Abstract
The aim of the present investigation was to verify the effect of H2O2-induced oxidative stress on SO4= uptake through Band 3 protein, responsible for Cl-/HCO3- as well as for cell membrane deformability, due to its cross link with cytoskeletal proteins. The role of cytoplasmic proteins binding to Band 3 protein has been also considered by assaying H2O2 effects on hemoglobin-free resealed ghosts of erythrocytes. Oxidative conditions were induced by 30 min exposure of human erythrocytes to different H2O2 concentrations (10 to 300 μM), with or without GSH (glutathione, 2 mM) or curcumin (10 μM), compounds with proved antioxidant properties. Since SO4= influx through Band 3 protein is slower and better controllable than Cl- or HCO3- exchange, the rate constant for SO4= uptake was measured to prove anion transport efficiency, while MDA (malondialdehyde) levels and –SH groups were estimated to quantify the effect of oxidative stress. H2O2 induced a significant decrease in rate constant for SO4= uptake at both 100 and 300 μM H2O2. This reduction, observed in erythrocytes but not in resealed ghosts and associated to increase in neither MDA levels nor in –SH groups, was impaired by both curcumin and GSH, whereas only curcumin effectively restored H2O2-induced changes in erythrocytes shape. Our results show that: i) 30 min exposure to 300 μM H2O2 reduced SO4= uptake in human erythrocytes; ii) oxidative damage was revealed by the reduction in rate constant for SO4= uptake, but not by MDA or –SH groups levels; iii) the damage was produced via cytoplasmic components which cross link with Band 3 protein; iv) the natural antioxidant curcumin may be useful in protecting erythrocytes from oxidative injury; v) SO4= uptake through Band 3 protein may be reasonably suggested as a tool to monitor erythrocytes function under oxidative conditions possibly deriving from alcohol consumption, use of drugs, radiographic contrast media administration, hyperglicemia or neurodegenerative diseases.
Introduction
The erythrocyte membrane consists of a phospholipid bilayer with integral proteins associated to cytoskeleton through a proteins network underlying the cytoplasmatic side of the membrane [1]. As frequently exposed to oxidative events, it represents a model to study the effect of oxidative stress. In this regard, H2O2, Cu2+-ascorbic acid, Fe2+-ascorbic acid, azocompounds, known to be oxidant substances, and their effects, such as methemoglobin production, lipid peroxidation and spectrin-hemoglobin (Hb) complexes, have been long investigated [2,3]. Membrane rigidity induced by oxidative stress has been also observed, mainly due to a reduction in mobility of the proteins embedded in the phospholipidic bilayer [4].
One of the most studied integral membrane proteins is Band 3 protein, particularly abundant in erythrocytes [5]. It is a Cl-/HCO3- exchanger responsible for gas exchange, ion balance across cell membrane, osmotic and mechanical properties of the erythrocyte, such as anchoring motifs for the glycolytic enzymes and cell shape maintaining [6]. These functions are mediated by two domains, a membrane domain for anion exchange and a cytoplasmic domain which mainly contributes to the protein–protein interactions, by coupling the lipid bilayer to the underlying cytoskeleton, through cysteine -SH groups [7]. It has been demonstrated that deficiencies in Band 3 protein are responsible for a reduced cohesion between the lipid bilayer and cytoskeleton, with consequent shape changes, leading to spherocytosis [8]. Band 3 protein interaction with intracellular components, namely hemoglobin [9,10,11], have been also described.
In an attempt to better clarify the response of Band 3 protein to external stressors, in line with what previously demonstrated [12,13], and since the study of H2O2 effects on human erythrocytes has been rather limited to a morphological level [2,14], the present investigation aims to demonstrate whether and how Band 3 protein function, monitored through SO4= uptake measurement, is affected by H2O2-induced oxidative stress in human erythrocytes, at concentrations below 1 mM considered physiological and nontoxic [15].
To accomplish this goal, the rate constant for SO4= transport, slower and better controllable than Cl- or HCO3- uptake and, hence, more easily estimated [16,17], has been measured by a turbidimetric method [12], to monitor Band 3 protein efficiency. Oxidative damage, induced by exposure of erythrocytes to H2O2 (10 to 300 μM), has been assessed by MDA (Malondialdehyde) assay and –SH group detection and verified after treatment with antioxidant compounds, such as GSH (Glutathione) and curcumin (Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1E,6E-heptadiene-3,5-dione), a yellow hydrophobic pigment deriving from the rhizome (turmeric) of Curcuma longa herb, frequently used in foods and known to have antioxidant properties [18]. The use of curcumin in therapeutics is also reported [19,20]. Furthermore, the involvement of intracellular content in H2O2–induced alterations of SO4= transport has been evaluated on hemoglobin-free resealed ghosts of erythrocytes [13,21].
Materials and Methods
Ethics statement
The study was conducted after informed written consent of healthy volunteers during routine medical purposes. The informed consent covered the use of blood for research scopes. P. Romano, MD, from Clinical Pathology of Ospedale Maggiore Modica (Ragusa, Italy) collected and anonymized blood samples, according to the local ethics committee guidelines(S1 text). Samples were collected during 2012 and handled as described in our previous investigations [12,22,23,24].
Erythrocytes preparation
Blood was collected in heparinized tubes, washed in an isotonic solution (145 mM NaCl, 20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), pH 7.4, osmotic pressure 300 mOsm), henceforth defined as isotonic medium, and centrifuged three times (Thermoscientific, 1200 g, 5 min) to remove plasma and buffy coat. Erythrocytes were then suspended to either 3% hematocrit (for SO4= uptake measurement and for obtaining hemoglobin-free resealed ghosts), while were suspended to 10% hematocrit for MDA assay and –SH groups estimation. Blood samples with normal hemoglobin were used.
SO4= uptake measurement in intact erythrocytes
SO4= uptake is used to study Band 3 protein functionality in erythrocytes washed and incubated with Cl--free buffer [16]. In these conditions, the intracellular Cl- content is reduced, while the influx of SO4= increases due to the absence of competing extracellular Cl- [25].
To measure SO4= uptake, erythrocytes were suspended to 3% hematocrit in 35 ml isotonic medium, henceforth defined as SO4= medium, containing 118 mM Na2SO4, 20 mM HEPES, 15 mM glucose, pH 7.4, osmotic pressure 300 mOsm. At specified time intervals (5-15-30-45-60-90-120 min), 5 ml samples of erythrocytes suspension were removed and added to a test tube containing 10 μM DIDS (4,4’-diisothiocyanato-stilbene-2,2’-disulfonate) stopping medium and kept under ice. DIDS blocks Band 3 protein by irreversibly and specifically binding its extracellular moiety [26]. Erythrocytes were observed under a light microscope (Leica DMLS, 400x) at 5 min and 90 min of incubation in SO4= medium and an estimation of damaged erythrocytes was provided by cell count per microscopic field (400x). Percentage of deformed erythrocytes, in both control and experimental conditions, was expressed as a mean value deriving from cell count per 5 fields.
After the last sample withdrawal, erythrocytes were washed three times in cold isotonic medium (4°C, 1200 g, 5 min) to remove SO4= from the external medium. Cells were then hemolysed by 1 ml distilled water and proteins were hydrolysed by 4% v/v Perchloric acid. Cell membranes were discarded by centrifugation (4°C, 2700 g, 10 min) and SO4= in the supernatant was precipitated by sequentially adding 1 ml glycerol and distilled water solution (1:1), 1 ml 4 M NaCl plus HCl (hydrochloric acid 37% v/v) solution (12:1) and 500 μl 1.24 M BaCl2•2H2O to 500 μl supernatant from each sample. Levels of SO4= were spectrophotometrically measured at 425 nm wavelength (Beckman DU 640). Using a calibrated standard curve obtained by precipitating known SO4= concentrations, the absorption was converted to mM of intracellular SO4=, necessary to calculate the rate constant in min-1, derived from the following equation: Ct = C∞ (1-e-rt) + C0, where Ct, C∞ and C0 represent the intracellular SO4= concentrations measured at time, t 0 and ∞ respectively; e indicates the Neper number (2.7182818), r is a constant accounting for specific velocity of the process and t is time at which intracellular SO4= concentration is measured [27]. SO4= uptake was measured as [SO4=] L cells x10-2.
Preparation of hemoglobin-free ghosts of erythrocytes and SO4= uptake measurement
Pink resealed ghosts of human erythrocytes were prepared as reported elsewhere [13], with slight modifications. In detail, erythrocytes, after washing, were re-suspended at 3% hematocrit in 35 ml hyposmotic medium (2.5 mM NaH2PO4, 5 mM HEPES, pH 7.4). After 10 min stirring at 0°C, hemoglobin and intracellular content were eliminated by repeated centrifugations (Beckman J2-21, 4°C, 17000 g, 20 min). After the last centrifugation, the supernatant was removed and replaced with 35 ml isotonic resealing medium (145 mM NaCl, 20 mM HEPES, 5 mM glucose, pH 7.4), pre-heated at 37°C. Membranes were incubated at 37°C for 45 min, to allow resealing. Then, pink resealed ghosts, containing about 10% of the original hemoglobin, were used for SO4= uptake measurement, following the protocol above described for intact erythrocytes.
SO4= uptake measurement after H2O2 treatment
After rate constant for SO4= transport assessment in control conditions the following experimental protocols were used:
i) intact erythrocytes, after washing, were diluted to 3% hematocrit in isotonic medium plus H2O2 at different concentrations (10-100-300 μM respectively). After 30 min incubation at 25°C, samples were centrifuged to remove the supernatant and erythrocytes re-suspended to 3% hematocrit in SO4= medium. SO4= uptake was measured following what described for control conditions. Erythrocytes were observed under a light microscope at 5 min and 90 min of incubation in SO4= medium and damaged cells counted per microscopic field at the end of incubation.
ii) intact erythrocytes, after washing, were diluted to 3% hematocrit in isotonic medium containing either 2 mM GSH or, alternatively, 10 μM curcumin [28], as antioxidant compounds. Higher concentrations were discarded from the experimental design because of the cell shape alterations observed in the absence of H2O2 (data not shown), not allowing to measure SO4= uptake. On the other hand, lower concentrations were not used as ineffective. provoked, they. After 30 min incubation at 25°C, 300 μM H2O2 was added to either GSH- or curcumin-containing tube. Such treated samples were incubated for further 30 min at 25°C, before re-suspension in SO4= medium. SO4= uptake was then measured as described for control conditions and erythrocytes checked under a light microscope at 5 min and 90 min of incubation in SO4= medium. Damaged erythrocytes were quantified by cell count per microscopic field at the end of incubation.
iii) Resealed ghosts of erythrocytes, once obtained, were re-suspended to 3% hematocrit in 35 ml isotonic medium plus 300 μM H2O2. After 30 min incubation at 25°C, samples were centrifuged to remove supernatant and resealed ghosts re-suspended to the same hematocrit in SO4= medium. SO4= uptake was then measured as described for control conditions.
MDA assay
To evaluate the oxidation status of membrane, in both intact erythrocytes and resealed ghosts, treated or not with H2O2, TBARS (thiobarbituric acid reactive species) levels, resulting from reaction between TBA (thiobarbituric acid) and MDA (malondialdehyde), the end product of the lipid peroxidation process, were measured. One molecule of MDA and two molecules of TBA make up a chromogen-condensing product, formed in acidic environment, spectrophotometrically read at 532 nm [29]. In detail, treated or untreated intact erythrocytes or, alternatively resealed ghosts (10% hematocrit), were centrifuged (2700 g, 5 min) and lysed by freezing/thawing procedure in presence of 200 μM GSH. Each sample was vigorously shaken and stored at -20°C overnight. After freezing/thawing cycles, 200 μl lysed sample were treated with 0.1% v/v TBA (500 μl), freshly prepared in 1 N HCl solution. Samples were then boiled at 95°C for 1 hour, cooled in ice and centrifuged (Eppendorf, 4°C, 10000g, 15 min). The supernatant, collected from each sample, was spectrophotometrically read at 532 nm. MDA levels were measured by comparing the absorbance with a standard curve previously obtained from known amounts of MDA (Sigma, Italy) and converted to μM.
Isolation of Band 3 and -SH groups estimation
-SH groups estimation was performed on both intact erythrocytes and resealed ghosts (untreated or treated with, alternatively, 300 μM H2O2 or 300 μM H2O2 plus antioxidants) after Band 3 protein isolation [30].
Intact erythrocytes or, alternatively, resealed ghosts, after washing with isotonic medium (10% hematocrit), were lysed by cold hypotonic buffer (2.5 mM NaH2PO4, 5 mM HEPES). After 10 min stirring at 0°C, hemoglobin and intracellular content were eliminated by repeated centrifugations (Beckman J2-21, 4°C, 17000 g, 20 min). The process, for intact erythrocytes, was repeated with the same hypotonic buffer to discard hemoglobin. One volume of membranes (from both lysed erythrocytes and lysed resealed ghosts) was then incubated with nine volumes of 0.1 M NaOH for 30 min at 0°C in presence of 200 μM DTT (dithiothreitol) and 20 μg/ml PMSF (Phenylmethylsulfonil fluoride). After incubation, samples were centrifuged (4°C, 35000 g, 45 min). The pellet, containing Band 3 protein, was washed thrice with 5 mM sodium phosphate (pH 8.0) and used for -SH groups determination. For this purpose, pellet (200 μl) was solubilized by incubating 300 μl of 20% v/v SDS (Sodium dodecyl sulphate) reagent in 3 ml of 100 mM sodium phosphate (pH 8.0), for 30 min at 37°C. Samples were further incubated with 100 μl of 10 mM DTNB (5,5'-dithiobis-(2-nitrobenzoic acid) in 100 mM sodium phosphate (pH 8.0), for 20 min at 37°C. DTNB reacts specifically with thiol groups resulting in a highly coloured yellow anion. NEM (N-ethilmaleimide, 2 mM) was used as a positive control to obtain complete oxidation of –SH groups [23]. Levels of -SH groups in the suspension were measured by spectrophotometry at 412 nm, using the molar extinction coefficient 13,600 [30], and % decrease of –SH groups with respect to untreated erythrocytes was considered.
Reagents
All chemicals were purchased from Sigma (Milan, Italy). H2O2 was freshly prepared and diluted from 30% w/w stock solution. DIDS and GSH were dissolved in distilled water and diluted from 10 mM stock solutions. NEM was dissolved in ethanol and diluted from 1 M stock solution. Curcumin (Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1E,6E-heptadiene-3,5-dione, MW = 368.4, “pro analysis” purity grade, Sigma, code C7727 (Milan, Italy) was kindly provided by Prof. S. Cuzzocrea (University of Messina, Italy) and dissolved in 0.5% v/v DMSO, as stock solution. Solvents (ethanol and DMSO) were preventively tested upon erythrocytes to exclude any damage.
Experimental data and statistics
All data are expressed as arithmetic means ± S.E.M. (standard error of the mean) for statistical analysis. GraphPad Prism software (version 5.00 for Windows; GraphPad software, San Diego, CA) was used. Statistically significant differences between means were tested by paired Student’s t test or one-way analysis of variance (ANOVA), followed by Bonferroni's post hoc test and were assumed significant at p<0.05 (*p<0.05, **<0.01, ***<0.001); N represents the number of independent experiments. Statistical analysis, reported in both text and figure legends, refers to rate constant values for SO4= uptake, unless differently stated.
Results
SO4= uptake measurement in intact erythrocytes
Control conditions
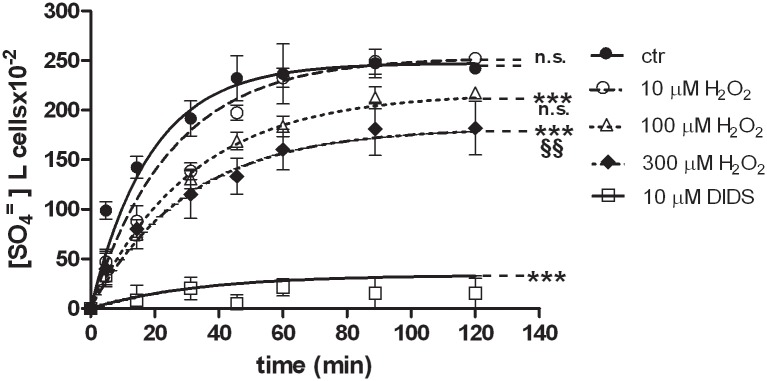
Fig 1 describes the time course for SO4= uptake in both treated and untreated erythrocytes. Treatment with 10 μM DIDS, applied at the beginning of incubation in SO4= medium, completely blocked SO4= uptake and, hence, considered as a positive control. SO4= transport in control erythrocytes (untreated) increased steeply at the initial stage and reached equilibrium within 30 min (Fig 1), exhibiting a rate constant of 0.053±0.001 (Table 1). Both SO4= concentrations at each time point and rate constant for SO4= uptake in control conditions were significantly different with respect to SO4= concentrations and rate constant of DIDS-treated cells (Fig 1, Table 1, ***p<0.001). Erythrocytes incubated in SO4= medium did not exhibit any morphological change, as shown in Fig 2A.
Fig 1. SO4= uptake in human erythrocytes under H2O2 treatment.
Time course of SO4= uptake in human erythrocytes measured in control conditions (untreated erythrocytes) or treated with 10 μM DIDS, applied at the beginning of incubation in SO4= medium. Points represent the mean ± SEM from separate experiments (see Table 1), where ***p<0.001 significant versus control and §§p<0.01 significant versus 100 μM H2O2, as determined by one way ANOVA followed by Bonferroni's post hoc test, by comparing all values of theoretical curves, at all time points.
Table 1. Rate constant for SO4= uptake in treated and untreated erythrocytes.
| Rate constant (min-1) | Time (min) | % decrease vs control | N | |
|---|---|---|---|---|
| control | 0.053±0.001 | 18 | 0 | 5 |
| 10 μM DIDS | 0.029±0.0015 *** | 33 | 45 | 3 |
| 10 μM H2O2 | 0.039±0.005 n. s. | 25 | 24 | 3 |
| 100 μ M H2O2 | 0.031±0.002 ***, n. s. | 31 | 40 | 4 |
| 300 μ M H2O2 | 0.032±0.001 ***,§§ | 30 | 40 | 6 |
| 2 mM GSH + 300 μ M H2O2 | 0.042±0.005 ***.¥¥ | 24 | 20 | 4 |
| 10 μM curcumin + 300 μ M H2O2 | 0.048±0.001 **,# | 20 | 10 | 4 |
Rate constant (min-1) of SO4= uptake in human erythrocytes measured in control conditions (untreated erythrocytes) or treated with either 10 μ M DIDS applied at the beginning of incubation in SO4= medium or H2O2 at different concentrations (10-100-300 μ M) or 300 μ M H2O2 preceded by antioxidant (2 mM GSH or 10 μ M curcumin). Data are presented as means ± SEM from separate experiments (see N column), where:
***p<0.001 significant versus control or
**p<0.01 significant versus control;
§§p<0.01 significant versus 100 μ M H2O2; n.s. not significantly different versus 100 μ M H2O2 or 10 μ M H2O2 versus control;
¥¥ p<0.01 significant versus 300 μ M H2O2 and
# p<0.05 versus 300 μ M H2O2, as determined by one way ANOVA followed by Bonferroni's post hoc test.
Fig 2. Erythrocytes morphology under H2O2 treatment.
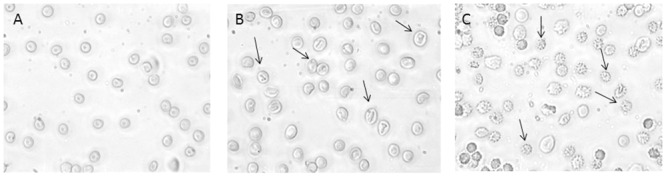
Light microscope observations (400x magnification) of: A) Untreated erythrocytes; B) 300 μM H2O2 treated erythrocytes at 5 min of SO4= medium incubation; C) 300 μM H2O2 treated erythrocytes at 90 min of SO4= medium incubation. Arrows indicate significant morphological changes in both B) and C) compared to the control (untreated) erythrocytes A).
H2O2 treatment
As depicted in Fig 1, SO4= uptake in erythrocytes exposed to 10 μM H2O2 did not significantly differ from SO4= uptake in untreated cells. Exposure to 100 μM H2O2 significantly reduced SO4= uptake when compared to untreated erythrocytes (Fig 1, ***p<0.001), while not significantly different with respect to what measured in 10 μM H2O2-treated erythrocytes (Fig 1). With regard to 300 μM H2O2 treatment, a significant SO4= uptake inhibition was observed when compared to both control (Fig 1, ***p<0.001) and 100 μM H2O2-treated erythrocytes (Fig 1, §§p<0.01). The rate constants for SO4= uptake, accounting for this inhibition, are reported in Table 1. Concentration of SO4= incorporated by the cells in both control and 10 μM H2O2-treated cells was significantly higher (p<0.001) than that one measured in 100 μM H2O2, 300 μM H2O2and DIDS treatment, respectively (Fig 1).
Fig 2 shows significant morphological changes in erythrocytes treated with 300 μM H2O2 at both 5 min (Fig 2B) and 90 min (Fig 2C) of SO4= medium incubation, with respect to untreated cells (Fig 2A). Lower H2O2 concentrations (both 10 and 100 μM) did not elicit any morphological change (data not shown). In control conditions (Fig 2 A), 1% damaged erythrocytes was observed, while, at 90 min of 300 μM H2O2 treatment, this percentage raised to 98% (Fig 2C).
On this basis, 300 μM H2O2 was used for further protocols on both intact erythrocytes and hemoglobin-free resealed ghosts of erythrocytes.
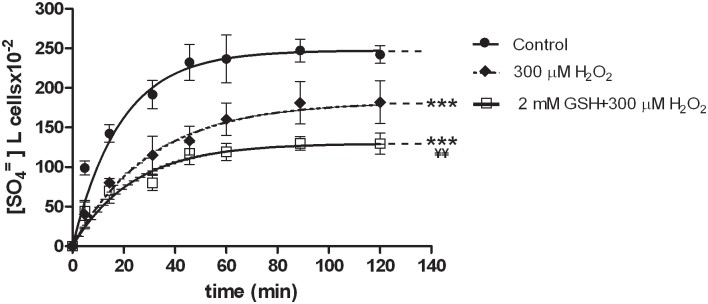
Erythrocytes, treated at first with 2 mM GSH and then with 300 μM H2O2 (Fig 3), exhibited a rate constant for SO4= uptake significantly higher (0.042±0.005, Table 1, ¥¥p<0.01) than what observed in 300 μM H2O2-treated erythrocytes (0.032±0.001, Table 1), albeit significantly lower than in untreated erythrocytes (Fig 3, ***p<0.001; 0.053±0.001, Table 1).
Fig 3. SO4= uptake in human erythrocytes under H2O2 plus GSH treatment.
Time course of SO4= uptake in human erythrocytes measured in control conditions (untreated erythrocytes) or treated with 300 μM H2O2, or treated with 2 mM GSH and then with 300 μM H2O2. Points represent the mean ± SEM from separated experiments (see Table 1), where ***p<0.001 significant versus control and ¥¥p<0.01 significant versus 300 μM H2O2, as determined by one way ANOVA followed by Bonferroni's post hoc test, by comparing all values of theoretical curves, at all time points.
Fig 4 shows that morphological changes induced by 300 μM H2O2 were impaired by 2 mM GSH neither after 5 min nor 90 min of incubation in SO4= medium, being the percentage of damaged cells unchanged after GSH plus H2O2 treatment.
Fig 4.
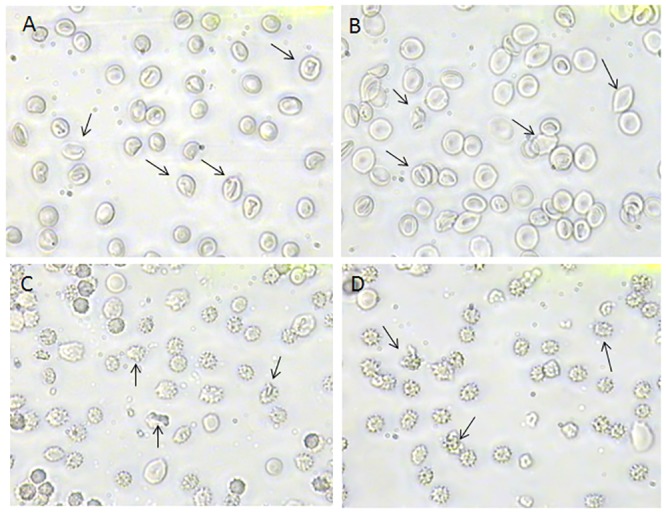
Erythrocytes morphology under H2O2 plus GSH treatment Light microscope observations (400x magnification) of: A) 300 μM H2O2 treated erythrocytes, observed after 5 min incubation in SO4= medium and B) erythrocytes treated with 2 mM GSH and then with 300 μM H2O2, observed after 5 min incubation in SO4= medium; C) 300 μM H2O2 treated erythrocytes, observed after 90 min incubation in SO4= medium and D) erythrocytes treated with 2 mM GSH and then with 300 μM H2O2 observed after 90 min incubation in SO4= medium. Arrows indicate that morphological changes after H2O2 treatment are not inhibited by GSH.
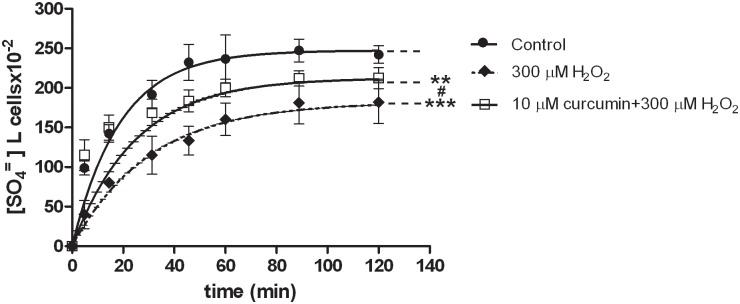
With regard to curcumin, treatment of erythrocytes with this natural antioxidant (10 μM), followed by exposure to 300 μM H2O2 (Fig 5), impaired the H2O2-induced inhibition of SO4= uptake, as shown by the rate constant (0.048±0.001, Table 1) significantly higher than that one measured in H2O2-treated erythrocytes (0.032±0.001, #p<0.05), while significantly lower than that one observed in untreated erythrocytes (0.053±0.001, **p<0.001).
Fig 5. SO4= uptake in human erythrocytes under H2O2 plus curcumin treatment.
Time course of SO4= uptake in human erythrocytes measured in control conditions (untreated erythrocytes) or treated with 300 μM H2O2 preceded or not by 10 μM curcumin application. Points represent the mean ± SEM from separate experiments (see Table 1), where ***p<0.001 significant versus control or **p<0.01 significant versus control and #p<0.05 significant versus 300 μM H2O2, as determined by one way ANOVA followed by Bonferroni's post hoc test, by comparing all values of theoretical curves, at all time points.
Fig 6 shows that morphological changes induced by 300 μM H2O2 treatment were prevented by 10 μM curcumin, at both 5 min and 90 min incubation in SO4= medium. Percentage of damaged cells after 10 μM curcumin plus 300 μM H2O2 treatment (Fig 6D) was significantly lower (3%) than that measured after exposure to 300 μM H2O2 (98%, Fig 6C), while comparable to what measured in untreated erythrocytes (1%, Fig 2A).
Fig 6. Erythrocytes morphology under H2O2 plus curcumin treatment.
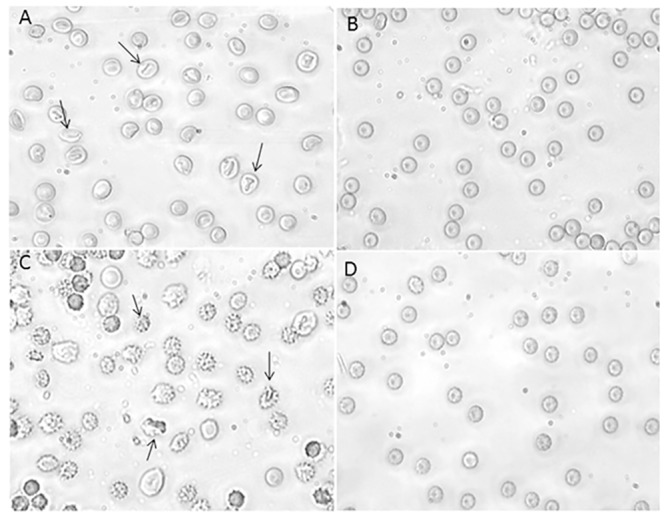
Light microscope observations (400x magnification) of: A) 300 μM H2O2 treated erythrocytes, observed after 5 min incubation in SO4= medium and B) erythrocytes treated with 10 μM curcumin followed by 300 μM H2O2, observed after 5 min incubation in SO4= medium; C) 300 μM H2O2 treated erythrocytes, observed after 90 min incubation in SO4= medium and D) erythrocytes treated with 10 μM curcumin followed by 300 μM H2O2, observed after 90 min incubation in SO4= medium. Arrows indicate morphological changes after H2O2 treatment, attenuated by curcumin treatment.
MDA assay and –SH groups estimation in erythrocytes
To estimate the oxidative damage induced by H2O2, MDA assay was performed. Treatment of erythrocytes with 300 μM H2O2 revealed MDA levels comparable to those observed in untreated cells (Fig 7A, n.s.). No MDA test was further carried out on erythrocytes treated with the antioxidant compounds chosen for the experimental design (GSH and curcumin).
Fig 7. MDA levels and –SH groups estimation under H2O2 treatment.
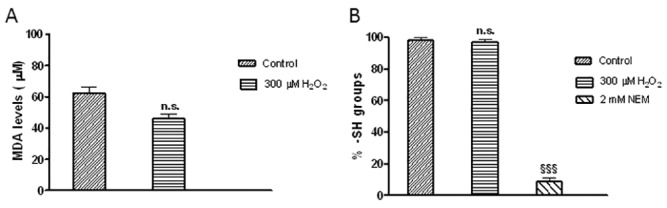
A) MDA levels observed in control (untreated erythrocytes) and in erythrocytes treated with 300 μM H2O2. Data are presented as means ± SEM from at least 3 experiments, where n.s. is not significantly different versus control, as determined by t-Student test. B) Percentage of –SH groups measured in control (untreated erythrocytes), in erythrocytes treated with either 300 μM H2O2 or 2 mM NEM. Bars represent the mean ± SEM from at least 3 experiments, where n.s. is not significant versus control, §§§ p<0.001 significant versus control and 300 μM H2O2-treated erythrocytes, as determined by one way ANOVA followed by Bonferroni's post hoc test.
As shown in Fig 7B, –SH groups levels measured in 300 μM H2O2-treated erythrocytes were comparable to those measured in untreated cells (Fig 7B, n.s.). These data were significantly different with respect to –SH groups levels measured in erythrocytes treated with 2 mM NEM, known as an oxidant compound and here assumed as a positive control (Fig 7B, §§§ p<0.001 versus control and 300 μM H2O2-treated erythrocytes).
SO4= uptake measurement in hemoglobin-free resealed ghosts of erythrocytes
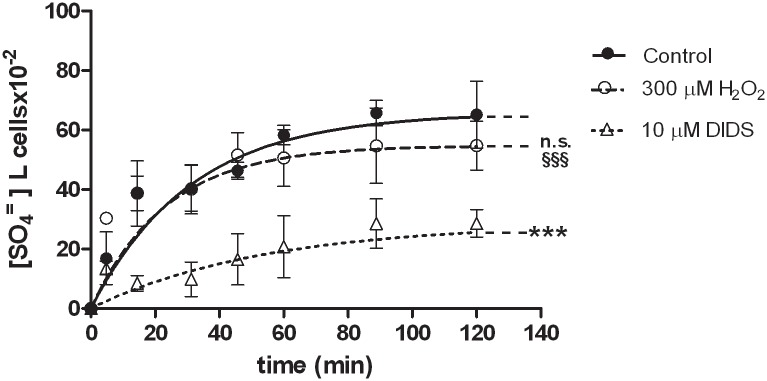
Fig 8 describes the time course for SO4= uptake in untreated resealed ghosts of erythrocytes. As a positive control, 10 μM DIDS was applied at the beginning of the incubation in SO4= medium. The rate constant for SO4= uptake in untreated resealed ghosts was significantly higher (0.063±0.001, Table 2) than that observed in presence of DIDS (0.018±0.002 ***p<0.001). This significant difference accounted for SO4= uptake efficiency after resealing procedures. After exposure to 300 μM H2O2, SO4= uptake was unchanged with respect to untreated erythrocytes (Fig 8, n.s.), while significantly higher (0.042±0.005, §§§ p<0.001) than in DIDS-treated cells. The rate constants for SO4= uptake in these experimental conditions are reported in Table 2.
Fig 8. SO4= uptake in human hemoglobin-free resealed ghosts of erythrocytes under H2O2 treatment.
Time course of SO4= uptake in hemoglobin-free resealed ghosts of erythrocytes measured in control conditions (untreated) or treated with either 300 μμM H2O2, or 10 μ M DIDS applied at the beginning of the incubation in SO4= medium. Points represent the mean ± SEM from separate experiments (see Table 2), where ***p<0.001 significant versus control or §§§p<0.001 significant versus 10 μ M DIDS and n.s. not significant versus control, as determined by one way ANOVA followed by Bonferroni's post hoc test, by comparing all values of theoretical curves, at all time points.
Table 2. Rate constant for SO4= uptake in treated and untreated resealed ghosts of erythrocytes.
| Rate constant (min-1) | Time (min) | % decrease vs control | N | |
|---|---|---|---|---|
| control | 0.063±0.001 | 16 | 0 | 5 |
| 10 μ M DIDS | 0.018±0.002*** | 55 | 72 | 5 |
| 300 μ M H2O2 | 0.042±0.005 §§§, n.s. | 24 | 34 | 5 |
Rate constant (min-1) of SO4= uptake in resealed ghosts of erythrocytes measured in control conditions (untreated ghosts) or treated with either or 10 μ M DIDS applied at the beginning of the incubation in SO4= medium or 300 μ M H2O2 Data are presented as means ± SEM of at least 5 experiments, where:
***p<0.001 significant versus control;
§§§ p<0.001 significant versus 10 μ M DIDS and n.s. not significant versus control, as determined by one way ANOVA followed by Bonferroni's post hoc test.
MDA assay and –SH groups estimation in resealed ghosts
In 300 μ M H2O2-treated resealed ghosts, MDA levels, as well as –SH groups quantity, were not significantly different with respect to those measured in untreated ghosts (data not shown). Also in this case, no further protocols with H2O2 treatment combined with antioxidants was used.
Discussion
Hydrogen peroxide (H2O2) is produced by O2.- dismutation, inducing oxidative modifications in oxyhemoglobin [31] and generating superoxide anion radical (O2.-), the main source of ROS in erythrocytes. It has been shown that the exposure of oxyhemoglobin to H2O2 leads to increased concentration of methemoglobin, lipid peroxidation and spectrin-hemoglobin complexes [2,14]. Nevertheless, the effect of H2O2 on Band 3 protein, the most abundant protein in human erythrocytes [5], which is linked to proteins underlying erythrocytes membrane (e.g. ankyrin, adducin, protein 4.1, glycophorin) [6], is still not completely clarified.
H2O2, being a cellular metabolite, may function as a signal in the regulation of its cellular levels and, owing to the variety of its effects, the study of its actions on cells, namely erythrocytes, may become quite difficult,. Moreover, that “H2O2 signaling” may be involved in both non beneficial effects, due to too low or high H2O2 levels, and possible cell adaptation is also worthy of note ([32] and ref therein). Nevertheless, this interesting issue still remains an open question.
The aim of the present study was to better verify, in human erythrocytes, the effect of H2O2 on Band 3 protein, whose function can be monitored by measuring the efficiency in SO4= uptake [12].
Our results show that cell shape of erythrocytes exposed for 30 min to 300 μ M H2O2 was, as expected, altered, accordingly to what already shown by Snyder and co-authors [2], who described echinocytes formation under H2O2 treatment. Furthermore, here we show a significant reduction in rate constant for SO4= uptake under μ M H2O2 treatment, in a dose dependent manner. To possibly explain at which level this alteration occurred, oxidative effects induced by 30 min exposure to 300 μ M H2O2 were estimated by two separate methods: the first one, MDA assay, to verify the involvement of lipoperoxidation events, and the second one, -SH group measurement, to verify whether the oxidative damage was inflicted at level of membrane proteins.
Both MDA assay and -SH groups measurement revealed neither lipoperoxidation events nor –SH groups oxidation, when compared to control conditions. Hence, based on MDA levels detected in 300 μ M H2O2-treated erythrocytes, we could exclude that lipoperoxidation was responsible for rate constant reduction. This result is interesting, if compared with what shown by Mendanha and co-authors [14], observing lipid peroxidation, revealed by MDA production, in erythrocytes exposed to 300 μ M H2O2 for 3 h, a longer time than that one chosen for our experiments. In addition, the same authors demonstrated lipid peroxidation for H2O2 concentrations from 300 to 1500 μ M.
Hence, the present investigation would add one more element to what reported by Mendanha and co-authors [14], that is oxidative stress induced by a short time exposure to H2O2, whose effects are not detectable by commonly used methods, including MDA assay, affects erythrocyte function and may be revealed by monitoring SO4= uptake through Band 3 protein. Therefore, this technique seems to be a more sensitive tool to evaluate the effects of oxidative stress on human erythrocytes function.
As far as –SH groups are concerned, we demonstrated that their levels after 300 μ M H2O2 treatment are unchanged if compared to control conditions. This observation is in line with findings from other authors [2,14], demonstrating, by means of electron spin resonance (EPR) spectroscopy, that the amount of accessible –SH groups gradually increases with H2O2 concentration, up to 300 μ M. What is more, Mendhana and co-workers [14] stated that, at H2O2 concentrations higher than 300 μ M, a decrease of –SH groups occurs, due to lipoperoxidation events causing –SH groups oxidation, as said above. So, according to these authors [14], we can suggest that in the H2O2-concentration range from 50 to 300 μM, proteins, putatively Hb molecules, bind to the membrane during oxidative stress and contribute additional -SH groups. At any rate, the reduction in rate constant for SO4= uptake observed in H2O2-treated erythrocytes, along with cell shape modifications, can be explained with the higher amount of free –SH groups in the membrane, correlating with degree of rigidity.
That Band 3 protein may be a target of oxidative stress has been already reviewed by Lutz and Bogdanova ([11] and ref therein), reporting about clusters of this protein, associated to oxidized and denatured Hb.
As a further step supported by the hypothesis that the intracellular content of erythrocytes is involved in the response to oxidative stress induced by 30 min exposure to 300 μM H2O2, we measured SO4= transport in H2O2-treated hemoglobin free resealed ghosts, consisting in re-constituted erythrocytes membranes deprived of intracellular content.
Resealed ghosts preparation is a validated method for drug delivery [33], for producing circulating blood analyte biosensor after entrapping fluorescence-dyes [34], and, closer to our purpose, a method to study Band 3 protein function (monitored by SO4= uptake measurement) and its cross linking with cytoplasmic proteins [13]. The use of resealed ghosts has been recently revealed as a promising technique to study Band 3 protein capacity to carry molecules, other than those currently known [21].
Coming back to our experiments, the exposure of ghosts for 30 min to 300 μM H2O2 did not inhibit the rate constant for SO4= uptake with respect to untreated ghosts, thus suggesting that the first target of H2O2 seems not to be the membrane, but the intracellular content, most likely Hb, which cross links with integral membrane protein, according to findings from other authors [11]. This link has been already shown, and, what is more, spectrin-Hb complex formation in human erythrocytes membrane after H2O2 treatment has been also demonstrated ([11] and ref therein), along with morphological changes and decreased cell deformability [2].
Moreover, in line with what described by Ivanov and collaborators [35] on erythrocytes exposed to an acidic medium, we may suggest that H2O2, permeating the membrane, may interfere with Hb, thus generating oxidative products, chemically reactive and capable to diffusing from cytosol to extracellular medium, thus inducing oxidative damage on membranes. In our case, H2O2, acting from the inside of the erythrocytes, would alter proteins conformation, resulting in more accessible –SH groups in the membrane. This effect would possibly obscure the oxidative damage inflicted by products deriving from Hb oxidation.
In an attempt to protect Band 3 protein from H2O2-induced oxidative stress, both GSH (2 mM) and curcumin (10 μM), have been used as antioxidant compounds. With regard to GSH experiments, the H2O2-induced reduction in rate constant for SO4= uptake was recovered, though not completely restored, by pre-treatment of erythrocytes with GSH, whereas cell shape alterations, due to exposure to H2O2, were not impaired. Hence, we may suggest that GSH, in these experimental conditions, is not completely effective in protecting erythrocytes from oxidative damage, not preserving cell shape, which is indeed critical for erythrocytes function.
It has been already shown that GSH is not able to cross the plasma membrane, so that the intracellular GSH is the only source for erythrocytes [11,36]. This can explain why pre-treatment with GSH fails in protecting H2O2-induced cell shape alterations, detected in our experiments. Moving from this consideration, curcumin, a natural antioxidant permeating cell membrane and already known for its beneficial properties [19,37,38], has been chosen to better focus on SO4= transport under H2O2-induced oxidative conditions in human erythrocytes.
Pre-treatment of erythrocytes with curcumin, significantly prevented both H2O2-induced cell shape alterations and reduction in rate constant for SO4= uptake. Therefore, we may hypothesize that, in the present experimental conditions, this natural antioxidant seems to be more effective in protecting both anatomical and functional properties of erythrocytes, when exposed to oxidative stressors.
This result could be explained with the antioxidant properties of flavonoids. Some flavonoids may incorporate into the hydrophobic membrane bilayer, thus reducing its fluidity and stability [39,40], which results in a more difficult diffusion of free radicals (deriving from the cytosolic environment) and, in turn, in a more effective antioxidant power of these natural compounds. As a matter of fact, that curcumin protects SO4= transport in human erythrocytes exposed to stressors in the external medium, like acid pH, has been recently demonstrated [23], supporting thus the hypothesis of beneficial effects of natural antioxidants.
Here we show similar effects, by proving that not only cell shape but also the rate constant for SO4= uptake of Band 3 protein is protected by curcumin, when erythrocytes are exposed to H2O2 induced-oxidative stress. This is in line with what recently shown by Yang and co-authors [41], reporting that curcuminoids supplementation may prevent membrane dysfunction of human erythrocytes due to hyperglycemia–induced oxidative conditions.
The beneficial action of curcumin on Band 3 protein function may also open the way to further considerations, taking into account the variety of oxidative stress sources affecting human tissues, including the above mentioned hyperglycemia [41]and even the administration of radiographic contrast media, a procedure which has become very common to date. In this latter regard, Iopromide administration [42] during coronary angiography in patients with coronary artery disease has been shown to induce Band 3 protein clustering, dissociation of spectrin from Band 3 protein, loss of homogeneity of the spectrin network, echinocytes formation, and, in turn, hypo-oxygenation of the tissue, as similarly observed in the myocardium of pigs [43]. Echinocytes formation has been already described in erythrocytes [2] exposed to H2O2.
On this basis and supported by the present results, we may underline the adjuvant role of natural antioxidants assumed with food, such as curcumin, in protecting Band 3 function from external stressors, such as increase in ROS levels, which correlate with high glucose blood levels, aging, physical exercise, use of drugs and their metabolites, neurodegenerative diseases and chronic alcohol consumption [32,37,41,44,45,46]. In this latter regard, increased blood viscosity, along with impaired erythrocyte flexibility and increased fragility, have been reported as a consequence of alcohol-induced oxidation [47].
Conclusions
Our results further confirm that, in human erythrocytes, the link between Band 3 protein and intracellular content is crucial for maintaining normal shape and, in turn, oxygen exchange efficiency; demonstrate that Band 3 protein function, monitored by SO4= uptake measurement, is affected by low concentrated H2O2, adding more information about the effect of oxidative stress at structural level, as already assessed by other authors [2], as well as at functional level, namely correlated to Band 3 protein; a short-time exposure to low concentrations of H2O2 induces oxidative damage not detectable by MDA assay or –SH group estimation and is sufficient to alter Band 3 protein efficiency, estimated by SO4= uptake measurement; natural antioxidants like curcumin are effective in protecting erythrocytes from this damage; Band 3 protein function assessment through SO4= uptake measurement can be confirmed as a tool to monitor oxidative stress effect on erythrocytes.
These findings open the way to further investigations, specifically addressed to evaluate, at level of erythrocytes function, the consequences of oxidative conditions possibly provoked by strenuous physical exercise, alcohol consumption, drugs administration or neurodegenerative diseases, which have nowadays become very common. Aged erythrocytes could be also considered for future study about the impact of aging on their function, monitored through SO4= transport measurement, related to hemoglobin modification [11,48]. The putative adaptation of erythrocytes, known as pre-conditioning response to a more or less prolonged oxidative stress [49], would be also a noteworthy issue.
Supporting Information
(PDF)
Acknowledgments
The authors acknowledge P. Romano, MD, from Clinical Pathology of Ospedale Maggiore Modica (Ragusa, Italy) for providing blood samples. The authors are also grateful to Prof. L. Romano for encouragement and helpful suggestions.
Data Availability
All relevant data are within the paper.
Funding Statement
Dr. Orazio Romano is affiliated to BromaTech s.r.l., Giarre (CT, Italy). Dr. O. Romano contributed to statistical analysis. The specific role of OR is reported in the ‘author contributions’ section. His voluntary contribution has been friendly and totally free of charge. The commercial company provided neither funding for the study design, data collection and analysis, page charges, or preparation of the manuscript nor financial support in the form of Dr. O. Romano’s salary and/or research materials.
References
- 1.Bennett V, Baines AJ (2001) Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev 81: 1353–1392. [DOI] [PubMed] [Google Scholar]
- 2.Snyder LM, Fortier NL, Trainor J, Jacobs J, Leb L, Lubin B, et al. (1985) Effect of hydrogen peroxide exposure on normal human erythrocyte deformability, morphology, surface characteristics, and spectrin-hemoglobin cross-linking. J Clin Invest 76: 1971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies KJ (1987) Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem 262: 9895–9901. [PubMed] [Google Scholar]
- 4.Soszynski M, Bartosz G (1997) Decrease in accessible thiols as an index of oxidative damage to membrane proteins. Free Radic Biol Med 23: 463–469. [DOI] [PubMed] [Google Scholar]
- 5.Steck TL (1974) The organization of proteins in the human red blood cell membrane. A review. J Cell Biol 62: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anong WA, Franco T, Chu H, Weis TL, Devlin EE, Bodine DM, et al. (2009) Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood 114: 1904–1912. 10.1182/blood-2009-02-203216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salhany JM, Sloan RL, Cordes KA (1990) In situ cross-linking of human erythrocyte band 3 by bis(sulfosuccinimidyl)suberate. Evidence for ligand modulation of two alternate quaternary forms: covalent band 3 dimers and noncovalent tetramers formed by the association of two covalent dimers. J Biol Chem 265: 17688–17693. [PubMed] [Google Scholar]
- 8.Stefanovic M, Markham NO, Parry EM, Garrett-Beal LJ, Cline AP, Gallagher PG, et al. (2007) An 11-amino acid beta-hairpin loop in the cytoplasmic domain of band 3 is responsible for ankyrin binding in mouse erythrocytes. Proc Natl Acad Sci U S A 104: 13972–13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulquiney PJ, Kuchel PW (1997) Model of the pH-dependence of the concentrations of complexes involving metabolites, haemoglobin and magnesium ions in the human erythrocyte. Eur J Biochem 245: 71–83. [DOI] [PubMed] [Google Scholar]
- 10.Alenghat FJ, Golan DE (2013) Membrane protein dynamics and functional implications in mammalian cells. Curr Top Membr 72: 89–120. 10.1016/B978-0-12-417027-8.00003-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutz HU, Bogdanova A (2013) Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol 4: 387 10.3389/fphys.2013.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morabito R, Marino A, Romano P, Rigano C, La Spada G (2013) Sulphate and chloride-dependent potassium transport in human erythrocytes are affected by crude venom from nematocysts of the jellyfish Pelagia noctiluca. Cell Physiol Biochem 32: 86–95. [DOI] [PubMed] [Google Scholar]
- 13.De Luca G, Gugliotta T, Scuteri A, Romano P, Rinaldi C, Sidoti A, et al. (2004) The interaction of haemoglobin, magnesium, organic phosphates and band 3 protein in nucleated and anucleated erythrocytes. Cell Biochem Funct 22: 179–186. [DOI] [PubMed] [Google Scholar]
- 14.Mendanha SA, Anjos JL, Silva AH, Alonso A (2012) Electron paramagnetic resonance study of lipid and protein membrane components of erythrocytes oxidized with hydrogen peroxide. Braz J Med Biol Res 45: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller S, Riedel HD, Stremmel W (1997) Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal Biochem 245: 55–60. [DOI] [PubMed] [Google Scholar]
- 16.Jennings ML (1976) Proton fluxes associated with erythrocyte membrane anion exchange. J Membr Biol 28: 187–205. [DOI] [PubMed] [Google Scholar]
- 17.Romano L, Passow H (1984) Characterization of anion transport system in trout red blood cell. Am J Physiol 246: C330–338. [DOI] [PubMed] [Google Scholar]
- 18.Glauert HP, Calfee-Mason K, Stemm DN, Tharappel JC, Spear BT (2010) Dietary antioxidants in the prevention of hepatocarcinogenesis: a review. Mol Nutr Food Res 54: 875–896. 10.1002/mnfr.200900482 [DOI] [PubMed] [Google Scholar]
- 19.Gupta SC, Patchva S, Aggarwal BB (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15: 195–218. 10.1208/s12248-012-9432-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelkel M, Jacob C, Dicato M, Diederich M (2010) Potential of the dietary antioxidants resveratrol and curcumin in prevention and treatment of hematologic malignancies. Molecules 15: 7035–7074. 10.3390/molecules15107035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vigliarolo T, Guida L, Millo E, Fresia C, Turco E, De Flora A, et al. (2015) Abscisic Acid transport in Human Erythrocytes. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marino A, Crupi R, Rizzo G, Morabito R, Musci G, La Spada G (2007) The unusual toxicity and stability properties of crude venom from isolated nematocysts of Pelagia noctiluca (Cnidaria, Scyphozoa). Cell Mol Biol (Noisy-le-grand) 53 Suppl: OL994–1002. [PubMed] [Google Scholar]
- 23.Morabito R, Falliti G, Geraci A, Spada GL, Marino A (2015) Curcumin Protects -SH Groups and Sulphate Transport after Oxidative Damage in Human Erythrocytes. Cell Physiol Biochem 36: 345–357. 10.1159/000430256 [DOI] [PubMed] [Google Scholar]
- 24.Marino A, Morabito R, La Spada G (2009) Factors altering the haemolytic power of crude venom from Aiptasia mutabilis (Anthozoa) nematocysts. Comp Biochem Physiol A Mol Integr Physiol 152: 418–422. 10.1016/j.cbpa.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 25.Jennings ML (2005) Evidence for a second binding/transport site for chloride in erythrocyte anion transporter AE1 modified at glutamate 681. Biophys J 88: 2681–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jessen F, Sjoholm C, Hoffmann EK (1986) Identification of the anion exchange protein of Ehrlich cells: a kinetic analysis of the inhibitory effects of 4,4'-diisothiocyano-2,2'-stilbene-disulfonic acid (DIDS) and labeling of membrane proteins with 3H-DIDS. The Journal of membrane biology 92: 195–205. [DOI] [PubMed] [Google Scholar]
- 27.Teti D, Crupi M, Busa M, Valenti A, Loddo S, Mondello M, et al. (2005) Chemical and pathological oxidative influences on band 3 protein anion-exchanger. Cell Physiol Biochem 16: 77–86. [DOI] [PubMed] [Google Scholar]
- 28.Kossler S, Nofziger C, Jakab M, Dossena S, Paulmichl M (2012) Curcumin affects cell survival and cell volume regulation in human renal and intestinal cells. Toxicology 292: 123–135. 10.1016/j.tox.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain SK, Levine SN, Duett J, Hollier B (1990) Elevated lipid peroxidation levels in red blood cells of streptozotocin-treated diabetic rats. Metabolism 39: 971–975. [DOI] [PubMed] [Google Scholar]
- 30.Roy SS, Sen G, Biswas T (2005) Role of sulfhydryl groups in band 3 in the inhibition of phosphate transport across erythrocyte membrane in visceral leishmaniasis. Arch Biochem Biophys 436: 121–127. [DOI] [PubMed] [Google Scholar]
- 31.Sadrzadeh SM, Graf E, Panter SS, Hallaway PE, Eaton JW (1984) Hemoglobin. A biologic fenton reagent. J Biol Chem 259: 14354–14356. [PubMed] [Google Scholar]
- 32.Liochev SI (2013) Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med 60: 1–4. 10.1016/j.freeradbiomed.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 33.Ihler GM (1983) Erythrocyte carriers. Pharmacol Ther 20: 151–169. [DOI] [PubMed] [Google Scholar]
- 34.Ritter SC, Milanick MA, Meissner KE (2011) Encapsulation of FITC to monitor extracellular pH: a step towards the development of red blood cells as circulating blood analyte biosensors. Biomed Opt Express 2: 2012–2021. 10.1364/BOE.2.002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov IT (1999) Low pH-induced hemolysis of erythrocytes is related to the entry of the acid into cytosole and oxidative stress on cellular membranes. Biochim Biophys Acta 1415: 349–360. [DOI] [PubMed] [Google Scholar]
- 36.Srivastava SK, Beutler E (1969) The transport of oxidized glutathione from the erythrocytes of various species in the presence of chromate. Biochem J 114: 833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu C, Mei XT, Zheng YP, Xu DH (2014) Zn(II)-curcumin protects against hemorheological alterations, oxidative stress and liver injury in a rat model of acute alcoholism. Environ Toxicol Pharmacol 37: 729–737. 10.1016/j.etap.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 38.Gupta SC, Patchva S, Koh W, Aggarwal BB (2012) Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol 39: 283–299. 10.1111/j.1440-1681.2011.05648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arora A, Byrem TM, Nair MG, Strasburg GM (2000) Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys 373: 102–109. [DOI] [PubMed] [Google Scholar]
- 40.Chaudhuri S, Banerjee A, Basu K, Sengupta B, Sengupta PK (2007) Interaction of flavonoids with red blood cell membrane lipids and proteins: antioxidant and antihemolytic effects. Int J Biol Macromol 41: 42–48. [DOI] [PubMed] [Google Scholar]
- 41.Yang W, Fu J, Yu M, Wang D, Rong Y, Yao P, et al. (2015) Effects of three kinds of curcuminoids on anti-oxidative system and membrane deformation of human peripheral blood erythrocytes in high glucose levels. Cell Physiol Biochem 35: 789–802. 10.1159/000369738 [DOI] [PubMed] [Google Scholar]
- 42.Franke RP, Scharnweber T, Fuhrmann R, Wenzel F, Kruger A, et al. (2014) Effect of radiographic contrast media on the spectrin/band3-network of the membrane skeleton of erythrocytes. PLoS One 9: e89512 10.1371/journal.pone.0089512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung F, Matschke K, Mrowietz C, Tugtekin SM, Geissler T, et al. (2003) Influence of radiographic contrast media on myocardial tissue oxygen tension: NaCl-controlled, randomised, comparative study of iohexol versus iopromide in an animal model. Clin Hemorheol Microcirc 29: 53–61. [PubMed] [Google Scholar]
- 44.Hsieh HL, Yang CM (2013) Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int 2013: 484613 10.1155/2013/484613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson's disease. J Parkinsons Dis 3: 461–491. 10.3233/JPD-130230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong Y, Li Y, Zhao Y, Tang F, Wang X (2013) Cluster of erythrocyte band 3: a potential molecular target of exhaustive exercise-induced dysfunction of erythrocyte deformability. Can J Physiol Pharmacol 91: 1127–1134. 10.1139/cjpp-2013-0145 [DOI] [PubMed] [Google Scholar]
- 47.Guillet R, Nalpas B, Perrotin P, Beuzard Y, Koutsouris D, et al. (1991) Increased Erythrocyte Rigidity in Chronic-Alcoholics without Cirrhosis—Deformability Improvement of Erythrocyte Subpopulations after Alcohol-Withdrawal. Clinical Hemorheology 11: 55–62. [Google Scholar]
- 48.Arashiki N, Kimata N, Manno S, Mohandas N, Takakuwa Y (2013) Membrane Peroxidation and Methemoglobin Formation Are Both Necessary for Band 3 Clustering: Mechanistic Insights into Human Erythrocyte Senescence. Biochemistry 52: 5760–5769. 10.1021/bi400405p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li D, Xu Y, Gao CY, Zhai YP (2014) Adaptive protection against damage of preconditioning human umbilical cord-derived mesenchymal stem cells with hydrogen peroxide. Genet Mol Res 13: 7304–7317. 10.4238/2014.February.21.9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper.