Abstract
Oligodendrocytes produce myelin, an insulating sheath required for the saltatory conduction of electrical impulses along axons. Oligodendrocyte loss results in demyelination, which leads to impaired neurological function in a broad array of diseases ranging from pediatric leukodystrophies and cerebral palsy, to multiple sclerosis and white matter stroke. Accordingly, replacing lost oligodendrocytes, whether by transplanting oligodendrocyte progenitor cells (OPCs) or by mobilizing endogenous progenitors, holds great promise as a therapeutic strategy for the diseases of central white matter. In this Primer, we describe the molecular events regulating oligodendrocyte development and how our understanding of this process has led to the establishment of methods for producing OPCs and oligodendrocytes from embryonic stem cells and induced pluripotent stem cells, as well as directly from somatic cells. In addition, we will discuss the safety of engrafted stem cell-derived OPCs, as well as approaches by which to modulate their differentiation and myelinogenesis in vivo following transplantation.
KEY WORDS: Myelin, Remyelination, Astrocyte, Stem cell, Neural stem cell, Glial progenitor cell, Multiple sclerosis, Leukodystrophy, White matter
Summary: This Primer article reviews the molecular events regulating oligodendrocyte development in vivo and discusses approaches to generate oligodendrocytes in vitro.
Introduction
Oligodendrocytes are the myelinating cells of the central nervous system (CNS), and the last major neural phenotype to form during development. They arise from oligodendrocyte progenitor cells (OPCs), which can generate either oligodendrocytes or astrocytes depending on context (ffrench-Constant and Raff, 1986; Raff et al., 1983a). Once an OPC commits to an oligodendroglial fate, it extends multiple processes that individually ensheath axons, and then proceeds to generate the concentric layers of modified cell membrane that compose myelin (Sherman and Brophy, 2005). By virtue of the dense fasciculation of axons in the subcortical white matter tracts, myelination is most notable in the white matter, but myelinating oligodendrocytes are present in the gray matter as well. In both compartments, myelin is necessary for the saltatory conduction of action potentials along axons, via sodium ion fluxes at the nodes of Ranvier. Oligodendrocytes also dictate the organization of those nodes as well as the sequestration of their ion channels (Kaplan et al., 1997; Susuki and Rasband, 2008). In addition to their contributions to neuronal signaling, oligodendrocytes provide trophic support to neurons, and especially to long axons that may not receive adequate support from intra-axonal trafficking alone (Nave, 2010a,b). As such, oligodendrocytes are crucial not only for the maintenance of neural transmission, but also of neurons themselves, as evidenced by the slow neuronal degeneration that accompanies stable demyelination in the adult CNS; hence the devastation wrought by demyelinating diseases of the brain and spinal cord.
The demyelinating diseases all involve the dysfunction or loss of oligodendrocytes, and hence the loss of central myelin. They include the acquired disorders of myelin in adults, such as multiple sclerosis, white matter stroke and age-related white matter loss, as well as the early myelination failure of cerebral palsy, and the hereditary and metabolic disorders of myelin loss (Helman et al., 2015; Powers, 2004). As a group, the myelin disorders are among the most prevalent and disabling conditions in neurology; multiple sclerosis alone is the most commonly diagnosed neurological disease in young adults (Rosati, 2001). Yet only a few of these disorders can be effectively managed, largely by the prevention of inflammatory or vascular demyelination (Franklin and Goldman, 2015), and no clinical treatment capable of achieving the remyelination of demyelinated axons has yet been developed. As myelin deficiency or loss contributes to a wide array of disorders, and both OPCs and oligodendrocytes are relatively homogeneous cell populations, cell replacement therapy may be an especially appropriate strategy by which to treat demyelinating disorders (Goldman et al., 2012). That said, the optimal cell type for transplantation-mediated clinical remyelination might not be the oligodendrocyte per se, but rather its progenitor: mature oligodendrocytes are fibrous and fragile, non-proliferative and non-migratory, limiting their practical utility in cell therapeutics. By contrast, OPCs are everything that their mature derivatives are not: they are highly migratory, actively proliferative, mechanically hardy, and robustly myelinogenic after their in vivo dispersal and maturation. As a result, OPCs – also referred to in the literature synonymously as glial progenitor cells – have become of great interest as potential vectors for the restoration of myelin in the demyelinated brain and spinal cord. In particular, the generation of myelinogenic OPCs from human pluripotent stem cells (hPSCs) might provide a common cellular reagent by which to treat the entire range of demyelinating disorders (Fox et al., 2014) (Fig. 1)
Fig. 1.
Derivation and use of pluripotent stem cell-derived and human fetal brain-derived oligodendrocyte progenitor cells. Oligodendrocyte progenitor cells (OPCs) can be readily derived from pluripotent stem cells, including both human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) (Wang et al., 2013b). In addition, they can be generated directly by reprogramming non-neural somatic cells to the oligodendroglial lineage (Najm et al., 2013; Yang et al., 2013), although this has not yet been accomplished in human cells (*). OPCs can also be purified directly from human brain tissue, as discussed elsewhere (Goldman et al., 2012). Regardless of their source, purified OPCs can then be transplanted to rectify the multicentric and diffuse demyelination seen in the neurodegenerative phase of multiple sclerosis, as well as in vascular demyelination and potentially white matter stroke. OPCs are highly migratory – they typically distribute throughout the neuraxis after perinatal graft, and can do so well into adulthood. Similarly, childhood disorders of myelin, including lysosomal storage disorders such as Krabbe disease, metachromatic leukodystrophy and Tay-Sachs disease, as well as vanishing white matter disease and its variants, might also be targets for OPC transplant-mediated remyelination. In those hereditary and metabolic disorders, allografted glial progenitors derived from human iPSCs (hiPSCs) might prove effective at concurrent metabolic correction and remyelination (Fox et al., 2014). In addition, when combined with gene editing technologies, such as zinc finger nuclease-, TALEN- and CRISPR/Cas9-mediated strategies, to correct underlying mutations before transplant (Li et al., 2014), one might anticipate the use of gene-edited hiPSCs and their derived OPCs as autologous cell therapeutics across the entire range of pediatric leukodystrophies (Fox et al., 2014; Helman et al., 2015). Besides their value as cell therapeutics, pluripotent stem cell-derived OPCs may also be used to model the effects of agents designed to influence myelination as well as glial function. Moreover, patient-derived hiPSC-derived OPCs can be engrafted into neonatal mice to produce human glial chimeric mice (Windrem et al., 2014), which can be used to assess the contributions of oligodendrocytes and astrocytes, as well as of residual OPCs, to diseases in which their respective roles had hitherto been unclear (Kondo et al., 2014). Such patient- and disease-specific human glial chimeric mice might allow the development of personalized glial-directed treatments. PSA-NCAM, polysialylated-neural cell adhesion molecule; FACS or MACS, fluorescence-activated or magnetic cell sorting.
On that basis, in this Primer we will address recent insights into OPC and oligodendrocyte development, how these data have guided the development of techniques by which to generate OPCs and oligodendrocytes from pluripotent cells, and how the cells thereby generated might be reasonably employed as clinical therapeutics.
How do OPCs arise and how are they maintained?
To make an OPC, or any other cell type that one wishes to produce from a pluripotent cell, one must be familiar with its normal ontogeny and developmental regulation. Following gastrulation and ectodermal specification, neuroepithelial cells form and rapidly expand along the neuraxis, remaining within what becomes the ventricular lining of the CNS (Schoenwolf and Alvarez, 1989). Neural stem cells initially expand within this layer, and serially give rise to the neurons, radial glia, astrocytes and bipotential astrocyte-oligodendrocyte progenitor cells that ultimately colonize the CNS. Neurons and their radial glial guides are the first cell types to be specified (Huttner and Brand, 1997; Jan and Jan, 2001), and only late in neurogenesis does significant gliogenesis and glial progenitor cell production begin. The timing of these events varies according to both species and region; in the human forebrain, oligodendrocyte-competent glial progenitor cells are not detected in appreciable numbers until well into the second trimester, at 16-18 weeks (Sim et al., 2011).
OPC generation in the spinal cord
OPC production begins in the spinal cord, in which it was first studied from a developmental perspective. This body of work has been expertly reviewed elsewhere (Gallo and Deneen, 2014; Rowitch, 2004; Rowitch and Kriegstein, 2010), so we will note only the major regulatory determinants revealed by those studies. Briefly, in mice OPCs begin to populate the developing spinal cord in three temporally distinct waves. The first of these originates at embryonic day (E) 12.5 from discrete domains in the ventral neural tube (Orentas et al., 1999), and occurs under the control of ventrally derived sonic hedgehog (SHH), which drives Nkx6.1- and Nkx6.2-regulated Olig1 and Olig2 transcription (Vallstedt et al., 2005). Accordingly, SHH signaling is both necessary and sufficient for Olig1 and Olig2 expression, and hence for OPC induction (Lu et al., 2000). Later in development, a second wave of OPCs originates from the dorsal neural tube at E15.5, in an SHH-independent manner (Cai et al., 2005; Vallstedt et al., 2005). The specification of these cells is transcriptionally regulated by Dbx1 and Ascl1, and requires a combination of fibroblast growth factor (FGF) signaling and reduced bone morphogenetic protein (BMP) signaling (Fogarty et al., 2005; Sugimori et al., 2008). In the spinal cord, dorsal OPCs tend to remain in the dorsolateral white matter, where they comprise less than 20% of the total OPC pool, while ventrally derived OPCs dominate and constitute the remainder (Fogarty et al., 2005; Vallstedt et al., 2005; Zhu et al., 2011). A third wave of OPC production occurs at birth, and appears to include local expansion of now resident progenitors as well as new immigrants from the central canal subependyma (Rowitch and Kriegstein, 2010).
Oligoneogenesis in the forebrain
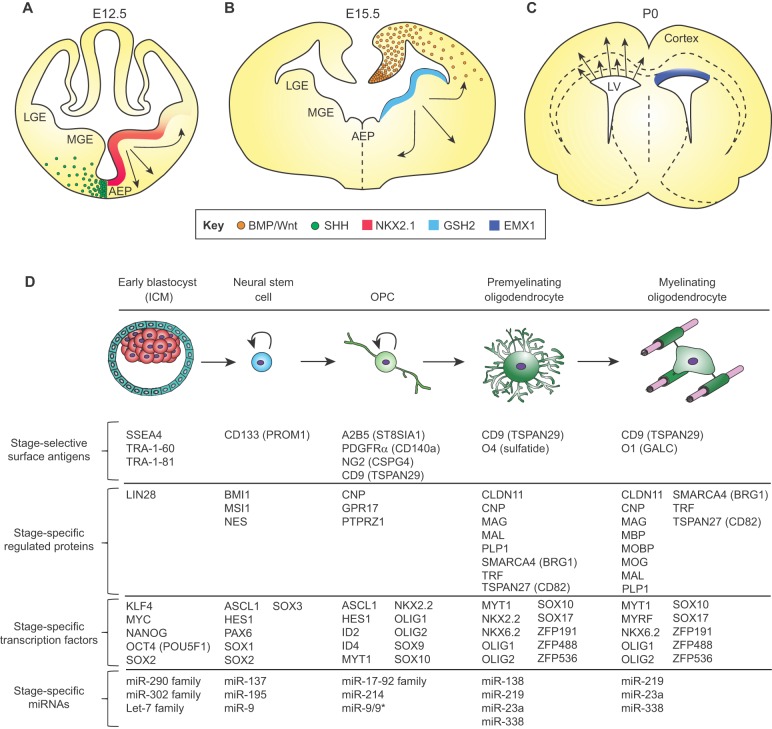
OPC production in the forebrain follows a similar pattern to that in the spinal cord (Rowitch and Kriegstein, 2010; Woodruff et al., 2001) (Fig. 2A-C). As in the spinal cord, following the initial period of neurogenesis, ventrally derived SHH drives the first phase of glial progenitor production in the first of three distinct waves of OPC generation (Kessaris et al., 2006; Nery et al., 2001; Orentas et al., 1999). The first wave arises in the medial ganglionic eminence and presumptive pre-optic area, and is under the transcriptional control of Nkx2.1 (Tekki-Kessaris et al., 2001). These OPCs migrate tangentially and dorsally to colonize the entire forebrain (Kessaris et al., 2006; Klambt, 2009) (Fig. 2A). Days later, a second wave of OPCs emerges from the developing medial forebrain, particularly from the lateral ganglionic eminence; these cells are under the transcriptional control of Gsh2 (also known as Gsx2) (Chapman et al., 2013) and migrate dorsally to the cortex, dispersing throughout the forebrain in the process (Kessaris et al., 2006; Klambt, 2009) (Fig. 2B). A third and final wave of OPC production and parenchymal infiltration occurs closer to birth, and projects radially from the dorsal subventricular zone (SVZ) and outer SVZ, directly subjacent to the developing corpus callosum; these cells express the homeobox transcription factor Emx1 (Kessaris et al., 2006) (Fig. 2C). These dorsally originating OPCs migrate locally to populate the corpus callosum and contribute to the overlying cortex as well, admixing with OPCs derived from the ganglionic eminences. In animal models, the early migrating OPCs from the first two waves that initially colonize the cortical mantle are later displaced by the third wave of pericallosal SVZ-derived OPCs, such that by postnatal day (P) 10, dorsally derived pericallosal OPCs make up most of the adult callosal and capsular white matter (Kessaris et al., 2006).
Fig. 2.
The transcription factor code and associated markers of oligodendroglial fate restriction. Oligodendrocytes arise from different regions of the developing forebrain (shown here) and spinal cord. (A) By E12.5 in the mouse, oligodendroglia are seen to arise ventrally from NKX2.1-expressing cells in the medial ganglionic eminence, which migrate to the dorsal forebrain. (B) By E15.5 in the mouse, cells generated under GSH2 control arise from the expanded ganglionic eminence and striatal anlagen to migrate broadly. (C) Around birth, the EMX1-expressing dorsal subcallosal ventricular wall shifts to OPC production. These distinct populations compete with one another as maturation proceeds, resulting in an admixture of OPCs and oligodendrocytes derived from different developmental germinal zones in the adult brain. See also Rowitch and Kriegstein (2010). (D) At the cellular level, either in vitro or in vivo, pluripotent stem cells progress through serial developmental stages: neuroepithelial stem cell, OPC, premyelinating and ultimately myelinating oligodendroglia. Such cardinal stages of oligodendroglial development are characterized by distinct signal effectors and transcription factors that regulate the expression of multiple stage-specific marker genes (Ahmed et al., 2009; Cahoy et al., 2008; Nishiyama et al., 2009; Sim et al., 2011; Young, 2011; Zhang, 2001; Zhang et al., 2014; Zuchero and Barres, 2013). At each stage, cells can be identified and isolated on the basis of temporally regulated surface antigens, which permit the selection of cells of defined mitotic and differentiation potential (Ishibashi et al., 2004; Nishiyama et al., 2009; Yuan et al., 2011; Zhang, 2001; Zhao et al., 2012). These discrete stages exhibit distinct miRNA profiles as well, that may serve to regulate translation, and thus sharpen phenotypic transitions during lineage progression (Barca-Mayo and Lu, 2012; Dugas et al., 2010; He et al., 2012; Lau et al., 2008; Mallanna and Rizzino, 2010; Shenoy and Blelloch, 2014; Shi et al., 2010; Zhao et al., 2010). AEP, anterior entopeduncular nucleus; ICM, inner cell mass; LGE, lateral ganglionic eminence; LV, lateral ventricle; MGE, medial ganglionic eminence.
Whether analogous developmental dynamics during oligodendrogenesis are manifest in the human brain is unknown. Yet these sequential waves of OPC generation and the different colonization patterns do inform us that there is more than one developmental program by which OPCs may be produced, and that we might need to consider the competitive interactions among OPCs and oligodendrocytes derived from different regions, particularly as we attempt to model their use as clinical therapeutics.
OPC maintenance in the mature brain
During development, OPCs disperse to achieve a relatively uniform distribution throughout both the adult white and gray matter, in both of which they integrate as mitotically competent progenitors occupying geographically distinct, non-overlapping domains (Hughes et al., 2013; Zhang and Miller, 1996). Once the cells reach their final destination, many exit the cell cycle to mature as myelinating oligodendroglia in an activity-dependent selection process, the proximal determinants of which remain unclear; many also persist as stably resident OPCs. Comprising ∼5% of all cells in the adult mouse brain and ∼3% of cells in the adult human brain (Roy et al., 1999; Scolding, 1998; Scolding et al., 1999), these parenchymal OPCs appear to provide the substrate by which normal myelin turnover is maintained (Franklin and ffrench-Constant, 2008). Importantly, although resident adult OPCs have been described as giving rise either predominantly or solely to oligodendrocytes (Kang et al., 2010; Rivers et al., 2008; Tripathi et al., 2010) or to both astrocytes and oligodendrocytes (Nishiyama et al., 2009) in vivo, they have also been reported to generate pyriform projection neurons in adult rodents, even in the absence of injury or exogenous manipulation (Guo et al., 2010; Rivers et al., 2008). Moreover, once removed from the brain, adult OPCs can also produce functional neurons of a variety of phenotypes, both in vitro and upon re-introduction into developing brains (Belachew et al., 2003; Nunes et al., 2003). Thus, whereas the normal in vivo fate of resident adult OPCs appears to be a function of injury and disease, brain region, assessment modality and perhaps species, it appears that these cells retain cell-autonomous multilineage competence, and may do so until the point of oligodendroglial commitment.
How are OPCs and oligodendrocytes specified and how can we identify them?
OPCs thus develop from multilineage competent neuroepithelial precursors in a stereotypic fashion, to ultimately give rise to myelinating oligodendrocytes (Fig. 2D). During this lineage progression, the cells sequentially express a number of transcription factors that specify their fate, while defining their real-time phenotype and lineage potential. The transcription factor code and associated intrinsic signals for oligodendroglial lineage restriction (Fig. 2D) have been extensively reviewed elsewhere (Rowitch and Kriegstein, 2010; Zuchero and Barres, 2013), but we will briefly discuss them here and highlight those that have been useful to isolate cells at various stages of oligodendroglial commitment.
In the mouse forebrain, several transcription factors, such as Znf488 (Zfp488) (Wang et al., 2006) and Znf536 (Zfp536) (Dugas et al., 2006; Qin et al., 2009; Yang et al., 2013), are involved in the early phase of oligodendroglial lineage restriction (Fig. 2D). This process is also mediated by the downregulation of early neuroepithelial transcripts, such as Sox2, which is associated with the sequential upregulation of glial lineage-associated Sox family members, including Sox8 and Sox9 (Wegner and Stolt, 2005). In turn, Sox8 and Sox9 expression correlate with that of transcription factors that include Nkx2.2, Olig1 and Olig2, and Sox10 (Kuhlbrodt et al., 1998), the expression of which precedes that of the platelet-derived growth factor (PDGF) α receptor (also known as CD140a), encoded by Pdgfra (Fig. 2D). The appearance of PDGFRα is a key event in OPC ontogeny, in that its expression heralds the acquisition of the OPC fate and PDGF responsiveness (Hart et al., 1989; Noble et al., 1988; Richardson et al., 1988). As such, it is an effective marker for the isolation of OPCs, from human as well as mouse cell preparations (Sim et al., 2011; Wang et al., 2013a). Indeed, PDGFRα is expressed only by OPCs in the human brain, in which PDGFRα-expressing cells account for all potentially myelinogenic cells (Sim et al., 2011).
During both development and adulthood, OPCs also express gangliosides recognized by the A2B5 antibody (Roy et al., 1999; Wolswijk and Noble, 1989), as well as the chondroitin sulfate proteoglycan type 4 (CSPG4; also known as NG2) (Chang et al., 2000; Dawson et al., 2000; Levine et al., 2001) (Fig. 2D). Importantly, although both A2B5 and NG2 have found broad use as phenotypic reporters for OPCs, neither is specific for the phenotype. The A2B5 antigen is expressed by neuronal as well as glial progenitors (Goldman, 1990; Raff et al., 1983b), requiring a secondary depletion of neurons in order to achieve selectivity for OPCs (Dietrich et al., 2002; Windrem et al., 2004). NG2 is expressed by pericytes, which are difficult to distinguish from perivascular OPCs because they are also localized around the vasculature. As a result, PDGFRα-directed isolation, whether by fluorescence-activated or magnetic cell sorting, seems to be the most effective current means of isolating human OPCs, whether from human tissue or pluripotent cell cultures (Sim et al., 2011).
Unlike their rodent counterparts, human oligodendroglia are postmitotic (Armstrong et al., 1992; Kirschenbaum et al., 1994) and, as such, oligodendroglial maturation and myelinogenesis can proceed only after OPCs exit the cell cycle. This occurs with the transcriptional repression of E2F1 and c-MYC, and requires the cell cycle inhibitor p27KIP1 (CDKN1B) (Casaccia-Bonnefil et al., 1999; Larocque et al., 2005). At the point of transition from OPC to immature oligodendrocyte, another set of transcription factors is induced. These include SOX17, a negative modulator of Wnt signaling that contributes to triggering the cell cycle exit of OPCs, a key event in their oligodendroglial lineage restriction (Chew et al., 2011). As OPCs transition to postmitotic oligodendrocytes, they undergo dramatic chromatin condensation with heterochromatin formation (Huang et al., 2015), which is associated with the silencing of many genes involved in their proliferative response to mitogenic cues, including Wnt, FGF and PDGF (Barca-Mayo and Lu, 2012; Yu et al., 2010), as well as that of their upstream regulator MYC (Magri et al., 2014). Yet despite chromatin condensation, Olig2 and Sox10 expression continues, and serves not only to direct oligodendrocyte fate commitment (Liu et al., 2007; Li et al., 2007; Xin et al., 2005), but also to consolidate phenotype by recruiting chromatin remodelers to oligodendrocyte-specific enhancers (Weider et al., 2013; Yu et al., 2013; Emery and Lu, 2015).
At this early stage of oligodendrocytic commitment, a number of miRNAs, including miR219, miR138 and miR338, which repress negative regulators of oligodendrocytic fate such as Sox6 and Hes5, are upregulated (Zhao et al., 2010) (Fig. 2D). In particular, miR219 represses the expression of PDGFRα, FOXJ3 and ZNF238 (ZBTB18), all of which contribute to the maintenance of mitotic OPCs. Accordingly, Barres and colleagues have reported that the induction of miR219 may be sufficient to mediate the transition from OPC to postmitotic myelinogenic oligodendrocyte (Dugas et al., 2010). During this progression from OPC to oligodendrocyte, the Olig1-regulated G protein-coupled receptor (GPCR) Gpr17 is itself downregulated, resulting in the suppression of its nuclear effectors Id2 and Id4, otherwise potent inhibitors of oligodendroglial commitment (Chen et al., 2009). Another GPCR, Gpr56 (Adgrg1), is expressed and appears to be required for oligodendroglial lineage progression at this point (Ackerman et al., 2015; Giera et al., 2015). As a result of this coordinated set of molecular events, OPCs commit to oligodendrocytic phenotype.
After oligodendrocytic specification, myelination is triggered by the transcriptional activation of myelin regulatory factor (MYRF) and its positively regulated targets, including myelin basic protein (MBP), proteolipid protein 1 (PLP1), myelin-associated glycoprotein (MAG), cyclic nucleotide phosphodiesterase (CNP) and myelin oligodendrocyte protein (MOG) (Emery et al., 2009), all of which are crucial in myelin formation (Fig. 2D). Importantly, the persistent transcription and enhancer occupancy of both Olig2 and Sox10 appear necessary for the maintenance of MYRF expression, and hence of its myelinogenic target genes. These newly lineage-restricted oligodendroglia also initiate expression of the surface lipid sulfatide, recognized by the O4 antibody (Sommer and Schachner, 1981). As the cells transition to myelinogenesis with the induction of Myrf expression and become more mature, their membrane lipid compositions change, and the cells begin to express galactocerebroside, recognized by the O1 antibody (Bansal et al., 1989; Keilhauer et al., 1985).
The stereotypic sequence of transcription factors and surface antigens expressed during oligodendroglial development allows us to follow that process in real time using transgenic reporters of gene expression as well as RNA-based detection methods. In addition, the sequential expression of stage-selective surface antigens, including, but not limited to, NG2, A2B5, PDGFRα, CD9, O4/sulfatide and O1/galactocerebroside, allows investigators to use immunoisolation-based strategies to isolate OPCs and their derived oligodendrocytes at prospectively defined stages in their development. These methods now include immunopanning (Cahoy et al., 2008; Dugas et al., 2006) and both immunomagnetic and fluorescence-activated cell sorting (Nunes et al., 2003; Sim et al., 2006; Windrem et al., 2004). Importantly, OPCs and oligodendroglia acquired at these stages differ in their mitotic and migratory competence, as well as in their maturation and myelination rates (Sim et al., 2011), allowing investigators to choose and isolate cells at specific stages appropriate for the experimental and therapeutic tasks at hand (Fig. 1).
How to make OPCs from pluripotent stem cells
Our nascent understanding of the cellular events and growth factors involved in neuroepithelial and oligodendrocyte differentiation led to the development of methods for instructing pluripotent cells to an oligodendroglial fate, first using mouse embryonic stem cells (ESCs), and, later, human (h) ESCs (Hu et al., 2009; Izrael et al., 2007; Liu et al., 2011; Nistor et al., 2005). These methods, some of which are summarized in Fig. 3, attempt to reproduce the temporal sequence of signals to which early neuroepithelial cells are exposed as they differentiate along the oligodendroglial lineage. In general, they include the sequential exposure to neuralizing FGFs and retinoids, followed by SHH as both a ventralizing morphogen and mitogen, with subsequent exposure to PDGF, which supports OPC expansion, and both insulin growth factor and thyroid hormone, which potentiate oligodendrocytic maturation. Yet, although efficient protocols have been developed that allow the high-yield production of hESC-derived OPCs and oligodendrocytes, ESC-based cell therapy suffers from the possibility of rejection due to incompatible immunophenotypes. Enthusiasm has thus developed for the potential use of autologous grafts of OPCs derived from induced pluripotent stem cells (iPSCs) for myelin repair (Yamanaka, 2007, 2008). iPSCs are generated by reprogramming somatic cells to a non-phenotypically committed pluripotent state by the forced expression of transcriptional regulators such as Oct4 (Pou5f1), Sox2, Myc, Klf4 and Nanog or, alternatively, with miRNAs that modulate the expression of these proteins, or small molecules that mimic their actions. Such treatment permits the re-emergence of the self-renewing stem cell phenotype (Lin et al., 2009; Stadtfeld and Hochedlinger, 2010), and may be accomplished using approaches that minimize the risk of later tumorigenesis (Lin and Ying, 2013). Yet however generated, iPSCs have the decided advantage over hESCs of permitting the autologous transplantation of their derived cell types of interest back into the subjects from which they were generated, potentially obviating the need for post-transplant immune suppression.
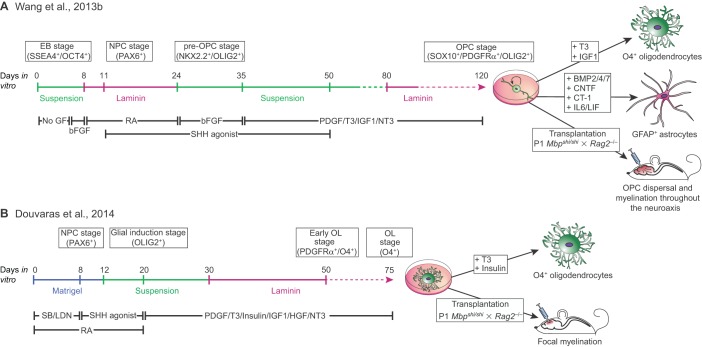
Fig. 3.
Comparative approaches towards producing OPCs and oligodendrocytes from pluripotent stem cells. Several approaches can be used to generate oligodendrocytes and their progenitors from human pluripotent cells (hPSCs), including both hiPSCs and hESCs. (A) The protocol developed by Wang and co-workers (Wang, 2013a,b; see also Hu et al., 2009 and Stacpoole et al., 2013) to generate stably maintained OPCs that can be instructed to adopt an oligodendrocyte phenotype at will by mitogen removal (see Box 2 for a more detailed breakdown of the specific differentiation steps). This method produces stable populations of mitotically competent OPCs that are especially amenable to in vivo engraftment and dispersal, but require long periods of time in culture before the acquisition of an oligodendrocyte phenotype. (B) The accelerated differentiation protocol developed by Douvaras and colleagues (Douvaras et al., 2014; Douvaras and Fossati, 2015; Piao et al., 2015) to generate oligodendroglia by rapidly transiting through the OPC stage. Compared with the protocol described in A, this approach yields postmitotic myelinogenic oligodendrocytes more quickly. Such cells can engraft and myelinate focal lesions but may be limited in their in vivo expansion and dispersal. These contrasting approaches might thus be most appropriately directed to distinct clinical situations and disease targets. bFGF, FGF2; CT-1, cardiotrophin 1 (CTF1); EB, embryoid body; GF, growth factor; LDN, LDN193189 (a BMP pathway antagonist); NPC, neural progenitor cell; NT3, neurotrophin 3 (NTF3); OL, oligodendrocyte; RA, retinoic acid; SB, SB431542 (a TGFβ antagonist); T3, thyroid hormone.
The methods first described for generating OPCs from ESCs have proven effective for iPSCs as well (Czepiel et al., 2011; Wang et al., 2013b) and yield both bipotential glial progenitors, which are able to give rise to astrocytes, as well as myelinogenic oligodendrocytes (Box 1). More recent advances in optimizing the methods for generating OPCs from hiPSCs and hESCs, which have incorporated more serially distinct stages of growth factor exposure and more extended periods of differentiation, have led to the production of highly enriched populations of human OPCs that are efficiently myelinogenic in vivo while manifesting no discernible tumor potential (Douvaras et al., 2014; Wang et al., 2013b) (Fig. 3). A number of different hPSC lines obtained from a variety of sources were used in the Wang et al. (2013b) study; these included WA09/H9 hESCs (Thomson et al., 1998) and both keratinocyte- and fibroblast-derived hiPSCs (Chambers et al., 2009; Maherali and Hochedlinger, 2008). The efficiency of hiPSC-derived OPC production, defined by the acquisition of an OLIG2+/NKX2.2+/SOX10+/PDGFRα+ phenotype prior to PDGFRα-directed sorting was uniformly over 70%. Moreover, the efficiency of OPC production rose with time in vitro, such that proportions of over 80% A2B5+/PDGFRα+ OPCs were first seen after 110 days in vitro, and rose further thereafter, becoming maximal only after 150 days in vitro (Wang et al., 2013b) (Fig. 3A, Box 2). As such, this study, as well as others, demonstrated that an in vitro recapitulation of the major developmental cues encountered by differentiating neuroepithelial cells during oligodendroglial development, applied serially over a time period analogous to that of late gestation, permitted the robust and sustained production of OPCs from human pluripotent cells.
Box 1. hiPSC-derived OPCs can produce astrocytes as well as oligodendrocytes
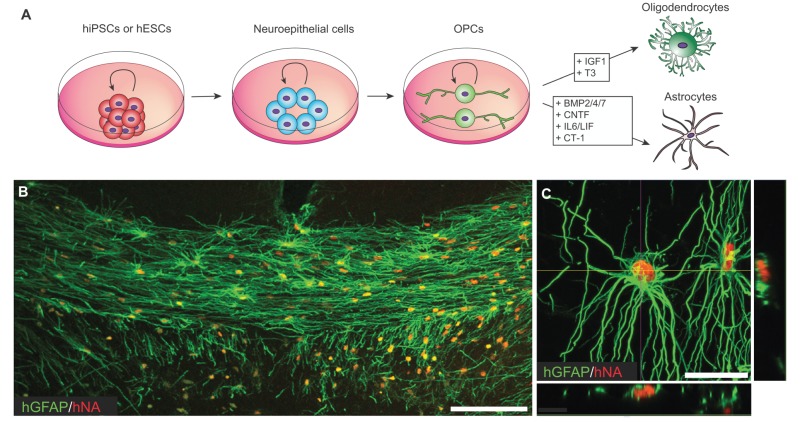
Human OPCs can produce astrocytes as well as oligodendrocytes until their terminal division; their daughter cells may be instructed to either cell fate in a largely context-dependent fashion (Nunes et al., 2003; Windrem et al., 2004). Astrocytic fate is potentiated by exposure to BMPs, whereas oligodendrocytic fate potential is preserved by BMP pathway suppression (Mabie et al., 1997; Namihira and Nakashima, 2013; Sim et al., 2006, 2011) (see A). By contrast, oligodendrocytic maturation is in turn facilitated by IGF1 and thyroid hormone (T3) receptor-dependent pathways, among others (Barres et al., 1994, 1993) (see A). Using current hESC and hiPSC astroglial differentiation protocols (Krencik et al., 2011; Wang et al., 2013b), a distinct population of GFAP+ astrocytes also appears in culture before OPCs, reflecting the heterogeneity of adult astrocytic phenotypes. When hiPSC-derived OPCs are grafted into the hypomyelinated shiverer mouse brain, astrocytes mature first (see B; human GFAP, green; human nuclear antigen, red), providing the scaffold upon which OPCs align and mature as myelinating oligodendroglia; together, the two daughter lineages achieve the reconstitution of the otherwise dysmyelinated white matter tracts (Wang et al., 2013b) in a manner identical to that accomplished by human fetal tissue-derived OPCs (Sim et al., 2011). Interestingly, white matter astrocytes derived from transplanted human OPCs develop the morphology typical of human fibrous astrocytes, preserving their characteristic human astrocytic morphology even in the context of the adult mouse brain (Wang et al., 2013b) (see C). Scale bars: 100 µm in B; 50 µm in C.
Box 2. Production of oligodendrocyte progenitors from hiPSCs to remyelinate the mouse brain

OPCs and oligodendrocytes can be generated from pluripotent stem cells using a multi-stage protocol involving extended periods of differentiation to produce highly enriched populations of human OPCs, that are efficiently myelinogenic in vivo while manifesting no evident tumorigenesis (see A). Following neonatal transplantation, hiPSC-derived OPCs can differentiate as oligodendrocytes and myelinate the brains of otherwise hypomyelinated shiverer mice (see B). Since these mice do not otherwise express MBP, the MBP immunostaining shown in B (green) is of human origin and hence donor-derived. hiPSC-derived OPCs typically mature as myelinogenic oligodendroglia by 3 months after neonatal graft (see C; human nuclear antigen is in red). The hiPSC-derived oligodendrocytes ensheath mouse axons (see D; neurofilament is in red) and generate ultrastructurally normal myelin that exhibits major dense lines and thick myelin sheaths with normal G-ratios (see E). Images are taken from Wang et al. (2013b). Scale bars: 100 µm in C; 10 µm in D; 100 nm in E. NSC, neural stem cell; NIM, neural induction medium; GIM, glial induction media (see Wang et al., 2013b for compositions).
How to use hPSC-derived OPCs to myelinate the brain
If hESC- and hiPSC-derived OPCs are bona fide oligodendroglial progenitor cells, they should be able to produce normally myelinogenic oligodendrocytes in vivo. To establish this capability, both hESC and hiPSC OPCs have been transplanted into neonatal immunodeficient shiverer mice, a hypomyelinated mutant that does not produce MBP. In these mice, any immunodetectable MBP must be of donor origin, and any functional benefits can be seen by the recovery and/or extended survival of the animals, which otherwise predictably die between 20 and 21 weeks of age, following progressive neurological deterioration. Wang et al. (2013,b) reported that hESC- and hiPSC-derived OPCs implanted in shiverer mice largely developed as oligodendrocytes in the white matter, produced functional myelin with normal ultrastructure and nodal reconstitution, and did so just as robustly as tissue-derived OPCs (Box 2). In the corpus callosum of shiverer recipients, hiPSC-OPC-derived oligodendrocytes typically ensheathed more than 10% of host axons by 12 weeks, and over half by 20 weeks, analogous to the myelination efficiency of primary human fetal OPCs. The resultant density and patterns of donor hiPSC-OPC-derived myelination compared favorably with those achieved using human fetal PDGFRα+ OPCs isolated from late second trimester human fetal brain (Sim et al., 2011). Most importantly, transplanted hiPSC-OPCs mediated the actual behavioral rescue of neonatally engrafted shiverer mice, allowing the long-term survival – and, in many cases, outright rescue – of mice that would otherwise have died by 21 weeks of age (Wang et al., 2013,b).
These results were analogous to the rescue of shiverer mice afforded by fetal human tissue-derived OPCs (Windrem et al., 2008); remarkably, the survival rate of hiPSC OPC-transplanted shiverer mice actually surpassed that of mice transplanted with fetal tissue-derived counterparts. These data indicated not only that congenitally hypomyelinated mice could be clinically rescued by hiPSC-derived OPC transplants, but also that the rescued mice can develop a completely humanized hiPSC OPC-derived white matter, in which human myelin maturation and ultrastructure, as well as pathophysiology (Kondo et al., 2014; Windrem et al., 2014), may be assessed in vivo.
How to make a better OPC…or at least to do so more quickly
The successful production of myelinogenic OPCs from hESCs and hiPSCs, and the subsequent rescue of animal models of congenital hypomyelination by their transplantation, suggested a potential clinical utility of these cells for treating a broad spectrum of myelin and other glial disorders (Fox et al., 2014) (Fig. 1). Yet, in all of these settings, the feasibility of using hESC- and hiPSC-derived oligodendroglia as donor cells, whether as therapeutic vectors or for animal modeling, has been limited by the slow rate of differentiation of the cells. Indeed, using the protocol of Wang et al. (2013,b), OPCs do not begin to appear in bulk until almost 3 months after initial induction, and 4-5 months in vitro are typically required for high-yield production of transplantable, robustly myelinogenic cells (Fig. 3A, Box 2). To address this limitation, several groups have refined the induction protocol so as to accelerate this process, while achieving higher proportions of OPCs and their derived oligodendrocytes at any given time point. Franklin and colleagues first published a low oxygen tension protocol (Stacpoole et al., 2013), which incorporates growth under relative hypoxia, a condition intended to mimic the environment of the developing forebrain. They reported significant improvement of the oligodendrocyte yield and an acceleration of the overall differentiation program using this approach. Fossati and colleagues (Douvaras et al., 2014) followed up that work with a modified procedure using the accelerated neuroepithelial differentiation strategy developed by the Studer lab (Chambers et al., 2009), which includes the small molecule-mediated suppression of SMAD signaling; this potentiates the rapid induction of neural stem cells and hence accelerates the timetable for glial induction (Fig. 3B). Douvaras et al. further refined the oligodendroglial induction procedure by sorting the cells with the oligodendrocytic surface lipid sulfatide recognized by the O4 antibody, rather than with an OPC antigen such as A2B5 or PDGFRα. The effect was to isolate more lineage-restricted oligodendroglia, with yields comparable to prior reports but in a more rapid fashion (Douvaras et al., 2014) (Fig. 3B).
Of note, although such accelerated induction strategies may allow the production of committed oligodendrocytes more quickly than previously reported approaches, it remains unclear whether the cells thereby generated can be as effective at in vivo dispersal or as efficient in myelinating broad regions as those isolated at the OPC stage. Comparison of the data reported by Douvaras et al. (2014) with those of Wang et al. (2013,b) suggests that immature oligodendroglia isolated on the basis of oligodendrocytic O4 might not be as capable of in vivo dispersal as earlier stage OPCs, since the O4-sorted cells generated with the Douvaras protocol are postmitotic and hence incapable of further expansion after implantation (Fig. 3). On the other hand, cells isolated on the basis of O4 expression might reasonably be expected to myelinate more rapidly and perhaps to have less potential for dysregulated expansion than their PDGFRα-sorted counterparts. Future studies will no doubt improve the balance of rapid oligodendroglial differentiation, safe in vivo expansion and facile migration, and robust myelinogenic competence.
In addition, future protocols will likely orient the production of OPCs and their derivatives towards particular regional, compartmental and segmental fates. Indeed, of the protocols thus far reported, most incorporate retinoic acid (RA), which biases cells towards a posterior fate, thus generating OPCs and oligodendroglia that express markers of segmental spinal and brainstem phenotype. By contrast, Stacpoole et al. (2013) and Piao et al. (2015) describe RA-independent protocols that favor telencephalic OPC production, which can be enhanced by antagonizing Wnt signaling with the tankyrase inhibitor XAV 939 (Piao et al., 2015). Further combination of efficient OPC induction protocols with regionalizing patterning factors will no doubt allow the refinement of such regionally specified progenitor production. Pending these anticipated advances, we can nonetheless take early advantage of the wealth of phenotypes already available for both experimental study and therapeutic evaluation of human oligodendrocytes and their progenitors.
How to make OPCs from other cells…skip the stem cells
OPCs may be generated not only from pluripotent cells, but also directly from somatic cells, using phenotype-defining transcription factors. Several recent studies have reported the direct induction of both OPCs and oligodendrocytes from fibroblasts, using targeted overexpression of defined pro-glial transcripts (Najm et al., 2013; Yang et al., 2013). These efforts have focused on skipping the pluripotent stage altogether, so as to accelerate the preparation of defined cell types of interest, while potentially mitigating the risk of tumorigenesis.
Wernig and colleagues (Yang et al., 2013) and Tesar and colleagues (Najm et al., 2013) both used the transduction of naïve mouse fibroblasts by progressively more refined sets of transcription factors to identify a core set of transcription factors sufficient to instruct oligodendrocytic development and maturation. Interestingly, while both groups derived myelinogenic oligodendroglia, they used only partially overlapping reprogramming gene combinations. Tesar and colleagues used a combination of eight transcription factors, from which a core set of three genes – Sox10, Nkx6.2 and Olig2 – proved sufficient to drive the production of induced oligodendrocyte progenitor cells (iOPCs). By contrast, Wernig and colleagues screened a distinct set of factors to identify Sox10, Olig2 and Zfp536 as sufficient to direct a glial progenitor cell phenotype. Yet, although each protocol gave rise to myelinogenic oligodendroglia, the two approaches proved distinct in terms of the lineage competence of the OPCs generated: the Tesar protocol yielded oligodendrocyte-restricted iOPCs, whereas the iOPCs produced with the Wernig protocol retained lineage competence for astrocytes as well as oligodendrocytes. The two approaches differ only in the substitution of Zfp536 for Nkx6.2, and it remains unclear whether that difference was alone sufficient to account for the difference in lineage commitment noted by the two groups (Goldman, 2013).
More broadly, since these data were obtained using rodent fibroblasts, it remains unclear whether the same transcription factor combination might prove competent to induce oligodendroglial differentiation from human somatic cells. Human oligodendroglia have been generated directly from neural stem cells by transduction with Sox10 alone (Wang et al., 2014), but efforts to directly instruct oligodendroglia from human fibroblasts have thus far proven more elusive. Once such direct induction of human oligodendroglia from non-neuroepithelial somatic cells is achieved, we can begin to critically assess the relative merits of pluripotent cell-derived OPCs and their directly induced counterparts, from the standpoints of their respective homologies to tissue-derived OPCs, their mitotic competence and lineage potential and, ultimately, their relative attributes as therapeutic agents.
How not to make tumors from hiPSC-derived OPCs
A number of studies have highlighted the risk of tumor formation from residual undifferentiated cells in hESC-derived neural transplants (Roy et al., 2006), as well as the trade-offs involved in mitigating that risk. Yet, published studies to date have reported no evidence of tumorigenesis or neuroepithelial overgrowth in the brains of mice engrafted with hESC- or iPSC-derived glial or OPC transplants, even among animals sacrificed as long as 2 years after neonatal implant of up to 3×105 hiPSC-derived OPCs (Wang et al., 2013b). It is possible that the prolonged differentiation protocols developed for OPC and oligodendrocytic differentiation are so robust as to effectively eliminate any residual undifferentiated cells prior to transplantation. By way of example, Wang et al. (2013b) found no evidence of detectable Oct4 mRNA or surface SSEA4 in either hESC- or hiPSC-derived OPC cultures after 3 months in vitro, at least a month before cells were harvested for either transplantation or further screening. Furthermore, these long culture protocols were not associated with any evidence of genomic or karyotypic instability. Thus, the safety profile of hiPSC-derived OPCs seems to be quite favorable.
That said, much longer survival time points, higher cell doses and more animals will need to be assessed, and an intensive search for any residual undifferentiated cells will have to be conducted in vivo, before these grafts can be deemed sufficiently safe for clinical assessment. Moreover, we believe that hiPSC OPCs will need to be sorted by negative selection or immunodepletion of less-differentiated stages, such as uncommitted CD133 (PROM1)+ neural progenitors, in order to purify the PDGFRα+ cells that are restricted to glial phenotype, since the dysregulated expansion of neuroepithelial cells may be as problematic as that of pluripotent cells (Roy et al., 2006). In that regard, we will need to compare the myelinogenic competence of grafts sorted to purity before transplantation with that of their unsorted counterparts, since the effects of admixed donor-derived astroglia on both the survival and the myelinogenic competence of OPCs has not yet been assessed in vivo. In addition, as the field moves towards both new small molecule-based reprogramming strategies and the accelerated differentiation protocols mentioned above, the potential risk of tumorigenesis by these more rapidly generated OPCs will need to be evaluated separately.
How to do even better…identify myelinogenic agents
As discussed earlier, the shiverer mouse model offers an unparalleled in vivo assay of the oligodendrocytic fate competence and myelinogenic ability of both tissue-derived and engineered OPCs. Yet, this in vivo assay is as involved and slow as it is powerful; it requires the maintenance and timed breeding of immunodeficient and myelin-deficient shiverer mice, their perinatal transplantation with cells of interest, and the maintenance of the resultant human glial chimeras for at least 3-4 months before sacrifice, with the in vivo administration of any test agents and extensive histological analysis thereafter. To develop more rapid and scalable models for screening large numbers of candidate myelinogenic agents, extensive efforts have thus been undertaken to achieve terminal oligodendrocyte differentiation and myelination in vitro, and to do so using testing platforms that may readily permit adoption across laboratories. These screening systems include high-content imaging of neuronal-OPC co-cultures (Abiraman et al., 2015; Deshmukh et al., 2013), and the imaging-assisted assessment of oligodendroglial interactions with inorganic substrates that permit myelinogenesis and fiber ensheathment (Lee et al., 2012; Mei et al., 2014). Each of these systems is designed to permit the screening of pharmacological agents for their ability to trigger oligodendrocytic maturation and myelin production, as well as for their abilities to modulate astrocyte production from the bipotential OPCs (Fig. 1).
These new testing platforms for myelinogenesis have allowed the identification of agents able to accelerate or otherwise potentiate the in vitro production of oligodendrocytes from OPCs and, most especially, their subsequent myelinogenesis and axonal ensheathment (Lee et al., 2012; Mei et al., 2014). Indeed, although this article has largely focused on the development and production of lineage-restricted oligodendrocytes, the induction of myelination by these oligodendroglia is a distinct step that requires its own strategies for exogenous potentiation. These approaches to induce de novo myelination are currently drawing significant interest, as a number of studies have highlighted the apparent inability of resident OPCs to mature and myelinate in some disease environments (Back and Rivkees, 2004; Fancy et al., 2009; Franklin, 2002; Franklin and ffrench-Constant, 2008).
As a result, a number of groups have taken the approach of identifying agents that are able to trigger terminal maturation and myelination from resident OPCs; indeed, several such agents able to potentiate oligodendrocytic myelination have been identified in just the past few years. These include small molecule inhibitors of tankyrase, which is an ADP-polyribosylating enzyme that stabilizes axin and thus releases OPCs from Wnt pathway-mediated differentiation block (Fancy et al., 2011); modulators of receptor tyrosine phosphatase β/ζ (PTPRZ1), which regulates β-catenin signaling and hence the Wnt pathway (McClain et al., 2012; Sim et al., 2006); agonists of retinoid X receptor RXR signaling (Huang et al., 2011); phosphodiesterase inhibition via rolipram (Syed et al., 2013); antagonists of the myelin inhibitory protein LINGO1 (Lee et al., 2014; Mi et al., 2013); the muscarinic cholinergic antagonists benztropine and solifenacin, which target CHRM1 and CHRM3 and were identified by an unbiased screen and OPC gene expression analysis, respectively (Abiraman et al., 2015; Deshmukh et al., 2013); and the imidazole and sterane representatives miconazole and clobetasol, which were similarly identified by unbiased screening of stem cell-derived OPCs (Najm et al., 2015). These pharmacological approaches towards potentiating myelination from resident progenitors have been extensively reviewed elsewhere (Fancy et al., 2010; Franklin and Goldman, 2015), and will not be further discussed here. Importantly, though, the same approaches as those now under development for potentiating myelination from endogenous OPCs might also be used to stimulate terminal differentiation and myelination by implanted stem cell-derived OPCs. One might reasonably anticipate that, as progress is made in identifying small molecules that potentiate myelinogenesis, these agents may be paired with pluripotent cell-derived OPCs to accomplish remyelination in increasingly challenging disease environments.
Conclusions
Our evolving understanding of oligodendroglial development has allowed us to design methods for producing these cells, as well as their immediate progenitors, from pluripotent stem cells, potentially obviating the need to return to tissue sources of these cells for therapeutic use. This advance has indeed been timely, as our recognition of the potential value of oligodendroglial replacement in myelin disorders has increased dramatically over the past few years, with the realization that oligodendrocyte loss is causally involved in a broad and increasingly bewildering spectrum of neurological diseases (Goldman et al., 2012).
The potential value of these cells as regenerative vectors has been established not only in experimental models of the hereditary leukodystrophies (Goldman, 2011; Windrem et al., 2008), but also in the adult rat brain, with the demonstration that hESC-derived oligodendroglia can remyelinate demyelinated white matter after radiation injury (Piao et al., 2015). In addition, the production of human glial chimeric mice, in which all resident OPCs as well as oligodendrocytes are human (Windrem et al., 2014), suggested the value of animals engrafted with these cells as models for human myelin disease as well (Goldman et al., 2015; Kondo et al., 2014). Thus, as techniques for the production, isolation and transplantation of OPCs have evolved, it has become increasingly realistic to consider these cells both as vectors for modeling the treatment of myelin diseases and, looking ahead, as scalable agents that might offer effective cell therapy to the multitude of patients with structural diseases of the brain and spinal cord. In so doing, this work might provide a playbook of sorts for how to use developmental information to design, produce and isolate specific cell types of interest, and how to employ these cells in the modeling and treatment of those diseases in which they are most deficient, and thus most needed.
Acknowledgements
We thank Drs Su Wang, Martha Windrem and Abdellatif Benraiss for their assistance with and contribution of images for this review.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
S.A.G. is supported by the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute of Mental Health (NIMH), the National Multiple Sclerosis Society, the CHDI Foundation, New York Stem Cell Science (NYSTEM), the Mathers Charitable Foundation, the Adelson Medical Research Foundation, the PML Consortium, the Lundbeck Foundation, and the Novo Nordisk Foundation. Deposited in PMC for release after 12 months.
References
- Abiraman K., Pol S. U., O'Bara M. A., Chen G.-D., Khaku Z. M., Wang J., Thorn D., Vedia B. H., Ekwegbalu E. C., Li J.-X. et al. (2015). Anti-muscarinic adjunct therapy accelerates functional human oligodendrocyte repair. J. Neurosci. 35, 3676-3688. 10.1523/JNEUROSCI.3510-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman S. D., Garcia C., Piao X., Gutmann D. H. and Monk K. R. (2015). The adhesion GPCR Gpr56 regulates oligodendrocyte development via interactions with Galpha12/13 and RhoA. Nat. Commun. 6, 6122 10.1038/ncomms7122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Gan H. T., Lam C. S., Poonepalli A., Ramasamy S., Tay Y., Tham M. and Yu Y. H. (2009). Transcription factors and neural stem cell self-renewal, growth and differentiation. Cell Adh. Migr. 3, 412-424. 10.4161/cam.3.4.8803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. C., Dorn H. H., Kufta C. V., Friedman E. and Dubois-Dalcq M. E. (1992). Pre-oligodendrocytes from adult human CNS. J. Neurosci. 12, 1538-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S. A. and Rivkees S. A. (2004). Emerging concepts in periventricular white matter injury. Semin. Perinatol. 28, 405-414. 10.1053/j.semperi.2004.10.010 [DOI] [PubMed] [Google Scholar]
- Bansal R., Warrington A. E., Gard A., Ranscht B. and Pfeiffer S. (1989). Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J. Neurosci. Res. 24, 548-557. 10.1002/jnr.490240413 [DOI] [PubMed] [Google Scholar]
- Barca-Mayo O. and Lu Q. R. (2012). Fine-tuning oligodendrocyte development by microRNAs. Front. Neurosci. 6, 13 10.3389/fnins.2012.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Schmid R., Sendnter M. and Raff M. C. (1993). Multiple extracellular signals are required for long-term oligodendrocyte survival. Development 118, 283-295. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Lazar M. A. and Raff M. C. (1994). A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120, 1097-1108. [DOI] [PubMed] [Google Scholar]
- Belachew S., Chittajallu R., Aguirre A. A., Yuan X., Kirby M., Anderson S. and Gallo V. (2003). Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J. Cell Biol. 161, 169-186. 10.1083/jcb.200210110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy J. D., Emery B., Kaushal A., Foo L. C., Zamanian J. L., Christopherson K. S., Xing Y., Lubischer J. L., Krieg P. A., Krupenko S. A. et al. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264-278. 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Qi Y., Hu X., Tan M., Liu Z., Zhang J., Li Q., Sander M. and Qiu M. (2005). Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron 45, 41-53. 10.1016/j.neuron.2004.12.028 [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P., Hardy R. J., Teng K. K., Levine J. M., Koff A. and Chao M. V. (1999). Loss of p27Kip1 function results in increased proliferative capacity of oligodendrocyte progenitors but unaltered timing of differentiation. Development 126, 4027-4037. [DOI] [PubMed] [Google Scholar]
- Chambers S. M., Fasano C. A., Papapetrou E. P., Tomishima M., Sadelain M. and Studer L. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275-280. 10.1038/nbt.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A., Nishiyama A., Peterson J., Prineas J. and Trapp B. D. (2000). NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 20, 6404-6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H., Waclaw R. R., Pei Z., Nakafuku M. and Campbell K. (2013). The homeobox gene Gsx2 controls the timing of oligodendroglial fate specification in mouse lateral ganglionic eminence progenitors. Development 140, 2289-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wu H., Wang S., Koito H., Li J., Ye F., Hoang J., Escobar S. S., Gow A., Arnett H. A. et al. (2009). The oligodendrocyte-specific G protein–coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci. 12, 1398-1406. 10.1038/nn.2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew L.-J., Shen W., Ming X., Senatorov V. V. Jr., Chen H.-L., Cheng Y., Hong E., Knoblach S. and Gallo V. (2011). SRY-box containing gene 17 regulates the Wnt/beta-catenin signaling pathway in oligodendrocyte progenitor cells. J. Neurosci. 31, 13921-13935. 10.1523/JNEUROSCI.3343-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czepiel M., Balasubramaniyan V., Schaafsma W., Stancic M., Mikkers H., Huisman C., Boddeke E. and Copray S. (2011). Differentiation of induced pluripotent stem cells into functional oligodendrocytes. Glia 59, 882-892. 10.1002/glia.21159 [DOI] [PubMed] [Google Scholar]
- Dawson M. R. L., Levine J. M. and Reynolds R. (2000). NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J. Neurosci. Res. 61, 471-479. [DOI] [PubMed] [Google Scholar]
- Deshmukh V. A., Tardif V., Lyssiotis C. A., Green C. C., Kerman B., Kim H. J., Padmanabhan K., Swoboda J. G., Ahmad I., Kondo T. et al. (2013). A regenerative approach to the treatment of multiple sclerosis. Nature 502, 327-332. 10.1038/nature12647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J., Noble M. and Mayer-Proschel M. (2002). Characterization of A2B5+ glial precursor cells from cryopreserved human fetal brain progenitor cells. Glia 40, 65-77. 10.1002/glia.10116 [DOI] [PubMed] [Google Scholar]
- Douvaras P. and Fossati V. (2015). Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells. Nat. Protoc. 10, 1143-1154. 10.1038/nprot.2015.075 [DOI] [PubMed] [Google Scholar]
- Douvaras P., Wang J., Zimmer M., Hanchuk S., O'Bara M. A., Sadiq S., Sim F. J., Goldman J. and Fossati V. (2014). Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Rep. 3, 250-259. 10.1016/j.stemcr.2014.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas J. C., Tai Y. C., Speed T. P., Ngai J. and Barres B. A. (2006). Functional genomic analysis of oligodendrocyte differentiation. J. Neurosci. 26, 10967-10983. 10.1523/JNEUROSCI.2572-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas J. C., Cuellar T. L., Scholze A., Ason B., Ibrahim A., Emery B., Zamanian J. L., Foo L. C., McManus M. T. and Barres B. A. (2010). Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron 65, 597-611. 10.1016/j.neuron.2010.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B. and Lu Q. R. (2015). Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the CNS. Cold Spring Harb. Perspect. Biol. 7, a020461 10.1101/cshperspect.a020461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B., Agalliu D., Cahoy J. D., Watkins T. A., Dugas J. C., Mulinyawe S. B., Ibrahim A., Ligon K. L., Rowitch D. H. and Barres B. A. (2009). Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 138, 172-185. 10.1016/j.cell.2009.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy S. P. J., Baranzini S. E., Zhao C., Yuk D.-I., Irvine K.-A., Kaing S., Sanai N., Franklin R. J. M. and Rowitch D. H. (2009). Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23, 1571-1585. 10.1101/gad.1806309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy S. P. J., Kotter M. R., Harrington E. P., Huang J. K., Zhao C., Rowitch D. H. and Franklin R. J. M. (2010). Overcoming remyelination failure in multiple sclerosis and other myelin disorders. Exp. Neurol. 225, 18-23. 10.1016/j.expneurol.2009.12.020 [DOI] [PubMed] [Google Scholar]
- Fancy S. P. J., Harrington E. P., Yuen T. J., Silbereis J. C., Zhao C., Baranzini S. E., Bruce C. C., Otero J. J., Huang E. J., Nusse R. et al. (2011). Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 14, 1009-1016. 10.1038/nn.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Constant C. and Raff M. C. (1986). Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature 319, 499-502. 10.1038/319499a0 [DOI] [PubMed] [Google Scholar]
- Fogarty M., Richardson W. D. and Kessaris N. (2005). A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development 132, 1951-1959. 10.1242/dev.01777 [DOI] [PubMed] [Google Scholar]
- Fox I. J., Daley G. Q., Goldman S. A., Huard J., Kamp T. J. and Trucco M. (2014). Use of differentiated pluripotent stem cells in replacement therapy for treating disease. Science 345, 1247391 10.1126/science.1247391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. J. M. (2002). Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 3, 705-714. 10.1038/nrn917 [DOI] [PubMed] [Google Scholar]
- Franklin R. J. M. and ffrench-Constant C. (2008). Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9, 839-855. 10.1038/nrn2480 [DOI] [PubMed] [Google Scholar]
- Franklin R. J. and Goldman S. A. (2015). Remyelination. In Glia (ed. Barres B.A. and Freeman M.R.). Cold Spring Harbor: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Gallo V. and Deneen B. (2014). Glial development: the crossroads of regeneration and repair in the CNS. Neuron 83, 283-308. 10.1016/j.neuron.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giera S., Deng Y., Luo R., Ackerman S. D., Mogha A., Monk K. R., Ying Y., Jeong S.-J., Makinodan M., Bialas A. R. et al. (2015). The adhesion G protein-coupled receptor GPR56 is a cell-autonomous regulator of oligodendrocyte development. Nat. Commun. 6, 6121 10.1038/ncomms7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. A. (1990). Neuronal development and migration in explant cultures of the adult canary forebrain. J. Neurosci. 10, 2931-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. A. (2011). Progenitor cell–based treatment of the pediatric myelin disorders. Arch. Neurol. 68, 848-856. 10.1001/archneurol.2011.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. A. (2013). White matter from fibroblasts. Nat. Biotechnol. 31, 412-413. 10.1038/nbt.2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. A., Nedergaard M. and Windrem M. S. (2012). Glial progenitor cell-based treatment and modeling of neurological disease. Science 338, 491-495. 10.1126/science.1218071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. A., Nedergaard M. and Windrem M. S. (2015). Modeling cognition and disease using human glial chimeric mice. Glia 63, 1483-1493. 10.1002/glia.22862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Maeda Y., Ma J., Xu J., Horiuchi M., Miers L., Vaccarino F. and Pleasure D. (2010). Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J. Neurosci. 30, 12036-12049. 10.1523/JNEUROSCI.1360-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart I. K., Richardson W. D., Heldin C. H., Westermark B. and Raff M. C. (1989). PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development 105, 595-603. [DOI] [PubMed] [Google Scholar]
- He X., Yu Y., Awatramani R. and Lu Q. R. (2012). Unwrapping myelination by microRNAs. Neuroscientist 18, 45-55. 10.1177/1073858410392382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman G., Van Haren K., Bonkowsky J. L., Bernard G., Pizzino A., Braverman N., Suhr D., Patterson M. C., Ali Fatemi S., Leonard J. et al. (2015). Disease specific therapies in leukodystrophies and leukoencephalopathies. Mol. Genet. Metab. 114, 527-536. 10.1016/j.ymgme.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B.-Y., Du Z.-W., Li X.-J., Ayala M. and Zhang S.-C. (2009). Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development 136, 1443-1452. 10.1242/dev.029447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. K., Jarjour A. A., Oumesmar B. N., Kerninon C., Williams A., Krezel W., Kagechika H., Bauer J., Zhao C., Evercooren A. B.-V. et al. (2011). Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 14, 45-53. 10.1038/nn.2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N., Niu J., Feng Y. and Xiao L. (2015). Oligodendroglial development: new roles for chromatin accessibility. Neuroscientist, pii: 1073858414565467 10.1177/1073858414565467 [DOI] [PubMed] [Google Scholar]
- Hughes E. G., Kang S. H., Fukaya M. and Bergles D. E. (2013). Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 16, 668-676. 10.1038/nn.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B. and Brand M. (1997). Asymmetric division and polarity of neuroepithelial cells. Curr. Opin. Neurobiol. 7, 29-39. 10.1016/S0959-4388(97)80117-1 [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Ding L., Ikenaka K., Inoue Y., Miyado K., Mekada E. and Baba H. (2004). Tetraspanin protein CD9 is a novel paranodal component regulating paranodal junctional formation. J. Neurosci. 24, 96-102. 10.1523/JNEUROSCI.1484-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrael M., Zhang P., Kaufman R., Shinder V., Ella R., Amit M., Itskovitz-Eldor J., Chebath J. and Revel M. (2007). Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol. Cell. Neurosci. 34, 310-323. 10.1016/j.mcn.2006.11.008 [DOI] [PubMed] [Google Scholar]
- Jan Y.-N. and Jan L. Y. (2001). Development: asymmetric cell division in the Drosophila nervous system. Nat. Rev. Neurosci. 2, 772-779. 10.1038/35097516 [DOI] [PubMed] [Google Scholar]
- Kang S. H., Fukaya M., Yang J. K., Rothstein J. D. and Bergles D. E. (2010). NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68, 668-681. 10.1016/j.neuron.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. R., Meyer-Franke A., Lambert S., Bennett V., Duncan I. D., Levinson S. R. and Barres B. A. (1997). Induction of sodium channel clustering by oligodendrocytes. Nature 386, 724-728. 10.1038/386724a0 [DOI] [PubMed] [Google Scholar]
- Keilhauer G., Meier D. H., Kuhlmann-Krieg S., Nieke J. and Schachner M. (1985). Astrocytes support incomplete differentiation of an oligodendrocyte precursor cell. EMBO J. 4, 2499-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N., Fogarty M., Iannarelli P., Grist M., Wegner M. and Richardson W. D. (2006). Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9, 173-179. 10.1038/nn1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum B., Nedergaard M., Preuss A., Barami K., Fraser R. A. R. and Goldman S. A. (1994). In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb. Cortex 4, 576-589. 10.1093/cercor/4.6.576 [DOI] [PubMed] [Google Scholar]
- Klambt C. (2009). Modes and regulation of glial migration in vertebrates and invertebrates. Nat. Rev. Neurosci. 10, 769-779. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Windrem M. S., Zou L., Chandler-Militello D., Schanz S. J., Auvergne R. M., Betstadt S. J., Harrington A. R., Johnson M., Kazarov A. et al. (2014). Human glial chimeric mice reveal astrocytic dependence of JC virus infection. J. Clin. Invest. 124, 5323-5336. 10.1172/JCI76629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R., Weick J. P., Liu Y., Zhang Z.-J. and Zhang S.-C. (2011). Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 29, 528-534. 10.1038/nbt.1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlbrodt K., Herbarth B., Sock E., Hermans-Borgmeyer I. and Wegner M. (1998). Sox10, a novel transcriptional modulator in glial cells. J. Neurosci. 18, 237-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocque D., Galarneau A., Liu H.-N., Scott M., Almazan G. and Richard S. (2005). Protection of p27(Kip1) mRNA by quaking RNA binding proteins promotes oligodendrocyte differentiation. Nat. Neurosci. 8, 27-33. 10.1038/nn1359 [DOI] [PubMed] [Google Scholar]
- Lau P., Verrier J. D., Nielsen J. A., Johnson K. R., Notterpek L. and Hudson L. D. (2008). Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J. Neurosci. 28, 11720-11730. 10.1523/JNEUROSCI.1932-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Leach M. K., Redmond S. A., Chong S. Y. C., Mellon S. H., Tuck S. J., Feng Z.-Q., Corey J. M. and Chan J. R. (2012). A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917-922. 10.1038/nmeth.2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee X., Shao Z., Sheng G., Pepinsky B. and Mi S. (2014). LINGO-1 regulates oligodendrocyte differentiation by inhibiting ErbB2 translocation and activation in lipid rafts. Mol. Cell. Neurosci. 60, 36-42. 10.1016/j.mcn.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Levine J. M., Reynolds R. and Fawcett J. W. (2001). The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 24, 39-47. 10.1016/S0166-2236(00)01691-X [DOI] [PubMed] [Google Scholar]
- Li H., Lu Y., Smith H. K. and Richardson W. D. (2007). Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes. J. Neurosci. 27, 14375-14382. 10.1523/JNEUROSCI.4456-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Suzuki K., Kim N. Y., Liu G.-H. and Izpisua Belmonte J. C. (2014). A cut above the rest: targeted genome editing technologies in human pluripotent stem cells. J. Biol. Chem. 289, 4594-4599. 10.1074/jbc.R113.488247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.-L. and Ying S.-Y. (2013). Mechanism and method for generating tumor-free iPS cells using intronic microRNA miR-302 induction. Methods Mol. Biol. 936, 295-312. 10.1007/978-1-62703-083-0_23 [DOI] [PubMed] [Google Scholar]
- Lin T., Ambasudhan R., Yuan X., Li W., Hilcove S., Abujarour R., Lin X., Hahm H. S., Hao E., Hayek A. et al. (2009). A chemical platform for improved induction of human iPSCs. Nat. Methods 6, 805-808. 10.1038/nmeth.1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A., Han Y. R., Li J., Sun D., Ouyang M., Plummer M. R. and Casaccia-Bonnefil P. (2007). The glial or neuronal fate choice of oligodendrocyte progenitors is modulated by their ability to acquire an epigenetic memory. J. Neurosci. 27, 7339-7343. 10.1523/JNEUROSCI.1226-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jiang P. and Deng W. (2011). OLIG gene targeting in human pluripotent stem cells for motor neuron and oligodendrocyte differentiation. Nat. Protoc. 6, 640-655. 10.1038/nprot.2011.310 [DOI] [PubMed] [Google Scholar]
- Lu Q. R., Yuk D.-i., Alberta J. A., Zhu Z., Pawlitzky I., Chan J., McMahon A. P., Stiles C. D. and Rowitch D. H. (2000). Sonic hedgehog–regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron 25, 317-329. 10.1016/S0896-6273(00)80897-1 [DOI] [PubMed] [Google Scholar]
- Mabie P. C., Mehler M. F., Marmur R., Papavasiliou A., Song Q. and Kessler J. A. (1997). Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J. Neurosci. 17, 4112-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri L., Gacias M., Wu M., Swiss V. A., Janssen W. G. and Casaccia P. (2014). c-Myc-dependent transcriptional regulation of cell cycle and nucleosomal histones during oligodendrocyte differentiation. Neuroscience 276, 72-86. 10.1016/j.neuroscience.2014.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N. and Hochedlinger K. (2008). Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell 3, 595-605. 10.1016/j.stem.2008.11.008 [DOI] [PubMed] [Google Scholar]
- Mallanna S. K. and Rizzino A. (2010). Emerging roles of microRNAs in the control of embryonic stem cells and the generation of induced pluripotent stem cells. Dev. Biol. 344, 16-25. 10.1016/j.ydbio.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain C. R., Sim F. J. and Goldman S. A. (2012). Pleiotrophin suppression of receptor protein tyrosine phosphatase-beta/zeta maintains the self-renewal competence of fetal human oligodendrocyte progenitor cells. J. Neurosci. 32, 15066-15075. 10.1523/JNEUROSCI.1320-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F., Fancy S. P. J., Shen Y.-A. A., Niu J., Zhao C., Presley B., Miao E., Lee S., Mayoral S. R., Redmond S. A. et al. (2014). Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 20, 954-960. 10.1038/nm.3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., Blake Pepinsky R. and Cadavid D. (2013). Blocking LINGO-1 as a therapy to promote CNS repair: from concept to the clinic. CNS Drugs 27, 493-503. 10.1007/s40263-013-0068-8 [DOI] [PubMed] [Google Scholar]
- Najm F. J., Lager A. M., Zaremba A., Wyatt K., Caprariello A., Factor D. C., Karl R. T., Maeda T., Miller R. H. and Tesar P. J. (2013). Transcription factor–mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat. Biotechnol. 31, 426-433. 10.1038/nbt.2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm F. J., Madhavan M., Zaremba A., Shick E., Karl R. T., Factor D. C., Miller T. E., Nevin Z. S., Kantor C., Sargent A. et al. (2015). Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 522, 216-220. 10.1038/nature14335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namihira M. and Nakashima K. (2013). Mechanisms of astrocytogenesis in the mammalian brain. Curr. Opin. Neurobiol. 23, 921-927. 10.1016/j.conb.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Nave K.-A. (2010a). Myelination and support of axonal integrity by glia. Nature 468, 244-252. 10.1038/nature09614 [DOI] [PubMed] [Google Scholar]
- Nave K.-A. (2010b). Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 11, 275-283. 10.1038/nrn2797 [DOI] [PubMed] [Google Scholar]
- Nery S., Wichterle H. and Fishell G. (2001). Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development 128, 527-540. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Komitova M., Suzuki R. and Zhu X. (2009). Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 10, 9-22. 10.1038/nrn2495 [DOI] [PubMed] [Google Scholar]
- Nistor G., Totoiu M., Haque N. S., Carpenter M. and Keirstead H. (2005). Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 49, 385-396. 10.1002/glia.20127 [DOI] [PubMed] [Google Scholar]
- Noble M., Murray K., Stroobant P., Waterfield M. D. and Riddle P. (1988). Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature 333, 560-562. 10.1038/333560a0 [DOI] [PubMed] [Google Scholar]
- Nunes M. C., Roy N. S., Keyoung H. M., Goodman R. R., McKhann G., Jiang L., Kang J., Nedergaard M. and Goldman S. A. (2003). Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat. Med. 9, 439-447. 10.1038/nm837 [DOI] [PubMed] [Google Scholar]
- Orentas D. M., Hayes J. E., Dyer K. L. and Miller R. H. (1999). Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development 126, 2419-2429. [DOI] [PubMed] [Google Scholar]
- Piao J., Major T., Auyeung G., Policarpio E., Menon J., Droms L., Gutin P., Uryu K., Tchieu J., Soulet D. et al. (2015). Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell 16, 198-210. 10.1016/j.stem.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J. (2004). The leukodystrophies: overview and classification. In Myelin Biology and Disorders (ed. Lazzarini R. A.), pp. 663-690. San Diego: Elsevier Academic Press. [Google Scholar]
- Qin Z., Ren F., Xu X., Ren Y., Li H., Wang Y., Zhai Y. and Chang Z. (2009). ZNF536, a novel zinc finger protein specifically expressed in the brain, negatively regulates neuron differentiation by repressing retinoic acid-induced gene transcription. Mol. Cell. Biol. 29, 3633-3643. 10.1128/MCB.00362-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R. and Noble M. (1983a). Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J. Neurosci. 3, 1289-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H. and Noble M. (1983b). A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303, 390-396. 10.1038/303390a0 [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Pringle N., Mosley M. J., Westermark B. and Dubois-Dalcg M. (1988). A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell 53, 309-319. 10.1016/0092-8674(88)90392-3 [DOI] [PubMed] [Google Scholar]
- Rivers L. E., Young K. M., Rizzi M., Jamen F., Psachoulia K., Wade A., Kessaris N. and Richardson W. D. (2008). PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392-1401. 10.1038/nn.2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati G. (2001). The prevalence of multiple sclerosis in the world: an update. Neurol. Sci. 22, 117-139. 10.1007/s100720170011 [DOI] [PubMed] [Google Scholar]
- Rowitch D. H. (2004). Glial specification in the vertebrate neural tube. Nat. Rev. Neurosci. 5, 409-419. 10.1038/nrn1389 [DOI] [PubMed] [Google Scholar]
- Rowitch D. H. and Kriegstein A. R. (2010). Developmental genetics of vertebrate glial–cell specification. Nature 468, 214-222. 10.1038/nature09611 [DOI] [PubMed] [Google Scholar]
- Roy N. S., Wang S., Harrison-Restelli C., Benraiss A., Fraser R. A., Gravel M., Braun P. E. and Goldman S. A. (1999). Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J. Neurosci. 19, 9986-9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N. S., Cleren C., Singh S. K., Yang L., Beal M. F. and Goldman S. A. (2006). Functional engraftment of human ES cell–derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 12, 1259-1268. 10.1038/nm1495 [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C. and Alvarez I. S. (1989). Roles of neuroepithelial cell rearrangement and division in shaping of the avian neural plate. Development 106, 427-439. [DOI] [PubMed] [Google Scholar]
- Scolding N. (1998). Glial precursor cells in the adult human brain. Neuroscientist 4, 264-272. [Google Scholar]
- Scolding N. J., Rayner P. J. and Compston D. A. S. (1999). Identification of A2B5-positive putative oligodendrocyte progenitor cells and A2B5-positive astrocytes in adult human white matter. Neuroscience 89, 1-4. 10.1016/S0306-4522(98)00548-X [DOI] [PubMed] [Google Scholar]
- Shenoy A. and Blelloch R. H. (2014). Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat. Rev. Mol. Cell Biol. 15, 565-576. 10.1038/nrm3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. L. and Brophy P. J. (2005). Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 6, 683-690. 10.1038/nrn1743 [DOI] [PubMed] [Google Scholar]
- Shi Y., Zhao X., Hsieh J., Wichterle H., Impey S., Banerjee S., Neveu P. and Kosik K. S. (2010). MicroRNA regulation of neural stem cells and neurogenesis. J. Neurosci. 30, 14931-14936. 10.1523/JNEUROSCI.4280-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim F. J., Lang J. K., Waldau B., Roy N. S., Schwartz T. E., Pilcher W. H., Chandross K. J., Natesan S., Merrill J. E. and Goldmanm S. A. (2006). Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation. Ann. Neurol. 59, 763-779. 10.1002/ana.20812 [DOI] [PubMed] [Google Scholar]
- Sim F. J., McClain C. R., Schanz S. J., Protack T. L., Windrem M. S. and Goldman S. A. (2011). CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nat. Biotechnol. 29, 934-941. 10.1038/nbt.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I. and Schachner M. (1981). Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev. Biol. 83, 311-327. 10.1016/0012-1606(81)90477-2 [DOI] [PubMed] [Google Scholar]
- Stacpoole S. R. L., Spitzer S., Bilican B., Compston A., Karadottir R., Chandran S. and Franklin R. J. (2013). High yields of oligodendrocyte lineage cells from human embryonic stem cells at physiological oxygen tensions for evaluation of translational biology. Stem Cell Rep. 1, 437-450. 10.1016/j.stemcr.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M. and Hochedlinger K. (2010). Induced pluripotency: history, mechanisms, and applications. Genes Dev. 24, 2239-2263. 10.1101/gad.1963910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimori M., Nagao M., Parras C. M., Nakatani H., Lebel M., Guillemot F. and Nakafuku M. (2008). Ascl1 is required for oligodendrocyte development in the spinal cord. Development 135, 1271-1281. 10.1242/dev.015370 [DOI] [PubMed] [Google Scholar]
- Susuki K. and Rasband M. N. (2008). Molecular mechanisms of node of Ranvier formation. Curr. Opin. Cell Biol. 20, 616-623. 10.1016/j.ceb.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Y. A., Baer A., Hofer M. P., González G. A., Rundle J., Myrta S., Huang J. K., Zhao C., Rossner M. J., Trotter M. W. B. et al. (2013). Inhibition of phosphodiesterase-4 promotes oligodendrocyte precursor cell differentiation and enhances CNS remyelination. EMBO Mol. Med. 5, 1918-1934. 10.1002/emmm.201303123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekki-Kessaris N., Woodruff R., Hall A. C., Gaffield W., Kimura S., Stiles C. D., Rowitch D. H. and Richardson W. D. (2001). Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development 128, 2545-2554. [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S. and Jones J. M. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145-1147. 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Tripathi R. B., Rivers L. E., Young K. M., Jamen F. and Richardson W. D. (2010). NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J. Neurosci. 30, 16383-16390. 10.1523/JNEUROSCI.3411-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallstedt A., Klos J. M. and Ericson J. (2005). Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron 45, 55-67. 10.1016/j.neuron.2004.12.026 [DOI] [PubMed] [Google Scholar]
- Wang S.-Z., Dulin J., Wu H., Hurlock E., Lee S.-E., Jansson K. and Lu Q. R. (2006). An oligodendrocyte-specific zinc-finger transcription regulator cooperates with Olig2 to promote oligodendrocyte differentiation. Development 133, 3389-3398. 10.1242/dev.02522 [DOI] [PubMed] [Google Scholar]
- Wang J., O'Bara M. A., Pol S. U. and Sim F. J. (2013a). CD133/CD140a-based isolation of distinct human multipotent neural progenitor cells and oligodendrocyte progenitor cells. Stem Cells Dev. 22, 2121-2131. 10.1089/scd.2013.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Bates J., Li X., Schanz S., Chandler-Militello D., Levine C., Maherali N., Studer L., Hochedlinger K., Windrem M. et al. (2013b). Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12, 252-264. 10.1016/j.stem.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Pol S. U., Haberman A. K., Wang C., O'Bara M. A. and Sim F. J. (2014). Transcription factor induction of human oligodendrocyte progenitor fate and differentiation. Proc. Natl. Acad. Sci. USA 111, E2885-E2894. 10.1073/pnas.1408295111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M. and Stolt C. S. (2005). From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 28, 583-588. 10.1016/j.tins.2005.08.008 [DOI] [PubMed] [Google Scholar]
- Weider M., Reiprich S. and Wegner M. (2013). Sox appeal – Sox10 attracts epigenetic and transcriptional regulators in myelinating glia. Biol. Chem. 394, 1583-1593. 10.1515/hsz-2013-0146 [DOI] [PubMed] [Google Scholar]
- Windrem M. S., Nunes M. C., Rashbaum W. K., Schwartz T. H., Goodman R. A., McKhann G., Roy N. S. and Goldman S. A. (2004). Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat. Med. 10, 93-97. 10.1038/nm974 [DOI] [PubMed] [Google Scholar]
- Windrem M. S., Schanz S. J., Guo M., Tian G.-F., Washco V., Stanwood N., Rasband M., Roy N. S., Nedergaard M., Havton L. A. et al. (2008). Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell 2, 553-565. 10.1016/j.stem.2008.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem M. S., Schanz S. J., Morrow C., Munir J., Chandler-Militello D., Wang S. and Goldman S. A. (2014). A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J. Neurosci. 34, 16153-16161. 10.1523/JNEUROSCI.1510-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G. and Noble M. (1989). Identification of an adult-specific glial progenitor cell. Development 105, 387-400. [DOI] [PubMed] [Google Scholar]
- Woodruff R. H., Tekki-Kessaris N., Stiles C. D., Rowitch D. H. and Richardson W. D. (2001). Oligodendrocyte development in the spinal cord and telencephalon: common themes and new perspectives. Int. J. Dev. Neurosci. 19, 379-385. 10.1016/S0736-5748(00)00083-6 [DOI] [PubMed] [Google Scholar]
- Xin M., Yue T., Ma Z., Wu F.-F., Gow A. and Lu Q. R. (2005). Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J. Neurosci. 25, 1354-1365. 10.1523/JNEUROSCI.3034-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. (2007). Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 1, 39-49. 10.1016/j.stem.2007.05.012 [DOI] [PubMed] [Google Scholar]
- Yamanaka S. (2008). Pluripotency and nuclear reprogramming. Philos. Trans. R. Soc. B Biol. Sci. 363, 2079-2087. 10.1098/rstb.2008.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Zuchero J. B., Ahlenius H., Marro S., Ng Y. H., Vierbuchen T., Hawkins J. S., Geissler R., Barres B. A. and Wernig M. (2013). Generation of oligodendroglial cells by direct lineage conversion. Nat. Biotechnol. 31, 434-439. 10.1038/nbt.2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A. (2011). Control of the embryonic stem cell state. Cell 144, 940-954. 10.1016/j.cell.2011.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Casaccia P. and Lu Q. R. (2010). Shaping the oligodendrocyte identity by epigenetic control. Epigenetics 5, 124-128. 10.4161/epi.5.2.11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Chen Y., Kim B., Wang H., Zhao C., He X., Liu L., Liu W., Wu L. M. N., Mao M. et al. (2013). Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell 152, 248-261. 10.1016/j.cell.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S. H., Martin J., Elia J., Flippin J., Paramban R. I., Hefferan M. P., Vidal J. G., Mu Y., Killian R. L., Israel M. A. et al. (2011). Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS ONE 6, e17540 10.1371/journal.pone.0017540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.-C. (2001). Defining glial cells during CNS development. Nat. Rev. Neurosci. 2, 840-843. 10.1038/35097593 [DOI] [PubMed] [Google Scholar]
- Zhang H. and Miller R. H. (1996). Density-dependent feedback inhibition of oligodendrocyte precursor expansion. J. Neurosci. 16, 6886-6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen K., Sloan S. A., Bennett M. L., Scholze A. R., O'Keeffe S., Phatnani H. P., Guarnieri P., Caneda C., Ruderisch N. et al. (2014). An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929-11947. 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., He X., Han X., Yu Y., Ye F., Chen Y., Hoang T., Xu X., Mi Q.-S., Xin M. et al. (2010). MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 65, 612-626. 10.1016/j.neuron.2010.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Ji X., Zhang F., Li L. and Ma L. (2012). Embryonic stem cell markers. Molecules 17, 6196-6236. 10.3390/molecules17066196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Whittemore S. R., Devries W. H., Zhao X., Kuypers N. J., and Qiu M. (2011). Dorsally-derived oligodendrocytes in the spinal cord contribute to axonal myelination during development and remyelination following focal demyelination. Glia 59, 1612-1621. [DOI] [PubMed] [Google Scholar]
- Zuchero J. B. and Barres B. A. (2013). Intrinsic and extrinsic control of oligodendrocyte development. Curr. Opin. Neurobiol. 23, 914-920. 10.1016/j.conb.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]