Abstract
Unlike adult damage, neonatal damage to the inferior prefrontal convexity (IC) in monkeys spares learning and performance on the delayed nonmatching-to-sample (DNMS) task ( Málková et al. 2000). We investigated whether this sparing was due to compensation by undamaged orbital frontal cortex (O), an area also critical for DNMS, by comparing combined IC and O damage (Neo-ICO) with damage to O alone (Neo-O). Group Neo-ICO was impaired on DNMS learning at 3 months and 2 years of age. In contrast, Group Neo-O was impaired at 3 months, but recovered this function by 2 years, compared with Neo-IC and controls (N). We propose that the intact IC assumed the function of learning the DNMS rule for Group Neo-O. The persistent impairment after Neo-ICO lesions suggests that whereas O may likely support the rule acquisition in the absence of IC, no compensatory mechanisms are available after the combined damage. For the memory of lists of items, all groups were impaired at 3 months. At 2 years, the performance of Groups N and Neo-IC dramatically improved, whereas that of groups with O damage (Neo-O and Neo-ICO) remained impaired, indicating a critical role for O in recognition memory that cannot be substituted by another area.
Keywords: lesion, memory, recognition, rhesus macaque
Introduction
In nonhuman primates, object recognition memory, as measured by the delayed nonmatching-to-sample (DNMS) task, critically depends on the interaction between temporal cortical areas (TE and perirhinal cortex) and frontal cortical areas. Anatomical studies have shown that TE and perirhinal cortex are strongly connected with the inferior prefrontal convexity (IC) and the orbital frontal cortex (O) (Cavada et al. 2000; Rempel-Clower Barbas 2000; Lavenex et al. 2002; Suzuki and Amaral 2004; Saunders et al. 2005). In addition, both electrophysiological and lesion studies have demonstrated that area TE and perirhinal cortex (Fuster et al. 1981; Miller et al. 1991; Li et al. 1993; Meunier et al. 1993; Suzuki et al. 1993) as well as prefrontal areas, IC and O (Bauer and Fuster 1978; Fuster et al. 1985; Kowalska et al. 1991; Meunier et al. 1997; Hirabayashi and Miyashita 2014), are critically involved in DNMS rule learning and memory. Furthermore, lesion studies disconnecting temporo-prefrontal regions severely impaired recognition memory (Parker and Gaffan 1998).
In monkeys, learning of the DNMS rule and performance on the task is developmentally protracted; neonatal and juvenile monkeys do not reach the adult level of proficiency until they are fully mature (i.e., 4–5 years; Bachevalier and Mishkin 1984; Bachevalier 1990). This protracted development is likely due to the prolonged postnatal maturation of the temporo-prefrontal network. In addition to immaturity early in life of several cortical areas within this network (e.g., TE, perirhinal cortex, prefrontal cortex; Goldman 1971; Berger and Alvarez 1994; Rodman 1994; Webster, Bachevalier, Ungerleider 1995; Webster, Ungerleider, Bachevalier 1995; Malkova et al. 2006; Tsujimoto 2008; Knickmeyer et al. 2010), a contributing factor may be the extended period of maturation of the uncinate fasciculus, which contains most of the temporo-frontal connections (Lebel et al. 2008). Although transection of this pathway once the network is mature does not impair visual recognition memory (Gaffan and Eacott 1995), it is possible that the protracted maturation of the uncinate fasciculus contribute to the late development of the whole temporo-prefrontal network. Further support for the delayed maturation of this network comes from the differential effects of damage to different nodes within this network occurring early when compared with later in life.
One of the proposed outcomes of the effects of early cortical lesions (Goldman 1971; Kolb et al. 2010) on cognitive development is as follows. If the immaturity of a given cortical area is a critical factor preventing normal infant monkeys from performing a task with adult proficiency, then infants with neonatal lesions of the area are expected to show no impairment compared with normal age-matched controls when tested early in life. As the cortical area matures, performance of normal monkeys is expected to gradually improve; however, the performance of monkeys with lesions of this cortical area is not expected to improve, rather, the monkeys are likely to demonstrate an impairment.
An alternative possible outcome is that damage to structures that are not functionally mature at the time of lesion results in long-term sparing of function. For example, neonatal damage to temporal cortical area TE produced long-term sparing in learning and performance on the DNMS task, whereas this same damage in adults severely impaired it (Mishkin and Philips 1990; Buffalo et al. 1999). A likely explanation for the described sparing is the existence of compensatory mechanisms, such as neural reorganization, that provide alternate circuitry to assume the function (e.g., Webster, Bachevalier, Ungerleider 1995; Webster, Ungerleider, Bachevalier 1995). Thus, the failure of neonatal TE lesions to produce a DNMS impairment might have resulted from compensatory mechanisms provided by other cortical areas in the temporal lobe and/or the spared prefrontal regions, such as the IC, which are also part of the adult network subserving this behavior.
To test this hypothesis, we previously compared neonatal damage to either IC or the medial temporal lobe structures on monkeys' rule learning and performance on DNMS at 3 months and 2 years of age (Málková et al. 2000). Neonatal damage to the medial temporal lobe (including the perirhinal and parahippocampal cortices) impaired learning and performance at both ages, consistent with earlier findings (Bachevalier and Mishkin 1994; Málková et al. 1995), indicating that compensatory mechanisms following these large medial temporal lesions are limited at best. In contrast, early lesions of the IC did not impair rule learning or performance at 3 months of age and only mildly affected rule learning at 2 years (Málková et al. 2000). This result was surprising, as damage to IC in adults markedly impaired the ability to learn the nonmatching rule (Kowalska et al. 1991). Thus, the functional sparing after the early IC lesions might be due to other, intact, prefrontal regions assuming the role of IC, reminiscent of previously observed compensatory mechanisms within the temporal cortex (Webster et al. 1991). One candidate for assuming the functions of the damaged IC is the O. Adult monkeys with damage to the O were severely impaired both on DNMS rule learning and the subsequent performance test (Meunier et al. 1997), suggesting that this area is critical for both learning (and/or retention) of the rule and recognition memory. Similarly, cooling of this region reduced performance on DNMS to chance levels even at short delays (Voytko 1985), a finding also consistent with difficulty in applying the DNMS rule. Thus, both the IC and O critically contribute to normal adult DNMS learning and performance.
In light of the above findings, recovery of function from neonatal damage to the IC might have been achieved by compensatory mechanisms in the O. This area matures earlier than the lateral prefrontal cortex (Goldman 1971; Orzhekhovskaya 1981; Fuster 2002; Tsujimoto 2008) and can potentially assume the rule learning and memory functions, even in the absence of IC. If this were the case, then the combined early damage to both areas (IC + O) would result in impairment. In the present study, we tested this hypothesis by assessing the effects of combined neonatal removals of IC + O when compared with those of neonatal IC damage alone from the previous study (Málková et al. 2000). We also aimed to determine whether neonatal damage to O alone would be sufficient to produce impairment.
Materials and Methods
Subjects
Subjects were 18 rhesus macaques (Macaca mulatta) of both sexes. All subjects were born at the National Institute of Health Veterinary Resources Branch (Bethesda, MD, USA). At birth, they were removed from their mothers and brought to the primate nursery of the Laboratory of Neuropsychology, NIMH, where they were raised. Data from 8 animals have already been published (Málková et al. 2000) and consisted of 4 cases in Group Neo-IC (IC-1–IC-4; 2 males and 2 females) that had received neonatal aspiration lesions of the inferior prefrontal convexity and 4 normal controls (N-1–N-4; 2 males and 2 females). They are used here for comparisons with 10 new monkeys prepared for the present study: 4 animals with combined neonatal lesions of the inferior convexity and orbital frontal cortex (Group Neo-ICO, 4 females), 4 animals with neonatal lesions of the orbital frontal cortex alone (Group Neo-O, 2 males and 2 females), and 2 naïve controls (N-5 and N-6, both females), which were added to the control group. Details of hand-raising and housing of these subjects have been described elsewhere (Málková et al. 2000) and were identical for the new animals added to the present study. All monkeys were trained and tested on the DNMS task at 3 months and re-tested on the same task at 2 years of age. One case in Group Neo-O (Neo-O4) had to be sacrificed due to illness before the 2-year retest; thus, data for this case are only included in the statistical analyses at 3 months.
The study was conducted under a protocol approved by the Institutional Animal Care and Use Committee at the National Institute of Mental Health and in accordance with the Guide for Care and Use of Laboratory Animals.
Surgery
As described previously (Málková et al. 2000), aspiration lesions were performed in 2 stages when the monkeys were 1 and 3 weeks old. All procedures were done under aseptic conditions with the aid of a surgical microscope while the animals were under anesthesia. Anesthesia was induced by a 1:10 mixture of acepromazine–ketamine (15 mg/kg, intramuscular), followed either by administration of diazepam (1 mg/kg, intramuscular) and ketamine to effect (all animals in the Group ICO) or by isoflurane gas (1–2% to effect; all animals in the Group O) administered via an endotracheal tube as this technique became available for surgeries in infant monkeys. Postoperative analgesia was given under the supervision of the institutional veterinarians. Removal of the orbital frontal cortex in Group Neo-O included all cortical areas located between the medial lip of the lateral orbital sulcus, laterally, and the ventral lip of the rostral sulcus, medially. The lesion extended posteriorly to the lateral olfactory stria and anteriorly to a line joining the anterior tips of the medial and lateral orbital sulci. Thus, the damage in this group included areas 10, 11, 13, 14, and 25 of the orbital frontal cortex (Fig. 1, intended damage). For Group Neo-ICO, the damage included the entire extent of the orbital frontal lesion and extended on the lateral surface to include the IC. The removal of the IC included the tissue bordered ventrally by the lateral orbital sulcus and dorsally by a line immediately below and parallel to the ventral lip of the principal sulcus. The lesion extended posteriorly to the fundus of the inferior arcuate sulcus, and anteriorly to the frontal pole. Thus, the damage included prefrontal areas 6, 8, 10, 12, and portions of ventral 46 (Fig. 2, intended damage).
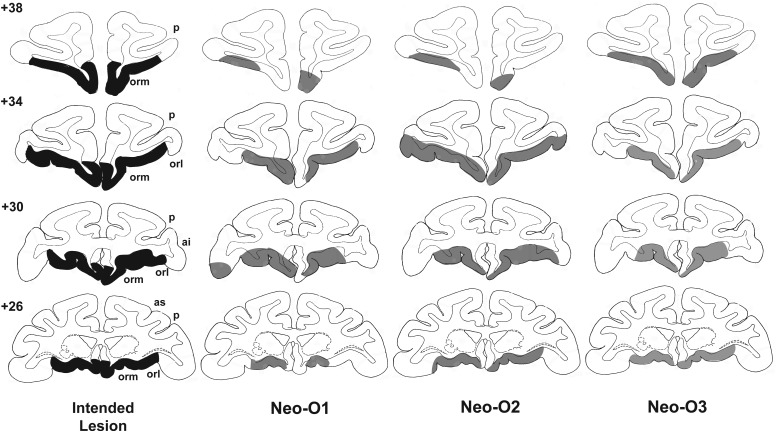
Figure 1.
Intended lesions of the orbital frontal cortex (shown in dark gray, left column) and reconstruction of the actual damage (in light gray) for the 3 cases in Group O onto standard coronal sections of a normal monkey brain. The numerals on the left of the coronal sections indicate the distance in millimeters from the interaural plane. ai, inferior arcuate sulcus; as, superior arcuate sulcus; orl, lateral orbital sulcus; orm, medial orbital sulcus; p, principal sulcus.
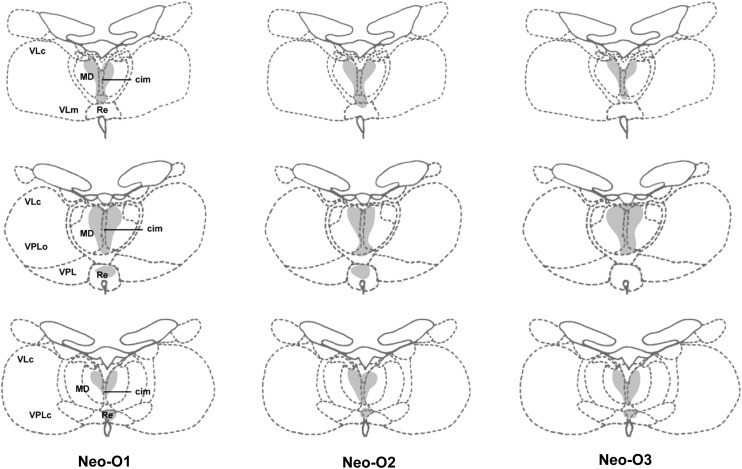
Figure 2.
Retrograde degeneration in the thalamus following orbital frontal cortex lesions for the 3 cases in Group O. Areas of moderate degeneration are shown in light shading. cim, central intermedial; MD, medial dorsal thalamus; Re, reuniens; VLc, ventral lateral caudal part; VLm, ventral lateral, medial part; VPLo, ventral posterior, lateral oral part; VPLc, ventral posterior, lateral caudal part; VPL, ventral posterior lateral.
Histology
On the completion of behavioral testing, subjects in Groups Neo-O and Neo-ICO were given a lethal dose of sodium pentobarbital and perfused intracardially with normal saline followed by 10% formalin. The brains were then removed, postfixed in 30% sucrose-formalin, frozen and sliced in a freezing microtome at 50 μm, mounted, and stained with thionin. Estimates of the extent of lesion were plotted at 1-mm intervals onto standardized, coronal drawings of the normal macaque brain. Surface reconstructions of the lesions on the lateral and ventral views of the brain were derived from the extent of lesion on each coronal section. Finally, the extent of retrograde thalamic degeneration was plotted on coronal sections of the normal macaque brain.
Apparatus
The testing apparatus and objects for DNMS training have been described in detail elsewhere (Málková et al. 2000). Briefly, DNMS training at 3 months of age was conducted in a reduced version of the standard Wisconsin General Testing Apparatus (WGTA) located in a darkened, sound-attenuated room with white noise. The testing tray contained 3 food wells, 10 cm apart, center-to-center, and aligned 6 cm from the front of the testing cage, and stimuli were drawn from a pool of 300 objects. Rewards were 190-mg banana pellets (P. J. Noyes, Lancaster, NH, USA). Training on DNMS at 2 years of age was conducted in the standard WGTA, with a pool of 2000 larger objects, which were all novel for the animals. The testing tray consisted of 3 food wells, 14 cm apart, and center-to-center. Rewards for 2-year-old monkeys were 300-mg banana pellets.
DNMS Training
As described in Málková et al. (2000), infant monkeys were first adapted to the WGTA and trained to displace objects to retrieve rewards hidden in the food wells. Formal training trials consisted of a sample phase, in which the monkey was presented with an object covering the central, baited food well, followed 5 s later by a choice phase. In the choice phase, the sample object covered one, unbaited, lateral well and was paired with a novel object covering the opposite, baited well. After the monkey made its choice, a 30-s intertrial interval preceded the next trial. Each animal received 20 such trials per day, using new stimuli for each trial, until the animal reached a performance criterion of 90 correct responses in 100 consecutive choices (across 5 days). Following a 2-week rest period, each animal was retrained to criterion on the DNMS rule, and then given a memory test in which increasing delays of 10, 30, 60, or 120 s separated the sample from the choice phases (100 trials at each delay). Finally, the animals were given lists of 3, 5, or 10 objects, with each object presented one at a time at 20 s intervals; each list item was then paired with a novel one. The pairs were also presented at 20 s intervals. Each list length was tested successively for a block of 150 trials.
Retraining at 2 years of age followed the same procedure until the monkeys reached again the 90% criterion. They were then given a 2-week rest period, retrained to criterion and given the memory performance test with the delays and list lengths.
Results
Lesion Assessment
Reconstructions of the lesions for Groups Neo-IC have been previously published (Málková et al. 2000, see Figs 1 and 2, Group IC). The neonatal IC lesions were largely as intended, with a minor unintended damage in a few cases dorsally, and unilaterally to area 46, and posteriorly and ventrally to area 6. Retrograde thalamic degeneration following Neo-IC lesions (Fig. 4 from Málková et al. 2000) included mainly the ventrolateral portion of the parvocellular division of the medial dorsal nucleus and minor cell loss in the midline nuclei (i.e., central inferior and caudal paraventricular).
Damage in Group Neo-O was largely as intended in all cases (Fig. 1). Minor unilateral sparing was found in the most medial portion of area 25/14 in one case (Neo-O1, levels +26 to +34) and in lateral area 13 in another (Neo-O3, level +30). In Neo-O2, the lesion encroached bilaterally on area 12, including some damage to the underlying white matter unilaterally (Fig. 1, level +34). Retrograde thalamic degeneration in the 3 cases was found in all cases (Fig. 2), with moderate bilateral cell loss in the dorsomedial portion of the magnocellular division of the medial dorsal nucleus. Partial cell loss could also be detected in all cases in the central intermedial nuclei as well as in the medial portion of the reuniens nucleus. The distribution of the retrograde degeneration in Group Neo-O thus corresponds to the nuclei that are known to be the main sources of thalamic inputs to orbital frontal cortex (Goldman-Rakic and Porrino 1985; Barbas et al. 1991; Morecraft et al. 1992; Ray and Price 1993).
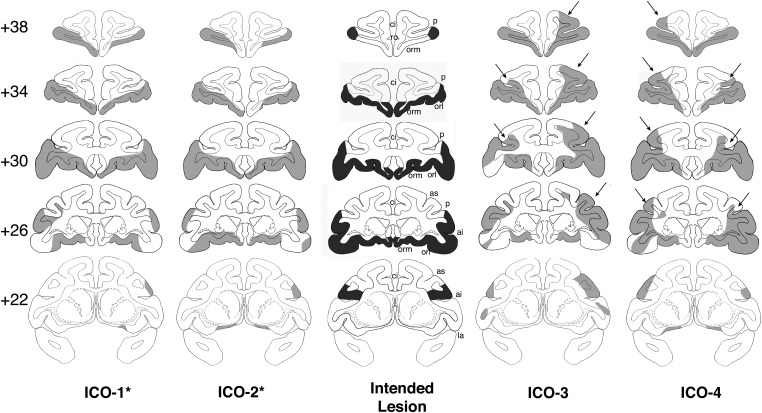
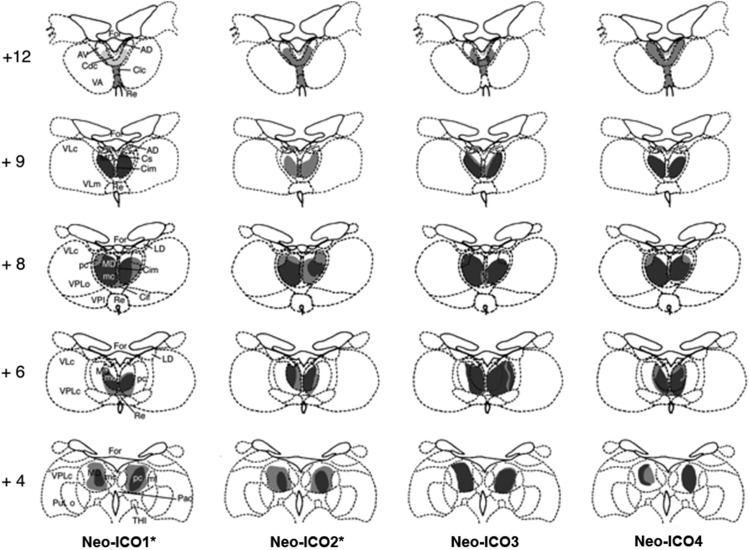
Damage in Group Neo-ICO was also largely as intended, although in 2 cases (Neo-ICO3 and Neo-ICO4) there was unintended damage to area 46, bilaterally (Fig. 3). Retrograde thalamic degeneration was also assessed for Group Neo-ICO. As shown in Figure 4, all subjects exhibited heavy cell loss in the magnocellular division of the medial dorsal nucleus of the thalamus and lighter cell loss in the centromedial portion of the anterior medial nucleus, the central intermedial nucleus, and the central superior nucleus. These nuclei are known to be sources of thalamic input to the IC and O (Goldman-Rakic and Porrino 1985; Barbas et al. 1991; Ray and Price 1993). In addition, cell loss extended to the parvocellular division of the medial dorsal nucleus posteriorly, consistent with damage to area 46. For subjects Neo-ICO1 and Neo-ICO2, this cell loss was light to moderate, reflecting the partial damage to area 46 on the ventral bank of the principal sulcus (Goldman-Rakic and Porrino 1985). However, for subjects Neo-ICO3 and Neo-ICO4, this degeneration was much heavier in the posterior parvocellular division of the medial dorsal nucleus, and extended anteriorly to the anterior ventral nucleus in case Neo-ICO4. The cell loss in this case was consistent with greater damage to area 46, extending to the dorsal bank of the principal sulcus.
Figure 3.
Intended combined lesions of the inferior prefrontal convexity and orbital frontal cortex (shown in dark gray, center column) and reconstruction of the actual damage (in light gray) for the 4 cases in Group ICO onto standard coronal sections of a normal monkey brain. The numerals on the left of the coronal sections indicate the distance in millimeters from the interaural plane. ai, inferior arcuate sulcus; as, superior arcuate sulcus; ci, cingulate sulcus; la, lateral fissure; orl, lateral orbital sulcus; orm, medial orbital sulcus; p, principal sulcus; ro, rostral sulcus. Asterisk indicates 2 subjects in Group ICO* with damage similar to the intended lesions. Cases ICO-3 and 4 had unintended damage to area 46 as indicated by the arrows.
Figure 4.
Retrograde degeneration in the thalamus following combined inferior prefrontal convexity and orbital frontal cortex lesions for the 4 cases in Group ICO. Areas of severe degeneration are shown in dark gray and areas of moderate degeneration are shown in light shading. AD, anterior dorsal; AV, anterior ventral; Cdc, central densocellular; Clc, central latocellular; Cif, central inferior; Cim, central intermedial; Cs, central superior; For, fornix; LD, lateral dorsal; MDmc, medial dorsal, magnocellular division; MDmf, medial dorsal multiform division; MDpc, medial dorsal, parvocellular division; Re, reuniens; THI, habenulo-interpeduncular tract; VLc, ventral lateral caudal part; VLm, ventral lateral, medial part; VPi, ventral posterior inferior; VPLo, ventral posterior, lateral oral part; VPLc, ventral posterior, lateral caudal part.
Acquisition and Retention of the DNMS Rule
Three months of age
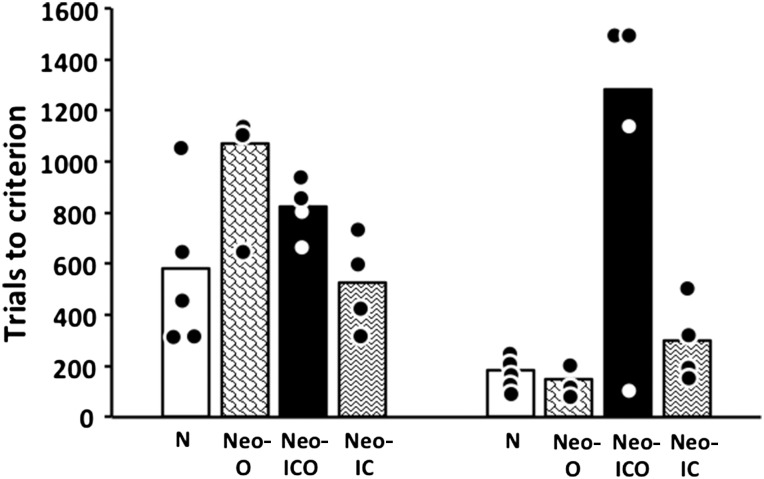
As shown in Table 1 and Figure 5, 3-month-old monkeys in Groups N and Neo-IC learned the DNMS rule with an average of 593 and 525 trials, respectively. Animals in Groups Neo-O and Neo-ICO, however, required more training to learn the DNMS rule, averaging 1069 and 825 trials, respectively. Group difference (Kruskal–Wallis, one-way analysis of variance, ANOVA) approached significance for trials to criterion [H(3) = 7.61, P = 0.055] and was significant for errors [H(3) = 10.40, P = 0.015]. For errors, paired-comparisons (Mann–Whitney U) confirmed that Groups N and Neo-IC did not differ from each other (Ps > 0.1). However, both Groups Neo-O and Neo-ICO differed from both Group N (both Ps < 0.038) and Group Neo-IC (both Ps < 0.029), but did not differ from each other (Ps > 0.1).
Table 1.
Learning and retention of delayed nonmatching-to-sample at 3 months of age
| Group/case | Sex | Learning |
Retention |
Percentage correct | ||
|---|---|---|---|---|---|---|
| Trials | Errors | Trials | Errors | |||
| N | ||||||
| N-1a | Male | 720 | 202 | 0 | 0 | 91 |
| N-2a | Male | 460 | 145 | 80 | 15 | 91 |
| N-3a | Female | 320 | 115 | 80 | 14 | 90 |
| N-4a | Female | 320 | 109 | 80 | 15 | 90 |
| N-5 | Female | 660 | 116 | 60 | 23 | 91 |
| N-6 | Female | 1080 | 300 | 20 | 4 | 90 |
| M | 593 | 165 | 53.3 | 11.83 | 90.5 | |
| IC | ||||||
| Neo-IC-1a | Female | 560 | 108 | 40 | 7 | 92 |
| Neo-IC-2a | Male | 760 | 228 | 0 | 0 | 90 |
| Neo-IC-3a | Female | 440 | 157 | 40 | 8 | 93 |
| Neo-IC-4a | Male | 340 | 127 | 0 | 0 | 90 |
| M | 525 | 143.3 | 20 | 3.75 | 91.3 | |
| ICO | ||||||
| Neo-ICO-1 | Female | 840 | 319 | 200 | 46 | 90 |
| Neo-ICO-2 | Female | 800 | 223 | 180 | 40 | 90 |
| Neo-ICO-3 | Female | 980 | 343 | 180 | 24 | 91 |
| Neo-ICO-4 | Female | 680 | 218 | 80 | 14 | 90 |
| M | 825 | 275.8 | 160 | 31 | 90.3 | |
| O | ||||||
| Neo-O-1 | Male | 1436 | 465 | 0 | 0 | 90 |
| Neo-O-2 | Female | 1100 | 317 | 140 | 25 | 90 |
| Neo-O-3 | Female | 1120 | 640 | 640 | 193 | 90 |
| Neo-O-4 | Male | 620 | 182 | 1160 | 226 | 90 |
| M | 1069 | 401 | 485 | 111 | 90.0 | |
Note: Scores are the number of trials and errors before reaching criterion to learn the DNMS rule and to retain it after a delay of 2 weeks. Percentage correct reflects performance during the 5-day criterion trials. M, mean; numbers in italics represent mean values for each group.
aAnimals that were reported in Málková et al. (2000).
Figure 5.
Mean number of trials to learn the delayed nonmatching-to-sample rule at 3 months (left panel) and 2 years of age (right panel). Circles indicate individual scores. Conventions: N, normal controls; Neo O, animals with neonatal damage to the orbital frontal cortex; Neo-ICO, animals with neonatal damage to the inferior convexity and the orbital frontal cortex; Neo-IC, animals with neonatal damage to the inferior convexity.
During the retention test (Table 1), animals in Groups N and Neo-IC retained the rule across the 2-week rest period, requiring on average less trials (53 and 20 trials, respectively) to reach criterion then they did to learn it for the first time. Similarly, Groups Neo-O and Neo-ICO showed substantial savings in the retention test, although these groups still took more trials (average of 485 and 160 trials, respectively) to relearn the DNMS rule than the other 2 groups. This group difference was significant for trials [H(3) = 8.34, P < 0.039] and approached the level of significance for errors [H(3) = 7.41, P < 0.06]. Thus, Group Neo-ICO required more trials (Ps < 0.03) than Groups N and Neo-IC, but not Group O; Group Neo-O did not differ from any other groups.
Two years of age
Maturation and prior experience improved acquisition of the DNMS rule for control (N) subjects when retested at 2 years of age (Table 2 and Fig. 5), since each animal of this group required fewer trials and made fewer errors to reach criterion at 2 years than they did at 3 months. Similarly, animals in Groups Neo-IC and Neo-O required fewer trials and made fewer errors to relearn the DNMS at 2 years of age. In contrast, animals in Group Neo-ICO required more trials and made more errors at 2 years than they did at 3 months, with cases Neo-ICO2 and Neo-ICO4 failing to reach criterion in 1000 trials and requiring an additional 500 training trials with correction (88 and 116 errors, respectively). Thus, at 2 years, as at 3 months, Group Neo-ICO was retarded in learning the DNMS rule when compared with the other 3 groups. A Group × Age ANOVA for trials yielded a significant effect of Group [F3, 13 = 8.95, P = 0.002] and Age [FHyunh-Feldt 1, 13 = 5.16, P < 0.05], and a significant Group × Age interaction [FHyunh-Feldt 3, 13 = 4.56, P < 0.03]. Post hoc analysis revealed that, although learning in Groups N, Neo-IC, and Neo-O improved with age, this change was significant for Group N only [FHyunh-Feldt 1, 5 = 12.01, P < 0.02 and FHyunh-Feldt 1, 2 = 61.7, P < 0.02, for trials and errors, respectively]. In addition, although the learning impairment in Group Neo-ICO became, on average, more severe at 2 years, the difference between the 2 ages was not significant (P > 0.05), most likely since one animal (Neo-ICO3) in the group improved. Finally, at 2 years, only Group Neo-ICO differed significantly from all 3 other groups (Ps < 0.05).
Table 2.
Relearning and retention of delayed nonmatching-to-sample at 2 years of age
| Group/case | Sex | Learning |
Retention |
Percentage correct | ||
|---|---|---|---|---|---|---|
| Trials | Errors | Trials | Errors | |||
| N | ||||||
| N-1a | Male | 220 | 67 | 0 | 0 | 90 |
| N-2a | Male | 180 | 51 | 0 | 0 | 97 |
| N-3a | Female | 160 | 47 | 0 | 0 | 90 |
| N-4a | Female | 120 | 46 | 0 | 0 | 96 |
| N-5 | Female | 240 | 52 | 0 | 0 | 94 |
| N-6 | Female | 180 | 56 | 0 | 0 | 95 |
| M | 183.3 | 53.17 | 0 | 0 | 93.7 | |
| IC | ||||||
| Neo-IC-1a | Female | 180 | 66 | 0 | 0 | 99 |
| Neo-IC-2a | Male | 200 | 55 | 0 | 0 | 95 |
| Neo-IC-3a | Female | 320 | 96 | 40 | 13 | 90 |
| Neo-IC-4a | Male | 500 | 120 | 80 | 14 | 91 |
| M | 300 | 84.5 | 30 | 6.75 | 93.8 | |
| ICO | ||||||
| Neo-ICO-1 | Female | 1000 | 313 | 640 | 138 | 91 |
| Neo-ICO-2 | Female | 1500 | 302 | - | - | 85 |
| Neo-ICO-3 | Female | 120 | 24 | 0 | 0 | 92 |
| Neo-ICO-4 | Female | 1500 | 387 | 480 | 62 | 90 |
| M | 1280 | 307.5 | 373.3 | 66.7 | 89.5 | |
| O | ||||||
| Neo-O-1 | Male | 100 | 32 | 20 | 4 | 90 |
| Neo-O-2 | Female | 220 | 55 | 40 | 7 | 90 |
| Neo-O-3 | Female | 120 | 32 | 60 | 10 | 90 |
| M | 148 | 64 | 40 | 7 | 90.0 | |
Note: Scores are the number of trials and errors before reaching criterion to learn the DNMS rule and to retain it after a delay of 2 weeks. Percentage correct reflects the performance during the 5-day criterion trials. M, mean; numbers in italics represent mean values for each group.
aAnimals that were reported in Málková et al. (2000). Note that Case O-4 was not tested at 2 years.
Memory Test
Three months of age
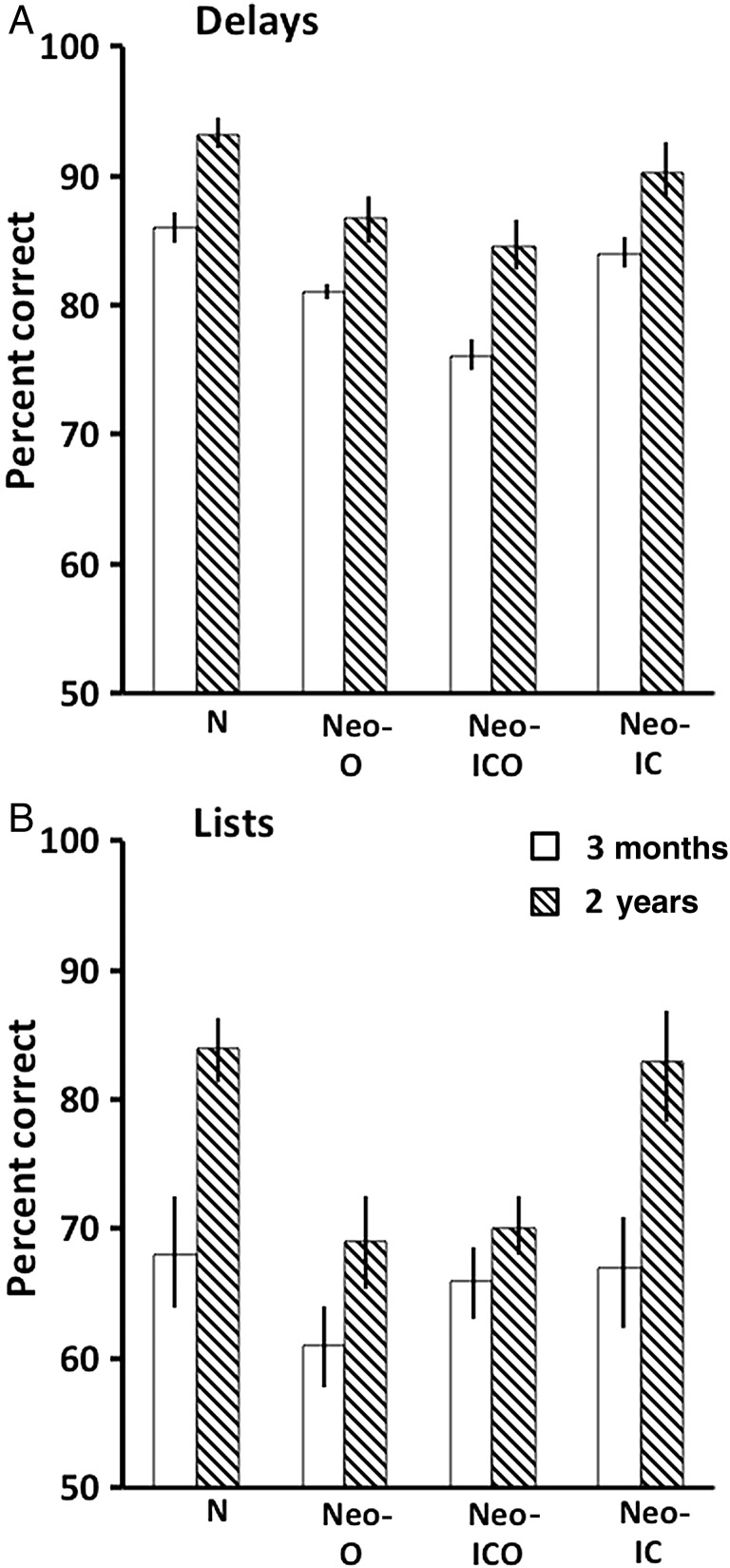
The results of the memory performance test indicated no effect from neonatal damage to either IC or O alone (Table 3 and Fig. 6), However, neonatal damage to ICO produced a mild deficit across all delays. All groups showed declining performance with increasing list lengths. Separate ANOVAs on the delay conditions yielded main effects of Group [F3, 14 = 7.24, P < 0.004] and Delay [FHyunh-Feldt 3, 42 = 14.41, P < 0.001], but no significant interaction. Post hoc analyses revealed that Group Neo-ICO differed from Groups N and Neo-IC (Ps < 0.03), but not from Group Neo-O. This latter group obtained an average performance score (81%) over the delays falling in between Groups N and Neo-IC (86 and 83.5%, respectively) on one side and Group Neo-ICO (76%) on the other. For the list conditions, there was no group difference and no interaction, but the effect of list length was significant [FHyunh-Feldt 2, 28 = 19.80, P < 0.001].
Table 3.
Performance scores across delays and lists at 3 months of age
| Group/case | 10s | 30s | 60s | 120s | LL3 | LL5 | LL10 | Average |
|---|---|---|---|---|---|---|---|---|
| N | ||||||||
| N-1a | 96 | 93 | 94 | 85 | 87 | 81 | 79 | 88 |
| N-2a | 86 | 84 | 80 | 78 | 74 | 57 | 43 | 72 |
| N-3a | 84 | 86 | 89 | 82 | 68 | 59 | 59 | 75 |
| N-4a | 84 | 81 | 84 | 83 | 65 | 55 | 39 | 70 |
| N-5 | 84 | 92 | 89 | 86 | 83 | 84 | 88 | 87 |
| N-6 | 88 | 84 | 82 | 81 | 83 | 66 | 57 | 77 |
| M | 87 | 86 | 86 | 82 | 76 | 67 | 61 | 78 |
| IC | ||||||||
| Neo-IC-1a | 86 | 91 | 86 | 93 | 77 | 75 | 67 | 82 |
| Neo-IC-2a | 85 | 90 | 79 | 77 | 59 | 59 | 55 | 72 |
| Neo-IC-3a | 88 | 81 | 81 | 78 | 79 | 80 | 75 | 80 |
| Neo-IC-4a | 84 | 83 | 83 | 71 | 58 | 53 | 64 | 71 |
| M | 86 | 86 | 82 | 80 | 68 | 67 | 65 | 76 |
| ICO | ||||||||
| Neo-ICO-1 | 86 | 79 | 68 | 69 | 73 | 68 | 52 | 71 |
| Neo-ICO-2 | 85 | 79 | 74 | 64 | 67 | 68 | 53 | 70 |
| Neo-ICO-3 | 71 | 68 | 75 | 75 | 76 | 81 | 74 | 74 |
| Neo-ICO-4 | 89 | 80 | 81 | 75 | 77 | 59 | 50 | 73 |
| M | 82 | 77 | 75 | 71 | 73 | 69 | 57 | 72 |
| O | ||||||||
| Neo-O-1 | 90 | 79 | 81 | 78 | 76 | 74 | 65 | 78 |
| Neo-O-2 | 86 | 84 | 80 | 76 | 62 | 54 | 51 | 70 |
| Neo-O-3 | 87 | 83 | 83 | 75 | 62 | 53 | 49 | 70 |
| Neo-O-4 | 89 | 83 | 74 | 70 | 76 | 61 | 52 | 72 |
| M | 88 | 82 | 80 | 75 | 69 | 61 | 54 | 73 |
Note: Scores are percent correct choices over 100 trials at each delay and over 150 trials at each list of the DNMS performance test. Average is the mean performance score across the 4 delays and 3 lists. M, mean; numbers in italics represent mean values for each group.
aAnimals that were already reported in Málková et al. (2000).
Figure 6.
Mean scores (+SEM) on the memory performance test across the 4 delay conditions (A) and the 3 list conditions (B) at 3 months (white bars) and 2 years (striped bars). Conventions: N, normal controls; Neo O, animals with neonatal damage to the orbital frontal cortex; Neo-ICO, animals with neonatal damage to the inferior convexity and the orbital frontal cortex; Neo-IC, animals with neonatal damage to the inferior convexity.
Two years of age
As shown in Table 4 and Figure 6, performance across delays improved with Age [|FHyunh-Feldt 1, 13 = 45.31, P < 0.000] for all animals. Although the performance in Group Neo-ICO also improved with age, they still performed more poorly than the other groups, but the difference reached significance only when compared with normal animals. Confirming this description, ANOVA revealed a main effect of Group [F3, 13 = 8.18, P < 0.003] and Delay [FHuynh-Feldt 3, 39 = 11.44, P < 0.000], but no interaction between Group × Delay and Group × Age × Delay.
Table 4.
Performance scores across delays and lists at 2 years of age
| Group/case | 10s | 30s | 60s | 120s | LL3 | LL5 | LL10 | Average |
|---|---|---|---|---|---|---|---|---|
| N | ||||||||
| N-1a | 92 | 98 | 98 | 91 | 97 | 91 | 83 | 93 |
| N-2a | 95 | 93 | 94 | 91 | 79 | 79 | 63 | 85 |
| N-3a | 100 | 95 | 94 | 93 | 97 | 98 | 67 | 92 |
| N-4a | 94 | 95 | 97 | 92 | 94 | 84 | 86 | 92 |
| N-5 | 90 | 88 | 91 | 92 | 93 | 85 | 79 | 88 |
| N-6 | 88 | 90 | 90 | 89 | 89 | 76 | 67 | 84 |
| M | 93 | 93 | 94 | 91 | 92 | 86 | 74 | 89 |
| IC | ||||||||
| Neo-IC-1a | 94 | 95 | 95 | 95 | 99 | 91 | 88 | 94 |
| Neo-IC-2a | 95 | 95 | 93 | 95 | 95 | 91 | 81 | 92 |
| Neo-IC-3a | 83 | 86 | 86 | 79 | 84 | 77 | 56 | 79 |
| Neo-IC-4a | 88 | 87 | 89 | 84 | 83 | 79 | 67 | 82 |
| M | 90 | 91 | 91 | 88 | 90 | 85 | 73 | 87 |
| ICO | ||||||||
| Neo-ICO-1 | 91 | 88 | 76 | 88 | 77 | 68 | 65 | 79 |
| Neo-ICO-2 | 82 | 85 | 81 | 75 | 70 | 65 | 58 | 74 |
| Neo-ICO-3 | 84 | 84 | 83 | 83 | 71 | 64 | 87 | 79 |
| Neo-ICO-4 | 93 | 83 | 88 | 82 | 80 | 79 | 79 | 84 |
| M | 88 | 85 | 82 | 82 | 75 | 69 | 65 | 79 |
| O | ||||||||
| Neo-O-1 | 88 | 95 | 93 | 89 | 92 | 73 | 67 | 85 |
| Neo-O-2 | 90 | 88 | 83 | 81 | 76 | 57 | 59 | 76 |
| Neo-O-3 | 84 | 83 | 80 | 85 | 75 | 69 | 52 | 75 |
| M | 87 | 89 | 85 | 85 | 81 | 66 | 59 | 79 |
Note: Scores are percent correct choices over 100 trials at each delay and over 150 trials at each list of the DNMS performance test. Percentage correct represents the average score across the 4 delays and 3 lists. M, mean; numbers in italics represent mean values for each group.
aAnimals that were already reported in Málková et al. (2000). Note that Case O-4 was not tested at 2 years of age.
Performance on increasing list lengths also improved with age for all 4 groups [Age effect: FHyung-Feldt 1, 13 = 11.87, P < 0.004]. There was also a list effect [FHyung-Feldt 2, 26 = 69.2, P < 0.000] reflecting decreasing performance levels across lists. However, although the Group × Age was not significant, the improvement in Groups N and Neo-IC was much greater than that of Groups Neo-O and Neo-ICO. Thus, at 2 years, the average scores across the 3 lists differed between groups [F3, 13 = 3.88, P < 0.05], with Groups Neo-O and Neo-ICO having lower scores than Group N (Ps < 0.05).
Effects of Timing on Lesions
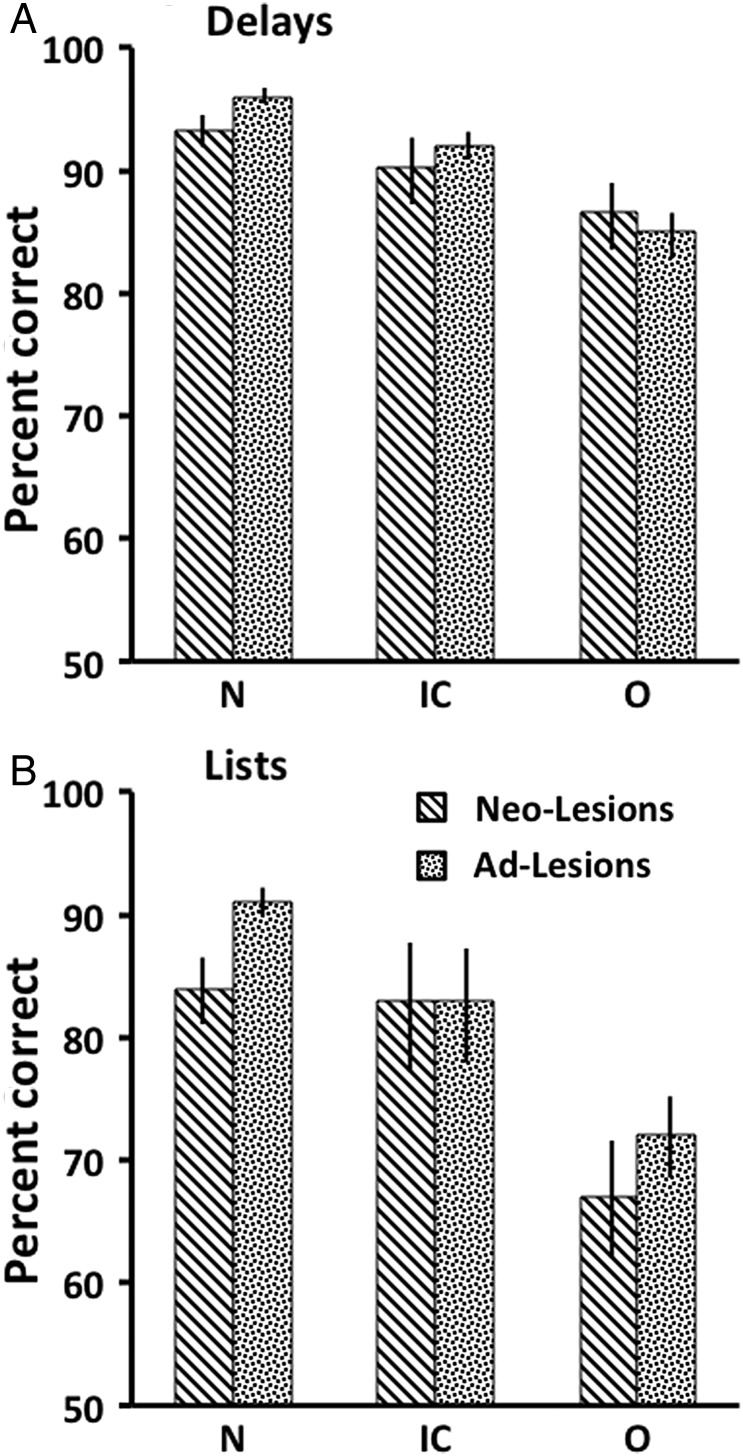
To assess whether functional compensation has occurred following the early prefrontal lesions, we compared learning and performance of animals with neonatal O lesions with that of animals that had received the same lesions in adulthood (Kowalska et al. 1991; Meunier et al. 1997). Since there were no data from animals with the combined ICO damage done in adulthood, animals with Neo-ICO were also compared with those with the O lesions done in adulthood. Note that for learning scores, the data for the early lesions were those the animals obtained after relearning the task at 2 years and the scores for the adult lesions were those the animals obtained at relearning the task immediately after surgery. Animals with adult O lesions required an average of 884 trials to reattain the DNMS criterion. This is significantly more than the number of trials required by the Neo-O group at 2 years (148; Mann–Whitney U = 0.0, P = 0.036). This finding demonstrates a clear sparing of the rule learning function after the early lesion when the animals reached adolescence. This functional sparing is similar to the sparing after the neonatal IC lesions we described previously (Málková et al. 2000).
Although animals in the Group Neo-ICO cannot be directly compared with the same lesion in adulthood, comparison with the adult O group is still informative. The average score of the Neo-ICO (1280 trials) was not significantly different from that of the group with adult O lesions (Mann–Whitney U = 8.0, P = 0.73), which was significantly impaired. Thus, in contrast to the Neo-O group, the combined damage results in no functional sparing.
For performance at 2 years on delays, although animals with neonatal O lesions showed only a mild impairment compared with their normal controls, they did not differ significantly from those with the adult O lesions (Mann–Whitney U = 6.0, P = 0.79), which showed a significant impairment (Meunier et al. 1997). For performance across the lists, animals with neonatal O lesions showed a significant impairment compared with their normal controls, as presented above (Fig. 7B). Moreover, Neo-O animals did not differ from those with the adult O lesions (Mann–Whitney U = 3.0, P = 0.25) that also showed a significant impairment (Meunier et al. 1997). This finding indicates that, with respect to memory, neonatal O lesions did not result in sparing of the function.
Figure 7.
Mean scores (+SEM) on the memory performance test across the 4 delay conditions (A) and the 3 list conditions (B) for animals with neonatal lesions (Neo-Lesions) tested at 2 years and animals with adult-onset lesions (Ad-Lesions). Conventions: N, normal controls; O, animals with damage to the orbital frontal cortex; IC, animals with damage to the inferior convexity.
Similarly, animals with neonatal ICO lesions were not significantly different from those with the adult O lesions, either on the delays (U = 7.5, P = 0.56) or the lists (U = 8.0, P = 0.73), indicating no sparing of the memory function after the combined lesions.
These results are in contrast with our previous findings that neonatal lesions limited to IC spared both the rule learning and performance on delays and lists of objects (Fig. 7A).
Discussion
The results from the present study yielded several novel findings: (1) Neonatal damage to O, separately or in combination with IC, impaired DNMS learning early in infancy. However, only the combined ICO lesions yielded impairment in adolescence; lesions of O alone resulted in a complete functional recovery. (2) For performance on the delay condition, only Neo-ICO lesions significantly impaired performance both early in infancy and in adolescence. (3) For performance on the list condition, neither Neo-O nor Neo-ICO lesions impacted performance in early infancy as all groups of animals, including normal performed equally poorly. However, in adolescence, the same neonatal lesions yielded significant impairment, since animals with Neo-O and Neo-ICO lesions did not improve their performance scores as did the normal controls. (4) The pattern of impairment described above for animals with Neo-O and Neo-ICO lesions contrasted with the significant sparing reported in animals with neonatal damage restricted to IC. (5) When compared with O and IC lesions performed in adulthood, those performed in infancy resulted in different patterns of sparing for acquisition of DNMS rule and memory performance (particularly on the list condition).
DNMS Rule Acquisition
DNMS rule acquisition has a protracted development with learning ability reaching adult levels of proficiency between 4 and 5 years of age in monkeys (Bachevalier and Mishkin 1984). Given that DNMS rule learning relies on temporo-prefrontal interactions (Kowalska et al. 1991; Meunier et al. 1997), we suggested that the protracted maturation of DNMS rule learning resulted from functional immaturity of the temporo-prefrontal circuit at this early age (Málková et al. 2000). This proposal was also based on the evidence that lesions of either TE or IC early in infancy resulted in a significant functional sparing, whereas the same lesions performed in adulthood resulted in DNMS learning impairment, indicating that this neural circuit is immature in infancy and compensatory mechanisms may be in operation. We hypothesized that the recovery of function after the neonatal damage to IC might have been achieved by compensatory mechanisms supported by the intact O, a prefrontal cortical area also critical for DNMS learning (Meunier et al. 1997). Our present findings support this proposal. First, the combined IC + O damage resulted in a severe impairment in both learning and memory, confirming our hypothesis that the O is likely to take over the function in case of damage to IC. However, when both IC and O are damaged, no compensatory mechanisms are available for the functional recovery. Secondly, neonatal lesions restricted to O impaired DNMS acquisition at 3 months, indicating that at this age no other brain region is available to compensate for damage to this cortical area. It is likely that O alone could support DNMS rule learning in normal infant animals at this early age and could also compensate following neonatal IC damage. The impairment after neonatal O lesions was, however, observed only in infancy, but not at adolescence at 2 years, suggesting that with further maturation IC could assume the function in the absence of O. This maturational pattern after early O lesions suggests that neural pathways supporting DNMS learning gradually incorporate the IC as it matures and perhaps shift certain functions to this region. A similar developmental shift was previously demonstrated for working memory processes, from the early developing caudate nucleus to the later developing dorsolateral prefrontal cortex (Goldman and Rosvold 1972).
DNMS Performance Test
When compared with normal adults, performance on the delay condition at 3 months was poorer in infants of all groups, including the normal controls. With further maturation, by the age of 2 years, all groups improved. This finding again indicates immaturity in the circuitry supporting recognition memory in infancy. Although animals in all groups improved in adolescence, animals with the combined ICO damage performed significantly worse than normal controls, providing additional evidence for the finding that compensatory mechanisms after the combined damage are significantly diminished or absent. In contrast to the delay conditions, where the improvement with age was evident in all groups, in the list condition, a dramatic improvement was observed only in Groups N and Neo-IC. The performance of animals with O damage, either alone or in combination with IC, remained on a similar level as in infancy showing no improvement with age. Thus, the performance results for group O show a dissociation between a substantial recovery of recognition memory when only one item has to be remembered, as in the delay condition, and no recovery when multiple items have to be remembered, as in the list condition. This finding indicates that orbital frontal cortex is critical to support recognition memory especially when many items need to be encoded and remembered and neonatal O damage may render the animals more susceptible to proactive interference. In addition, given that the recognition memory impairment seen after Neo-O lesions is similar to that seen after adult O lesions, the data indicate that there is no or little recovery of function following early O damage. Finally, contrary to what was described above for learning the DNMS rule, our results demonstrate that the IC cannot compensate after neonatal O damage with respect to recognition memory. Taken together, our findings demonstrated that orbital frontal cortex contributes critically to recognition memory.
It is important to note that neonatal orbital frontal lesions resulted in thalamic degeneration that showed a pattern similar to that found after adult O lesions (Meunier et al. 1997). Because damage to these thalamic nuclei results in recognition memory impairment (Aggleton and Mishkin 1983a; 1983b), we cannot rule out the possibility that degeneration of these nuclei following Neo-O lesions may have contributed to the impairment.
It is also important to point out that although 2 cases with the combined IC + O lesions (ICO-3 and ICO-4) sustained a substantial unintended damage to the dorsolateral prefrontal cortex, this additional cortical damage did not result in more severe impairment either in learning or memory performance. This result is consistent with previous finding that lesions of the dorsolateral prefrontal cortex in adult monkeys produce no impairment in DNMS (Bachevalier and Mishkin 1986). Interestingly, 1 of the 2 animals (ICO-3), which obtained performance scores within the range of all other animals in the ICO group, relearned the task at 2 years with the lowest number of trials and errors of all subjects, indicating a full recovery of the learning ability. This outcome remains somewhat puzzling since the intended damage in this animal was comparable to those in the same lesion group, suggesting possible individual differences in the learning ability and/or the plasticity underlying functional recovery.
Conclusions
We have demonstrated that the previously found sparing of DNMS learning and performance following neonatal IC damage can be accounted for by the intact orbital frontal cortex since the combined damage to the two cortical areas impaired acquisition and performance of DNMS. The results suggest that, by 3 months of age, the orbital frontal cortex is at least partially functional, although not fully mature, and can support DNMS learning in the absence of the IC. They also indicate that the IC is not functional at birth, but rather shows a protracted postnatal development, reaching maturity before the second year of life.
These findings provide further evidence that the neural circuitry mediating a given memory process may shift during ontogeny. Thus, the neural circuit in the adult may not be identical to that in the infant, even though the basic behavioral outcome is the same. The present findings are consistent with recent literature in humans, indicating that the prefrontal cortex undergoes considerable maturation during early childhood and becomes functionally organized into dissociable, specialized systems that underlie the age-related improvement of cognitive abilities dependent on the prefrontal cortex (see for review Tsujimoto 2008). In addition, given the critical role of the orbital frontal cortex not only in memory processes but also in inhibitory cognitive control, the long-term cognitive deficits observed after the Neo-O and Neo-ICO lesions in monkeys might also provide insights on the source of maladaptative behavior reported in human cases with early prefrontal damage, including the orbital frontal cortex (Anderson et al. 2000; Estlinger et al. 2004; Happaney et al. 2004; Anderson et al. 2009).
Funding
This work was supported in part by the National Institute of Mental Health Intramural Research Program, the National Institute of Mental Health (MH58848 to J.B.) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD35471 to J.B. and KO2 HD42269 to L.M.), all at the National Institutes of Health.
Notes
The authors thank Dr Mortimer Mishkin for his continuous support throughout the entire study. The authors also thank Dr Maree Webster for performing some of the surgeries and Norma Minters, Eugena Pixley, Carolyn Lex, and John Sewell III for technical support.
Conflict of Interest: None declared.
References
- Aggleton JP, Mishkin M. 1983a. Memory impairments following restricted medial thalamic lesions in monkeys. Exp Brain Res. 52:199–209. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Mishkin M. 1983b. Visual recognition impairment following medial thalamic lesions in monkeys. Neuropsychologia. 21:189–197. [DOI] [PubMed] [Google Scholar]
- Anderson AW, Wisnowski JL, Barrash J, Damasio H, Tranel D. 2009. Consistency of neuropsychological outcome following damage to prefrontal cortex in the first years of life. J Clin Exp Neuropsychol. 31:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SW, Damasio H, Tranel D, Damasio AR. 2000. Long-term sequelae of prefrontal cortex damage acquired in early childhood. Dev Neuropsychol. 18:281–296. [DOI] [PubMed] [Google Scholar]
- Bachevalier J. 1990. Ontogenetic development of habit and memory formation in primates. Ann N Y Acad Sci. 608:457–477. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. 1984. An early and late developing system for learning and retention in infant monkeys. Behav Neurosci. 98:770–778. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. 1986. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 20:249–261. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. 1994. Effects of selective neonatal temporal lobe lesions on visual recognition memory in rhesus monkeys. J Neurosci. 14:2128–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Henion THH, Dermon CR. 1991. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 313:65–94. [DOI] [PubMed] [Google Scholar]
- Bauer RH, Fuster JM. 1978. Effects of d-amphetamine and prefrontal cortical cooling on delayed matching to sample behavior. Pharmacol Biochem Behav. 8:243–249. [DOI] [PubMed] [Google Scholar]
- Berger B, Alvarez C. 1994. Neurochemical development of the hippocampal region in the fetal rhesus monkey. II. Immunocytochemistry of peptides, calcium-binding proteins, DARPP-32, and monoamine innervation in the entorhinal cortex by the end of gestation. Hippocampus. 4(1):85–114. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire LR, Zola SM. 1999. Dissociation between the effects of damage to perirhinal cortex and area TE. Learn Mem. 6:572–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. 2000. The anatomical connections of the macaque monkey orbitofrontal cortex. A review . Cereb Cortex. 10:220–242. [DOI] [PubMed] [Google Scholar]
- Estlinger PJ, Flaherty-Craig CV, Benton AL. 2004. Developmental outcomes after early prefrontal cortex damage. Brain Cogn. 55:84–103. [DOI] [PubMed] [Google Scholar]
- Fuster JM. 2002. Frontal lobe and cognitive development. J Neurocytol. 31:373–385. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bauer RH, Gervey JP. 1981. Effects of cooling inferotemporal cortex on performance of visual memory tasks. Exp Neurol. 71:398–409. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bauer RH, Gervey JP. 1985. Functional interactions between inferotemporal and prefrontal cortex in a cognitive task. Brain Res. 338:299–307. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Eacott MJ. 1995. Uncinate fascicle section leaves delayed matching-to-sample intact, with both large and small stimulus sets. Exp Brain Res. 105(1):175–180. [DOI] [PubMed] [Google Scholar]
- Goldman PS. 1971. Functional development of the prefrontal cortex in early life and the problem of neuronal plasticity. Exp Neurol. 32:366–387. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. 1972. The effects of selective caudate lesions in infant and juvenile rhesus monkeys. Brain Res. 43:53–66. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Porrino LJ. 1985. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol. 242:535–560. [DOI] [PubMed] [Google Scholar]
- Happaney K, Zelazo PD, Stuss DT. 2004. Development of the orbitofrontal function: current themes and future directions. Brain Cogn. 55:1–10. [DOI] [PubMed] [Google Scholar]
- Hirabayashi T, Miyashita Y. 2014. Computational principles of microcircuits for visual object processing in the macaque temporal cortex. Trends Neurosci. 37:178–187. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Styner M, Short SJ, Lubach GR, Kang C, Hamer R, Coe CL, Gilmore JH. 2010. Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cereb Cortex. 20(5):1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Halliwell C, Gibb R. 2010. Factors influencing neocortical development in the normal and injured brain. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press. [Google Scholar]
- Kowalska DM, Bachevalier J, Mishkin M. 1991. The role of the inferior prefrontal convexity in performance of delayed non-matching-to-sample. Neuropsychologia. 29:583–600. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. 2002. Perirhinal and parahippocampal cortices of the macaque monkey: projections to the neocortex. J Comp Neurol. 447:394–420. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. 2008. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 40:1044–1055. [DOI] [PubMed] [Google Scholar]
- Li L, Miller EK, Desimone R. 1993. The representation of stimulus familiarity in anterior inferior temporal cortex. J Neurophysiol. 69:1918–1929. [DOI] [PubMed] [Google Scholar]
- Málková L, Bachevalier J, Webster MJ, Mishkin M. 2000. Effects of neonatal inferior prefrontal and medial temporal lesions on learning the rule for delayed nonmatching-to-sample. Dev Neuropsychol. 18:399–421. [DOI] [PubMed] [Google Scholar]
- Malkova L, Heuer E, Saunders RC. 2006. Longitudinal magnetic resonance imaging study of rhesus monkey brain development. Eur J Neurosci. 24(11):3204–3212. [DOI] [PubMed] [Google Scholar]
- Málková L, Mishkin M, Bachevalier J. 1995. Long-term effects of selective neonatal temporal lobe lesions on learning and memory in monkeys. Behav Neurosci. 109:212–226. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. 1997. Effects of orbital frontal and anterior cingulated lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 35:999–1015. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. 1993. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortices in rhesus monkeys. J Neurosci. 13:5418–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. 1991. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 254:1377–1379. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Phillips RR. 1990. A corticolimbic path revealed through its disconnection. In: Trevarthen C, editor. Brain circuitr and the functions of the mind. Essays in honor of Roger W. Sperry. New York. Cambridge University Press. p. 196–210. [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. 1992. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 323:341–358. [DOI] [PubMed] [Google Scholar]
- Orzhekhovskaya NS. 1981. Fronto-striatal relationships in primate ontogeny. Neurosci Behav Physiol. 11:379–384. [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. 1998. Interaction of frontal and perirhinal cortices in visual object recognition memory in monkeys. Eur J Neurosci. 10:3044–3057. [DOI] [PubMed] [Google Scholar]
- Ray JP, Price JL. 1993. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 337:1–31. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. 2000. The laminar pattern of connections between prefrontal and anterior temporal cortices in the Rhesus monkey is related to cortical structure and function. Cereb Cortex. 10:851–865. [DOI] [PubMed] [Google Scholar]
- Rodman 1994. Development of inferior temporal cortex in the monkey. Cereb Cortex. 4(5):484–498. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Mishkin M, Aggleton JP. 2005. Projections from the entorhinal cortex, perirhinal cortex, presubiculum, and parasubiculum to the medial thalamus in macaque monkeys: identifying different pathways using disconnection techniques. Exp Brain Res. 167:1–16. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. 2004. Functional neuroanatomy of the medial temporal lobe memory system. Cortex. 40:220–222. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Zola-Morgan S, Squire LR, Amaral DG. 1993. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J Neurosci. 13:2430–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S. 2008. The prefrontal cortex: functional neural development during early childhood. Neuroscientist. 14:345–358. [DOI] [PubMed] [Google Scholar]
- Voytko ML. 1985. Cooling orbital frontal cortex disrupts matching-to-sample and visual discrimination learning in monkeys. Physiol Psychol. 13:219–229. [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. 1995. Transient subcortical connections of inferior temporal areas TE and TEO in infant macaque monkeys. J Comp Neurol. 352:213–226. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Ungerleider LG, Bachevalier J. 1991. Lesions of inferior temporal area TE in infant monkeys alter cortico-amygdalar projections. Neuroreport. 2:769–772. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Ungerleider LG, Bachevalier J. 1995. Development and plasticity of the neural circuitry underlying visual recognition memory. Can J Physiol Pharmacol. 73:1364–1371. [DOI] [PubMed] [Google Scholar]