Abstract
PURPOSE
We aimed to evaluate the survival benefit achieved with radiofrequency (RF) ablation of primary and metastatic lung tumors and determine significant prognostic factors for recurrence-free survival.
METHODS
Forty-nine patients with lung cancer (10 primary and 39 metastatic) underwent computed tomography-guided percutaneous RF ablation between June 2005 and October 2013. A total of 112 tumors (101 metastatic and 11 primary non-small cell lung cancer) were treated with RF ablation. Tumor diameter ranged from 0.6 to 4 cm (median 1.5 cm). Effectiveness of treatment, complications, and survival were analyzed.
RESULTS
Primary success rate was 79.5% and local tumor progression occurred in 23 tumors. Among tumors showing progression, 10 were re-treated with RF ablation and secondary success rate was 87.5%. One-, two-, and three-year overall survival rates of 10 patients with primary lung cancer were 100%, 86%, and 43%, respectively. One-, two-, three-, four-, and five-year overall survival rates for 39 patients with metastatic lung tumors were 90%, 73%, 59%, 55%, and 38%, respectively. One-, two-, three-, and four-year overall survival rates for 16 patients with colorectal pulmonary metastases were 94%, 80%, 68%, and 23%, respectively. Complications occurred in 30 sessions (24.6%). Pneumothorax occurred in 19 sessions with seven requiring image-guided percutaneous chest tube drainage. Tumor status (solitary or multiple) and presence of extrapulmonary metastasis at initial RF ablation were significant prognostic factors in terms of recurrence-free survival.
CONCLUSION
RF ablation is a safe and effective treatment with a survival benefit for selected patients with primary and secondary lung tumors.
Primary lung cancer is the leading cause of cancer-related death worldwide (1). Treatment of primary lung cancers includes surgical resection, radiation therapy, chemotherapy, and thermal ablation. Surgical resection remains the treatment of choice for patients with early stage non-small cell lung cancer (NSCLC) (2). However, primary lung cancers are generally diagnosed in advanced stages. Moreover, due to the high incidence of associated comorbidities and limited pulmonary reserve, most patients are considered ineligible for surgery (3, 4).
In addition to primary cancers, lungs are the second most frequent site of metastatic disease. In selected patients with metastatic lung cancer, surgical resection is the preferred treatment. However, even patients who have undergone a complete resection have a high incidence of recurrence and may require multiple surgeries (5). Repeat thoracotomy leads to further removal of functional pulmonary tissue. Surgical resection might not be possible in patients with certain comorbidities and limited pulmonary reserve.
Patients with pulmonary colorectal metastases constitute a significant portion of metastatic lung tumor group. Approximately 10% of patients with colorectal cancer develop pulmonary metastases during the course of disease (6). It has been reported that in patients with limited colorectal pulmonary metastases and no extrapulmonary disease, five-year survival following surgical resection is approximately 35%–45% (7). However, many patients are not suitable candidates for surgery.
Percutaneous image-guided radiofrequency (RF) ablation is a minimally invasive technique established in the treatment of solid tumors. Since Dupuy et al. (8) reported the first clinical use of RF ablation to treat lung cancer in 2000, it has been increasingly used as a treatment option for patients with primary and secondary lung tumors, who are not surgical candidates. RF ablation offers reduced morbidity and mortality, and allows preservation of pulmonary functions because surrounding uninvolved lung parenchyma is preserved (9, 10). It is very useful for patients who have limited pulmonary reserve or with multifocal or bilateral metastatic disease. It is performed with computed tomography (CT) guidance and avoids thoracotomy in patients with significant comorbidities or in patients who refuse surgery. Repeatability of the procedure is a great advantage (11). It can be performed on an outpatient basis or with a minimum hospital stay (12).
The aim of this study was to evaluate the survival benefit achieved with RF ablation for primary and secondary lung tumors and determine significant prognostic factors in terms of recurrence-free survival.
Methods
All patients provided written informed consent for RF ablation procedure. Institutional ethics committee approval was obtained for retrospective data analysis.
Before being considered for RF ablation, all cases were discussed with a multidisciplinary team including an interventional radiologist, a thoracic surgeon, a radiotherapist, and a medical oncologist. RF ablation was performed in patients who were considered ineligible for surgery due to poor cardiopulmonary reserve or other comorbidities (38 patients), patients in whom prior treatments had failed and who had recurrence after previous lung surgery (10 patients), and patients who refused surgery (one patient). Seven patients who had tumors larger than 4 cm ablated for palliative therapy were not included in the study. Four patients whose follow-up clinical and imaging findings were not accessible from the electronic database or hospital archive were also excluded.
All patients underwent a cross-sectional examination for tumor assessment at a median of 28 days (range, 7–35 days) before ablation: 48 patients had a CT scan and one patient with colorectal carcinoma had a positron emission tomography (PET)/CT scan.
Before the procedure, all patients had laboratory tests, including liver and kidney function tests, coagulation parameters, and complete blood count. A platelet count of higher than 75,000/mm3 and international normalized ratio less than 1.3 were required to undergo ablation.
Patient characteristics
Between June 2005 and October 2013, 49 patients (30 males and 19 females; median age, 63 years; range, 13–85 years) with primary (10 patients, 20%) or metastatic (39 patients, 80%) lung cancer underwent CT-guided RF ablation. A total of 112 tumors, of which 101 (90%) were metastatic and 11 (10%) were primary (NSCLC), were treated in 122 RF ablation sessions. Twenty-nine patients (59%) were older than 60 years. Cancer diagnosis of 49 patients included colorectal carcinoma (n=16, 33%), NSCLC (n=10, 20%), sarcoma (n=9, 18%), adenoid cystic carcinoma (n=3, 6%), breast carcinoma (n=2, 4%), gastric carcinoma (n=2, 4%), esophagus carcinoma (n=1, 2%), renal cell carcinoma (n=1, 2%), prostate carcinoma (n=1, 2%), malignant melanoma (n=1, 2%), hepatocellular carcinoma (n=1, 2%), bladder carcinoma (n=1, 2%), and thyroid Hurthle cell carcinoma (n=1, 2%). Ten patients (20%) had prior lung surgery. Thirty-five patients (71%) had a solitary lung tumor at the initial RF ablation. Twelve patients (25%) had a history of extrapulmonary RF ablation either before lung RF ablation or during the follow-up period. Nine patients underwent RF ablation for liver tumors, one patient underwent RF ablation for kidney tumors, and one patient underwent RF ablation both for kidney, liver and gluteal subcutaneous metastases. One patient underwent RF ablation for liver metastases and had irreversible electroporation for adrenal tumors.
Ablated index tumors had a median diameter of 1.5 cm (range, 0.6–4 cm); 109 tumors were ≤3 cm, while three tumors ranged 3.1–4 cm. The 112 index tumors were identified as colorectal (n=36, 32.1%), adenoid cystic carcinoma (n=31, 27.7%), sarcoma (n=13, 11.6%), NSCLC (n=11, 9.8%), bladder carcinoma (n=4, 3.6%), hepatocellular carcinoma (n=4, 3.6%), gastric carcinoma (n=3, 2.7%), prostate carcinoma (n=3, 2.7%), malignant melanoma (n=2, 1.8%), breast carcinoma metastases (n=2, 1.8%), esophagus carcinoma (n=1, 0.9%), renal cell carcinoma (n=1, 0.9%), and thyroid Hurthle cell carcinoma metastasis (n=1, 0.9%). Sixty-four tumors (57%) were located in the right lung.
RF ablation technique
Procedures were performed using CT guidance (Siemens Somatom Volume Zoom). Thirty-four patients (69%) received conscious sedation and 15 patients (31%) received general anesthesia. Midazolam and fentanyl were used for conscious sedation. Dispersive grounding pads were placed on each patient’s thighs. The tumor was localized on the initial CT scan that was obtained just before the procedure and then a grid was superimposed on the CT image at the level of anticipated needle entry to assess the shortest and safest entry site. The skin entry site that avoided interlobar fissures, bullae, or pulmonary vessels was chosen. Optimal electrode positioning was confirmed using CT and the relationship of the electrode needle with the tumor was assessed in different planes using appropriate image reconstructions.
RF ablation was performed using either the RITA (RITA Medical Systems, AngioDynamics) or the Cool-tip (Covidien) systems powered by 200 W or 250 W generators. A 17-gauge multitined expandable electrode which could be deployed up to 4 cm (The RITA StarBurst Talon or StarBurst Talon Semi-Flex RF ablation electrodes) was used in 115 procedures. The tumors were ablated at 80°C for 5 min if their deployed diameter was 3 cm or for 9 min if their deployed diameter was 4 cm. Saline infusion during RF ablation provided lower impedance, higher power delivery and larger tumor size. A single 17-gauge internally cooled electrode with a 2 or 3 cm noninsulated tip (Cool-tip system) was used in six procedures. Internal cooling was achieved by continuous perfusion with chilled saline. Ablation was achieved at around 10–12 min. The UniBlate RF ablation electrode (The RITA StarBurst) was used in only one procedure. After the ablation was completed, tract ablation was performed until 1–2 cm outside of the ablated tumor in all patients.
The aim was to obtain an ablation margin of at least 0.5 cm around the tumor. An internally cooled electrode was generally preferred for tumors located adjacent to the pleura or pulmonary hilum to avoid the unpredictable expansion of multitined electrodes that could damage the pleura or large vessels. Tumors located in different lungs were preferably treated at separate times.
Postprocedural care
Chest radiographs were performed two hours after RF ablation to exclude pneumothorax. Patients were observed overnight and generally discharged the next day if there were no complications. As defined by American College of Chest Physicians (13), patients with small (<3 cm apex-to-cupola distance) and asymptomatic pneumothoraces were observed, whereas large (≥3 cm apex-to-cupola distance) or symptomatic pneumothoraces underwent chest tube drainage.
Follow-up exam was performed at a median of 30 days (range, 22–38 days) after RF ablation: 44 patients had contrast-enhanced CT, four patients with impaired renal function had unenhanced CT, and one patient had a PET/CT scan to simultaneously evaluate the growth of liver metastases. Subsequent imaging controls were performed every 3–6 months to assess recurrence. In addition to CT scans, five patients had PET/CT scans (Fig. 1) and three patients had magnetic resonance imaging (MRI, Siemens Magnetom Avanto 1.5T) at one of the follow-ups. PET/CT was performed between 5 and 17 months (median 12 months) postablation. PET/CT scans were applied to evaluate the response to treatment as well as to search for extrapulmonary metastases. MRI was used for confirmation in cases where CT scans suggested local tumor progression (LTP).
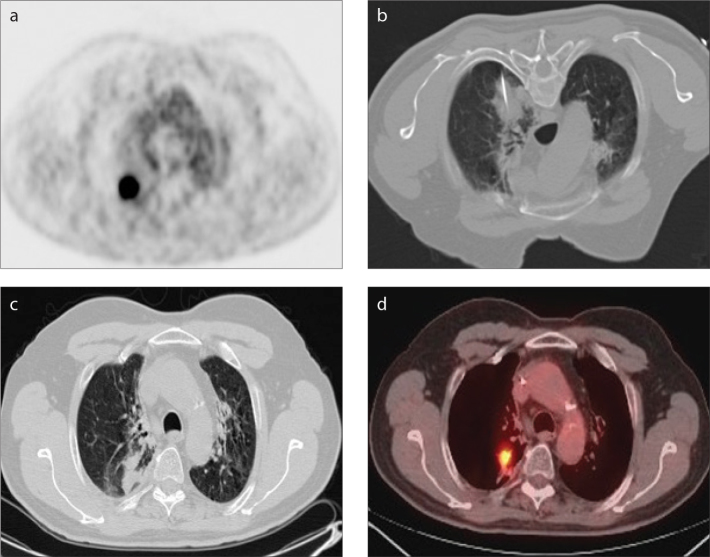
Figure 1 a–d.
A 65-year-old male with NSCLC treated using Cool-tip system (Covidien) and a single electrode with a 3 cm noninsulated tip for 12 minutes. Axial 18F-FDG PET image (a) obtained before RF ablation shows a 2.5 cm tumor in the right upper lobe. Axial CT scan (b) obtained at prone position during RF ablation. Axial CT scan (c) obtained two months follow up demonstrates parenchymal opacification at the ablation zone. Axial 18F-FDG PET/CT image (d) obtained one year after RF ablation demonstrates increased uptake in tumor compared with previous PET/CT obtained at two-month follow-up. Findings are consistent with local tumor progression.
Ten tumors (9%) were treated with a second RF ablation session due to LTP (Fig. 2). During a median of 27 months of follow-up, 22 of 49 patients (45%; 4 patients with NSCLC and 18 patients with secondary lung tumors) died.
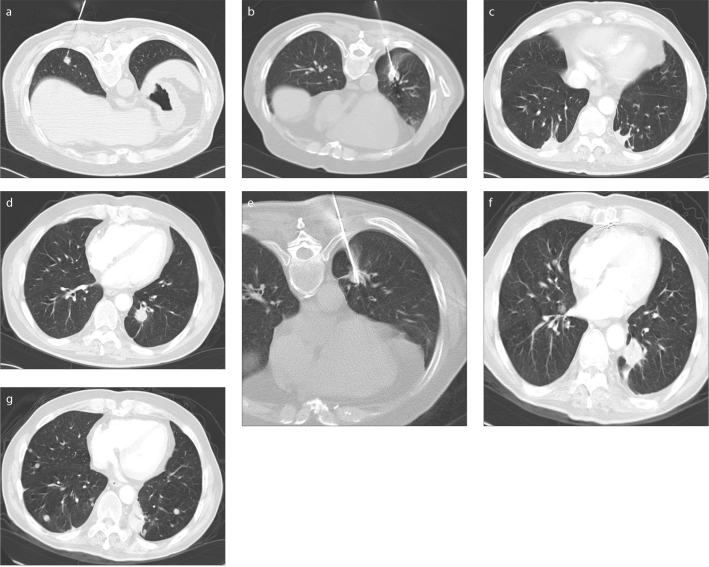
Figure 2 a–g.
A 75-year-old male with colorectal lung metastases treated using RITA system (RITA Medical Systems, AngioDynamics) and StarBurst Talon electrode with a 3 cm array. Axial CT scan (a) obtained during RF ablation of a metastasis with a size of 1 cm located at the lower lobe of the right lung. Axial CT scan (b) obtained during RF ablation of a 1.5 cm-sized metastasis located at the lower lobe of the left lung, which occurred 11 days after the first RF ablation procedure. Axial CT scan (c) obtained seven months later shows involution of the ablation zones. Axial CT scan (d) obtained 19 months later demonstrates local tumor progression in the metastasis located at the left lung. Axial CT scan (e) obtained during the second RF ablation session. Axial CT scans obtained 36 months after the first procedure show local tumor progression in the metastasis located at the left lung (f) and multiple lung metastases (g).
Data collection
Patients who had undergone RF ablation for primary and secondary lung tumors were identified by performing a retrospective search from electronic database and hospital archive. The following characteristics were recorded: patient’s history, demographic findings, number of lung tumors (solitary/multiple) at initial ablation, maximum longitudinal diameter of the tumor, presence of extrapulmonary metastasis at initial ablation, technique of RF ablation, total number of procedures, total number of lung tumors ablated, complications, presence of extrapulmonary ablation, presence of recurrence. Phone calls were made with patients or their relatives to assess the exact time of overall survival. Patient characteristics and tumor characteristics were described and summarized.
Ablation of the index tumor was considered complete when the index tumor was covered by the ablation zone including at least a 5 mm margin all around it, in addition to observing the involution of the ablation zone throughout follow-up imaging.
Survival end-points of interest included overall survival and recurrence-free survival. For overall survival, time from the initial RF ablation to last follow-up visit or death from any cause was used. For recurrence-free survival, time from the initial RF ablation to diagnosis of recurrence, if there was any, was used. Recurrence was described as follows: LTP (growth of the tumor, focal or nodular enhancement within or around the tumor in CT scans or MRI, and increased fluorodeoxyglucose (FDG) uptake in PET/CT scans were considered to be the signs of LTP), intrapulmonary recurrence (intrapulmonary new tumor other than the target tumor detected during follow-up), and extrapulmonary recurrence (extrapulmonary tumor detected during follow-up).
Statistical analysis
Survival analysis was conducted by Kaplan–Meier probability curves and the log-rank test was used for comparison. Statistical analyses were performed with the Statistical Package for the Social Sciences Version 18.0 (SPSS Inc.). A P value of <0.05 was considered significant.
Results
Primary success rate was 79.5% as the complete ablation of the index tumor was obtained in 89 tumors (82 metastatic and 7 primary) after the first RF ablation procedure. LTP at the initial RF ablation site occurred in 23 tumors (4 primary and 19 metastatic). LTP was detected 1–20 months (median, 8 months) after the initial ablation. Among the progressed tumors, 10 (2 primary, 8 metastatic) were re-treated with RF ablation. Complete ablation occurred in 9 of 10 tumors (90%) after the first LTP. Overall complete ablation was obtained in 98 tumors (90 metastatic and 8 primary) including tumors that underwent successful repeat ablation procedures and secondary success rate was 87.5%. One patient with progressed NSCLC, who had a repeat ablation, underwent lung surgery due to LTP that occurred after the second RF ablation procedure. A second RF ablation procedure was not performed for 13 patients with LTP because these patients developed extrapulmonary and/or intrapulmonary metastases during the follow-up. These patients were referred to the oncology department for consideration of systemic chemotherapy.
Eleven patients (24%) had not developed any form of recurrence by the time of the last follow-up. During the follow-up, nine patients (18%) developed intrapulmonary recurrence and LTP, eight patients (16%) developed intrapulmonary and extrapulmonary recurrence, seven patients (14%) developed intrapulmonary recurrence only, six patients (12%) developed intrapulmonary recurrence, extrapulmonary recurrence, and LTP, three patients (6%) developed LTP only, three patients (6%) developed extrapulmonary recurrence only, and two patients (4%) developed extrapulmonary recurrence and LTP.
Median overall and recurrence-free survival for 10 patients with NSCLC were 27 months (standard error (SE), 6.5 months) and 11 months (SE, 5.6 months), respectively. Median overall survival for 39 patients with secondary lung tumors was 50 months (SE, 2.7 months) and median recurrence-free survival was 5 months (SE, 0.9 months). Due to the limited number of patients with NSCLC, it was not possible to evaluate further survival rates.
When colorectal cancer metastases were separately evaluated as a group, median overall and recurrence-free survival for 16 patients were 50 months (SE, 5.1 months) and 4 months (SE, 1.0 months), respectively. One-, two-, three-, and four-year overall survival rates were 94%, 80%, 68%, and 23%, respectively. One-, two-, and three-year recurrence-free survival rates were 32%, 19%, and 12%, respectively.
Table 1 summarizes the association of individual variables correlating with overall and recurrence-free survival for the whole patient group. Age, sex, tumor status (solitary or multiple) at the initial RF ablation, presence of extrapulmonary metastasis at the initial RF ablation were not statistically significant for overall survival. Patients who had a solitary tumor at the initial RF ablation had a higher recurrence-free survival rate at one, two, and three years compared with patients who had multiple tumors (54% vs. 26%, 43% vs. 11%, and 36% vs. 11%, respectively; P = 0.002). Moreover, patients who had extrapulmonary metastasis at the initial RF ablation of lung tumors had a significantly lower recurrence-free survival rate at one and two years than patients who did not have extrapulmonary metastasis (12% vs. 50% and 12% vs. 25%, respectively; P = 0.020).
Table 1.
Variables correlating with overall and recurrence-free survival
| Overall survival rate (%) | Recurrence-free survival rate (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | n | 1-year | 2-year | 3-year | P | 1-year | 2-year | 3-year | P |
| Sex | 0.188 | 0.534 | |||||||
| Male | 30 | 89 | 70 | 47 | 33 | 13 | 6 | ||
| Female | 19 | 94 | 70 | 62 | 28 | 24 | 24 | ||
|
| |||||||||
| Age | 0.845 | 0.521 | |||||||
| ≤60 years | 20 | 95 | 72 | 58 | 28 | 20 | 13 | ||
| >60 years | 29 | 96 | 69 | 55 | 35 | 14 | 14 | ||
|
| |||||||||
| Tumor status | 0.198 | 0.002 | |||||||
| Solitary | 35 | 94 | 69 | 63 | 54 | 43 | 36 | ||
| Multiple | 14 | 90 | 71 | 53 | 26 | 11 | 11 | ||
|
| |||||||||
| Presence of EPM | 0.253 | 0.020 | |||||||
| Yes | 13 | 92 | 71 | 60 | 12 | 12 | 12 | ||
| No | 36 | 94 | 80 | 68 | 50 | 25 | 12 | ||
EPM, extrapulmonary metastasis at the time of radiofrequency ablation; n, number of patients.
There was no procedure-related mortality (SIR classification F). Complications occurred in 30 of 122 sessions (24.6%). Pneumothorax occurred in 19 sessions (15.6%). Pneumothorax requiring image-guided percutaneous chest tube drainage was considered as a major complication (SIR classification C) and occurred in 7 of 122 procedures (5.7%). There were 23 minor complications (SIR classification B) including pneumothoraces without a need of drainage (n=12), pain (n=6), fever (n=3), and self-limiting parenchymal hemorrhage (n=2; Table 2).
Table 2.
Complications classified according to SIR Standards of Practice Committee
| Complications | n | SIR classification | Comments |
|---|---|---|---|
| Minor | |||
| Pain | 6 | B | Analgesic treatment |
| Fever | 3 | B | Analgesic treatment |
| Hemorrhage | 2 | B | Self-limiting, no treatment |
| Pneumothorax (asymptomatic) | 12 | B | No treatment; observation |
|
| |||
| Major | |||
| Pneumothorax (requiring drainage) | 7 | C | Image-guided chest tube drainage |
n, number of procedures; SIR, Society of Interventional Radiology.
Median hospital stay was one day (range, 1–22 days). A prolonged hospital stay of 22 days occurred in one patient due to comorbidities and added urinary tract infection.
Discussion
Our overall survival rates for patients with NSCLC are similar to previously published studies. Indeed, in the review study of Hiraki et al. (14), one-, two-, three-, and five-year overall survival rates after RF ablation of stage I NSCLC were reported as 78%–100%, 53%–86%, 36%–88%, and 25%–61%, respectively. In another previously published systematic review of RF ablation for lung tumors including 17 studies, Zhu et al. (15) reported that one-, two-, and three-year survival rates ranged between 63%–85%, 55%–65%, and 15%–46%, respectively. Lencioni et al. (16) showed one- and two-year overall survival rates of 70% and 48%, respectively in a prospective, multicenter clinical trial of 33 patients with NSCLC. One-year recurrence-free survival rate of NSCLC patients was 34% in our study. Due to the number of NSCLC patients in our study, it was not possible to assess recurrence-free survival rate after one year.
For the group of lung metastases (including colorectal and noncolorectal metastases), our one-, two-, three-, four-, and five-year overall survival rates of 90%, 73%, 59%, 55%, and 38%, were comparable with the results of previous series. Lencioni et al. (16) reported that overall survival rate was 92% at one year and 64% at two years for a group of 20 patients with noncolorectal pulmonary metastases. In another study that demonstrated long-term outcome of RF ablation of lung metastases, three- and five-year overall survival rates were 60% and 45%, respectively (17).
Similarly, our overall survival rates for the subgroup of colorectal lung metastases of 16 patients were parallel to those of many other reports in the literature. A recent study reported that the estimated overall survival rates were 95.2%, 65%, and 51.6% at one, three, and five years, respectively, for 84 patients with 172 colorectal lung metastases (18). In another study, Gillams et al. (19) performed 256 RF ablation procedures in 122 patients with a total of 398 colorectal pulmonary metastases. They found that overall median survival was 41 months and three-year survival rate was 57%. Petre et al. (20) reported the results of 45 patients with colorectal pulmonary metastases. One-, two-, and three-year overall survival rates were 95%, 72%, and 50%, respectively. In a review of eight published studies of colorectal pulmonary metastases, Hiraki et al. (21) reported one- and three-year survival rates as approximately 85%–95% and 45%–55%, respectively.
In several published studies, significant prognostic factors associated with survival of patients treated with RF ablation are reported. However, separate evaluation of factors correlating with overall survival and with recurrence-free survival, as shown in our study, is not common. In our study, age, sex, tumor status, presence of extrapulmonary metastasis at the initial RF ablation were not statistically significant in the assessment of overall survival. Many studies have shown that tumor size is a significant prognostic factor for survival of patients treated with RF ablation. These studies indicate that there is significant difference between the survival outcomes of tumors <3 cm and >3 cm (22–24). However, tumor size was not found to be a contributory prognostic factor in our study. This should be explained by the predominance of small tumors in our study as majority of the tumors were ≤3 cm (only three tumors’ longest diameters were >3 cm), with a median size of 1.5 cm.
LTP is an important factor in determining the effectiveness of RF ablation. In our study, primary success rate was 79.5% and secondary success rate was 87.5%. Similarly, Thanos et al. (25) observed a total necrosis rate of 79.1% in a series of 48 malignant pulmonary lesions. Our findings were parallel to those of a systematic review (15), in which the rates of complete tumor necrosis achieved by RF ablation were reported to range between 38% and 97%.
CT is the most commonly used modality of imaging in the follow-up. In our study, we used PET/CT and MRI in the follow-up of a limited number of patients. Although CT is more easily applied in the follow-up, MRI combined with diffusion-weighted imaging and PET/CT may be useful in depicting LTP. However, these results must be confirmed in larger series.
In our study there was no mortality associated with the procedure. Pneumothorax was the most frequent complication as it occurred after 19 procedures (15.6%). Of the cases of pneumothorax, seven (5.7% of all sessions) required image-guided percutaneous chest tube drainage, which was considered as a major complication (SIR classification C). In several studies, pneumothorax rate ranged between 4.5% and 61.1% (26, 27), whereas 3.9%–28.6% of RF ablation procedures required chest tube placement for drainage (28, 29). Similar to our study, Garetto et al. (30) reported a pneumothorax rate of 14% in 100 RF ablation procedures. Our incidence of pneumothorax requiring chest tube drainage was relatively low compared with the previous studies. We speculate that this could be due to a careful planning of the electrode trajectory in order to minimize the amount of aerated lung that needs to be traversed and avoid interlobar fissures.
For early stage NSCLC, RF ablation is an alternative treatment to other local therapies such as sublobar resection and stereotactic body radiation therapy (SBRT). In a study comparing the selection criteria and short-term outcomes among three prospective clinical trials using SBRT (Radiation Therapy Oncology Group [RTOG] trial 0236), sublobar resection (American College of Surgeons Oncology Group [ACOSOG] trial Z4032), and RF ablation (ACOSOG trial Z4033), mortality rates were not significantly different between the three modalities (31). A few series reporting the outcomes of SBRT in patients with early stage NSCLC showed that one- and three-year overall survival rates ranged 80%–95% and 43%–85%, respectively (32–34). Although our study consisted of limited number of patients with NSCLC, our overall survival outcomes were similar to those reports. LTP after RF ablation was reported to be higher than after sublobar resection and SBRT (14), which was similar to our study.
Although surgery remains as the standard choice in suitable patients with lung metastases, RF ablation can be beneficial for patients who are ineligible for surgery. In a study including 708 lung metastasectomies, the overall survival of patients after a complete resection was reported to be 74% at two years and 46% at five years whereas the overall survival for patients with incomplete resection was 47% at two years and 20% at five years (35). Similarly, our overall survival rate of patients with lung metastases as a whole group was 73% at two years and 38% at five years. SBRT is another treatment method for patients with lung metastases with survival outcomes parallel to metastasectomy (36).
Our study has several limitations. First, the number of NSCLC patients was limited; therefore, it was not possible to evaluate long-term outcomes of RF ablation in this patient subgroup. Second, our study was retrospectively assessed, similar to most of the previously published studies. In the literature, there are only a few prospective studies accomplished in this field. This might be due to RF ablation being generally performed in patients who are not candidates for curative surgical resection as a result of associated comorbidities or insufficient lung function. Therefore, it is difficult to design a prospective randomized study for this group of patients. Another limitation was the relatively small size of tumors in our study.
In conclusion, RF ablation is a safe and effective treatment with a survival benefit for selected patients with primary and secondary lung tumors. Tumor status (solitary or multiple) and presence of extrapulmonary metastasis at the initial RF ablation were significant prognostic factors for recurrence-free survival. Prospective randomized trials comparing RF ablation with SBRT and other adjuvant treatment methods will be beneficial for patients with lung tumors.
Main points.
Percutaneous image-guided radiofrequency (RF) ablation has been increasingly used as a treatment option for patients with primary and metastatic lung tumors.
RF ablation is a safe and effective treatment with a survival benefit for selected patients with primary and metastatic lung tumors.
RF ablation offers reduced morbidity, mortality and allows preservation of pulmonary functions through protection of surrounding uninvolved lung parenchyma.
Tumor status (solitary or multiple) and presence of extrapulmonary metastasis at the initial RF ablation were found to be the two main prognostic factors for recurrence-free survival in our study.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J Thorac Oncol. 2008;3:819–831. doi: 10.1097/JTO.0b013e31818020eb. http://dx.doi.org/10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 2.Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of non-small cell lung cancer stage I and stage II. ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:S234–242. doi: 10.1378/chest.07-1378. [DOI] [PubMed] [Google Scholar]
- 3.Dienemann H. Principles of surgical treatment in localized non-small cell lung cancer. Lung Cancer. 2001;1:S3–8. doi: 10.1016/s0169-5002(01)00296-3. http://dx.doi.org/10.1016/S0169-5002(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 4.Pearson FG. Current status of surgical resection for lung cancer. Chest. 1994;106:S337–339. doi: 10.1378/chest.106.6_supplement.337s. http://dx.doi.org/10.1378/chest.106.6.337S. [DOI] [PubMed] [Google Scholar]
- 5.Ketchedjian A, Daly B, Luketich J, Fernando HC. Minimally invasive techniques for managing pulmonary metastases: video-assisted thoracic surgery and radiofrequency ablation. Thorac Surg Clin. 2006;16:157–165. doi: 10.1016/j.thorsurg.2005.11.002. http://dx.doi.org/10.1016/j.thorsurg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Mitry E, Guiu B, Cosconea S, Jooste V, Faivre J, Bouvier AM. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 2010;59:1383–1388. doi: 10.1136/gut.2010.211557. http://dx.doi.org/10.1136/gut.2010.211557. [DOI] [PubMed] [Google Scholar]
- 7.Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007;84:324–338. doi: 10.1016/j.athoracsur.2007.02.093. http://dx.doi.org/10.1016/j.athoracsur.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 8.Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–59. doi: 10.2214/ajr.174.1.1740057. http://dx.doi.org/10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- 9.Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities-part II. J Vasc Interv Radiol. 2001;12:1135–1148. doi: 10.1016/s1051-0443(07)61670-4. http://dx.doi.org/10.1016/S1051-0443(07)61670-4. [DOI] [PubMed] [Google Scholar]
- 10.Pereira PL, Masala S. Standards of practice: guidelines for thermal ablation of primary and secondary lung tumors. Cardiovasc Intervent Radiol. 2012;35:247–254. doi: 10.1007/s00270-012-0340-1. http://dx.doi.org/10.1007/s00270-012-0340-1. [DOI] [PubMed] [Google Scholar]
- 11.Hiraki T, Mimura H, Gobara H, et al. Repeat radiofrequency ablation for local progression of lung tumors: does it have a role in local tumor control? J Vasc Interv Radiol. 2008;19:706–711. doi: 10.1016/j.jvir.2007.12.441. http://dx.doi.org/10.1016/j.jvir.2007.12.441. [DOI] [PubMed] [Google Scholar]
- 12.Tatli S, Tapan U, Morrison PR, Silverman SG. Radiofrequency ablation: technique and clinical applications. Diagn Interv Radiol. 2012;18:508–516. doi: 10.4261/1305-3825.DIR.5168-11.1. [DOI] [PubMed] [Google Scholar]
- 13.Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590–602. doi: 10.1378/chest.119.2.590. http://dx.doi.org/10.1378/chest.119.2.590. [DOI] [PubMed] [Google Scholar]
- 14.Hiraki T, Gobara H, Iguchi T, Fujiwara H, Matsui Y, Kanazawa S. Radiofrequency ablation for early-stage nonsmall cell lung cancer. Biomed Res Int. 2014;2014:152087. doi: 10.1155/2014/152087. http://dx.doi.org/10.1155/2014/152087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol. 2008;15:1765–1774. doi: 10.1245/s10434-008-9848-7. http://dx.doi.org/10.1245/s10434-008-9848-7. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9:621–628. doi: 10.1016/S1470-2045(08)70155-4. http://dx.doi.org/10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 17.Chua TC, Sarkar A, Saxena A, Glenn D, Zhao J, Morris DL. Long-term outcome of image-guided percutaneous radiofrequency ablation of lung metastases: an open-labeled prospective trial of 148 patients. Ann Oncol. 2010;21:2017–2022. doi: 10.1093/annonc/mdq098. http://dx.doi.org/10.1093/annonc/mdq098. [DOI] [PubMed] [Google Scholar]
- 18.Matsui Y, Hiraki T, Gobara H, et al. Long-term survival following percutaneous radiofrequency ablation of colorectal lung metastases. J Vasc Interv Radiol. 2015;26:303–310. doi: 10.1016/j.jvir.2014.11.013. http://dx.doi.org/10.1016/j.jvir.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Gillams A, Khan Z, Osborn P, Lees W. Survival after radiofrequency ablation in 122 patients with inoperable colorectal lung metastases. Cardiovasc Intervent Radiol. 2013;36:724–730. doi: 10.1007/s00270-012-0500-3. http://dx.doi.org/10.1007/s00270-012-0500-3. [DOI] [PubMed] [Google Scholar]
- 20.Petre EN, Jia X, Thornton RH, et al. Treatment of pulmonary colorectal metastases by radiofrequency ablation. Clin Colorectal Cancer. 2013;12:37–44. doi: 10.1016/j.clcc.2012.07.003. http://dx.doi.org/10.1016/j.clcc.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Hiraki T, Gobara H, Iguchi T, Fujiwara H, Matsui Y, Kanazawa S. Radiofrequency ablation as treatment for pulmonary metastasis of colorectal cancer. World J Gastroenterol. 2014;20:988–996. doi: 10.3748/wjg.v20.i4.988. http://dx.doi.org/10.3748/wjg.v20.i4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan TD, King J, Sjarif A, et al. Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol. 2006;13:1529–1537. doi: 10.1245/s10434-006-9101-1. http://dx.doi.org/10.1245/s10434-006-9101-1. [DOI] [PubMed] [Google Scholar]
- 23.Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long term safety and efficacy in 153 patients. Radiology. 2007;243:268–275. doi: 10.1148/radiol.2431060088. http://dx.doi.org/10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 24.Yamakado K, Hase S, Matsuoka T, et al. Radiofrequency ablation for the treatment of unresectable lung metastases in patients with colorectal cancer: a multicentre study in Japan. J Vasc Interv Radiol. 2007;18:393–398. doi: 10.1016/j.jvir.2006.11.003. http://dx.doi.org/10.1016/j.jvir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Thanos L, Mylona S, Ptohis N, et al. Percutaneous radiofrequency thermal ablation in the management of lung tumors: presentation of clinical experience on a series of 35 patients. Diagn Interv Radiol. 2009;15:290–296. doi: 10.4261/1305-3825.DIR.1828-08.2. [DOI] [PubMed] [Google Scholar]
- 26.Thanos L, Mylona S, Pomoni M, et al. Percutaneous radiofrequency thermal ablation of primary and metastatic lung tumors. Eur J Cardiothorac Surg. 2006;30:797–800. doi: 10.1016/j.ejcts.2006.08.015. http://dx.doi.org/10.1016/j.ejcts.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Suh RD, Wallace AB, Sheehan RE, Heinze SB, Goldin JG. Unresectable pulmonary malignancies: CT-guided percutaneous radiofrequency ablation--preliminary results. Radiology. 2003;229:821–829. doi: 10.1148/radiol.2293021756. http://dx.doi.org/10.1148/radiol.2293021756. [DOI] [PubMed] [Google Scholar]
- 28.Yamagami T, Kato T, Hirota T, Yoshimatsu R, Matsumoto T, Nishimura T. Pneumothorax as a complication of percutaneous radiofrequency ablation for lung neoplasms. J Vasc Interv Radiol. 2006;17:1625–1629. doi: 10.1097/01.RVI.0000236607.05698.4A. http://dx.doi.org/10.1097/01.RVI.0000236607.05698.4A. [DOI] [PubMed] [Google Scholar]
- 29.Nour-Eldin NE, Naguib NN, Saeed AS, et al. Risk factors involved in the development of pneumothorax during radiofrequency ablation of lung neoplasms. AJR Am J Roentgenol. 2009;193:W43–48. doi: 10.2214/AJR.08.1457. http://dx.doi.org/10.2214/AJR.08.1457. [DOI] [PubMed] [Google Scholar]
- 30.Garetto I, Busso M, Sardo D, et al. Radiofrequency ablation of thoracic tumours: lessons learned with ablation of 100 lesions. Radiol Med. 2014;119:33–40. doi: 10.1007/s11547-013-0308-5. http://dx.doi.org/10.1007/s11547-013-0308-5. [DOI] [PubMed] [Google Scholar]
- 31.Crabtree T, Puri V, Timmerman R, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033) J Thorac Cardiovasc Surg. 2013;145:692–699. doi: 10.1016/j.jtcvs.2012.10.038. http://dx.doi.org/10.1016/j.jtcvs.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann FB, Geinitz H, Schill S, et al. Stereotactic hypofractionated radiation therapy for stage I non-small cell lung cancer. Lung Cancer. 2005;48:107–114. doi: 10.1016/j.lungcan.2004.10.015. http://dx.doi.org/10.1016/j.lungcan.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. http://dx.doi.org/10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 34.Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:348–353. doi: 10.1016/j.ijrobp.2011.06.2003. http://dx.doi.org/10.1016/j.ijrobp.2011.06.2003. [DOI] [PubMed] [Google Scholar]
- 35.Casiraghi M, De Pas T, Maisonneuve P, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the “international registry of lung metastases”. J Thorac Oncol. 2011;6:1373–1378. doi: 10.1097/JTO.0b013e3182208e58. http://dx.doi.org/10.1097/JTO.0b013e3182208e58. [DOI] [PubMed] [Google Scholar]
- 36.Widder J, Klinkenberg TJ, Ubbels JF, Wiegman EM, Groen HJ, Langendijk JA. Pulmonary oligometastases: metastasectomy or stereotactic ablative radiotherapy? Radiother Oncol. 2013;107:409–413. doi: 10.1016/j.radonc.2013.05.024. http://dx.doi.org/10.1016/j.radonc.2013.05.024. [DOI] [PubMed] [Google Scholar]