WT. 2005. Evidence for a selective prefrontal cortical GABA(B) receptor-mediated inhibition of glutamate release in the ventral tegmental area: a dual probe microdialysis study in the awake rat. Neuroscience. 130:215–222. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. 2007. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 27:11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. 2004. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci USA. 101:8467–8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. 2007. Glutamate and schizophrenia: phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 78:69–108. [DOI] [PubMed] [Google Scholar]
- Javitt DC. 2010. Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci. 47:4–16. [PubMed] [Google Scholar]
- Jedema HP, Moghddam B. 1996. Characterization of excitatory amino acid modulation of dopamine release in the prefrontal cortex of conscious rats. J Neurochem. 66:1448–1453. [DOI] [PubMed] [Google Scholar]
- Jin S. 1997. AMPA- and kainate-receptors differentially mediate excitatory amino acid-induced dopamine and acetylcholine release from rat striatal slices. Neuropharmacology. 36:1503–1510. [DOI] [PubMed] [Google Scholar]
- Kajta M, Budziszewska B, Marszal M, Lason W. 2001. Effects of 17-beta estradiol and estriol on NMDA-induced toxicity and apoptosis in primary cultures of rat cortical neurons. J Physiol Pharmacol. 52:437–446. [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. 2010. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 83:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer C, Maziashvili N, Dugladze T, Gloveli T. 2008. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci. 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostakis E, Smith C, Jang MK, Martin SC, Richards KG, Russek SJ, Gibbs TT, Farb DH. 2013. The neuroactive steroid pregnenolone sulfate stimulates trafficking of functional N-methyl d-aspartate receptors to the cell surface via a noncanonical, G protein, and Ca2+-dependent mechanism. Mol Pharmacol. 84:261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM. 2008. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 28:9525–9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG, Killiany RJ, Rosene DL, Moss MB. 1999. Spatial cognition in rhesus monkeys: male superiority declines with age. Horm Behav. 36:70–76. [DOI] [PubMed] [Google Scholar]
- Lai DC, Tseng YC, Hou YM, Guo HR. 2012. Gender and geographic differences in the prevalence of autism spectrum disorders in children: analysis of data from the national disability registry of Taiwan. Res Dev Disabil. 33:909–915. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ. 2009. Striatal dopamine and working memory. Cereb Cortex. 19:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Frankle WG, Narendran R, Kegeles LS, Abi-Dargham A. 2005. Mechanism of action of antipsychotic drugs: from dopamine D(2) receptor antagonism to glutamate NMDA facilitation. Clin Ther. 27(Suppl A):S16–S24. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. 2004. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 24:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. 2003. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 23:1588–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Chue P. 2000. Sex differences in schizophrenia: a review of the literature. Acta Psychiatr Scand Suppl. 401:3–38. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. 2012. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. 2006. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 63:1372–1376. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. 1999. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry. 46:616–626. [DOI] [PubMed] [Google Scholar]
- Lu WY, Jackson MF, Bai D, Orser BA, MacDonald JF. 2000. In CA1 pyramidal neurons of the hippocampus protein kinase C regulates calcium-dependent inactivation of NMDA receptors. J Neurosci. 20:4452–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. 2002. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 62:609–614. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. 2006. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci USA. 103:2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masana M, Santana N, Artigas F, Bortolozzi A. 2012. Dopamine neurotransmission and atypical antipsychotics in prefrontal cortex: a critical review. Curr Top Med Chem. 12:2357–2374. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Stip E. 2011. Sexual dimorphism in schizophrenia: is there a need for gender-based protocols? Expert Rev Neurother. 11:951–959. [DOI] [PubMed] [Google Scholar]
- Miller IN, Cronin-Golomb A. 2010. Gender differences in Parkinson's disease: clinical characteristics and cognition. Mov Disord. 25:2695–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. 2012. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 37:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. 1996. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 93:1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Rodnitzky RL, Uc EY. 2013. Prefrontal dopamine signaling and cognitive symptoms of Parkinson's disease. Rev Neurosci. 24:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ. 2009. Dihydrotestosterone activates CREB signaling in cultured hippocampal neurons. Brain Res. 1298:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, Lu L, Tomisato S, Jaaro-Peled H, Seshadri S, et al. 2010. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 65:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. 2010. Adolescent maturation of cortical dopamine. Neurotox Res. 18:306–312. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. 2003. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 17:429–435. [DOI] [PubMed] [Google Scholar]
- Ortiz-Gil J, Pomarol-Clotet E, Salvador R, Canales-Rodriguez EJ, Sarro S, Gomar JJ, Guerrero A, Sans-Sansa B, Capdevila A, Junque C, et al. 2011. Neural correlates of cognitive impairment in schizophrenia. Br J Psychiatry. 199:202–210. [DOI] [PubMed] [Google Scholar]
- Ott BR, Tate CA, Gordon NM, Heindel WC. 1996. Gender differences in the behavioral manifestations of Alzheimer's disease. J Am Geriatr Soc. 44:583–587. [DOI] [PubMed] [Google Scholar]
- Overman WH, Bachevalier J, Schuhmann E, Ryan P. 1996. Cognitive gender differences in very young children parallel biologically based cognitive gender differences in monkeys. Behav Neurosci. 110:673–684. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1998. The rat brain in stereotaxic coordinates. San Diego: Academic Press. [DOI] [PubMed] [Google Scholar]
- Pericic D, Manev H, Bujas M. 1996. Gonadal hormones and picrotoxin-induced convulsions in male and female rats. Brain Res. 736:174–179. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. 2002. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol. 63:83–90. [PubMed] [Google Scholar]
- Povysheva NV, Johnson JW. 2012. Tonic NMDA receptor-mediated current in prefrontal cortical pyramidal cells and fast-spiking interneurons. J Neurophysiol. 107:2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Inoue T, Murphy DD. 1999. Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices. J Neurophysiol. 81:1404–1411. [DOI] [PubMed] [Google Scholar]
- Retaux S, Besson MJ, Penit-Soria J. 1991. Opposing effects of dopamine D2 receptor stimulation on the spontaneous and the electrically evoked release of [3H]GABA on rat prefrontal cortex slices. Neuroscience. 42:61–71. [DOI] [PubMed] [Google Scholar]
- Roof RL, Zhang Q, Glasier MM, Stein DG. 1993. Gender-specific impairment on Morris water maze task after entorhinal cortex lesion. Behav Brain Res. 57:47–51. [DOI] [PubMed] [Google Scholar]
- Santiago M, Machado A, Cano J. 1993. Regulation of the prefrontal cortical dopamine release by GABAA and GABAB receptor agonists and antagonists. Brain Res. 630:28–31. [DOI] [PubMed] [Google Scholar]
- Scott L, Aperia A. 2009. Interaction between N-methyl-d-aspartic acid receptors and D1 dopamine receptors: an important mechanism for brain plasticity. Neuroscience. 158:62–66. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. 2001. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 21:3628–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. 2004. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 74:1–58. [DOI] [PubMed] [Google Scholar]
- Seeman P. 2009. Glutamate and dopamine components in schizophrenia. J Psychiatry Neurosci. 34:143–149. [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. 2006. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 26:8517–8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. 2010. Duration of estrogen deprivation, not chronological age, prevents estrogen's ability to enhance hippocampal synaptic physiology. Proc Natl Acad Sci USA. 107:19543–19548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan A, Lewis DA. 2012. Altered cortical GABA neurotransmission in schizophrenia: insights into novel therapeutic strategies. Curr Pharm Biotechnol. 13:1557–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. 1982. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 9:321–353. [DOI] [PubMed] [Google Scholar]
- Szymanski S, Lieberman J, Pollack S, Kane JM, Safferman A, Munne R, Umbricht D, Woerner M, Masiar S, Kronig M. 1996. Gender differences in neuroleptic nonresponsive clozapine-treated schizophrenics. Biol Psychiatry. 39:249–254. [DOI] [PubMed] [Google Scholar]
- Taherianfard M, Sharifi M, Tadjali M, Kohkiloezadeh M. 2012. Modulation of NR1 subunit of N-methyl-d-aspartate receptor by ovariectomy and passive avoidance learning. Iran J Neurol. 11:140–145. [PMC free article] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. 1998. Glutamatergic regulation of basal and stimulus-activated dopamine release in the prefrontal cortex. J Neurochem. 71:1443–1449. [DOI] [PubMed] [Google Scholar]
- Tandon PN. 2013. Not so "silent": the human prefrontal cortex. Neurol India. 61:578–580. [DOI] [PubMed] [Google Scholar]
- Tanila H, Taira T, Piepponen TP, Honkanen A. 1994. Effect of sex and age on brain monoamines and spatial learning in rats. Neurobiol Aging. 15:733–741. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. 1996. Antagonism of NMDA receptors but not AMPA/kainate receptors blocks bursting in dopaminergic neurons induced by electrical stimulation of the prefrontal cortex. J Neural Transm. 103:889–904. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. 2004. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 24:5131–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. 2007. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 61:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usall J, Suarez D, Haro JM. 2007. Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Res. 153:225–231. [DOI] [PubMed] [Google Scholar]
- Vedder LC, Smith CC, Flannigan AE, McMahon LL. 2013. Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus. 23:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. 1996. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 16:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Neubauer FB, Luscher HR, Thurley K. 2010. GABAB receptor-dependent modulation of network activity in the rat prefrontal cortex in vitro. Eur J Neurosci. 31:1582–1594. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. 2006. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 139:263–276. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 336:293–306. [DOI] [PubMed] [Google Scholar]
- Woolley DG, Vermaercke B, Op de Beeck H, Wagemans J, Gantois I, D'Hooge R, Swinnen SP, Wenderoth N. 2010. Sex differences in human virtual water maze performance: novel measures reveal the relative contribution of directional responding and spatial knowledge. Behav Brain Res. 208:408–414. [DOI] [PubMed] [Google Scholar]
- Wu WR, Li N, Sorg BA. 2002. Regulation of medial prefrontal cortex dopamine by alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptors. Neuroscience. 114:507–516. [DOI] [PubMed] [Google Scholar]
- Xu TX, Sotnikova TD, Liang C, Zhang J, Jung JU, Spealman RD, Gainetdinov RR, Yao WD. 2009. Hyperdopaminergic tone erodes prefrontal long-term potential via a D2 receptor-operated protein phosphatase gate. J Neurosci. 29:14086–14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H. 1998. Involvement of gamma-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol. 341:45–56. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. 1997. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 17:8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. 2003. A review of the cognitive and behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry. Cogn Behav Neurol. 16:193–210. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX. 1999. Opposite modulation of cortical N-methyl-d-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience. 91:527–535. [DOI] [PubMed] [Google Scholar]