Abstract
PURPOSE
We aimed to demonstrate the success and reliability of a novel puncture, aspiration, injection, and reaspiration (PAIR) technique in liver hydatid cysts.
METHODS
Percutaneous treatment with ultrasonographic guidance was performed in 493 hepatic hydatid cysts in 374 patients. Patients were treated with a new PAIR technique by single puncture method using a 6F trocar catheter. The results of this novel technique were evaluated with regards to efficacy and safety of the procedure and complication rates.
RESULTS
Out of 493 cysts, 317 were Gharbi type I (WHO CE 1) and 176 were Gharbi type II (WHO CE 3A). Of all cysts, 13 were referred to surgery because of cystobiliary fistulization. Recurrence was observed in 11 cysts one month later. Therefore, the success rate of the PAIR technique was 97.7% (469/480). Minor complications (fever, urticaria-like reactions, biliary fistula) were seen in 44 treated patients (12%, 44/374); the only major complication was reversible anaphylactic shock which was observed in two patients (0.5%, 2/374).
CONCLUSION
This novel modified PAIR technique may be superior to catheterization by Seldinger technique due to its efficiency, easier application, lower severe complication rate, and lower cost. Further comparative studies are required to confirm our observations.
Hydatid disease is a parasitic infection caused by the larval stage of the tapeworm Echinococcus. E. granulosus is the most common cause of hydatid disease in humans and is found throughout the world. It is endemic in large sheep raising areas like the Mediterranean region, the Middle East, Southeast and Central Russia, Northern China, South America, Australia, and New Zealand (1). Hydatid disease usually affects the liver (50%–70%) and less frequently lung, peritoneum, kidney, brain, mediastinum, heart, bone, soft tissues, spinal cord, spleen, pleura, adrenal glands, bladder, ovary, scrotum, and thyroid gland (2). Treatment approaches include medical, surgical, and minimally invasive procedures. Medical treatment with albendazole or mebendazole alone has a low rate of success and high rate of relapse, making this treatment option controversial (3). The surgical approach has been the gold standard therapy for the hydatid disease for a long time (4). However, in recent years, percutaneous treatment of the hydatid cyst emerged as a potential alternative to surgery, because of its efficiency, reliability, and low morbidity and mortality rates. The puncture, aspiration, injection, and reaspiration (PAIR) technique, which involves puncture of the cyst wall, aspiration of cyst contents, instillation, and reaspiration of the scolicidal agent, has gained international recognition. PAIR technique can be achieved by using a coaxial catheter system to aspirate the cyst content and infuse scolicidal agent at the same time (5). Another technique can be performed by catheterization (3, 6). In our study, we performed the PAIR procedure by directly entering into the hydatid cyst cavity through a single puncture using a trocar catheter instead of placing a catheter through stiff wire after puncturing with a Seldinger needle. The primary goal of the current study was to determine the success and reliability of this technique in patients with hydatid disease.
Methods
Patients
Data from patients who underwent percutaneous treatment for hydatid liver cysts between January 2008 and December 2013 from two institutions were retrospectively reviewed. The local ethics committee approved the protocol. Our series included 374 patients and a total of 493 cysts of Gharbi type 1 (WHO CE 1, i.e., unilocular anechoic lesions with double line sign) or Gharbi type 2 (WHO CE 3A, i.e., cysts with detached membranes). All patients recruited in this study were diagnosed with hydatid disease by radiologic and serologic evaluation. Immunohemagglutination positivity was questioned in routine serologic investigation. Radiologic modalities (ultrasonography [US], computed tomography, and magnetic resonance imaging) were utilized to differentiate Gharbi type 1 hydatid cyst from simple liver cyst. In contrast to simple liver cysts, which often have lobulated contours, type 1 hydatid cysts are round or ellipsoid due to their hydrostatic pressure and their walls are thicker than of simple cysts. If the differentiation of hydatid cyst from simple cyst of the liver could not be made by radiologic studies, a more sensitive serologic test, i.e., IgE test specific for the hydatid cyst, was utilized.
Preprocedure
Written informed consent was obtained from all participants. All patients, except six with albendazole intolerance, were given 10 mg/kg per day albendazole orally for three weeks before the procedure to prevent dissemination during the procedure. Each patient was examined by an anesthesiologist during preoperative evaluation and underwent US examination to determine cyst type, location, number, and size. Complete blood count and coagulation parameters were checked before the intervention, an INR value less than 1.5 and platelet count higher than 100 000/mL were considered appropriate.
Heart rate, arterial oxygen saturation, and noninvasive blood pressure were monitored. After catheterization of a peripheral vein, lactated Ringer solution 5 mL/kg was administered, and oxygen 4 L/min was applied via face mask. Diphenhydramine HCl 20 mg and methyl prednisolone 1 mg/kg were applied intravenously to all patients for prevention of allergic reactions and decreasing the anaphylaxis risk. Because of an anaphylaxis risk, epinephrine was also prepared. Midazolam 0.03 mg/kg and fentanyl 50 μg were used for initial sedation and additional midazolam 0.01 mg/kg and fentanyl 25 μg were applied when needed.
Procedure
All procedures were performed by two radiologists who have at least three years of experience in nonvascular interventional radiology. Patients were positioned in supine or lateral decubitus position on a fluoroscopy table with C-arm equipment. Povidone iodine and appropriate draping were used to attain a sterile condition after shaving of the abdominal wall covering hepatic region. Local anesthesia (prilocaine hydrochloride) was administered before the puncture of the cyst. We used number 11 scalpel blade for skin incision after local anesthesia. A 6F trocar type all-purpose drainage catheter (Skater™, Angiotech Pharmaceuticals) was then placed into the cyst through US guidance. A stopcock was attached to the catheter to prevent air escape into the cyst during aspiration and injection. All cyst content was aspirated and the cyst was filled with a mixture of nonionic contrast medium and saline solution (½ contrast medium + ½ saline solution [0.09% NaCl]) equal to the amount of aspirate. A cystogram was then obtained in two planes (anteroposterior and lateral) to check any communication between the cyst cavity and the biliary tract. If cystography showed communication between the biliary tract and the hepatic hydatid cyst cavity, alcohol was not used because of the risk of inducing secondary sclerosing cholangitis. The content of the cyst cavity was aspirated again after the cystograms, followed by injection of absolute alcohol (98% ethyl alcohol) in a volume equaling two-thirds of the aspirate. After 20–30 minutes, the procedure was completed by aspiration of the alcohol from the cyst cavity and fixation of the catheter to the skin.
Postprocedure
After the procedure, all patients were monitored and observed for early complications in the interventional radiology observation unit. Patients were reevaluated 24 hours later, and if their vital signs and laboratory tests were normal, they were discharged from the hospital after catheter removal. Removal of the catheters during the procedure was thought to increase the anaphylaxis risk; therefore, all catheters were removed one day after the procedure. Control cystograms were not obtained prior to catheter removal. In the follow-up, the cysts were evaluated by US examination in the first month, sixth month, first year, and second year of the procedure. The success criteria of the percutaneous hydatid cyst treatment included considerable reduction in size and volume of the cyst as well as the fluid component of the cavity, irregularity and thickening of the cyst wall, disappearance of the fluid component and eventual solidification of the cyst, and no increase in size during follow-up.
Results
Our study included 374 patients (161 males and 213 females; mean age, 48 years; age range, 7–81 years) with a total of 493 cysts. Of the cysts, 317 were Gharbi type I (WHO CE 1) and 176 were Gharbi type II (WHO CE 3A). All patients were treated with albendazole for prevention of peritoneal dissemination of the cyst content, except six patients who were intolerant to the drug.
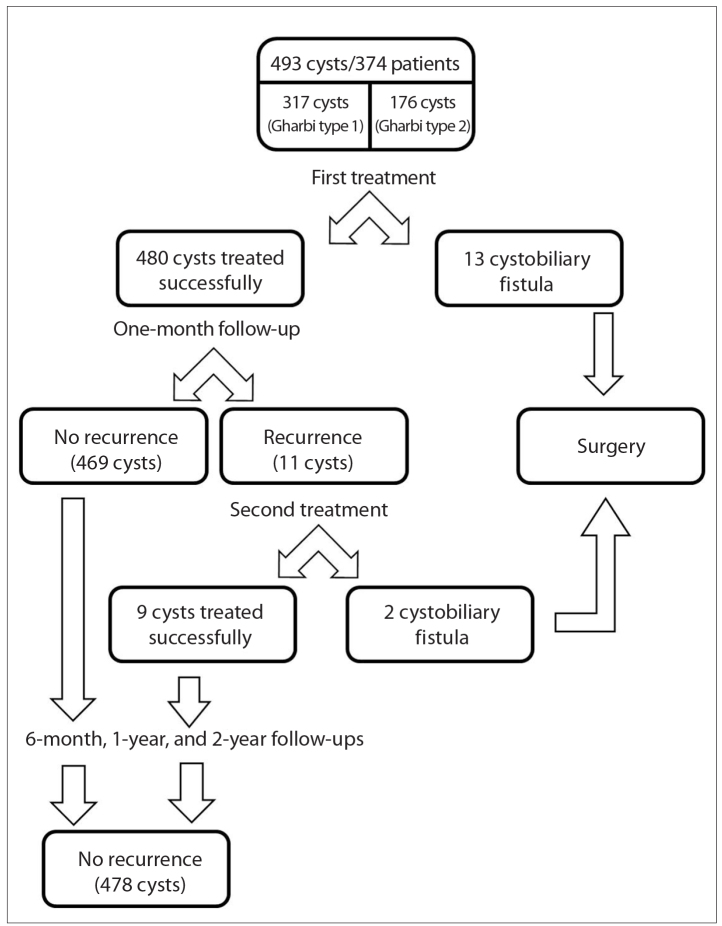
Trocar catheter was applied successfully in all cysts. Mean diameter of the cysts was 76 mm, ranging from 33 mm to 221 mm. In cystograms obtained from 493 cysts, cystobiliary fistulization was observed in 13 cases (Fig. 1). These cysts were considered inappropriate for alcohol injection and referred to surgery. In the remaining 480 cysts of 361 patients, 469 cysts (97.7%) were treated successfully (Figs. 2, 3), while 11 cysts (2.2%) recurred. A distended appearance or lack of reduction in cyst size was considered as a sign of recurrence. All recurrences were detected in the first month. A second percutaneous intervention was performed for all of the 11 recurrent cysts and nine of them were treated successfully. Cystobiliary fistulas were detected during the second intervention in the remaining two recurrent cysts and these cases were referred to surgery (Fig. 4). Cysts with reduced size and loss of distended appearance after the first month showed no increase in size at six-month, one-year, or two-year follow-up exams. Mean follow-up time was 21.6±1.8 months and there were no further follow-ups after two years. During the follow-up the patients were evaluated by US (additionally by MRI for multiple cysts), and serologic tests were not utilized.
Figure 1.

Cystogram in the anteroposterior plane obtained during the procedure shows cystobiliary communication.
Figure 2 a–c.
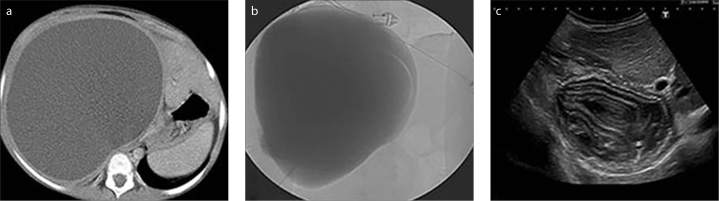
A 13-year-old girl with hepatomegaly. CT image obtained before the intervention (a) shows a giant type I hydatid cyst of the liver. Cystogram in the anteroposterior plane obtained during the procedure (b) shows no cystobiliary communication. Sonogram obtained at six-month follow-up (c) shows increasingly settling solid component.
Figure 3 a–d.
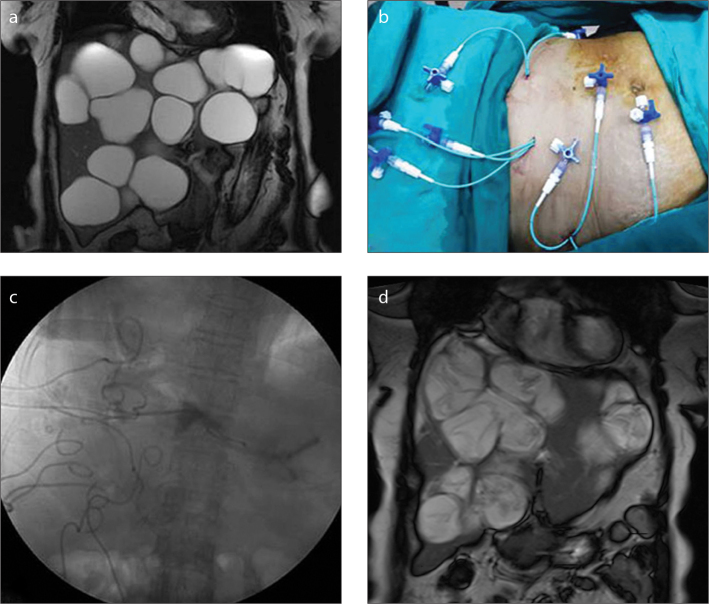
A 63-year-old woman with twenty-three hydatid cysts in the liver and one in the spleen. All cysts were treated in four sessions. Coronal T2-weighted image (a) shows multiple cysts in the liver. In every session, the patient was given a maximum of 30 mL prilocaine hydrochloride prophylactically for the prevention of methemoglobinemia related to local anesthetics overdose (b). After administration of local anesthesia in three different regions, eight cysts were treated using trocar. Fluoroscopic image (c) shows the ablation of the cysts with alcohol. Size reduction in all cysts and detachment of membranes are seen in coronal T2-weighted image (d) obtained one year later.
Figure 4.
Patient and hydatid cysts flowchart. Of 374 patients with 493 hydatid cysts enrolled in the study, fifteen were not able to receive PAIR treatment and were redirected to surgery. Recurrence was observed in 11 cysts at one month. There was no recurrence in 478 cysts at six-month, one-year, and two-year follow-ups.
Complications were usually minor and transient. Minor complications were fever (n=14, 3.7%), urticaria-like reactions (pruritus, mild skin rash, transient allergic reaction) (n=23, 6.1%), and biliary fistula (n=7, 1.9%). The only major complication was anaphylaxis observed in two cases (0.55%), which quickly resolved by immediate intervention of the anesthesiology team. These two patients had been given albendazole prophylactically. No deaths occurred related to percutaneous treatment per se or its complications. No alcoholemia was seen in any of the cases during or after the procedure.
All patients were hospitalized overnight because of catheterization; 46 patients were hospitalized 2–17 days (mean, 1.6 days) due to complications. Prolonged hospitalization of the patient who stayed in the hospital for 17 days was due to patient’s multiple cysts which required four sessions of the procedure. This patient had urticaria as well.
Discussion
Our results suggest that our novel modified PAIR technique may have easy application, high success rate, lower severe complication rate, and lower cost. In addition, to the best of our knowledge, this is the largest study on percutaneous treatment of hydatid cysts in the literature.
The gold standard treatment of the hydatid cyst remains controversial. In WHO informal working group on echinococcosis 2010 guidelines, it is stated that possible methods for the treatment of the hydatid cyst have not been compared and there is no “best treatment option” (7). Traditional treatment is surgical; however, mortality (0%–6.3%), complication (12.5%–80%), and recurrence (2.2%–22%) rates of this modality are high (8–10). Medical treatment alone is not efficient, but it can be used as an adjunct before or after the percutaneous treatment as a prophylaxis for abdominal dissemination (9, 11). Percutaneous treatment of the hydatid cyst is a reliable, efficient, and comfortable option, which has been used widely in the last two decades. In our study, treatment of the hydatid cyst was performed effectively by single puncture catheterization as a modification of the PAIR procedure.
In a meta-analysis, the success rate of hydatid cyst treatment by the PAIR procedure with albendazole or mebendazole prophylaxis was found to be higher than that of surgery. The success rates of the PAIR and surgery were reported as 95.8% (737/769) and 89.8% (855/952), respectively, and the difference was statistically significant. The success rate was 97.7% (469/480) in our study. In addition, lower recurrence rates were reported in percutaneous treatment compared with surgery (12). In our study, the recurrence rate was 2.2%.
Previous studies in which the patients were given albendazole or mebendazole preoperatively and postoperatively varied in terms of dose and duration of the medication. In studies reporting the length of medical treatment period precisely, patients were given albendazole or mebendazole for a median of 7 days (range, 4 hours to 15 days) before the drainage and for a median of 28 days (range, 4 hours to 6 months) after the procedure. Albendazole 10–20 mg/kg per day or mebendazole 10–50 mg/kg per day have been used in different studies. In our study, patients received albendazole 10 mg/kg per day before the procedure, for three weeks. Hypertonic saline or absolute alcohol is frequently used as scolicidal agents. Administration of silver nitrate, mebendazole, albendazole, and polidocanol has been reported in individual studies (12–14). Absolute alcohol was the scolicidal agent in our study. No scolicidal agents were used in patients with cystobiliary fistulas due to possible chemical sclerosing effects of these compounds (13).
In the Seldinger technique, after the puncture of the cyst using a Seldinger, Chiba, or guiding needle, the cyst is drained to a certain extent to reduce the inner hydrostatic pressure and prevent dissemination during catheterization. Then the tract is dilated with fascial dilator and the catheter is placed. Manipulation of the dilator and catheter over the wire during the procedure may cause leakage of the cyst content into the abdominal cavity and this can result in anaphylaxis. In the trocar technique, the catheter is placed into the cyst cavity instantly with direct single puncture and PAIR is performed. This technique allows catheterization and PAIR procedure to be performed in an easy manner.
Among percutaneous treatment options, PAIR procedure performed with Chiba, Seldinger, or guiding needle is more cost-effective compared with catheterization. However, it has some disadvantages such as the difficulty of keeping the tip of the needle steady within the cavity of the cysts that are located in regions hard to reach such as the dome of the liver, or clogging of the needle tip by membranes during the PAIR steps. Catheterization by Seldinger technique is a more controlled and comfortable method than the PAIR procedure (15). Our method may be superior to catheterization by Seldinger technique (via Seldinger needle, stiff wire, dilator, catheter) owing to its easier application and lower cost.
The potential complications of pre- and postprocedure include fever, hypotensive reaction, vasovagal reaction, nausea, vomiting, biliary fistula/rupture, cavity infection/abscess, peritoneal leakage, subcapsular hematoma, active arterial hemorrhage, intracystic bleeding or gallbladder hemorrhage, pleural effusion/pneumothorax, transient hypernatremia, and other unclassified reversible complications. Lethal anaphylactic shock is the most feared complication of the percutaneous treatment of the hydatid disease. The rates of reversible and irreversible anaphylactic shock were reported as 1.67% (99/5943) and 0.03% (2/5943), respectively in a meta-analysis by Neumayr et al. (16). No fatal anaphylactic shock occurred in our study, but reversible anaphylactic shock was encountered in two cases (0.55%, 2/370).
Surgical treatment of the hydatid cyst is associated with prolonged hospitalization. The length of stay in hospital was reported to be 10 days on average (range, 4.6–15 days) (17, 18). Laparoscopic surgery of the hydatid cyst, which requires a shorter hospitalization than laparotomy, became popular in recent years and the length of hospitalization for this method was reported as 4.7 days (range, 2–8 days) in a meta-analysis (19). The mean length of hospital stay in the literature for percutaneous treatment of the hydatid cyst is 2.4 days (range, 1.6–4.2 days); however, outpatient percutaneous treatment has also been reported (12, 15). In our study, the mean length of hospitalization was 1.6 days (range, 1–17 days), consistent with the previous literature.
The larger series in the literature mostly discuss surgical treatment options, whereas percutaneous treatment series include fewer patients. To the best of our knowledge, our study is the largest series reporting percutaneous treatment in the literature (20).
Our study has some limitations. The main limitation of this study is its retrospective and nonrandomized nature. The fact that we included consecutive subjects with Gharbi type 1 and 2 cysts, but not subjects with Gharbi type 3 or 4 is another limitation. Exclusion of patients with cystobiliary fistulas from PAIR procedure can be considered as another limitation as well.
In conclusion, this study confirms that it is possible to treat hydatid disease in a minimally invasive manner with a new modified PAIR technique. Percutaneous treatment of hydatid cyst with a trocar catheter may be at least as effective and applicable as the standard catheterization technique and it may be an effective alternative to conventional PAIR procedure or standard catheterization method, considering its cost-effectiveness and low complication rates.
Main points.
Percutaneous treatment of the hydatid cyst emerged as an alternative to surgery with low morbidity and mortality rates.
The PAIR technique involves puncture of the cyst wall, aspiration of cyst contents, instillation and reaspiration of the scolicidal agent.
In our study, we performed the PAIR procedure by directly entering into the hydatid cyst cavity through a single puncture using a trocar catheter instead of placing a catheter through stiff wire after puncturing with a Seldinger needle.
This novel modified PAIR technique may be superior to catheterization by Seldinger technique due to its efficiency, easier application, lower severe complication rate, and lower cost.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Safioleas MC, Misiakos EP, Kouvaraki M, Stamatakos MK, Manti CP, Felekouras ES. Hydatid disease of the liver: a continuing surgical problem. Arch Surg. 2006;141:1101–1108. doi: 10.1001/archsurg.141.11.1101. http://dx.doi.org/10.1001/archsurg.141.11.1101. [DOI] [PubMed] [Google Scholar]
- 2.Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics. 2003;23:475–494. doi: 10.1148/rg.232025704. http://dx.doi.org/10.1148/rg.232025704. [DOI] [PubMed] [Google Scholar]
- 3.Akhan O, Ozmen MN. Percutaneous treatment of liver hydatid cysts. Eur J Radiol. 1999;32:76–85. doi: 10.1016/s0720-048x(99)00116-3. http://dx.doi.org/10.1016/S0720-048X(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 4.Yagci G, Ustunsoz B, Kaymakcioglu N, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg. 2005;29:1670–1679. doi: 10.1007/s00268-005-0058-1. http://dx.doi.org/10.1007/s00268-005-0058-1. [DOI] [PubMed] [Google Scholar]
- 5.Gabal AM, Khawaja FI, Mohammad GA. Modified PAIR technique for percutaneous treatment of high-risk hydatid cysts. Cardiovasc Intervent Radiol. 2005;28:200–208. doi: 10.1007/pl00021047. http://dx.doi.org/10.1007/PL00021047. [DOI] [PubMed] [Google Scholar]
- 6.Giorgio A, Di Sarno A, de Stefano G, et al. Percutaneous treatment of hydatid liver cyst. Recent Pat Antiinfect Drug Discov. 2009;4:29–36. doi: 10.2174/157489109787236274. http://dx.doi.org/10.2174/157489109787236274. [DOI] [PubMed] [Google Scholar]
- 7.Brunetti E, Kern P, Vuitton DA. Writing Panel for the W-I. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. doi: 10.1016/j.actatropica.2009.11.001. http://dx.doi.org/10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Mentes A. Hydatid liver disease: a perspective in treatment. Dig Dis. 1994;12:150–160. doi: 10.1159/000171448. http://dx.doi.org/10.1159/000171448. [DOI] [PubMed] [Google Scholar]
- 9.Akhan O, Ozmen MN, Dincer A, Sayek I, Gocmen A. Liver hydatid disease: long-term results of percutaneous treatment. Radiology. 1996;198:259–264. doi: 10.1148/radiology.198.1.8539390. http://dx.doi.org/10.1148/radiology.198.1.8539390. [DOI] [PubMed] [Google Scholar]
- 10.Dawson JL, Stamatakis JD, Stringer MD, Williams R. Surgical treatment of hepatic hydatid disease. Br J Surg. 1988;75:946–950. doi: 10.1002/bjs.1800751004. http://dx.doi.org/10.1002/bjs.1800751004. [DOI] [PubMed] [Google Scholar]
- 11.Akhan O, Gumus B, Akinci D, Karcaaltincaba M, Ozmen M. Diagnosis and percutaneous treatment of soft-tissue hydatid cysts. Cardiovasc Intervent Radiol. 2007;30:419–425. doi: 10.1007/s00270-006-0153-1. http://dx.doi.org/10.1007/s00270-006-0153-1. [DOI] [PubMed] [Google Scholar]
- 12.Smego RA, Bhatti S, Khaliq AA, Beg MA. Percutaneous aspiration-injection-reaspiration drainage plus albendazole or mebendazole for hepatic cystic echinococcosis: a meta-analysis. Clin Infect Dis. 2003;37:1073–1083. doi: 10.1086/378275. http://dx.doi.org/10.1086/378275. [DOI] [PubMed] [Google Scholar]
- 13.Paksoy Y, Odev K, Sahin M, Arslan A, Koc O. Percutaneous treatment of liver hydatid cysts: comparison of direct injection of albendazole and hypertonic saline solution. AJR Am J Roentgenol. 2005;185:727–734. doi: 10.2214/ajr.185.3.01850727. http://dx.doi.org/10.2214/ajr.185.3.01850727. [DOI] [PubMed] [Google Scholar]
- 14.Ormeci N, Soykan I, Bektas A, et al. A new percutaneous approach for the treatment of hydatid cysts of the liver. Am J Gastroenterol. 2001;96:2225–2230. doi: 10.1111/j.1572-0241.2001.03886.x. http://dx.doi.org/10.1111/j.1572-0241.2001.03886.x. [DOI] [PubMed] [Google Scholar]
- 15.Koroglu M, Erol B, Gurses C, et al. Hepatic cystic echinococcosis: Percutaneous treatment as an outpatient procedure. Asian Pac J Trop Med. 2014;7:212–215. doi: 10.1016/S1995-7645(14)60023-7. http://dx.doi.org/10.1016/S1995-7645(14)60023-7. [DOI] [PubMed] [Google Scholar]
- 16.Neumayr A, Troia G, de Bernardis C, et al. Justified concern or exaggerated fear: the risk of anaphylaxis in percutaneous treatment of cystic echinococcosis-a systematic literature review. PLoS Negl Trop Dis. 2011;5:1154. doi: 10.1371/journal.pntd.0001154. http://dx.doi.org/10.1371/journal.pntd.0001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta N, Javed A, Puri S, Jain S, Singh S, Agarwal AK. Hepatic hydatid: PAIR, drain or resect? J Gastrointest Surg. 2011;15:1829–1836. doi: 10.1007/s11605-011-1649-9. http://dx.doi.org/10.1007/s11605-011-1649-9. [DOI] [PubMed] [Google Scholar]
- 18.Birnbaum DJ, Hardwigsen J, Barbier L, Bouchiba N, Le Treut YP. Is hepatic resection the best treatment for hydatid cyst? J Gastrointest Surg. 2012;16:2086–2093. doi: 10.1007/s11605-012-1993-4. http://dx.doi.org/10.1007/s11605-012-1993-4. [DOI] [PubMed] [Google Scholar]
- 19.Citgez B, Battal M, Cipe G, Karatepe O, Muslumanoglu M. Feasibility and safety of laparoscopic hydatid surgery: a systematic review. Hepatogastroenterology. 2013;60:784–788. doi: 10.5754/hge12527. [DOI] [PubMed] [Google Scholar]
- 20.Agaev R. Surgical treatment of hepatic echinococcosis and its complications. Khirurgiia. 2000;2:32–36. [PubMed] [Google Scholar]