Abstract
BACKGROUND
Idiopathic nephrotic syndrome (NS) is one of the most common glomerular disorders of childhood and is associated with increased urinary vitamin D-binding protein (uVDBP) excretion. We tested the hypothesis that uVDBP represents a biomarker to differentiate steroid-resistant nephrotic syndrome (SRNS) from the more benign forms of steroid-sensitive nephrotic syndrome (SSNS).
METHODS
This cross-sectional study included children with SRNS (n = 24), SSNS (n = 28), and normal controls (n = 5). Urine and clinical data were collected from patients. Measurements of uVDBP were performed with a commercially available ELISA kit and normalized to urine creatinine.
RESULTS
Concentrations of uVDBP were significantly higher (P < 0.001) in patients with SRNS (13,659 ng/mL, interquartile range [IQR] 477–22,979) than in patients with SSNS (94 ng/mL, IQR 53–202) and normal controls (23 ng/mL, IQR 22–99, P = 0.002). Significance did not change when the results were corrected for urine creatinine. uVDBP was significantly negatively correlated with estimated glomerular filtration rate (eGFR; R = −0.76, P = 0.03). However, uVDBP was still markedly elevated in patients with SRNS with eGFR >100 mL/minute/1.73 m2. There was a positive correlation between microalbuminuria (MALB/Cr) and uVDBP (R = 0.67, P < 0.001). However, uVDBP displayed a much higher discriminatory ability for distinguishing SRNS than MALB/Cr (area under the curve = 0.92 vs 0.67, respectively). An uVDBP cutoff of 362 ng/mL yielded the optimal sensitivity (80%) and specificity (83%) to distinguish SRNS from SSNS.
CONCLUSIONS
In this preliminary study, uVDBP represents a noninvasive biomarker that could distinguish SRNS from the more benign SSNS with high discriminatory power.
Keywords: nephrotic syndrome, steroid resistance, vitamin D, focal segmental glomerulosclerosis, minimal change disease, biomarkers
Introduction
Nephrotic syndrome (NS) is one of the most common glomerular diseases in children. The disease is characterized by relapsing episodes of edema, proteinuria, and hypoalbumine-mia.1 The two most common histopathological findings on invasive biopsy are minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS). Children with MCD most commonly display a steroid-sensitive course, whereas those with FSGS are often steroid resistant. The prognosis of children with NS depends on the underlying histopathol-ogy and the response to steroid treatment. Steroid-resistant nephrotic syndrome (SRNS) is significantly associated with poor outcome when compared to steroid-sensitive nephrotic syndrome (SSNS).2–5 The incidence of SRNS is on the rise, as marked by the increase in incidence of FSGS in children.6–8 FSGS is the most common primary glomerular disease leading to end-stage renal disease (ESRD) in children.9 An additional complication in patients with FSGS is the high rate of recurrence (30%–40%) following kidney transplant.10
A renal biopsy remains the standard of diagnosis in adults. Children, however, do not undergo an invasive biopsy at presentation, unless they have atypical features, because the response to steroids is a better predictor of long-term prognosis than is histology.11 In children, FSGS is often underdiagnosed by a single biopsy due to the smaller core size and focal nature of the disease, and the biopsy’s effectiveness in influencing outcome is questionable.12 What is greatly needed is an effective, noninvasive means of diagnosing SRNS to avoid the unnecessary effects of high-dose corticosteroids and allow earlier administration of alternative treatments for SRNS.
Both SSNS and SRNS are associated with a deficiency in vitamin D, attributed largely to the loss of its carrier, vitamin D-binding protein (VDBP), in the urine.13–15 Sato et al.16 demonstrated higher excretion of urinary vitamin D in patients with NS than in control patients. They found that the urinary vitamin D was unconjugated and likely excreted along with VDBP, which has a molecular weight (VDBP – 58 kDa vs albumin 66 kDa) and isoelectric point (VDBP – variants range from pI 4.8–5.2 vs albumin – pI 4.8) similar to albumin.17,18 Grymonprez et al.19 found that serum levels of VDBP were below control levels in children with NS and that serum levels of VDBP were negatively correlated with urinary excretion of VDBP. They also found that urinary VDBP (uVDBP) excretion was positively correlated with albumin excretion, but they did not distinguish between patients with SSNS and SRNS. Weng et al.20 showed that vitamin D deficiency was associated to a greater degree with SRNS than SSNS. We therefore hypothesized that the more pronounced vitamin D deficiency in SRNS is the result of increased loss of uVDBP in comparison with SSNS. In this cross-sectional pilot study of pediatric patients with idiopathic NS, we set out to determine the ability of uVDBP levels to differentiate SRNS from SSNS.
Methods
Patients and study design
This study complied with the principles of the Declaration of Helsinki. Under a protocol approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board, urine and clinical data were collected from patients, aged 2–19 years, who were diagnosed with idiopathic NS at Cincinnati Children’s Hospital Medical Center. The samples were collected over a period of 24 months. The study included 23 patients with SRNS (19 of whom had biopsy-proven FSGS), 28 patients with SSNS, and 5 healthy controls. Informed consent was recorded from all participants and/or their legal guardians. Exclusion criteria included history of gross hematuria, active or recurrent urinary tract infection, or NS secondary to systemic disease. Urine was collected as part of a standard clinical visit, centrifuged at 5,000 × g for five minutes, aliquoted, and stored at −80 °C. No more than two freeze–thaw cycles were used per sample. Demographic and clinical data, including urinalysis, steroid-response history, most recent serum creatinine, and current remission/relapse status, were recorded at the time of patient enrollment. Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the new Schwartz formula21 and classified to chronic kidney disease (CKD) stage.22 SSNS was defined as the ability to reach remission within eight weeks after initial diagnosis in response to steroid treatment, as evidenced by normalization of protein urine reading to a negative reading on a urine dipstick. SRNS was defined as a failure to respond to standard steroid treatment (2 mg/kg/day) for at least eight weeks. Normal controls were defined as children who had no history or evidence of renal disease.
Urine measurements
Urine VDBP was measured using a commercially available ELISA (R&D Systems). Intra- and interassay coefficient of variations were 5.9% and 6.2%, respectively. Data were analyzed raw and normalized to urine creatinine. Urine creatinine measurements were made using a modified Jaffe reaction, and microalbumin (MALB) was measured by immunoturbidimetry, both on a Dimension Xpand plus HM Clinical Analyzer (Siemens). Coefficients of variability for the creatinine measurements were 2.4% (intra) and 4.2% (total), and 2.9% (intra) and 5.9% (inter) for MALB. Urinalysis by dipstick (Multistix 10 SG, Siemens) was performed as part of a routine clinical care and read on a Clinitek+ Status Analyzer (Siemens).
Statistical analysis
Statistics were analyzed using Sig-maplot 12.0 (Systat Software Inc.). Medians and interquartile ranges (IQRs) were calculated and compared between groups using nonparametric Mann–Whitney rank sum analysis with P-values < 0.01 considered significant. A receiver operating characteristic (ROC) curve was also calculated to determine the specificity and sensitivity of VDBP to distinguish patients with SRNS from patients with SSNS. Subgroup analysis on the SSNS group was also preformed based on their positive/negative urine dipstick protein test to determine if VDBP levels were different between patients in remission (negative reading) or relapse (positive reading). Correlation data were generated using the Spearman’s rank correlation test; categorical demographic and clinical data were generated using Fisher’s exact tests.
Results
Fifty-two patients were enrolled over a 24-month period. Of those 52 patients, 24 had SRNS, 19 of which had biopsy-proven FSGS. Twenty-eight patients responded to steroid treatment and were labeled SSNS. At the time of urine collection, 10 patients with SSNS were in relapse and 18 were in remission. All patients with SRNS and the patients with active SSNS had 4+ proteinuria readings by dipstick at the time of collection. The 4+ reading is indicative of a protein concentration >2,000 mg/dL. Table 1 displays the patient demographics. SRNS differed from SSNS in terms of age (11.3 vs 6.6 years, P < 0.001), pathology (FSGS vs no biopsy, respectively, P < 0.001), presence of hypertension (56.5% vs 21.4%, respectively, P < 0.01), treatment (SRNS calcineurin inhibitor [CNI] 53.3%; SRNS mycophenolate mofetil [MMF] 30.4%; SRNS angiotensin-converting enzyme inhibition [ACEI] 39.1%), serum creatinine (1.30 ± 0.32 vs 0.42 ± 0.02 mg/dL, respectively, P < 0.001), and eGFR (SRNS 82.9 ± 7.9 vs SSNS 128 ± 7.1 mg/dL, P < 0.001).
Table 1.
Patient demographics.
| VARIABLE | SRNS (n = 24) | SSNS (n = 28) | P-VALUE |
|---|---|---|---|
| Age (years; mean ± SD) | 11.3 ± 1.2 | 6.6 ± 0.6 | 0.001 |
| Sex (%) Male | 15(65.2) | 17 (60.7) | NS |
| Race (%) African American Caucasian Other |
8 (34.87) 14 (60.9) 1 (4.3) |
12 (42.9) 14 (50) 2 (7.1) |
NS |
| Pathology (%) FSGS MCD Other No biopsy |
19 (82.6) 2 (8.7) 2 (8.7) 1 (4.3) |
1 (3.6) 6 (21.4) 0 21 (75) |
NA |
| Hypertension (%) | 13 (56.5) | 6 (21.4) | 0.01 |
| Immunosuppressant (%) Steroid CNI MMF |
9 (39) 8 (53.3) 7 (30.4) |
22 (77.8) 0 0 |
NA |
| ACEI/ARB (%) | 9 (39.1) | 3 (10.7) | NA |
| Cr (mg/dl) | 1.30 + 0.32 | 0.42 + 0.02 | 0.001 |
| GFR (ml/min/1.73 m2) | 82.0 + 7.91 | 128.0 + 7.12 | 0.001 |
| MALB/Cr (mg/mg; ± SD) | 1.98 ± 2.04 | 1.43 ± 1.43 | 0.59 |
Median biomarker levels were subjected to Mann–Whitney rank sum analysis. uVDBP was significantly higher (P < 0.001) in patients with SRNS (13,659 ng/mL, IQR 477–22,979) than in both patients with SSNS (94 ng/mL, IQR 53–202) and controls (23 ng/mL, IQR 22–99, P = 0.002), as shown in Figure 1. Statistical significance did not change when uVDBP levels were corrected for urine creatinine. Subgroup analysis (Fig. 2) showed that while trending higher, VDBP was not significantly different between patients with SSNS with active proteinuria (203.7 ng/mL; IQR 39.7–717.9) and those in remission (42.1 ng/mL; IQR 15–144). VDBP levels were significantly higher in patients with SRNS than in patients with SSNS both with active disease (P = 0.007) and in remission (P < 0.001).
Figure 1.
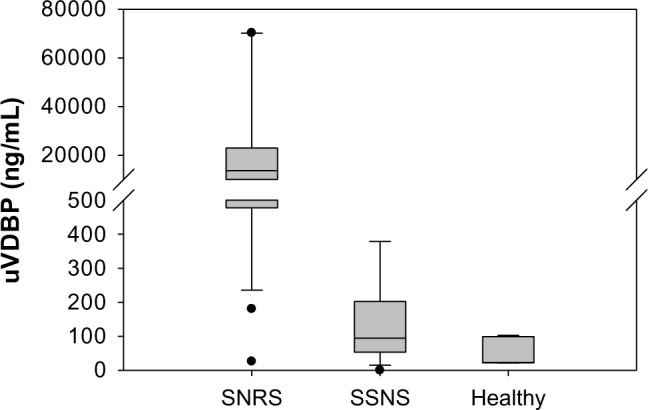
Urine VDBP measurements. Median urine VDBP was significantly higher in patients with SRNS than in both patients with SSNS and healthy controls (P < 0.001).
Figure 2.
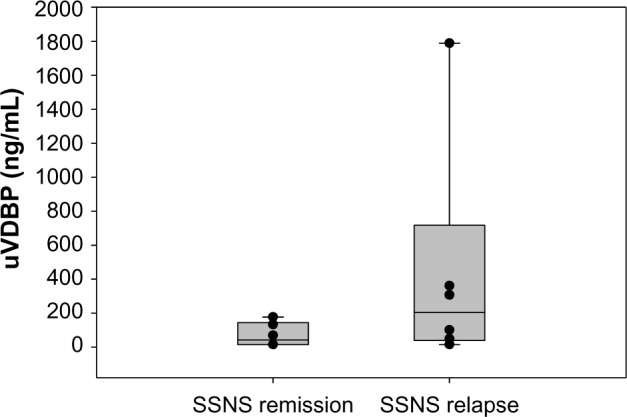
VDBP subgroup analysis. While trending higher, VDBP was not significantly higher in patients with active proteinuric SSNS than those in remission.
To test the discriminatory power of VDBP to distinguish patients with SRNS from patients with SSNS, we ran a ROC curve analysis (Fig. 3A). The area under the curve (AUC) for VDBP to distinguish SRNS from SSNS was 0.87 (P = 0.0001; 95% CI 78.8–0), indicative of a good discriminatory biomarker. The cutoff for optimum sensitivity (80%) and specificity (83%) was 362 ng/mL. Since previous studies had linked uVDBP excretion to proteinuria, we tested whether urinary protein excretion itself was a good predictor of SRNS versus SSNS. We first tested whether uVDBP excretion was correlated with proteinuria as was previously reported.19 Spear-man’s rank correlation analysis revealed that MALB/Cr was positively correlated with VDBP excretion with an R-value of 0.66 (P < 0.0001), consistent with previous findings (Fig. 4). To control for low levels of protein in patients in remission, only patients with active disease were compared to determine if MALB/Cr was different between SRNS and SSNS. There was a trend toward increased MALB/Cr in the urine of patients with SRNS with active proteinuria versus patients with SSNS with active proteinuria, but it did not reach signifi-cance (1.6 mg/mg, IQR 0.7–2.3, to 0.9 mg/mg, IQR 0.6–2.8, P = 0.3). ROC analysis revealed that MALB/Cr had an AUC of 0.67 to distinguish SRNS from SSNS but once again did not reach significance (P = 0.07, Fig. 3B). These findings indicate that uVDBP can distinguish SRNS from SSNS independent of proteinuria.
Figure 3.
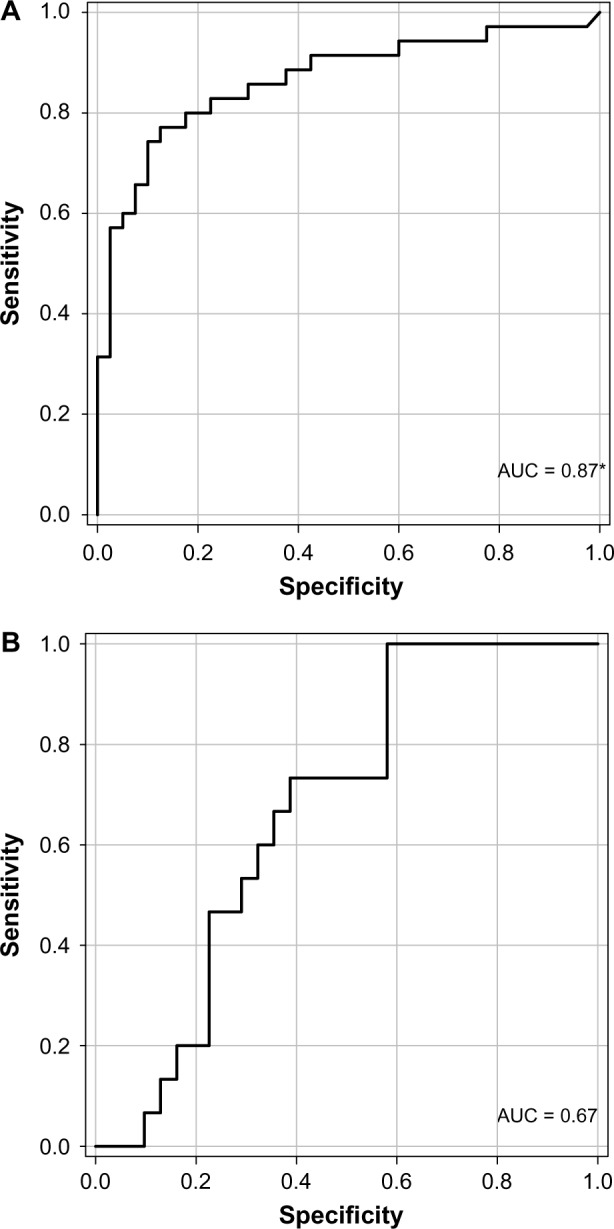
Urine VDBP ROC analysis. (A) VDBP shows good discriminatory power to distinguish SRNS from SSNS (AUC = 0.87; P = 0.0001). (B) MALB/Cr shows mild discriminatory power to distinguish SRNS from SSNS, but it did not reach significance (AUC = 0.67; P = 0.07).
Note: *indicates significance.
Figure 4.
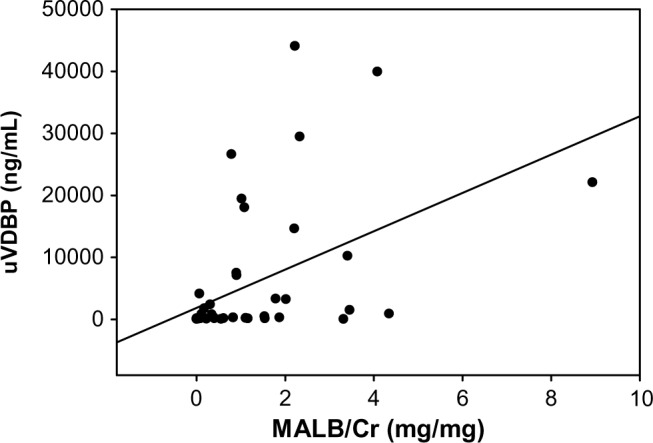
MALB/Cr is positively correlated with uVDBP excretion. R = 0.66, P < 0.001.
To determine if increased uVDBP in SRNS may be related to worse tubular injury from more advanced disease, we examined uVDBP levels as a function of eGFR (Fig. 5). VDBP in patients with SRNS was significantly negatively correlated with GFR (r = −0.76; P = 0.03). However, even SRNS with eGFRs well over the normal 100 mL/minute/1.73 m2 range displays very high levels of uVDBP (10,000–20,000 ng/mL). This indicates that while uVDBP does seem to increase with diminished eGFR, there is still a marked increase in the excretion of uVDBP in patients with SRNS early in the disease course who still have well-preserved kidney function.
Figure 5.
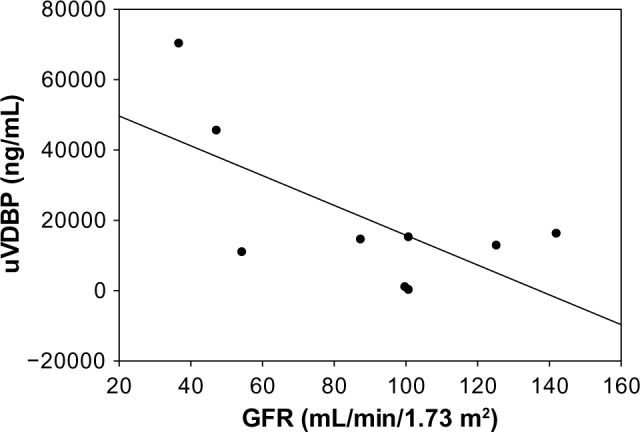
VDBP correlates with disease severity in SRNS as evidenced by GFR. R = −0.76, P = 0.03.
Discussion
Steroid resistance in idiopathic NS is strongly associated with poor outcomes, including progression to ESRD. Currently no validated diagnostic markers exist to distinguish SSNS from SRNS, and patients often must endure high-dose corticosteroid therapy prior to invasive kidney biopsy and histopathologic diagnosis. In this pilot study, our objective was to determine if uVDBP measurements could be used as a noninvasive biomarker to distinguish SRNS, which has generally poor prognosis and a high risk for progression, from the more benign SSNS.
Our results show that uVDBP concentrations are markedly increased in patients with SRNS versus patients with SSNS and healthy controls (P < 0.001). These results remained significant after correcting for urine creatinine. Previous studies investigating VDBP in NS showed strong correlations between uVDBP and proteinuria.19,23 These earlier studies, however, did not investigate differences between SRNS and SSNS. Our study confirmed the strong positive correlation between uVDBP excretion and proteinuria (R = 0.66, P < 0.001). However, proteinuria did not fully account for the higher levels of uVDBP in patients with SRNS since the AUC for MALB/Cr was only 0.67 and failed to reach significance. In contrast, uVDBP by itself showed high discriminatory power (AUC = 0.87, P < 0.0002) between patients with SRNS and SSNS, independent of proteinuria, indicating that there may be a disease-specific process leading to increased uVDBP in patients with SRNS. One plausible explanation relates to the fact that reabsorption of any filtered VDBP requires the integrity of megalin and cubulin receptors in the proximal tubule. Thus, any form of chronic tubular injury, as would be expected in SRNS, could result in increased uVDBP excretion.
Indeed, uVDBP excretion has recently been demonstrated to be a potential marker of renal interstitial damage and fibrosis.24 This study demonstrated elevated urinary excretion of uVDBP very early in a rat model of adriamycin-induced nephrosis, before onset of fibrosis and inflammatory interstitial renal damage. In humans, uVDBP was increased in subjects with microalbuminuria as well as in patients with CKD from diverse etiologies with overt proteinuria. In patients with CKD, urine VDBP levels diminished to a degree in response to renoprotective therapy (ACEI) but remained 100-fold increased during maximal therapy versus controls (P < 0.001), demonstrating that levels persist independent of proteinuria. This would be consistent with our findings that even between patients with SRNS and SSNS with active high-grade (>2,000 mg/dL) proteinuria, uVDBP levels are much more elevated in patients with SRNS than could be explained by the degree of proteinuria alone. This could render uVDBP as a good potential marker to monitor TI damage and fibrosis in patients with NS, if its relationship to these histological features can be further validated. Previous attempts to find markers to track fibrotic features in NS have been unsuccessful. Valles et al.25 examined urinary levels of the tubular damage biomarkers beta 2-microglobulin (beta 2M) and N-acetyl-beta-d-glucosaminidase (NAG) during active disease and remission over a follow-up period of three years in patients diagnosed with FSGS. Although urinary NAG was found to be elevated in patients with FSGS compared to patients with SSNS and steroid-dependent nephrotics, there was no link between levels of NAG or beta 2M and histo logical evidence of tubulointerstitial (TI) damage in these patients.
Previous studies have examined urinary concentrations of neutrophil gelatinase-associated lipocalin (NGAL), an established biomarker for acute kidney injury, in children with NS.26 Urinary NGAL levels were shown to be significantly higher in patients with SRNS than in patients with SSNS (P < 0.001) with a similar discriminatory power as VDBP to distinguish SRNS from SSNS (NGAL – AUC 0.91; VDBP – AUC 0.87). To our knowledge, uVDBP and NGAL are the first two identified markers to successfully distinguish SRNS from SSNS with a high degree of reliability. However, both are markers of tubular damage and are unlikely to add to each other’s discriminatory power. One earlier study investigated whether a high-throughput cytokine panel could distinguish SRNS from SSNS or FSGS from MCD.27 In this study, although there was a 5.5-fold increase in overall cytokine expression in patients with NS than control subjects, they failed to find a specific marker that could distinguish steroid responsiveness. Another group discovered a proteomic signature that could discriminate SRNS from SSNS using surface-enhanced laser desorption ionization time of flight mass spectrometry.28 While the authors found that the panel had a positive predictive value of 96% and a negative predictive value of 88%, this panel has limited clinical utility because the peptides were not identified and this type of mass spectrometry is not readily available in most clinical laboratories. In the clinic, it is likely that multiple biomarkers will be needed in a panel to provide the best discriminatory and predictive power for steroid responsiveness in NS.
Our study is not without limitations. First, this was a single center, cross-sectional pilot study with a small group of patients who had already begun treatment at enrollment. This limits the conclusions we can draw about the value of urine VDBP to predict steroid responsiveness in NS patients. Since the data are not normally distributed, we used nonparametric statistics to analyze our data. This variability is inherent when studying steroid responsiveness in NS and can be magnified in a small patient population. There is also a significant age difference between our patients with SRNS and our patients with SSNS. This is inherent in any study of NS since the majority of patients with SSNS have MCD and the majority of patients with SRNS have FSGS. Approximately 70% of children with MCD are under five years of age, while primary FSGS is typically not diagnosed until after the age of six years.11 However, with such a high discriminatory power (AUC 0.87, P < 0.0001), our results are unlikely to represent an artifact of age differences. Since serum samples were not available to our research team, we were unable to determine if the massive loss of VDBP in the urine had a potential effect on serum levels of vitamin D. In future expanded studies, this will be an important addition to our protocol as it has been demonstrated that a large percentage of children are vitamin D deficient at diagnosis and steroid treatment can exacerbate the effects of vitamin D deficiency.29 Lastly, it must be acknowledged that uVDBP may not be specific to SRNS and may simply reflect proximal tubular injury that can occur in many forms of CKD. However, within the clinical context of a child with NS, our results indicate promising utility of uVDBP for the discrimination of the steroid-resistant form of the disease.
Our results call for a multicenter, prospective longitudinal study to capture a larger number of patients with new onset NS prior to administration of treatment. This would allow us to determine whether uVDBP levels could predict response to treatment and obviate the need for unnecessary exposure to high-dose corticosteroids and other powerful immunosuppressants in patients who are unlikely to respond. Additionally, it would be valuable to determine if VDBP levels could be used to monitor response to treatment. Biomarkers that can be used as surrogate end points are valuable in clinical trials and can allow for more rapid drug development. The discovery of a urine biomarker that could predict response to treatment in NS could aid the physician in developing an individualized treatment plan that could potentially lead to better care for patients with this serious and progressive disease.
Footnotes
ACADEMIC EDITOR: Karen Pulford, Editor in Chief
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1420 words, excluding any confidential comments to the academic editor.
FUNDING: This work was funded by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 8UL1TR000077-04 to MRB and by NIH P50 DK096418 to MRB and PD. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: MRB discloses a patent pending for compositions and methods for treating steroid resistant nephrotic syndrome, 14/838,551. Other authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Study design, data collection, statistical analysis, result interpretation, and manuscript preparation: MRB. Data collection and manuscript preparation: AP, CH, LP, QM. Study design, result interpretation, and manuscript preparation: PD. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Lewis JB, Nielson EG. Glomerular diseases. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw Hill; 2012. p. 283. [Google Scholar]
- 2.Cattran DC, Rao P. Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis. 1998;32(1):72–9. doi: 10.1053/ajkd.1998.v32.pm9669427. [DOI] [PubMed] [Google Scholar]
- 3.Hari P, Bagga A, Jindal N, et al. Treatment of focal glomerulosclerosis with pulse steroids and oral cyclophosphamide. Pediatr Nephrol. 2001;16(11):901–5. doi: 10.1007/s004670100680. [DOI] [PubMed] [Google Scholar]
- 4.Gipson DS, Chin H, Presler TP, et al. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol. 2006;21(3):344–9. doi: 10.1007/s00467-005-2097-0. [DOI] [PubMed] [Google Scholar]
- 5.Roberti I, Vyas S. Long-term outcome of children with steroid-resistant nephrotic syndrome treated with tacrolimus. Pediatr Nephrol. 2010;25(6):1117–24. doi: 10.1007/s00467-010-1471-8. [DOI] [PubMed] [Google Scholar]
- 6.Gulati S, Sharma AP, Sharma RK, et al. Changing trends of histopathology in childhood nephrotic syndrome. Am J Kidney Dis. 1999;34(4):646–50. doi: 10.1016/S0272-6386(99)70388-4. [DOI] [PubMed] [Google Scholar]
- 7.Bonilla-Felix M, Parra C, Dajani T, et al. Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int. 1999;55(5):1885–90. doi: 10.1046/j.1523-1755.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava T, Simon SD, Alon US. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatr Nephrol. 1999;13(1):13–8. doi: 10.1007/s004670050555. [DOI] [PubMed] [Google Scholar]
- 9.Smith JM, Stablein DM, Munoz R, et al. Contributions of the transplant registry: the 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Pediatr Transplant. 2007;11(4):366–73. doi: 10.1111/j.1399-3046.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 10.Korbet SM. Clinical picture and outcome of primary focal segmental glomerulosclerosis. Nephrol Dial Transplant. 1999;14(suppl 3):68–73. doi: 10.1093/ndt/14.suppl_3.68. [DOI] [PubMed] [Google Scholar]
- 11.Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362(9384):629–39. doi: 10.1016/S0140-6736(03)14184-0. [DOI] [PubMed] [Google Scholar]
- 12.Gulati S, Sharma AP, Sharma RK, et al. Do current recommendations for kidney biopsy in nephrotic syndrome need modifications? Pediatr Nephrol. 2002;17(6):404–8. doi: 10.1007/s00467-002-0840-3. [DOI] [PubMed] [Google Scholar]
- 13.Barragry JM, France MW, Carter ND, et al. Vitamin-D metabolism in nephrotic syndrome. Lancet. 1977;2(8039):629–32. doi: 10.1016/s0140-6736(77)92498-9. [DOI] [PubMed] [Google Scholar]
- 14.Chesney RW, Hamstra A, Rose P, et al. Vitamin D and parathyroid hormone status in children with the nephrotic syndrome and chronic mild glomerulonephritis. Int J Pediatr Nephrol. 1984;5(1):1–4. [PubMed] [Google Scholar]
- 15.Yousefzadeh P, Shapses SA, Wang X. Vitamin D binding protein impact on 25-hydroxyvitamin D levels under different physiologic and pathologic conditions. Int J Endocrinol. 2014;2014:6. doi: 10.1155/2014/981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato KA, Gray RW, Lemann J., Jr Urinary excretion of 25-hydroxyvitamin D in health and the nephrotic syndrome. J Lab Clin Med. 1982;99(3):325–30. [PubMed] [Google Scholar]
- 17.Vlasova IM, Saletsky AM. Study of the denaturation of human serum albumin by sodium dodecyl sulfate using the intrinsic fluorescence of albumin. J Appl Spectrosc. 2009;76(4):536–41. [Google Scholar]
- 18.Tang WX, Bazaraa HM, Magiera H, et al. Electrophoretic mobility shift assay identifies vitamin D binding protein (Gc-globulin) in human, rat, and mouse sera. Anal Biochem. 1996;237(2):245–51. doi: 10.1006/abio.1996.0236. [DOI] [PubMed] [Google Scholar]
- 19.Grymonprez A, Proesmans W, Van Dyck M, et al. Vitamin D metabolites in childhood nephrotic syndrome. Pediatr Nephrol. 1995;9(3):278–81. doi: 10.1007/BF02254183. [DOI] [PubMed] [Google Scholar]
- 20.Weng FL, Shults J, Herskovitz RM, et al. Vitamin D insufficiency in steroid-sensitive nephrotic syndrome in remission. Pediatr Nephrol. 2005;20(1):56–63. doi: 10.1007/s00467-004-1694-7. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Kidney F K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–266. [PubMed] [Google Scholar]
- 23.Doorenbos CR, de Cuba MM, Vogt L, et al. Antiproteinuric treatment reduces urinary loss of vitamin D-binding protein but does not affect vitamin D status in patients with chronic kidney disease. J Steroid Biochem Mol Biol. 2012;128(1–2):56–61. doi: 10.1016/j.jsbmb.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Mirkovic K, Doorenbos CR, Dam WA, et al. Urinary vitamin D binding protein: a potential novel marker of renal interstitial inflammation and fibrosis. PLoS One. 2013;8(2):e55887. doi: 10.1371/journal.pone.0055887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valles P, Peralta M, Carrizo L, et al. Follow-up of steroid-resistant nephrotic syndrome: tubular proteinuria and enzymuria. Pediatr Nephrol. 2000;15(3–4):252–8. doi: 10.1007/s004670000472. [DOI] [PubMed] [Google Scholar]
- 26.Bennett MR, Piyaphanee N, Czech K, et al. NGAL distinguishes steroid sensitivity in idiopathic nephrotic syndrome. Pediatr Nephrol. 2012;27(5):807–12. doi: 10.1007/s00467-011-2075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woroniecki RP, Shatat IF, Supe K, et al. Urinary cytokines and steroid responsiveness in idiopathic nephrotic syndrome of childhood. Am J Nephrol. 2008;28(1):83–90. doi: 10.1159/000109396. [DOI] [PubMed] [Google Scholar]
- 28.Woroniecki RP, Orlova TN, Mendelev N, et al. Urinary proteome of steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome of childhood. Am J Nephrol. 2006;26(3):258–67. doi: 10.1159/000093814. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen CA, Jensen JE, Cortes D. Vitamin D status is insufficient in the majority of children at diagnosis of nephrotic syndrome. Danish Med J. 2015;62(2) pii: A5017. [PubMed] [Google Scholar]


