Abstract
The genetic code, initially thought to be universal and immutable, is now known to contain many variations, including biased codon usage, codon reassignment, ambiguous decoding and recoding. As a result of recent advances in the areas of genome sequencing, biochemistry, bioinformatics and structural biology, our understanding of genetic code flexibility has advanced substantially in the past decade. In this Review, we highlight the prevalence, evolution and mechanistic basis of genetic code variations in microorganisms, and we discuss how this flexibility of the genetic code affects microbial physiology.
Pioneering work with Escherichia coli in the 1960s1,2 ‘cracked’ the genetic code, which constitutes a cornerstone of molecular biology. The genetic code is established through accurate ligation of each amino acid to its correct (cognate) tRNA by a specific aminoacyl-tRNA synthetase (aaRS). The resulting aminoacyl-tRNA (aa-tRNA) products of the aaRSs read codons by codon–anticodon pairing between mRNA and tRNA on the ribosome, allowing precise translation of the genetic information from mRNA to protein (FIG. 1).
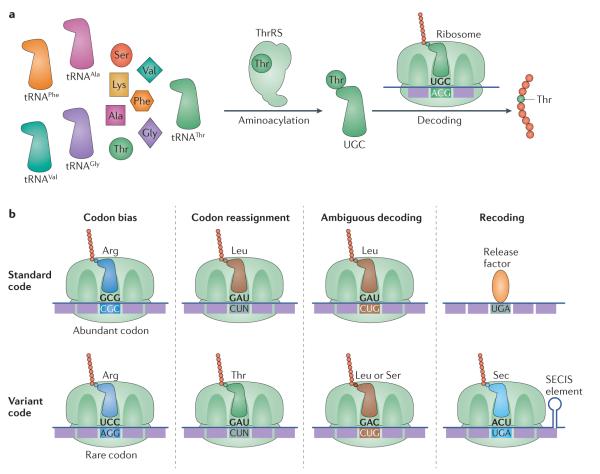
Figure 1. Mechanisms of genetic code flexibility.
a ∣ Each amino acid is attached to the corresponding tRNA by a specialized aminoacyl-tRNA synthetase (aaRS), in a reaction called aminoacylation. For example, threonyl-tRNA synthetase (ThrRS) selects Thr out of the amino acid pool and ligates it on to the 3′ end of tRNAThr. The resulting aminoacyl-tRNAs are then delivered to the ribosome by initiation or elongation factors to decode the matching codon. b ∣ There are multiple mechanisms of genetic code flexibility. Codon bias refers to selective usage of synonymous codons to encode the same amino acid. The frequency of codons in a given organism typically matches the cellular abundance of the corresponding tRNA. Codon reassignment requires evolution of a new tRNA to decode sense codons with a new amino acid, or a new tRNA that can decode stop codons with an amino acid. Ambiguous decoding refers to simultaneous decoding of the same codon by two or more amino acids in one cellular compartment; this could be caused by recognition of the same tRNA by more than one aaRS, by misacylation of a tRNA or by ribosomal decoding errors. Recoding traditionally refers to partial codon reassignment that is context dependent. For instance, in certain bacteria and eukaryotes, a subset of UGA stop codons with a nearby selenocysteine (Sec) insertion sequence (SECIS) element, in the presence of SelB, are recoded to Sec, whereas other UGA stop codons retain their ability to signal translational termination.
Originally, it was thought that all living organisms used a universal set of codons, with 61 of the possible 64 nucleotide triplets translating 20 amino acids (termed sense codons) and the 3 remaining codons (UAA, UAG and UGA) being responsible for termination of protein synthesis (termed stop codons). However, it was later discovered that the mitochondrial genetic code of yeast deviates from the standard code, with CUN codons assigned to Thr instead of Leu, and UGA used to encode Trp3,4. Later sequencing, bioinformatics and biochemical studies of the protein synthesis machinery in microorganisms provided further insights into genetic code variations5 and revealed deviations from the standard genetic code in various microorganisms, including bacteria, archaea, fungi and viruses (see Supplementary information S1 (table)). These genetic code variants retain many features of the standard code, but the known exceptions are diverse, and they have evolved through distinct biochemical mechanisms (see Supplementary information S1 (table)). In this Review, we have broadly categorized these mechanisms into four types: biased codon usage, codon reassignment, ambiguous decoding and natural genetic code expansion (FIG. 1).
Codon degeneracy allows each amino acid to be decoded by more than one codon. These synonymous codons can be recognized by different tRNA isoacceptors. Some synonymous codons are preferentially used over others at higher frequencies, leading to biased codon usage, which is found in almost all sequenced genomes. Optimal codon usage typically correlates with high protein synthesis rates, particularly for highly transcribed genes6. Below, we provide updated views of how codon usage affects translation and the resulting biological consequences that are only beginning to be understood7.
Codon reassignment completely changes the meaning of a codon throughout the transcriptome. The most common example of codon reassignment occurs in microorganisms in which stop codons were reassigned to encode amino acids, but sense codons have also been reassigned. Codon reassignment events have been identified in viruses and in a wide range of microorganisms, including microbial eukaryotes and their mitochondria. Below, we focus on recently characterized codon reassignment mechanisms in yeast and bacterial systems.
Ambiguous decoding (also known as mistranslation) refers to simultaneous decoding of the same codon by two or more amino acids in one cellular compartment. Ambiguous decoding can be caused by recognition of the same tRNA by more than one aaRS or by misacylation of a tRNA with a non-cognate amino acid, which both result in different amino acids being loaded onto tRNAs recognizing the same codon. It can also be caused by ribosomal decoding errors, in which tRNAs are mismatched to their assigned codons. Because ambiguous decoding can lead to errors in protein synthesis, it is usually regarded as deleterious, but increasing evidence suggests that this is a widespread mechanism in nature and may increase microbial fitness under certain conditions, such as during stress8. Recent advances uncovering the causes and physiological impact of ambiguous decoding are discussed below.
Natural genetic code expansion refers to genetic codes that enable protein synthesis with more than the 20 canonical amino acids. The two known naturally evolved examples include selenocysteine (Sec) and pyrrolysine (Pyl). Whereas codon reassignment occurs at a genome-wide level, recoding refers to site-specific codon re-definition that is dependent on the mRNA sequence context9. One of the earliest recognized deviations of the standard genetic code involves recoding by frameshifting10, which changes the readout of mRNAs (for an extensive review, see REF. 9). Below, we discuss the mechanisms and physiological consequences of reassigning the stop codon UAG to Pyl and recoding the UGA codon to Sec in archaea and bacteria.
In this Review, we provide an update on recent studies of genetic code flexibility in microorganisms, with a focus on novel insights into the mechanisms of biased codon usage, codon reassignment, ambiguous decoding and recoding, and we discuss how these evolutionary events affect cellular fitness.
Biased codon usage
The most widespread mechanism of genetic code flexibility is the biased usage of synonymous codons, which is a universal feature of all microbial genomes (FIG. 2). Biased codon usage was first observed in a comparison of the degenerate codons of sequenced mRNAs, revealing that each gene in a genome exhibited similar preference for certain synonymous codons over others11. For example, in E. coli, Arg codons CGC and CGU occur over ten times more frequently than AGA and AGG. Later studies show that codon bias is present in almost all sequenced genomes6. Both mutation (neutral) and selection hypotheses have been proposed to explain codon usage bias (these hypotheses are reviewed in REFS 6,12). According to the mutation hypothesis, codon bias results from mutational pressure that may vary in different organisms, leading to different codon usage across various genomes. By contrast, the selection hypothesis proposes that codon bias provides a selective advantage by optimizing the level and accuracy of protein expression. A combination of both mutation and selection hypotheses has led to a more widely accepted mutation–selection–drift model13, which posits that major (abundant) codons that are frequently used are preferred during selection, whereas mutational pressure keeps the minor (rare) codons in the genome.
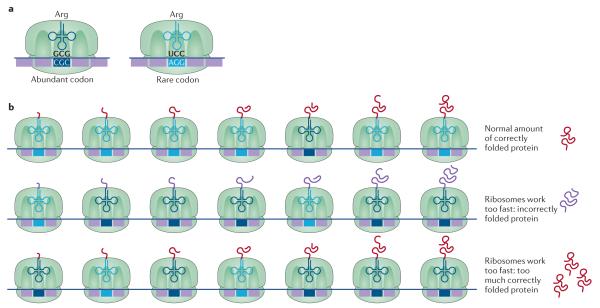
Figure 2. Biased codon usage.
a ∣ Biased codon usage occurs when synonymous codons are decoded by different tRNA isoacceptors. As some synonymous codons are used at higher frequencies than others, this leads to biased codon usage. b ∣ Although the optimized usage of synonymous codons increases the protein synthesis rate under most conditions, non-optimal codon usage can improve bacterial fitness under certain conditions. For example, codon optimization of the frq gene in Neurospora crassa results in a loss of fitness. frq controls the circadian clock function, and optimizing the codons of frq increases the expression of Frq but leads to defects in folding of this protein. These folding defects impair Frq function in the regulation of the circadian feedback loop (middle panel). Similarly, the kaiB and kaiC genes in the cold-adapted cyanobacterium Synechococcus elongatus are also enriched for non-optimal codons. The kaiB and kaiC genes are critical for regulation of circadian rhythms, and codon optimization increases the protein levels of KaiB and KaiC and prevents the circadian rhythm from switching off, resulting in loss of fitness by the cyanobacteria (bottom panel). Part b of the image adapted from REF. 144, Nature Publishing Group.
Biased codon usage has been shown to affect the expression levels of endogenous and heterologous proteins, and usage of rare codons is generally considered to slow down translation6. However, several recent studies have reshaped our view of ‘optimal’ codon usage and protein expression. Ribosome profiling, which measures ribosomal densities at all codons in the transcriptome, of E. coli and Bacillus subtilis grown in rich media showed that rare codons recognized by tRNAs with low cellular abundance are translated at the same speed as abundant codons (FIG. 2). Instead of stalling at rare codons, ribosomes stall most often at Shine–Dalgarno-like sequences that may pair with the anti-Shine–Dalgarno sequence of the 16S ribosomal RNA14. Another study used a yellow fluorescent protein in E. coli to show that the protein synthesis rate is not affected by synonymous changes in abundant or rare codons in bacteria growing in amino acid-rich media15. High-throughput translation assays showed that the presence of rare codons located near the 5′ end of an open reading frame surprisingly increase protein expression about fourfold in E. coli16; this is primarily due to a decrease in the number of mRNA secondary structures, which facilitates the travelling of the ribosome along the mRNA.
Under amino acid starvation conditions, however, synonymous codons are indeed translated at distinct rates owing to the different aminoacylation levels of the corresponding tRNA isoacceptors15 (FIG. 2). It seems that the abundance of aa-tRNA, rather than the total level of tRNA (either charged or uncharged with amino acids), correlates better with the decoding rates in vivo. Under nutrient-rich conditions, the supply of aa-tRNAs meets the demand of protein synthesis, and all synonymous codons appear to be translated at similar speeds. By contrast, during amino acid starvation, tRNA isoacceptors are charged at various levels, leading to distinct translation rates of synonymous codons. Similarly, other conditions that perturb the relative abundances of charged tRNA isoacceptors, such as alterations in the tRNA expression level and aminoacylation efficiency, may also affect the translation rates of synonymous codons. In addition, non-optimal codons in over-expressed genes could sequester rare tRNAs, therefore reducing the charging levels of such tRNAs to decrease total protein expression. Given that microorganisms in the natural environment are not often supplied with sufficient nutrients, maintaining optimal codons with biased usage of synonymous codons probably has an important role in maximizing the overall translational speed and growth.
Although optimized usage of synonymous codons increases the protein synthesis rate under certain conditions, non-optimal codons used at specific locations sometimes lead to increased bacterial fitness. For example, in B. subtilis, non-optimal Ser codons are preferentially used in the gene encoding SinR, which inhibits biofilm formation17. Experimental substitution of non-optimal Ser codons with optimal Ser codons increases the synthesis rate of SinR, which leads to increased protein levels of SinR and suppresses biofilm development. Under physiological conditions, depletion of cellular Ser levels during stationary phase reduces translation of the native SinR to trigger biofilm synthesis. Therefore, the apparent non-optimal Ser codons in SinR serve as a molecular sensor for the cellular levels of Ser, and this strategy enables B. subtilis to control the expression of biofilm-associated genes in response to environmental conditions.
In the cold-adapted cyanobacterium Synechococcus elongatus, the kaiB and kaiC genes are critical for the regulation of circadian rhythms, and these genes are usually highly expressed18. The codon adaptation index of kaiB and kaiC is below average, which means that kaiB and kaiC are enriched in non-optimal codons. Notably, experimental codon optimization of kaiB and kaiC increases the protein levels of KaiB and KaiC at low temperature, which prevents the circadian rhythm from switching off, resulting in loss of fitness. Therefore, these data demonstrate that S. elongatus uses non-optimal codons as a mechanism to regulate bacterial fitness (FIG. 2). Similarly, in Neurospora crassa, the frq gene, which controls the circadian clock function, also exhibits non-optimal codon usage19. Optimization of the codons of frq increases the protein levels of Frq but also leads to protein folding defects and impairs its function in the circadian feedback loop. This suggests that the non-optimal codons serve as checkpoints to slow down translation of certain regions in Frq, allowing proper folding of the protein (FIG. 2). In these examples, non-optimal codons actually provide microorganisms with selective advantages for adaptation to environmental changes.
Codon reassignment
Codon reassignment events include stop-to-sense, sense-to-stop and sense-to-sense codon changes, and occur through multiple mechanisms (see Supplementary information S1 (table)). Whereas sense-to-stop reassignments only require loss of the cognate tRNA, stop-to-sense and sense-to-sense changes require evolution of new tRNAs. Such new tRNAs may arise from modification of an existing tRNA or by tRNA duplication20. For example, reassignment of the UGA codon from stop to Trp is accompanied by either a mutation21 or post-transcriptional editing (C34→U34)22 that converts tRNATrpCCA to tRNATrpUCA, which reads both the UGA and UGG codons as Trp. In bacteria and archaea, both tRNAMetCAU and tRNAIleCAU contain the same CAU anticodon. Whereas tRNAMetCAU recognizes the AUG codon, tRNAIleCAU is modified at position C34 by addition of lysidine23 or agmatidine24,25. As a result, the modified tRNAIleCAU specifically recognizes AUA instead of AUG. In the mitochondria, tRNAIleCAU is absent and tRNAMetCAU contains a formylcytidine (f5C) modification at C34 (REF. 26), which allows tRNAMetCAU to recognize A at the third position (wobble position). A recent structural study confirmed that f5C of tRNAMetCAU pairs with both A and G at the wobble position, enabling it to read both AUA and AUG codons as Met27. Consequently, AUA codons are reassigned from Ile to Met in the mitochondria.
A more complicated scenario involves tRNA evolution from duplication. For example, CUN codons are reassigned from Leu to Thr in Saccharomyces cerevisiae mitochondria owing to the loss of tRNALeuUAG and the presence of tRNAThrUAG, which has an enlarged eight-nucleotide (as opposed to seven-nucleotide) anticodon loop3. An unmodified U at the wobble position in mitochondrial tRNAs allows pairing with all four nucleotides28. Interestingly, phylogenetic analyses reveal that tRNAThrUAG is not related to either the ancestral tRNALeuUAG or the isoacceptor tRNAThrUGU but shows high similarity to the mitochondrial tRNAHisGUG (REF. 29). Further biochemical studies verify that two nucleotide changes are sufficient to switch the tRNAHisGUG from a His-accepting to Thr-accepting molecule29. These results suggest that the tRNAThrUAG is evolutionarily derived from a precursor tRNAHisGUG. In support of this model, a duplicated copy of tRNAHisGUG is found in the mitochondrial genome of Candida albicans and Kluyveromyces lactis, which are closely related to S. cerevisiae (FIG. 3).
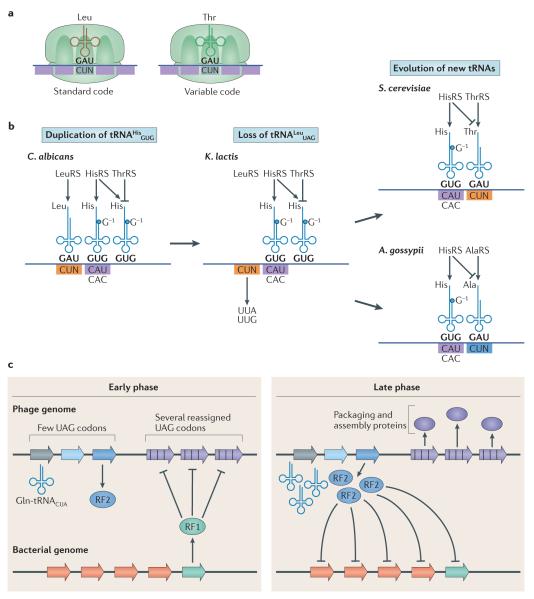
Figure 3. Codon reassignment.
a ∣ CUN codons are read as Leu in the standard genetic code through the use of tRNALeuUAG. However, in some microorganisms, CUN codons have been reassigned to Thr. b ∣ CUN reassignment in mitochondria involved several steps. In the mitochondrial genome of some Saccharomycetaceae species (for example, Candida albicans), the tRNAHisGUG gene was duplicated. The CUN codons and a tRNALeuUAG that decodes CUN then disappeared, leading to a reduced genetic code, as in Kluyveromyces lactis. In Saccharomyces cerevisiae, one copy of tRNAHisGUG evolved to carry an anticodon UAG that reads CUN codons (tRNAHisUAG). The threonyl-tRNA synthetase (ThrRS) co-evolved with the tRNAHisUAG to recognize it as a tRNAThrUAG. CUN codons then reappeared to complete the codon reassignment from Leu to Thr. In Ashbya gossypii, secondary mutations enabled the new tRNAHisUAG to be recognized by AlaRS instead of HisRS or ThrRS, therefore generating tRNAAlaUAG and reassigning CUN codons to Ala in this species. c ∣ Codon reassignment can affect bacteria and phage physiology. In a phage from the human oral cavity, reassigning UAG codons to Gln may allow the phage to interfere with translation of host genes without affecting translation of the phage genes. In the early stages of infection, the phage early genes contain few in-frame UAG codons and are therefore efficiently translated by the host machinery. Among these genes, the phage expresses release factor 2 (RF2), which suppresses translation of UGA codons, including recoded ones. As the bacterial protein RF1, which suppresses translation of UAG codons, contains multiple in-frame UGA codons, expression of RF2 by the phage inhibits translation of host RF1. This inhibition allows the phage to translate late-stage phage genes, which are enriched in recoded UAG codons, while modifying the translation of host genes. Part b is adapted from Su, D., Lieberman, A., Lang, B. F., Simonovic, M., Söll, D. & Ling, J., An unusual tRNAThr derived from tRNAHis reassigns in yeast mitochondria the CUN codons to threonine, Nucleic Acids Res., 2011, 39, 11, 4866–4874, by permission of Oxford University Press. Part c is adapted from Ivanova, N. N. et al. Stop codon reassignments in the wild. Science 344, 909–913 (2014). Reprinted with permission from AAAS.
In another closely related species, Ashbya gossypii, a tRNAAlaUAG derived from either tRNAHisGUG or tRNAThrUAG reassigns mitochondrial CUA and CUU codons to Ala, and CUC and CUG codons are absent in mitochondrial genes30. This leaves UUG and UUA as the only Leu codons in the A. gossypii mitochondrial genome.
In addition to tRNA, aaRSs have evolved to enable codon reassignment, as highlighted by CUN reassignment in S. cerevisiae mitochondria. The S. cerevisiae threonyl-tRNA synthetase (ThrRS) recognizes both tRNAThrUAG and tRNAThrUGU (REF. 31), whereas E. coli and the mitochondrial ThrRSs from Schizosaccharomyces pombe and C. albicans do not recognize tRNAThrUAG, suggesting that the mitochondrial ThrRS from S. cerevisiae has co-evolved with tRNAThrUAG to allow for CUN recoding (FIG. 3).
Together, these data suggest that CUN reassignment in S. cerevisiae and in A. gossypii involved several steps. First, in the mitochondrial genome of some Saccharomycetaceae species (such as C. albicans), the tRNAHisGUG gene was duplicated. The CUN codons and a tRNALeuUAG that decodes CUN then disappeared, leading to a reduced genetic code that does not involve CUN, such as in K. lactis. Following this reduction, in S. cerevisiae, one copy of tRNAHisGUG evolved to carry a UAG anticodon that reads CUN codons (tRNAHisUAG). The ThrRS co-evolved with the tRNAHisUAG to recognize it as a tRNAThrUAG. CUN codons then reappeared to complete the codon reassignment from Leu to Thr. In A. gossypii, secondary mutations enabled the new tRNAHisUAG to be recognized by AlaRS instead of HisRS or ThrRS, thereby generating tRNAAlaUAG and reassigning CUN codons to Ala in this species (FIG. 3).
The driving forces and physiological consequences of codon reassignment are not completely understood. For example, reassignment of CUN codons in yeast mitochondria is considered to be neutral because the original CUN codons had been lost, new CUN codons appeared at non-conserved positions, and a new tRNA evolved, which apparently did not involve a period of ambiguous translation of mitochondrial proteins. However, a recent bioinformatic analysis of >5 trillion bp of metagenomic data revealed that stop codon reassignment is widespread in microorganisms and that differential reassignment between bacteria and phages could have physiological consequences32. For example, the authors found a phage from the human oral cavity that reassigned the UAG stop codon to Gln. Interestingly, the phage genes expressed at early stages contain few in-frame UAG codons and can therefore be efficiently translated by the host machinery. However, one of the phage proteins expressed during the early stages is release factor 2 (RF2), which suppresses translation of UGA codons. As the bacterial protein RF1, which suppresses translation of UAG codons, contains multiple in-frame UGA codons, expression of RF2 by the phage inhibits translation of host RF1. Inhibition of host RF1 allows the phage to translate late-stage phage genes, which are enriched in recoded UAG codons (FIG. 3). This intriguing model suggests that phages can use codon reassignment to interfere with translation of host genes without affecting translation of the phage genes, but this model needs to be experimentally validated in future studies.
Insight into the consequences of codon reassignment also comes from studies of microorganisms in which the genetic code has been re-engineered (BOX 1). Several groups have deleted RF1 in an attempt to reassign UAG to a sense codon in E. coli by subsequently introducing an orthogonal aaRS–tRNA33–38 pair to incorporate a non-canonical amino acid. Notably, when the native UAG codons are present, deleting RF1 and reassigning UAG as a sense codon causes severe growth defects34,35. Converting all endogenous UAG codons to UAA rescues the growth phenotype36, suggesting that the observed toxicity is due to extension of proteins beyond their intended stop. In the RF1 deletion mutant, UAG is also translated by natural amino acids (predominantly Gln, Tyr and Lys), which compete with the non-canonical amino acid insertions, resulting in impure proteins39. These studies indicate that radical codon reassignment may decrease fitness in some aspects, such as growth and proteome stability. Therefore, during the evolution of codon reassignment, the gained benefits may need to outweigh the potential negative effects. In one such example, phages growing in E. coli cells that reassign UAG from stop to a non-canonical amino acid (3-iodotyrosine) rapidly accumulate in-frame UAG codons from mutations; such speeded codon reassignment in the phage provides benefits to the phage by increasing its titre owing to the presence of the non-canonical amino acid in the proteome40. An expanded genetic code can thus increase the evolvability of codons. Future studies are needed to identify the phenotypic and proteomic changes in organisms in which the genetic code has been re-engineered and to clarify the underlying molecular mechanisms of how a cell adapts to genetic code expansion.
Box 1. Emerging biotechnology for engineering the genetic code.
The flexibility of the genetic code as illustrated by natural codon reassignments has prompted researchers to expand the genetic code with non-canonical amino acids108. Both site-specific109,110 and residue-specific111 approaches have been developed to co-translationally insert non-canonical amino acids into proteins of interest. The site-specific approach uses an orthogonal pair of aminoacyl-tRNA synthetase (aaRS)–tRNA to reassign a stop or quadruplet codon to a non-canonical amino acid. By contrast, the residue-specific approach allows ambiguous decoding of a sense codon by both the native amino acid and a non-canonical amino acid. Site-specific sense codon recoding is also being explored as a means to further expand the genetic code62. Genetic code expansion with non-canonical amino acids has been applied to site-specifically label proteins with fluorophores, study post-translational modifications and identify newly synthesized proteins109. Given the flexibility of the active site and orthogonality in bacteria and eukaryotes, tyrosyl-tRNA synthetase (TyrRS) and PylRS have been most widely used for site-specific insertion of non-canonical amino acids (reviewed in REFS 108,112). Recently, non-canonical amino acids have been used as safeguards to contain recoded bacteria113,114. In such synthetic microorganisms, stop codons are introduced into essential genes that require translation with a non-canonical amino acid. Therefore, the viability of the recoded bacteria depends on the presence of the non-canonical amino acid, preventing them from proliferating in natural ecosystems. As an additional safety layer, stop-codon-interrupted genes should be less susceptible to horizontal gene transfer (HGT), thus helping to prevent transmission of genes from synthetic organisms to natural genomes. A similar mechanism may also occur in nature. The bacterium SR1 encodes Gly with five codons, including the UGA ‘stop’ codon. This genetic code variation was hypothesized to bias HGT, because 85% of SR1’s genes contain UGA sense codons, which would be interpreted as stop codons by recipient microorganisms115.
Recent advances in genome editing and DNA synthesis provide powerful tools to engineer the genetic code and understand the evolution of codon reassignments. The goal of these efforts is to remove codons from the genome so that they can be reassigned to new and non-canonical amino acids. Multiplex automated genome engineering (MAGE)116 and conjugative assembly genome engineering (CAGE)117 approaches were developed to allow simultaneous and genome-wide editing of the Escherichia coli genome. MAGE uses synthetic single-stranded DNA fragments to simultaneously target multiple chromosomal sites and introduce precise genomic changes by recombination-based genetic engineering, whereas CAGE uses a conjugation strategy to move large regions of the chromosome between different strains. A combination of MAGE and CAGE has allowed complete reassignment of UAG to UAA in an E. coli strain36. Another potent genome-editing tool is based on the CRISPR system, which permits convenient genome editing in bacterial and eukaryotic cells118,119. Complete de novo synthesis of a Mycoplasma genome120 and a yeast chromosome121 have also been achieved. Together, these technological advances set the stage for synthetic organisms (with potentially radically different genetic codes) that will provide novel opportunities for biotechnology and new insights into the evolution of the genetic code.
Ambiguous decoding
In contrast to biased codon usage in which a set of codons are translated by the same amino acid, a specific codon can also be ambiguously decoded by more than one amino acid41. Ambiguous decoding can result from multiple aaRSs recognizing the same tRNA, which leads to different amino acids being loaded onto tRNAs recognizing the same codon; from errors in the aminoacylation reaction carried out by a specific aaRS, which loads a tRNA with a non-cognate amino acid; or from ribosomal decoding errors (FIG. 4). Ambiguous decoding leads to a statistical pool of protein products with amino acid substitutions at various positions (statistical proteome). High levels of ambiguous translation lead to accumulation of misfolded proteins that are toxic to cells.
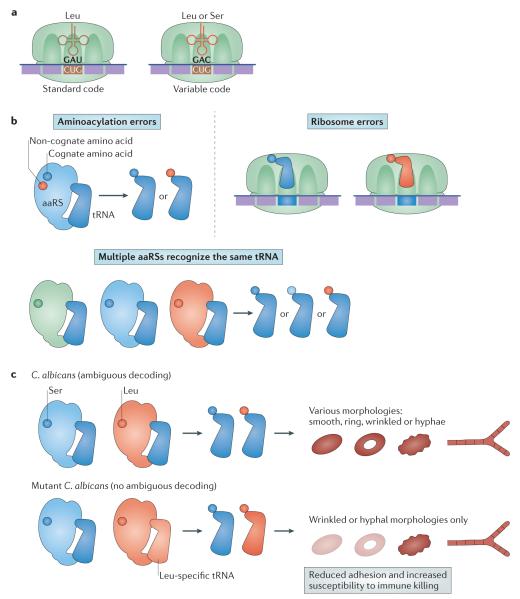
Figure 4. Ambiguous decoding.
a ∣ Ambiguous decoding refers to the process by which the same codon gives rise to incorporation of different amino acids in a nascent polypeptide chain. b ∣ Ambiguous decoding can result from errors in the aminoacylation reaction carried out by a specific aminoacyl-tRNA synthetase (aaRS), which loads a tRNA with a non-cognate amino acid; by multiple aaRSs recognizing the same tRNA, which leads to different amino acids being loaded onto tRNAs recognizing the same codon; or by ribosomal decoding errors. c ∣ In Candida albicans, altering the ratio of Ser to Leu incorporation at CUG codons introduces diverse cell and colony morphologies. Wild-type C. albicans encodes a CUG-decoding tRNACAG that is recognized by both seryl- and leucyl-tRNA synthetases. This ability of ambiguous decoding enables wild-type cells to display different colony morphologies, including smooth, ring, wrinkled and hyphae. Eliminating the ambiguity by the substitution of the tRNACAG with a Leu-specific tRNA results in loss of the smooth and ring morphologies. These changes also reduce cell adhesion and increase fungal susceptibility to macrophage killing by immune cells.
Multiple quality control mechanisms are used to ensure fidelity during protein synthesis. For instance, some aaRSs use editing sites to hydrolyse mismatched aa-tRNAs42. Similarly, the ribosome selects the cognate aa-tRNA based on both preferential binding of the cognate aa-tRNA at the decoding centre and kinetic proofreading of non-cognate aa-tRNAs43,44. However, despite the existence of these complex mechanisms that maintain translational fidelity (reviewed in REFS 42,44), ambiguous decoding can still result from reduced fidelity during protein synthesis owing to either mutations44 or stress45. For instance, mutations in the ribosome allow mismatch between the mRNA codon and tRNA anticodon, and oxidative stress decreases fidelity during aminoacylation.
Increasing evidence suggests that ambiguous decoding may be used as an adaptive mechanism by microorganisms to survive harsh environmental conditions and gain a selective advantage during evolution. Some aaRSs use editing to ensure correct pairing between amino acids and tRNAs46, but the editing domains of ThrRS, LeuRS and PheRS are either lost or defunct in Mycoplasma spp. and in mitochondria from yeasts47–49, indicating that ambiguous translation occurs in these organisms and organelles. Indeed, analyses of mass spectrometry data reveal that the intracellular bacterium Mycoplasma mobile encodes a statistical proteome48, which was suggested to benefit this organism by increasing the proteomic and phenotypic diversity of the bacterium, enabling it to escape host defences48.
Direct experimental evidence of phenotypic diversity created by a statistical proteome comes from elegant studies of CUG codon ambiguity in C. albicans. Altering the ratio of Ser to Leu incorporation at CUG codons introduces diverse cell and colony morphologies, as well as distinct antifungal and immune responses50,51. Wild-type C. albicans encodes a CUG-decoding tRNACAG that is recognized by both SerRS and LeuRS (FIG. 1b). The wild-type cells display colony morphologies, including smooth, ring, wrinkled and hyphae50. Substituting the ambiguous tRNACAG with a Leu-specific tRNA abolishes CUG ambiguity as well as the smooth and ring morphologies50 (FIG. 4). Enhanced CUG ambiguity increases cell adhesion by ambiguous translation of adhesins and reduces susceptibility to macrophage killing by decreasing surface exposure of β-glucans51. It has also been shown that, in Mycobacterium smegmatis, the level of ambiguous decoding at Asn codons increases during stationary phase and under low pH conditions52. Ambiguous translation increases resistance against rifampicin by producing RpoB variant proteins that are no longer recognized by this antibiotic, therefore epigenetically enhancing the fitness of M. smegmatis52 (FIG. 4).
Ambiguous decoding not only increases the diversity of the proteome but may also activate stress responses. A recent study reveals that ambiguous translation caused by a mutation in the ribosome activates the bacterial general stress response and enhances tolerance to hydrogen peroxide53. In E. coli, oxidative stress induces ambiguous decoding at ACN codons, which are recognized by tRNAThr. ThrRS uses editing to hydrolyse misacylated Ser-tRNAThr, and the editing site of ThrRS contains an activated Cys that is hypersensitive to oxidation54. Oxidative stress thus impairs ThrRS editing and causes incorporation of both Thr and Ser into ACN codons (FIG. 4). The reactive Cys and activating residues at the ThrRS editing sites are conserved among bacteria, suggesting that oxidative-stress-induced ambiguous decoding is a highly conserved mechanism. Switching between accurate and ambiguous decoding may allow bacteria to adapt to different environmental conditions quickly, but this possibility requires additional testing in future studies.
Natural genetic code expansion
In 1966, Crick stated: “A more serious problem is whether in a normal cell a triplet can be read in more than one way.” (REF. 55). Natural genetic code expansion encompasses changes in the genetic code — which evolved in natural organisms — that enable protein synthesis with more than the 20 canonical amino acids. Sec and Pyl are the only known cases of natural genetic code expansion. The mechanisms of Sec and Pyl translation are distinct and rarely co-occur in the same organism (BOX 2).
Box 2. Natural genetic code expansion.
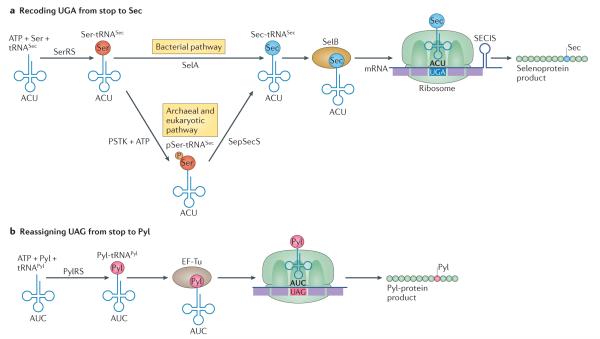
Selenocysteine (Sec) biosynthesis evolved twice independently122 (FIG. 5). This is not in itself a unique feature of Sec, as two independently evolved aminoacylation systems exist for Lys, Gln, Asn, Gly, Cys123 and possibly Ser124. The fact that Sec is exclusively biosynthesized on its tRNA, however, is unusual. Sec is also the only amino acid for which a specific aminoacyl-tRNA synthetase (aaRS) does not exist. Initially, the normal seryl-tRNA synthetase (SerRS) ligates Ser onto tRNASec, and the Ser-tRNASec product is converted to Sec-tRNASec (FIG. 5). This reaction is catalysed by a single pyridoxal-phosphate (PLP)-dependent enzyme in bacteria (SelA), which forms a stunning decameric ring structure that simultaneously binds to ten tRNASec molecules125. In archaea126 and eukaryotes127, a unique kinase (phosphoseryl(Sep)-tRNASec kinase (PSTK)) phosphorylates the tRNA-bound Ser to form phosphoseryl (Sep)-tRNASec, and a distant relative of SelA, a dimeric enzyme known as Sep-tRNASec:Sec-tRNASec synthetase (SepSecS), produces the Sec-tRNASec substrate required to insert Sec into proteins. SepSecS is dimeric, like most members of the PLP enzyme family, and binds to two tRNASec molecules128. Both SelA and SepSecS require the selenium donor selenophosphate, which is the product of selenophosphate synthase (SelD), to complete the Sec synthesis reaction.
Adding to the complexity of selenoprotein biosynthesis, a specialized elongation factor (SelB in Escherichia coli, EF-Sec in eukaryotes) and a RNA recoding signal (Sec insertion sequence (SECIS)) are also required to convert the meaning of UGA from stop to Sec. In archaea and bacteria, the SECIS is found in the reading frame of the selenoprotein transcript, within a few nucleotides of the recoded UGA codon. In eukaryotes, the SECIS (of which there are two structurally divergent types) is found in the 3′ untranslated region (UTR), and additional proteins are required for Sec insertion (including SECIS-binding protein 2 (REF. 129)) or for regulation of the recoding event (including ribosomal protein L30 (REF. 130) and initiation factor 4a3 (REF. 122)). Despite numerous biochemical and structural studies on Sec synthesis and recoding in bacteria61 and eukaryotes131, the structural basis of UGA recoding on the ribosome remains unresolved. A structural model of the bacterial system132 indicates that the SECIS–SelB–Sec-tRNASec complex may fit between the ‘body’ and ‘head’ regions of the 30S ribosomal subunit, which could alter the conformation of the decoding centre on the translating ribosome.
Following the discovery of Sec, pyrrolysine (Pyl) is the second known case of natural genetic code expansion. Genetic code expansion with Sec and Pyl arose by distinct evolutionary and biochemical mechanisms133. Unlike Pyl, which appears in proteins as either a catalytic84 or non-catalytic93 residue, Sec nearly always has essential roles in the active sites of redox enzymes28. Another feature that distinguishes Pyl from Sec is that incorporation of Sec requires a SECIS mRNA structure, whereas Pyl can effectively provide informational suppression134 of UAG codons without a co-evolved mRNA secondary structure97. The flexibility of tRNAPyl in UAG translation135, together with the natural orthogonality of the PylRS–tRNAPyl pair in bacterial and eukaryotic hosts, and the large amino acid-binding pocket of PylRS107,136, prompted multiple groups to engineer PylRS as a facile system for genetic incorporation of non-canonical amino acids in bacterial and mammalian cells109,112,137–141. The Sec system was recently shown to be a powerful mechanism for recoding sense codons62. We anticipate that both systems will continue to drive further genetic code engineering that is inspired by nature (BOX 1).
Fascinatingly, Sec and Pyl may coexist in some organisms. Two genome sequences, that of Acetohalobium arabaticum and one from metagenomic sequencing of a bacterial symbiont of the worm Olavius algarvensis142 show evidence of genetic codes with 22 amino acids. Indeed, two Gly reductase selenoprotein B (grdB) genes in A. arabaticum contain both a SECIS-recoded UGA codon for Sec incorporation as well as one or two UAG codons, which may be translated as Pyl in the presence of trimethylamine. A. arabaticum was shown to decode UAG as Pyl104 and because Sec in GrdB is required for Gly reductase activity143, this organism may produce the only known natural proteins that contain 22 genetically encoded amino acids.
Recoding UGA to Sec
Most UGA codons signal termination of protein synthesis. Certain organisms, including E. coli and humans, have a naturally expanded genetic code and recode some UGA codons to insert Sec into selenoproteins. Therefore, UGA takes on two meanings in the same cell and sometimes even in the same open reading frame56, illustrating the “more serious problem” foreshadowed by Crick55. These naturally expanded genetic codes specify 21 rather than the ‘usual’ 20 amino acids.
Sec is chemically similar to Cys, but the lower redox potential and higher nucleophilicity of Sec explain why some selenoenzymes are far more reactive than their Cys-containing counterparts57. For example, mammalian Met-R-sulfoxide reductases containing Sec in the active site are 100-fold more active than the Cys-containing variants58. Sec is also found to be more resistant to irreversible oxidation than Cys59 and has utility as a probe for protein structure and function60.
Sec biosynthesis occurs on its cognate tRNA, beginning with a Ser-tRNASec precursor (reviewed in REF. 61) (BOX 2). Particular UGAs are selected for recoding to Sec by the presence of a downstream Sec insertion sequence (SECIS) (FIG. 5). A special elongation factor (SelB) that binds to both the SECIS and Sec-tRNASec on the ribosome is also required (BOX 2). Given the complexity of UGA recoding to Sec, it is not surprising that codon recoding is rare in nature. The benefit (and significance) of recoding, as opposed to codon reassignment, is that nature found a way to expand the genetic code that does not interfere with normal protein synthesis.
Figure 5. Expanding the genetic code with Sec and Pyl.
a ∣ Protein synthesis with selenocysteine (Sec). Sec is biosynthesized on its tRNA. This occurs in multiple steps, beginning with Ser-tRNASec formation catalysed by the normal seryl-tRNA synthetase (SerRS). In bacteria, Ser-tRNASec is converted to Sec-tRNASec by the Sec synthase (SelA). In archaea and eukaryotes, Ser-tRNASec is first phosphorylated by phosphoseryl(Sep)-tRNASec kinase (PSTK) to generate pSer-tRNASec and then an enzyme related to SelA known as Sep-tRNASec:Sec-tRNASec synthase (SepSecS) converts pSer-tRNASec species to Sec-tRNASec. A specialized elongation factor (SelB) simultaneously binds to Sec-tRNASec as well as the Sec insertion sequence (SECIS) element to direct recoding on the ribosome of specific UGA codons in selenoprotein mRNAs. b ∣ Protein synthesis with pyrrolysine (Pyl). Pyl is biosynthesized as a free amino acid in the cell. PylRS ligates Pyl to tRNAPyl, which contains an anticodon (5′-CUA-3′) that reads UAG codons. Like canonical aminoacyl-tRNAs, Pyl-tRNAPyl is bound by elongation factor Tu (EF-Tu), enabling UAG translation with Pyl on the ribosome. The Pyl system does not require a specialized elongation factor.
Sec is not hardwired to UGA codons. Recent experiments demonstrate that the Sec machinery is able to recode nearly every codon in the genetic code table62. A tRNA molecule was engineered to insert Sec at the UAG codon using elongation factor Tu (EF-Tu), but even with optimal expression of the Sec synthesis components, the precursor Ser-tRNASec was incorporated only 30% of the time63. Ambiguous decoding of Ser and Sec in this SECIS-independent route to selenoprotein synthesis supports the notion that the elaborate Sec recoding machinery may have evolved to enhance the fidelity of Sec insertion into proteins64. Further tRNA refinements created synthetic tRNAs65,66 that, together with EF-Tu67 mutants, led to efficient site-specific Sec incorporation64.
Furthermore, Sec-tRNASec is capable of wobble decoding, and a Sec-tRNASecCCA mutant has been shown to efficiently decode the UGA codon in a thioredoxin reductase68. In E. coli, mutation of the tRNASec to each of the other 63 anticodons shows that Sec can be inserted in response to 60 of the codons in the genetic code62,66. In fact, for 15 codons, Sec-tRNASec can completely out-compete cognate aa-tRNA and provide selenoprotein yields that are tenfold greater than when Sec is encoded by UGA62. These findings indicate that microorganisms could encode Sec with a codon other than UGA and bring into question as to why UGA is the ‘chosen’ Sec codon. It is, therefore, plausible that even in nature Sec is not hardwired to UGA.
Interestingly, Sec recoding is found in all three domains of life, but not in all organisms. Sec is normally found in redox enzymes, and these selenoproteins have important roles in central energy metabolism in archaea and bacteria as well as in cellular defence against reactive oxygen species.
Approximately 20% of sequenced bacterial taxa encode Sec69. Some bacteria encode only a single selenoprotein, whereas others such as the δ-proteobacterium Syntrophobacter fumaroxidans encode 31 (REF. 69). Bacterial selenoproteins belong to 58 protein families70. The number of selenoprotein genes in bacterial species differs greatly, in part because replacement of Sec with Cys is common in the evolution of bacterial selenoproteins69. The majority of bacterial selenoproteins, including formate dehydrogenase-α subunit (FdhA), Gly reductase B and glutathione peroxidase, are homologous to thiol-based redox enzymes69,71. The most common bacterial selenoproteins are selenophosphate synthase (SelD) (BOX 2) and FdhA. E. coli formate dehydrogenase (FDHH) is one of the most studied selenoproteins72. FDHH is a component of the formate hydrogen lyase complex and functions to reduce formate and shuttle electrons to the respiratory chain. The Sec residue in FDHH is essential for its function, and replacement with Cys reduces the catalytic efficiency of the enzyme 200-fold73.
Bacterial selenoproteins are involved in other central metabolic pathways, including purine degradation, and the energy-conserving acetogenesis pathway74. Environmental sequencing of microbial genomes revealed many new potential selenoproteins and novel selenoprotein families, such as Ser proteases and formamidase regulatory proteins, in which the role of Sec is unknown70.
Early work demonstrated that Sec is dispensable in E. coli and Salmonella enterica subsp. enterica serovar Typhimurium75, but our understanding of how natural expansion of the genetic code with Sec affects bacterial physiology is still limited. A recent report found that concentrations of sodium selenite (a necessary precursor of Sec biosynthesis) in the millimolar range were detrimental to E. coli growth, but concentrations of 1–10 nM stimulated cell growth76. Certain bacterial pathogens (such as Staphylococcus aureus) rely completely on the selenoprotein thioredoxin reductase to survive oxidative stress77. Further work will be required to understand the selective value of genetic code expansion with Sec in bacteria.
Sec-decoding archaea are confined to closely related species78, including Methanopyrus kandleri and all members of the order Methanococcales. The archaeal selenophosphate synthase is a selenoprotein, but most archaeal selenoproteins are involved in methanogenesis, which is the main energy production pathway for these microorganisms78. Sec is found in FDHH, formylmethano furan dehydrogenase, F420 reducing and non-reducing hydrogenases, heterodisulfide reductase and a HesB-like protein, which has been implicated in iron–sulfur cluster assembly79–81. These selenoproteins are involved in redox reactions and cofactor regeneration, facilitating the transfer of electrons in the reduction of formate to produce methane.
Genetic experiments in Methanococcus maripaludis indicate that archaeal selenoproteins are more active than Cys-containing homologues and that the metabolic range and efficiency of the organism decreases when their genetic code is reduced from 21 to 20 amino acids82,83. In M. maripaludis JJ, genetic inactivation of the Sec-specific elongation factor SelB leads to overexpression of Cys-containing versions of each of its selenoproteins, suggesting that the Sec version is catalytically more active82. M. maripaludis JJ cannot sustain growth on formate because there is no Cys paralogue for FDHH, and a 15% decrease in growth rate was observed in cells consuming H2 and CO2.
Reassigning UAG to Pyl
Pyl was first discovered in methanogenic archaea and identified as the twenty-second genetically encoded amino acid84,85 (BOX 2). A recent search of the US National Center for Biotechnology Information and the US Joint Genome Institute sequence databases shows that there are 46 microbial species (21 archaea and 25 bacteria) that contain all of the genes that are necessary to synthesize Pyl-containing proteins. Recently, a new clade of Pyl-decoding methanogens was discovered in Methanomassiliicoccales species related to the euryarchaeal order Thermoplasmatales86. The sequences hint at potential new roles for Pyl in CRISPR-associated protein Cas1 and digeranylgeranylglyceryl phosphate synthase87, but it is still debatable whether all UAG codons in Pyl-decoding organisms lead to Pyl insertion.
The biochemical mechanism required to genetically encode Pyl is distinct and orthogonal to the system that evolved to insert Sec into proteins. Free Pyl is biosynthesized in the cell from two molecules of Lys by three enzymes that are the products of the pylB, pylC and pylD genes88. Structural and biochemical work on Pyl biosynthesis was recently reviewed89. Pyl is then ligated onto tRNAPyl by PylRS90,91. The CUA anticodon of tRNAPyl reads the UAG codon and reassigns the meaning of UAG from stop to Pyl (FIG. 5).
Natural incorporation of Pyl was first experimentally verified in a monomethylamine methyltransferase (MtmB)85, when crystallographic structures of Pyl-containing MtmB (purified from Methanosarcina acetivorans) provided an atomic-level view of Pyl in the active site84. A hypothetical structure-based reaction mechanism suggested that Pyl may function to properly orient the methylamine substrate for transfer of the methyl group to a cognate corrinoid protein (MtmC). Related bacterial methyltransferases that lack Pyl are now known to use Gly betaine as a substrate92, a quaternary amine and a precursor of the tertiary amine trimethylamine (TMA). The authors argue that, because Gly betaine is a quaternary amine, it is already an ‘activated’ methyl donor, possibly providing a clue as to the role of Pyl in the methylamine methyltransferases. In the proposed reaction scheme84, Pyl may form an adduct with TMA, converting TMA into a quaternary amine intermediate that is activated for methyl transfer.
Pyl is, however, not restricted to the active site of methyltransferases and was also identified and characterized in the tRNA-editing enzyme tRNAHis guanylyltransferase (Thg1) from M. acetivorans93, in which Pyl is only required to read through an in-frame UAG codon in the Thg1 mRNA to generate a full-length protein; thus, Pyl serves as a ‘normal’ amino acid without a catalytic role. Furthermore, a recent proteomic investigation confirmed UAG translation or Pyl insertion in eight additional proteins in M. acetivorans94. These proteins included methylcorrinoid:coenzyme M (CoM) methyltransferase (MtaA), His kinase, tRNA endonuclease, hypothetical proteins and methylornithine synthase (PylB), which is the enzyme that catalyses the initial step of Pyl biosynthesis. PylB is folded95 and active without Pyl88, but the 21 amino acid extension following Pyl readthrough may modulate PylB activity or participate in sensing or regulating Pyl synthesis.
Translation of UAG codons in M. acetivorans may involve competition between release factor and PyltRNAPyl readthrough, and it is possible that some UAG codons are translated more efficiently as Pyl than others. In a M. acetivorans tRNAPyl deletion mutant, three instances of UAG codons serving as stop codons were detected by mass spectrometry, with none detected in the wild-type strain94. The fact that UAG codes for stop in the tRNAPyl deletion mutant indicates that the M. acetivorans release factor has activity towards the UAG codons. Indeed, an in vitro study using chimeric Methanosarcina barkeri–human release factor in an in vitro translation system found that the M. barkeri release factor had activity towards all three stop codons (UAG, UAA and UGA), but significantly reduced activity for UAG96. Although no natural M. acetivorans transcripts have been shown to use UAG as a stop codon in wild-type cells, previous work using an E. coli β-glucuronidase gene reporter with a UAG codon showed translation with Pyl (20%) and stopping at UAG (80%) in wild-type M. acetivorans97. Competition with release factor is not unique to Pyl. Trp decoding at UGG is well known to compete with release factor62,98, and release factor is also active at SECIS-recoded UGA codons that specify Sec62,99. Despite the fact that competition exists between translational readthrough and termination, Trp and Sec codons are not considered to be stop codons. The above studies indicate that UAG codons are not recoded to Pyl at specific loci, as is the case for UGA codons recoded to Sec (see above). Future studies will provide a more detailed picture of Pyl decoding, but the preponderance of the evidence indicates that in native M. acetivorans transcripts UAG is reassigned to Pyl.
Pyl recoding has physiological impacts on both archaea and bacteria. For example, in genetic studies, a M. acetivorans strain lacking tRNAPyl was unable to grow on TMA94,100. This observation reaffirmed the notion that Pyl is evolutionarily connected to methylamine metabolism101. The fact that all Pyl-decoding organisms encode at least one methylamine methyltransferase with an in-frame UAG codon (placing Pyl in the active site of these enzymes)84 suggests that the selective value of Pyl may be related to its role in methylamine metabolism. However, all organisms that putatively encode Pyl also have UAG codons in mRNAs that direct production of other proteins. Protein families encoded in the M. acetivorans genome and enriched with Pyl residues include transposases, recombinases, methylamine and methylcorrinoid:CoM methyltransferases, methyltransferases (with unknown substrates), radical S-adenosylmethionine (SAM) enzymes and His kinases involved in two-component signalling94.
In M. acetivorans, there are 267 in-frame UAG codons in annotated genes, and Pyl is intricately linked with the proteome, cellular metabolism and fitness. Growth of a tRNAPyl deletion strain in minimal medium containing methanol as the sole carbon source resulted in significant increases in generation and lag time compared with the wild-type strain94. This phenotypic defect corresponded to ~350 differentially abundant (mostly non-Pyl-containing) proteins. In the deletion mutant, the abundance of several enzymes required for methanogenesis from methanol were reduced 20-fold, indicating lower metabolic efficiency with methanol. Methyltransferases involved in methanogenesis from dimethylsulfides102 increased 100-fold, suggesting an altered or compensatory metabolism94. Although anabolic or other defects in the deletion mutant cannot be ruled out, the data show Pyl has a far-reaching impact on the composition of the proteome and suggest that M. acetivorans is metabolically less efficient when the organism has a reduced genetic code.
The Pyl-decoding trait exists in 25 anaerobic bacteria from the Firmicutes phylum (in classes Clostridia and Negativicutes) and the δ-Proteobacteria class. Early work in the field showed that E. coli expressed Pyl-containing proteins with a recombinant Pyl-decoding system, indicating that bacteria are capable of decoding UAG codons as Pyl90,103. However, there has been little work to show whether Pyl is actively decoded in bacteria in which the Pyl trait naturally occurs.
An investigation into several of these bacteria uncovered a novel genetic code variation referred to as dynamic genetic code expansion104. Several bacterial strains thought to genetically encode Pyl and at least one TMA methyltransferase (MttB) with one in-frame UAG codon were examined for the ability to produce Pyl-tRNAPyl and Pyl-containing MttB. The Pyl-decoding bacterium Acetohalobium arabaticum genetically encodes 20 amino acids when grown on pyruvate; however, when grown on TMA, the cells dynamically expand their genetic code to 21 amino acids with Pyl104. Only in the presence of TMA did A. arabaticum synthesize Pyl-tRNAPyl, produce Pyl-containing MttB and consume TMA. In the absence of TMA, A. arabaticum transcriptionally silences the gene encoding PylRS and downregulates pylB, pylC and pylD transcription. The mechanism by which A. arabaticum senses TMA and initiates the expression of the Pyl-decoding system remains unknown. Interestingly, despite encoding a functional PylRS105,106 and tRNAPyl pair107, Desulfitobacterium hafniense did not show detectable production of Pyl-tRNAPyl or expression of MttB. Desulfitobacterium dehalogenans expressed transcripts for tRNAPyl and PylRS, but no detectable transcription of the Pyl biosynthesis genes (pylB, pylC and pylD) was observed. This suggests that Pyl is either not decoded at all or simply not decoded under the conditions tested. Collectively, these data show two different ways that codon usage can be adapted to genetic code expansion with Pyl. Pyl-decoding archaea severely limit their use of UAG codons (~5% of all annotated genes ‘stop’ at a UAG codon)87,104, whereas many other organisms, including all Pyl-decoding bacteria104, use UAG codons abundantly (~25% of all annotated genes ‘stop’ at a UAG codon). Furthermore, the recently sequenced Thermoplasmatales-related methanogens display an extremely reduced use of UAG codons, as low as 1.6%87. The Pyl machinery is thought to be the evolutionary driving force that maintains low UAG codon usage in these species87. The archaeal strategy seems to be to limit UAG codons to locations where Pyl is required or at least tolerated. By contrast, the bacteria examined so far seem to be able to silence Pyl when Pyl-containing proteins are not needed, which may allow these species to use higher levels of UAG codons.
Conclusion and outlook
Recent developments in genome sequencing, biochemistry, bioinformatics and structural biology have facilitated the discovery of new genetic codes and provided crucial tools to analyse and gain insight into the physiological impact of genetic code flexibility. In this Review, we categorized the mechanisms of genetic code variation into four types — biased codon usage, codon reassignment, ambiguous decoding and natural genetic code expansion (FIG. 1b) — but this is certainly an incomplete list of the genetic code diversity in the living world. Increasing evidence suggests that genetic code flexibility is a selected trait during evolution to benefit microorganisms under certain conditions. However, little is known about the molecular mechanisms that lead to the improved fitness as a result of genetic code variation.
Our improved understanding of natural genetic code variations has also fostered a new exciting research area in expanding the genetic code with non-canonical amino acids and engineering synthetic organisms with new genetic codes (BOX 1). Single-cell model microorganisms with advanced genetic systems, such as E. coli and S. cerevisiae, serve as major vehicles for such engineering efforts. However, even in these well-studied organisms, we are only beginning to understand the physiological changes brought about by engineered genetic code variations.
For each genetic code variant described above, far more is known about how the genetic code changed (in terms of evolutionary or biochemical mechanisms) than about why the genetic code changed or the potential selection pressures that drive and maintain these changes. Therefore, these are areas where there is still great potential for future experimentation. Molecular genetic approaches have already revealed much about genetic code variants in terms of effects on growth, proteome status and cellular metabolism. Furthermore, engineered synthetic organisms with very different genetic codes may lay the basis for systematic experiments to explore the selective value and physiological impact of genetic code evolution.
Our knowledge of decoding mechanisms is far from complete. These gaps in knowledge have presented major obstacles to understanding the evolution of the genetic code and engineering synthetic cells with expanded codes. We envision that with advances in single-cell genome sequencing and systems biology, further genetic code variations will be uncovered. This will provide new insights into genetic code flexibility and novel tools for engineering organisms with altered genetic codes.
Supplementary Material
Acknowledgements
Work in the authors’ laboratories was supported by grants from the US National Institute of General Medical Sciences (GM022854 to D.S.; and GM115431 to J.L.), from the Natural Sciences and Engineering Research Council of Canada (RGPIN 04282–2014 to P.O.), from the Canadian Institutes of Health Research Tier 2 Canada Research Chair (950-229917 to P.O.), and from The University of Texas Health Science Center at Houston start-up fund (to J.L.). The authors are grateful to I. Heinemann for discussions and a critical reading of the manuscript.
Glossary
- Aminoacyl-tRNA
(aa-tRNA). A tRNA molecule with an amino acid attached to the 3′ end. It is used as a substrate by the ribosome to synthesize proteins.
- Codon–anticodon pairing
During translation, the bases of the mRNA codon and the tRNA anticodon need to match each other. Watson–Crick pairing (A–U and G–C) in the first and second positions of the codon is required for efficient decoding, whereas the third position allows more flexible pairing, for example, between G and U or using modified bases.
- Synonymous codons
Different triplet nucleotide sequences that decode the same amino acid.
- tRNA isoacceptors
Different tRNA species recognized by the same aminoacyl-tRNA synthetase and ligated with the same amino acid.
- Misacylation
Incorrect pairing of an amino acid and tRNA by an aminoacyl-tRNA synthetase. Errors resulting from misacylation, if left uncorrected, reduce the overall translational fidelity.
- Frameshifting
Change in the reading frame during translation due to mutations in the DNA, errors during transcription or translation or specific mRNA structures, leading to new protein sequences.
- Shine–Dalgarno-like sequences
mRNA sequences that share high similarity with the Shine–Dalgarno sequence, which pairs with the anti-Shine–Dalgarno sequence of the ribosomal RNA.
- Codon adaptation index
A method for analysing usage bias of synonymous codons using a set of highly expressed genes from a species as a reference to assign a score to each gene.
- Wobble position
The third position of a codon, which is more flexibly recognized by the tRNA compared with other positions.
- Phages
(Also called bacteriophages). Viruses that infect and propagate within bacteria. Phages contain their own genome but hijack the translational machinery of the bacterial host for protein synthesis.
- RpoB
The β-subunit of the bacterial RNA polymerase and target of the antibiotic rifampicin.
- Nucleophilicity
The property to donate an electron in chemical reactions.
- Elongation factor Tu
(EF-Tu). A bacterial elongation factor that delivers aminoacyl-tRNAs to the ribosome during peptide synthesis. The counterpart of EF-Tu in archaea and eukaryotes is EF-1A
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Nirenberg M, et al. RNA codewords and protein synthesis, VII. On the general nature of the RNA code. Proc. Natl Acad. Sci. USA. 1965;53:1161–1168. doi: 10.1073/pnas.53.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Söll D, et al. Studies on polynucleotides, XLIX. Stimulation of the binding of aminoacyl-sRNA’s to ribosomes by ribotrinucleotides and a survey of codon assignments for 20 amino acids. Proc. Natl Acad. Sci. USA. 1965;54:1378–1385. doi: 10.1073/pnas.54.5.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, Tzagoloff A. Assembly of the mitochondrial membrane system: sequences of yeast mitochondrial valine and an unusual threonine tRNA gene. Cell. 1979;18:47–53. doi: 10.1016/0092-8674(79)90352-0. [DOI] [PubMed] [Google Scholar]
- 4.Macino G, Coruzzi G, Nobrega FG, Li M, Tzagoloff A. Use of the UGA terminator as a tryptophan codon in yeast mitochondria. Proc. Natl Acad. Sci. USA. 1979;76:3784–3785. doi: 10.1073/pnas.76.8.3784. First discovery of codon reassignment in microorganisms.
- 5.Ambrogelly A, Palioura S, Söll D. Natural expansion of the genetic code. Nat. Chem. Biol. 2007;3:29–35. doi: 10.1038/nchembio847. [DOI] [PubMed] [Google Scholar]
- 6.Plotkin JB, Kudla G. Synonymous but not the same: the causes and consequences of codon bias. Nat. Rev. Genet. 2011;12:32–42. doi: 10.1038/nrg2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quax TE, Claassens NJ, Söll D, van der Oost J. Codon bias as a means to fine-tune gene expression. Mol. Cell. 2015;59:149–161. doi: 10.1016/j.molcel.2015.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan T. Adaptive translation as a mechanism of stress response and adaptation. Annu. Rev. Genet. 2013;47:121–137. doi: 10.1146/annurev-genet-111212-133522. Excellent review on benefits of ambiguous decoding under stress conditions.
- 9.Atkins JF, Baranov PV. The distinction between recoding and codon reassignment. Genetics. 2010;185:1535–1536. doi: 10.1534/genetics.110.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkins JF, Gesteland RF, Reid BR, Anderson CW. Normal tRNAs promote ribosomal frameshifting. Cell. 1979;18:1119–1131. doi: 10.1016/0092-8674(79)90225-3. One of the first studies to demonstrate frameshifting and genetic code variability.
- 11.Grantham R, Gautier C, Gouy M, Mercier R, Pave A. Codon catalog usage and the genome hypothesis. Nucleic Acids Res. 1980;84:r49–r62. doi: 10.1093/nar/8.1.197-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shabalina SA, Spiridonov NA, Kashina A. Sounds of silence: synonymous nucleotides as a key to biological regulation and complexity. Nucleic Acids Res. 2013;41:2073–2094. doi: 10.1093/nar/gks1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulmer M. The selection-mutation-drift theory of synonymous codon usage. Genetics. 1991;129:897–907. doi: 10.1093/genetics/129.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li GW, Oh E, Weissman JS. The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature. 2012;484:538–541. doi: 10.1038/nature10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramaniam AR, Pan T, Cluzel P. Environmental perturbations lift the degeneracy of the genetic code to regulate protein levels in bacteria. Proc. Natl Acad. Sci. USA. 2013;110:2419–2424. doi: 10.1073/pnas.1211077110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman DB, Church GM, Kosuri S. Causes and effects of N-terminal codon bias in bacterial genes. Science. 2013;342:475–479. doi: 10.1126/science.1241934. [DOI] [PubMed] [Google Scholar]
- 17.Subramaniam AR, et al. A serine sensor for multicellularity in a bacterium. eLife. 2013;2:e01501. doi: 10.7554/eLife.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, et al. Non-optimal codon usage is a mechanism to achieve circadian clock conditionality. Nature. 2013;495:116–120. doi: 10.1038/nature11942. Suggested that non-optimal codons are used as a regulatory mechanism.
- 19.Zhou M, et al. Non-optimal codon usage affects expression, structure and function of clock protein FRQ. Nature. 2013;495:111–115. doi: 10.1038/nature11833. Suggested that non-optimal codons are used as a regulatory mechanism.
- 20.Lang BF, Lavrov D, Beck N, Steinberg SV. In: Organelle Genetics. Bullerwell CE, editor. Springer; 2012. pp. 431–474. [Google Scholar]
- 21.Sengupta S, Yang X, Higgs PG. The mechanisms of codon reassignments in mitochondrial genetic codes. J. Mol. Evol. 2007;64:662–688. doi: 10.1007/s00239-006-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alfonzo JD, Blanc V, Estevez AM, Rubio MA, Simpson L. C to U editing of the anticodon of imported mitochondrial tRNATrp allows decoding of the UGA stop codon in Leishmania tarentolae. EMBO J. 1999;18:7056–7062. doi: 10.1093/emboj/18.24.7056. Demonstrated that RNA editing is responsible for UGA reassignment in Leishmania tarentolae mitochondria.
- 23.Muramatsu T, et al. A novel lysine-substituted nucleoside in the first position of the anticodon of minor isoleucine tRNA from Escherichia coli. J. Biol. Chem. 1988;263:9261–9267. doi: 10.1351/pac198961030573. [DOI] [PubMed] [Google Scholar]
- 24.Mandal D, et al. Agmatidine, a modified cytidine in the anticodon of archaeal tRNAIle, base pairs with adenosine but not with guanosine. Proc. Natl Acad. Sci. USA. 2010;107:2872–2877. doi: 10.1073/pnas.0914869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeuchi Y, et al. Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea. Nat. Chem. Biol. 2010;6:277–282. doi: 10.1038/nchembio.323. [DOI] [PubMed] [Google Scholar]
- 26.Tomita K, et al. Codon reading patterns in Drosophila melanogaster mitochondria based on their tRNA sequences: a unique wobble rule in animal mitochondria. Nucleic Acids Res. 1999;27:4291–4297. doi: 10.1093/nar/27.21.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantara WA, Murphy FV, Demirci H, Agris PF. Expanded use of sense codons is regulated by modified cytidines in tRNA. Proc. Natl Acad. Sci. USA. 2013;110:10964–10969. doi: 10.1073/pnas.1222641110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marniemi J, Parkki MG. Radiochemical assay of glutathione S-epoxide transferase and its enhancement by phenobarbital in rat liver in vivo. Biochem. Pharmacol. 1975;24:1569–1572. doi: 10.1016/0006-2952(75)90080-5. [DOI] [PubMed] [Google Scholar]
- 29.Su D, et al. An unusual tRNAThr derived from tRNAHis reassigns in yeast mitochondria the CUN codons to threonine. Nucleic Acids Res. 2011;39:4866–4874. doi: 10.1093/nar/gkr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling J, Daoud R, Lajoie MJ, Söll D, Lang BF. Natural reassignment of CUU and CUA sense codons to alanine in Ashbya mitochondria. Nucleic Acids Res. 2014;42:499–508. doi: 10.1093/nar/gkt842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling J, et al. Yeast mitochondrial threonyl-tRNA synthetase recognizes tRNA isoacceptors by distinct mechanisms and promotes CUN codon reassignment. Proc. Natl Acad. Sci. USA. 2012;109:3281–3286. doi: 10.1073/pnas.1200109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanova NN, et al. Stop codon reassignments in the wild. Science. 2014;344:909–913. doi: 10.1126/science.1250691. Uncovered widespread stop codon reassignment events in microorganisms.
- 33.Mukai T, et al. Codon reassignment in the Escherichia coli genetic code. Nucleic Acids Res. 2010;38:8188–8195. doi: 10.1093/nar/gkq707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson DB, et al. Release factor one is nonessential in Escherichia coli. ACS Chem. Biol. 2012;7:1337–1344. doi: 10.1021/cb300229q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinemann IU, et al. Enhanced phosphoserine insertion during Escherichia coli protein synthesis via partial UAG codon reassignment and release factor 1 deletion. FEBS Lett. 2012;586:3716–3722. doi: 10.1016/j.febslet.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lajoie MJ, et al. Genomically recoded organisms expand biological functions. Science. 2013;342:357–360. doi: 10.1126/science.1241459. Created the first synthetic microorganism with complete removal of UAG stop codons.
- 37.Mukai T, et al. Highly reproductive Escherichia coli cells with no specific assignment to the UAG codon. Sci. Rep. 2015;5:9699. doi: 10.1038/srep09699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukai T, et al. Reassignment of a rare sense codon to a non-canonical amino acid in Escherichia coli. Nucleic Acids Res. 2015;43 doi: 10.1093/nar/gkv787. http://dx.doi.org/10.1093/nar/gkv787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aerni HR, Shifman MA, Rogulina S, O’Donoghue P, Rinehart J. Revealing the amino acid composition of proteins within an expanded genetic code. Nucleic Acids Res. 2015;43:e8. doi: 10.1093/nar/gku1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammerling MJ, et al. Bacteriophages use an expanded genetic code on evolutionary paths to higher fitness. Nat. Chem. Biol. 2014;10:178–180. doi: 10.1038/nchembio.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz DW, Yarus M. Transfer RNA mutation and the malleability of the genetic code. J. Mol. Biol. 1994;235:1377–1380. doi: 10.1006/jmbi.1994.1094. [DOI] [PubMed] [Google Scholar]
- 42.Ling J, Reynolds N, Ibba M. Aminoacyl-tRNA synthesis and translational quality control. Annu. Rev. Microbiol. 2009;63:61–78. doi: 10.1146/annurev.micro.091208.073210. [DOI] [PubMed] [Google Scholar]
- 43.Rodnina MV, Wintermeyer W. Ribosome fidelity: tRNA discrimination, proofreading and induced fit. Trends Biochem. Sci. 2001;26:124–130. doi: 10.1016/s0968-0004(00)01737-0. [DOI] [PubMed] [Google Scholar]
- 44.Zaher HS, Green R. Fidelity at the molecular level: lessons from protein synthesis. Cell. 2009;136:746–762. doi: 10.1016/j.cell.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Netzer N, et al. Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature. 2009;462:522–526. doi: 10.1038/nature08576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mascarenhas AP, An S, Rosen AE, Martinis SA, Musier-Forsyth K. In: Protein Engineering. RajBhandary UL, Köhrer C, editors. Springer; 2008. pp. 153–200. [Google Scholar]
- 47.Roy H, Ling J, Alfonzo J, Ibba M. Loss of editing activity during the evolution of mitochondrial phenylalanyl-tRNA synthetase. J. Biol. Chem. 2005;280:38186–38192. doi: 10.1074/jbc.M508281200. [DOI] [PubMed] [Google Scholar]
- 48.Li L, et al. Naturally occurring aminoacyl-tRNA synthetases editing-domain mutations that cause mistranslation in Mycoplasma parasites. Proc. Natl Acad. Sci. USA. 2011;108:9378–9383. doi: 10.1073/pnas.1016460108. Suggested that Mycoplasma spp. may use ambiguous decoding to defend against the host immune response.
- 49.Yadavalli SS, Ibba M. Selection of tRNA charging quality control mechanisms that increase mistranslation of the genetic code. Nucleic Acids Res. 2013;41:1104–1112. doi: 10.1093/nar/gks1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bezerra AR, et al. Reversion of a fungal genetic code alteration links proteome instability with genomic and phenotypic diversification. Proc. Natl Acad. Sci. USA. 2013;110:11079–11084. doi: 10.1073/pnas.1302094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miranda I, et al. Candida albicans CUG mistranslation is a mechanism to create cell surface variation. mBio. 2013;4:e00285–13. doi: 10.1128/mBio.00285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Javid B, et al. Mycobacterial mistranslation is necessary and sufficient for rifampicin phenotypic resistance. Proc. Natl Acad. Sci. USA. 2014;111:1132–1137. doi: 10.1073/pnas.1317580111. Demonstrated that ambiguous decoding increases resistance to an antibiotic in mycobacteria.
- 53.Fan Y, et al. Protein mistranslation protects bacteria against oxidative stress. Nucleic Acids Res. 2015;43:1740–1748. doi: 10.1093/nar/gku1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu J, Fan Y, Ling J. Mechanism of oxidant-induced mistranslation by threonyl-tRNA synthetase. Nucleic Acids Res. 2014;42:6523–6531. doi: 10.1093/nar/gku271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crick FH. The Croonian lecture, 1966. The genetic code. Proc. R. Soc. Lond. B. 1967;167:331–347. doi: 10.1098/rspb.1967.0031. [DOI] [PubMed] [Google Scholar]
- 56.Turanov AA, et al. Genetic code supports targeted insertion of two amino acids by one codon. Science. 2009;323:259–261. doi: 10.1126/science.1164748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arner ES. Selenoproteins — what unique properties can arise with selenocysteine in place of cysteine? Exp. Cell Res. 2010;316:1296–1303. doi: 10.1016/j.yexcr.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 58.Kim HY, Gladyshev VN. Different catalytic mechanisms in mammalian selenocysteine- and cysteine-containing methionine-R-sulfoxide reductases. PLoS. Biol. 2005;3:e375. doi: 10.1371/journal.pbio.0030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snider GW, Ruggles E, Khan N, Hondal RJ. Selenocysteine confers resistance to inactivation by oxidation in thioredoxin reductase: comparison of selenium and sulfur enzymes. Biochemistry. 2013;52:5472–5481. doi: 10.1021/bi400462j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Metanis N, Hilvert D. Natural and synthetic selenoproteins. Curr. Opin. Chem. Biol. 2014;22:27–34. doi: 10.1016/j.cbpa.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Yoshizawa S, Böck A. The many levels of control on bacterial selenoprotein synthesis. Biochim. Biophys. Acta. 2009;1790:1404–1414. doi: 10.1016/j.bbagen.2009.03.010. An excellent review of bacterial selenoproteins.
- 62.Bröcker MJ, Ho JM, Church GM, Söll D, O’Donoghue P. Recoding the genetic code with selenocysteine. Angew. Chem. Int. Ed. Engl. 2014;53:319–323. doi: 10.1002/anie.201308584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aldag C, et al. Rewiring translation for elongation factor Tu-dependent selenocysteine incorporation. Angew. Chem. Int. Ed. Engl. 2013;52:1441–1445. doi: 10.1002/anie.201207567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su D, et al. How an obscure archaeal gene inspired the discovery of selenocysteine biosynthesis in humans. IUBMB Life. 2009;61:35–39. doi: 10.1002/iub.136. [DOI] [PubMed] [Google Scholar]
- 65.Thyer R, Robotham SA, Brodbelt JS, Ellington AD. Evolving tRNASec for efficient canonical incorporation of selenocysteine. J. Am. Chem. Soc. 2015;137:46–49. doi: 10.1021/ja510695g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller C, et al. A synthetic tRNA for EF-Tu mediated selenocysteine incorporation in vivo and in vitro. FEBS Lett. 2015;589:2194–2199. doi: 10.1016/j.febslet.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haruna K, Alkazemi MH, Liu Y, Söll D, Englert M. Engineering the elongation factor Tu for efficient selenoprotein synthesis. Nucleic Acids Res. 2014;42:9976–9983. doi: 10.1093/nar/gku691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J, Croitoru V, Rutishauser D, Cheng Q, Arner ES. Wobble decoding by the Escherichia coli selenocysteine insertion machinery. Nucleic Acids Res. 2013;41:9800–9811. doi: 10.1093/nar/gkt764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Romero H, Salinas G, Gladyshev VN. Dynamic evolution of selenocysteine utilization in bacteria: a balance between selenoprotein loss and evolution of selenocysteine from redox active cysteine residues. Genome Biol. 2006;7:R94. doi: 10.1186/gb-2006-7-10-r94. Although many new sequences are now available, this is still the definitive resource that documents the evolutionary replacement of Cys with Sec residues in bacterial proteins.
- 70.Zhang Y, Gladyshev VN. Trends in selenium utilization in marine microbial world revealed through the analysis of the global ocean sampling (GOS) project. PLoS Genet. 2008;4:e1000095. doi: 10.1371/journal.pgen.1000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stadtman TC. Selenocysteine. Annu. Rev. Biochem. 1996;65:83–100. doi: 10.1146/annurev.bi.65.070196.000503. [DOI] [PubMed] [Google Scholar]
- 72.Jormakka M, Byrne B, Iwata S. Formate dehydrogenase — a versatile enzyme in changing environments. Curr. Opin. Struct. Biol. 2003;13:418–423. doi: 10.1016/s0959-440x(03)00098-8. [DOI] [PubMed] [Google Scholar]
- 73.Axley MJ, Böck A, Stadtman TC. Catalytic properties of an Escherichia coli formate dehydrogenase mutant in which sulfur replaces selenium. Proc. Natl Acad. Sci. USA. 1991;88:8450–8454. doi: 10.1073/pnas.88.19.8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stock T, Rother M. Selenoproteins in Archaea and Gram-positive bacteria. Biochim. Biophys. Acta. 2009;1790:1520–1532. doi: 10.1016/j.bbagen.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 75.Stadtman TC, Davis JN, Zehelein E, Böck A. Biochemical and genetic analysis of Salmonella typhimurium and Escherichia coli mutants defective in specific incorporation of selenium into formate dehydrogenase and tRNAs. Biofactors. 1989;2:35–44. [PubMed] [Google Scholar]
- 76.Tetteh AY, et al. Transcriptional response of selenopolypeptide genes and selenocysteine biosynthesis machinery genes in Escherichia coli during selenite reduction. Int. J. Microbiol. 2014;2014:394835. doi: 10.1155/2014/394835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 78.Rother M, Krzycki JA. Selenocysteine, pyrrolysine, and the unique energy metabolism of methanogenic archaea. Archaea. 2010;2010:453642. doi: 10.1155/2010/453642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stock T, Selzer M, Rother M. In vivo requirement of selenophosphate for selenoprotein synthesis in archaea. Mol. Microbiol. 2010;75:149–160. doi: 10.1111/j.1365-2958.2009.06970.x. [DOI] [PubMed] [Google Scholar]
- 80.Kryukov GV, Gladyshev VN. The prokaryotic selenoproteome. EMBO Rep. 2004;5:538–543. doi: 10.1038/sj.embor.7400126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cobucci-Ponzano B, Rossi M, Moracci M. Translational recoding in archaea. Extremophiles. 2012;16:793–803. doi: 10.1007/s00792-012-0482-8. [DOI] [PubMed] [Google Scholar]
- 82.Rother M, Mathes I, Lottspeich F, Böck A. Inactivation of the selB gene in Methanococcus maripaludis: effect on synthesis of selenoproteins and their sulfur-containing homologs. J. Bacteriol. 2003;185:107–114. doi: 10.1128/JB.185.1.107-114.2003. A seminal study on the phenotypic impact of removing Sec from the genetic code of the model archaeaon M. maripaludis.
- 83.Hohn MJ, Palioura S, Su D, Yuan J, Söll D. Genetic analysis of selenocysteine biosynthesis in the archaeon Methanococcus maripaludis. Mol. Microbiol. 2011;81:249–258. doi: 10.1111/j.1365-2958.2011.07690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hao B, et al. A new UAG-encoded residue in the structure of a methanogen methyltransferase. Science. 2002;296:1462–1466. doi: 10.1126/science.1069556. Discovery of the twenty-second genetically encoded amino acid, Pyl.
- 85.Srinivasan G, James CM, Krzycki JA. Pyrrolysine encoded by UAG in Archaea: charging of a UAG-decoding specialized tRNA. Science. 2002;296:1459–1462. doi: 10.1126/science.1069588. [DOI] [PubMed] [Google Scholar]
- 86.Borrel G, et al. Genome sequence of ‘Candidatus Methanomethylophilus alvus’ Mx1201, a methanogenic archaeon from the human gut belonging to a seventh order of methanogens. J. Bacteriol. 2012;194:6944–6945. doi: 10.1128/JB.01867-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borrel G, et al. Comparative genomics highlights the unique biology of Methanomassiliicoccales, a Thermoplasmatales-related seventh order of methanogenic archaea that encodes pyrrolysine. BMC Genomics. 2014;15:679. doi: 10.1186/1471-2164-15-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gaston MA, Zhang L, Green-Church KB, Krzycki JA. The complete biosynthesis of the genetically encoded amino acid pyrrolysine from lysine. Nature. 2011;471:647–650. doi: 10.1038/nature09918. Elucidated activities of the biosynthetic route to Pyl.
- 89.Krzycki JA. The path of lysine to pyrrolysine. Curr. Opin. Chem. Biol. 2013;17:619–625. doi: 10.1016/j.cbpa.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 90.Blight SK, et al. Direct charging of tRNACUA with pyrrolysine in vitro and in vivo. Nature. 2004;431:333–335. doi: 10.1038/nature02895. Elucidated the mechanism of Pyl decoding.
- 91.Polycarpo C, et al. An aminoacyl-tRNA synthetase that specifically activates pyrrolysine. Proc. Natl Acad. Sci. USA. 2004;101:12450–12454. doi: 10.1073/pnas.0405362101. Elucidated the mechanism of Pyl decoding.
- 92.Ticak T, Kountz DJ, Girosky KE, Krzycki JA, Ferguson DJ., Jr. A nonpyrrolysine member of the widely distributed trimethylamine methyltransferase family is a glycine betaine methyltransferase. Proc. Natl Acad. Sci. USA. 2014;111:e4668–e4676. doi: 10.1073/pnas.1409642111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heinemann IU, et al. The appearance of pyrrolysine in tRNAHis guanylyltransferase by neutral evolution. Proc. Natl Acad. Sci. USA. 2009;106:21103–21108. doi: 10.1073/pnas.0912072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Donoghue P, et al. Reducing the genetic code induces massive rearrangement of the proteome. Proc. Natl Acad. Sci. USA. 2014;111:17206–17211. doi: 10.1073/pnas.1420193111. Provided proteome-level view of the phenotypic impact of removing Pyl from the genetic code of M. acetivorans.
- 95.Quitterer F, List A, Eisenreich W, Bacher A, Groll M. Crystal structure of methylornithine synthase (PylB): insights into the pyrrolysine biosynthesis. Angew. Chem. Int. Ed. Engl. 2012;51:1339–1342. doi: 10.1002/anie.201106765. [DOI] [PubMed] [Google Scholar]
- 96.Alkalaeva E, et al. Translation termination in pyrrolysine-utilizing archaea. FEBS Lett. 2009;583:3455–3460. doi: 10.1016/j.febslet.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Longstaff DG, Blight SK, Zhang L, Green-Church KB, Krzycki JA. In vivo contextual requirements for UAG translation as pyrrolysine. Mol. Microbiol. 2007;63:229–241. doi: 10.1111/j.1365-2958.2006.05500.x. [DOI] [PubMed] [Google Scholar]
- 98.Freistroffer DV, Kwiatkowski M, Buckingham RH, Ehrenberg M. The accuracy of codon recognition by polypeptide release factors. Proc. Natl Acad. Sci. USA. 2000;97:2046–2051. doi: 10.1073/pnas.030541097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mansell JB, Guevremont D, Poole ES, Tate WP. A dynamic competition between release factor 2 and the tRNASec decoding UGA at the recoding site of Escherichia coli formate dehydrogenase H. EMBO J. 2001;20:7284–7293. doi: 10.1093/emboj/20.24.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mahapatra A, et al. Characterization of a Methanosarcina acetivorans mutant unable to translate UAG as pyrrolysine. Mol. Microbiol. 2006;59:56–66. doi: 10.1111/j.1365-2958.2005.04927.x. [DOI] [PubMed] [Google Scholar]
- 101.Krzycki JA. Function of genetically encoded pyrrolysine in corrinoid-dependent methylamine methyltransferases. Curr. Opin. Chem. Biol. 2004;8:484–491. doi: 10.1016/j.cbpa.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 102.Oelgeschlager E, Rother M. In vivo role of three fused corrinoid/methyl transfer proteins in Methanosarcina acetivorans. Mol. Microbiol. 2009;72:1260–1272. doi: 10.1111/j.1365-2958.2009.06723.x. [DOI] [PubMed] [Google Scholar]
- 103.Polycarpo CR, et al. Pyrrolysine analogues as substrates for pyrrolysyl-tRNA synthetase. FEBS Lett. 2006;580:6695–6700. doi: 10.1016/j.febslet.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Prat L, et al. Carbon source-dependent expansion of the genetic code in bacteria. Proc. Natl Acad. Sci. USA. 2012;109:21070–21075. doi: 10.1073/pnas.1218613110. Demonstrated natural Pyl decoding in bacteria and revealed first example of dynamic genetic code expansion.
- 105.Jiang R, Krzycki JA. PylSn and the homologous N-terminal domain of pyrrolysyl-tRNA synthetase bind the tRNA that is essential for the genetic encoding of pyrrolysine. J. Biol. Chem. 2012;287:32738–32746. doi: 10.1074/jbc.M112.396754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Katayama H, Nozawa K, Nureki O, Nakahara Y, Hojo H. Pyrrolysine analogs as substrates for bacterial pyrrolysyl-tRNA synthetase in vitro and in vivo. Biosci. Biotechnol. Biochem. 2012;76:205–208. doi: 10.1271/bbb.110653. [DOI] [PubMed] [Google Scholar]
- 107.Nozawa K, et al. Pyrrolysyl-tRNA synthetase-tRNAPyl structure reveals the molecular basis of orthogonality. Nature. 2009;457:1163–1167. doi: 10.1038/nature07611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Donoghue P, Ling J, Wang YS, Söll D. Upgrading protein synthesis for synthetic biology. Nat. Chem. Biol. 2013;9:594–598. doi: 10.1038/nchembio.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu CC, Schultz PG. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 2010;79:413–444. doi: 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]
- 110.Chin JW. Reprogramming the genetic code. EMBO J. 2011;30:2312–2324. doi: 10.1038/emboj.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnson JA, Lu YY, Van Deventer JA, Tirrell DA. Residue-specific incorporation of non-canonical amino acids into proteins: recent developments and applications. Curr. Opin. Chem. Biol. 2010;14:774–780. doi: 10.1016/j.cbpa.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chin JW. Expanding and reprogramming the genetic code of cells and animals. Annu. Rev. Biochem. 2014;83:379–408. doi: 10.1146/annurev-biochem-060713-035737. An excellent review on engineering protein synthesis for genetic code expansion in diverse expression systems.
- 113.Rovner AJ, et al. Recoded organisms engineered to depend on synthetic amino acids. Nature. 2015;518:89–93. doi: 10.1038/nature14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mandell DJ, et al. Biocontainment of genetically modified organisms by synthetic protein design. Nature. 2015;518:55–60. doi: 10.1038/nature14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Campbell JH, et al. UGA is an additional glycine codon in uncultured SR1 bacteria from the human microbiota. Proc. Natl Acad. Sci. USA. 2013;110:5540–5545. doi: 10.1073/pnas.1303090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang HH, et al. Programming cells by multiplex genome engineering and accelerated evolution. Nature. 2009;460:894–898. doi: 10.1038/nature08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Isaacs FJ, et al. Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science. 2011;333:348–353. doi: 10.1126/science.1205822. First example of a genome engineered with 62 codons by mutation of all TAGs to TAA.
- 118.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- 120.Gibson DG, et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329:52–56. doi: 10.1126/science.1190719. First demonstration of genome transplantation with a synthetic genome.
- 121.Annaluru N, et al. Total synthesis of a functional designer eukaryotic chromosome. Science. 2014;344:55–58. doi: 10.1126/science.1249252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Budiman ME, et al. Eukaryotic initiation factor 4a3 is a selenium-regulated RNA-binding protein that selectively inhibits selenocysteine incorporation. Mol. Cell. 2009;35:479–489. doi: 10.1016/j.molcel.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hohn MJ, Park HS, O’Donoghue P, Schnitzbauer M, Söll D. Emergence of the universal genetic code imprinted in an RNA record. Proc. Natl Acad. Sci. USA. 2006;103:18095–18100. doi: 10.1073/pnas.0608762103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bilokapic S, et al. Structure of the unusual seryl-tRNA synthetase reveals a distinct zinc-dependent mode of substrate recognition. EMBO J. 2006;25:2498–2509. doi: 10.1038/sj.emboj.7601129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Itoh Y, et al. Decameric SelA•tRNASec ring structure reveals mechanism of bacterial selenocysteine formation. Science. 2013;340:75–78. doi: 10.1126/science.1229521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sherrer RL, O’Donoghue P, Söll D. Characterization and evolutionary history of an archaeal kinase involved in selenocysteinyl-tRNA formation. Nucleic Acids Res. 2008;36:1247–1259. doi: 10.1093/nar/gkm1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carlson BA, et al. Identification and characterization of phosphoseryl-tRNA[Ser]Sec kinase. Proc. Natl Acad. Sci. USA. 2004;101:12848–12853. doi: 10.1073/pnas.0402636101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Palioura S, Sherrer RL, Steitz TA, Söll D, Simonovic M. The human SepSecS–tRNASec complex reveals the mechanism of selenocysteine formation. Science. 2009;325:321–325. doi: 10.1126/science.1173755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Copeland PR, Fletcher JE, Carlson BA, Hatfield DL, Driscoll DM. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. EMBO J. 2000;19:306–314. doi: 10.1093/emboj/19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bifano AL, Atassi T, Ferrara T, Driscoll DM. Identification of nucleotides and amino acids that mediate the interaction between ribosomal protein L30 and the SECIS element. BMC Mol. Biol. 2013;14:12. doi: 10.1186/1471-2199-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Allmang C, Wurth L, Krol A. The selenium to selenoprotein pathway in eukaryotes: more molecular partners than anticipated. Biochim. Biophys. Acta. 2009;1790:1415–1423. doi: 10.1016/j.bbagen.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 132.Yoshizawa S, et al. Structural basis for mRNA recognition by elongation factor SelB. Nat. Struct. Mol. Biol. 2005;12:198–203. doi: 10.1038/nsmb890. [DOI] [PubMed] [Google Scholar]
- 133.Yuan J, et al. Distinct genetic code expansion strategies for selenocysteine and pyrrolysine are reflected in different aminoacyl-tRNA formation systems. FEBS Lett. 2010;584:342–349. doi: 10.1016/j.febslet.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eggertsson G, Söll D. Transfer ribonucleic acid-mediated suppression of termination codons in Escherichia coli. Microbiol. Rev. 1988;52:354–374. doi: 10.1128/mr.52.3.354-374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ambrogelly A, et al. Pyrrolysine is not hardwired for cotranslational insertion at UAG codons. Proc. Natl Acad. Sci. USA. 2007;104:3141–3146. doi: 10.1073/pnas.0611634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kavran JM, et al. Structure of pyrrolysyl-tRNA synthetase, an archaeal enzyme for genetic code innovation. Proc. Natl Acad. Sci. USA. 2007;104:11268–11273. doi: 10.1073/pnas.0704769104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wan W, et al. A facile system for genetic incorporation of two different noncanonical amino acids into one protein in Escherichia coli. Angew. Chem. Int. Ed. Engl. 2010;49:3211–3214. doi: 10.1002/anie.201000465. [DOI] [PubMed] [Google Scholar]
- 138.Neumann H, Peak-Chew SY, Chin JW. Genetically encoding Nε-acetyllysine in recombinant proteins. Nat. Chem. Biol. 2008;4:232–234. doi: 10.1038/nchembio.73. [DOI] [PubMed] [Google Scholar]
- 139.Umehara T, et al. N-acetyl lysyl-tRNA synthetases evolved by a CcdB-based selection possess N-acetyl lysine specificity in vitro and in vivo. FEBS Lett. 2012;586:729–733. doi: 10.1016/j.febslet.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 140.Yanagisawa T, Umehara T, Sakamoto K, Yokoyama S. Expanded genetic code technologies for incorporating modified lysine at multiple sites. Chembiochem. 2014;15:2181–2187. doi: 10.1002/cbic.201402266. [DOI] [PubMed] [Google Scholar]
- 141.Guo LT, et al. Polyspecific pyrrolysyl-tRNA synthetases from directed evolution. Proc. Natl Acad. Sci. USA. 2014;111:16724–16729. doi: 10.1073/pnas.1419737111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lobanov AV, et al. Evolutionary dynamics of eukaryotic selenoproteomes: large selenoproteomes may associate with aquatic life and small with terrestrial life. Genome Biol. 2007;8:r198. doi: 10.1186/gb-2007-8-9-r198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grobe T, Reuter M, Gursinsky T, Sohling B, Andreesen JR. Peroxidase activity of selenoprotein GrdB of glycine reductase and stabilisation of its integrity by components of proprotein GrdE from Eubacterium acidaminophilum. Arch. Microbiol. 2007;187:29–43. doi: 10.1007/s00203-006-0169-6. [DOI] [PubMed] [Google Scholar]
- 144.Hurley JM, Dunlap JC. Cell biology: a fable of too much too fast. Nature. 2013;495:57–58. doi: 10.1038/nature11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.