Abstract
Despite substantial advances in early diagnosis, breast cancer (BC) still remains a clinical challenge. Most BC models use complex in vivo models and two-dimensional monolayer cultures that do not fully mimic the tumor microenvironment. The integration of cancer biology and engineering can lead to the development of novel in vitro approaches to study BC behavior and quantitatively assess different features of the tumor microenvironment that may influence cell behavior. In this review, we present tissue engineering approaches to model BC in vitro. Recent advances in the use of three-dimensional cell culture models to study various aspects of BC disease in vitro are described. The emerging area of studying BC dormancy using these models is also reviewed.
Keywords: spheroids, microfluidics, scaffolds, dormancy, three-dimensional culture, tissue engineering, tumor engineering
Introduction
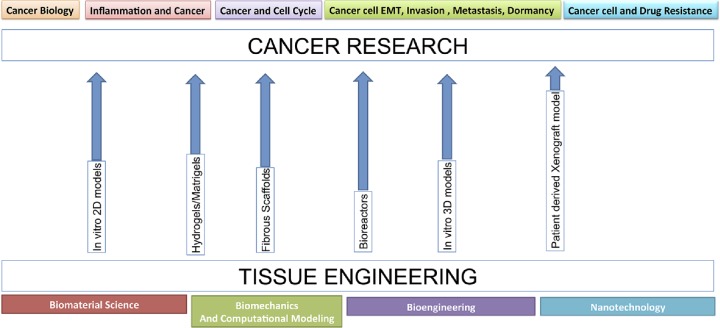
Despite significant improvements in research and development in the cancer field, about 95% of oncology drugs in clinical trials fail to receive Food and Drug Administration approval.1 Part of the issue is the lack of suitable preclinical models that take into account the complexity of the disease and accurately represent disease progression. A growing need exists for technologies in research that can accurately recapitulate the in vivo environment. These models can represent the biological, physical, and biochemical environment of the natural extracellular matrix (ECM). Several innovations in tissue engineering have led to the design of scaffold- or matrix-based culture systems that more closely mimic the native ECM. For many years, cancer researchers have relied on two-dimensional (2D) monolayer culture studies and small animal models to study the complex tumorigenic mechanisms of angiogenesis, invasion, and metastasis. However, 2D cell culture models lack the structure for proper cell–cell and cell–matrix interactions and are not able to replicate an in vivo phenotype.2–5 Multiple studies have used small animal models for conducting cancer research. However, there exist major differences between cancer progression in humans and animals.3,6 Also, using animals can be very costly, laborious, and requires animal facilities as well as Institutional Animal Care and Use Committee approval.7 Moreover, understanding specific factors such as chemical, cellular, and mechanical cues in animal models can be difficult to discern due to their complexity.8,9 Recently, there has been promising published work on three-dimensional (3D) cell culture models developed to study breast cancer (BC) tumor progression in vitro. Results show that these models have the capability of re-establishing the cellular morphologies and phenotypes present during in vivo tumor development.10–15 The 3D cell culture has been shown to impact cell morphology, gene/protein expression, signal transduction, proliferation, migration, polarization, and drug tolerance.16–19 As shown in Figure 1, cancer biology combined with tissue engineering strategies (scaffolds, microfabrication, and biologically inspired culture models) enable studies of various aspects of disease dynamics across different scales.20,21 For instance, at the tissue level, factors such as cell–cell and cell–ECM interactions, culture dimensionality, and soluble factor transport and signaling can be explored using biomaterials, scaffold fabrication techniques, and bioreactors. Moreover, at the cellular level, features such as topography and mechanical properties can be tailored using microfluidic channels.22,23 In addition, with the integration of cancer biology and engineering, novel in vitro approaches can be used to quantitatively assess different features of the tumor microenvironment.24–26 In this review, we discuss the recent literature using tissue engineering approaches in developing in vitro models for BC research and drug discovery. The 3D in vitro models and their applications with an emphasis on studying dormancy are described.
Figure 1.
Tissue engineering concepts offer a powerful toolbox for cancer research areas. Modified from Hutmacher et al.63 Reused with permission from Elsevier.
Three-dimensional Culture Systems
The 3D culture models are important tools in the advancement of basic cancer research. Nelson and Bissell11 have been one of the pioneers to use 3D to model the murine mammary gland in both its normal and diseased state. They also showed that the design criteria to model BC in vitro in 3D are similar to those used in tissue engineering. Other groups have also been able to adopt their techniques to study cancer in 3D and further develop matrices to sustain organoid growth in vitro. Based on the current literature, next steps are to utilize them as predictive models in massive drug screening processes, shifting from academia to the pharmaceutical industry.
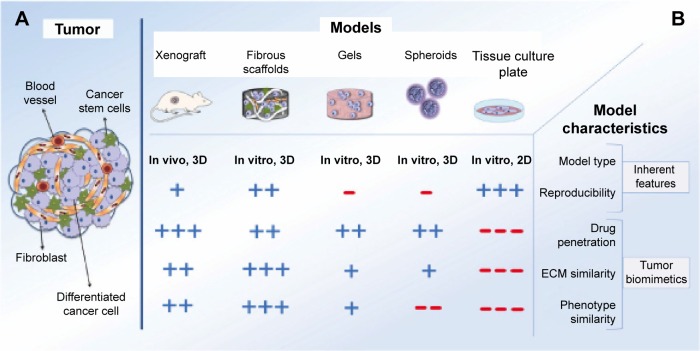
As shown in Figure 2, 3D in vitro culture systems to model tumors can be mainly categorized into cells cultured as multicellular aggregates (spheroids) and cells embedded in constructs made of natural or synthetic matrices.27 In addition, in vitro systems have been developed to model tumors and the influence of the microenvironment using high-throughput technologies such as microfluidic devices. These systems are described in more detail in the following sections.
Figure 2.
Schematic picture of (A) a tumor and (B) different tumor models. Modified from Ricci et al.27 Reused under the terms of a CC-BY license.
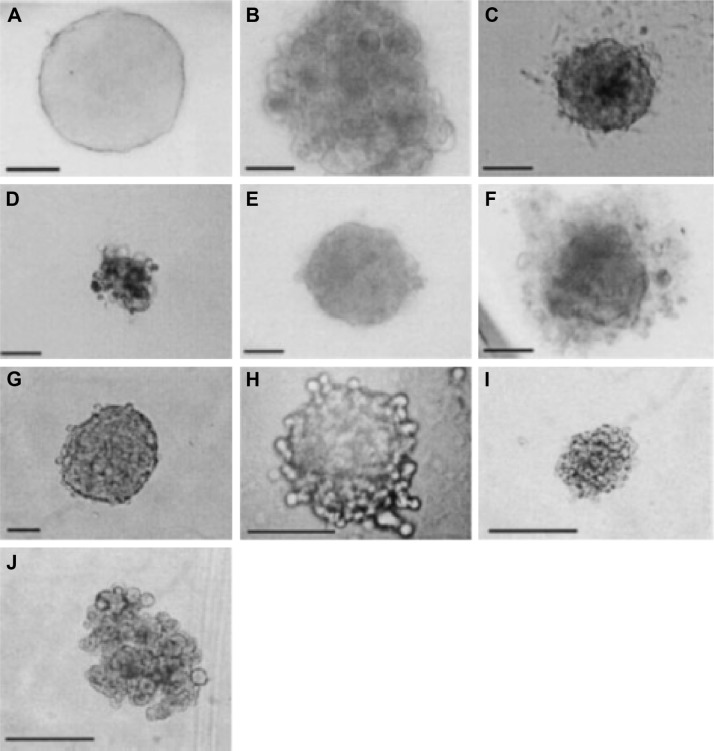
Figure 3.
Spheroids of various cell lines generated by the hanging drop method (bar = 100 μm). (A) MCF-7 (mammary gland adenocarcinoma); (B) WW; (C) MM483 (human multiple myeloma); (D) MM239 (human multiple myeloma); (E) ME1402 (human melanoma); (F) MCF-10a (mammary gland fibrocystic disease – nontumorigenic); (G) DU-145 (human prostate carcinoma); (H) HT-1080 (fibrosarcoma); (I) HeLa (human cervical carcinoma); and (J) Caco-2 (human colon adenocarcinoma). Reused from Kelm JM et al31 with permission from the publisher, John Wiley and Sons. Copyright 2003 Wiley Periodicals, Inc.
Spheroids
Despite advances in the treatment of BC, mortality rates of this disease still remain very high, primarily due to metastases in other organs such as bone, brain, and lungs.1 As depicted by Talmadge,28 these metastases start as single cell that detaches from the primary breast tumor and travel through the bloodstream or the lymphatic system to a secondary site, where at first they form micrometastases that stay undetected. Current therapies using cytotoxic drugs are delivered systemically causing serious side effects for patients and frequently do not offer prevention of long-term metastases.29,30 Spheroids have been widely used in the cancer field as a model system in several studies involving 3D cell culture for drug screening predominantly for high-throughput applications (Fig. 3).31 Spheroids are 20–1000 μm diameter clusters of cells that self-aggregate when cultured in rotary wall vessels or spinner flasks. Spheroid cultures have been described to generate heterogeneous cell populations that vary in response to diffusion limitations similar to the in vivo environment and unlike cells seeded on 2D monolayers.32,33 Spheroids have been shown to preserve the physiological shape of the tumor, respond to chemotherapy and radiation therapy,17,20,34 and maintain tissue-specific properties of the primary tissue.33,35,36 Moreover, the 3D arrangements of the spheroids facilitate differentiation to support expansion of heterogeneous subpopulations similar to that observed in vivo.18,37 Several techniques have been presented in the literature for culturing spheroids. The most widely used techniques involve growing cells on plates coated with low attachment substrates to prevent cell–substrate interaction,38,39 in hanging drops,31 or in a continually rotated suspension such as a spinner flask.40 As shown in Figures 4 and 5, Markovitz-Bishitz et al41 also were able to grow mature spheroids in microchambers as a drug screening tool. However, these techniques are time consuming and hard to standardize because they often produce spheroids with a nonuniform range of shapes and sizes with the lack of control in the cell aggregation process. To address some limitations of traditional spheroids, recent reports in the field have shown controlled 3D culture in Matrigel microbeads to analyze clonal acinar development.42 For instance, Dolega et al have established and optimized a technique for microfluidic Matrigel droplet formation where epithelial cells are encapsulated, so that on an average a single acinus is formed per single Matrigel bead. The group also mentions that with their approach acini culture homogeneity is conserved, and 3D structures are recapitulated for further analysis such as fundamental genomics and flow-based high-throughput analysis.42
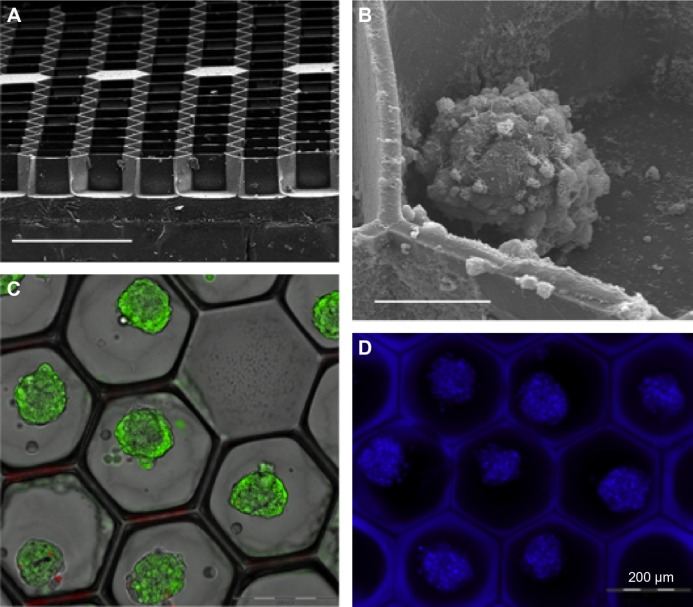
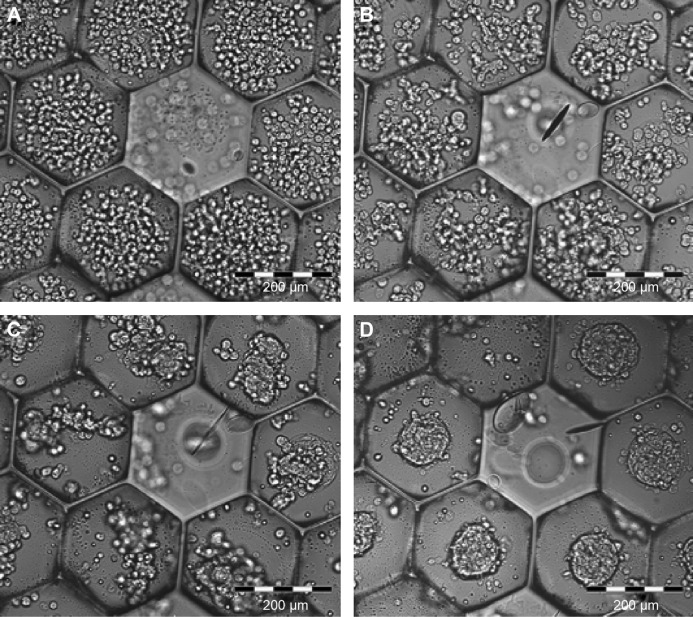
Figure 4.
Spheroids cultured in microchambers. (A) Scanning electron microscopy (SEM) micrograph of the microstructure array embossed on a glass surface. Note the dense honeycomb structure, the sharp edges between the microchambers and the filled microchambers that form built-in points of origin on the array. Scale bar: 500 μm. (B) SEM micrograph of one spheroid in the microchamber. Scale bar: 50 μm. (C) Structured illumination images of live dead staining of spheroids after 72 hours, overlapped with transmission images. Green staining (FDA) indicates live cells, while red (PI) indicates dead cells. Note that dead cells are rarely observed. Scale bar: 200 μm. (D) Fluorescence image of Hoechst 33342-stained spheroids in the microchamber array. Scale bar: 200 μm. Reused from Markovitz-Bishitz Y et al,41 with permission from Elsevier.
Figure 5.
Assembly process of the individual cells on the microstructure. (A) Initial distribution immediately after seeding. (B) Six hours after seeding. (C) Eighteen hours after seeding (note that by this time most cells in each microchamber are already arranged in one amorphous cluster). (D) Mature spheroids, 48 hours after seeding. Reused from Markovitz-Bishitz Y et al,41 with permission from Elsevier.
Microfluidics
Recent advances in microfluidic technology have made it possible to develop innovative assays that enable accurate control of the cellular microenvironment,43 thus addressing limitations of several assays that fail to allow user-defined microenvironments where chemical, physical, and mechanical stimuli can be accurately controlled. Microfluidic assays are highly beneficial toward clinical applications since they are high throughput and automated, thus requiring minimal manual operations during measurements.44 Cell seeding in microfluidic systems is usually done by loading cells suspended in fluid or hydrogel solution, with cell movement being monitored after establishment of chemokine gradient or flow conditions.45–48 For instance, Polacheck et al48 established a microfluidic cell culture system to investigate the effects of interstitial flow on tumor cell migration (Figs. 6 and 7) and found that breast cancer cells (BCCs) migrated in an organized fashion with interstitial flow as compared to control devices without flow where cells migrated randomly. Similar to spheroid cultures, several 3D models have been established using microfluidic devices49,50 to study metastasis initiation and progression. Some of the leading fabrication techniques have been proposed by Stroock and Fischbach51 to address the drawbacks of conventional in vitro technologies lacking variation of soluble chemicals or a representative model of in vivo mass transport. Moreover, more recently, several investigators have applied microfabrication technologies to obtain engineered biomimetic vasculature in order to simulate physiological transport phenomena within these microfluidic conduits.23,52,53 The main objective of these studies is to characterize the processes activated by cancer cells under shear stress conditions: adhesion with endothelial cells (ECs) and degradation of the basement membrane to undergo metastatic growth. Furthermore, in these devices, 3D cultures made with Matrigel54,55 or collagen48,56–58 scaffolds are subjected to a continuous flow (shear stress, interstitial fluid flow). The main goals are to allow for the analysis of epithelial–mesenchymal transition (EMT) processes as a function of fluid forces during tumorigenesis. However, a major disadvantage of these high-throughput microfluidic assays is that the information content is too simplistic and does not fully depict the complexity of a biological phenomenon. For instance, microfluidic culture systems are constrained to very small artificial environments in the order of few hundreds of microns, which fail to mimic the heterogeneous complexity of breast metastatic niches. In addition, these small environments with relatively low seeding densities can be challenging for some biochemical assays.38,59–62
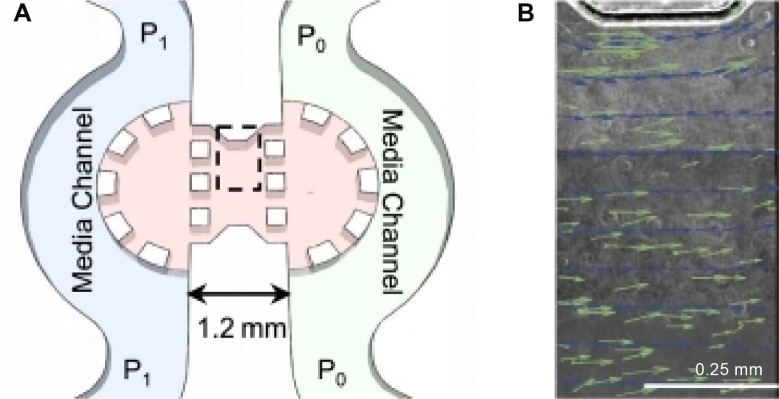
Figure 6.
Microfluidic cell culture system for investigating the effects of interstitial flow on tumor cell migration. (A) Schematic of the microfluidic device. The device consists of two channels (P1 and P0) separated by a region in which cells are suspended in collagen type I gel. By applying a pressure gradient across the gel, a consistent flow field is generated. To validate the flow field, fluorescent microspheres were introduced into the bulk media and time-lapse images were taken to track the beads. (B) Velocity vectors observed by tracking the fluorescent microspheres (green) superimposed on streamline vectors for a computation model (blue) and on a composite phase contrast image of the region of the device indicated by the dashed line in A. The composite phase contrast image is comprised of subregions that were imaged sequentially to measure velocity throughout the whole gel region. Reused from Polacheck WJ et al.48 Copyright is retained by the authors of the original work.
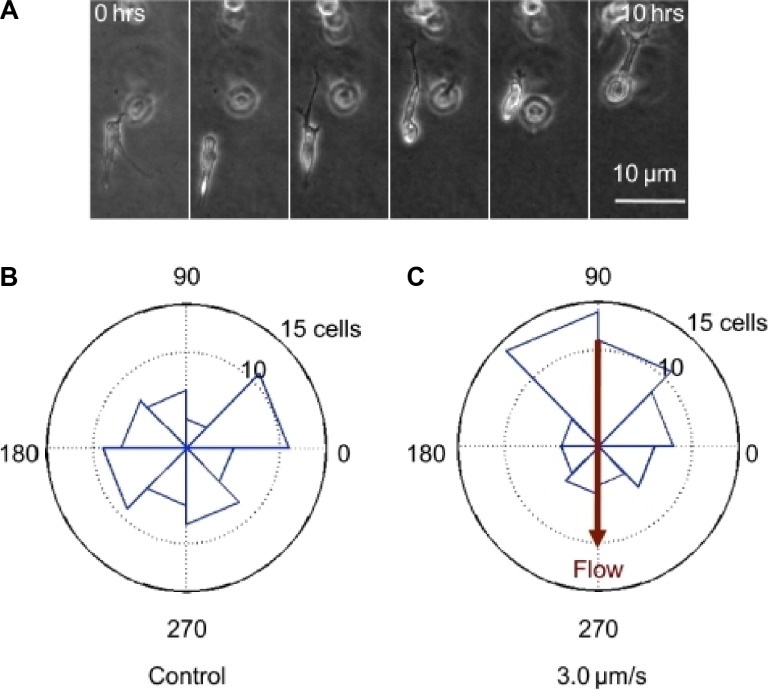
Figure 7.
Interstitial flow influences direction of cell migration. (A) Sample time-lapse images of a cell migrating in an interstitial flow field. Flow is 3.0 μm/s from top to bottom in the image. (B) Sample data from one control device. The polar histogram demonstrates distribution of angles of net migration vectors for cells in a population in one device. Cells in control devices without flow migrate randomly. (C) Flow changes the distribution of migration vector angles. Reused from Polacheck WJ et al.48 Copyright is retained by the authors of the original work.
Scaffolds
The 3D culture techniques usually include adding cell suspension to matrices such as type I collagen or Matrigel or culturing cells on biomaterial scaffolds that can be fabricated into various desired architectures from different materials.20,25,63–65 Hydrogel matrices, such as type I collagen and Matrigel, and synthetic matrices have been widely used to investigate how the physical properties of ECM modulates tumor cell invasion.66,67 Generally, in these studies, tumor cells are uniformly seeded inside a homogeneous 3D ECM and their phenotypic characteristics are monitored in real time or after a given time period.67,68 Many groups have shown the benefits of using 3D scaffolds over 2D tissue culture polystyrene (TCP) for obtaining an in vivo phenotype.2–5,26 In general, studies comparing 3D models using biomaterials to 2D monolayer cultures using cell lines across a range of cancer types have demonstrated in vitro proliferation rates closer to those found in vivo,10,69 increased gene expression, especially upregulation of angiogenic factors,10,69–71 and enhanced drug resistance (Fig. 8).5,10,69,72
Figure 8.
Breast cancer cells cultured on collagen I hydrogels. MDA-MB-231 cells were cultured in collagen I hydrogels for one, three, five, and seven days (A–D, respectively), exhibiting the typical cell–matrix and cell–cell interactions observed in vivo. Cells developed an elongated morphology over seven days with visible processes, demonstrating cell–matrix interactions. As the cells began to proliferate, they aggregated into 3D clusters, demonstrating cell–cell interactions. Scale bars are (A, B) 10 μm and (C, D) 20 μm. Reused from Szot CS et al,71 with permission from Elsevier.
Natural biomaterials
Matrigel is a basement membrane formulation derived from the Engelbreth–Holm–Swarm mouse sarcoma. The major ECM components of Matrigel include laminin-1 (α1β1γ1), collagen IV, enactin (nidogen-1), and perlecan (heparin sulfate proteoglycan). Moreover, type I collagen is used as a substrate for cell culture and tissue engineering applications since it contains the tripeptide RGD (Arg–Gly–Asp), a short amino acid sequence that binds to receptors on cell surfaces.73 Type I collagen hydrogels possess 3D architecture and biocompatibility that are ideal properties to mimic some of the conditions of the tumor microenvironment such as cell-mediated degradation of collagen allowing for remodeling of the matrix during proliferation, migration, and infiltration.74 Type I collagen-based hydrogels can promote cell adhesion, proliferation, and the formation of large cell clusters.2,26
Matrigel and type I collagen isolated from animal tissues75 possess unique characteristics that can be modulated by adjusting gelling conditions such as gel thickness, temperature, and concentration.76–78 However, when using Matrigel with cells, several studies have also identified batch-to-batch variations that cannot be controlled and can be problematic when interpreting results from these studies,79–81 suggesting that improved 3D systems are still needed.
Synthetic biomaterials
To address the shortcomings of natural biomaterials, synthetic materials have been widely used by cancer biologists and engineers. Synthetic biomaterials can be fabricated in a reproducible manner and in large quantities. Some of the commonly used synthetic biomaterials to generate BC models include poly(lactide-co-glycolide) (PLG),10 poly(lactic acid) (PLA),5,82 and poly(ethylene glycol).83 Paszek et al84 found that combining polyacrylamide gels with ECM components and changing the elastic moduli can disrupt epithelial tissue homeostasis, potentially leading to malignant behavior. Recently, Ghajar and Bissell20 have described tumor engineering as “the construction of complex in vitro culture models that mimic aspects of the in vivo tumor microenvironment to study the dynamics of tumor development, progression, and therapy happening in multiple scales.” Fischbach et al,10 Cross et al,13 and Szot71 have engineered 3D in vitro tumor models using both natural and synthetic polymeric scaffolds, respectively, showing that ECs can remodel dense type I collagen matrices in response to angiogenic factors from cancer cells (Fig. 9) and the different effects of these angiogenic factors on drug responsiveness. Embedding cancer cells in 3D hydrogels may induce stress, such as limited oxygen and nutrient transport, as compared to 2D cultures and may induce the expression of responsive genes, such as hypoxia-inducible factor and vascular endothelial growth factor. Findings, therefore, demonstrating cancer cells cultured in 3D better recapitulate in vivo behaviors than 2D cultures.2,85 Synthetic biomaterial scaffolds offer better outcomes for studies that require tailoring of the tumor microenvironment (eg, mechanical properties and surface chemistry).4
Figure 9.
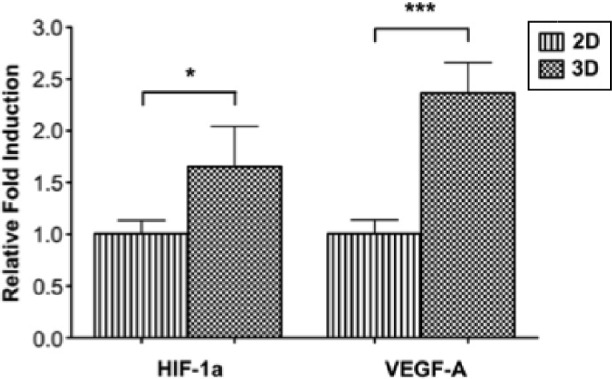
Hypoxia-inducible factor-1 alpha (HIF-1α) and vascular endothelial growth factor-A (VEGF-A) gene expression of MDA-MB-231 cells cultured in 3D type I collagen hydrogels in comparison to cells cultured on 2D tissue culture polystyrene. Gene expressions were significantly upregulated when MDA-MB-231 cells were cultured in 3D type I collagen hydrogels as compared to cells cultured in a monolayer on 2D tissue culture polystyrene. Gene expression was compared on day 0 to determine the specific effect of 3D culture without the contribution of cell proliferation or the development of hypoxia. *P < 0.05 and ***P < 0.001, respectively. Reused from Szot CS et al,71 with permission from Elsevier.
Other Cancer Models
Despite advances in drug development, one of the main issues that still remains is the lack of correlation between preclinical findings and clinical trial results.86 Thus, over recent years, several groups have established BC patient-derived xenograft (PDXs) models as main tools for translational research.87–95 For these PDX models, the tumors of the patient, acquired via biopsy or surgical resection, are sliced and transplanted in immunodeficient mice and left to grow without any in vitro manipulation. Consequently, generations of mice are used for drug-testing purposes in an effort to advance patient therapy.96 These models are also known as personalized mouse models or patient-derived tumor xenografts models to acquire further understanding on tumor progression, metastasis, and eradication via key targets. PDXs generated from BC tumor samples have been shown to mimic several tumor behaviors and characteristics of the original tumor.87,89,95 Moreover, in the past 20 years, there have been several improvements in BC PDXs. For instance, DeRose et al89 incorporated the addition of mesenchymal stem cells (MSCs) to modify the microenvironment, while other groups such as Kabos et al97 incorporated Matrigel with the PDXs models. Additionally, studies by Liu et al98 were able to show that CD44+ cancer stem cells (CSCs) directly metastasize to the lungs and lymph nodes using PDXs indicating that breast cancer stem cells (BCSCs) characterization could be performed using PDXs models.
Unfortunately, PDXs have several limitations. One issue is the rapid loss of human stroma that becomes replaced by murine stroma following engraftment,89 potentially leading to change in the tumor biology.99 Considering the importance of immune cells during tumorigenesis and BC metastasis, another main issue with PDXs is the need to use immunocompromised mice. Moreover, even with the immunocompromised mice, PDXs models have low engraftment rates and require a long time (several months) to be established. Additionally, in comparison to regular tissue culture techniques, PDX models are significantly more costly since they require production of genetically engineered mice and their maintenance. Thus, overall PDXs have not led to major enhancement in the survival rate of patients with cancer and needs further improvements.100
Applications
Many cancers have been shown to metastasize to bone, and this topic has been intensely raised in the current research leading to novel models to establish the multiple stages of metastasis.101,102 It is now well known and accepted that in the primary stages of metastasis, disseminated tumor cells in the bone undergo a prolonged period of growth arrest in response to cues from the bone marrow (BM) microenvironment, following the successful removal of the primary tumor, this is also known as “dormancy”.28 Dormancy has been implicated with cell cycle arrest and drug resistance, and engineering of dormant stage has emerged as a novel clinical approach to tackle BC. This clinical behavior is frequently observed in cancers of the breast,103 along with other cancers (skin104 and prostate105) with relapse time varying from years to decades. In the case of BC, bone is the most common site of metastasis, affecting up to 70% of women with advanced disease.106 The progression of this disease results in severe skeletal complications with an average 5-year survival of only 20% of the patients.107
Despite several improvements, the mechanisms underlying BC homing to bone remains poorly understood. Primary breast tumors can transform to invasive BC. This transformation of the cells is also known as EMT.108,109 During EMT, the BCCs lose their epithelial phenotypes including their polarity and specialized E-cadherin-based cell–cell contacts, and they acquire a migratory phenotype, which is associated with an increase in metastatic potential.110,111 Recently, CSCs have been described as inducers of EMT in tumor cells.112 Furthermore, Phillips et al113 have demonstrated that cells undergoing EMT may be chemoresistant and have linked this resistance to the presence of CSCs within this population of transformed cells. Additionally, the EMT process allows CSCs to maintain their self-renewal abilities leading to a more invasive phenotype capable of initiating secondary tumors at distant sites.114 Studies by Louie et al115 have linked BC with EMT by using BCSCs conferring enhanced invasiveness, conserved EMT properties, and stem-like properties in an immune-deficient mouse model. It is generally established that dormant tumor cells can stay in a nondividing phase for many years as single cells with chemoresistance and radiation resistance characteristics.116–119 Hence, BC resurgence is believed to be a result of BCSCs remaining dormant within the bone microenvironment. BCSCs, as described by Al-Hajj et al,87 possess several other characteristics such as long-term self-renewal, chemoresistance, and ability to initiate distant metastatic disease.120 There is a growing need to understand this mechanism of dormancy in order to develop therapies to target these dormant cells.3,63,87,117,121–137 In the literature, there are two categories of dormancy. First is cellular dormancy, where single cells enter into a nonproliferative state. Cells are described to be quiescent and can show G0/G1 cell cycle arrest in response to microenvironmental signals or stresses.102 Second is tumor mass dormancy in which the growth of the tumor mass is limited by a state of matched turnover between proliferative and apoptotic cells.138 BC bone dormancy in vitro models have fundamental limitations with regard to reproducibility and flexibility of design. To date, many 2D in vitro models and in vivo models have been used to investigate the tumor microenvironment;139,140 however, the complexity of human bone is difficult to recapitulate.141,142
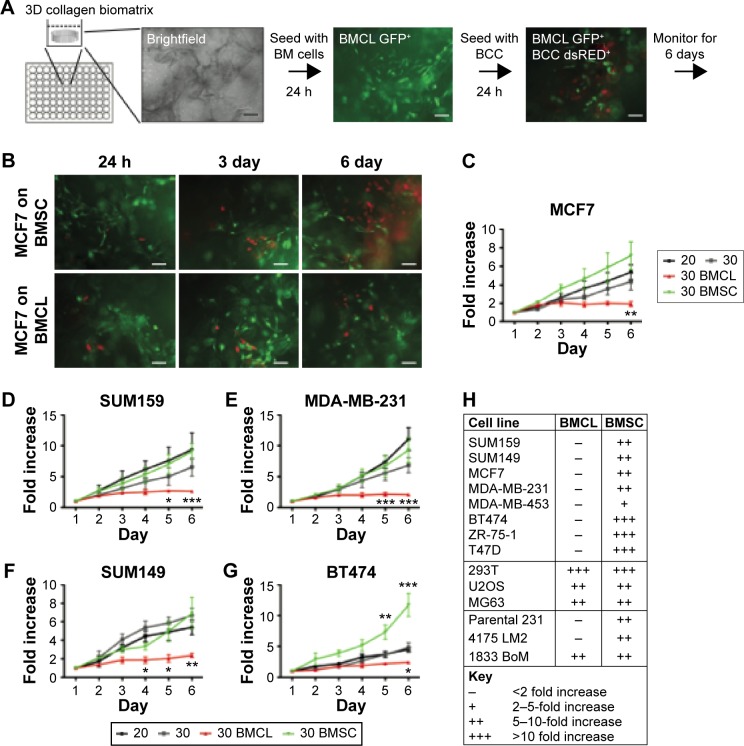
Although 2D models present easy and powerful methods for investigating BCC behavior in vitro, a 2D cancer postmetastasis model oversimplifies the native 3D microenvironment due to the lack of spatial cues.50 In addition, a 2D cell–cell in vitro model often fails to mimic cellular interaction with native ECM. This lack of cell–matrix interactions can affect gene expression of both normal and cancerous cells.143 Some groups have demonstrated 3D models of cancer cell dormancy (Table 1). For instance, Weaver et al144 showed that blocking β1 integrins can lead to BCCs entering a nonproliferative phase. Similarly, to represent the premetastatic niche, Ghajar et al145 have recently fabricated microvascular constructs made of stromal cells derived from the BM and ECs in order to have a variety of cell populations present in their model to represent the metastatic niche. Their findings suggest that the presence of the ECs reduced proliferation of cancer cells by fivefold. Moreover, dormant cells versus active cells were found to have different proliferation potential with active cells being able to proliferate more. Moreover, progress has been made in fabricating bioreactors with the potential of mimicking the architecture observed in the bone.20,146,147 In these studies, BCCs were cultured and observed to form a “single cell file” that is known to be characteristic of metastatic cancer in vivo. Findings also showed that the growth rate of BCCs was reduced in this 3D environment, while osteoblasts in the coculture altered the phenotype in response to the metastatic invasion, adopting a more cuboidal morphology.148 As a model of cancer dormancy, Marlow et al fabricated a 3D coculture model by culturing MSCs together with ECs and BCCs in a 3D collagen matrix. BCCs in cocultures proliferated less than in monocultures and appeared to be cell cycle arrested (Fig. 10).149
Table 1.
A summary of models studying breast cancer dormancy.
| MODELS FOR BREAST CANCER DORMANCY | SIGNIFICANT FINDINGS AND LIMITATIONS | REFERENCE |
|---|---|---|
| Investigated dormancy of BCCs in cocultures with bone marrow cells on 3D collagen porous scaffold (Gelfoam) both in vitro and in vivo. | Identified bone marrow stromal cells in co-culture with BCCs supported proliferation whereas other bone marrow cell lines were inhibitory. Validated findings in vivo. Bone marrow cell lines were derived from other sources—HUVEC, immortalized fetal osteoblasts and stromal cells, which may not be representative of the cells in the native adult bone marrow microenvironment. | 149 |
| Fabricated 3D scaffolds consisting of micron-sized random and aligned fibers to mimic the orientation and size of collagen fibers in the native ECM. | Investigated proliferation, viability and cell cycle analysis of BCCs on electrospun fibrous scaffold and determined the aggressive BCCs adopt a dormant phenotype, while chemoresistant BCCs retained their dormant phenotype. Co-cultures with other cells types were not examined. | 150 |
| Examined the influence of β1- and β4-integrins on BCC behavior in a 3D basement membrane (Matrigel). | Demonstrated that integrins regulated the level of the acini organization and reverted the malignant phenotype to a normal phenotype. Matrigel is derived from tumor basement membrane and can vary in protein/growth factor content. | 144 |
| Investigated BCC behavior in metastasis assay in mice and in an organotypic microvascular culture. | Determined dormant BCCs reside upon microvasculature of lung, bone marrow and brain in vivo and endothelial cells via thrombospondin-1 induces sustained BCC quiescence. HUVEC cells used in in vitro model may not represent native adult endothelium. | 145 |
Figure 10.
Bone marrow (BM) metastatic niche models: primary bone marrow mesenchymal stem cell (BMSC) supported proliferation of breast cancer cells (supportive niche), whereas a mix of osteoblasts, mesenchymal, and endothelial cell lines (BMCLs) did not support proliferation of breast cancer cells (inhibitory niche). (A) Diagram of bone marrow niche model setup. The 3D biomatrix is seeded with GFP+ BMCL or BMSC when bone marrow cells reach subconfluency; dsRED+ BCCs are seeded in low numbers. (B) Representative images of dsRED+ MCF7 cells grown into GFP+ BMCL (bottom) or BMSC (top) over six days. Scale, 50 μm. (C–G) Total fluorescence of BCCs (MCF7, SUM159, MDA-MB-231, SUM149, and BT474) was monitored. BCCs seeded in 3D biomatrix without stromal cells (3D monoculture, 3D) and BCCs plated in standard 2D conditions (2D) were used as controls. Fluorescence is expressed as the fold increase from 24 hours after seeding (n = 6 experiments, five replicates in each experiment). Error bars, standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001; two-way analysis of variance with Bonferroni posttest. (H) Proliferation rates of cell lines in 3D coculture. Only BCCs were found to be growth arrested in the inhibitory niche (BMCL). ER+ BCCs are indicated in bold. The fetal kidney cell line, 293t, and the osteosarcoma cell lines, U2OS and MG63, proliferate in the BMCL coculture. The subline of MDA-MB-231 1833 BoM proliferates in BMCL coculture, the parental and the 4175 LM2 lines do not.149
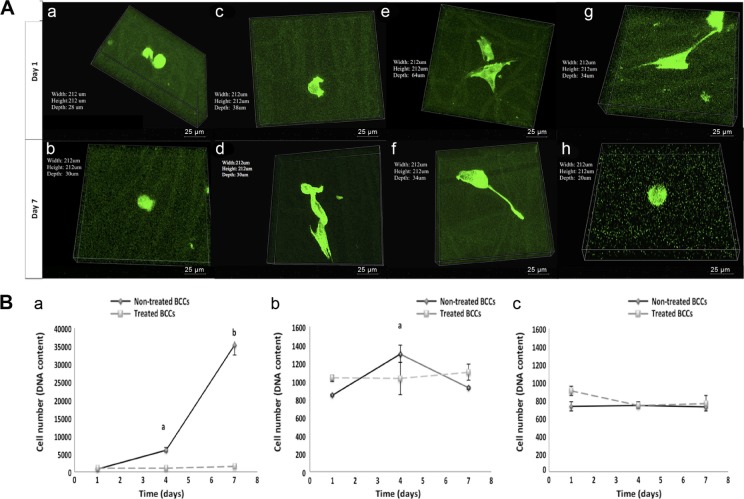
Recently, others and we have shown that 3D in vitro models using synthetic scaffolds can overcome the limitations of 2D models.150,151 Our studies using MDA-MB-231 BCCs seeded on the 3D scaffolds showed changes in cell morphology, adherence, and growth. Unlike cells on fibrous scaffolds, cells seeded on TCP surfaces displayed confluency by day 7 (Fig. 11A). Furthermore, the aggressive MDA-MB-231 cells showed little to no change in cell number over time on the scaffolds, whereas on TCP, they displayed a significant increase in cell number at days 4 and 7 as compared to day 1 (P < 0.05) (Fig. 11B), indicating that the scaffolds may support dormancy. Pathi et al152 fabricated a 3D PLG scaffold and illustrated that hydroxyapatite nanoparticles play a crucial role in regulating BC bone metastasis. Moreover, the ability of malignant cells to grow and home to the BM in vivo depends upon specific cell–cell and cell–ECM interactions, many of which are absent when cells are cultured on conventional 2D tissue culture plastic.153 Currently, the cell-based 2D monolayer cultures used as in vitro models present several limitations that 3D tissue engineering scaffolds/models can address.17,34,154,155
Figure 11.
BCCs morphology and growth on polycaprolactone (PCL) random and aligned scaffolds. (A) Confocal fluorescent microscope images of MDA-MB-231 cells on the PCL random and aligned fibrous scaffolds and TCP control. Volume view of MDA-MB-231 BCCs, green indicates F-actin. On random fibers, nontreated cells at (a) day 1 and (b) day 7 and treated cells at (e) day 1 and (f) day 7. On aligned fibers, nontreated cells at (c) day 1 and (d) day 7 and treated cells at (g) day 1 and (h) day 7. 60× objective. Scale bar is 25 μm. The arrows show the cell body orientation along the fibers. (B) BCC growth on random and aligned fibrous scaffolds in comparison to TCP. (a) TCP. aP < 0.05, significant increase in growth of nontreated BCCs at day 4 as compared to day 1. bP < 0.05, significant increase in growth of nontreated BCCs at day 7 as compared to day 1 and day 4. (b) Random fibers. aP < 0.05, significant increase in growth of nontreated BCCs at day 4 as compared to day 1 and day 7. (c) Aligned fibers. Values are mean ± standard deviation.150
Conclusion
A growing need exists for the development of novel in vitro systems to facilitate the discovery of innovative therapies for BC. In order to realistically mimic the tumor microenvironment, 3D systems can be designed to allow for complex interactions between multiple cells and cell–ECM. As mentioned in this review, current animal models have several limitations that 3D tissue engineered scaffold models can potentially address. It is feasible to develop 3D culture systems that can be specifically designed to recapitulate key characteristics of the tumor tissue to model specific disease stages of BC (EMT, metastasis, invasion, dormancy) and important BC hallmarks including several layers of complexities (coculture with immune cells, bone cells, growth factors, etc). The 3D scaffolds can also be used in conjunction with high-tech microfluidic system devices to acquire highly effective models to screen and target CSCs within different milieu. These efforts would most likely be less expensive than the cost of animal studies. The 3D culture systems have been established and shown to be an improvement over 2D monolayer cultures in several aspects (ie, drug response, model of invasion and metastasis, model of EMT, and recently model of dormancy). Thus, tissue engineering can advance the development of relevant in vitro models for BC research.
Abbreviations
- BC
breast cancer
- BCCs
breast cancer cells
- BCSCs
breast cancer stem cells
- BM
bone marrow
- CSCs
cancer stem cells
- EC
endothelial cells
- ECM
extracellular matrix
- EMT
epithelial to mesenchymal transition
- HUVEC
human umbilical vein endothelial cells
- MSCs
mesenchymal stem cells
- PLG
poly(lactide-co-glycolide)
- TCP
tissue culture polystyrene
- 3D
three-dimensional
- 2D
two-dimensional
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 960 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: KG and TLA. Contributed to the writing of the manuscript: KG and TLA. Agree with manuscript results and conclusions: KG and TLA. Jointly developed the structure and arguments for the paper: KG and TLA. Made critical revisions and approved final version: TLA. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 2.Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol. 2005;15(5):365–377. doi: 10.1016/j.semcancer.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130(4):601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Hutmacher DW, Horch RE, Loessner D, et al. Translating tissue engineering technology platforms into cancer research. J Cell Mol Med. 2009;13(8a):1417–1427. doi: 10.1111/j.1582-4934.2009.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horning JL, Sahoo SK, Vijayaraghavalu S, et al. 3-D tumor model for in vitro evaluation of anticancer drugs. Mol Pharm. 2008;5(5):849–862. doi: 10.1021/mp800047v. [DOI] [PubMed] [Google Scholar]
- 6.Rangarajan A, Weinberg RA. Opinion: comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer. 2003;3(12):952–959. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- 7.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9(11):4227–4239. [PubMed] [Google Scholar]
- 8.Johnson JI, Decker S, Zaharevitz D, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84(10):1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JB, Stein R, O’Hare MJ. Three-dimensional in vitro tissue culture models of breast cancer—a review. Breast Cancer Res Treat. 2004;85(3):281–291. doi: 10.1023/B:BREA.0000025418.88785.2b. [DOI] [PubMed] [Google Scholar]
- 10.Fischbach C, Chen R, Matsumoto T, et al. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4(10):855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 11.Nelson CM, Bissell MJ. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol. 2005;15(5):342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbridge SS, Choi NW, Zheng Y, Brooks DJ, Stroock AD, Fischbach C. Oxygen-controlled three-dimensional cultures to analyze tumor angiogenesis. Tissue Eng Part A. 2010;16(7):2133–2141. doi: 10.1089/ten.tea.2009.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross VL, Zheng Y, Won Choi N, et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31(33):8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson CM, Inman JL, Bissell MJ. Three-dimensional lithographically defined organotypic tissue arrays for quantitative analysis of morphogenesis and neoplastic progression. Nat Protoc. 2008;3(4):674–678. doi: 10.1038/nprot.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raof NA, Raja WK, Castracane J, Xie Y. Bioengineering embryonic stem cell microenvironments for exploring inhibitory effects on metastatic breast cancer cells. Biomaterials. 2011;32(17):4130–4139. doi: 10.1016/j.biomaterials.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 16.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14(5):633–639. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 17.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8(10):839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, Spagnoli GC, Martin I, et al. Three-dimensional culture of melanoma cells profoundly affects gene expression profile: a high density oligonucleotide array study. J Cell Physiol. 2005;204(2):522–531. doi: 10.1002/jcp.20320. [DOI] [PubMed] [Google Scholar]
- 19.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1(1):46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghajar CM, Bissell MJ. Tumor engineering: the other face of tissue engineering. Tissue Eng Part A. 2010;16(7):2153–2156. doi: 10.1089/ten.tea.2010.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wlodkowic D, Cooper JM. Tumors on chips: oncology meets microfluidics. Curr Opin Chem Biol. 2010;14(5):556–567. doi: 10.1016/j.cbpa.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21(12):745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker GM, Zeringue HC, Beebe DJ. Microenvironment design considerations for cellular scale studies. Lab Chip. 2004;4(2):91–97. doi: 10.1039/b311214d. [DOI] [PubMed] [Google Scholar]
- 24.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103(8):2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burdett E, Kasper FK, Mikos AG, Ludwig JA. Engineering tumors: a tissue engineering perspective in cancer biology. Tissue Eng Part B Rev. 2010;16(3):351–359. doi: 10.1089/ten.TEB.2009.0676. [DOI] [PubMed] [Google Scholar]
- 26.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7(3):211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 27.Ricci C, Moroni L, Danti S. Cancer tissue engineering-new perspectives in understanding the biology of solid tumours-a critical review. OA Tissue Eng. 2013;1(1):4. [Google Scholar]
- 28.Talmadge JE. Clonal selection of metastasis within the life history of a tumor. Cancer Res. 2007;67(24):11471–11475. doi: 10.1158/0008-5472.CAN-07-2496. [DOI] [PubMed] [Google Scholar]
- 29.Allan AL, Vantyghem SA, Tuck AB, Chambers AF. Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis. 2006;26:87–98. doi: 10.3233/bd-2007-26108. [DOI] [PubMed] [Google Scholar]
- 30.Riethdorf S, Pantel K. Disseminated tumor cells in bone marrow and circulating tumor cells in blood of breast cancer patients: current state of detection and characterization. Pathobiology. 2008;75(2):140–148. doi: 10.1159/000123852. [DOI] [PubMed] [Google Scholar]
- 31.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003;83(2):173–180. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- 32.Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3(9–10):1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 33.Kunz-Schughart LA. Multicellular tumor spheroids: intermediates between monolayer culture and in vivo tumor. Cell Biol Int. 1999;23(3):157–161. doi: 10.1006/cbir.1999.0384. [DOI] [PubMed] [Google Scholar]
- 34.Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010;148(1):3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi H, Man S, Graham CH, Kapitain SJ, Teicher BA, Kerbel RS. Acquired multicellular-mediated resistance to alkylating agents in cancer. Proc Natl Acad Sci U S A. 1993;90(8):3294–3298. doi: 10.1073/pnas.90.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutherland RM, Durand RE. Radiation response of multicell spheroids—an in vitro tumour model. Curr Top Radiat Res Q. 1976;11(1):87–139. [PubMed] [Google Scholar]
- 37.Hauptmann S, Gebauer-Hartung P, Leclere A, et al. Induction of apoptosis in the centre of multicellular tumour spheroids of colorectal adenocarcinomas—involvement of CD95 pathway and differentiation. Apoptosis. 1998;3(4):267–279. doi: 10.1023/a:1009613325845. [DOI] [PubMed] [Google Scholar]
- 38.Carlsson J, Yuhas JM. Liquid-overlay culture of cellular spheroids. Recent Results Cancer Res. 1984;95:1–23. doi: 10.1007/978-3-642-82340-4_1. [DOI] [PubMed] [Google Scholar]
- 39.Ivascu A, Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen. 2006;11(8):922–932. doi: 10.1177/1087057106292763. [DOI] [PubMed] [Google Scholar]
- 40.Sutherland RM, McCredie JA, Inch WR. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst. 1971;46(1):113–120. [PubMed] [Google Scholar]
- 41.Markovitz-Bishitz Y, Tauber Y, Afrimzon E, et al. A polymer microstructure array for the formation, culturing, and high throughput drug screening of breast cancer spheroids. Biomaterials. 2010;31(32):8436–8444. doi: 10.1016/j.biomaterials.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 42.Dolega ME, Abeille F, Picollet-D’hahan N, Gidrol X. Controlled 3D culture in Matrigel microbeads to analyze clonal acinar development. Biomaterials. 2015;52:347–357. doi: 10.1016/j.biomaterials.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 43.Salieb-Beugelaar GB, Simone G, Arora A, Philippi A, Manz A. Latest developments in microfluidic cell biology and analysis systems. Anal Chem. 2010;82(12):4848–4864. doi: 10.1021/ac1009707. [DOI] [PubMed] [Google Scholar]
- 44.Gossett DR, Tse HT, Lee SA, et al. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc Natl Acad Sci U S A. 2012;109(20):7630–7635. doi: 10.1073/pnas.1200107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Philipsborn AC, Lang S, Loeschinger J, et al. Growth cone navigation in substrate-bound ephrin gradients. Development. 2006;133(13):2487–2495. doi: 10.1242/dev.02412. [DOI] [PubMed] [Google Scholar]
- 46.Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009;9(2):269–275. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- 47.Murrell M, Kamm R, Matsudaira P. Tension, free space, and cell damage in a microfluidic wound healing assay. PLoS One. 2011;6(9):e24283. doi: 10.1371/journal.pone.0024283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polacheck WJ, Charest JL, Kamm RD. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc Natl Acad Sci U S A. 2011;108(27):11115–11120. doi: 10.1073/pnas.1103581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sung KE, Yang N, Pehlke C, et al. Transition to invasion in breast cancer: a microfluidic in vitro model enables examination of spatial and temporal effects. Integr Biol. 2011;3(4):439–450. doi: 10.1039/c0ib00063a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nyga A, Cheema U, Loizidou M, editors. 3D tumour models: novel in vitro approaches to cancer studies. J Cell Commun Signal. 2011;5(3):239–248. doi: 10.1007/s12079-011-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stroock AD, Fischbach C. Microfluidic culture models of tumor angiogenesis. Tissue Eng Part A. 2010;16(7):2143–2146. doi: 10.1089/ten.tea.2009.0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 53.Tourovskaia A, Figueroa-Masot X, Folch A. Differentiation-on-a-chip: a microfluidic platform for long-term cell culture studies. Lab Chip. 2005;5(1):14–19. doi: 10.1039/b405719h. [DOI] [PubMed] [Google Scholar]
- 54.Rizvi I, Gurkan UA, Tasoglu S, et al. Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc Natl Acad Sci U S A. 2013;110(22):E1974–E1983. doi: 10.1073/pnas.1216989110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang CP, Lu J, Seon H, et al. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab Chip. 2009;9(12):1740–1748. doi: 10.1039/b818401a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hernández Vera R, Genové E, Alvarez L, et al. Interstitial fluid flow intensity modulates endothelial sprouting in restricted Src-activated cell clusters during capillary morphogenesis. Tissue Eng Part A. 2009;15(1):175–185. doi: 10.1089/ten.tea.2007.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vickerman V, Blundo J, Chung S, Kamm R. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip. 2008;8(9):1468–1477. doi: 10.1039/b802395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semino CE, Kamm RD, Lauffenburger DA. Autocrine EGF receptor activation mediates endothelial cell migration and vascular morphogenesis induced by VEGF under interstitial flow. Exp Cell Res. 2006;312(3):289–298. doi: 10.1016/j.yexcr.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 59.Yamada S, Wirtz D, Kuo SC. Mechanics of living cells measured by laser tracking microrheology. Biophys J. 2000;78(4):1736–1747. doi: 10.1016/S0006-3495(00)76725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33(1):15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- 61.Crocker JC, Valentine MT, Weeks ER, et al. Two-point microrheology of inhomogeneous soft materials. Phys Rev Lett. 2000;85(4):888–891. doi: 10.1103/PhysRevLett.85.888. [DOI] [PubMed] [Google Scholar]
- 62.Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys J. 2005;89(4):2855–2864. doi: 10.1529/biophysj.105.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hutmacher DW, Loessner D, Rizzi S, Kaplan DL, Mooney DJ, Clements JA. Can tissue engineering concepts advance tumor biology research? Trends Biotechnol. 2010;28(3):125–133. doi: 10.1016/j.tibtech.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008;14(1):61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 65.Hutmacher DW. Biomaterials offer cancer research the third dimension. Nat Mater. 2010;9(2):90–93. doi: 10.1038/nmat2619. [DOI] [PubMed] [Google Scholar]
- 66.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185(1):11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HD, Peyton SR. Bio-inspired materials for parsing matrix physicochemical control of cell migration: a review. Integr Biol. 2012;4(1):37–52. doi: 10.1039/c1ib00069a. [DOI] [PubMed] [Google Scholar]
- 69.Leung M, Kievit FM, Florczyk SJ, et al. Chitosan-alginate scaffold culture system for hepatocellular carcinoma increases malignancy and drug resistance. Pharm Res. 2010;27(9):1939–1948. doi: 10.1007/s11095-010-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kievit FM, Florczyk SJ, Leung MC, et al. Chitosan-alginate 3D scaffolds as a mimic of the glioma tumor microenvironment. Biomaterials. 2010;31(22):5903–5910. doi: 10.1016/j.biomaterials.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szot CS, Buchanan CF, Freeman JW, Rylander MN. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 2011;32(31):7905–7912. doi: 10.1016/j.biomaterials.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dhiman HK, Ray AR, Panda AK. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials. 2005;26(9):979–986. doi: 10.1016/j.biomaterials.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 73.Saltzman WM. Tissue Engineering: Engineering Principles for the Design of Replacement Organs and Tissues. New York: Oxford University Press; 2004. [Google Scholar]
- 74.Ala-aho R, Kahari VM. Collagenases in cancer. Biochimie. 2005;87(3–4):273–286. doi: 10.1016/j.biochi.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 75.Patterson J, Martino MM, Hubbell JA. Biomimetic materials in tissue engineering. Mater Today. 2010;13(1):14–22. [Google Scholar]
- 76.Wheeldon I, Ahari AF, Khademhosseini A. Microengineering hydrogels for stem cell bioengineering and tissue regeneration. JALA Charlottesville Va. 2010;15(6):440–448. doi: 10.1016/j.jala.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roy R, Boskey A, Bonassar LJ. Processing of type I collagen gels using nonenzymatic glycation. J Biomed Mater Res A. 2010;93(3):843–851. doi: 10.1002/jbm.a.32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carey SP, Kraning-Rush CM, Williams RM, Reinhart-King CA. Biophysical control of invasive tumor cell behavior by extracellular matrix microarchitecture. Biomaterials. 2012;33(16):4157–4165. doi: 10.1016/j.biomaterials.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 80.Miron-Mendoza M, Lin X, Ma L, Ririe P, Petroll WM. Individual versus collective fibroblast spreading and migration: regulation by matrix composition in 3D culture. Exp Eye Res. 2012;99:36–44. doi: 10.1016/j.exer.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Savina IN, Dainiak M, Jungvid H, Mikhalovsky SV, Galaev IY. Biomimetic macroporous hydrogels: protein ligand distribution and cell response to the ligand architecture in the scaffold. J Biomater Sci Polym Ed. 2009;20(12):1781–1795. doi: 10.1163/156856208X386390. [DOI] [PubMed] [Google Scholar]
- 82.Sahoo SK, Panda AK, Labhasetwar V. Characterization of porous PLGA/PLA microparticles as a scaffold for three dimensional growth of breast cancer cells. Biomacromolecules. 2005;6(2):1132–1139. doi: 10.1021/bm0492632. [DOI] [PubMed] [Google Scholar]
- 83.Weiss MS, Bernabé BP, Shikanov A, et al. The impact of adhesion peptides within hydrogels on the phenotype and signaling of normal and cancerous mammary epithelial cells. Biomaterials. 2012;33(13):3548–3559. doi: 10.1016/j.biomaterials.2012.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 85.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5(9):675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 86.Gillet JP, Calcagno AM, Varma S, et al. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci U S A. 2011;108(46):18708–18713. doi: 10.1073/pnas.1111840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beckhove P, Schütz F, Diel IJ, et al. Efficient engraftment of human primary breast cancer transplants in nonconditioned NOD/Scid mice. Int J Cancer. 2003;105(4):444–453. doi: 10.1002/ijc.11125. [DOI] [PubMed] [Google Scholar]
- 89.DeRose YS, Wang G, Lin YC, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17(11):1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fiebig HH, Maier A, Burger AM. Clonogenic assay with established human tumour xenografts: correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur J Cancer. 2004;40(6):802–820. doi: 10.1016/j.ejca.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 91.Marangoni E, Vincent-Salomon A, Auger N, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007;13(13):3989–3998. doi: 10.1158/1078-0432.CCR-07-0078. [DOI] [PubMed] [Google Scholar]
- 92.Petrillo LA, Wolf DM, Kapoun AM, et al. Xenografts faithfully recapitulate breast cancer-specific gene expression patterns of parent primary breast tumors. Breast Cancer Res Treat. 2012;135(3):913–922. doi: 10.1007/s10549-012-2226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reyal F, Guyader C, Decraene C, et al. Molecular profiling of patient-derived breast cancer xenografts. Breast Cancer Res. 2012;14(1):R11. doi: 10.1186/bcr3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Visonneau S, Cesano A, Torosian MH, Miller EJ, Santoli D. Growth characteristics and metastatic properties of human breast cancer xenografts in immunodeficient mice. Am J Pathol. 1998;152(5):1299–1311. [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, Claerhout S, Prat A, et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 2013;73(15):4885–4897. doi: 10.1158/0008-5472.CAN-12-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9(6):338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kabos P, Finlay-Schultz J, Li C, et al. Patient-derived luminal breast cancer xenografts retain hormone receptor heterogeneity and help define unique estrogen-dependent gene signatures. Breast Cancer Res Treat. 2012;135(2):415–432. doi: 10.1007/s10549-012-2164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu H, Patel MR, Prescher JA, et al. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci U S A. 2010;107(42):18115–18120. doi: 10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hidalgo M, Amant F, Biankin AV, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rogosin S, Sandler AB. Beyond bevacizumab: antiangiogenic agents. Clin Lung Cancer. 2012;13(5):326–333. doi: 10.1016/j.cllc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 101.Karrison TG, Ferguson DJ, Meier P. Dormancy of mammary carcinoma after mastectomy. J Natl Cancer Inst. 1999;91(1):80–85. doi: 10.1093/jnci/91.1.80. [DOI] [PubMed] [Google Scholar]
- 102.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Retsky MW, Demicheli R, Hrushesky WJ, Baum M, Gukas ID. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS. 2008;116(7–8):730–741. doi: 10.1111/j.1600-0463.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 104.Ossowski L, Aguirre-Ghiso JA. Dormancy of metastatic melanoma. Pigment Cell Melanoma Res. 2010;23(1):41–56. doi: 10.1111/j.1755-148X.2009.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morgan TM, Lange PH, Porter MP, et al. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15(2):677–683. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10(2):169–180. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- 107.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 108.Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial–mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101(2):293–299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 110.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14(1):29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 111.Xu J, Lamouille S, Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Du Z, Qin R, Wei C, et al. Pancreatic cancer cells resistant to chemoradiotherapy rich in “stem-cell-like” tumor cells. Dig Dis Sci. 2011;56(3):741–750. doi: 10.1007/s10620-010-1340-0. [DOI] [PubMed] [Google Scholar]
- 113.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 114.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Louie E, Nik S, Chen JS, et al. Identification of a stem-like cell population by exposing metastatic breast cancer cell lines to repetitive cycles of hypoxia and reoxygenation. Breast Cancer Res. 2010;12(6):R94. doi: 10.1186/bcr2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patel SA, Ramkissoon SH, Bryan M, et al. Delineation of breast cancer cell hierarchy identifies the subset responsible for dormancy. Sci Rep. 2012;2:906. doi: 10.1038/srep00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 118.Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106(33):13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Botchkina IL, Rowehl RA, Rivadeneira DE, et al. Phenotypic subpopulations of metastatic colon cancer stem cells: genomic analysis. Cancer Genomics Proteomics. 2009;6(1):19–29. [PubMed] [Google Scholar]
- 121.Ponti D, Costa A, Zaffaroni N, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65(13):5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 122.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 123.Diehn M, Cho RW, Clarke MF. Therapeutic implications of the cancer stem cell hypothesis. Semin Radiat Oncol. 2009;19(2):78–86. doi: 10.1016/j.semradonc.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Braun S, Auer D, Marth C. The prognostic impact of bone marrow micrometastases in women with breast cancer. Cancer Invest. 2009;27(6):598–603. doi: 10.1080/07357900802574496. [DOI] [PubMed] [Google Scholar]
- 125.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 126.Morris SR, Carey LA. Molecular profiling in breast cancer. Rev Endocr Metab Disord. 2007;8(3):185–198. doi: 10.1007/s11154-007-9035-3. [DOI] [PubMed] [Google Scholar]
- 127.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10(10):712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 128.Box C, Rogers SJ, Mendiola M, Eccles SA. Tumour-microenvironmental interactions: paths to progression and targets for treatment. Semin Cancer Biol. 2010;20(3):128–138. doi: 10.1016/j.semcancer.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 129.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 130.Benton G, George J, Kleinman HK, Arnaoutova IP. Advancing science and technology via 3D culture on basement membrane matrix. J Cell Physiol. 2009;221(1):18–25. doi: 10.1002/jcp.21832. [DOI] [PubMed] [Google Scholar]
- 131.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9(9):665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 132.Zhang S. Beyond the Petri dish. Nat Biotechnol. 2004;22(2):151–152. doi: 10.1038/nbt0204-151. [DOI] [PubMed] [Google Scholar]
- 133.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462(7272):433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463(7280):485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Uhr JW, Pantel K. Controversies in clinical cancer dormancy. Proc Natl Acad Sci U S A. 2011;108(30):12396–12400. doi: 10.1073/pnas.1106613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khanna C, Hunter K. Modeling metastasis in vivo. Carcinogenesis. 2005;26(3):513–523. doi: 10.1093/carcin/bgh261. [DOI] [PubMed] [Google Scholar]
- 140.Kaemmerer E, Melchels FP, Holzapfel BM, Meckel T, Hutmacher DW, Loessner D. Gelatine methacrylamide-based hydrogels: an alternative three-dimensional cancer cell culture system. Acta Biomater. 2014;10(6):2551–2562. doi: 10.1016/j.actbio.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 141.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328(3):679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 142.Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;18(5):311–321. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro—a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15(5):405–412. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 144.Weaver VM, Petersen OW, Wang F, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137(1):231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15(7):807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mastro AM, Vogler EA. A three-dimensional osteogenic tissue model for the study of metastatic tumor cell interactions with bone. Cancer Res. 2009;69(10):4097–4100. doi: 10.1158/0008-5472.CAN-08-4437. [DOI] [PubMed] [Google Scholar]
- 147.Weigelt B, Ghajar CM, Bissell MJ. The need for complex 3D culture models to unravel novel pathways and identify accurate biomarkers in breast cancer. Adv Drug Deliv Rev. 2014;6(9–70):42–51. doi: 10.1016/j.addr.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krishnan V, Shuman LA, Sosnoski DM, Dhurjati R, Vogler EA, Mastro AM. Dynamic interaction between breast cancer cells and osteoblastic tissue: comparison of two- and three- dimensional cultures. J Cell Physiol. 2011;226(8):2150–2158. doi: 10.1002/jcp.22550. [DOI] [PubMed] [Google Scholar]
- 149.Marlow R, Honeth G, Lombardi S, et al. A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res. 2013;73(23):6886–6899. doi: 10.1158/0008-5472.CAN-13-0991. [DOI] [PubMed] [Google Scholar]
- 150.Guiro K, Patel SA, Greco SJ, Rameshwar P, Arinzeh TL. Investigating breast cancer cell behavior using tissue engineering scaffolds. PLoS One. 2015;10(3):e0118724. doi: 10.1371/journal.pone.0118724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Foroni L, Vasuri F, Valente S, et al. The role of 3D microenvironmental organization in MCF-7 epithelial-mesenchymal transition after 7 culture days. Exp Cell Res. 2013;319(10):1515–1522. doi: 10.1016/j.yexcr.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 152.Pathi SP, Lin DD, Dorvee JR, Estroff LA, Fischbach C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials. 2011;32(22):5112–5122. doi: 10.1016/j.biomaterials.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cichon MA, Gainullin VG, Zhang Y, Radisky DC. Growth of lung cancer cells in three-dimensional microenvironments reveals key features of tumor malignancy. Integr Biol. 2012;4(4):440–448. doi: 10.1039/c1ib00090j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Justice BA, Badr NA, Felder RA. 3D cell culture opens new dimensions in cell-based assays. Drug Discov Today. 2009;14(1–2):102–107. doi: 10.1016/j.drudis.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 155.Mazzoleni G, Di Lorenzo D, Steimberg N. Modelling tissues in 3D: the next future of pharmaco-toxicology and food research? Genes Nutr. 2009;4(1):13–22. doi: 10.1007/s12263-008-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]