Abstract
Background
Triple-negative breast cancer (TNBC) has negative expression of progesterone receptor (PR) and estrogen receptor (ER), and low expression of human epithelial growth factor receptor-2 (HER-2). This study aimed to investigate the expressional profile of cytokines in TNBC patients with significant expression of macrophages.
Material/Methods
Immunohistochemical (IHC) S-P staining method was used to detect the tumor-associated macrophages (TAMs) marker CD68 expression in 48 cases of TNBC samples. The correlation between CD68 expression and prognosis was analyzed. Expressions of key cytokines – interleukin-6 (IL-6), IL-10, IL-12, IL-1β, chemokine (C-C motif) ligand-5 (CCL-5), and macrophage inflammatory protein-2 (MIP-2) – were quantified by RT-PCR and enzyme-linked immunosorbent assay (ELISA).
Results
Thirty-four out of 48 TNBC samples (71.4%) had CD68-positive expression. IL-6 and CCL-5 were up-regulated in high-infiltrated tumors when compared to low-infiltrated samples. Other cytokines had no significant difference regarding the expression level across groups.
Conclusions
TAMs were up-regulated in most TNBC patients after the surgery. Its expression suggested unfavorable prognosis, especially in the high-infiltrated group. Those tumors with more macrophage also had elevated expression of cytokine IL-6 and chemotactic factor CCL-5, both of which have potency to be clinical index and drug target for TNBC.
MeSH Keywords: Adrenal Gland Neoplasms, Macrophages, Occlusal Adjustment
Background
Triple-negative breast cancer (TNBC) has negative expression of progesterone receptor (PR) and estrogen receptor (ER), and low expression of human epithelial growth factor receptor-2 (HER-2) [1,2]. Due to its strong invasiveness, unfavorable prognosis, high malignancy, and high reoccurrence, TNBC has relatively lower overall survival rate compared to other types of breast cancer [3–5]. Recent studies revealed the infiltration of immune cells in TNBC, accompanied with the features of stem cell and epithelial-mesenchymal transition [6,7].
Tumor-associated macrophages (TAMs) are infiltrated macrophages inside or adjacent to the tumor tissues and are major infiltrated cells in the micro-environment of tumors. Recent discoveries indicate a significant correlation between TAMs infiltration and cancer prognosis [8,9]. TAMs have been confirmed to facilitate the progression of tumors via up-regulating tumor infiltration and migration. As a specific marker of macrophages, CD68 can be used to detect the presence of TAMs [10,11]. A recent study suggested the potency of M2 type macrophage, which was often identified in TNBC, as a novel drug target for those breast cancers that are insensitivity to hormonal and HER2-target therapy [12].
Interleukin-6 (IL-6) facilitates the proliferation and differentiation of bone marrow-derived cells, in addition to potentiating the cell lysis ability of natural killer (NK) cells, via its synergistic effect on colony-stimulating factor (CSF). IL-6, as a pluripotent cytokine, modulates various cellular functions, including proliferation, differentiation, and immune defense. It is also involved in the progression of tumors by interference in the expression of cell adhesion and surface antigen molecules [13]. Chemokine (C-C motif) ligand-5 (CCL-5) is the most widely studied chemotactic factor and plays a critical role in recruiting leukocytes to inflammatory sites. CCL-5 is believed to facilitate metastasis of breast tumors, along with its receptor CCR5 [14]. IL-10 is an important factor in mononuclear macrophage-involved body immune processes, and IL-12 can suppress tumor growth via inducing strong cell immunity response. IL-1β can interfere with normal T-cell mediated immune response, thus causing the release of IL-17, which has oncogenic effects in the sense of tumor angiogenesis. Macrophage inflammatory protein-2 (MIP-2), also referred to as CCL-9, has been reported to be involved in liver metastasis of intestinal tumors. Current studies, however, have not revealed the expressional profiles of all those abovementioned cytokines/chemotactic factors as a whole in TNBC, especially those in patients with higher TAMs expression. This study thus investigated the expression of TAMs in 48 TNBC patients, followed by the quantification of related cytokines in CD68 high infiltration and low infiltration groups.
Material and Methods
Patient information
A total of 48 TNBC patients were recruited in this study. Inclusion criteria were: (1) With complete clinical records including tumor TNM staging, pathological diagnosis, post-operative follow-up and treatment plant; (2) With full follow-up record including the time and location of metastasis (if any) and clinical examination results. Patients had an average age at 48.4 years (range, 34~58 years). TNBC diagnosis was made based on negative test results in ER, PR, and HER-2 from biopsy samples. No significant difference existed in sex, age, and body weight between CD68 high expression and low expression group. The study protocol was approved by the Research Ethics Committee of our hospital, and all patients gave their informed consent before study commencement.
Immunohistochemical (IHC) staining
Tumor samples were firstly fixed in 10% formalin overnight, followed by dehydration in gradient ethanol (70% for 3 hours, 80% for 3 hours, 95% for 2 hours, 100% for 1.5 hours, two changes) and processed in xylene (0.5 hour, two changes) and paraffin (1 hour + 2 hours). After embedding in paraffin, tissues were prepared for 3 μm slices, which were de-waxed in routine way (xylene followed by gradient ethanol). Antigen retrieval was performed by hot citric acid buffer (pH 6) for 5 min. Non-specific binding sites were blocked by 10% bovine serum albumin (BSA) for 30 min. Primary antibody against CD68 (Santa Cruz, US) was applied for overnight incubation. On the next day, anti-mouse IgG was added for 1-hour incubation at 37ºC. DAB substrate was then used to develop the tissue slide, followed by hematoxylin counter-staining (3 min). Images were captured under a light-field microscope. The determination of CD68 high infiltration and low infiltration was made according to the infiltration of positive cells across the basal membrane as previously described [15].
RT-PCR
Equal volumes of tumor tissues were rinsed in Trizol reagents (Invitrogen, US) for 10 min, followed by 10-min centrifugation at 10 000 g. Supernatants were transferred into a new tube for lysing on ice, then 0.2 mL chloroform was added to extract nucleic acids by mixture, incubation on ice, and centrifugation (15 min at 10 000 g). The upper phase was saved with equal volume of isopropanol for precipitation at room temperature and 10 000 g centrifugation. RNA pellets were washed in 75% ethanol (in DEPC-treated water) and dissolved in 20 μL DEPC-treated water. RT-PCR was performed using reverse transcription kit (TaKaRa, Japan) using specific primers (as shown in Table 1) under the following parameters: 95ºC for 3 min, followed by 35 cycles each containing 95ºC denaturing for 30 s, 58ºC annealing for 40 s, and 72ºC elongation for 50 s, and ended with 72ºC elongation for 10 min.
Table 1.
Primer sequence.
| Gene | Forward primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| CCL5 | GGAACACGTCGTGGGATAATG | GGCAGACTTTGGATGCTTCTT |
| IL-1β | AGCTTCAGGCAGGCAGTATC | CATCTCGGAGCCTGTAGTGC |
| Mip-2 | AGTTTGCCTTGACCCTGAAG | GCACATCAGGTACGATCCAG |
| IL-6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| IL-10 | AGCCTTATCGGAAATGATCCAGT | GGCCTTGTAGACACCTTGGT |
| IL-12 | AACCTCACCTGTGACACGCC | CAAGTCCATGTTTCTTTGCACC |
| GAPDH | GGTGAAGGTCGGTGTGAACG | CTCGCTCCTGGAAGATGGTG |
Enzyme-linked immunosorbent assay (ELISA)
Test kits for human IL-6, IL-10, Il-12 and IL-1β were purchased from Ruiboao Biotech, Guangzhou, China. Test kits for human CCL-5 and MIP-2 were products of Hengyuan Biotech, Shanghai, China. ELISA was performed according to the manual instructions. In brief, total proteins were extracted from both CD68 high infiltration and low infiltration groups. Both standard samples and tissue samples were added into 96-well plates, followed by the addition of 0.1 mL biotin-labelled antibody and 60-min incubation at 37ºC. After gentle washing in 0.01 M TBS, ABC reagents were added into each well for further 30-min incubation. The color was developed in the dark by TMB reagents. After stopping, a microplate reader quantified the optical density value at 470 nm for further analysis.
Statistical analysis
SPSS 11.0 software package was used to process all collected data. Student’s t-test was used to compare means between groups. A statistical significance was defined when p<0.05.
Results
CD68 positive rate and prognosis
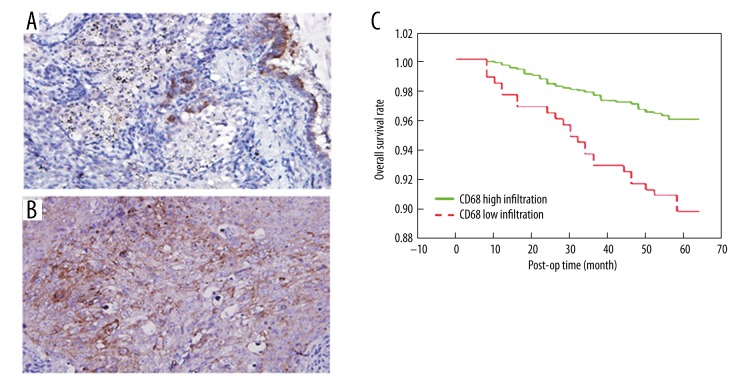
We found 34 out of 48 TNBC samples were positive for CD68 based on IHC staining. The positive signal was determined as brown granules inside cytoplasm of macrophages (Figure 1). Five fields were randomly selected from each slice. Using software statistics, CD68-positive tissues were further divided into 14 cases of low infiltration and 20 cases of high infiltration (Figure 1A, 1B). When combined with the post-operative follow-up, we found significantly higher survival rate of low-infiltration patients when compared to high-infiltration patients, as more than 95% of CD68 low-infiltration patients survived after 5 years, while less than 90% of high-infiltration individuals had 5-year survival (p<0.05, Figure 1C).
Figure 1.
CD68 expression and prognosis. (A) IHC staining images of CD68 low infiltration group; (B) Staining of CD68 high infiltration group; (C) Overall survival rate in low infiltration patients was significantly higher than the high infiltration group.
Elevated expression of IL-6 and CCL-5 in high infiltration tumors
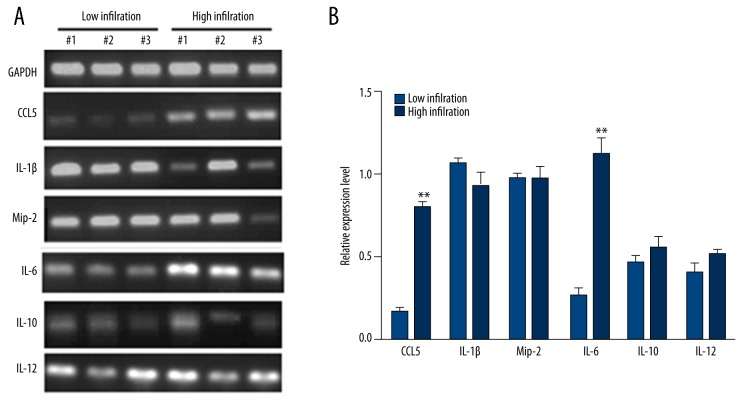
We measured the expression levels of IL-6, CCL-5, IL-1β, MIP-2, IL-10, and IL-12 mRNA in both groups. Results showed significantly elevated expression of CCL-5 and IL-6 in the high infiltration group (Figure 2).
Figure 2.
Cytokine expressions in tumors. (A) Representative DNA bands in both low infiltration and high infiltration groups. (B) Quantitative results of (A). **, p<0.01 compare to low infiltration group.
Elevated IL-6 and CCL-5 proteins in high infiltration group
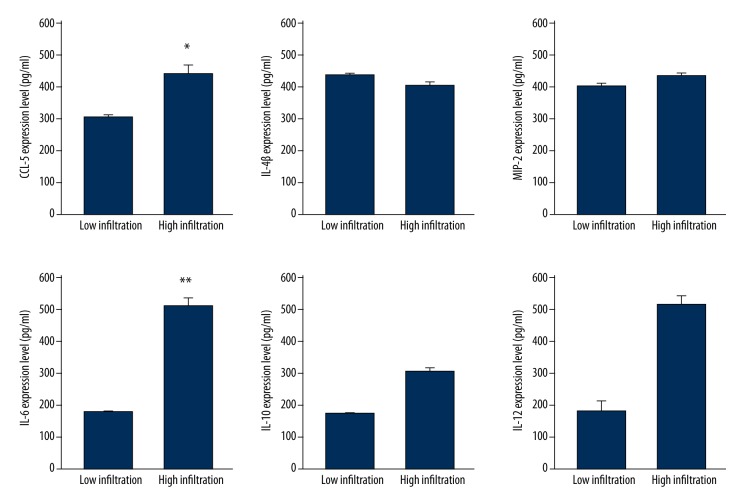
By ELISA approach, we obtained consistent results, as only IL-6 and CCL-5 proteins were up-regulated in high infiltration tumor samples (p<0.05, Figure 3).
Figure 3.
ELISA results of cytokines and chemotactic factors. *, p<0.05; **, p<0.01 compared to low infiltration group.
Discussion
The incidence of breast cancer has been increasing in recent years. As a type of high-risk breast cancer, TNBC has its unique immune phenotype, pathological features, and clinical progress, all of which make it insensitive to hormonal or target treatment [16]. Specific markers of TNBC have been studied previously. For example, RUNX1 protein has been shown to be correlated with TNBC to some extent, as patients with RUNX1 positive expression had shorter survival time compared to those with RUNX1 negative individuals [17]. TNBC has unfavorable prognosis and lower 3-year survival rate compared to other subtypes of breast cancer. Recent findings showed the activation of JAK/STAT signaling pathway in TNBC, and its inhibitor CYT387, can somehow inhibit the tumor growth [18,19]. Other studies indicated the potential correlation between CD44+/CD24− cells and TNBC, although the role of related cytokines requires further research [20–22].
As a specific marker for TAMs, the infiltration of CD68+ cells was positively related with tumor severity. Our study agreed with this opinion by showing significantly elevated 5-year survival rate of CD68 high-infiltration patients. Such differential patterns of CD68+ cells may contribute to various cytokine expressions. CCL-5 was firstly identified in breast cancer based on its expression or secretion from tumor cells [23]. IL-6 is a classical inflammatory factor with well-known function. Its expression in breast cancer, however, has received little research attention, especially in TNBC patients. On the other hand, IL-6 and IL-8 have been found to co-exist in various tumors. IL-6 has been reported to facilitate tumor infiltration and development of drug resistance by up-regulating X-linked inhibitor of apoptosis (XIAP) gene [24]. Other reports indicated the up-regulation of IL-6 on multidrug resistance receptor 1 (MDR1) gene expression as chemotherapy agents can facilitate the accumulation of IL-6 and IL-8, although the details of the mechanism require further study [25].
This study investigated the expression of cytokines, especially classical inflammatory cytokine IL-6 and the well-studied chemotactic factor CCL-5, in CD68+ TNBC patients. We found significantly elevated expression of those 2 factors in high infiltrated cases compared to other cytokines examined. ELISA results agreed that IL-6 and CCL-5 proteins were up-regulated in high infiltrated TNBC patients. These results provide new insights on the etiology of TNBC, in addition to providing a novel target, against which drugs targeting TNBC may be developed. The detailed reason for such high-expression and the effect of suppressing those factors on the tumor progression, however, requires further investigations.
Conclusions
In summary, this study provided evidences that CD68+ TNBC patients with high infiltration had lower survival rates compared to the low infiltration group. CD68 high infiltration patients had higher IL-6 and CCL-5 expression levels, suggesting the potential role of IL-6 and CCL-5 antagonist in suppressing tumor formation and development.
Footnotes
Source of support: Departmental sources
References
- 1.Youlden DR, Cramb SM, Dunn NA, et al. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36(3):237–48. doi: 10.1016/j.canep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Markkula A, Bromée A, Henningson M, et al. Given breast cancer, does breast size matter? Data from a prospective breast cancer cohort. Cancer Causes Control. 2012;23(8):1307–16. doi: 10.1007/s10552-012-0008-9. [DOI] [PubMed] [Google Scholar]
- 3.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106(33):13820–25. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69(10):4116–24. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evers B, Drost R, Schut E, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res. 2008;14(12):3916–25. doi: 10.1158/1078-0432.CCR-07-4953. [DOI] [PubMed] [Google Scholar]
- 6.Markkula A, Hietala M, Henningson M, et al. Clinical profiles predict early nonadherence to adjuvant endocrine treatment in a prospective breast cancer cohort. Cancer Prev Res (Phila) 2012;5(5):735–45. doi: 10.1158/1940-6207.CAPR-11-0442. [DOI] [PubMed] [Google Scholar]
- 7.Lundin KB1, Henningson M, Hietala M, et al. Androgen receptor genotypes predict response to endocrine treatment in breast cancer patients. Br J Cancer. 2011;105(11):1676–83. doi: 10.1038/bjc.2011.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith AK, Conneely KN, Pace TW, et al. Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy. Brain Behav Immun. 2014;38:227–36. doi: 10.1016/j.bbi.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambarus CA, Krausz S, van Eijk M, et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012;375(1–2):196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Multhoff G, Radons J. Radiation, inflammation, and immune responses in cancer. Front Oncol. 2012;2:58. doi: 10.3389/fonc.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 12.Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605–20. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Huang M, Wang L, Ma H, et al. Lack of an association between interleukin-6–174G/C polymorphism and circulating interleukin-6 levels in normal population: a meta-analysis. DNA Cell Biol. 2013;32(11):654–64. doi: 10.1089/dna.2013.2148. [DOI] [PubMed] [Google Scholar]
- 14.Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16(Suppl 1):1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 15.Tiainen S, Tumelius R, Rilla K, et al. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology. 2015;66(6):873–83. doi: 10.1111/his.12607. [DOI] [PubMed] [Google Scholar]
- 16.Stormes KA, Lemken CA, Lepre JV, et al. Inhibition of metastasis by inhibition of tumor-derived CCL5. Breast Cancer Res Treat. 2005;89(2):209–12. doi: 10.1007/s10549-004-5328-3. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari N, Mohammed ZM, Nixon C, et al. Expression of RUNX1 correlates with poor patient prognosis in triple negative breast cancer. PLoS One. 2014;9(6):e100759. doi: 10.1371/journal.pone.0100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyner JW, Bumm TG, Deininger J, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 2010;115(25):5232–40. doi: 10.1182/blood-2009-05-223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27(6):1322–17. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marotta LL, Almendro V, Marusyk A, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(–) stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121(7):2723–35. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiezzi DG, Valejo FA, Marana HR, et al. CD44+/CD24- cells and lymph node metastasis in stage I and II invasive ductal carcinoma of the breast. Med Oncol. 2012;29(3):1479–85. doi: 10.1007/s12032-011-0014-x. [DOI] [PubMed] [Google Scholar]
- 22.Britschgi A1, Andraos R, Brinkhaus H, et al. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell. 2012;22(6):796–811. doi: 10.1016/j.ccr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Thomas S, Baumgart DC. Targeting leukocyte migration and adhesion in Crohn’s disease and ulcerative colitis. Inflammopharmacology. 2012;20(1):1–18. doi: 10.1007/s10787-011-0104-6. [DOI] [PubMed] [Google Scholar]
- 24.Empana JP, Jouven X, Canouï-Poitrine F, et al. C-reactive protein, interleukin 6, fibrinogen and risk of sudden death in European middle-aged men: the PRIME study. Arterioscler Thromb Vasc Biol. 2010;30(10):2047–52. doi: 10.1161/ATVBAHA.110.208785. [DOI] [PubMed] [Google Scholar]
- 25.Anderson DR, Poterucha JT, Mikuls TR, et al. IL-6 and its receptors in coronary artery disease and acute myocardial infarction. Cytokine. 2013;62(3):395–400. doi: 10.1016/j.cyto.2013.03.020. [DOI] [PubMed] [Google Scholar]