Abstract
Context
Tetrahydro iso-α acids (THIAAs), derived from Humulus lupulus (hops), have demonstrated anti-inflammatory effects in vitro and in an animal model of rheumatoid arthritis (RA). Undenatured type 2 collagen has been found to be effective in clinical studies in RA and osteoarthritis (OA).
Objective
The study intended to evaluate the efficacy and safety of a proprietary tablet containing 150 mg of n-enriched THIAA (nTHIAA) and 10 mg of undenatured type 2 collagen (UC-II) (containing 25% UC-II) in patients with arthritis.
Design
The study was an open-label case series. This article also includes a case history for 1 participant.
Setting
The study was conducted at the Functional Medicine Research Center (FMRC) in Gig Harbor, WA, USA, from February 2013–June 2013.
Participants
Participants were 17 adults, 12 women, and 5 men aged 39–69 y, who had chronic joint pain involving various joints, 13 with probable OA and 4 with possible RA.
Intervention
Participants took 2 tablets of nTHIAA + UC-II 2 ×/d with meals for 12 wk.
Outcome Measures
Participants completed arthritis-related and quality-of-life questionnaires, at weeks 2, 4, 8, and 12: (1) the visual analog scale for pain (VAS-P); (2) the medical symptoms questionnaire (MSQ), with the analysis particularly focusing on the joint/muscle subscale and total scores; (3) the health and wellness outcome questionnaire (MOS-SF36), with the analysis particularly focusing on the physical and mental subscales; (4) the arthritis impact questionnaire (AIQ), with the analysis particularly focusing on the arthritis symptoms and daily living subscales; (5) the health assessment questionnaire (HAQ-DI) with the analysis particularly focusing on question 26 (Q26), which indicates overall pain during the week prior to the survey; and (6) the arthritis impact measurement scales 2 (AIMS2). At 12 wk, participants also completed the visual analog scale for efficacy (VAS-E).
Results
All participants completed the 12-wk evaluation, and all reported improvements in pain. Significant improvements in scores on the questionnaires were observed as early as 2 wk. For example, the total score on the MSQ was significantly decreased from a mean of 20.76 ± 2.90 (SE) at baseline to 12.24 ± 2.81 after 2 wk (P < .001). At 12 wk, the participants rated the supplement’s efficacy at 7.6 ± 0.6 of 10. At baseline, 13 of the 17 participants were using analgesics for joint pain, compared with only 4 participants at 12 wk. Two of those 4 had reduced their analgesic dosages. The studied supplement was well tolerated, and no serious side effects occurred.
Conclusions
The supplement containing nTHIAA and UC-II is safe and efficacious in participants with chronic joint pain.
According to the National Health Interview Survey in 2006, nearly 30% of US adults ≥18 years have reported experiencing some type of joint pain during the preceding 30 days.1 Reported by 18% of respondents, knee pain was the most common, followed by pain in the shoulders, fingers, and hips.1 Chronic pain is typically caused by arthritis, a debilitating inflammatory condition of the joints and the leading cause of disability in the United States.2 Two of the most common types of arthritis, in which the main feature is joint pain, are osteoarthritis (OA) and rheumatoid arthritis (RA).2
OA is a degenerative, localized joint disease characterized by active bone remodeling, degradation of articular cartilage, joint-space narrowing, and synovial inflammation, resulting in a loss of joint function and angular deformity or malalignment.3 RA is a chronic, inflammatory, systemic autoimmune disease that is characterized by symmetric inflammation of synovial joints, leading to a progressive erosion of cartilage and bone.4 Although OA and RA are defined and diagnosed differently, similarities exist in the symptoms (eg, joint pain and cartilage destruction). Recent research also has found common underlying causes contributing to the pathogenesis of both conditions, such as synovial inflammation, immune-cell infiltration, and chondrocyte pathophysiology.5–8
Patients with OA or RA routinely seek pain relief with medications to resume normal daily activities.9 Although many palliative medications are available for arthritis, currently no effective strategy exists to prevent disease progression, and nearly all medications have unwanted side effects.10,11 The most commonly used class of medications, nonsteroidal anti-inflammatory drugs (NSAIDs), has been found to have serious health consequences when used long term.12 Without a targeting of underlying causes, symptoms generally worsen. In severe clinical disease, joint replacement surgery is often implemented. Therefore, newer and safer management approaches for joint health are needed.
Tetrahydro iso-α acids (THIAAs), derived from Humulus lupulus (hops), are a dietary ingredient containing a mixture of 3 major (co-, n-, and ad-) congeners. THIAA has been shown to have anti-inflammatory properties in vitro and to reduce bone and cartilage degradation in a mouse model of RA.13–16 The n-enriched THIAA (nTHIAA) consists of the same congeners, but the proportion of n- and ad-congeners is increased. An in vivo study has found that the n- and ad-congeners provided higher anti-inflammatory activity than the co-congener.17
Undenatured type 2 collagen, a dietary ingredient derived from chicken-sternum cartilage, has been shown to ameliorate symptoms in individuals with RA, without treatment-related adverse events.18–20 Purportedly, the effects work via the mechanism of oral tolerance, the ability to induce antigen-specific, peripheral immune tolerance by oral administration of antigens.21 More recently, undenatured type 2 collagen was studied in individuals with OA22 and in normal participants who had experienced joint discomfort after exercise. In both, symptom alleviation was reported, and the intervention was well tolerated, without serious adverse events.
In view of those encouraging findings, a combination of nTHIAA and undenatured type 2 collagen was formulated as a nutritional supplement. The current case series investigated the efficacy and safety of that combination in participants with chronic joint pain, probably secondary to OA and/or RA.
Methods
Participants
This open-label case series was conducted at the Functional Medicine Research Center (FMRC) (Gig Harbor, WA, USA) from February to June 2013. Potential participants were self-referred in response to a newspaper advertisement or the FMRC mailing list. They were then phone screened by study staff. Eligible individuals were nonsmoking adults with chronic joint pain, including pain from symptomatic OA that involved any joint or combination of joints and from RA, and were invited to the clinic.
At the clinic visit, the study’s clinician assessed participants’ pain level using the visual analog scale for pain (VAS-P).23 The research team enrolled those individuals who had joint pain that was rated to be at least 4/10 in intensity and who agreed to participate.
Procedures
The studied supplement contained 300 mg of nTHIAA (Metagenics, Inc) and 20 mg of UC-II, a natural collagen concentrate that contained 25% undenatured type 2 collagen (InterHealth Inc, Benecia, CA, USA). Both Metagenics’ and InterHealth’s manufacturing facilities are certified for current good manufacturing practices (GMP). Each batch of the studied supplement went through microbiology testing to detect contaminants and analytical testing to confirm the amount of actives at Metagenics’ quality assurance/quality control (QA/QC) department.
Intervention
Each participant received the supplement twice daily for 12 weeks and returned to FMRC at 2, 4, 8, and 12 weeks for visits with the clinician. At each visit, participants were given enough tablets to use until the next visit, and patients were asked to bring back any remaining tablets. Concomitant use of over-the-counter (OTC) analgesics was allowed. After 12 weeks, the study’s participants were given the option to continue use of the supplement.
Outcome Measures
At baseline and at each clinical visit, participants completed a series of quality-of-life and arthritis-related questionnaires and were evaluated by a staff physician. The questionnaires included the (1) VAS-P; (2) medical symptoms questionnaire (MSQ) with the analysis particularly focusing on the joint/muscle subscale and total scores24; (3) the health and wellness outcome questionnaire (MOS-SF36), with the analysis particularly focusing on the physical and mental subscales25; (4) the arthritis impact questionnaire (AIQ), with the analysis particularly focusing on the arthritis symptoms and daily living subscales; (5) the health assessment questionnaire (HAQ-DI), with the analysis particularly focusing on question 26 (Q26), which indicates overall pain during the week previous to the survey26; and (6) the arthritis impact measurement scales 2 (AIMS2).27 At 12 weeks, each participant also rated the supplement’s efficacy using the visual analog scale for efficacy (VAS-E).23 The analgesic use by participants at baseline and at 12 weeks was also recorded.
An adverse event was defined as any unanticipated or unintended medical occurrence or worsening of a sign or symptom, including any abnormal laboratory finding, or disease in the study’s participants. Information about such events was collected via a questionnaire and via an interview with the clinician at every visit. Fasting blood samples were obtained at baseline and also at 12 weeks to monitor any abnormal change in safety biomarkers. Serious adverse events, according to the Food and Drug Administration (FDA) definition, would have been reported to FDA within 15 days, if they had occurred. The current case series was conducted in accordance with the Declaration of Helsinki of 1975, as revised in 2000.
Statistical Analyses
Statistical analyses were performed on the entire dataset of 17 participants with probable OA + possible RA and on the dataset of 13 participants with probable OA only, using Excel (Microsoft, Redmond, WA, USA). Twosided paired t tests were used to compare the data from each clinical visit to that of the baseline. Data are expressed as mean ± standard error of the mean (SE). Data from the 4 possible RA participants were summarized without statistical analysis due to the small sample size. P < .05 was considered statistically significant.
Results
Of the 29 prospective participants who had visited the clinic, 18 were enrolled in the study; one participant dropped out early on due to relocation. The 17 patients, including 12 women and 5 men ranging in age from 39 to 69 years, had joint pain, presumably due to OA. Four of the 17 patients had possible RA or a combination of probable OA and possible RA. All participants were Caucasians.
A diagnosis of RA was considered on the basis of positive testing for the rheumatoid factor. Participants’ joint symptoms had been present for 9 months to 30 years. All 17 participants were followed for 12 weeks. Compliance, assessed by questionnaire and interviews with participants, was considered to have been high. The nTHIAA-and-UC-II combination was well tolerated, and no serious adverse events occurred in the course of the case series.
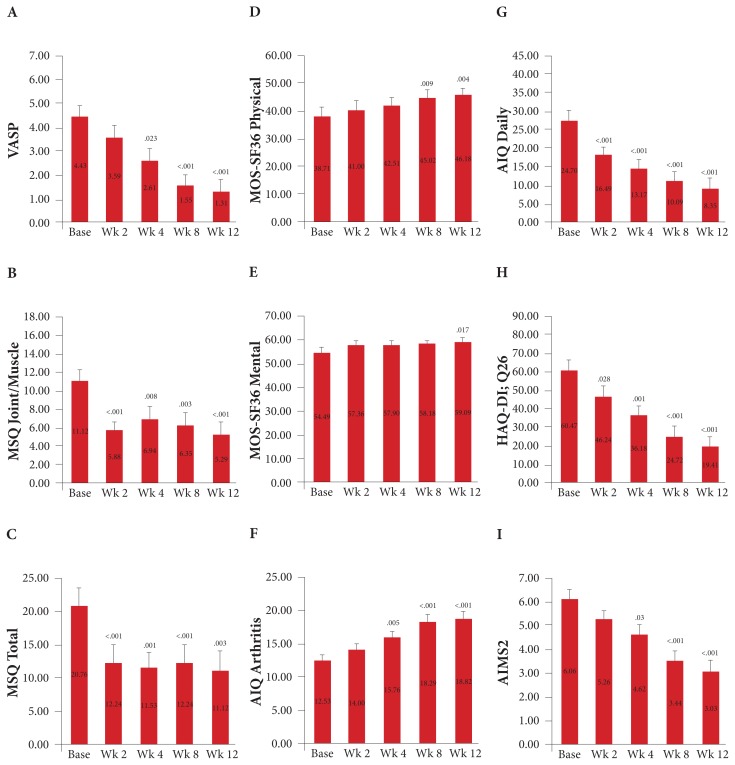
Among the participants, significant improvements compared with baseline were observed for every questionnaire measured (Figure 1). Statistically significant changes in scores occurred at 2 weeks, 4 weeks, 8 weeks, and 12 weeks after baseline for the (1) joint/muscle subscale on the MSQ questionnaire—P < .001, P = .008, P = .003, and P < .001, respectively; (2) MSQ’s total score— P < .001, P = .001, P < .001, and P = .003, respectively; (3) daily living subscale on the AIQ questionnaire— P < .001, P < .001, P < .001, and P < .001, respectively; and (4) Q26 on the HAQ-DI questionnaire—P = .028, P = .001, P < .001, and P < .001, respectively.
Figure 1.
Mean changes in a series of quality-of-life and arthritis-related questionnaires from baseline to 12 wk among 17 participants with chronic joint pain. Figure 1A shows the scores on the VAS-P. Figure 1B shows the joint/muscle scores and Figure 1C shows the total scores on the MSQ. Figure 1D shows the scores for the physical subscale and Figure 1E shows the scores for the mental subscale on the MOS-SF36. Figure 1F shows the scores for the arthritis symptoms subscale and Figure 1G shows the scores for the daily living subscale on the AIQ. Figure 1H shows the scores for Q26 on the HAQ-DI; Q26, which indicates participants’ overall pain during the week prior to the questionnaire. Figure 1I shows the scores on the AIMS2. Mean values are displayed in the midbar, and the error bars indicate the standard error. P values indicate a significant difference compared with baseline.
Abbreviations: VAS-P, visual analog scale for pain; MSQ, medical symptoms questionnaire; MOS-SF36, health and wellness outcome questionnaire; AIQ, arthritis impact questionnaire; Q26, question 26; HAQ-DI, health assessment questionnaire; AIMS2, arthritis impact measurement scales 2.
Statistically significant changes in scores occurred at 4 weeks, 8 weeks, and 12 weeks after baseline for the (1) VAS-P questionnaire—P = .023, P < .001, and P < .001, respectively; (2) arthritis symptoms subscale on the AIQ questionnaire—P = .005, P < .001, and P < .001, respectively; and (3) AIMS2 questionnaire—P = .03, P < .001, and P < .001, respectively.
Statistically significant changes in scores occurred at 8 weeks and 12 weeks for the physical subscale on the MOS-SF36 questionnaire—P = .009 and P = .004, respectively, and at 12 weeks after baseline for the mental subscale on the MOS-SF36 questionnaire—P = .017.
At 12 weeks, the 17 participants rated the supplement’s self-assessed efficacy using VAS-E at 7.8 ± 0.47 of 10. At baseline, 13 of the 17 participants were using analgesics for joint pain. At 12 weeks, only 4 participants, 3 with probable OA and 1 with possible RA, were using OTC analgesics, and 2 of the 4 had reduced their dosages.
A separate analysis for 13 of the participants with probable OA showed similar findings (Table 1). Compared with baseline, significant improvements were observed as early as 2 weeks, and by 12 weeks, improvements were seen for all questionnaires. At 12 weeks, the participants with probable OA rated the supplement’s efficacy on the VAS-E at 7.6 ± 0.59 of 10. At baseline, 10 of the 13 participants were using analgesics for joint pain. By 12 weeks, only 3 remained on analgesics; one of the 3 had reduced the dosage.
Table 1.
Changes in Quality-of-life and Arthritis-related Questionnaires From Baseline to 12 Weeks Among 13 Participants With Probable OA
| Questionnaire | Baseline | Week 2 | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|---|
| VAS-P | 4.18 ± 0.61 | 3.48 ± 0.60 | 2.68 ± 0.61 | 1.69 ± 0.58b | 1.43 ± 0.68a |
| MSQ joint/muscle | 11.08 ± 1.42 | 5.15 ± 1.01b | 6.00 ± 1.65a | 5.62 ± 1.53b | 4.69 ± 1.57b |
| MSQ total | 18.38 ± 2.87 | 9.31 ± 2.18b | 8.77 ± 2.27b | 9.31 ± 2.68b | 7.85 ± 2.05b |
| MOS-SF36 physical | 42.75 ± 3.03 | 45.55 ± 2.30 | 46.38 ± 2.71 | 48.22 ± 2.84a | 49.17 ± 2.55a |
| MOS-SF36 mental | 54.64 ± 2.00 | 56.76 ± 1.66 | 57.81 ± 1.42 | 59.05 ± 1.42a | 58.78 ± 1.35a |
| AIQ arthritis symptoms | 12.31 ± 0.97 | 14.08 ± 0.98 | 15.85 ± 1.18a | 18.23 ± 1.12c | 18.46 ± 1.30b |
| AIQ daily living | 23.79 ± 2.55 | 15.95 ± 2.76b | 11.67 ± 2.56c | 10.09 ± 2.65c | 8.36 ± 2.99c |
| HAQ-DI Q26 | 60.23 ± 6.47 | 46.62 ± 6.98 | 35.62 ± 6.22b | 25.41 ± 6.58c | 20.54 ± 6.38c |
| AIMS2 | 6.08 ± 0.54 | 5.35 ± 0.47 | 4.54 ± 0.55 | 3.54 ± 0.58b | 3.27 ± 0.70b |
Note: Data are mean ± SE.
Abbreviations: OA, osteoarthritis; SE, standard error of the mean; VAS-P, visual analog scale for pain; MSQ, medical symptoms questionnaire; MOS-SF36, health and wellness outcome questionnaire; AIQ, arthritis impact questionnaire; HAQ-DI Q26, health assessment questionnaire question 26; AIMS2, arthritis impact measurement scales 2.
P < .05 compared with baseline.
P < .01 compared with baseline.
P < .001 compared with baseline.
Data from 4 participants with possible RA suggested a trend toward improvements on the VAS-P, on the physical subscale of the MOS-SF36, on the arthritis symptoms and the daily living subscales of the AIQ, on Q26 of the HAQ-DI, and on the AIMS2, although the sample size was not sufficient for statistical analysis to determine significance (Table 2).
Table 2.
Changes in Quality-of-life and Arthritis-related Questionnaires From Baseline to 12 Weeks Among 4 Participants With Possible RA
| Questionnaire | Baseline | Week 2 | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|---|
| VAS-P | 5.23 ± 0.66 | 3.93 ± 0.41 | 2.40 ± 0.75 | 1.08 ± 0.63 | 0.90 ± 0.52 |
| MSQ joint/muscle | 11.25 ± 2.29 | 8.25 ± 2.59 | 10.00 ± 1.78 | 8.75 ± 2.59 | 7.25 ± 2.25 |
| MSQ total | 28.50 ± 7.59 | 21.75 ± 8.81 | 20.50 ± 5.33 | 21.75 ± 6.54 | 21.75 ± 10.13 |
| MOS-SF36 physical | 25.60 ± 2.84 | 26.23 ± 4.20 | 29.90 ± 2.30 | 34.63 ± 6.56 | 36.48 ± 2.52 |
| MOS-SF36 mental | 54.03 ± 6.26 | 59.30 ± 5.66 | 58.20 ± 4.84 | 55.35 ± 2.91 | 60.10 ± 1.95 |
| AIQ arthritis symptoms | 13.25 ± 1.11 | 13.75 ± 1.44 | 15.50 ± 1.32 | 18.50 ± 2.40 | 20.00 ± 1.47 |
| AIQ daily living | 27.65 ± 5.40 | 18.25 ± 2.06 | 18.05 ± 3.66 | 10.10 ± 3.44 | 8.33 ± 4.03 |
| HAQ-DI Q26 | 61.25 ± 10.87 | 45.00 ± 3.54 | 38.00 ± 6.31 | 22.50 ± 10.31 | 15.75 ± 3.61 |
| AIMS2 | 6.00 ± 0.54 | 5.00 ± 0.20 | 4.88 ± 0.43 | 3.13 ± 0.77 | 2.25 ± 0.60 |
Note: Data are mean ± SE. No statistical analysis was performed.
Abbreviations: RA, rheumatoid arthritis; SE, standard error of the mean; VAS-P, visual analog scale for pain; MSQ, medical symptoms questionnaire; MOS-SF36, health and wellness outcome questionnaire; AIQ, arthritis impact questionnaire; HAQ-DI Q26, health assessment questionnaire question 26; AIMS2, arthritis impact measurement scales 2.
Illustrative Case Report
Participant
Presenting Concern
The 39-year-old, white male was an education specialist who came to the FMRC with chronic joint pain, probably due to OA. He was first diagnosed with OA by his primary physician in June 2008. At his first visit to the FMRC on January 13, 2013, he was experiencing hip pain upon sitting and needed to shift positions frequently. Pain was generally worse in the evenings and with inactivity. Sleep was difficult for him due to the aching in his hands and knees. His knees and thumbs were the most symptomatic, with his knee pain generally rating 6–7/10 and his intermittent thumb pain rating 8/10 at times.
Medical History
Approximately 5 years prior to his first visit, the participant had noted the onset of knee discomfort with running and with ascending and descending stairs, primarily in the deep anterior aspect of his knees, and he had noted reduced stamina. Vigorous physical exercise became limited due to his painful knees, hips, and hands. He had no history of knee trauma. He was evaluated by an orthopedic surgeon in 2008; knee X-rays revealed mild height narrowing of his medial articular cartilage and early osteophyte formation in his patella-femoral compartments bilaterally. He was referred for physical therapy and was instructed to avoid running and to do low-impact activities. Four years prior to his first visit to the clinic, he noted a gradual onset of intermittent pain in his fingers, mostly in the proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints, and also intermittent pain and stiffness in his hips. X-rays of both hands were essentially normal, but X-rays of his hips revealed very mild narrowing of the hip joints.
Relevant Past Interventions
The participant had tried glucosamine/chondroitin sulfate with a methylsulfonylmethane supplement as well as naproxen, without significant benefits. Acetaminophen had provided minimal pain relief.
Comorbidities
The participant had experienced the onset of migraine headaches in childhood, had sleep apnea and seasonal allergies, and had suffered from seasonal affective disorder (SAD) with depression since moving to the Pacific Northwest.
Family History
Both parents had OA.
Current Medications and Supplements; Diet
The participant (1) was using Tylenol Arthritis (acetaminophen) twice daily, 2 to 3 d/wk; (2) had been taking Maxalt-MLT (rizatriptan benzoate) PRN for 15 years for migraine; (3) was taking a multivitamin once daily; (4) was using a light box regularly for SAD; and (5) was taking Allegra (fexofenadine) PRN in the spring. His diet consisted of the “total crappy American diet.” He also had undergone juice fasting 3 years prior to his first visit. Upon food reintroduction after the fasting, he had noted tomatoes and potatoes as causing worsening of joint pain and had eliminated them from his diet.
Diagnostic Assessment
Physical Evaluation
The participant’s height was 68 in (173 cm), and his weight was 209 lb (95 kg). His body mass index (BMI) was 31.8 kg/m2; his blood pressure (BP) was 119/68, and his pulse was 60 beats per minute. He was a pleasant, healthy-appearing, mildly-obese male in no acute distress; he was alert, cooperative, and well oriented. The general examination was unremarkable, except for bilateral knee tenderness. No swelling, warmth, erythema, or effusions were noted.
Laboratory Testing
The participant’s CBC and CMP were within reference ranges (reference ranges in parentheses). His other laboratory tests showed the following results: (1) sedimentation rate—1 mm/h (≤15 mm/h); (2) hs-CRP—0.5 mg/L; (3) rheumatoid factor—7 IU/mL (<14 IU/mL); (4) CCP—Ab < 16 U (negative < 20 U); (5) ANA screen—negative; (6) 25(OH) vitamin D—26 ng/mL (30–100 ng/mL); and (7) homocysteine—16.7 umol/L (5.4–11.9 umol/L). The participant’s immunoglobulin G (IgG) and immunoglobulin A (IgA) for the tissue transglutaminase antibody and for the deaminated gliadin antibody were within normal reference ranges.
Initial Impression
The clinician’s initial evaluation showed that the participant had chronic joint pain, probably secondary to OA; migraine headaches; obstructive sleep apnea; obesity; SAD; hypovitaminosis D; and hyperhomocysteinemia.
Intervention
On the participant’s return visit on February 12, 2013, the combination of nTHIAA at 600 mg/d and UC-II at 40 mg/d in 2 divided doses daily with meals was begun.
Outcomes
Two-week Visit on February 27, 2013
Overall, the participant’s joint pain was similar. He had stopped taking acetaminophen to be able to better evaluate the effects of the intervention. Although his knee pain had bothered him for a couple of days, at the time of the visit, he had no knee pain. His thumb pain was 5/10 in intensity and annoying (ie, preventing him from gripping things). His energy level had improved (Table 3).
Table 3.
Changes in Quality-of-life and Arthritis-related Questionnaires From Baseline to 6 Months in the Male Participant Described in the Case Report
| Questionnaire | Baseline (02/12/13) | Week 2 (02/27/13) | Week 5 (03/20/13) | Week 8 (04/11/13) | 3 Months (05/02/13) | 6 Months (08/14/13) |
|---|---|---|---|---|---|---|
| VAS-P | 7.5 | 6.9 | 2.0 | 0.4 | 0 | 0 |
| MSQ joint/muscle | 10 | 8 | 6 | 10 | 3 | 2 |
| MSQ total | 49 | 32 | 23 | 36 | 14 | 11 |
| MOS-SF36 physical | 25.2 | 32.4 | 37.3 | 44.6 | 48.8 | 48.3 |
| MOS-SF36 mental | 48 | 44 | 52.5 | 48.8 | 52.8 | 54.4 |
| AIQ arthritis symptoms | 14 | 15 | 17 | 20 | 24 | 24 |
| AIQ daily living | 43 | 28 | 18 | 19 | 8.2 | 6.2 |
| HAQ-DI Q26 | 65 | 65 | 35 | 20 | 10 | 5 |
| AIMS2 | 5.0 | 5.0 | 4.5 | 2.5 | 0.5 | 0.5 |
Abbreviations: VAS-P, visual analog scale for pain; MSQ, medical symptoms questionnaire; MOS-SF36, health and wellness outcome questionnaire; AIQ, arthritis impact questionnaire; HAQ-DI Q26, health assessment questionnaire question 26; AIMS2, arthritis impact measurement scales 2.
Five-week Visit on March 20, 2013
The participant was cautiously optimistic and stated: “My knees haven’t really bothered me at all.” He was a bit more active and had stiffness associated with that increased activity. His hips were not bothering him, and he did not notice them. His thumbs had improved, but he still noted some pain (2.5/10).
Eight-week Visit on April 11, 2013
Overall, the participant had noted less pain and stiffness and almost never noted knee pain. His hands were much improved. He was doing lots of yard work and had noted improved dexterity. He no longer had trouble gripping a shovel, etc. He had no tender joints.
Twelve-week Visit on May 2, 2013
The participant had no knee pain and only an occasional twinge of hand pain. His sleep was not being interrupted. Except for the rizatriptan benzoate for migraines, he had not taken any medications. Since starting the intervention, he had had only 2 migraines, fewer than one-half of what was usual for him. Vitamin D (5000 IU) once daily was begun due to hypovitaminosis D and the nTHIAA + UC-II twice daily was continued.
Four-month Visit on May 28, 2013
The participant continued to do well. If he did a lot of walking or yard work, he noted a slight twinge of pain.
Lab testing found that his vitamin B12 was 354 pg/mL (200–1100 pg/mL) and that his serum folate was 10.9 ng/mL (>5.4 ng/mL). A vitamin-B complex supplement containing methylated folate and vitamin B12 was added once daily due to hyperhomocysteinemia.
Five-month Visit on June 25, 2013
The participant had no arthritis pain except for 1 to 2 days after driving for 4 hours. He had a migraine headache and took 1 OTC medication for his migraine and obtained relief.
Six-month Visit on August 14, 2013
The participant remained pain-free except for a couple of days when he forgot to take the nTHIAA + UC-II when going camping. On the second day without it, he noted low-grade hand soreness (1/10). Within a day of restarting it, the pain resolved. At the visit, his BP was 113/55, and his pulse was 57 beats per minute. His laboratory testing showed (1) 25(OH) vitamin D—59 ng/mL (30–100 ng/mL) and (2) homocysteine—10.4 umol/L (5.4–11.9 umol/L). He continued taking the nTHIAA + UC-II twice daily, the vitamin D3 (5000 IU) once daily, and the vitamin B-complex containing methylated folate and vitamin B12 once daily.
Seven-month Visit on September 26, 2013
The participant continued to do well, saying “I haven’t recognized any pain in the last 6 weeks,” and “Everything’s going fine.” He had been more active, hiking, hunting, and fishing. In addition, he walked for approximately 45 minutes per day, 5 days per week, at work. He no longer took analgesics or NSAIDs for joint pain. He had “a ton of allergy issues” in the month prior to the visit and had taken fexofenadine as needed. The allergies had led to tiredness. Upon the physical examination, his weight was 216.0 lb (98 kg); his BP was 121/46; and his pulse was 61 beats per minute.
Seven-month Assessment
The clinician’s evaluation showed that the participant had (1) OA but was symptom free; (2) sleep apnea; (3) seasonal allergies; (4) hyperhomocysteinemia, which had been resolved; (5) hypovitaminosis D, which had been resolved; and (6) obesity.
Case Summary
At the start of the intervention, the 39-year-old white male, with a 5-year history of chronic joint pain involving his knees, hips, and hands, had joint pain with an intensity of approximately 7/10. He also had migraine headaches, and he had had an inadequate response to acetaminophen. He was placed on nTHIAA + UC-II twice daily, with no changes to diet or exercise, and he stopped taking acetaminophen. No other nutritional supplements were added until his 12-week visit.
At 2 weeks, he had noted some minor improvements, but by 5 weeks, he had noted significant pain reduction. By 12 weeks, he was essentially pain-free, and vitamin D (5000 IU) once daily was started in view of his hypovitaminosis D. At his 4-month visit, a methylated folate and vitamin B complex once daily was started in view of his hyperhomocysteinemia. At 6 months, his 25(OH)-vitamin-D level and homocysteine were normal.
He had forgotten to take the study’s supplement for a couple of days when camping and had noted mild hand pain, which resolved rapidly on restarting the nutritional supplement. He was followed for 7 months, showing continued improvement and complete pain relief. At 7 months, he reported that he had become more active, in addition to walking for approximately 45 minutes, 5 days per week, at work.
His improvement was documented by multiple questionnaires evaluating joint symptoms and quality of life. The frequency of his migraines was significantly lower. He had had no adverse events. The case was not considered representative of all participants but was among the best observed over a 12-week period, during which no other new nutritional supplements or medications were taken and no dietary changes were begun.
Discussion
In the current exploratory, 12-week case series, the research team evaluated the responses of 17 participants with chronic joint pain to a novel nutritional supplement that contained nTHIAA and UC-II, providing a respective daily dose of 600 mg and 40 mg. At the conclusion of the case series, the participants reported favorable outcomes, as indicated by improvements in their scores on arthritis-related and quality-of-life questionnaires, absence of serious adverse reactions, and discontinuation or reductions in use of analgesics.
Under mechanical stress or chronic wear and tear, degraded cartilage fragments in the joint trigger the innate immune response, leading to production of proinflammatory cytokines and chemokines as well as chondrocyte-mediated degradation of cartilaginous-matrix components.5,6 The inflamed synovial membrane also facilitates recruitment of immune cells that produce cytokines, perpetuating a vicious cycle.
In synovial fibroblasts in human RA, THIAA has been shown to inhibit interleukin 1β (IL-1β)-activated prostaglandin E2 (PGE2), matrix metalloproteinase 3 (MMP-3), IL-6, IL-8, and monocyte chemoattractant protein 1 (MCP-1) significantly.16 In a mouse model of acute inflammation, THIAA was shown to reduce paw swelling in a way similar to the effects of aspirin. In mice with collagen-induced arthritis in an animal model of RA, THIAA significantly reduced the arthritis index and decreased bone, joint, and cartilage degradation. Serum IL-6 concentrations in those mice were inhibited in a dose-dependent manner.16
Mechanistically, THIAA targets inflammatory signal transduction by modulating multiple kinases involved in the nuclear factor κB (NF-κB) pathway, without affecting constitutive cyclooxygenase 2 (COX-2) enzymes.14 THIAA contains a mixture of 3 major congeners (co-, n-, and ad-) that share a substituted 1,3-cyclopentadione motif. The ratio of co:n:ad congeners in THIAA can vary, depending on the climate, location, and condition where the hops have been grown. The nTHIAA comes from an extract, with the composition of the n + ad:co congeners at a 2.1:1 ratio. Experimental studies at the FMRC have indicated that n-congeners are more active than co-congeners for kinase inhibition and for anti-inflammatory properties.17 Thus, nTHIAA was chosen for the current case series.
In articular cartilage, type 2 collagen is the most abundant structural protein, making it a candidate autoantigen for RA. Because oral administration of autoantigens suppresses experimentally induced autoimmune pathologies, such as experimental allergic encephalomyelitis and antigen(s)-induced arthritis,28–31 researchers have been interested in orally administered, undenatured type 2 collagen as a potential remedy for RA.18–20 As ingested, undenatured type 2 collagen reaches the small intestine, where it interacts with Peyer’s patches in gut-associated lymphoid tissue.32 Regulatory T cells in Peyer’s patches become activated and produce the anti-inflammatory cytokines IL-4, IL-10, and transforming growth factor β (TGF-β), which help turn off the T-cell attack on type 2 joint collagen, thereby reducing pain and inflammation.33,34 Also, those inhibitory cytokines are important in restoring the cytokine balance and in shifting chondrocyte metabolism toward extracellular-matrix replenishment.35–37 Undenatured type 2 collagen retains its original triple-helical form and high molecular weight, making it resistant to digestion and absorption.21 That structure is essential for the interaction with Peyer’s patches and the development of oral tolerance. In contrast, the denatured (hydrolyzed) form of type 2 collagen no longer retains the tertiary structure, does not induce immunological hyporesponsiveness upon ingestion, and has not demonstrated an effect on reducing pain and inflammation.29
In the 1990s when undenatured type 2 collagen was evaluated for its efficacy in RA, OA was still viewed as an aging condition due to mechanical wear and tear to the joint. Researchers have since then recognized that synovial inflammation and the immune system are important factors in the development and progression of both OA and RA, although the number of infiltrating immune cells and the expression of proinflammatory cytokines are higher in RA than in OA synovial tissue.8
A recent clinical trial in individuals with OA was conducted with UC-II-treated participants, indicating a significant enhancement in daily activities and improvements in quality of life after 90 days.22 Later, another clinical trial investigated the efficacy of UC-II in healthy participants who developed joint pain while undergoing strenuous exercise, based on the rationale that the symptom might be indicative of possible future joint problems.38 That study found that daily supplementation with UC-II improved joint function and flexibility. Because of their differences in the mechanisms of action, nTHIAA and UC-II together may form a unique, possibly complementary, approach to managing arthritis and joint discomfort. The current 12-week case series demonstrated that the combination was safe and efficacious.
Several limitations of any case series must be recognized. A selection bias can occur even when the inclusion and exclusion criteria are explicitly stated. Without a randomized study including a control arm, the observed efficacy cannot be attributed to the studied supplement alone. A potential placebo effect, seasonal effect, and Hawthorne effect (ie, the case in which a study’s participants modify or improve their behavior or performance due to their awareness of being studied) also cannot be ruled out. The current study’s small sample size and short duration also limited the generalizability of the findings. The study included enough OA participants but not enough RA participants for subgroup analysis; therefore, only descriptive statistics have been provided for the latter. Further, the effect from each individual ingredient cannot be assessed. Nevertheless, the current exploratory case series offers encouraging insight into the clinical use of the 2 dietary ingredients in management of chronic joint pain and provides a rationale for future clinical trials.
Conclusions
In summary, participants with chronic joint pain received nTHIAAs at 600 mg per day and undenatured type 2 collagen as UC-II at 40 mg per day and experienced improvements as early as their initial return visit at 2 weeks. By 12 weeks, statistically significant improvements compared with baseline were observed in every questionnaire used in the case series. The 2 ingredients in the supplement may offer a unique and safe approach in managing chronic joint discomfort.
Acknowledgments
The research team would like to thank Joseph Lamb, md; Matthew Tripp, phd; and Kimberly Koch for their involvement in the case series.
Footnotes
Author Disclosure Statement
The supplement discussed in this article was developed by Metagenics, Inc, in Gig Harbor, WA, USA, and the study was funded by that company.
References
- 1.QuickStats: percentage of adults reporting joint pain or stiffness--National Health Interview Survey, United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(17):467. [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation--United States, 2010–2012. MMWR Morb Mortal Wkly Rep. 2013;62(44):869–873. [PMC free article] [PubMed] [Google Scholar]
- 3.Racine J, Aaron RK. Pathogenesis and epidemiology of osteoarthritis. R I Med J (2013) 2013;96(3):19–22. [PubMed] [Google Scholar]
- 4.Doan T, Massarotti E. Rheumatoid arthritis: an overview of new and emerging therapies. J Clin Pharmacol. 2005;45(7):751–762. doi: 10.1177/0091270005277938. [DOI] [PubMed] [Google Scholar]
- 5.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21(1):16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Hoff P, Buttgereit F, Burmester GR, et al. Osteoarthritis synovial fluid activates pro-inflammatory cytokines in primary human chondrocytes. Int Orthop. 2013;37(1):145–151. doi: 10.1007/s00264-012-1724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otero M, Goldring MB. Cells of the synovium in rheumatoid arthritis: chondrocytes. Arthritis Res Ther. 2007;9(5):220. doi: 10.1186/ar2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20(12):1484–1499. doi: 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Hauber AB, Arden NK, Mohamed AF, et al. A discrete-choice experiment of United Kingdom patients’ willingness to risk adverse events for improved function and pain control in osteoarthritis. Osteoarthritis Cartilage. 2013;21(2):289–297. doi: 10.1016/j.joca.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 10.O’Neil CK, Hanlon JT, Marcum ZA. Adverse effects of analgesics commonly used by older adults with osteoarthritis: focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother. 2012;10(6):331–342. doi: 10.1016/j.amjopharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh JA, Cameron DR. Summary of AHRQ’s comparative effectiveness review of drug therapy for rheumatoid arthritis (RA) in adults--an update. J Manag Care Pharm. 2012;18(4 suppl C):S1–S18. doi: 10.18553/jmcp.2012.18.s4-c.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15(suppl 3):S2. doi: 10.1186/ar4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai A, Darland G, Bland JS, Tripp ML, Konda VR. META060 attenuates TNF-alpha-activated inflammation, endothelial-monocyte interactions, and matrix metalloproteinase-9 expression, and inhibits NF-kappaB and AP-1 in THP-1 monocytes. Atherosclerosis. 2012;223(1):130–136. doi: 10.1016/j.atherosclerosis.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Desai A, Konda VR, Darland G, et al. META060 inhibits multiple kinases in the NF-kappaB pathway and suppresses LPS-mediated inflammation in vitro and ex vivo. Inflamm Res. 2009;58(5):229–234. doi: 10.1007/s00011-008-8162-y. [DOI] [PubMed] [Google Scholar]
- 15.Tripp M, Darland G, Lerman R, Lukaczer D, Bland J, Babish J. Hop and modified hop extracts have potent in vitro anti-inflammatory properties. Acta Hortic. 2005;668:217–228. [Google Scholar]
- 16.Konda VR, Desai A, Darland G, Bland JS, Tripp ML. META060 inhibits osteoclastogenesis and matrix metalloproteinases in vitro and reduces bone and cartilage degradation in a mouse model of rheumatoid arthritis. Arthritis Rheum. 2010;62(6):1683–1692. doi: 10.1002/art.27441. [DOI] [PubMed] [Google Scholar]
- 17.Konda VR, Desai A, Darland G, Carroll BJ, Bland JS, Tripp ML. Multi-target kinase inhibitors co- & n-humulones from hops differentially inhibit inflammation in vitro and collagen-induced arthritis in mice. Paper presented at: Inflammation 2010: Inflammatory Cell Signaling Mechanisms; January 27–30, 2010; Luxembourg. [Google Scholar]
- 18.Barnett ML, Combitchi D, Trentham DE. A pilot trial of oral type II collagen in the treatment of juvenile rheumatoid arthritis. Arthritis Rheum. 1996;39(4):623–628. doi: 10.1002/art.1780390413. [DOI] [PubMed] [Google Scholar]
- 19.Barnett ML, Kremer JM, St Clair EW, et al. Treatment of rheumatoid arthritis with oral type II collagen: results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998;41(2):290–297. doi: 10.1002/1529-0131(199802)41:2<290::AID-ART13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Trentham DE, Dynesius-Trentham RA, Orav EJ, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261(5129):1727–1730. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 21.Bagchi D, Misner B, Bagchi M, et al. Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: a mechanistic exploration. Int J Clin Pharmacol Res. 2002;22(3–4):101–110. [PubMed] [Google Scholar]
- 22.Crowley DC, Lau FC, Sharma P, et al. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: a clinical trial. Int J Med Sci. 2009;6(6):312–321. doi: 10.7150/ijms.6.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goddard G, Karibe H, McNeill C. Reproducibility of visual analog scale (VAS) pain scores to mechanical pressure. Cranio. 2004;22(3):250–256. doi: 10.1179/crn.2004.030. [DOI] [PubMed] [Google Scholar]
- 24.Jones DS, Quinn S, editors. Textbook of Functional Medicine. Gig Harbor, WA: The Institute for Functional Medicine; 2005. Medical symptoms questionnaire; pp. 784–785. [Google Scholar]
- 25.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 26.Sousa KH, Kwok OM, Ryu E, Cook SW. Confirmation of the validity of the HAQ-DI in two populations living with chronic illnesses. J Nurs Meas. 2008;16(1):31–42. doi: 10.1891/1061-3749.16.1.31. [DOI] [PubMed] [Google Scholar]
- 27.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2: the content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis Rheum. 1992;35(1):1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 28.Higgins PJ, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein and its fragments. J Immunol. 1988;140(2):440–445. [PubMed] [Google Scholar]
- 29.Nagler-Anderson C, Bober LA, Robinson ME, Siskind GW, Thorbecke GJ. Suppression of type II collagen-induced arthritis by intragastric administration of soluble type II collagen. Proc Natl Acad Sci U S A. 1986;83(19):7443–7446. doi: 10.1073/pnas.83.19.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshino S, Quattrocchi E, Weiner HL. Suppression of antigen-induced arthritis in Lewis rats by oral administration of type II collagen. Arthritis Rheum. 1995;38(8):1092–1096. doi: 10.1002/art.1780380811. [DOI] [PubMed] [Google Scholar]
- 31.Bitar DM, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. Cell Immunol. 1988;112(2):364–370. doi: 10.1016/0008-8749(88)90305-x. [DOI] [PubMed] [Google Scholar]
- 32.Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18(7):335–343. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 33.Tong T, Zhao W, Wu YQ, et al. Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm Res. 2010;59(5):369–377. doi: 10.1007/s00011-009-0109-4. [DOI] [PubMed] [Google Scholar]
- 34.Solinger AM, Bhatnagar R, Stobo JD. Cellular, molecular, and genetic characteristics of T cell reactivity to collagen in man. Proc Natl Acad Sci U S A. 1981;78(6):3877–3881. doi: 10.1073/pnas.78.6.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Meegeren ME, Roosendaal G, Jansen NW, et al. IL-4 alone and in combination with IL-10 protects against blood-induced cartilage damage. Osteoarthritis Cartilage. 2012;20(7):764–772. doi: 10.1016/j.joca.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Roman-Blas JA, Stokes DG, Jimenez SA. Modulation of TGF-beta signaling by proinflammatory cytokines in articular chondrocytes. Osteoarthritis Cartilage. 2007;15(12):1367–1377. doi: 10.1016/j.joca.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Boehmer H. Oral tolerance: is it all retinoic acid? J Exp Med. 2007;204(8):1737–1739. doi: 10.1084/jem.20071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lugo JP, Saiyed ZM, Lau FC, et al. Undenatured type II collagen (UC-II(R)) for joint support: a randomized, double-blind, placebo-controlled study in healthy volunteers. J Int Soc Sports Nutr. 2013;10(1):48. doi: 10.1186/1550-2783-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]