Abstract
Intracranial hemorrhage (ICH) is associated with great morbidity and mortality in patients with acute leukemia. We identified 118 patients with ICH from a total of 2,421 patient with leukemia who were treated at our institution between 2005–2009, and assessed the prognostic factors for mortality in the ICH cohort. Median age at time of ICH was 58 years, 49% were male, and 60% had acute myeloid leukemia. The relative incidence of ICH was highest in patients with chronic myeloid leukemia in blast crisis (12.1%). Mental status changes were the most common symptom which prompted work-up for ICH. Median survival for all patients after onset of ICH was 20 days. In multivariate analyses, four clinical characteristics were identified as independent adverse factors for mortality in patients with ICH: albumin <3.5 g/dL, lactate dehydrogenase (LDH) >835 U/L, age > 60 years, and relapsed disease status. Based on the number of risk factors, mortality after ICH was: 25% (0 risk factor), 47% (1), 78% (2), and >97% (3 or 4). In conclusion, patients with leukemia who develop ICH do not have universally poor outcomes, and a simple scoring system could serve to advise physicians and families of the prognosis and the potential benefit of aggressive treatment options.
Introduction
Intracranial hemorrhage (ICH) has been identified as a major cause of morbidity and mortality in patients with acute leukemia [1–4]. Whereas very early studies had to rely on autopsies to establish a diagnosis of ICH [3], newer imaging modalities such as computed tomography (CT) have made it possible to diagnose ICH more quickly and of smaller sizes. Several studies so far have tried to describe the clinical characteristics of patients with ICH [2–4], but predictors of mortality in patients with leukemia who develop ICH are not available. It is generally believed that once ICH occurs, prognosis is dismal [2–4]. Better characterization and description of prognosis after ICH would help to risk stratify patients with ICH and possibly guide aggressiveness of therapy once ICH is diagnosed.
Thus, the goal of our study was to evaluate the clinical characteristics and outcomes in a large cohort of contemporary patients who developed ICH at MD Anderson Cancer Center (MDACC) and define a risk model that would predict the likelihood of death after ICH occurs.
Patients and Methods
Patient selection
The Institutional Review Board at MDACC approved this retrospective analysis. All adults with a diagnosis of acute myeloid leukemia (AML; n = 1,265), acute lymphoblastic leukemia (ALL; n = 304), myelodysplastic syndrome (MDS; n = 728), or blast-crisis chronic myelogenous leukemia (BC-CML; n = 124) referred from 2005–2009 to MDACC (n = 2,421) were retrospectively reviewed. All patients with at least one documented scan of the brain [either by CT or magnetic resonance imaging (MRI)] showing hemorrhage were included. If several images at different time points were obtained, the date of the first scan demonstrating ICH was used for analysis. Chart review was performed by three investigators (FD, SSM, and KN). A total of n = 118 patients met the inclusion criteria. Clinical, pathological, and laboratory information were retrieved from a database maintained by the Department of Leukemia at MDACC. Morphology was based on the French–American–British system [5].
Statistical analysis
Survival was calculated from the time of first diagnosis of ICH (by either CT or MRI brain) until death from any cause. Survival probabilities were estimated by the Kaplan-Meier method, and compared by the log-rank test. Differences among variables were evaluated by the chi-square test and Mann Whitney U test for categorical and continuous variables, respectively. Univariate and multivariate analyses were performed to identify potential prognostic factors associated with mortality after ICH (defining death from any cause as an event). Factors retaining significance in the multivariate model were interpreted as being independently predictive of mortality. Multivariate analysis of mortality used logistic regression model.
RESULTS
Clinical characteristics
Among 2,421 patients with acute leukemias and MDS referred to MDACC from 2005–2009, a total of 118 patients (4.9%) developed ICH, as diagnosed by either CT or MRI of the brain (AML, n = 71/1265, 5.7%; ALL, n = 19/304, 6.3%; MDS, n = 13/728, 1.6%; CML-BC, n = 15/124, 12.1%). The clinical and laboratory characteristics at the time of the bleed are shown in Table I. The localization of the bleed was intraparenchymal (IPH) in 58%, subdural (SDH) in 30%, subarachnoid (SAH) in 12%, and epidural (EDH) in 2.5%. Three patients had two different types of bleed. Median time from presentation to MDACC to ICH was 210 days (range 0–1953). Only 22 of 118 patients (18.6%) diagnosed with ICH bled within 30 days of diagnosis of leukemia. Among all bleeders, three patients carried a diagnosis of acute promyelocytic leukemia (APL), and two of them bled within 2 days of diagnosis. Table II describes the clinical indications leading to head imaging and diagnosis of ICH. As shown in Table II, the majority of the patients were symptomatic at the time of the hemorrhage, which subsequently promted the treating physicians to obtain head imaging. The majority of the patients were thrombocytopenic at the time of ICH diagnosis (median platelet count 19,000/μL; range: 0–318,000/μL). In general, patients with diagnosed ICH were transferred to the intensive care unit with neurosurgical consultation. The transfusion parameters for patients with ICH at our institution are platelet count goal of >80,000/μL, and International Normalized Ratio (INR) <1.5. The majority of the patients were not candidates for surgical interventions due to various factors such as depressed mental status, inability to achieve adequate platelet counts with transfusion, prolonged time from ICH to diagnosis, and location of the hemorrhage.
TABLE I.
Clinical Characteristics of Patients with Intracranial Hemorrhage (ICH)
| Median | Range | |
|---|---|---|
| Age (years) | 58 | 14–81 |
| Male (%) | N = 58 | (49%) |
| WBC (x103/uL) | 2.2 | 0–186 |
| Plt (x103/uL) | 19 | 0–318 |
| Hb (g/dL) | 9 | 4.5–14.7 |
| Peripheral Blasts (%) | 0 | 0–92 |
| INR | 1.4 | 0.4–12 |
| PTT (sec) | 29 | 17–66 |
| Fibrinogen (mg/dL) | 456 | 6–897 |
| Creatinine (mg/dL) | 0.9 | 0.3–3.8 |
| Albumin (g/dL) | 3 | 1.4–4.9 |
| LDH (IU/L) | 840 | 49–36082 |
WBC, White blood cell count; Plt, Platelet Count; Hb, Hemoglobin; INR, International Normalized Ratio; PTT, Partial thromboplastin time; LDH, Lactate dehydrogenase
TABLE II.
Presenting Signs and Symptoms which Prompted Brain Imaging
| N | (%) | |
|---|---|---|
| Mental status changes | 59 | 49.6 |
| Headache | 24 | 20.2 |
| Localized neurologic deficits | 11 | 9.2 |
| Fall/trauma | 10 | 8.4 |
| Others | 14 | 11.8 |
Overall survival and mortality after ICH onset
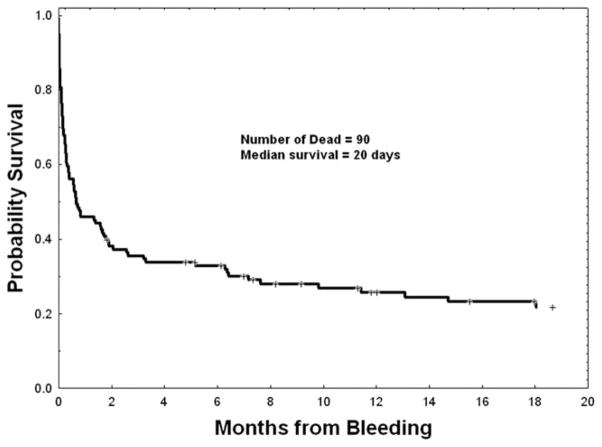
Median overall survival (OS) from the onset of ICH for all 118 patients was 20 days, and the time of last analysis (January 2011), 90 of 118 had died (Fig. 1). For the entire cohort, the 7-day and 30-day mortality were 35% (41 of 118) and 54% (64 of 118). The mortality rates based on the anatomic site of ICH (EDH vs. SDH vs. SAH vs. IPH vs. two or more sites) varied from 58 to 100% (Table III), but were not statistically significant, likely due to the small number of events at certain sites (e.g. epidural). Likewise, there was no statistical difference in the mortality rates based on the leukemia subtype (for ALL, AML, BC-CML and MDS the rates were 63, 78, 93, and 69%, respectively; P = 0.19). Less than 10% of the patients underwent a surgical intervention for ICH. The median time from diagnosis of ICH to surgical intervention was 0 days (range, 0–11 days). The mortality among patients who underwent surgery (11 of 12) vs. those who did not undergo surgery (79 of 106) was not statistically different (P = 0.29).
Figure 1.
OS for all 118 leukemia patients with ICH.
TABLE III.
Patient and Disease Characteristics at Bleeding in Association with Mortality
| Variable | Dead | Median (range) | N | No. dead (%) | P |
|---|---|---|---|---|---|
| Age (years), bleed | Y | 60 (21–80) | 0.005 | ||
| N | 48 (14–78) | ||||
| Wbc, bleed | Y | 3.25 (0–185.6) | 0.30 | ||
| N | 1.55 (0.1–116.4) | ||||
| ANC, bleed | Y | 0.65 (0–27.73) | 0.98 | ||
| N | 1.03 (0–27.7) | ||||
| Hgb, bleed | Y | 8.9 (4.5–14.6) | 0.27 | ||
| N | 9.4 (4.5–14.7) | ||||
| Plt, bleed | Y | 21 (0–318) | 0.55 | ||
| N | 16 (1–208) | ||||
| Blast% bleed | Y | 2 (0–99) | 0.02 | ||
| N | 0 (0–94) | ||||
| PTT, bleed | Y | 29.6 (16.8–66.2) | 0.22 | ||
| N | 28.9 (21.9–38.8) | ||||
| INR, bleed | Y | 1.48 (0.36–12.3) | 0.01 | ||
| N | 1.27 (0.95–3.23) | ||||
| Fibrinogen, bleed | Y | 494 (6–768) | 0.15 | ||
| N | 628 (190–897) | ||||
| Days from DX to bleed | Y | 208 (4–1923) | 0.11 | ||
| N | 106 (0–1099) | ||||
| Creat, bleed | Y | 0.9 (0.3–3.8) | 0.09 | ||
| N | 0.75 (0.4–1.7) | ||||
| Bili, bleed | Y | 0.8 (0.1–18.2) | 0.21 | ||
| N | 0.6 (0.1–23.3) | ||||
| Albumin, bleed | Y | 3 (1.4–4.9) | 0.003 | ||
| N | 3.6 (2–4.4) | ||||
| LDH, bleed | Y | 968 (101–36082) | 0.005 | ||
| N | 524 (49–5294) | ||||
| Sex | |||||
| Female | 60 | 44 (73) | 0.52 | ||
| Male | 58 | 46 (79) | |||
| Bleed onset | |||||
| Ind CR | 18 | 6 (33) | < 0.001 | ||
| No RX | 3 | 2 (67) | |||
| On ind | 39 | 30 (77) | |||
| Relapsed/multirx | 58 | 52 (90) | |||
| Leukemia subtype | |||||
| ALL | 19 | 12 (63) | 0.19 | ||
| AML | 71 | 55 (78) | |||
| CML | 15 | 14 (93) | |||
| MDS | 13 | 9 (69) | |||
| Prior malignancy, | |||||
| Yes | 26 | 22 (85) | 0.31 | ||
| No | 92 | 68 (74) | |||
| Rx, prior malignancy | |||||
| Chemo+X | 9 | 7 (78) | 0.48 | ||
| Chemo only | 7 | 7 (100) | |||
| X-ray only | 3 | 3 (100) | |||
| No rx (inc. no prior malignancy) | 99 | 73 (74) | |||
| Bleeding site | |||||
| ED | 2 | 2 (100) | 0.53 | ||
| IP | 67 | 52 (78) | |||
| SD | 34 | 26 (77) | |||
| SAH | 12 | 7 (58) | |||
| Two | 3 | 3 (100) | |||
| Surgery Intervention | |||||
| No | 106 | 79 (75) | 0.29 | ||
| Yes | 12 | 11 (92) | |||
Prognostic model for mortality after ICH
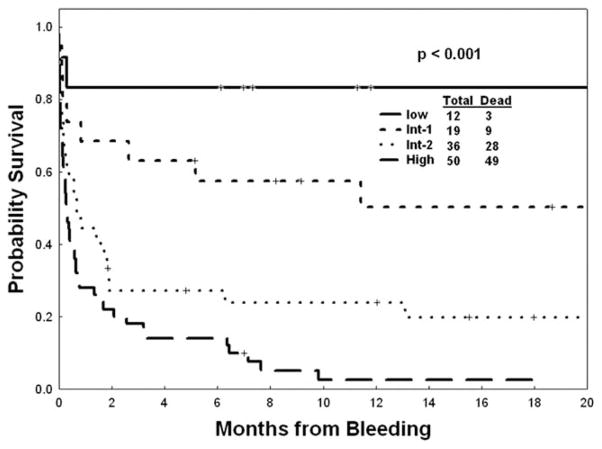
Since the anatomic sites of ICH and leukemia subtype were not prognostic for mortality after ICH, we next analyzed a range of patient and disease characteristics at the time of the diagnosis of ICH (+/− 1 day) for association with mortality (Table III). In univariate analysis, several factors (albumin, LDH, age, INR, peripheral blast count, and relapsed disease/multiple lines of therapy) were found to be associated with death after ICH as shown in Table III. In multivariate analysis using a logistic regression model, INR and blast count were not confirmed as independent significant predictors of mortality in ICH. Thus, following four independent variables were used for our prediction model: albumin <3.5 g/dL (P = 0.004), LDH ≥ 835 U/L (P = 0.017), age ≥60 years, and relapsed disease/status post multiple lines of therapy (vs. no prior therapy, ICH during induction therapy, or complete remission after induction therapy; P = 0.007). The risk ratios for mortality for each of these independent predictors are shown in Table IV. Using these four risk factors, mortality rates for patients with zero, one, two, three, and four of the above risk factors were 3 of 12 (25%), 9 of 19 (47%), 28 of 36 (78%), 37 of 38 (97%), and 12 of 12 (100%) (P < 0.001), respectively. Based on these findings, four risk levels predicting mortality in ICH were obtained: low (0 risk factor), intermediate-1 (one risk factor), intermediate-2 (two risk factors), and high (greater than two risk factors). The corresponding mortality rates ranged from 25 to 98% (Table V). The survival curves for the patients with ICH according to their risk group are shown in Fig. 2.
TABLE IV.
Patient and Disease Characteristics in Association with Mortality (Multivariate Analysis)
| Variable | Risk ratio | P value | Assigned score | |
|---|---|---|---|---|
| Albumin at bleed, g/dL | < 3.5 | 5.05 | 0.004 | 1 |
| Bleed onset: Relapsed/multiple Rx | Yes (vs. othersa) | 4.8 | 0.007 | 1 |
| LDH at bleed, U/L | > 835 (median) | 3.96 | 0.017 | 1 |
| Age at bleed, years | > 60 | 3.93 | 0.019 | 1 |
Others: In complete remission after induction chemotherapy, no chemotherapy yet, bleeding during induction therapy. Rx: Therapy.
TABLE V.
Estimated Outcomes Within Each Proposed Risk Category
| Category | No. of patients | Median survival (weeks) | 18-month survival (%) | Risk factors |
|---|---|---|---|---|
| Low | 12 | Not reached | 75 | 0 |
| Intermediate-1 | 19 | 25 | 53 | 1 |
| Intermediate-2 | 36 | 2 | 22 | 2 |
| High | 50 | 1 | 2 | 3, 4 |
Figure 2.
OS by mortality risk model in leukemia patients with ICH.
Discussion
To our knowledge, we report here the largest cohort of contemporary patients with leukemia who develop ICH. The incidence of ICH at our institution, as diagnosed by CT or MRI, was 5%. This is in concordance with previously published series [2–4,6,7], indicating that the occurrence of ICH in patients with leukemia has remained relatively constant over the past four decades. Although most previous studies highlight APL as the premier cause of ICH, in our cohort of over 2,400 patients only three patients with APL-developed ICH, likely due to heightened awareness for bleeding complications in these patients resulting in aggressive transfusion and early treatment with differentiating agents (all-trans retinoic acid and/or arsenic trioxide). We also presented the incidence of ICH by leukemia subtype, and found the highest incidence of ICH in patients in BC-CML, implicating a low threshold for head imaging in these patients. In patients with lymphoid BC-CML, the occurrence of bleeding has been linked to vincristine/prednisone induced dysregulation in fibrinogen levels and other coagulation factors [8]. Although other series have reported the onset of the majority of ICH cases during the first 30 days of diagnosis [2,4], in our cohort only 18.5% of cases occurred within 30 days of presentation, likely reflecting the patient pool of a large cancer center, where early death at diagnosis of leukemia precludes a referral to the tertiary center. Previously published reports identified low fibrinogen levels as a risk factor for ICH [2,4,9–12]. Many of these studies included patients with APL. However, in our series, only three patients with ICH carried a diagnosis of APL, and fibrinogen levels did not predict for mortality, as confirmed by a previous analysis [4]. Thrombocytopenia and elevated INR levels have been significantly associated with the incidence of ICH in published reports [2,4]. In our series, the majority of patients who developed ICH were thrombocytopenic (medial platelet count 19,000/μL), thus while a low platelet count predicts for increase ICH risk, in our model it did not predict mortality after ICH. An elevated INR was confirmed in univariate analysis to be associated with mortality (P = 0.01), but in multivariate this association was no longer significant, again emphasizing that previously recognized risk factors for ICH are different from factors that actually predict mortality after ICH.
Based on four clinico-laboratory parameters, we identified a risk model for mortality in patients with ICH, which distinguishes four subgroups with significantly different mortality rates, ranging from 25 to 100%. A serum albumin level below normal has been linked to mortality in leukemias before [13–15] and in our series was associated with a five-fold higher risk of death in patients with ICH (P = 0.004). Likewise, elevated serum LDH levels, previously found to be prognostic in acute leukemias is previous series [16–18], portended a fourfold higher risk for death (P = 0.017). We also found that age > 60 years, one of the poor prognostic factors for AML [19], was associated with an almost fourfold higher risk of death (P = 0.019). Finally, we established a 4.8-fold higher risk of death in patients with relapsed leukemia or those who were treated with more than one previous therapies when compared with patients with no prior chemotherapy, undergoing first induction therapy, or patients in complete remission after induction (P = 0.007).
Ideally, it would be desirable to develop a model that could accurately predict for the incidence of ICH in patients with leukemia. Such a model would likely help in prevention and or early intervention for ICH and possibly improve outcomes. Unfortunately, such a model is almost impossible to create since, as shown above, the patients with leukemia do not develop ICH at defined time points during the course of diagnosis and treatment. Therefore, it is very difficult to determine the temporal relationship between laboratory characteristics and bleed onset as these would have to be compared with patients who did not bleed, and it is not clear what time point to choose for the control cohort. Our analysis examined the mortality after ICH onset, thus identifying for both groups (dead vs. alive) a clearly defined time point: the onset of the ICH.
There are limitations to our study, mainly based on the retrospective nature of this work. For example, it would be helpful to determine the Glasgow Coma Scale [20] at the time of ICH onset to correlate the neurological status with survival, but unfortunately such information is not routinely documented in the charts. For our analysis, we had to rely on objective parameters which are routinely obtained and recorded. Also, the patients with ICH were not all uniformly treated, and the prognosis of the underlying leukemia might have guided the aggressiveness of the therapeutic approach to ICH. Nevertheless, in this contemporary cohort, the majority of the patients were transferred to the intensive care unit (see results) and similar transfusions parameters were applied to most of them. Ultimately, our prognostic model will aide in decision making but should be used within the greater context of the patients’ condition and overall prognosis.
In summary, our risk model based on the largest database of contemporary patients with leukemia, demonstrates that using four routine and simple clinical variables, it is possible to distinguish highly varying outcomes in leukemia patients with ICH, a condition that in general was believed to be fatal [2,4]. This risk prediction model should help in individualizing aggressive treatment (e.g., early surgical intervention) in patients with ICH based on their predicted prognosis.
Footnotes
Author Contributions
FD designed concept, analyzed data, wrote, and approved the manuscript. FR, JC, JW, EJ, SF, WW, DT, SOB, and HK provided materials and approved the manuscript. SSM, KN, JS, and SP analyzed data and approved the manuscript. GGM designed concept, wrote, and approved the manuscript.
Conflict of interest: Nothing to report.
References
- 1.Chang HY, Rodriguez V, Narboni G, et al. Causes of death in adults with acute leukemia. Medicine. 1976;55:259–268. doi: 10.1097/00005792-197605000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Chen CY, Tai CH, Tsay W, et al. Prediction of fatal intracranial hemorrhage in patients with acute myeloid leukemia. Ann Oncol. 2009;20:1100–1104. doi: 10.1093/annonc/mdn755. [DOI] [PubMed] [Google Scholar]
- 3.Groch SN, Sayre GP, Heck FJ. Cerebral hemorrhage in leukemia. Arch Neurol. 1960;2:439–451. doi: 10.1001/archneur.1960.03840100077011. [DOI] [PubMed] [Google Scholar]
- 4.Kim H, Lee JH, Choi SJ, et al. Risk score model for fatal intracranial hemorrhage in acute leukemia. Leukemia. 2006;20:770–776. doi: 10.1038/sj.leu.2404148. [DOI] [PubMed] [Google Scholar]
- 5.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Brit J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 6.Creutzig U, Ritter J, Budde M, et al. Early deaths due to hemorrhage and leukostasis in childhood acute myelogenous leukemia. Associations with hyperleukocytosis and acute monocytic leukemia. Cancer. 1987;60:3071–3079. doi: 10.1002/1097-0142(19871215)60:12<3071::aid-cncr2820601235>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Feldman EJ, Arlin ZA, Ahmed T, et al. Acute promyelocytic leukemia: a 5-year experience with new antileukemic agents and a new approach to preventing fatal hemorrhage. Acta Haematologica. 1989;82:117–121. doi: 10.1159/000205321. [DOI] [PubMed] [Google Scholar]
- 8.Sunder-Plassmann G, Speiser W, Korninger C, et al. Disseminated intravascular coagulation and decrease in fibrinogen levels induced by vincristine/prednisolone therapy of lymphoid blast crisis of chronic myeloid leukemia. Ann Hematol. 1991;62:169–173. doi: 10.1007/BF01703143. [DOI] [PubMed] [Google Scholar]
- 9.Sarris A, Cortes J, Kantarjian H, et al. Disseminated intravascular coagulation in adult acute lymphoblastic leukemia: frequent complications with fibrinogen levels less than 100 mg/dl. Leukemia Lymphoma. 1996;21:85–92. doi: 10.3109/10428199609067584. [DOI] [PubMed] [Google Scholar]
- 10.Sarris AH, Kempin S, Berman E, et al. High incidence of disseminated intra-vascular coagulation during remission induction of adult patients with acute lymphoblastic leukemia. Blood. 1992;79:1305–1310. [PubMed] [Google Scholar]
- 11.Ventura GJ, Hester JP, Dixon DO, et al. Analysis of risk factors for fatal hemorrhage during induction therapy of patients with acute promyelocytic leukemia. Hematol Pathol. 1989;3:23–28. [PubMed] [Google Scholar]
- 12.Wilde JT, Davies JM. Haemostatic problems in acute leukaemia. Blood Rev. 1990;4:245–251. doi: 10.1016/0268-960x(90)90004-c. [DOI] [PubMed] [Google Scholar]
- 13.Giles F, O’Brien S, Cortes J, et al. Outcome of patients with acute myelogenous leukemia after second salvage therapy. Cancer. 2005;104:547–554. doi: 10.1002/cncr.21187. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien S, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113:3186–3191. doi: 10.1002/cncr.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preisler H. Poor prognosis acute myelogenous leukemia. Leukemia Lymphoma. 1993;9:273–283. doi: 10.3109/10428199309148524. [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian HM, Thomas D, Ravandi F, et al. Outcome of adults with acute lymphocytic leukemia in second or subsequent complete remission. Leukemia Lymphoma. 2010;51:475–480. doi: 10.3109/10428190903503412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krug U, Rollig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: A web-based application for prediction of outcomes. Lancet. 2010;376:2000–2008. doi: 10.1016/S0140-6736(10)62105-8. [DOI] [PubMed] [Google Scholar]
- 18.Rollig C, Thiede C, Gramatzki M, et al. A novel prognostic model in elderly patients with acute myeloid leukemia: results of 909 patients entered into the prospective AML96 trial. Blood. 2010;116:971–978. doi: 10.1182/blood-2010-01-267302. [DOI] [PubMed] [Google Scholar]
- 19.Foran JM. New prognostic markers in acute myeloid leukemia: Perspective from the clinic. Hematology/Am Soc Hematol Educ Program. 2010;2010:47–55. doi: 10.1182/asheducation-2010.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]