Abstract
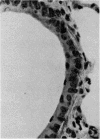
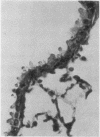
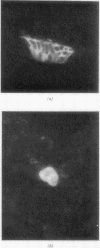
A comparative study of the Falck-Hillarp Technique, a modification of Eaton-Fedde procedure and silver staining of aldehyde-fixed tissue was carried out to determine the most efficient procedure to demonstrate neuroendocrine cells of the hamster and rat lung. The modified Eaton-Fedde procedure is the most efficient method of observing these cells, and is also the easiest to perform. With this method, the normal hamster lung contains a total of 2.00 x 10(-1) to 3.00 x 10(-1) neuroendocrine cells/mm in the small and large bronchioles. In the larger airways approximately 3.51 x 10(-1) neuroepithelial bodies (NEB)/mm are observed. Immediately after 24-hour exposure to NO2 the number of APUD cells dropped to approximately 25% of the control levels. These cells were decreased to 50% of the control levels throughout the 28 days of exposure. The number of NEB decreased transiently after 24 hours of NO2 but returned to normal numbers by day 14. We recommend the application of fluorescence techniques coupled with standardised sections and quantitative methods of study for analysis of APUD cells and NEB.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CORRODI H., HILLARP N. A., JONSSON G. FLUORESCENCE METHODS FOR THE HISTOCHEMICAL DEMONSTRATION OF MONOAMINES. 3. SODIUM BOROHYDRIDE REDUCTION OF THE FLUORESCENT COMPOUNDS AS A SPECIFICITY TEST. J Histochem Cytochem. 1964 Aug;12:582–586. doi: 10.1177/12.8.582. [DOI] [PubMed] [Google Scholar]
- Cutz E., Chan W., Wong V., Conen P. E. Ultrastructure and fluorescence histochemistry of endocrine (APUD-type) cells in tracheal mucosa of human and various animal species. Cell Tissue Res. 1975 May 20;158(4):425–437. doi: 10.1007/BF00220210. [DOI] [PubMed] [Google Scholar]
- Eaton J. A., Jr, Fedde M. R. Identification of two populations of biogenic amine-containg cells in the mouse lung. Cell Tissue Res. 1977 Jan 12;176(2):243–249. doi: 10.1007/BF00229465. [DOI] [PubMed] [Google Scholar]
- FEYRTER F. Zur Pathologie des argyrophilen Helle-Zellen-Organes im Bronchialbaum des Menschen. Virchows Arch Pathol Anat Physiol Klin Med. 1954;325(6):723–732. doi: 10.1007/BF00955103. [DOI] [PubMed] [Google Scholar]
- Fernandez Pascual J. S. A new method for easy demonstration of argyrophil cells. Stain Technol. 1976 Jul;51(4):231–235. doi: 10.3109/10520297609116708. [DOI] [PubMed] [Google Scholar]
- Grimelius L. The argyrophil reaction in islet cells of adult human pancreas studies with a new silver nitrate procedure. Acta Soc Med Ups. 1968;73(5-6):271–294. [PubMed] [Google Scholar]
- HAMBERGER B., MALMFORS T., SACHS C. STANDARDIZATION OF PARAFORMALDEHYDE AND OF CERTAIN PROCEDURES FOR THE HISTOCHEMICAL DEMONSTRATION OF CATECHOLAMINES. J Histochem Cytochem. 1965 Feb;13:147–147. doi: 10.1177/13.2.147. [DOI] [PubMed] [Google Scholar]
- Hage E. Histochemistry and fine structure of endocrine cells in foetal lungs of the rabbit, mouse and guinea-pig. Cell Tissue Res. 1974 Jun 24;149(4):513–524. doi: 10.1007/BF00223029. [DOI] [PubMed] [Google Scholar]
- Hernandez-Vasquez A., Will J. A., Quay W. B. Quantitative characteristics of the Feyrter (APUD) cells of the neonatal rabbit lung in normoxia and chronic hypoxia. Thorax. 1977 Aug;32(4):449–456. doi: 10.1136/thx.32.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkanson R., Lilja B., Owman C. Cellular localization of histamine and monoamines in the gastric mucosa of man. Histochemie. 1969 Apr 17;18(1):74–86. doi: 10.1007/BF00309904. [DOI] [PubMed] [Google Scholar]
- Kleinerman J., Rynbrandt D. Lung proteolytic activity and serum protease inhibition after NO2 exposure. Arch Environ Health. 1976 Jan-Feb;31(1):37–41. doi: 10.1080/00039896.1976.10667187. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Cokelaere M., Deleersynder M., Liebens M. Intrapulmonary neuro-epithelial bodies in newborn rabbits. Influence of hypoxia, hyperoxia, hypercapnia, nicotine, reserpine, L-DOPA and 5-HTP. Cell Tissue Res. 1977 Sep 5;182(4):425–440. doi: 10.1007/BF00219827. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Goddeeris P. Neuroepithelial bodies in the human child and adult lung. Am Rev Respir Dis. 1975 Apr;111(4):469–476. doi: 10.1164/arrd.1975.111.4.469. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Peuskens J. C. Argyrophil (kinin and amine producing?) cells in human infant airway epithelium. Life Sci. 1969 Jun 1;8(11):577–585. doi: 10.1016/0024-3205(69)90019-8. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Peuskens J. C., Cokelaere M. Argyrophil, fluorescent and granulated (peptide and amine producing?) AFG cells in human infant bronchial epithelium. Light and electron microscopic studies. Life Sci I. 1970 Dec 15;9(24):1417–1429. doi: 10.1016/0024-3205(70)90136-0. [DOI] [PubMed] [Google Scholar]
- Moosavi H., Smith P., Heath D. The Feyrter cell in hypoxia. Thorax. 1973 Nov;28(6):729–741. doi: 10.1136/thx.28.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse A. G. Common cytochemical properties of cells producing polypeptide hormones, with particular reference to calcitonin and the thyroid C cells. Vet Rec. 1966 Nov 19;79(21):587–590. doi: 10.1136/vr.79.21.587. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M. Cytochemical evidence for the neural crest origin of mammalian ultimobranchial C cells. Histochemie. 1971;27(2):96–102. doi: 10.1007/BF00284951. [DOI] [PubMed] [Google Scholar]
- SEVIER A. C., MUNGER B. L. TECHNICAL NOTE: A SILVER METHOD FOR PARAFFIN SECTIONS OF NEURAL TISSUE. J Neuropathol Exp Neurol. 1965 Jan;24:130–135. doi: 10.1097/00005072-196501000-00012. [DOI] [PubMed] [Google Scholar]
- Tateishi R. Distribution of argyrophil cells in adult human lungs. Arch Pathol. 1973 Sep;96(3):198–202. [PubMed] [Google Scholar]
- Taylor W. Pulmonary argyrophil cells at high altitude. J Pathol. 1977 Jul;122(3):137–144. doi: 10.1002/path.1711220304. [DOI] [PubMed] [Google Scholar]
- Terzakis J. A., Sommers S. C., Andersson B. Neurosecretory appearing cells of human segmental bronchi. Lab Invest. 1972 Jan;26(1):127–132. [PubMed] [Google Scholar]