Abstract
Oxytocin and vasopressin modulate a range of species typical behavioral functions that include social recognition, maternal-infant attachment, and modulation of memory, offensive aggression, defensive fear reactions, and reward seeking. We have employed novel functional magnetic resonance mapping techniques in awake rats to explore the roles of these neuropeptides in the maternal and non-maternal brain. Results from the functional neuroimaging studies that are summarized here have directly and indirectly confirmed and supported previous findings. Oxytocin is released within the lactating rat brain during suckling stimulation and activates specific subcortical networks in the maternal brain. Both vasopressin and oxytocin modulate brain regions involved unconditioned fear, processing of social stimuli and the expression of agonistic behaviors. Across studies there are relatively consistent brain networks associated with internal motivational drives and emotional states that are modulated by oxytocin and vasopressin.
Keywords: Functional MRI, BOLD fMRI, Rat, Awake Rat Imaging, Oxytocin, Vasopressin, Maternal Rat, Maternal Attachment, Fear, Anxiety, Aggression, Aggressive Behavior, Autism, Addiction
1. Introduction
It is well established that the neuropeptides oxytocin (OT) and vasopressin (AVP) play major roles in what many consider to be vital behavioral functions (Carter et al., 2008). The range of functions encompasses a spectrum of emotional and motivational mechanisms that include social recognition, modulation of social memory, maternal-infant attachment, offensive aggression, defensive and fear reactions, and reward seeking behavior. Consistent with the range of neurobehavioral roles that have been discovered for oxytocin and AVP, the receptor distribution for these neuropeptides is observed to include various subcortical forebrain and midbrain areas of rats, which subserve the aforementioned behaviors. In non-human primates the expression of their receptors may include regions of the neocortex (Young et al., 1999). Among the challenges for studying these integrative brain functions using a systems-level approach is the limited amount of non-invasive methods that are available. Our group has used functional magnetic resonance mapping techniques to explore the modulatory roles of these neuropeptides in maternal and non-maternal brain networks. The present review offers a summary of these experiments in awake rats and puts forth several interpretations that could guide future fMRI studies in this area.
2. Functional Magnetic Resonance Imaging of Awake Rats
Functional magnetic resonance methods developed by Ferris and colleagues have allowed fMRI studies in awake restrained rats (Ferris et al., 2005). There are obvious advantages to this experimental paradigm that is often overlooked by many in the neuroscience community. Most of the available fMRI studies in rodents are carried out while animals are under anesthesia. This is done despite the anticipation of studying neural circuits that underlie or drive distinct motivational and cognitive states. This, of course, is impossible when the awake state is suppressed by anesthetics. Experiments that employ the awake rat methods require acclimation to restraint and MR sound prior to experiments. We have been able to successfully implement these procedures in our studies. This requires the animals be placed under transient stress over the span of 5 days or so. Results from previous studies show promising outcomes in which animals show reductions in physiological and motor perturbations that may hinder the quality of the collected data (King et al., 2005). Moreover, the behavioral outcomes of restraint acclimation for fMRI do not appear to be permanent (Reed et al., 2013). Thus, the studies cited in the present review are all collected while rats are in the awake, unanesthetized state. Details of the experimental paradigms used, especially pre-processing strategies to screen for and to minimize the impact of motion artifact have been published (Ferris et al., 2008).
The non-invasive measurement of neural activity through the blood oxygenation level dependent (BOLD) signal is key to the enthusiasm in pursuing fMRI studies rodents and other species. To date, there are no neuroscience techniques that can supersede and replace this feature of fMRI. The BOLD signal arises from changes in tissue oxy-to-deoxyhemoglobin ratio and. Microvascular magnetic field gradients that are sensitive to changes in paramagnetic deoxy- and diamagnetic oxy-hemoglobin concentrations near brain areas of altered neuronal metabolism are a likely source of the BOLD fMRI signal. Changes in neuronal activity, and the accompanying compensatory adjustments in blood flow, in blood volume and in the cerebral consumption rates for oxygen, underlie the mapping of the measured BOLD signal changes. A variety of experimental paradigms have been developed over the years in order to examine brain function with fMRI. The premise in all of the paradigms is the external delivery of a sensory-driving stimulus, be it drug, autonomic, primary sensory, or a higher order complex stimulus.
Despite its advantages, it is important to keep in mind shortfalls of fMRI in awake rats. First, the quantitative assessment of excitatory/inhibitory neuronal firing as recorded using other invasive techniques is limited in fMRI. Second, restraint used for fMRI experiments precludes behavioral tests during fMRI scanning. There are always concerns about the effects of repeated exposure to transient, but significant, levels of stress. This is an important and ongoing area of study by our laboratories.
3. Affiliation: Imaging Oxytocin and Maternal-Offspring Interactions
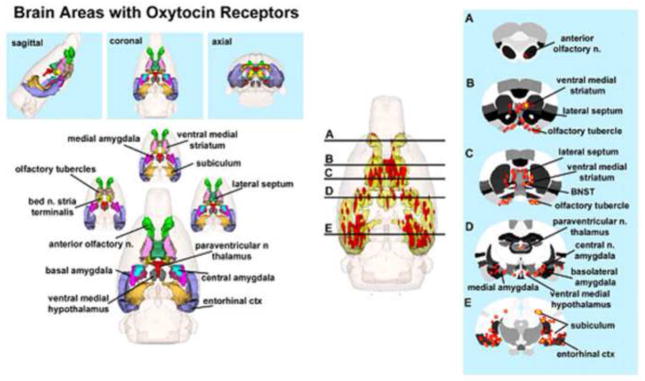
OT acts within the lactating brain to provide a major signaling mechanism that strengthens the mother-offspring bond early in life. Its synthesis mainly occurs in neurons of paraventricular (PVN) and supraoptic nucleus (SON) of hypothalamus. Suckling stimulates the release of OT into the bloodstream via the neurohypophyseal portal system and in the central nervous system of postpartum rats (Neumann et al., 1993b). Systemically, OT stimulates smooth muscle contraction, which is important for milk ‘let-down’ during nursing and for uterine contraction during parturition. OT release within the CNS during parturition initiates maternal behaviors and may act to coordinate emotion, social and cognitive networks necessary for maternal care. Many of the brain areas involved in maternal behavior in rat and other species of mammals express moderate-to-high levels of OT receptors (Tribollet et al., 1988a; Tribollet et al., 1988b; Vaccari et al., 1998; Veinante and Freund-Mercier, 1997; Yoshimura et al., 1993), suggesting a convergence of maternal and OT pathways. Brain regions include amygdala, dorsal hippocampus, hypothalamic paraventricular, ventromedial, and preoptic nuclei, lateral septum, olfactory structures, nucleus accumbens, substantia nigra (SN), ventral tegmental area (VTA), bed nucleus of stria terminalis (BNST), among others (See Fig. 1 and Table 1). Elevated OT receptor density in areas such as the preoptic area (mPOA) has been correlated with greater levels of pup licking and grooming in postpartum dams (Champagne et al., 2001; Francis et al., 2000). It is in these structures that OT may contribute to strengthening of mother-infant interactions. OT release in response to suckling has been measured using microdialysis in the substantia nigra, olfactory bulbs, mediobasal hypothalamus, BNST, MPOA and septum of parturient ewes (Levy et al., 1995), as well as in sites of origin, PVN and SON, of rat (Neumann et al., 1993a; Neumann et al., 1993b). Administration of an OT receptor antagonist locally within the latter two nuclei reduced OT release, suggesting that a positive feedback mechanism is involved (Neumann et al., 1994). Moreover, administration of an OT antagonist locally within the mPOA reduces arched back nursing in the rat (Bosch et al., 2010).
Figure 1. Neural Circuitry of High Density OT Receptor Binding Sites.
The 3D color model on the top depicts the brain areas in the rat with a high density of OT receptor. These areas are coalesced into a single volume (yellow) within a translucent shell of the brain, as shown in the lower 3D images for ICV. Areas colored red are the localization of activated voxels representing the composite average of nine rat brain fMRI scans. Once fully registered, and segmented, the statistical responses for each animal are averaged on a voxel-by-voxel basis. The averaged voxels that are significantly different from baseline, and exceed a 2.0% threshold, are show in their appropriate spatial location. The 2D images appearing to the right of each 3D image show the location of interpolated voxels of positive BOLD activation registered onto coronal sections of the segmented rat atlas. The approximate location of these brain slices are depicted by the black lines shown in the 3D ICV image.
Table 1. Brain Activation with Intraventricular Oxytocin.
Shown is a truncated list from 152 brain areas and their median (med), number of voxels activated at 30 min post ICV injection of OT in doses of 1.0, 0.1 and 0.01 ug or vehicle. The brain areas are rank order for their significance. The voxel numbers for each treatment were analyzed using a Newman-Keuls multiple comparisons test statistic. P values are presented on the far right column. The yellow highlight denotes brain areas with high OT receptor binding and association with OT mediated behaviors.
| Positive BOLD-Volume of Activation (voxel numbers)
| |||||
|---|---|---|---|---|---|
| REGION OF INTEREST | 1.0 ug | 0.1 ug | 0.01 ug | vehicle | |
|
| |||||
| med | med | med | med | P value | |
| retrosplenial cortex | 101 | 42 | 49 | 53 | 0.001 |
| subiculum hippocampus | 94 | 43 | 48 | 46 | 0.001 |
| substantia nigra reticularis | 28 | 10 | 9 | 9 | 0.001 |
| substantia nigra compacta | 8 | 3 | 4 | 5 | 0.001 |
| medial preoptic area | 20 | 8 | 7 | 7 | 0.001 |
| olfactory tubercles | 73 | 20 | 56 | 55 | 0.001 |
| medial dorsal thalamus | 10 | 6 | 4 | 4 | 0.001 |
| dorsal medial hypothalamus | 7 | 2 | 1 | 1 | 0.002 |
| orbital cortex | 23 | 5 | 6 | 6 | 0.003 |
| CA1 dorsal hippocampus | 46 | 14 | 26 | 20 | 0.003 |
| ventral tegmental area | 17 | 4 | 6 | 5 | 0.004 |
| piriform cortex | 96 | 52 | 67 | 47 | 0.006 |
| dorsal lateral striatum | 37 | 20 | 11 | 12 | 0.007 |
| ventral medial hypothalamus | 14 | 6 | 7 | 10 | 0.008 |
| glomerular layer | 8 | 2 | 0 | 0 | 0.01 |
| anterior cingulated cortex | 64 | 27 | 42 | 42 | 0.01 |
| outer plexiform layer | 13 | 2 | 0 | 0 | 0.01 |
| lateral preoptic area | 12 | 4 | 3 | 3 | 0.013 |
| lateral septal nucleus | 42 | 16 | 24 | 23 | 0.021 |
| ventral medial striatum | 21 | 5 | 4 | 4 | 0.022 |
| granular cell layer | 5 | 2 | 0 | 0 | 0.028 |
| basal amygdala | 14 | 7 | 2 | 2 | 0.031 |
| medial amygdala | 16 | 5 | 7 | 5 | 0.033 |
| ventral lateral striatum | 16 | 6 | 10 | 10 | 0.039 |
| dorsal medial striatum | 42 | 11 | 17 | 17 | 0.04 |
| insular cortex | 17 | 6 | 19 | 18 | 0.041 |
| CA1 ventral hippocampus | 31 | 14 | 9 | 9 | 0.042 |
| bed nucleus stria terminalis | 10 | 7 | 4 | 2 | 0.047 |
| parietal cortex | 29 | 10 | 23 | 13 | 0.05 |
In one of the first experiments investigating the putative functional neural circuits of lactating rat brain, we presented fMRI evidence that suckling-induced OT release into central neural sites increases the BOLD signal. This effect on the BOLD signal was reported for PVN, olfactory tubercle, anterior olfactory nucleus (AON), insular cortex, piriform cortex, cortical amygdala, mPOA and prefrontal cortex. For several of the regions, suckling and central OT administration increased the BOLD signal in a strikingly similar pattern. The promising results were not unexpected if the technique were to reliably show lactation neural circuits, and the contribution of OT release into these sites. In addition, central administration of an OT receptor antagonist (d(CH2)5-[Tyr(Me)2, Thr4, Tyr-NH29]-Ornithine Vasotocin) partly blocked the suckling-induced increase in the BOLD signal in cortical, midbrain and olfactory regions (Febo et al., 2005). OT antagonist blockade in olfactory regions suggests that OT release during nursing contributes to olfactory-related neural activity. The results of the above-cited study (Febo et al., 2005) illustrate a common theme present through several of the subsequent neuroimaging studies in rat. There is a close functional interaction between OT and AVP with olfactory function. This may be particularly important for enhancing the perception or facilitation of the registration of socially relevant stimuli into short- and long-term memory circuits of rats. We have observed that neuranatomical substrates activated by central OT administration in the lactating rat closely paralleled that observed with suckling stimulation alone. OT given intracerebroventricularly (ICV) showed a dose-dependent BOLD activation of brain areas co-localized with OT receptors, or that are associated with OT-mediated behaviors (Table 1). Centrally administered OT activated areas associated with maternal, and other reproductive behaviors, e.g. the primary olfactory system, the ventral medial hypothalamus, mPOA and BNST. The 3D color model on the top of Fig. 1 depicts the brain areas in the rat that are reported to display a high density of OT receptor binding (De Kloet et al., 1985; Freund-Mercier et al., 1987; Tribollet et al., 1988c; van Leeuwen et al., 1985). As there are no reported differences in OT receptor binding sites between males and female rats (Barberis and Tribollet, 1996), this 3D model is representative of both sexes. These areas have been coalesced into a single volume (yellow), as shown in the lower 3D images for ICV administration. Areas in red are from composite images of BOLD signal changes collected in nine rats, each given OT ICV (1.0 ug). The localization of BOLD activation is clearly shown on the 2D axial sections of the MR atlas of rat. Blockade of OT receptors with a specific antagonist selectively reduced brain activity in many of these areas, evidence that endogenous OT has a role in the neurobiology of nursing. There were non-overlapping brain regions, namely the caudate-putamen, septum, and thalamus that might involve other neurotransmitter systems aside from OT.
As noted above, there are numerous studies showing that the main olfactory system, indeed the olfactory bulb itself, is critical for processing and remembering odors that relevant for social recognition (Sanchez-Andrade and Kendrick, 2009). Volatile chemical signals from the environment interact with odorant receptors on the olfactory epithelium. In the rat, there are approximately 1200 genes coding for odorant receptors (Quignon et al., 2005). These olfactory sensory neurons project to mitral cells in the glomerular layer of the olfactory bulb. Olfactory information is processed at the level of olfactory bulbs and conveyed to the olfactory cortex and amygdala. Natural maternal behavior in rats and mice (Fleming and Rosenblatt, 1974; Gandelman et al., 1971), maternal recognition of offspring in ewe (Baldwin and Shillito, 1974) and social recognition in male rats (Dantzer et al., 1990), require the olfactory bulbs. OT plays a significant role in each. During parturition and vaginal-cervical stimulation, OT levels increase in the olfactory bulbs (Kendrick et al., 1988a; Kendrick et al., 1988b; Larrazolo-Lopez et al., 2008). Blocking OT receptors in the olfactory bulbs immediately after parturition delays maternal behavior in rats (Yu et al., 1996). Conversely, OT injections into the olfactory bulbs induces maternal behavior in virgin rats (Yu et al., 1996). The memory preservation of social recognition of juvenile female conspecifics is reduced with injection of OT receptor antagonist into the olfactory bulb (Larrazolo-Lopez et al., 2008), while OT infused in the olfactory bulbs of male rats preserves the memory of prior social interactions (Dluzen et al., 1998). Indeed, the olfactory bulb, and its downstream connections to cortical amygdala, medial amygdala, BNST and medial preoptic areas, are all integrated in social recognition and all are sensitive to OT neurotransmission.
4. Aggression: Imaging Vasopressin and Intruder Aggression
4.1 AVP V1a Modulation of Maternal Neural Correlates of Aggression
AVP varies from its cousin nonapeptide OT by way of substitution of the amino acids Phe for Ile in the 3rd position, and Arg for Leu in the 8th position (Gimpl and Fahrenholz, 2001). It is implicated in the regulation of numerous social and behavioral processes such as aggression, social bonding, and maternal behavior (Goodson and Bass, 2001). Within the mammalian central nervous system, the synaptic actions of AVP are mediated to a large degree by the V1a receptor subtype (Tribollet et al., 1988a), which is found throughout the rodent brain (Ostrowski et al., 1994), and the V1b receptor subtype. AVP is important for the modulation of aggressive behaviors in males and females. Ferris and Potegal (Ferris and Potegal, 1988) demonstrated that microinjection of a V1a receptor antagonist in the anterior hypothalamus reduced resident male hamster aggression toward a male intruder. Similarly, a microinjection of AVP into the lateral ventricle causes an increased BOLD signal in regions of the brain involved in aggression and that are known to contain V1a receptors (Ferris et al., 2008). In microtine rodents, AVP also has been observed to promote offensive aggression, as well as partner preference (Winslow et al., 1993). Both the aggressive and affiliative responses may be mediated by V1a receptors in this species of rodent (Lim et al., 2004; Young, 1999). AVP is also important in maternal behavior, as chronic AVP treatment in lactating rats increases maternal care (Bosch and Neumann, 2008), and V1a antagonists impair maternal memory (Nephew and Bridges, 2008) and reduce nursing and pup retrieval (Pedersen et al., 1994).
One specific type of maternal behavior is maternal aggression, which is a robust form of aggression most evident within the first two weeks of lactation (Erskine et al., 1978). This aggressive response can be eliminated by bilateral olfactory bulbectomy (Kolunie and Stern, 1995); however, deprivation of auditory and visual inputs has no effect on maternal aggression (Kolunie et al., 1994), indicating that olfaction, but not auditory or visual stimulation, is essential for maternal aggression. Several brain regions have been associated with maternal aggression, including regions of the limbic system such as the amygdala, nucleus accumbens and BNST (Nephew et al., 2009; Numan and Numan, 1996). The hypothalamus is also associated with the onset of maternal behavior, particularly the VMH (Bridges and Mann, 1994). Lesions to the VMH advance the onset of maternal behavior in primigravid rats (Mann and Babb, 2004), suggesting that this region may be inhibitory toward maternal behavior. Interestingly, anxiogenic state may influence how OT and AVP modulate maternal aggression. Low anxiety behavior (LAB) dams reportedly show lower basal levels of attacks towards a nets intruder than high anxiety behavior (HAB) dams (Bosch and Neumann, 2010). Blocking OT receptors in LAB dams increases the number of attacks, whereas it decreases maternal aggression in HAB dams (Bosch and Neumann, 2010). Similarly, blocking vasopressin V1a receptors also increases maternal aggression in LAB dams and reduces it in HAB dams (Bosch and Neumann, 2010). The similar outcomes of these two peptides might be the result of ‘cross-talk’ via their receptors.
Cortical regions involved in maternal aggression are less well understood. Conscious lactating female rats were injected ICV with either a V1a antagonist or saline, and presented with a novel male intruder in the presence of her pups. Brain activation was measured using BOLD fMRI to determine the role of V1a receptors on maternal brain activation during presentation of a novel male intruder. It was shown that central blockade of V1a receptors modulated BOLD signal responses in primiparous dams (Caffrey et al., 2010). The differential BOLD responses were not generalized across the maternal rat brain, but were site specific. V1a receptor antagonist enhanced the volume of BOLD activation in the AON, and reduced it in the cortical amygdala and VMH. Greater percentage increases in BOLD signal were observed across several brain areas in response to V1a antagonist treatment, including the AON, gustatory cortex, infralimbic area, substantia innominata (which expresses V1a receptors), and the somatosensory cortex. An unexpected finding of the study was that V1a receptor blockade significantly enhanced BOLD signal in somatosensory areas during intruder presentation. The greater BOLD signal response occurred in both primary and supplemental areas, but was not generalized to the entire cortical mantle. The heightened BOLD response observed in this region of the cortex was not observed in primary or secondary motor cortical areas, or in parietal or temporal cortices. Thus, somatosensory modulation by V1a receptors appears to be selective for this cortical region. The only region that showed a lower percentage change in BOLD with V1a receptor blockade was the VMH. The results indicated that V1a receptors modulate neural processing in specific neural circuits recruited during a timeframe corresponding to the initial phases of maternal aggressive motivation. The V1a sensitive circuits include structures which are involved in sensory processing, the control of visceral responses, and emotional memory. One interesting feature of the brain areas that were modulated by V1a receptors in our imaging studies is that some of these share connectivity with the orbital prefrontal cortex in rats (Ongur and Price, 2000). Gustatory, infralimbic, olfactory, somatosensory and amygdalar networks are processed through this limbic cortical structure that plays an important role in sensory and visceromotor associations (Gabbott et al., 2005; Ongur and Price, 2000; Vertes, 2004). Although V1a receptors in rat are not localized in the various cortical regions, the observed enhancement might be achieved through indirect subcortical actions of this receptor antagonist. It remains to be determined whether or not this is the case. Activation of the somatosensory cortex and its modulation by V1a receptors is interesting in light of the fact that AVP receptors have not been detected in this brain region, and that a somatosensory stimulus was not presented to dams. Thalamic sensory relay nuclei were unaffected by antagonist treatment, and do not contain V1a receptors. However, the substantia innominata, which has been shown to contain an understudied population of V1a receptors (Ostrowski et al., 1994), sends major cholinergic projections to the cortical mantle, in particular the somatosensory and prefrontal cortices. Lesions of these cholinergic projections differ dramatically from sensory cortical ablations, and alter emotional reactivity in rats (Knox et al., 2008; Wozniak et al., 1989). Indeed, it is possible that V1a receptors in this basal forebrain area, and its interaction with the somatosensory cortex, may control aspects of behavioral reactivity, and perception of internal autonomic/visceral states. Due to the transient nature of the BOLD changes in the substantia innominata, it is postulated that this nucleus may mediate the immediate response to the male intruder, perhaps mapping maternal autonomic bodily reactions to possible aggression towards pups (Caffrey et al., 2010). Though the above interpretations appear plausible in light of previous literature and our reported data, they remain highly speculative at the moment.
4.2 Male Aggression: Role of AVP V1a Neurotransmission
With regards to the actions of AVP on aggression in rat and in other rodent models, it appears to partly mediate its effect via interactions with serotonin (5-HT) at the synaptic level. There is a body of literature reporting that blockade of V1a receptors in a variety of animal models suppresses aggression (Ferris, 2005). Consequently, drugs that target, and block, the V1a receptors are being developed as potential therapeutics for the treatment of impulsivity and violence. Recently, a new class of non-peptidic compounds targeting the human V1a receptor was developed using a monocyclic beta lactam platform (Guillon et al., 2006). One of these potential drugs, SRX251, was tested for serenic activity in the hamster (Ferris et al., 2006). It was shown that oral administration of SRX251 caused a dose-dependent decrease in several measures of aggressive behavior, without affecting motor activity, olfactory communication, and sexual motivation.
Precisely how and where 5-HT and AVP interact to affect the organization, and expression, of aggressive behavior is unclear. Normal aggressive behaviors, and aggression characterized by impulsivity and violence, are envisioned to be organized and controlled by a distributed neural circuit. These include subsets of interconnected neurons conveying sensory and motor information to and from sites of integration (Ferris et al., 2008). With functional magnetic resonance imaging (fMRI) it has been possible to identify the neural circuitry involved in aggressive motivation using an experimental paradigm that drives an agonistic state just prior to the onset of attack. As part of the ethogram of aggression, resident male rats housed with a female cage will piloerect along the dorsal midline when in the presence of a male intruder. The piloerection is unique to offensive aggression, and is not seen in other behaviors signaling an impending attack (Blanchard and Blanchard, 1977). Using a novel aggression model adapted to the neuroimaging conditions, we discovered that even though a resident male is confined to a restraining device for an imaging session, placing an intruder into the vivarium with its cage mate induces piloerection. Our results identified distributed putative neural circuits associated with the genesis of attack behavior and their differential modulation but AVP and 5-HT acting compounds.
Enhanced 5HT neurotransmission is associated with a reduction in aggressive responding via interaction with 5-HT1a and 5-HT1b receptors (Simon et al., 1998; Grimes and Melloni, 2005). Oral fluoxetine suppressed aggression and diminished BOLD activation across the putative neural circuit of aggressive motivation (Ferris et al., 2008). Conversely, AVP neurotransmission promotes aggression by interacting with V1a receptors. Oral SRX251, a V1a receptors antagonist, suppressed aggression and produced a general reduction in BOLD activation in the neural circuitry of aggression similar to that seen with fluoxetine (Ferris et al., 2008). The observation that fluoxetine and SRX251 are similar in their fMRI profile during suppression of aggressive motivation was anticipated. There is evidence that the stimulation of aggression by AVP is regulated by 5-HT. The hypothalamus, the primary site of AVPergic facilitation of aggression, has a high density of 5-HT1a and 5-HT1b binding sites and receives a dense innervation of 5-HT fibers and terminals (Delville et al., 2000; Ferris et al., 1997; Grimes and Melloni, 2002; Ricci et al., 2006). Hypothalamic AVP neurons implicated in the control of aggression appear to be preferentially innervated by 5-HT (Ferris et al., 1991). Fluoxetine blocks resident-intruder aggression that is facilitated by the microinjection of AVP in the hypothalamus (Delville et al., 1996; Ferris, 1996; Ferris et al., 1997). Fluoxetine elevates 5-HT and reduces AVP levels in hypothalamic tissue in hamsters (Ferris, 1996) and rats (Altemus et al., 1992). Serotonin can also block the activity of AVP following its release in the hypothalamus as evidenced by the dose-dependent reduction of aggression with injections combining AVP and 5-HT1a receptor agonists. Enhanced aggression caused by activation of V1a receptors in the hypothalamus is suppressed by the simultaneous activation of 5-HT1a receptors in the same site (Ferris et al., 1997). Personality disordered subjects with a history of fighting and assault show a negative correlation for prolactin release in response to D-fenfluramine challenge, indication of a hyposensitive 5-HT system (Coccaro et al., 1998). These same subjects show a positive correlation between CSF levels of vasopressin and aggression (Coccaro et al., 1998). Thus, in humans a hyposensitive 5-HT system may result in enhanced CNS levels of AVP, with a consequent facilitation of aggressive behavior.
While fluoxetine and SRX251 have similar effects on the putative neural circuitry of aggressive motivation, a markedly different fMRI signature was observed with each compound when treated males were challenged with sexual motivating stimuli (Ferris et al., 2008). With V1a receptor blockade there was activation of the SN, VTA, and their afferent projections to the forebrain limbic cortex, as well as the dorsal and ventral striatum. Measures of sexual activity in the home environment were unaffected by SRX251 treatment. Treatment with fluoxetine, on the other hand, resulted in a diminished BOLD activation in response to sexual motivating stimuli. It also caused inhibition of sexual behavior in the home environment. These opposite effects point to a difference in drug specificity, and underscore the serenic properties of SRX251, specifically its ability to block aggression without affecting other appetitive behaviors.
5. Anxiety: Imaging Oxytocin and Vasopressin in Unconditioned Fear
5.1 OT and Unconditioned Fear in the Lactating Rat Brain
Attending and responding effectively to environmental dangers is important during the postpartum period. Lactating rats show aggression to conspecific males and are generally less fearful than virgin rats. Central release of OT and AVP during lactation may contribute to heightened maternal aggression and nest defense, and lower fearfulness (Bale et al., 2001; Blume et al., 2008; Bosch and Neumann, 2008; Francis et al., 2000; Windle et al., 1997a; Windle et al., 1997b). In the case of OT, it is released in the PVN and supraoptic nucleus during lactation (Neumann et al., 1993b) and nest defense (Bosch et al., 2005), respectively. Blocking OT receptors reduces lactation-related behaviors (Bosch and Neumann, 2008; Pedersen and Boccia, 2003), increases anxiety related behavior in an elevated plus maze (Bosch and Neumann, 2008), and affects maternal offensive and defensive behaviors (Bosch et al., 2005). The actions of OT on aggression and fear are perhaps exerted through PVN, central amygdala and septum (Bosch et al., 2005; Heinrichs et al., 2008); however, it is quite possible that a wider circuitry including other limbic and forebrain sites contributes to OT’s role in fear reactivity during the postpartum period.
We investigated the effect of centrally administered OT on BOLD activation in response to an unconditioned fear stimulus in dams. Trimethylthiazoline (TMT), a chemical extract of fox feces, has been shown to evoke fear-associated freezing behavior (Wallace and Rosen, 2001), and it also has been observed to elevate plasma levels of corticosterone (Chen et al., 2009; Febo et al., 2009; Morrow et al., 2000). TMT-associated increase in corticosterone in lactating rats is enhanced when pups are in the nest (Deschamps et al., 2003). The physiological and behavioral signs of stress and fear in response to TMT are distinguishable from responses to novel acrid odors (Morrow et al., 2000; Staples et al., 2008), and considered innate since laboratory rodents have no prior exposure to predator odors.
Our fMRI findings showed that OT treatment modulates BOLD signal increases and decreases in response to TMT in lactating rats. OT enhanced TMT-induced positive BOLD signal changes in the anterior cingulate, a brain region involved in emotional expression and fear conditioning. While the BNST, which was previously shown to be responsive to TMT (Day et al., 2004), and also constitute part of the neural circuitry mediating behavioral responses to this predator scent (Fendt et al., 2003; Fendt et al., 2005), also showed greater BOLD signal as compared to vehicle controls. The latter basal forebrain region is interesting in light of the fact that it also modulates VTA dopamine neuron activity (Georges and Aston-Jones, 2001; Georges and Aston-Jones, 2002), thus providing a neurobiological interface between distinct motivation and emotional states. Interestingly, a wider array of structures showed negative BOLD signal changes in response to TMT following OT administration. The anterior olfactory nucleus, which serves as an initial relay site for olfactory-processing, showed primarily signal decreases. This was observed in the prelimbic prefrontal cortex, orbital cortex, mammillary bodies, secondary motor cortex and the gustatory cortex (Febo et al., 2009). The pattern of reduced brain activity (negative BOLD) underscores the importance of OT in regulating fear and anxiogenic responses during the lactation period. Moreover, the pattern varies from findings in male rats (see below). It is tempting to speculate on whether this represents an underlying physiological mechanism where activity in the anterior cingulate during exposure to an innate fear stimulus occurs in parallel with a lack of neuronal activity or inhibition in other adjacent limbic cortical regions controlling approach behavior and autonomic responses. Negative BOLD has, however, less support from the literature on its role in neuronal processing than positive BOLD (Logothetis, 2003). Although somewhat controversial, there are indications that negative BOLD correlates with reductions in synaptic activity (Shmuel et al., 2006).
Our results for OT and fear are in agreement with a previous study looking at the BOLD response to OT administration in lactating rats (Febo et al., 2005). ICV OT administration was observed to activate the anterior cingulate, parietal cortex, somatosensory, temporal cortices, dorsal striatum, preoptic area and ventral tegmental area. The actions of OT administration on BOLD responses within these regions may hold importance to emotional reactivity, particularly to innate fear reactions. For instance, the anterior cingulate is involved in the initiation of goal directed behaviors (Devinsky et al., 1995), as well as the emotional control of visceral, skeletal, and endocrine functions (Vogt et al., 1992).
OT receptor binding sites, and its receptor-encoding mRNA, have been detected in the BNST, lateral septum, posterior cortical amygdala, anterior olfactory nucleus, piriform cortex, main olfactory bulbs, central amygdala, subiculum, and dispersed cortical regions (Veinante and Freund-Mercier, 1997; Yoshimura et al., 1993). The observed TMT-induced BOLD responses were in partial agreement with areas of the rat brain expressing OT receptors. The BNST is particularly interesting. This region is part of the extended amygdala and not only participates in modulating stress and fear responses, but also expresses both OT and V1a receptors. We observed a greater volume of activation in this area with OT pretreatment, which suggests a greater neuronal activity in response to TMT. Recent report shows that TMT exposure elevates extracellular norepinephrine levels in the BNST (Fendt et al., 2005). Blocking norepinephrine receptors with clonidine results in less time spent freezing in response to TMT (Fendt et al., 2005). Although electrolytic lesions of the lateral amygdala block conditioned fear, but not innate fear (Wallace and Rosen, 2001), the opposite has been shown for the BNST (Fendt et al., 2003). This suggests an essential role of the BNST in unconditioned fear response (Fendt et al., 2003). It might be interesting to examine the interactions between noradrenergic inputs and OT inputs to this region. It should be noted that the BNST also is important in behavioral and endocrine responses to contextual fear stimuli (Sullivan et al., 2004). Therefore, the BNST may be an important brain area where OT exerts its effect on TMT-induced freezing in lactating rats. Although there is no evidence showing OT receptors in the anterior cingulate, this region shares synaptic communication with the septum and amygdala, which might indirectly have regulated TMT-induced BOLD activation. Indeed, previous reports indicate that the anterior cingulate cortex plays a role in memory of emotional and fear-inducing events (Malin and McGaugh, 2006; Malin et al., 2007).
5.2 OT and AVP Modulation of Unconditioned Fear in the Male Rat Brain
Brain regions that are associated with unconditioned fear partly overlap with receptors for the neuropeptides OT and AVP. Therefore, it is important to consider the role of these neuromodulators as potential pharmaceutical targets for treatment of generalized anxiety disorders. The central distribution of OT and AVP suggest that they play a role in modulating olfactory processing, social behavior, as well as cognitive, emotion-related, and goal-directed behavioral functions (Huber et al., 2005; Ostrowski et al., 1994; Szot et al., 1994; Tribollet et al., 1988a; Tribollet et al., 1988b; Veinante and Freund-Mercier, 1997). There is growing evidence that OT-mediated effects result in anxiolysis, while AVP actions through the V1a receptor promote aggression and heightened anxiety (Appenrodt et al., 1998; Knobloch et al., 2012; Murgatroyd et al., 2004; Waldherr and Neumann, 2007). Not only do these neuropeptides control anxiety, but also their presence in amygdala and hippocampus suggests a prominent role in cognitive mechanisms, such as conditioned fear learning processes (De Wied et al., 1975; de Wied et al., 1984; Vawter et al., 1997). Based on their location within the components of the olfactory system, such as AON and the olfactory tubercles, as well as central amygdala and BNST, these peptides may control olfactory perception and the association between smell, social recognition and emotionality (Ferris, 2008).
We used functional MRI to investigate the role of OT and V1a receptors in modulating odor-evoked brain activity in the awake male rat (Reed et al., 2013). A novel repulsive odor for the rats, butyric acid (BA), and unknown chemical constituents present in cat fur were chosen in order to examine unconditioned fear and anxiety responses. The results indicated that BA-induced BOLD signal increases were significantly greater than with cat fur. This presented challenges to the final interpretations of the data since the fear-eliciting chemicals in cat fur are unclear. BA activated several olfactory system structures and areas of the brain involved in the neural processing of smell. Cat fur odor caused modest activation in limbic structures compared to BA. We observed a significant effect of cat fur in anterior thalamic nucleus, BNST, ventral CA3, cortical amygdala, lateral amygdala, medial amygdala, olfactory tubercle, posterior amygdala, PAG, posterior hypothalamus, dorsomedial, dorsolateral and ventromedial regions of the striatum, nucleus accumbens core, subiculum, and ventral tegmental area (Reed et al., 2013). In spite of the shortfalls, an important finding was that OT and V1a receptors differentially modulated BA- and cat fur-induced BOLD signal responses in several brain areas. BA-induced BOLD signal changes were curtailed in the cortical amygdala by the OT receptor antagonist (d(CH2)5-[Tyr(Me)2, Thr4, Tyr-NH29]Ornithine vasotocin). V1a receptor blockade using [β-Mercapto-β, β-cyclopentamethylenepropionyl1, O-me-Tyr2, Arg8]-Vasopressin reduced cat fur-induced BOLD signal changes in the amygdala, hippocampus and PAG. OT blockade had no effect on the response to cat fur in these regions. In the central amygdala both OT and V1a antagonists curtailed the BOLD response to cat fur.
The findings suggested that OT-mediated neurotransmission is important in responding to a novel odor such as BA. V1a-mediated neurotransmission appeared more closely associated with cat fur induced BOLD activation across several brain regions. This could indicate a closer association between V1a receptors and unconditioned fear than shown by OT receptors. However, the results only partly support this notion, and require improvements in the experimental model. The incorporation of conditioned fear stimuli, which holds significance to generalized anxiety and posttraumatic stress states, should provide the needed improvements.
It is noteworthy that we initially expected regions such as the ventral BNST, amygdala, ventral hippocampus, lateral septum, would distinguish the effects of cat odor from BA. Using c-fos immunolabeling, Staples et al. (2007) identified regions of rat brain that responded selectively to cat and TMT over no odor and formalin odor. They reported selective cat-induced activation of c-fos labeling in VTA, striatum (dorsal and ventral), basal and medial amygdaloid nuclei, subareas of AON, medial prefrontal cortex, anterior and ventromedial hypothalamic nuclei, BNST and dorsal premammilary nucleus (Staples et al., 2008). TMT had only modest effects on cellular activation, increasing activity in the cortical amygdala (anterior portion), piriform and ventral orbital cortices. Rosen et al. (2005) reported increased mRNA expression of egr-1 in the hypothalamic paraventricular nucleus (PVN) of rats following exposure to a cat (Rosen et al., 2005). This is interesting since this region contains OT and V1a receptors that control the central release of their corresponding neuropeptides via magnocellular neurons (Knobloch et al., 2012). Several lesion studies have provided evidence of a role for the ventral hippocampus and BNST in unlearned fear reactivity. Inactivation of the ventral BNST or blockade of norepinephrine α2 receptors reduces freezing in response to TMT (Fendt et al., 2003; Fendt et al., 2005). Similar effects are observed with lateral septum inactivation (Endres and Fendt, 2008). These areas express V1a receptor mRNA (Veinante and Freund-Mercier, 1997). In the case of the ventral hippocampus, lesions to this site result in significantly more time spent in the open arms of an elevated plus maze (Kjelstrup et al., 2002). The ventral hippocampus also shows significant V1a receptor mRNA expression (Ostrowski et al., 1994). We observed significant effects of V1a blockade on cat but not BA induced BOLD activation (Reed et al., 2013), which is consistent with some of the aforementioned studies.
In sum, investigating the neural circuitry of unconditioned fear and the modulatory role of OT and AVP may be improved by the development of alternative models for eliciting signs of fear that may be measurable from the rats. Something along the lines of what Ferris and colleagues reported for the resident-intruder model mentioned in the preceding section (Ferris et al., 2008). The use of olfactory-mediated fear is complicated by the ambiguous outcomes of TMT (is it stress responsiveness or true innate anxiety/fear circuits) and the lack of detail on the chemical components of cat fur and feces that may trigger fear and defense reactions in rats. Moreover, the use of centrally administered neuropeptides should be improved by the administration of orally active agents as well as using state of the art genetic tools to remotely drive activation of selective OT and AVP neurons in hypothalamic nuclei. Our first studies, however, indicate that OT and AVP may modulate fear and/or stress both centrally and during the initial processing stages in the olfactory system. This outcome is again consistent with the distribution of their corresponding receptors within olfactory and limbic structures (Fig. 1).
8. Potential Role of V1a Receptors In Social Neural Processing In Autism Spectrum Disorders
Original studies of AVP linked this peptide neuromodulator to learning and memory functions (De Wied et al., 1975; De Wied et al., 1993; Vawter et al., 1997). Although this was a large focus of classical studies it has subsided somewhat, but there is an important neuroanatomical and neurophysiological association between OT and AVP V1a/V1b receptor expression in the hippocampus and amygdala. Therefore, diseases in which the functional activities of these peptides are dysregulated could lead to impairments not only associated with anxiety, mood, violence, but also can potentially underlie memory/learning impairments. The neurophysiology underlying this possibility is studied very scarcely. One such area involving complex deficits including social deficits, emotional and cognitive disturbances, is autism spectrum disorders (ASD).
A pilot study to examine the possible role of V1a receptors was carried out in a well-studied animal model of ASD (Felix-Ortiz and Febo, 2012). ASD’s, which includes autism, Asperger’s syndrome, and pervasive developmental disorder not otherwise specified (PD-NOS), are characterized by deficits in verbal and non-verbal communication, reduced social interactions, and restricted range of interests and motor stereotypies. Genome-wide screening has identified numerous gene mutations that might underlie ASD (Abrahams and Geschwind, 2008). Among the candidate genes for ASD are those encoding the V1a receptor (Kim et al., 2002), and the OT receptor (Abrahams and Geschwind, 2008), which are known to modulate social behaviors in rodent models (Bielsky et al., 2004; Israel et al., 2008).
Genes encoding the V1a receptor have been linked to ASD (Kim et al., 2002). The role of AVP in modulating the neural response to a social stimulus was assessed in adult rats exposed prenatally to valproic acid (VPA). VPA is a mood stabilizer and anticonvulsant, given during pregnancy to women suffering from epilepsy. Fetal exposure at embryonic day E20–E24 can produce neural tube defects and ASD-like deficits. In rats, teratologic effects similar to those reported in humans are observed when exposure occurs between E9.5–12.5 (Arndt et al., 2005; Miyazaki et al., 2005; Rodier et al., 1996; Stodgell et al., 2006). Reports indicate that VPA treated rats show early signs of developmental abnormalities (Markram et al., 2008; Rodier et al., 1996; Schneider et al., 2001; Schneider and Przewlocki, 2005) and these are consistent with motor and cognitive behavioral features partially resembling ASD. Neurobiological changes have also been reported and (Arndt et al., 2005; Ingram et al., 2000; Rinaldi et al., 2008; Rodier et al., 1996), which parallels postmortem pathological findings in autistics. Recent evidence indicates that neonatal VPA exposure can influence AVP immunolabeling in several regions of the rodent brain such as the anterior hypothalamic area and mediodorsal thalamus, and influences olfactory-based social behavior in rodents (Murray et al., 2011). We tested whether prenatally exposed rats show abnormal neurophysiological responses to social and non-social (visual) stimuli (Felix-Ortiz and Febo, 2012).
The experiments were designed to track the developmental progress of various social behaviors from the early postnatal period to adulthood. It was anticipated that gestational VPA would dramatically alter the way the specific brain regions of the rat respond to a social stimulus. Our neuroimaging data provided evidence of a lasting effect of prenatal VPA on social neural processing (Felix-Ortiz and Febo, 2012). VPA-exposed rats show significantly greater BOLD signal responses to a stimulus juvenile rat than saline control animals. Even though the observed behavioral changes suggested a deficit, the differences in neural responses to a social stimulus in VPA compared to controls were of greater magnitude. Differences were observed in cortical and subcortical limbic regions, including temporal, secondary motor and entorhinal cortices, basal region of the amygdala, medial genticulate, posterior hypothalamus and mammillary nuclei, and the substantia nigra pars reticulata. We also provided a primary sensory stimulus to study three brain regions of the visual pathway to examine whether differences in social neural processing could be attributable to changes in primary sensory processing, albeit through a specific sensory system. Interestingly, we observed that the greater activation found with the social stimulus was not observed with the visual stimulus. Instead, the visual cortex of VPA rats showed a lower percentage BOLD signal responses that perhaps occurs as a result of lower neural activity in this region. The effects of VPA on primary sensory systems were thus distinct from subcortical and higher order cortical processing of a socially salient stimulus.
In order to examine whether VPA-induced effects on social neural processing is associated with altered functionality activity through the V1a receptor, we included subgroups of animals that received an ICV injection of a V1a antagonist. A reduction in BOLD activity in these regions was observed with V1a blockade. The antagonist on its own had no effect on the pattern of brain activation shown in control animals. The effect of the antagonist was not found to affect primary sensory processing (visual). Therefore, the actions of the antagonist could be interpreted as occurring in conjunction with VPA-induced deficits in higher order processing, but not primary visual processing.
The role of V1a receptors in modulating socially-relevant neural circuits was influenced by prenatal VPA exposure. This is consistent with the role of the V1a receptor subtype in social behaviors, and might point to V1a receptors as a substrate affected by prenatal VPA treatment. Thus, in a teratologic model recapitulating severe ASD-like disturbances in rodents, we find a pattern of social responding that is partly rescued by V1a blockade. These results are encouraging and support recent clinical trials examining the efficacy and safety of a selective and orally active AVP antagonist (RG7314) in individuals with autism spectrum disorders (http://clinicaltrials.gov/show/NCT01793441) (Nightingale, 2012). According to our data obtained in a rat model of VPA-associated social deficits, blocking V1a receptors may potentially be effective at ameliorating the social and anxiogenic deficits observed in autistic individuals. However, more animal imaging studies are needed to examine specific mechanisms of action. Further data using neurochemical techniques are needed to confirm whether AVP, or V1a receptors, or both, are affected. The role of V1a receptors, and also OT receptors, in the regulation of emotion, cognition and social behavior merits further investigation, within the context of the neurobiology of ASD. Our results show a greater BOLD response to a social stimulus in the VPA rat. The V1a receptor antagonist blocks this. Therefore, the greater ‘sensitivity’ to social stimuli could in part be mediated by excitatory AVP neurotransmission.
7. Conclusions
Over the past decade or so, we have taken a novel and non-invasive approach to studying the varied roles of OT and AVP in modulating maternal, aggression, fear and social brain networks. The initial results are promising and call for furthering creative ways to examine vital emotional and motivation states. Our neuroimaging findings skim the surface of the underlying cellular and molecular mechanisms and really provide what might be considered as ‘integrated network states’. That is, maps of how the brain under the tested conditions, initiates an effectively patterned response involving not just one region, but multiple disparate regions across the brain. The integration of the multiple (otherwise unassociated) brain regions in these states is perhaps an area that deserves deeper consideration. A resetting or synchronization of population firing across distributed brain regions may underlie the observed putative network states under the influence of the triggering sensory stimuli selected for each study. fMRI in such a case is ideally suited for reliably examining the patterned neural responses in rats.
We find that across the integrated network states (which involve many regions that have been associated indirectly, or casually, to cognitive, emotional and motivational mechanisms), OT and AVP exhibit modulatory functions. The observation of these modulatory functions indirectly link these neuropeptides to the brain networks and may be a rationale for targeting these substrates when the ‘network states’ fail, as in psychiatric illnesses. Under normal conditions, we observe that OT is released within the lactating rat brain during suckling stimulation and therefore activates particular networks of regions in the maternal brain (Febo et al., 2005). AVP and OT play major roles in modulating brain regions involved emotional states (Reed et al., 2013) and agonistic behaviors (Ferris et al., 2008). Across several of these studies, there are relatively consistent networks of brain regions that arise within the context of their role in internal motivational and emotional states that are modulated by OT and AVP and their receptors. Most of these are summarized in Table 1 and Fig. 1. The brain regions include as center points nuclei in the basal forebrain, amygdalar nuclei, midbrain regions and also include subregions of the cortex that were not previously associated with aggression, maternal care, and unconditioned fear. The results also point to possible differences according to reproductive status. Although this was not directly examined in the above-cited studies, there are clear differences between the studies in male rodents versus those including lactating rats. This warrants further investigation (Carter, 2007), as it might be important from a drug discovery and treatment development standpoint.
Highlights.
Reviews state of the art functional magnetic resonance imaging studies investigating the neuromodulation by oxytocin and vasopressin.
Discusses rat brain networks relevant to the maternal-offspring bond, intermale aggression, unconditioned fear, and processing of social stimuli in autism.
Offers insight into the fMRI data interpretations from these fMRI studies in awake rats.
According to the summarized results, there appears to be a link between oxytocin and vasopressin and the modulation of brain networks controlling olfaction and social behaviors in rats.
Acknowledgments
Support was provided in part by NIH grant DA019946. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health. The NIH and NIDA had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
The authors have no financial, commercial or personal conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Cited References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M, Cizza G, Gold PW. Chronic fluoxetine treatment reduces hypothalamic vasopressin secretion in vitro. Brain Res. 1992;593:311–3. doi: 10.1016/0006-8993(92)91326-a. [DOI] [PubMed] [Google Scholar]
- Appenrodt E, Schnabel R, Schwarzberg H. Vasopressin administration modulates anxiety-related behavior in rats. Physiol Behav. 1998;64:543–7. doi: 10.1016/s0031-9384(98)00119-x. [DOI] [PubMed] [Google Scholar]
- Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. Int J Dev Neurosci. 2005;23:189–99. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Baldwin BA, Shillito EE. The effects of ablation of the olfactory bulbs on parturition and maternal behaviour in Soay sheep. Anim Behav. 1974;22:220–3. doi: 10.1016/s0003-3472(74)80072-2. [DOI] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10:119–54. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–93. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Aggressive behavior in the rat. Physiol Behav. 1977;1:197–224. doi: 10.1016/s0091-6773(77)90308-x. [DOI] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. European Journal of Neuroscience. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–15. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc Natl Acad Sci U S A. 2008;105:17139–44. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. Eur J Neurosci. 2010;31:883–91. doi: 10.1111/j.1460-9568.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Pfortsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J Neuroendocrinol. 2010;22:420–29. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Mann PE. Prolactin-brain interactions in the induction of maternal behavior in rat. Psychoneuroendocrinology. 1994;19:611–622. doi: 10.1016/0306-4530(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Caffrey MK, Nephew BC, Febo M. Central vasopressin V1a receptors modulate neural processing in mothers facing intruder threat to pups. Neuropharmacology. 2010;58:107–16. doi: 10.1016/j.neuropharm.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176:170–86. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–6. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–41. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Shields J, Huang W, King JA. Female fear: influence of estrus cycle on behavioral response and neuronal activation. Behav Brain Res. 2009;201:8–13. doi: 10.1016/j.bbr.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry. 1998;55:708–14. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Tazi A, Bluthe RM. Cerebral lateralization of olfactory-mediated affective processes in rats. Behav Brain Res. 1990;40:53–60. doi: 10.1016/0166-4328(90)90042-d. [DOI] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–51. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Rotteveel F, Voorhuis TA, Terlou M. Topography of binding sites for neurohypophyseal hormones in rat brain. Eur J Pharmacol. 1985;110:113–9. doi: 10.1016/0014-2999(85)90036-6. [DOI] [PubMed] [Google Scholar]
- De Wied D, Bohus B, Van Wimersma BTB. Memory deficit in rats with hereditary diabetes insipidus. Brain Res. 1975;85:152–156. doi: 10.1016/0006-8993(75)91022-7. [DOI] [PubMed] [Google Scholar]
- de Wied D, Gaffori O, van Ree JM, de Jong W. Central target for the behavioural effects of vasopressin neuropeptides. Nature. 1984;308:276–8. doi: 10.1038/308276a0. [DOI] [PubMed] [Google Scholar]
- De Wied D, Diamant M, Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol. 1993;14:251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Serotonin blocks vasopressin-facilitated offensive aggression: interactions within the ventrolateral hypothalamus of golden hamsters. Physiol Behav. 1996;59:813–6. doi: 10.1016/0031-9384(95)02166-3. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Deschamps S, Woodside B, Walker CD. Pups Presence Eliminates the Stress Hyporesponsiveness of Early Lactating Females to a Psychological Stress Representing a Threat to the Pups. Journal of Neuroendocrinology. 2003;15:486–497. doi: 10.1046/j.1365-2826.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, Landgraf R. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998;19:999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- Endres T, Fendt M. Inactivation of the lateral septum blocks fox odor-induced fear behavior. Neuroreport. 2008;19:667–70. doi: 10.1097/WNR.0b013e3282fb78d9. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behavioral Biology. 1978;23:206–218. doi: 10.1016/s0091-6773(78)91814-x. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–44. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Shields J, Ferris CF, King JA. Oxytocin modulates unconditioned fear response in lactating dams: an fMRI study. Brain Res. 2009;1302:183–93. doi: 10.1016/j.brainres.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Febo M. Gestational Valproate Alters BOLD Activation in Response to Complex Social and Primary Sensory Stimuli. PLoS One. 2012;7:e37313. doi: 10.1371/journal.pone.0037313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–8. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. Functional magnetic resonance imaging and the neurobiology of vasopressin and oxytocin. In: Neumann ID, Landgraf R, editors. Progress in Brain Research. Vol. 170. 2008. pp. 305–319. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol and Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Pilapil CG, Hayden-Hixson D, Wiley R, Koh ET. Evidence for two functionally and anatomically distinct populations of magnocellular neurons in the golden hamster. J Neuroendocrinol. 1991;4:193–205. doi: 10.1111/j.1365-2826.1992.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF. Serotonin inhibits vasopressin facilitated aggression in the Syrian hamster. In: Ferris C, Grisso T, editors. Understanding aggressive behavior in children. Vol. 794. New York Academy of Sciences; New York: 1996. pp. 98–103. [Google Scholar]
- Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–40. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF. Vasopressin/oxytocin and aggression. In: Bock G, Goode J, editors. Molecular Mechanisms Influencing Aggressive Behaviours. Vol. Novartis Foundation Symposium 268. Wiley; Chichester, UK: 2005. pp. 190–200. [Google Scholar]
- Ferris CF, Lu S-F, Messenger T, Guillon CD, Koppel GA, Miller MJ, Heindel ND, Simon NG. Orally active vasopressin V1a receptor antagonist, SRX251, selectively blocks aggressive behavior. Pharm, Biochem Behav. 2006;83:169–174. doi: 10.1016/j.pbb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon aNG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neuroscience. 2008;9 doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats. I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J Comp Physiol Psychol. 1974;86:221–32. doi: 10.1037/h0035937. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Stoeckel ME, Palacios JM, Pazos A, Reichhart JM, Porte A, Richard P. Pharmacological characteristics and anatomical distribution of [3H]oxytocin-binding sites in the Wistar rat brain studied by autoradiography. Neuroscience. 1987;20:599–614. doi: 10.1016/0306-4522(87)90113-8. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–77. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gandelman R, Zarrow MX, Denenberg VH, Myers M. Olfactory bulb removal eliminates maternal behavior in the mouse. Science. 1971;171:210–1. doi: 10.1126/science.171.3967.210. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–87. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin system in vertebrates. Brain Research Reviews. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Melloni RH., Jr Serotonin modulates offensive attck in adolescent anabolic steroid-treated hamsters. Pharm Biochem Behav. 2002;73:713–721. doi: 10.1016/s0091-3057(02)00880-8. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Melloni RH., Jr Serotonin-1B receptor activity and expression modulate the aggression-stimulating effects of adolescent anabolic steroid exposure in hamsters. Behav Neurosci. 2005;119:1184–94. doi: 10.1037/0735-7044.119.5.1184. [DOI] [PubMed] [Google Scholar]
- Guillon CD, Koppel GA, Brownstein MJ, Chaney MO, Ferris CF, Lu S-F, Fabio KM, Miller MJ, Heindel ND, Hunden DC, Cooper RDG, Kaldor SW, Skelton JJ, Dressman BA, Clay MP, Steinberg MI, Bruns RF, Simon NG. Azetidinones as vasopressin V1a antagonists. Bioorganic Med Chem. 2006;15:2054–2080. doi: 10.1016/j.bmc.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Domes G, Inga DN, Rainer L. Progress in Brain Research. Vol. 170. Elsevier; 2008. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans; pp. 337–350. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–8. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Ingram JL, Peckham SM, Tisdale B, Rodier PM. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol. 2000;22:319–24. doi: 10.1016/s0892-0362(99)00083-5. [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, Uzefovsky F, Reibold M, Bachner-Melman R, Granot R, Bornstein G, Knafo A, Yirmiya N, Ebstein RP. Molecular genetic studies of the arginine vasopressin 1a receptor (AVPR1a) and the oxytocin receptor (OXTR) in human behaviour: from autism to altruism with some notes in between. Prog Brain Res. 2008;170:435–49. doi: 10.1016/S0079-6123(08)00434-2. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Chapman C, Baldwin BA. Microdialysis measurement of oxytocin, aspartate, gamma-aminobutyric acid and glutamate release from the olfactory bulb of the sheep during vaginocervical stimulation. Brain Res. 1988a;442:171–4. doi: 10.1016/0006-8993(88)91447-3. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Chapman C, Baldwin BA. Intracranial dialysis measurement of oxytocin, monoamine and uric acid release from the olfactory bulb and substantia nigra of sheep during parturition, suckling, separation from lambs and eating. Brain Res. 1988b;439:1–10. doi: 10.1016/0006-8993(88)91455-2. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchesne R, Courchesne E, Lord C, Leventhal BL, Cook EH, Jr, Insel TR. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry. 2002;7:503–7. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148:154–60. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–30. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–66. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Knox D, Brothers H, Norman GJ, Berntson GG. Nucleus basalis magnocellularis and substantia innominata corticopetal cholinergic lesions attenuate freezing induced by predator odor. Behav Neurosci. 2008;122:601–10. doi: 10.1037/0735-7044.122.3.601. [DOI] [PubMed] [Google Scholar]
- Kolunie JM, Stern JM, Barfield RJ. Maternal aggression in rats: effects of visual or auditory deprivation of the mother and dyadic pattern of ultrasonic vocalizations. Behav Neural Biol. 1994;62:41–9. doi: 10.1016/s0163-1047(05)80057-3. [DOI] [PubMed] [Google Scholar]
- Kolunie JM, Stern JM. Maternal Aggression in Rats: Effects of Olfactory Bulbectomy, ZnSO4-Induced Anosmia, and Vomeronasal Organ Removal. Hormones and Behavior. 1995;29:492–518. doi: 10.1006/hbeh.1995.1285. [DOI] [PubMed] [Google Scholar]
- Larrazolo-Lopez A, Kendrick KM, Aburto-Arciniega M, Arriaga-Avila V, Morimoto S, Frias M, Guevara-Guzman R. Vaginocervical stimulation enhances social recognition memory in rats via oxytocin release in the olfactory bulb. Neuroscience. 2008;152:585–93. doi: 10.1016/j.neuroscience.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Levy F, Kendrick KM, Goode JA, Guevara-Guzman R, Keverne EB. Oxytocin and vasopressin release in the olfactory bulb of parturient ewes: changes with maternal experience and effects on acetylcholine, gamma-aminobutyric acid, glutamate and noradrenaline release. Brain Res. 1995;669:197–206. doi: 10.1016/0006-8993(94)01236-b. [DOI] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) The Journal of Comparative Neurology. 2004;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–71. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proc Natl Acad Sci U S A. 2006;103:1959–63. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin EL, Ibrahim DY, Tu JW, McGaugh JL. Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: interaction with the basolateral amygdala. Neurobiol Learn Mem. 2007;87:295–302. doi: 10.1016/j.nlm.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann PE, Babb JA. Disinhibition of maternal behavior following neurotoxic lesions of the hypothalamus in primigravid rats. Brain Research. 2004;1025:51–58. doi: 10.1016/j.brainres.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology. 2008;33:901–12. doi: 10.1038/sj.npp.1301453. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Narita N, Narita M. Maternal administration of thalidomide or valproic acid causes abnormal serotonergic neurons in the offspring: implication for pathogenesis of autism. Int J Dev Neurosci. 2005;23:287–97. doi: 10.1016/j.ijdevneu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Redmond AJ, Roth RH, Elsworth JD. The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat. Brain Res. 2000;864:146–51. doi: 10.1016/s0006-8993(00)02174-0. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Wigger A, Frank E, Singewald N, Bunck M, Holsboer F, Landgraf R, Spengler D. Impaired repression at a vasopressin promoter polymorphism underlies overexpression of vasopressin in a rat model of trait anxiety. J Neurosci. 2004;24:7762–70. doi: 10.1523/JNEUROSCI.1614-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EK, Varnum MM, Fernandez JL, de Vries GJ, Forger NG. Effects of neonatal treatment with valproic acid on vasopressin immunoreactivity and olfactory behaviour in mice. J Neuroendocrinol. 2011;23:906–14. doi: 10.1111/j.1365-2826.2011.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiology & Behavior. 2008;95:182–186. doi: 10.1016/j.physbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Caffrey MK, Felix-Ortiz AC, Ferris CF, Febo M. Blood oxygen level-dependent signal responses in corticolimbic ‘emotions’ circuitry of lactating rats facing intruder threat to pups. Eur J Neurosci. 2009;30:934–45. doi: 10.1111/j.1460-9568.2009.06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993a;58:637–45. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993b;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Neumann I, Koehler E, Landgraf R, Summy-Long J. An oxytocin receptor antagonist infused into the supraoptic nucleus attenuates intranuclear and peripheral release of oxytocin during suckling in conscious rats. Endocrinology. 1994;134:141–8. doi: 10.1210/endo.134.1.8275928. [DOI] [PubMed] [Google Scholar]
- Nightingale S. From the analyst’s couch: autism spectrum disorders. Nature Rev Drug Discovery. 2012;11:745–46. doi: 10.1038/nrd3771. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ostrowski NL, Lolait SJ, Young WS., 3rd Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994;135:1511–28. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- Pedersen C, Caldwell J, Walker C, Ayers G, Mason G. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behavioral Neuroscience. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin antagonism alters rat dams’ oral grooming and upright posturing over pups. Physiol Behav. 2003;80:233–41. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Quignon P, Giraud M, Rimbault M, Lavigne P, Tacher S, Morin E, Retout E, Valin AS, Lindblad-Toh K, Nicolas J, Galibert F. The dog and rat olfactory receptor repertoires. Genome Biol. 2005;6:R83. doi: 10.1186/gb-2005-6-10-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MD, Pira AS, Febo M. Behavioral effects of acclimatization to restraint protocol used for awake animal imaging. J Neurosci Methods. 2013;217:63–6. doi: 10.1016/j.jneumeth.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MD, Price KE, Archbold J, Moffa A, Febo M. Predator odor-evoked BOLD activation in the awake rat: modulation by oxytocin and V(1)a vasopressin receptor antagonists. Brain Res. 2013;1494:70–83. doi: 10.1016/j.brainres.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci LA, Rasakham K, Grimes JM, Melloni RH., Jr Serotonin-1A receptor activity and expression modulate adolescent anabolic/androgenic steroid-induced aggression in hamsters. Pharmacol, Biochem Behav. 2006;85:1–11. doi: 10.1016/j.pbb.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Rinaldi T, Silberberg G, Markram H. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb Cortex. 2008;18:763–70. doi: 10.1093/cercor/bhm117. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370:247–61. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Adamec RE, Thompson BL. Expression of egr-1 (zif268) mRNA in select fear-related brain regions following exposure to a predator. Behav Brain Res. 2005;162:279–88. doi: 10.1016/j.bbr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andrade G, Kendrick KM. The main olfactory system and social learning in mammals. Behav Brain Res. 2009;200:323–35. doi: 10.1016/j.bbr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Schneider T, Labuz D, Przewlocki R. Nociceptive changes in rats after prenatal exposure to valproic acid. Pol J Pharmacol. 2001;53:531–4. [PubMed] [Google Scholar]
- Schneider T, Przewlocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30:80–9. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–77. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Simon NG, Cologer-Clifford A, Lu SF, McKenna SE, Hu S. Testosterone and its metabolites modulate 5HT1A abd 5HT1B agonist effects on internale aggression. Neurosci Biobehav Rev. 1998;23:325–36. doi: 10.1016/s0149-7634(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008;151:937–47. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Stodgell CJ, Ingram JL, O’Bara M, Tisdale BK, Nau H, Rodier PM. Induction of the homeotic gene Hoxa1 through valproic acid’s teratogenic mechanism of action. Neurotoxicol Teratol. 2006;28:617–24. doi: 10.1016/j.ntt.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Szot P, Bale TL, Dorsa DM. Distribution of messenger RNA for the vasopressin V1a receptor in the CNS of male and female rats. Brain Res Mol Brain Res. 1994;24:1–10. doi: 10.1016/0169-328x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Dreifuss JJ, Jard S. Autoradiographic localization of vasopressin and oxytocin binding sites in rat kidney. Kidney Int. 1988a;33:959–65. doi: 10.1038/ki.1988.94. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Jard S, Dubios-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Research. 1988b;442:105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Jard S, Dubois-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988c;442:105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139:5015–33. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FW, van Heerikhuize J, van der Meulen G, Wolters P. Light microscopic autoradiographic localization of [3H]oxytocin binding sites in the rat brain, pituitary and mammary gland. Brain Res. 1985;359:320–5. doi: 10.1016/0006-8993(85)91443-x. [DOI] [PubMed] [Google Scholar]
- Vawter MP, De Wied D, Van Ree JM. Vasopressin fragment (4–8), improves long-term and short-term memory in the hole board search task. Neuropeptides. 1997;31:489–94. doi: 10.1016/s0143-4179(97)90044-5. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383:305–25. [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–43. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci U S A. 2007;104:16681–4. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. J Neurosci. 2001;21:3619–27. doi: 10.1523/JNEUROSCI.21-10-03619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997a;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Wood S, Shanks N, Perks P, Conde GL, da Costa APC, Ingram CD, Lightman SL. Endocrine and Behavioural Responses to Noise Stress:Comparison of Virgin and Lactating Female Ratsduring Non-Disrupted Maternal Activity. Journal of Neuroendocrinology. 1997b;9:407–414. doi: 10.1046/j.1365-2826.1997.00587.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Stewart GR, Finger S, Olney JW. Comparison of behavioral effects of nucleus basalis magnocellularis lesions and somatosensory cortex ablation in the rat. Neuroscience. 1989;32:685–700. doi: 10.1016/0306-4522(89)90290-x. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Kiyama H, Kimura T, Araki T, Maeno H, Tanizawa O, Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993;133:1239–46. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]
- Young LJ. Oxytocin and vasopressin receptors and species typical social behaviors. Horm and Behav. 1999;36:212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J Neuroendocrinol. 1999;11:291–7. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]
- Yu GZ, Kaba H, Okutani F, Takahashi S, Higuchi T. The olfactory bulb: a critical site of action for oxytocin in the induction of maternal behaviour in the rat. Neuroscience. 1996;72:1083–8. doi: 10.1016/0306-4522(95)00600-1. [DOI] [PubMed] [Google Scholar]