Long time readers of IMCJ will know that I have been studying the field and caring for patients suffering diseases due to toxicity. The more research I read, the more convinced I am that toxicity—exogenous, endogenous, and toxins of choice—are now the primary causes of most chronic disease in industrialized countries.
I am currently working to create a comprehensive database on toxins to track where they come from; the amount of each that enters the average person every day from food, water, air, health and beauty aids, etc; half-lives; and the primary mechanisms for their detoxification/excretion. As I totaled the amount of each toxin, I was stunned to see that many of these are now entering our bodies at higher dosages than the trace mineral and vitamins required for health. Considering that enzymes are basically special proteins requiring cofactors—almost all of which are vitamins or minerals—to work and that the mechanism by which many toxins cause damage is by displacing cofactors from enzymes, is it any wonder that we are seeing increasing incidence of almost all chronic diseases?
I am getting to the point of recommending that if a patient has high toxic load, deal with that first and then see what is left needing to be treated. This provides further support to the concept that symptom-relief-based medicine is not only not dealing with the true causes of disease and ill health, but exacerbating the situation through the use of toxic drugs. As most readers know, most drugs are basically enzyme poisons—which means they actually often make patients less healthy by adding to their already heavy toxic load. Don’t get me wrong, conventional medicine has many benefits and at times drugs are lifesaving. But for everyday health problems, we simply must stop using them as the first line of intervention. Treating the causes of disease is the only solution to the health care crisis, and toxins are a—if not the—primary cause of disease.
So, how do we determine a patient’s toxic load?
Determining a Patient’s Toxic Load
With toxic load becoming an ever-more serious clinical problem, accurate assessment is very important for not only determining if this needs to be addressed for the patient but also for tracking efficacy of intervention. There are a number of accepted tests for metal toxicity, but they rely mostly on blood and urine, which are known to be primarily useful only for acute exposure and are unreliable for body load. The standards for chemical toxin load are basically population based (ie, unless a patient is in the top 5% of blood levels, they are not considered toxic). Obviously the huge problem with this is the assumption that those with lower levels are healthy and not being damaged by their toxic load.
My editorial in IMCJ 14.4 analyzed the research evaluating the validity of “unconventional” challenge testing to assess body load of toxic metals. Space and time limitations resulted in my only looking at the research evaluating the validity of challenge testing with dimercaptosuccinic acid (DMSA) to assess body load of mercury. My conclusion was that challenge testing looks good for following patients over time, but there is simply not enough research to determine if it is an accurate assessment of body load.
Long-time readers will remember that in my glutathione editorial (IMCJ 13.1) I noted that the conventional liver enzyme test γ-glutamyltransferase (GGTP) increases—within the normal range—in response to chemical toxic load. I also mentioned the research showing that as GGTP goes up—again within the normal range—rate of death increases proportionately. I thought this was quite intriguing and decided to look into the research to see if any other conventional lab tests were also showing toxic load correlations within the supposed normal range. I was quite surprised to find quite a lot.
Conventional Laboratory Tests That Correlate With Toxic Load
So far I have found that a many conventional laboratory tests increase within the “normal” range in proportion to toxin load: (1) complete blood count (CBC), which includes a red blood cell (RBC) count, white blood cell (WBC) count, platelet count, hemoglobin, and basophilic stippling; (2) liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and GGTP; (3) inflammatory markers, including C-reactive protein (CRP); (4) lipids, including low-density lipoprotein (LDL), oxLDL, and triglycerides; (5) blood sugar, including insulin, fasting blood sugar (FBS), and 2-hour postprandial (2-h PP) blood sugar; and (6) metabolites (bilirubin, uric acid, and 8-OHdG). Unfortunately, these tests are not very specific as they tend to go up with total toxic load and, for some, with classes of toxins, rather than with specific toxins. Nonetheless, this looks very, very useful and I suspect this is just the tip of the iceberg. Normal isn’t healthy.
Complete Blood Count
It’s hard to find a cheaper and more readily available test than the good old CBC, which can easily be done in the office. How many of you have seen a patient’s CBC come back with a low WBC count or platelets at the bottom edge of normal and wondered what was happening as they did not have any of the diseases typically associated with low levels? Turns out these can be early indicators of toxin exposure.
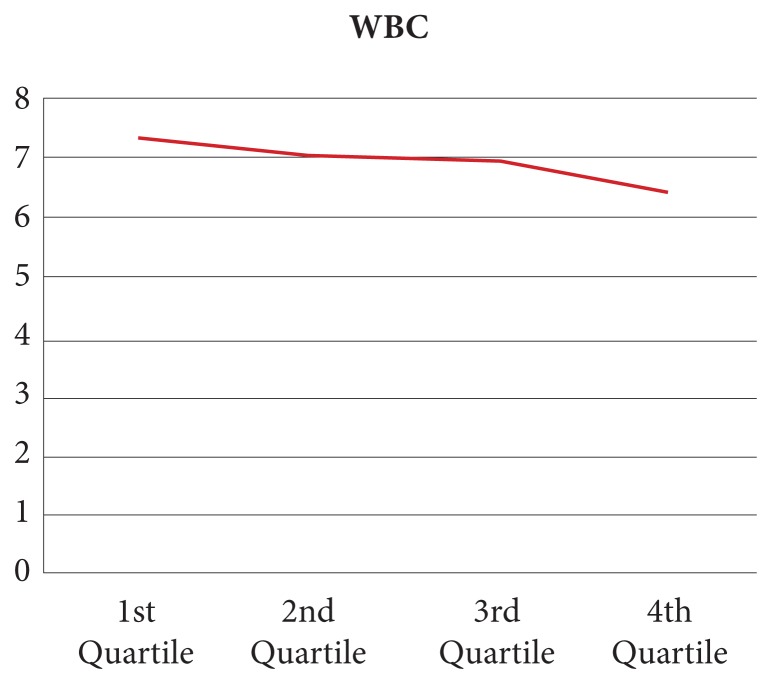
As can be seen in Figure 1, the WBC count decreases in proportion to body load of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs).1 Note the 14% drop in the WBC count—again within the “normal” range. One of the challenges is that although total PCBs and OCPs correlate well, the correlation with specific chemicals in these classes is quite inconsistent.
Figure 1.
Decrease in WBC count in proportion to exposure to PCBs and OCPs.
Abbreviations: WBC, white blood cell; PCBs, polychlorinated biphenyls; OCPs, organochlorine pesticides.
Chronic low-level exposure to solvents decreases platelet count. A study of auto repair workers found that even though they wore masks and protective gear, when compared with office workers in the same facility (which we must assume were also exposed, albeit at lower levels), they had a 14% lower platelet count (216 000 versus 252 000 per mL). As “normal” is 150 000 to 450 000, they are still in the normal range—but are clearly experiencing measurable physiological effects. Interestingly, the researchers also looked at neuropsychological factors and found a 4.13-fold increased risk of impaired sympathetic function and a 6.94-fold increased risk for neuropsychiatric dysfunction.
Another useful indication from the standard CBC is basophilic stippling of the RBCs, which occurs in both arsenic and lead poisoning. I had a patient with basophilic stippling who helped me determine she was suffering from lead poisoning. Her presenting complaint was sudden loss of vision. I looked in her eyes and saw retinal nerve swelling. A bit of detective work determined that she lived on a house boat at the end of a long dock. A few months previously, a new water line had been installed—copper pipe using leaded solder. She was a heavy tea drinker—refilling rather than replacing the water in her kettle, thus concentrating the lead. Happily, her symptoms began resolving within a few days of removing all sources of lead and she eventually fully regained her eyesight. Her case left quite an impression on me.
Liver Enzymes
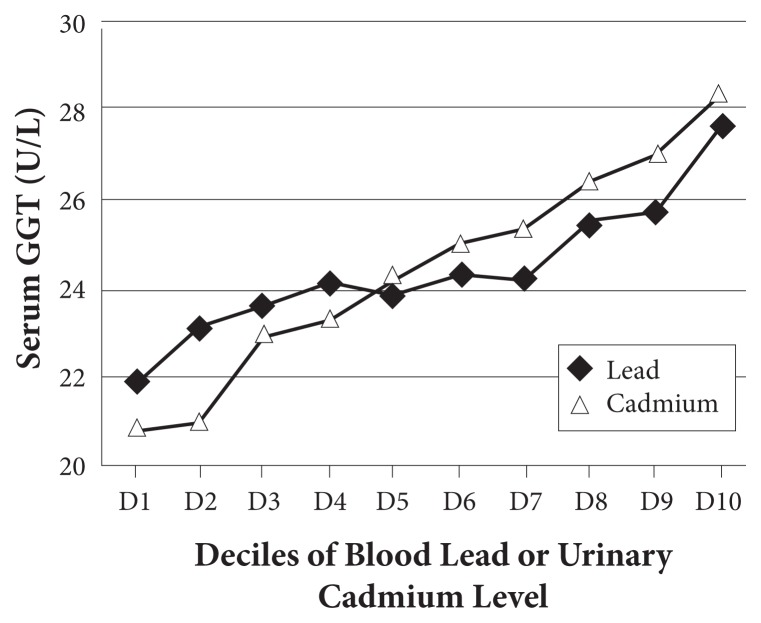
Liver enzymes are typically measured to detect hepatitis. Research is now showing that several of them increase in proportion to the load of specific classes of toxins. This is not surprising considering that many liver enzymes play important roles in detoxification and are induced as needed. Perhaps most useful is GGTP, a key enzyme in glutathione recycling. It is induced to provide more glutathione, presumably for phase 2 conjugation, as well as for oxidative stress. As discussed in my earlier editorial, elevations are strongly associated with several chronic diseases, especially diabetes.2 I was quite intrigued to find that GGTP increases with toxic metals as well as persistent organic pollutants (POPs), as shown in Figure 2, again, within “normal” range.3
Figure 2.
GGTP increases in proportion to toxic metal load.
Abbreviation: GGTP, γ-glutamyltransferase.
ALT catalyzes the transfer of an amino group from l-alanine to α-ketoglutarate, the products of this transamination reaction being pyruvate and l-glutamate. ALT increases in a dose-dependent manner with body load of blood cadmium, lead, mercury, and PCBs within and above the normal range.4 In the adult population, 10.4% have an elevated ALT, typically due to nonalcoholic fatty liver disease (NAFLD). A strong case can be made that NAFLD is caused by toxin exposure and excessive consumption of high-fructose corn syrup.
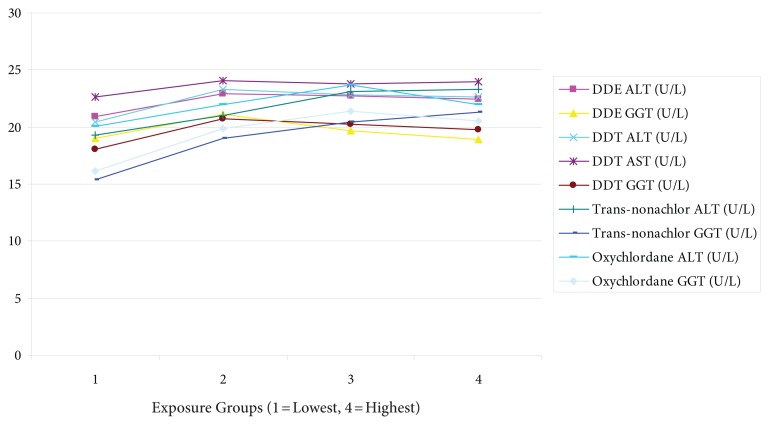
Another study found AST, ALT, and GGT increase with body load of PCBs and OCPs. As can be seen in Figure 4, these increase in proportion to serum levels of these toxins. Note, however, the nonlinearity of these relationships. Those in the top quartile of serum pesticide oxychlordane had a 10% increase in ALT and 25% increase in GGT.1
Figure 4.
Increase of liver enzymes in proportion to serum OCP levels.
Abbreviations: OCP, organochlorine pesticide; DDE, dichlorodiphenyldichloroethylen; ALT, alanine aminotransferase; GGT, γ-glutamyltransferase; DDT, dichlorodiphenyltrichloroethane; AST, aspartate aminotransferase.
Inflammatory Markers
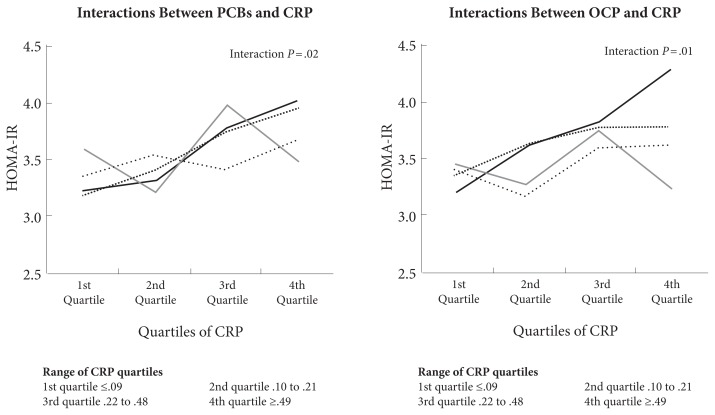
Most environmental toxins increase inflammation and oxidative stress. Figure 5 shows how CRP and body load of PCBs and OCPs interact to increase risk of metabolic syndrome.5
Figure 5.
Association between CRP, body load of PCBs and OCPs, and metabolic syndrome.
Abbreviations: CRP, C-reactive protein; PCBs, polychlorinated biphenyls; OCPs, organochlorine pesticides; HOMA-IR, homeostatic model assessment-insulin resistance.
Lipids
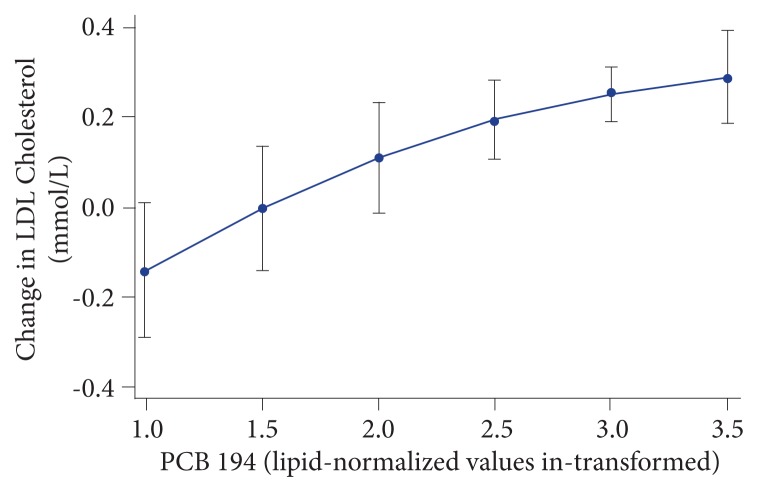
An intriguing prospective study evaluated if PCB levels could predict future cholesterol levels over time. They found huge variation with some PCBs having little effect, whereas some had a substantial impact. Nonetheless, the overall effect was quite clear. As can be seen in Figure 6, PCB 194 alone predicted a remarkable 10% of the elevation of LDL cholesterol during a 5-year period.6 This is particularly interesting as most reports of POP levels in the blood standardize according to serum lipid levels. As cholesterol levels increase with age for most people, this may suggest the research is underestimating actual body load.
Figure 6.
PCB 194 predictive of LDL-cholesterol elevation.
Note: Relationship between PCB 194 levels (lipid-normalized (ng/g lipid) and in-transformed) and the change in LDL cholesterol between age 70 and 75 y using predictive margins with 95% CI (P = 8.1 ×10−10).
Abbreviations: PCB, polychlorinated biphenyl; LDL, low-density lipoprotein; CI, confidence interval.
Perhaps the most important aspect of this is that PCBs oxidize cholesterol—the most artery damaging form.7 As would be expected, they also show a direct correlation between PCBs and the ratio of glutathione disulfide (GSSG) to reduced glutathione (GSH), a definitive measure of oxidative status.
Metabolites
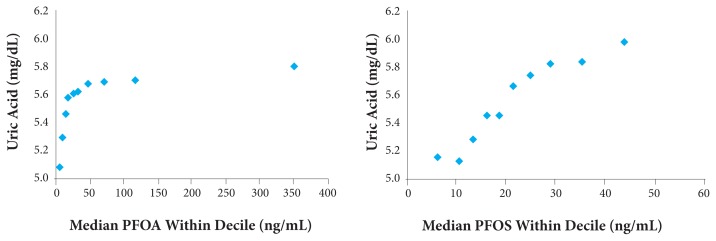
As can be seen in Figure 7, serum uric acid increases in proportion to body load of polyfluorinated hydrocarbons (PFOA, or perfluorooctanoic acid; and PFOS, or perfluorooctane sulfonate). Notice the very interesting threshold effect where low levels show little effect and then rapid elevation.8
Figure 7.
Serum uric acid increases in proportion to body load of PFOA and PFOS.
Abbreviations: PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate.
Bilirubin levels go up in proportion to the level of various PCBs.9 This is particularly interesting as bilirubin is currently considered the best prognostic measure of chronic liver dysfunction.10
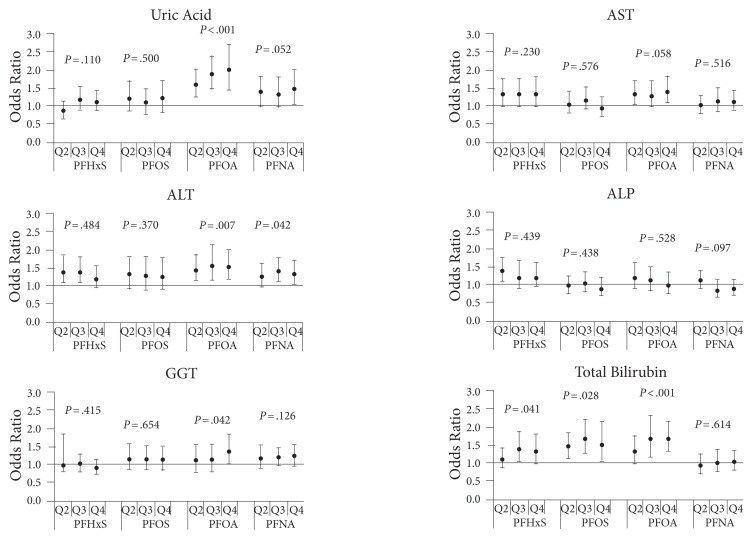
Finally, one useful study looked at the association of perfluorinated chemicals with a wide range of liver-associated enzymes and molecules. Note in Figure 8 the huge variability, with different measures responding to different chemicals.11
Figure 8.
Serum measures showing response to perfluorinated chemical exposure.
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyltransferase; Q2, quartile 2; Q3, quartile 3; Q4, quartile 4; PFHxS, perfluorohexane sulfonate; PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoic acid; PFNA, perfluorononanoic acid.
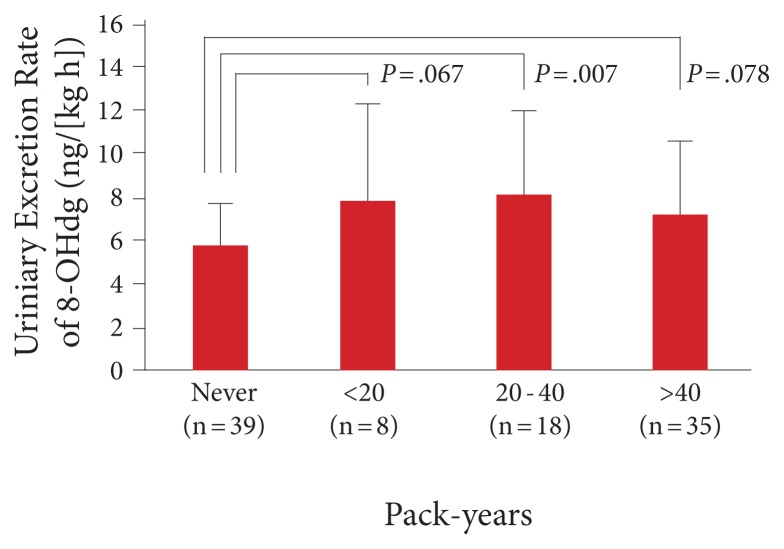
Another useful metabolite is urinary 8-OHdG. Basically, as DNA becomes damaged, it is repaired and the oxidized nucleosides appear in the urine. Thus, these urinary nucleoside metabolites are a direct measure of DNA damage and an indirect measure of oxidative stress and toxin load. It correlates with multiple cancers, mitochondrial damage, rate of aging, smoking, mercury levels, etc. As can be seen in Figure 9, 8-OHdG increases directly with pack years of smoking.12 Interestingly, those who have smoked the most have a slightly lower level—which can be easily interpreted as those with the highest levels have already died from lung cancer.
Figure 9.
Urinary 8-OHdg correlates with pack-years of smoking.
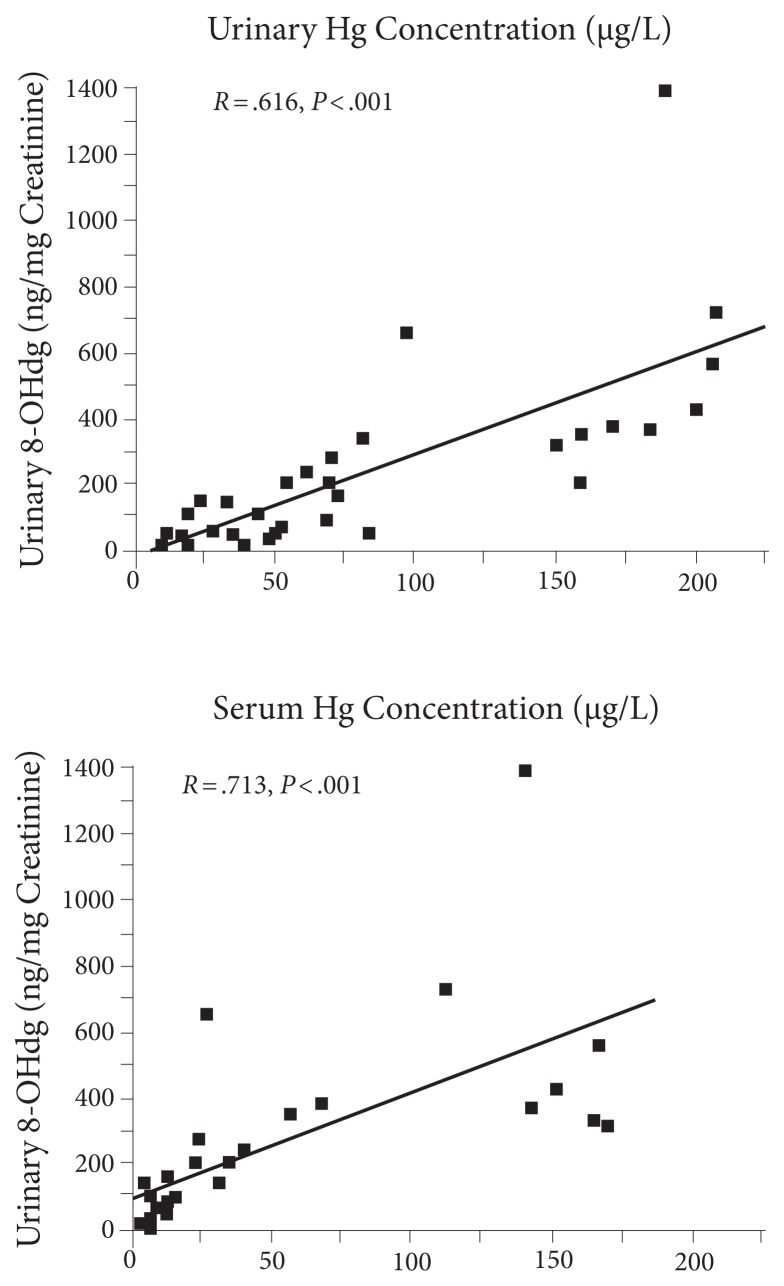
Urinary 8-OHdG (as well as other DNA repair metabolites) clearly correlates with toxic load, such as mercury as shown in Figure 10.13 Note the very strong correlations with both blood and urine as well as the occasional substantial outlier. It seems obvious to speculate that those above the line suffer the greatest damage from mercury.
Figure 10.
Mercury levels correlate with 8-OHdG.
Walter Crinnion, nd, suggests using 8-OHdG for monitoring total toxic load and as a measure of efficacy of intervention (American Association of Naturopathic Physicians [AANP], 2015 conference). I think this an excellent clinical pearl.
Blood Sugar Regulation
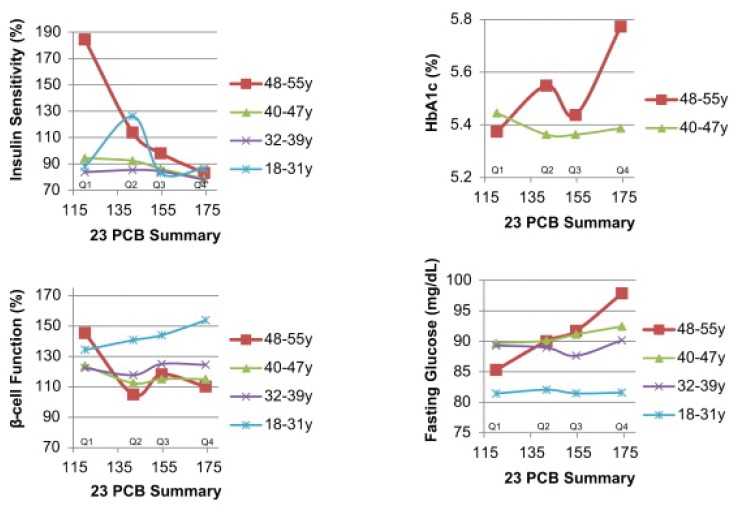
The greatest amount of research on potential disease causation has shown very strong correlations between toxic load and virtually every measure of blood sugar regulation: FBS, 2-h PP sugar, HbA1c, insulin levels, metabolic syndrome, and diabetes. This is not surprising considering that most of these chemicals are insulin receptor site poisons. I think Figure 11 provides extremely important insights. It shows the results of a 23-year prospective study.14 Looking at those who were not diabetic at the start of the study, there are several critical observations:
Figure 11.
A 23-year prospective study on effects of PCBs on blood sugar regulation.
Abbreviation: PCB, polychlorinated biphenyl.
Until the age of 50 years, there is essentially no difference in the several blood sugar regulation measures between those with the lowest and highest PCB levels suggesting there is apparently little impact of the increasing toxin load in younger people.
In the youngest group, insulin production increases in response to toxin level, as would be expected because the blocking of insulin receptor sites by PCBs requires more insulin. That adaptive ability decreases with aging.
At age 50 years, all the measures show very strong toxin-dose response suggesting that the body’s adaptive capabilities have become inadequate or are overwhelmed by ever-increasing toxic load.
Conclusion
Looking at all this research, it seems quite clear to me that what is defined as “normal” is actually mostly toxic with the body showing measurable signs of adaptation and metabolic dysfunction. The study shown in Figure 11 is particularly illustrative of deep principles. I interpret their results to mean that our bodies have tremendous ability to heal and respond to physiological insults. However, with aging, damage accumulates (especially to the DNA) and, as enzymes are made progressively less optimally, the body in time loses its ability to adapt to the toxic environment, nutrient deficiencies, etc.
The apparent transition age of around 50 years especially resonates with me. Long-time readers will know I am an avid basketball player. My core game has now been running twice per week for more than 25 years. No one in my age group is left of the original players. I have noticed that almost every guy I have played with hits a wall at about age 50. Yes, some of their loss in skill and increased susceptibility to injury is due to decreased testosterone. But I am beginning to think that perhaps the main reason is that their toxic load finally caught up to them. It’s truly fascinating to look into how health devolves to disease due to cumulative damage from toxins. (BTW, we have also had women play with us and, in fact, have helped the recovery of several Seattle Storm players coming back from injury. However, none of them have played with us long enough for me to see if the women experience the same problems at age 50.)
The final question now is, “What is the optimal range for each of these conventional laboratory tests?” Except for CBC and platelet count, it looks to me like we want our patients in the lowest quartile of the range of each of these tests. Note especially in Figure 11 that in the older, 48- to 55-year-old range, those in the bottom quartile of PCB exposure have excellent blood sugar regulation by all measures. Seems to me a good place to be.
In This Issue
I am delighted welcome to IMCJ 2 new associate editors: Jeffrey Bland, phd, and David S. Riley, md. I suspect all readers of this journal know of Jeff’s seminal work and have likely heard him lecture. As cofounder of the Institute for Functional Medicine and Founder of the Personalized Lifestyle Medicine Institute, his work has been transformative to tens of thousands of health care professionals, including me. He will be writing a commentary each issue sharing his latest thoughts. His “Functional Medicine as the Operating System for Integrative Medicine” takes us on a journey showing how the concepts of functional medicine were founded and have evolved. Having been there since day one, I can say it has been quite a ride!
David S. Riley, md, has been active for decades in holistic/integrative medicine research, journals, and leadership. He is involved in the governance of the Academy for Integrative Health and Medicine (AIHM), where he has founded the AIHM Journal Club and is on faculties of the National College of Natural Medicine and Southwest College of Naturopathic Medicine. I am delighted he is joining IMCJ to advance case reports in integrative medicine, with at least 1 planned for every issue. As he states so well in his accompanying article, a lot of the true breakthroughs in medicine often come from clinical observation. We are posting at http://imjournal.com 2 companion documents he wrote (“Case Report Author Guidelines” and “Timeline Instructions”) to help clinicians create valid case reports that can help advance our medicine. Even those not interested in submitting case reports will find the guidelines he suggests a useful exercise in rigorous case taking.
How often in medical journals do we hear about stories of failure rather than success? I applaud the bravery of Linda L. Isaacs, md, who frankly discusses the huge challenges of engaging in research of nonorthodox therapies and concepts. I especially appreciate her guidance on how we can do better by learning from her hard-earned experience. This seems a particularly apt opportunity to recognize now-deceased cancer research pioneer, Nicholas Gonzalez, md. His courage in taking on the conventional medical oncology establishment was historic, but virtually all of us involved in integrative cancer research are quite frustrated that the attempt to objectively research his work was so badly handled and an important option for pancreatic cancer patients seemingly lost. Those wanting to learn more about the life and accomplishments of this remarkable man are encouraged to read the nice tribute written by Kelly Brogan, md, abihm and available on our Web site.
I have known Deborah Kesten, mPh, and Larry Scherwitz, phd, for almost 2 decades and have been so impressed by their pioneering work in understanding how patients interact with food and eating. As we all know, health is fundamentally determined by how and what we eat (all nutrients, but also 80% of toxins, come from food). Clinicians well understand the huge challenge of helping patients change their dietary behaviors is extremely difficult. Thank you Deborah and Larry for providing such useful insight.
IMCJ editorial board member Robert H. Lerman, md, phd; Jyh-Lurn Chang, phd; Veera Konda, phd; Anuradha Desai, phd; and Michael B. Montalto, phd, provide us original research on the use of an Humulus lupulus (hops) extract and undenatured type 2 collagen as anti-inflammatory agents for patients with rheumatoid arthritis. This is an intriguing and novel application of natural health products to promote health and decrease disease burden.
Khara Lucius, nd, fabno; and Kristen Trukova, ms, rd, cso, cnsc, ldn, provide us the second and concluding article in their “Integrative Therapies and Cardiovascular Disease in the Breast Cancer Population.” Here they focus on nutritional interventions. These are experienced clinicians providing us great guidance for complicated patients.
Managing editor, Craig Gustafson, interviews a heroine of our time, Barbara Dossey, phd, rn. As a pioneer in the holistic nursing and nurse coaching movements, she has worked to advance the practice and philosophies of holistic care, both within the health professions and in the lives of lay people. What an inspiring team she and Larry Dossey, md, make!
Craig also interviewed for us Dale E. Bredesen, md of the Buck Institute. I have been following with considerable interest his team’s work on reversing cognitive decline. They have clearly now documented that, as IMCJ readers know, Alzheimer’s disease is multifactorial and expecting good results from a drug or other monotherapy is not just wishful thinking but naïve denial of the reality confronting us every day in practice. Not sure I agree with his brain “downsizing” hypothesis, but quite interesting and important nonetheless.
As could be logically inferred from my editorial above, I fully agree with John Weeks’s assertion that most of the determinants of health occur outside the time patients spend in our offices. We all need to be involved in public health, in its broadest sense. Having been actively involved in the legislative process to mandate that the National Center for Complementary and Alternative Medicine (NCCAM) research systems of healing and not isolated therapies, I share John’s dismay that so little of their funding goes to nonconventional medical institutions. They have the deepest understanding of the proper use of these interventions and have recruited skilled researchers. Come on NCCAM, what exactly are you afraid of? Congratulations to Michael Murray, nd, for being only the third recipient of the Benedict Lust Lifetime Achievement Award by the AANP (an organization I helped cofound 3 decades ago). As the first recipient of this award named after the founder of naturopathic medicine, I had the honor of presenting this award with Kasra Pournadeali, nd, president of the AANP. I think few people appreciate how huge an impact Michael has had on advancing integrative/natural medicine. To name but a few: The Textbook of Natural Medicine we coauthored and later invited contributing authors was his idea. Michael brought standardized herbal medicines to the United States from Europe. The first articles recommending glucosamine sulphate to consumers and health care professionals were written by Michael. Astoundingly, he has authored more than 30 consumer books and professional textbook advancing this medicine. The list of his accomplishments is long and impact immense.
Every issue, I open Bill Benda, md’s BackTalk with considerable anticipation. I don’t know if he is going to provide an insight into why conventional medicine is failing in some area or chastise those of us who choose the nonconventional route for some hypocrisy or foolish wishful thinking. Truly, I am writing this before I even open the file he sent me. Almost always a fun treat to indicate completion of my editorial responsibilities so another issue can go to press. Hmm, okay, none of the above.
Joseph Pizzorno, nd, Editor in Chief
drpizzorno@innovisionhm.com
Figure 3.
Risk of elevation of ALT with body load of toxic metals and PCBs.
Abbreviations: ALT, alanine aminotransferase; PCBs, polychlorinated biphenyls.
References
- 1.Serdar B, LeBlanc WG, Norris JM, Dickinson LM. Potential effects of polychlorinated biphenyls (PCBs) and selected organochlorine pesticides (OCPs) on immune cells and blood biochemistry measures: a cross-sectional assessment of the NHANES 2003–2004 data. Environ Health. 2014 Dec;13:114. doi: 10.1186/1476-069X-13-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DH, Steffes MW, Jacobs DR., Jr Can persistent organic pollutants explain the association between serum gamma-g.lutamyltransferase and type 2 diabetes? Diabetologia. 2008;51(3):402–407. doi: 10.1007/s00125-007-0896-5. [DOI] [PubMed] [Google Scholar]
- 3.Lee DH, Lim JS, Song K, Boo Y, Jacobs DR., Jr Graded associations of blood lead and urinary cadmium concentrations with oxidative-stress-related markers in the U.S. population: results from the third National Health and Nutrition Examination Survey. Environ Health Perspect. 2006;114(3):350–354. doi: 10.1289/ehp.8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect. 2010;118(12):1735–1742. doi: 10.1289/ehp.1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim KS, Hong NS, Jacobs DR, Jr, et al. Interaction between persistent organic pollutants and C-reactive protein in estimating insulin resistance among non-diabetic adults. J Prev Med Public Health. 2012;45(2):62–69. doi: 10.3961/jpmph.2012.45.2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penell J, Lind L, Salihovic S, et al. Persistent organic pollutants are related to change in circulating lipid levels during a 5 year follow-up. Environ Res. 2014;134:190–197. doi: 10.1016/j.envres.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Kumar J, Lind PM, Salihovic S, et al. Influence of persistent organic pollutants on oxidative stress in population-based samples. Chemosphere. 2014;114:303–309. doi: 10.1016/j.chemosphere.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Steenland K, Tinker S, Shankar A, Ducatman A. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ Health Perspect. 2010 Feb;118:229–233. doi: 10.1289/ehp.0900940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar J. Persistent organic pollutants and liver dysfunction biomarkers in a population-based human sample of men and women. Environ Res. 2014;134:251–256. doi: 10.1016/j.envres.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46(12):2050–2068. doi: 10.1093/clinchem/46.12.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleason JA, Post GB, Fagliano JA. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environ Res. 2015;136:8–14. doi: 10.1016/j.envres.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Yano T, Shoji F, Baba H, et al. Significance of the urinary 8-OHdG level as an oxidative stress marker in lung cancer patients. Lung Cancer. 2009;63(1):111–114. doi: 10.1016/j.lungcan.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Chen C. Increased oxidative DNA damage, as assessed by urinary 8-hydroxy-2′-deoxyguanosine concentrations, and serum redox status in persons exposed to mercury. Clin Chem. 2005;51(4):759–767. doi: 10.1373/clinchem.2004.042093. [DOI] [PubMed] [Google Scholar]
- 14.Suarez-Lopez JR, Lee DH, Porta M, Steffes MW, Jacobs DR., Jr Persistent organic pollutants in young adults and changes in glucose related metabolism over a 23-year follow-up. Environ Res. 2015 Feb;137:485–494. doi: 10.1016/j.envres.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]