One of the challenges for integrative/functional/natural medicine is that we are generally not aligned with academic medicine. Typically, this means that the kinds of questions we think are important for clinical practice are usually not being researched. Worse, when some of our ideas are addressed by conventional research centers, the intent appears to be invalidation rather than objective inquiry. A clear example of this problem is assessing body load of toxins as well as their clinical effects and subacute levels of poisoning. With a few interesting exceptions, the standard of assessment for toxic metals is blood with the arbitrary criterion that only those in the top 5% are considered toxic. The problem with this method, of course, is that blood primarily indicates current exposure, not body load, and does not at all consider the huge variation in individual susceptibility to specific toxins as well as total body load of toxins. I am confident that all clinicians reading this journal have seen remarkable patient improvement from reducing toxin load, even when the standard tests indicated they were in the “normal” range. Another very big problem is the definition of normal. An important point I will make below is that not only is normal no longer healthy, but environmental toxicity has become so prevalent that we no longer even know what totally nontoxic looks like.
Please note that for the following review, I used only PubMed-indexed studies. I realize there may be some good research available to document this assessment methodology, but if it is not apparently good enough to make it into a peer-reviewed journal, I am not going to publish it here. Another challenge is that there may be studies published before PubMed’s electronic indexing, so those will be missed as well. Finally, due to time and space limitations, I looked at only the mercury and dimercaptosuccinic acid (DMSA) research, though some dimercaptopropane sulfonate (DMPS) research showed up as well.
The 95% Standard Is Not Valid
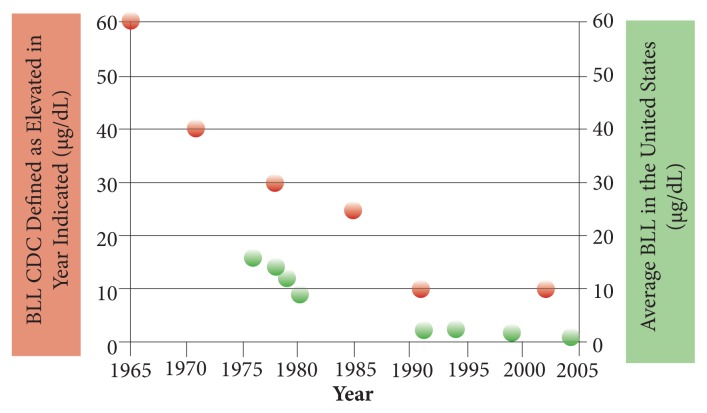
Although the definition of only the top 5% of exposure as being toxic is the standard for most pollutants, there is considerable reason to question its validity. This is quite well documented, for example, by looking at how the “safe” level of lead has changed so dramatically from the original 95% standard. As can be seen in Figure 1, the “safe” indicated by the red spots has decreased dramatically as research has shown strong disease correlations and causations within the “normal” range. Even the current 10 μg/dL is too high, with research showing that a blood lead level (BLL) of 5 to 9 μg/dL has been associated with an increased risk of death from all causes, cardiovascular disease, and cancer.1 In 2012, the Centers for Disease Control (CDC) decreased the “safe” level to 5 μg/dL for children and added commentary that no level of lead is apparently safe for children.2 Unfortunately, still 2.5% of children are above this level, which is known to decrease their intelligence quotient (IQ), as noted in the CDC report. BTW, this is a good example of a public health initiative that was effective in promoting health and decreasing disease. As can be seen clearly in the figure, removing lead from gasoline in the 1970s greatly decreased the lead levels in the general population.
Figure 1.
Declining CDC “safe” level for blood lead.3
Abbreviations: CDC, Centers for Disease Control; BLL, blood lead level.
The Case Against Challenge Testing for Toxic Metal Load
In December 2013, Anne-Michelle Ruha, md, facmt, at the University of Arizona College of Medicine, published an articulate review asserting that “Urine mobilization test,” “challenge test,” and “provoked urine test” are not valid measures of toxic metal body load.4 The challenge agents she challenged included DMSA, DMPS, and ethylenediaminetetraacetic acid (EDTA). Her argument is essentially the following:
Diverse routes of administration of chelating agents (oral, transdermal, intravenous, suppositories, etc).
Inconsistent dosages.
Variation in urine collection time.
Inappropriate reference ranges.
No established reference ranges for provoked urine samples in healthy people.
Creatinine correction of urine.
Mercury is detected in the urine of most people even in the absence of known exposure or chelator administration.
Urinary mercury excretion rises after administration of a chelator, regardless of exposure history and in an unpredictable fashion.
Challenge testing fails to reveal a “body burden” of mercury due to exposure in the past.
Chelating agents have themselves been associated with adverse reactions.
Clinicians involved in this field will, of course, agree that many of these concerns do indeed need to be addressed. In fact, we would all much prefer a well characterized methodology that is fully validated with healthy and toxic individuals. However, that does not mean this testing is invalid or not clinically useful. I do not have the space or time to address every issue she raised. However, there are many problems with most of the research she quotes as invalidating challenge testing. Following are a few. Again, this does not mean challenge testing is valid; rather, the criticisms have huge methodological problems themselves.
“Normal” is not healthy. In fact, the majority of the population has chronic disease or is chronically unwell. So the conventional “standards” are simply not valid as they include mostly unhealthy people. More than 50% of the US population now suffers at least 1 diagnosed chronic disease (the number is even higher considering the high percentage with disease not yet diagnosed) and 25% have 2 or more.5 In addition, at least 16% of the population describes themselves as chronically unwell. In fact, according to a recent study, only 4.3% of the world population is fully healthy.6 Were only these 4.3% used in the studies as the normal? Abnormal is the new normal.
The “normal” population carries a high toxin load, which has both strong association with disease and a growing body of research showing causation (see my many past editorials in IMCJ on mercury, persistent organic pollutants, glutathione, etc). Table 1 shows the toxin/disease correlations I have found so far comparing those in the top quintile of exposure to those with the lowest quintile of body load. This is only the tip of the iceberg with just a few of the thousands of toxins in our environment and food. In fact, I now assert in my lectures to health care professionals literally all over the world that toxicity is the primary cause of chronic disease and ill health in industrialized countries. Obviously, we cannot make the assumption that only those in the top quintile suffer disease while those with less exposure have no increased disease risk. So her assertion that challenge testing is not valid as it increases metal excretion in “healthy” people with no known exposure is simply invalid.
There is huge individual variation in ability to remove toxins as well as sensitivity to toxins. I will, in an upcoming editorial, review the research on genomics and detoxification. Looking at just CYP2D6 (which detoxifies approximately 25% of prescription drugs), there is a 1000-fold variation in its function!7
Table 1.
Toxin Load and Disease Risk
| Toxin | Disease | Risk | Reference |
|---|---|---|---|
| Arsenic | Diabetes | 3.6 | Navas-Acien et al8 (2008) |
| Lung cancer | 3.0–5.0 | Heck et al9 (2009) | |
| Cadmium | Myocardial infarction | 1.8 | Everett et al10 (2008) |
| Osteoporosis | 1.4 | Gallagher et al11 (2008) | |
| Obstructive lung disease | 2.52 (top decile) | Yoon et al12 (2014) | |
| Lead | Gout | 3.6 | Krishnan et al13 (2012) |
| Obstructive lung disease | 2.37 (top decile) | Rokadia et al14 (2013) | |
| Organochlorine pesticides | Diabetes | 9.1 | Kim et al15 (2014) |
| Rheumatoid arthritis | 3.5 | Lee et al16 (2007) | |
| Hyperuricemia | 2.5 | Lee et al17 (2013) | |
| Organophosphate pesticides | IQ in children according to OPs in mother | 7.1-point decrease in IQ | Bouchard et al18 (2010) |
| ADHD | 2.0 | Bouchard et al19 (2011) | |
| PCBs | ADHD | >3.0 | Boersma et al20 (2000) |
| Rheumatoid arthritis | 8.5 | Lee et al16 (2007) | |
| Bisphenol A | Prediabetes | 1.34 (top tertial) | Sabanayagam et al21 (2013) |
| Metabolic syndrome | 1.51 | Tepalla et al22 (2012) | |
| Obesity (children) | 2.55 | Bhandari et al23 (2013) | |
| Polybrominated diphenyl ethers | Diabetes | 2.0–3.0 | Lim et al24 (2008) |
| Phthalates | Osteoporosis | 14.1-fold (MCPP) 5.9 (MCOP) 5.9 (MBzP) |
Min et al25 (2014) |
| Obesity | 1.62 (DEHP, adults) 1.77 (HMW, adults) 2.84 (LMW, children) 4.29 (MiBP, male children) |
Buser et al26 (2014) |
Abbreviations: IQ, intelligence quotient; OP, organophosphate pesticides; ADHD, attention-deficit/hyperactivity disorder; PCB, polychlorinated biphenyl; MCPP, mono-(3-carboxypropyl) phthalate; MCOP, monocarboxyoctyl phthalate; MBzP, mono benzylbutyl phthalate; DEHP, di(2-ethylhexyl)phthalate; HMW, high molecular weight phthalates; LMW, low molecular weight phthalates; MiBP, mono-isobutyl phthalate.
Bottom line, we have an almost universally sick population, so normal is not actually healthy and the normal is indeed usually toxic.
(BTW, if you read her article, please note her calculation showing that creatinine correction for metal reporting is invalid makes no sense. Her math is fine, but the result is meaningless. Correcting for creatinine clearance may indeed result in higher numbers, but this is a necessary part of the standardization process and is the methodology widely used in the published research. Most important, however, is that she ignores the intent of the correction is to improve reproducibility by adjusting for changing urine volume. If this correction were not made, she would have the valid objection that not correcting for urine volume decreases reliability. This portion of her article should not have been allowed by the editor.)
A 2004 study reported no difference between elevation in urinary mercury after challenge between self-reported “healthy” controls and levels found in those with purported mercury toxicity symptoms.27 They used high dosages (30 mg/kg) of DMSA with urine collected for 3 hours. One of the unreported number of subjects suffered a serious (though not described) adverse drug reaction (ADR). As only a very skimpy abstract was available, I am unable to analyze this study.
A large 2001 study comparing 119 previous employees at a chloralkali plant with 101 “unexposed” controls found statistically insignificant differences in postchallenge results.28 The huge problem with this study is that it was done several years after the plant closed and used community members as controls. The reported levels of mercury, both provoked and unprovoked, were above normal, suggesting that the whole community was exposed, invalidating the control group.
A 1994 study (which I had read several years ago) still makes me worry about science. These researchers provided patients 20 mg/kg of DMSA or placebo for 14 days.29 After treatment they found that the DMSA had decreased blood mercury (0.04 μg/L) and increased urinary mercury excretion by 65%. Three months after cessation of treatment, they were retested and found no differences from pretreatment levels. They then concluded that DMSA was ineffective diagnostically or therapeutically. They did not remove the dental amalgams. All they proved is that if the amalgams are not removed, the blood and urine levels go back up to where they were before treatment.
As can be seen from the above, despite the problems with the validity of the negative studies, there are real issues with challenge testing which must be addressed. Of course, we have patients today who need our help, most of whom have been failed by conventional medicine. Unfortunately, the problems noted above with the critiques does not mean challenge testing is valid or clinically relevant. So, is there any research to support this methodology?
The Case for Challenge Testing for Toxic Metal Load
First, let’s be clear that there is no scientific disagreement about DMSA (Hg, Pb), DMPS (Hg), and EDTA (Pb) increasing toxic metal excretion—literally hundreds of articles dating back to the 1950s document this. Rather, the question is whether the increased excretion of these metals in the urine after providing a challenge molecule correlates with body burden and whether this correlation is clinically relevant. Unfortunately, there is a dearth of articles directly addressing this question.
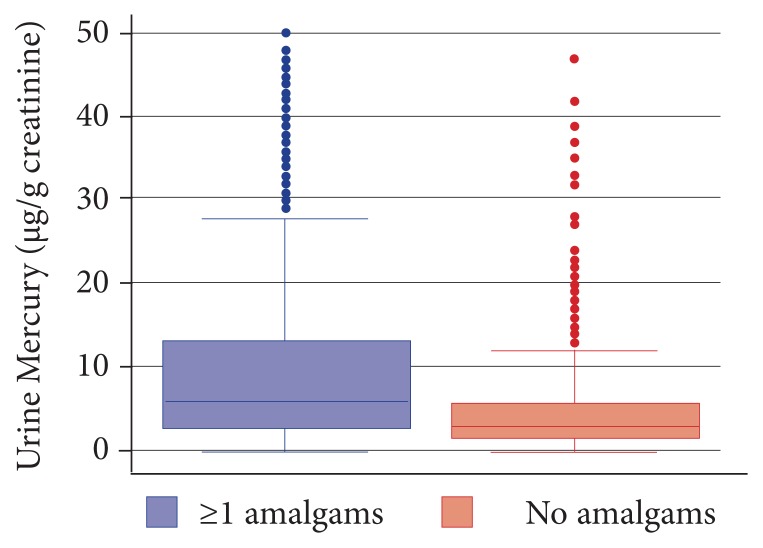
The largest study (2137 subjects) was published in 2013. (Full disclosure, I played a leadership role in the design of the corporate wellness program from which the data for this study were derived. However, I was not involved in the data analysis, which occurred after I left the project.) Comparing those with 1 or more amalgam fillings (which are typically 55% mercury) with those with no fillings, the researchers found a statistically significant difference in mercury excretion in both unprovoked and after challenge with DMPS (300 mg) plus DMSA (500 mg) and urine collection for 6 hours.30 They found in nonchallenge urinary mercury that those with fillings had 1.1 μg/g creatinine versus 0.6 μg/g creatinine for those with no amalgams. Challenge testing found urinary mercury of 6.3 μg/g creatinine versus 2.75 μg/g creatinine for those with no amalgam surfaces. This is important as the number of fillings (as opposed to number of teeth with fillings) shows strong correlation with brain and kidney levels of mercury (see my earlier IMCJ editorial for a review of this research). On the surface, this would appear to validate challenge testing. However, some significant concerns exist:
As can be seen from Figure 2, there is huge overlap in mercury excretion between those with fillings and those without.
Unchallenged mercury excretion was almost as good in differentiation as challenged mercury excretion.
Why didn’t they report the correlation between number of fillings and urinary mercury excretion? As this would have been a far more significant finding, my assumption is the results were not as good.
Figure 2.
Urinary mercury after challenge according to presence of amalgams.
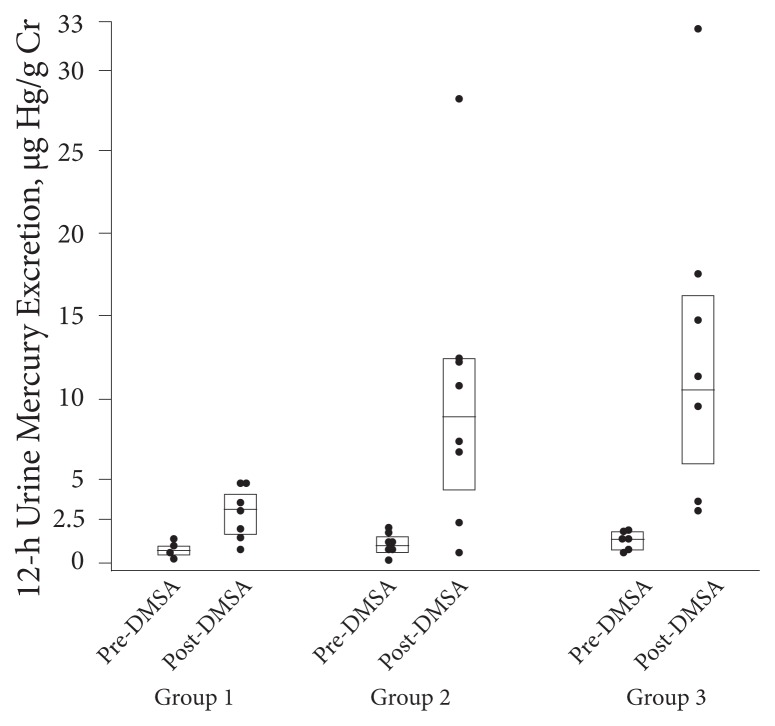
A small study looked at blood and urinary mercury, with and without challenge, and correlated it with fish consumption.31 Fish consumption correlates well with body levels of mercury (see my earlier editorial). They found an increase in blood mercury in proportion to the amount of fish eaten but no difference in baseline between those who ate no fish, those who ate 1 to 2 servings per week, and those who ate 3 or more servings per week. However, as can be seen in Figure 3, they found significant differences after challenge testing with DMSA at 30 mg/kg in all groups with a significantly larger increase in proportion to fish consumption. They then inexplicably reported that the significant differences disappeared when they did a multiple linear regression (whose variables were not listed in the study). Finally, they inexplicably reported in their conclusion, “A simple rise in chelated mercury excretion over baseline excretion is not a reliable diagnostic indicator of mercury poisoning, since healthy adults without mercury poisoning will demonstrate a rise in urine mercury levels following a single dose of DMSA.” Huh? Their study clearly demonstrated that DMSA was much more effective in differentiating those who ate fish in a dose-dependent manner than is unprovoked urine (which is why we need to read studies, not just abstracts). The astute amongst you may have noticed that the lead author also published the above negative review of challenge testing.
Figure 3.
Results of challenge testing according to amount of fish eaten.
Abbreviation: DMSA, dimercaptosuccinic acid. 12-h Urine Mercury Excretion, μg Hg/g Cr
An intriguing report of a single patient who had self-injected 3.52 g of elemental mercury found that there was big elevation in urinary mercury after 5 days of oral DMSA and DMPS—8.0 and 3.0 mg, respectively. However, the report described this as negligible in comparison to the high body load.32 Unfortunately, the authors did not report the dosage used in the abstract and I could not access the full article. A possible interpretation is that the kidney’s ability to excrete DMSA and DMPS bound to mercury is limited—which may also help explain the big variations in challenge testing results.
An encouraging study, but old (1991) and not reproduced, looked at urinary excretion of mercury various lengths of time after cessation of occupational exposure to mercury vapor.33 The authors found clear correlation between exposure currency and mercury levels before and after DMSA (2000 mg) challenge. Their conclusion was that DMSA results were indicative of exposure but most likely most accurately reflected kidney load.
That’s it folks. I looked at every study in PubMed that came up for “dimercaptosuccinic acid mercury” (196 hits) and for which there was either a comprehensive abstract or access to the full article (in addition to the freely available publications, I can access all Elsevier journals).
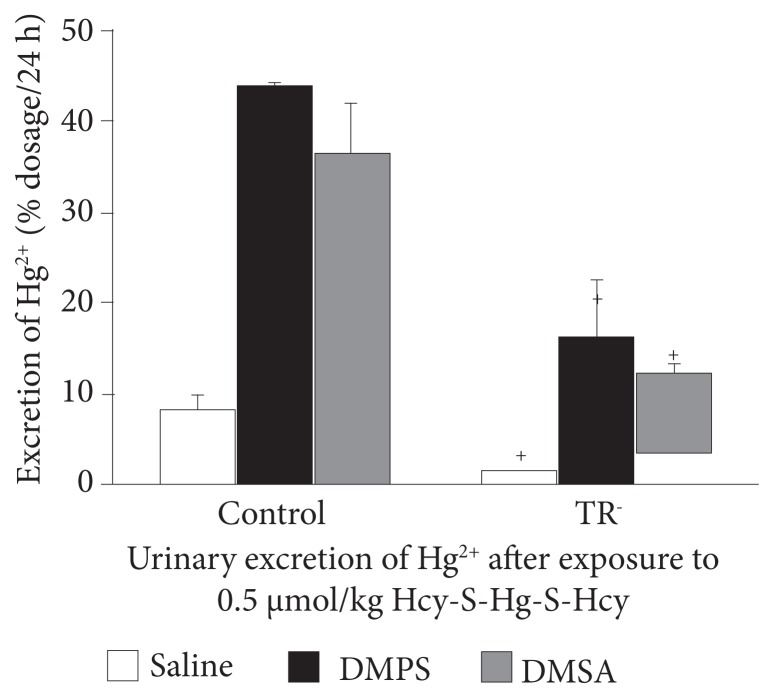
There is some intriguing animal research. One study looked at placental and fetal tissue mercury levels after the pregnant rats were injected with mercury. They then injected saline, DMPS, or DMSA. The chelating agents significantly decreased the mercury levels in the placenta and multiple fetal tissues.34 Presumably, that removed mercury showed up in the urine but was not measured. Nonetheless, this is not a direct study of accuracy for diagnostic reliability. However, an earlier animal study found a strong correlation between mercury excreted in the urine and mercury exposure in animals when a chelating agent is injected.35 As can be seen in Figure 4, both DMPS and DMSA are far more accurate at predicting body load than saline. Interesting, the TR− (Mrp2-deficient) rats are deficient in the multidrug resistance protein and thus less able to excrete the mercury-bound DMSA and DMPS. This may help explain the great variation in mercury excretion in humans after DMSA or DMPS as well as ability to detoxify these damaging metals. These same researchers have published other research with similar results.
Figure 4.
DMPS and DMSA increase urinary mercury excretion in proportion to recent exposure.
Abbreviations: DMPS, dimercaptopropane sulfonate; DMSA, dimercaptosuccinic acid; TR−, Mrp2-deficient.
Conclusion
After looking at the available research, I tentatively conclude that DMPS and DMSA challenge testing does to an unclear degree correlate with body load of mercury. However, there are serious challenges as aptly noted above. Most important:
We do not know the optimal dosage for either DMSA or DMPS. Note that the animal studies and the fish study used far higher dosages than are typically being used.
We have no standard for toxic versus “safe” levels. Obviously, the less the better, but when does the toxic metal become a priority and explain patient symptoms?
We do not know how well urine challenge results correlate with actual body load. Is the R value 0.1 or 0.75? Big difference.
High dosages of DMSA, even for a short period of time, may result in ADRs. The dosages typically currently used diagnostically (500 mg) are extremely unlikely to cause problems.
There are very serious confounding factors in determining body load of mercury and its clinical significance. Number of amalgams, amount and type of fish, living near coal burning or cement manufacturing facilities, genomic variation in glutathione production, level of homocysteine (which transports mercury!), MP2 status—this is probably only part of the list of factors we need to better understand.
Bottom line, at this time I am confident in using challenge testing to monitor patient response to intervention. Not at all clear however, are the reliability, sensitivity, or specificity of challenge testing as a diagnostic tool. However, it appears to be the best we currently have available, recognizing the serious limitations.
In This Issue
IMCJ is now PubMed indexed!!! Hard to describe the joy of our team that finally after 14 years of hard work and dedication we have achieved this recognition. I would like to express my deep appreciation to our publisher, Dick Benson, for his effective leadership; managing editor, Craig Gustafson; editorial assistant, Katie Tholkes; editor, Michael Miller; and production editor, John Benson, for their excellent work producing our high-quality publication. Perhaps most important, I want to recognize our associate editors and editorial board for their excellence in ensuring the quality of our science, which ultimately is the key criterion for our achievement.
Our presenter interview is Andrew Campbell, md, editor in chief of our sister publication, Alternative Therapies in Health and Medicine. His topic is incredibly important: the gut/brain connection. The more I look at the research, the clearer how critical gut function is to health and disruption is to disease.
Khara Lucius, nd, fabno; and Kristen Trukova, ms, rd, cso, cnsc, ldn, provide us a timely 2-part review of the role of integrative medicine in helping patients with breast cancer deal with the adverse effects of chemotherapy, especially for the heart. I believe strongly that integrative medicine is the future of cancer care.
Editorial board member Clyde B. Jensen, phd, is unique, the only person to have served as the president of colleges of allopathic, osteopathic, naturopathic, and oriental medicine and as vice president of a chiropractic college. His perspectives and experience are remarkable, broad, and deep. His concept of the “Health Professions’ Continuum Cleft” provides us great insight into challenges and potential benefits in education and collaboration.
My dear wife and editorial board member Lara Pizzorno, mdiv, ma, lmt, continues her deep review of the osteoporosis research. In this issue, she presents what she has learned about boron, both for bone health, but also for a surprising range of other roles ranging from wound healing to sex-hormone metabolism.
Managing editor, Craig Gustafson, presents his multipart interview with Michael Smith, md, on weight health (full text available on the IMCJ Web site). I think his “pillars” are consistent with my clinical experience helping patients achieve optimal weight.
I don’t think I can overstate the immense role of my friend Mark Blumenthal—founder and president of the American Botanical Council—in advancing herbal medicine. He lectures literally worldwide on the science and quality control of herbal medicine to legislators, health care professionals, media, and consumers. Extremely important is the ABC-AHP-NCNPR Botanical Adulterants Program he initiated in 2010. As long-time IMCJ readers will know, we have published close to 50 articles and editorials on product quality. We health care professionals have an important responsibility pressuring manufacturers to credibly document the safety and efficacy of their products we prescribe our patients.
Welcome back to Seattle, John Weeks! (For those unaware, John and his family have lived in Puerto Rico the past several years.) A lot of interesting reports in this issue. John documents the continuing inroads integrative medicine has made into ever-more conventional settings. I especially appreciate that the term health creation is gaining traction. Especially intriguing is the growing research documenting the efficacy of whole-practice research in naturopathic medicine. As I have stated here before, I don’t think green drug research—for example comparing St John’s wort to Prozac—will do much to solve the health care crisis. Rather, we need to think differently about health and treat people comprehensively rather than just the symptoms of their disease. Whole-system research is critical to advance this understanding. Finally, welcome Charles “Mac” Powell, phd, to the presidency of Bastyr University! Near and dear to me.
Bill Benda, md, has the last word, as usual. And, as usual, I can’t add anything to his brilliant insights. Be careful of what you wish for, indeed.
Joseph Pizzorno, nd, Editor in Chief
drpizzorno@innovisionhm.com
References
- 1.Schober SE, Mirel LB, Graubard BI, Brody DJ, Flegal KM. Blood lead levels and death from all causes, cardiovascular disease, and cancer: results from the NHANES III mortality study. Environ Health Perspect. 2006;114(10):1538–1541. doi: 10.1289/ehp.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control What do parents need to know to protect their children? http://www.cdc.gov/nceh/lead/ACCLPP/blood_lead_levels.htm. Updated June 19, 2014Accessed July. 29, 2015
- 3.Iqbal S, Muntner P, Batuman V, Rabito FA. Estimated burden of blood lead levels 5 microg/dl in 1999–2002 and declines from 1988 to 1994. Environ Res. 2008;107(3):305–311. doi: 10.1016/j.envres.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Ruha AM. Recommendations for provoked challenge urine testing. J Med Toxicol. 2013 Dec;9(4):318–325. doi: 10.1007/s13181-013-0350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. Chronic disease overview. [Accessed July 30, 2015]. http://www.cdc.gov/chronicdisease/overview. Updated May 18, 2015.
- 6.Science Daily. Over 95% of the world’s population has health problems, with over a third having more than five ailments. [Accessed July 30, 2015]. http://www.sciencedaily.com/releases/2015/06/150608081753.htm. Published June 8, 2015.
- 7.Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352(21):2211–2221. doi: 10.1056/NEJMra032424. [DOI] [PubMed] [Google Scholar]
- 8.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300(7):814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 9.Heck JE, Andrew AS, Onega T, et al. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ Health Perspect. 2009;117(11):1718–1723. doi: 10.1289/ehp.0900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett CJ, Frithsen IL. Association of urinary cadmium and myocardial infarction. Environ Res. 2008;106(2):284–286. doi: 10.1016/j.envres.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher CM, Kovach JS, Meliker JR. Urinary cadmium and osteoporosis in U.S. Women >or= 50 years of age: NHANES 1988–1994 and 1999–2004. Environ Health Perspect. 2008;116(10):1338–1343. doi: 10.1289/ehp.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon JH, Kim I, Kim HR, et al. The association between blood cadmium level and airflow obstruction in Korean men. Ann Hum Biol. 2014 Dec;26:1–7. doi: 10.3109/03014460.2014.990512. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan E, Lingala B, Bhalla V. Low-level lead exposure and the prevalence of gout: an observational study. Ann Intern Med. 2012;157(4):233–241. doi: 10.7326/0003-4819-157-4-201208210-00003. [DOI] [PubMed] [Google Scholar]
- 14.Rokadia H, Agarwal S. Serum heavy metals and obstructive lung disease: results from the National Health and Nutrition Examination Survey. Chest. 2013;143(2):388–397. doi: 10.1378/chest.12-0595. [DOI] [PubMed] [Google Scholar]
- 15.Kim KS, Lee YM, Kim SG, et al. Associations of organochlorine pesticides and polychlorinated biphenyls in visceral vs. subcutaneous adipose tissue with type 2 diabetes and insulin resistance. Chemosphere. 2014 Jan;94:151–172. doi: 10.1016/j.chemosphere.2013.09.066. [DOI] [PubMed] [Google Scholar]
- 16.Lee DH, Steffes M, Jacobs DR. Positive associations of serum concentration of polychlorinated biphenyls or organochlorine pesticides with self-reported arthritis, especially rheumatoid type, in women. Environ Health Perspect. 2007;115(6):883–888. doi: 10.1289/ehp.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YM, Bae SG, Lee SH, et al. Persistent organic pollutants and hyperuricemia in the U.S. general population. Atherosclerosis. 2013;230(1):1–5. doi: 10.1016/j.atherosclerosis.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125(6):e1270–e1277. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchard MF, Chevrier J, Harley KG, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year old children. Environ Health Perspect. 2011;119(8):1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boersma ER, Lanting CI. Environmental exposure to polychlorinated biphenyls (PCBs) and dioxins. Consequences for long term neurological and cognitive development of the child lactation. Adv Exp Med Biol. 2000;478:271–287. [PubMed] [Google Scholar]
- 21.Sabanayagam C, Teppala S, Shankar A. Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetol. 2013;50(4):625–631. doi: 10.1007/s00592-013-0472-z. [DOI] [PubMed] [Google Scholar]
- 22.Teppala S, Madhavan S, Shankar A. Bisphenol A and Metabolic Syndrome: Results from NHANES. Int J Endocrinol. 2012;2012:598180. doi: 10.1155/2012/598180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhandari R, Xiao J, Shankar A. Urinary bisphenol A and obesity in U.S. children. Am J Epidemiol. 2013;177(11):1263–1270. doi: 10.1093/aje/kws391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim JS, Lee DH, Jacobs DR., Jr Association of brominated flame retardants with diabetes and metabolic syndrome in the U.S. population, 2003–2004. Diabetes Care. 2008;31(9):1802–1807. doi: 10.2337/dc08-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min KB, Min JY. Urinary phthalate metabolites and the risk of low bone mineral density and osteoporosis in older women. J Clin Endocrinol Metab. 2014;99(10):E1997–E2003. doi: 10.1210/jc.2014-2279. [DOI] [PubMed] [Google Scholar]
- 26.Buser MC, Murray HE, Scinicariello F. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007–2010. Int J Hyg Environ Health. 2014;217(6):687–694. doi: 10.1016/j.ijheh.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Archbold GP, McGuckin RM, Campbell NA. Dimercaptosuccinic acid loading test for assessing mercury burden in healthy individuals. Ann Clin Biochem. 2004;41(Pt 3):233–236. doi: 10.1258/000456304323019622. [DOI] [PubMed] [Google Scholar]
- 28.Frumkin H, Manning CC, Williams PL, et al. Diagnostic chelation challenge with DMSA: a biomarker of long-term mercury exposure? Environ Health Perspect. 2001;109(2):167–171. doi: 10.1289/ehp.01109167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Englund SG, Dahlqvist R, Lindelöf B, et al. DMSA administration to patients with alleged mercury poisoning from dental amalgams: a placebo-controlled study. J Dent Res. 1994;73(3):620–628. doi: 10.1177/00220345940730030701. [DOI] [PubMed] [Google Scholar]
- 30.Dutton DJ, Fyie K, Faris P, Brunel L, Emery JH. The association between amalgam dental surfaces and urinary mercury levels in a sample of Albertans, a prevalence study. J Occup Med Toxicol. 2013;8(1):22. doi: 10.1186/1745-6673-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruha AM, Curry SC, Gerkin RD, Caldwell KL, Osterloh JD, Wax PM. Urine mercury excretion following meso-dimercaptosuccinic acid challenge in fish eaters. Arch Pathol Lab Med. 2009;133(1):87–92. doi: 10.5858/133.1.87. [DOI] [PubMed] [Google Scholar]
- 32.Eyer F, Felgenhauer N, Pfab R, Drasch G, Zilker T. Neither DMPS nor DMSA is effective in quantitative elimination of elemental mercury after intentional IV injection. Clin Toxicol (Phila) 2006;44(4):395–397. doi: 10.1080/15563650600671795. [DOI] [PubMed] [Google Scholar]
- 33.Roels HA, Boeckx M, Ceulemans E, Lauwerys RR. Urinary excretion of mercury after occupational exposure to mercury vapour and influence of the chelating agent meso-2,3-dimercaptosuccinic acid (DMSA) Br J Ind Med. 1991;48(4):247–253. doi: 10.1136/oem.48.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bridges CC, Joshee L, Zalups RK. Effect of DMPS and DMSA on the placental and fetal disposition of methylmercury. Placenta. 2009;30(9):800–805. doi: 10.1016/j.placenta.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bridges CC, Joshee L, Zalups RK. MRP2 and the DMPS- and DMSA-mediated elimination of mercury in TR(−) and control rats exposed to thiol S-conjugates of inorganic mercury. Toxicol Sci. 2008;105(1):211–220. doi: 10.1093/toxsci/kfn107. [DOI] [PMC free article] [PubMed] [Google Scholar]