ABSTRACT
CD98 heavy chain (SLC3A2) facilitates lymphocyte clonal expansion that enables adaptive immunity; however, increased expression of CD98 is also a feature of both lymphomas and leukemias and represents a potential therapeutic target in these diseases. CD98 is transcriptionally regulated and ectopic expression of the membrane-associated RING-CH (MARCH) E3 ubiquitin ligases MARCH1 or MARCH8 leads to ubiquitylation and lysosomal degradation of CD98. Here, we examined the potential role of ubiquitylation in regulating CD98 expression and cell proliferation. We report that blocking ubiquitylation by use of a catalytically inactive MARCH or by creating a ubiquitylation-resistant CD98 mutant, prevents MARCH-induced CD98 downregulation in HeLa cells. March1-null T cells display increased CD98 expression. Similarly, T cells expressing ubiquitylation-resistant CD98 manifest increased proliferation in vitro and clonal expansion in vivo. Thus, ubiquitylation and the resulting downregulation of CD98 can limit cell proliferation and clonal expansion.
KEY WORDS: CD98, Clonal expansion, Immunobiology, Integrin signaling
Summary: Ubiquitylation of CD98 regulates its expression, and is a mechanism to limit the clonal expansion of T lymphocytes and potentially of other normal and transformed cells.
INTRODUCTION
In vertebrates, adaptive immunity provides for protection against a remarkably diverse collection of pathogens. During an adaptive immune response, a few lymphocytes with a particular antigenic specificity can rapidly proliferate upon exposure to cognate antigen, resulting in a large population of effector cells that can eliminate pathogens bearing the antigen (Boehm, 2011). Termed clonal expansion, this explosive proliferation of few antigen-specific cells from a large and diverse repertoire is crucial to preserving the specificity and diversity of lymphocytes and also enables the memory arm of adaptive immunity upon re-exposure (Sprent and Surh, 2002). Although of great benefit to vertebrates, clonal expansion can also be co-opted by cancer cells. Following a similar paradigm, clonal expansion allows a few cancer-initiating cells to rapidly proliferate resulting in tumorigenesis (Cantor and Ginsberg, 2012). CD98 heavy chain (SLC3A2), which is a lymphocyte activation antigen (Haynes et al., 1981), enables clonal expansion of normal and malignant cell types including lymphocytes (Cantor et al., 2009, 2011), vascular smooth muscle cells (Fogelstrand et al., 2009), squamous cell carcinomas (Estrach et al., 2014) and teratomas (Feral et al., 2005) by amplifying integrin signaling and supporting amino acid transport as a heterodimer with a light chain such as SLC7A5.
High expression of CD98 in lymphomas and acute myeloid leukemia (AML) correlates with negative outcomes (Holte et al., 1987, 1989; Nikolova et al., 1998; Salter et al., 1989). Thus, CD98 expression levels could determine adaptive immune responses or the progression of malignancies. Rapid CD98 upregulation is under transcriptional control (Gottesdiener et al., 1988; Karpinski et al., 1989; Lindsten et al., 1988). Ectopic expression of membrane-associated RING-CH (MARCH) ubiquitin ligases can reduce surface CD98 protein (Eyster et al., 2011) by marking it for lysosomal degradation. We hypothesized that CD98 ubiquitylation regulates its expression to limit cell proliferation and clonal expansion. Here, we report that CD98 expression is regulated by ubiquitylation. Furthermore, we find that T cells expressing ubiquitylation-resistant CD98 display increased antigen-driven proliferation and clonal expansion. Thus, ubiquitylation and the resulting downregulation of CD98 provides a new mechanism to limit cell proliferation and clonal expansion.
RESULTS AND DISCUSSION
Ubiquitylation of CD98 controls its expression in model systems
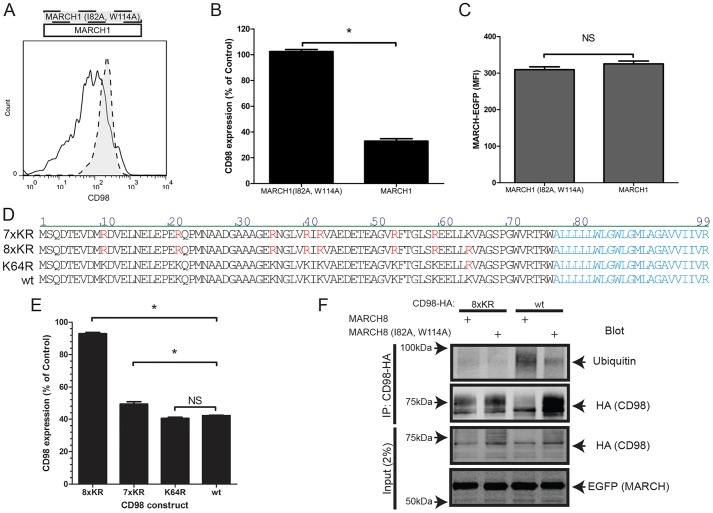
To assess whether the ubiquitin ligase activity of MARCH1 is required to regulate CD98 surface expression, we used catalytically inactive mutants in the RING domain (Baravalle et al., 2011). Expression of MARCH1 reduced surface CD98 threefold in HeLa cells, whereas expression of a catalytically inactive mutant [MARCH1 (I82A, W114A)] had no effect when expressed at similar levels (Fig. 1A–C). The MARCH8 ubiquitylation function was also required to reduce CD98 (data not shown, but see Fig. 1F). MARCH proteins typically modify cytoplasmic Lys residues (Bartee et al., 2004; Fujita et al., 2013; Oh et al., 2013). There are eight cytoplasmic Lys residues within the CD98 tail (Fig. 1D). Mutation of these Lys residues to Arg [CD98 (8×KR)], resulted in CD98 that was not downregulated by MARCH8 (Fig. 1E). Mutation of either the seven most membrane-distal [CD98 (7×KR)] or the most membrane-proximal Lys residues [CD98 (K64R)], did not prevent downregulation by MARCH8 (Fig. 1E). Therefore, mutation of multiple Lys residues, including Lys64, is required to render CD98 resistant to MARCH-mediated downregulation (Fig. 1E). Furthermore, expression of MARCH8 increased CD98 ubiquitylation, whereas mutant MARCH8 [MARCH8 (I82A, W114A)] had no effect (Fig. 1F). Western blotting of the CD98 heavy chain revealed a low-molecular-mass (∼60 kDa) band and a higher molecular mass component (70–80 kDa); expression of the low molecular mass component was not affected by ectopic MARCH8 expression. This MARCH8-resistant component is likely to correspond with unglycosylated intracellular CD98 (Bartee et al., 2004; Eyster et al., 2011). In contrast to wild-type CD98, the higher molecular mass component of CD98 (8×KR) was resistant to MARCH8-mediated ubiquitylation (Fig. 1F). Preventing ubiquitylation and downregulation of other MARCH substrates, such as major histocompatibility complex (MHC) class II molecules, CD86, and CD71 (also known as TFRC) also requires mutation of all membrane proximal cytoplasmic Lys residues (Baravalle et al., 2011; Fujita et al., 2013; Oh et al., 2013). Taken together, these results indicate that MARCH proteins, which are tethered to the membrane, ubiquitylate Lys residues in their vicinity in a relatively non-selective manner. The data reported here define a CD98 mutant that is resistant to MARCH-mediated ubiquitylation and downregulation, and can therefore be used as a tool to probe the role of this modification in clonal expansion.
Fig. 1.
CD98 is downregulated following ubiquitylation of its cytoplasmic lysines. (A) Expression of wild-type (wt) MARCH1–EGFP (white histogram) but not the catalytically inactive mutant of MARCH1 (I82A, W114A)–EGFP (gray histogram) downregulates surface expression of CD98. HeLa cells were transfected with the indicated MARCH1–EGFP expression vectors. After 24 h, surface expression of endogenous human CD98 in EGFP-expressing cells was assayed by flow cytometry. A representative histogram from one of three experiments is shown. (B) Quantification of MARCH1-mediated downregulation of CD98. Surface expression of CD98 upon transfection of MARCH1–EGFP or MARCH1 (I82A, W114A)–EGFP was assayed as in A. The geometric mean fluorescence intensity (MFI) of surface CD98 was expressed as a percentage of that of an EGFP transfected control. The mean±s.e.m. of three experiments is depicted. *P<0.01 (unpaired t-test). (C) Similar expression of wild-type and mutant MARCH1. The MFI of each of the EGFP–MARCH constructs in EGFP-positive cells from the experiments depicted in B is shown. The mean±s.e.m. of three experiments is depicted. NS, not significant (P>0.01 by unpaired t-test). (D) The sequence of mouse CD98 cytoplasmic domain (wt) joined to its transmembrane domain (in cyan) is depicted. Above that are shown the three sequences of mutants in which Lys residues were mutated to Arg (red). (E) Mutation of all cytoplasmic Lys residues renders CD98 resistant to downregulation by MARCH8. HeLa cells were co-transfected with the indicated CD98 expression vectors and MARCH8–EGFP. Surface expression of the indicated CD98 construct was assessed by flow cytometry in MARCH8–EGFP-expressing cells and is shown as the percentage of expression in EGFP-transfected control cells. The mean±s.e.m. of triplicate experiments is shown. *P<0.05; NS, not significant (by one way ANOVA and Tukey's post test). (F) Mutation of all cytoplasmic Lys residues renders CD98 resistant to ubiquitylation by MARCH proteins. HeLa cells were co-transfected with either wild-type or 8×KR CD98 and MARCH8–EGFP or MARCH8 (I82A, W114A)–EGFP. After 24 h, HA-tagged CD98 was isolated by anti-HA antibody immunoprecipitation (IP), fractionated by SDS-PAGE and immunoblotted with the indicated antibodies. The input (2%) is also shown.
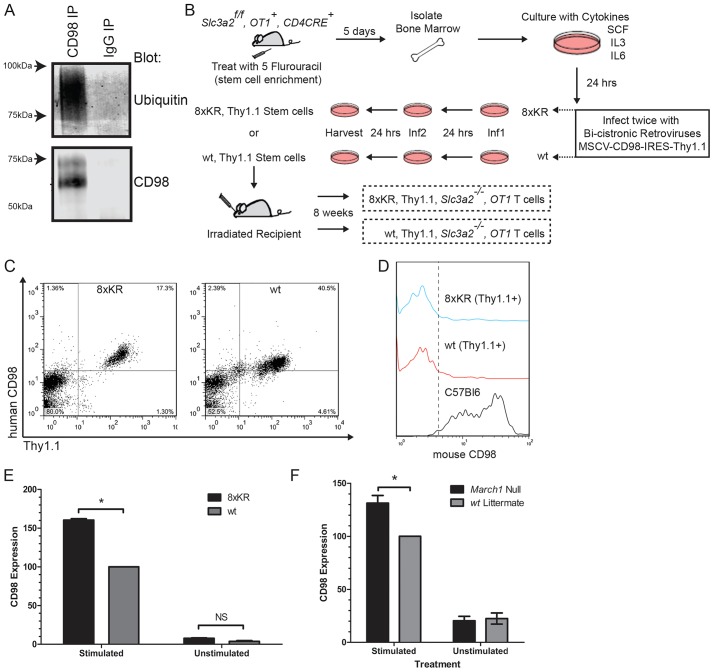
Because CD98 is essential for lymphocyte clonal expansion (Cantor et al., 2009, 2011; Gottesdiener et al., 1988), we used T cells to test the biological relevance of CD98 ubiquitylation in the physiological regulation of CD98 expression and cell proliferation. Immunoprecipitation of CD98 from Jurkat T cells followed by immunoblotting for ubiquitin demonstrated that endogenous T-cell CD98 could be ubiquitylated (Fig. 2A). To examine the role of CD98 ubiquitylation in primary T cells, we introduced CD98 (8×KR) into naive CD8+ T cells using retrogenic bone marrow reconstitution (Cantor et al., 2011). Human CD98 or CD98 (8×KR), was introduced into OT-1, Cd4Cre, Slc3a2f/f murine bone marrow cells using retroviral transduction. Transduced cells were marked by surface expression of human CD98 and Thy1.1 expressed from the retroviral vector. By virtue of the Cd4CRE, Slc3a2f/f background, T cells will delete the endogenous mouse Slc3a2 during thymic development, leaving only the retrovirally introduced human CD98 or CD98 (8×KR) (Fig. 2B). At 8 weeks post transplant, human CD98+, Thy1.1+, CD8+ T cells were readily detectable in the peripheral blood (Fig. 2C) and were null for mouse CD98 (Fig. 2D). A pronounced increase in expression of CD98 (8×KR) compared to wild-type CD98 was observed in splenic OT-1 cells following activation with anti-CD3 and anti-CD28 monoclonal antibodies (mAbs) in vitro (Fig. 2E). March1 is known to be expressed at low levels in resting splenic T cells (Matsuki et al., 2007), and an increase in CD98 surface expression was observed upon activation of March1-null T cells compared to wild-type littermate control T cells (Fig. 2F). Therefore, ubiquitylation of CD98 by MARCH1 limits its surface expression in activated CD8 T cells.
Fig. 2.
Surface expression of T-cell CD98 is limited by ubiquitylation. (A) CD98 is ubiquitylated in T cells. Endogenous human CD98 was immunoprecipitated (IP) from 107 Jurkat cells, fractionated by SDS-PAGE and immunoblotted for ubiquitin. (B) Schematic of retrogenic reconstitution of CD98. The indicated donor animals were treated with 5-Flurouracil (FU) (4 mg/mouse). Five days later, bone marrow was harvested and after 24 h, stem cells were infected with bi-cistronic retroviral expression vectors for human CD98 and Thy1.1 (wt, wild type). After 24 h, cells were re-infected with the same vector and 24 h later were transferred to irradiated recipients. Circulating cells were analyzed by flow cytometry after 8 weeks. (C) Surface expression of human CD98 and Thy1.1 marker on circulating donor-derived CD8+ T cells. (D) Mouse CD98 is deleted from donor T cells. Mouse CD98 expression on circulating CD8+, Thy1.1+ T cells and normal (C57BL6) CD8+ T cells as assessed by flow cytometry. (E) Surface expression of ubiquitylation-resistant CD98 8×KR is increased in activated CD8+ T cells. Purified splenic OT-1 T cells were stimulated with plate bound anti-CD3 and anti-CD28 mAbs for 48 h. The geometric mean fluorescence intensity (MFI) of CD98 from stimulated and unstimulated cells was assayed by flow cytometry and converted into CD98 expression level defined as the percentage compared with stimulated wild-type control. The mean±s.e.m. CD98 expression is depicted (n=3 mice per group). *P<0.05; NS, not significant (paired t-test). (F) T cells were purified from splenocytes of naive March1-null, OT-1 mice and March1 wild-type, OT-1 mice (n=3 mice per group) and cultured with irradiated APCs, IL-2 and antigen (1000 nM SIINFEKL peptide) for 48 h. The MFI of CD98 from stimulated and unstimulated CD8+ cells was assayed by flow cytometry. CD98 expression was calculated as in E and is depicted as mean±s.e.m. *P<0.05 (paired t-test).
T-cell clonal expansion is limited by ubiquitylation-mediated downregulation of CD98
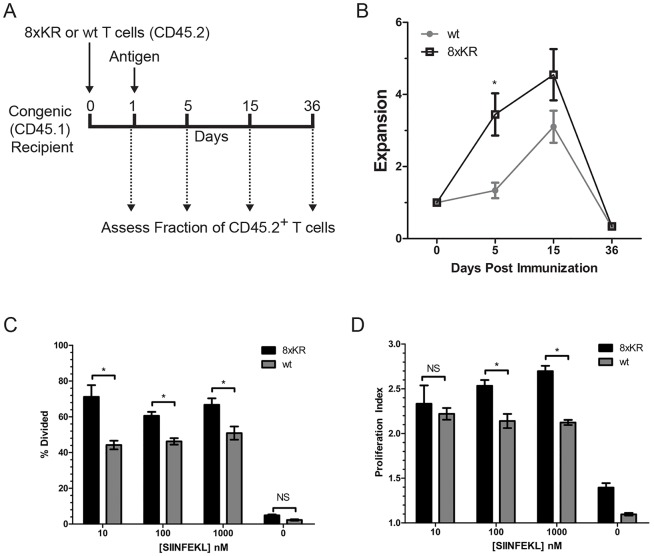
To directly test whether CD98 ubiquitylation controls clonal expansion of T cells in vivo, naive mouse CD98 null, CD8+ T cells expressing either human CD98 or human CD98 (8×KR) were adoptively transferred into congenic recipient mice. Recipients were subsequently immunized with antigen under conditions that provide modest stimulation (Beacock-Sharp et al., 2003; Hommel and Kyewski, 2003; Li et al., 2006; Oh et al., 2015; Parish et al., 2001; Teague et al., 2004) and permit sequential assay of the fraction of circulating donor-derived T cells in an individual mouse over time. Clonal expansion was monitored by assaying blood at 0, 5, 15 or 36 days after antigen stimulation (Fig. 3A). Upon antigen challenge, CD98 (8×KR)-bearing T cells appeared earlier and in greater numbers compared to cells bearing wild-type human CD98 (Fig. 3B). Thus, ubiquitylation of CD98 limits clonal expansion of T cells in vivo. To confirm the effect of blocking CD98 ubiquitylation on T-cell proliferation in a more precisely controlled setting, mouse CD98-null, OT-1 T cells bearing either human CD98 (8×KR) or human CD98 were purified, labeled with the division dye CFSE and mixed with irradiated mouse splenocytes [used as antigen-presenting cells (APCs)] and SIINFEKL peptide antigen; proliferation was measured by CFSE dilution. At all doses of cognate peptide tested, more CD98 (8×KR)-bearing T cells divided compared to control cells (Fig. 3C). The rate of proliferation of CD98 (8×KR) cells was also increased compared to CD98 wild-type control cells at higher doses of cognate peptide (Fig. 3D). These data show that ubiquitylation of CD98 regulates T-cell proliferation in vivo and in vitro.
Fig. 3.
T-cell proliferation and clonal expansion are limited by ubiquitylation of CD98. (A) Schematic of in vivo clonal expansion assay. Naive human CD98 wild-type (wt) or 8×KR, Thy1.1+, Slc3a2−/−, OT-1 splenic T cells, as described in Fig. 2, were purified and transferred to congenic recipients. After 1 day, recipients were immunized with OVA peptide in CFA. Expansion of CD8+, CD45.2+ OT-1 T cells (congenically marked donor cells) was monitored in peripheral blood on day 0 prior to immunization and on day 5, 15 and 36. Expansion was calculated using the formula E=FN/F1, where E is expansion, F1 is the fraction of CD8 T cells that are CD45.2+ on day 1 and FN is the fraction of CD8 T cells that are CD45.2+ on day 5, 15 or 36. (B) Increased in vivo clonal expansion of T cells bearing ubiquitylation-resistant CD98. Depicted is the mean±s.e.m. expansion of wild-type CD98 (n=3 recipients) and CD98 (8×KR) (n=6 recipients) as described in A. *P<0.05 (unpaired t-test). (C,D) Ubiquitylation-resistant CD98 increases antigen-dependent proliferation of CD8+ T cells. Naive human CD98-bearing wild-type or 8×KR OT-1 T cells were purified from splenocytes and labeled with CFSE. CFSE-labeled T cells were cultured with irradiated APCs, IL-2 and antigen (SIINFEKL peptide at 10, 100 or 1000 nM) for 72 h. Proliferation of wild-type CD98 or 8xKR CD8+, OT-1 T cells was assayed by monitoring CFSE dye dilution. The percentage (C) of cells that had divided and (D) the proliferation index were calculated using the proliferation module of Flowjo®. Depicted are mean±s.e.m. values for each condition from three experiments. *P<0.05; NS, not significant (one way ANOVA and Tukey's post test).
Our findings that CD98 ubiquitylation can modulate its expression on primary cells and limit clonal expansion provides a new mechanism for regulation of CD98 and show how this particular post-translational modification can control cell proliferation. CD98 levels can be transcriptionally controlled (Gottesdiener et al., 1988; Karpinski et al., 1989; Lindsten et al., 1988) and our data indicate post-translational mechanisms also contribute to CD98 regulation. Together, CD98 regulatory pathways can govern adhesive signaling (Cantor et al., 2011; Feral et al., 2005) and light-chain amino acid transporters such as SLC7A5 (Sinclair et al., 2013; Verrey et al., 2004) to support proliferation (Cantor et al., 2009, 2011; Sinclair et al., 2013). The increase in expression of the CD98 8×KR mutant can therefore enable increased cellular transport of amino acids or integrin signaling; however, it is unlikely to alter those functions on a per molecule basis. Specifically, the 8×KR mutations do not affect the extracellular Cys residue that couples CD98 to the amino acid transporters or the transmembrane and membrane proximal cytoplasmic domain residues that support integrin signaling (Henderson et al., 2004). Genetic ablation of CD98 protects from autoimmunity and several cancers (Cantor and Ginsberg, 2012) and blocking CD98 can inhibit leukemias and lymphomas (Hayes et al., 2015). Thus, our finding that ubiquitylation regulates CD98 expression and clonal expansion pinpoints a new locus for intervention in either autoimmunity or hematologic malignancies.
MATERIALS AND METHODS
Reagents
Phusion polymerase (Thermo), the In-Fusion cloning system (Clontech) and FuGENE-HD (Clontech) were used according to manufacturer's directions. Fluorophore-conjugated antibodies were purchased as follows: anti-human CD98 (clone UM7F8, BD Biosciences), anti-mouse CD98 (clone RL388, Biolegend), anti-mouse CD8a (clone 53-6.7, Biolegend), anti-mouse CD45.2 (clone A20, Biolegend) and anti-Thy1.1 (clone OX-7, Biolegend). Unconjugated antibodies were purchased as follows: anti-mouse CD98 (clone RL388, Biolegend), anti-HA (clone 16B12, Biolegend), anti-EGFP rabbit polyclonal (Clontech), anti-ubiquitin (P4D1, Santa Cruz Biotechnology), anti-human CD98 (C-20, Santa Cruz Biotechnology), anti-CD3 and anti-CD28 (Bio X Cell). The mouse untouched T-cell kit was from Thermo. Ly5.1+(CD45.1) congenic mice were purchased from the Jackson Laboratory (Bar Harbor, ME). March1-null mice (Matsuki et al., 2007) were provided by Satoshi Ishido (Laboratory of Integrative Infection Immunity, Showa Pharmaceutical University, Machida, Tokyo, Japan) and bred to the OT-1 background. All mice were housed at the University of California San Diego animal facility, and all experiments were approved by the Institutional Animal Care and Use Committee.
Plasmids and DNA manipulations
pEGFP-N1 was purchased from Clontech, pcDNA3.1ZEO was purchased from Thermo, pBKS-MARCH8 was purchased from Open Biosystems (Thermo) and MSCV-IRES-Thy1.1 DEST was a gift from Anjana Rao (Department of Pharmacology, University of California, San Diego, La Jolla, CA). All sub-cloned vectors and point mutations were generated using PCR amplification and In-Fusion cloning according to manufacturer's directions. pEGFP-N1-MARCH1 was generated by PCR amplification of human MARCH1 from HeLa cell cDNA using specific primers. pEGFP-N1-MARCH1 (I82A, W114A) and pEGFP-N1-MARCH8 (I82A, W114A) mutants were generated by site-directed mutagenesis. To generate pcDNA3.1-mouse CD98 (8×KR), the 5′ residues of mouse CD98 encoding the cytoplasmic domain was synthesized as two overlapping ultramers (IDT, Coralville, IA) with all eight Lys residues mutated to Arg (8×KR) that were then fused by PCR. The cDNA encoding the transmembrane and ectodomains of mouse CD98 were amplified as a separate fragment and cloned together with the 8×KR fragment into pcDNA3.1ZEO. pcDNA3.1-mouse CD98 (7×KR) was generated by site-directed mutagenesis of R64K from mouse CD98_8×KR and mouse CD98 (K64R) was generated from wild-type CD98 by site-directed mutagenesis. The retroviral expression vector for human CD98 (8×KR) was generated using overlapping ultramers (IDT) with all 8 Lys to Arg mutations as described above, and was cloned into MSCV-IRES-Thy1.1 DEST. All expression vectors were verified by direct sequencing.
Cells
HeLa CCL-2 cells were purchased from ATCC (Manassas, VA) and maintained as described previously (Bartee et al., 2004). Primary mouse T cells were maintained as described previously (Cantor et al., 2011). Flow cytometry and immunoblotting were performed as described previously (Cantor et al., 2011; Eyster et al., 2011).
Proliferation assays
For in vivo T-cell clonal expansion, mouse CD98-null, human CD98+ (wild-type or 8×KR) T cells were purified from spleens of OT-1, Slc3a2f/f, Cd4Cre retrogenic mice by magnetic depletion using the mouse untouched T-cell kit with anti-mouse CD98 antibody, clone RL388, to remove any remaining mouse CD98+ cells. Purified T cells (106) were injected into Ly5.1+(CD45.1) congenic recipients. After 1 day, recipient mice containing ‘parked’ CD45.2+ OT-1 T cells were immunized intraperitoneally with 75 μg SIINFEKL peptide in complete Freund's adjuvant (CFA). At 5, 15 and 36 days after immunization, blood samples were collected, treated with RBC lysis buffer, stained with antibodies specific for mouse CD8 and CD45.2, and analyzed by flow cytometry for CD45.2+ as a fraction of total CD8+ cells. For in vitro antigen-dependent proliferation assays, mouse CD98-null, human CD98+ (wild-type or 8×KR) T cells (1×105) were purified as described above, labeled in 5 µM CFSE, and mixed with 5×105 irradiated (3500 rad) wild-type C57/BL6 splenocytes as APCs, 10 U/ml recombinant IL2 and SIINFEKL peptide. After 72 h of culture, cells were stained with antibodies against CD8 and mouse CD98 and assayed by flow cytometry. Proliferation was determined by CFSE dye dilution of cells in the CD8+ and mouse CD98− gate using the proliferation module of FlowJo® software (Treestar). For antigen-independent stimulation of T cells, human CD98+ or CD98 (8×KR)+ cells were purified as above, and stimulated with plate bound anti-CD3 and anti-CD28 as described previously (Cantor et al., 2011). After 72 h cells were stained for human CD98, mouse CD98, and mouse CD8 and analyzed by flow cytometry for the surface level of human CD98 in the CD8+ and mouse CD98− gate using FlowJo® software (Treestar).
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
J.N.G.A., J.M.C., J.T.C., and M.H.G. conceived the project, analyzed data, and wrote and edited the paper. Experiments were performed by J.N.G.A., J.M.C. and P.J.M.
Funding
This work was supported by the National Institutes of Health (NIH) [grant numbers HL31950 and HL106489 to M.H.G., K01-DK090416 and P30 DK063491 to J.M.C., and OD008469 and AI095277 to J.T.C.]. J.N.G.A. is a recipient of a post-doctoral fellowship award from the Arthritis Foundation, and J.M.C. is partly funded by a Melanoma Research Alliance Young Investigator Award. Deposited in PMC for release after 12 months.
References
- Baravalle G., Park H., McSweeney M., Ohmura-Hoshino M., Matsuki Y., Ishido S. and Shin J.-S. (2011). Ubiquitination of CD86 is a key mechanism in regulating antigen presentation by dendritic cells. J. Immunol. 187, 2966-2973. 10.4049/jimmunol.1101643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartee E., Mansouri M., Hovey Nerenberg B. T., Gouveia K. and Fruh K. (2004). Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J. Virol. 78, 1109 10.1128/JVI.78.3.1109-1120.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacock-Sharp H., Donachie A. M., Robson N. C. and Mowat A. M. (2003). A role for dendritic cells in the priming of antigen-specific CD4+ and CD8+ T lymphocytes by immune-stimulating complexes in vivo. Int. Immunol. 15, 711-720. 10.1093/intimm/dxg067 [DOI] [PubMed] [Google Scholar]
- Boehm T. (2011). Design principles of adaptive immune systems. Nat. Rev. Immunol. 11, 307-317. 10.1038/nri2944 [DOI] [PubMed] [Google Scholar]
- Cantor J. M. and Ginsberg M. H. (2012). CD98 at the crossroads of adaptive immunity and cancer. J. Cell Sci. 125, 1373-1382. 10.1242/jcs.096040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J., Browne C. D., Ruppert R., Féral C. C., Fässler R., Rickert R. C. and Ginsberg M. H. (2009). CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat. Immunol. 10, 412-419. 10.1038/ni.1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J., Slepak M., Ege N., Chang J. T. and Ginsberg M. H. (2011). Loss of T cell CD98 H chain specifically ablates T cell clonal expansion and protects from autoimmunity. J. Immunol. 187, 851-860. 10.4049/jimmunol.1100002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrach S., Lee S.-A., Boulter E., Pisano S., Errante A., Tissot F. S., Cailleteau L., Pons C., Ginsberg M. and Feral C. C. (2014). CD98hc (SLC3A2) loss protects against Ras-driven tumorigenesis by modulating integrin-mediated mechanotransduction. Cancer Res. 74, 6878-6889. 10.1158/0008-5472.CAN-14-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyster C. A, Cole N. B., Petersen S., Viswanathan K., Früh K. and Donaldson J. G. (2011). MARCH ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation. Mol. Biol. Cell 22, 3218-3230. 10.1091/mbc.E10-11-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feral C. C., Nishiya N., Fenczik C. A., Stuhlmann H., Slepak M. and Ginsberg M. H. (2005). CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. USA 102, 355-360. 10.1073/pnas.0404852102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelstrand P., Feral C. C., Zargham R. and Ginsberg M. H. (2009). Dependence of proliferative vascular smooth muscle cells on CD98hc (4F2hc, SLC3A2). J. Exp. Med. 206, 2397-2406. 10.1084/jem.20082845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H., Iwabu Y., Tokunaga K. and Tanaka Y. (2013). Membrane-associated RING-CH (MARCH) 8 mediates the ubiquitination and lysosomal degradation of the transferrin receptor. J. Cell Sci. 126, 2798-2809. 10.1242/jcs.119909 [DOI] [PubMed] [Google Scholar]
- Gottesdiener K. M., Karpinski B. A., Lindsten T., Strominger J. L., Jones N. H., Thompson C. B. and Leiden J. M. (1988). Isolation and structural characterization of the human 4F2 heavy-chain gene, an inducible gene involved in T-lymphocyte activation. Mol. Cell. Biol. 8, 3809-3819. 10.1128/MCB.8.9.3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes G. M., Chinn L., Cantor J. M., Cairns B., Levashova Z., Tran H., Velilla T., Duey D., Lippincott J., Zachwieja J. et al. (2015). Antitumor activity of an anti-CD98 antibody. Int. J. Cancer 137, 710-720. 10.1002/ijc.29415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L. and Fauci A. S. (1981). Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J. Immunol. 126, 1409-1414. [PubMed] [Google Scholar]
- Henderson N. C., Collis E. A., Mackinnon A. C., Simpson K. J., Haslett C., Zent R., Ginsberg M. and Sethi T. (2004). CD98hc (SLC3A2) interaction with beta 1 integrins is required for transformation. J. Biol. Chem. 279, 54731-54741. 10.1074/jbc.M408700200 [DOI] [PubMed] [Google Scholar]
- Holte H., Davies C. D. L., Kvaløy S., Smeland E. B., Foss-Abrahamsen A., Kaalhus O., Marton P. F. and Godal T. (1987). The activation-associated antigen 4f2 predicts patient survival in low-grade b-cell lymphomas. Int. J. Cancer 39, 590-594. 10.1002/ijc.2910390508 [DOI] [PubMed] [Google Scholar]
- Holte H., De Lange Davies C., Beiske K., Stokxe T., Marton P. F., Smeland E. B., Høie J. and Kvaløy S. (1989). Ki67 and 4F2 antigen expression as well as dna synthesis predict survival at relapse/tumour progression in low-grade B-cell lymphoma. Int. J. Cancer 44, 975-980. 10.1002/ijc.2910440605 [DOI] [PubMed] [Google Scholar]
- Hommel M. and Kyewski B. (2003). Dynamic changes during the immune response in T cell-antigen-presenting cell clusters isolated from lymph nodes. J. Exp. Med. 197, 269-280. 10.1084/jem.20021512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski B. A, Yang L. H., Cacheris P., Morle G. D. and Leiden J. M. (1989). The first intron of the 4F2 heavy-chain gene contains a transcriptional enhancer element that binds multiple nuclear proteins. Mol. Cell. Biol. 9, 2588-2597. 10.1128/MCB.9.6.2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li O., Chang X., Zhang H., Kocak E., Ding C., Zheng P. and Liu Y. (2006). Massive and destructive T cell response to homeostatic cue in CD24-deficient lymphopenic hosts. J. Exp. Med. 203, 1713-1720. 10.1084/jem.20052293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T., June C. H., Thompson C. B. and Leiden J. M. (1988). Regulation of 4F2 heavy-chain gene expression during normal human T-cell activation can be mediated by multiple distinct molecular mechanisms. Mol. Cell. Biol. 8, 3820-3826. 10.1128/MCB.8.9.3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki Y., Ohmura-Hoshino M., Goto E., Aoki M., Mito-Yoshida M., Uematsu M., Hasegawa T., Koseki H., Ohara O., Nakayama M. et al. (2007). Novel regulation of MHC class II function in B cells. EMBO J. 26, 846-854. 10.1038/sj.emboj.7601556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova M., Guenova M., Taskov H., Dimitrova E. and Staneva M. (1998). Levels of expression of CAF7 (CD98) have prognostic significance in adult acute leukemia. Leuk. Res. 22, 39-47. 10.1016/S0145-2126(97)00129-X [DOI] [PubMed] [Google Scholar]
- Oh J., Wu N., Baravalle G., Cohn B., Ma J., Lo B., Mellman I., Ishido S., Anderson M. and Shin J.-S. (2013). MARCH1-mediated MHCII ubiquitination promotes dendritic cell selection of natural regulatory T cells. J. Exp. Med. 210, 1069-1077. 10.1084/jem.20122695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H. S., Choi B. K., Kim Y. H., Lee D. G., Hwang S., Lee M. J., Park S. H., Bae Y.-S. and Kwon B. S. (2015). 4-1BB signaling enhances primary and secondary population expansion of CD8+ T cells by maximizing autocrine IL-2/IL-2 receptor signaling. PLoS ONE 10, e0126765 10.1371/journal.pone.0126765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C. R., Glidden M. H., Quah B. J. C. and Warren H. S. (2001). Use of the intracellular fluorescent dye CFSE to monitor lymphocyte migration and proliferation. In Current Protocols in Immunology. John Wiley & Sons, Inc. [DOI] [PubMed] [Google Scholar]
- Salter D. M., Krajewski A. S., Sheehan T., Turner G., Cuthbert R. J. and McLean A. (1989). Prognostic significance of activation and differentiation antigen expression in B-cell non-Hodgkin's lymphoma. J. Pathol. 159, 211-220. 10.1002/path.1711590307 [DOI] [PubMed] [Google Scholar]
- Sinclair L. V., Rolf J., Emslie E., Shi Y.-B., Taylor P. M. and Cantrell D. A. (2013). Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500-508. 10.1038/ni.2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J. and Surh C. D. (2002). T cell memory. Annu. Rev. Immunol. 20, 551-579. 10.1146/annurev.immunol.20.100101.151926 [DOI] [PubMed] [Google Scholar]
- Teague R. M., Tempero R. M., Thomas S., Murali-Krishna K. and Nelson B. H. (2004). Proliferation and differentiation of CD8+ T cells in the absence of IL-2/15 receptor beta-chain expression or STAT5 activation. J. Immunol. 173, 3131-3139. 10.4049/jimmunol.173.5.3131 [DOI] [PubMed] [Google Scholar]
- Verrey F., Closs E. I., Wagner C. A., Palacin M., Endou H. and Kanai Y. (2004). CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 447, 532-542. 10.1007/s00424-003-1086-z [DOI] [PubMed] [Google Scholar]