Abstract
Clinically significant hypersensitivity reactions (HSRs) to the chemotherapy drug L-asparaginase are reported in humans and dogs, but frequency in small animals is not well-defined. This study retrospectively evaluated the frequency of HSR to L-asparaginase given by IM injection to dogs and cats with lymphoid malignancies. The medical records of all dogs and cats treated with at least 1 dose of L-asparaginase chemotherapy over a 5-year period were reviewed. A total of 370 doses of L-asparaginase were administered to the dogs, with 88 of 142 dogs receiving multiple doses, and 6 dogs experiencing an HSR. A total of 197 doses were administered to the cats, with 33 of 68 cats receiving multiple doses, and no cats experiencing an HSR. Hypersensitivity reactions were documented in 4.2% of dogs, and in association with 1.6% of L-asparaginase doses administered. These results show that HSRs occur uncommonly among dogs and cats, even with repeated dosing.
Résumé
Réactions d’hypersensibilité associées à l’administration de L-asparaginase chez 142 chiens et 68 chats atteints de tumeurs malignes lymphoïdes: 2007–2012. Des réactions d’hypersensibilité cliniquement significatives (HCS) au médicament de chimiothérapie L-asparaginase sont signalées chez les humains et les chiens, mais leur fréquence chez les petits animaux n’est pas bien définie. Cette étude a évalué rétrospectivement la fréquence des HCS au médicament L-asparaginase administré par injection IM aux chiens et aux chats atteints de tumeurs malignes lymphoïdes. On a examiné les dossiers médicaux de tous les chiens et chats traités avec au moins une dose de chimiothérapie au médicament L-asparaginase pendant une période de 5 ans. Un total de 370 doses de L-asparaginase a été administré aux chiens, 88 des 142 chiens ont reçu des doses multiples et 6 chiens ont manifesté des HCS. Un total de 197 doses ont été administrées aux chats, 33 des 68 chats ont reçu des doses multiples et aucun chat n’a manifesté des HCS. Les HCS ont été documentées chez 4,2 % des chiens et en association avec 1,6 % des doses de L-asparaginase administrées. Ces résultats indiquent que les HCS se produisent rarement chez les chiens et les chats, même avec des doses répétées.
(Traduit par Isabelle Vallières)
Introduction
The tumor inhibitory properties of L-asparaginase were discovered in the 1960’s (1). L-asparaginase hydrolyzes asparagine to aspartic acid leading to decreased systemic L-asparagine levels. This, coupled with the limited asparaginase synthetase activity in malignant lymphoid cells, decreases protein synthesis leading to cell death. In contrast, non-cancerous cells are capable of synthesizing asparagine by transamination of L-aspartic acid with glutamine through the action of asparagine synthetase (2).
Asparaginase isolated from bacteria is FDA-approved for IV or IM administration in the treatment of childhood and adult acute lymphoblastic leukemia (ALL) as part of a multi-agent chemotherapy protocol (1,3). In dogs and cats, L-asparaginase is used in the treatment of ALL (4,5), in front-line therapy for lymphoma (6–9), and in rescue therapy for lymphoma (10,11). Escherichia coli derived (native) L-asparaginase (Elspar; Lundbeck, Deerfield, Illinois, USA) is the most commonly used formulation in veterinary medicine in the United States. The plasma half-life in dogs is relatively short at 12 to 40 h (median: 14 h) (6,12). In Canada, native L-asparaginase is available (Kidrolase; EUSA Pharma SAS, Limonest, France). Another formulation called pegaspargase (PEG) (Oncaspar; Enzon Pharmaceuticals, Piscataway, New Jersey, USA) conjugates L-asparaginase and polyethylene glycol leading to less immunogenicity and a longer half-life (12,13). It has limited use in clinical veterinary medicine due to cost (6). Erwinia derived L-asparaginase (Erwinaze; EUSA Pharma, Langhorne, Pennsylvania, USA) was approved in 2011 to treat humans who develop hypersensitivity to native L-asparaginase or pegaspargase (3). Erwinaze is designated as an orphan drug (6,14) and is, therefore, not commercially available for use in veterinary medicine.
Toxicities related to L-asparaginase fall into 2 categories: those related to hypersensitivity to the foreign protein and those related to decreased asparagine and protein synthesis inhibition (2,9). Fatal hypersensitivity reactions (HSRs) occur in less than 1% of humans treated (2). Reactions to a single administration are rare (2), but since native L-asparaginase is isolated from E. coli which is present in the normal intestinal flora, antibodies to the enzyme can be present at the first injection (15). Types of hypersensitivity reactions include urticarial, true anaphylaxis with hypotension, and delayed hypersensitivity with proteinuria and fever (2). All formulations of L-asparaginase are immunogenic because they are foreign proteins (9).
Review of the veterinary literature reveals 29 dogs that experienced an HSR to L-asparaginase administered IM or SQ (7,10,16–20) and 13 dogs that experienced an HSR to L-asparaginase administered IP (12,21) (Table 1). The number of dogs receiving L-asparaginase in these 9 publications was 588, for a frequency of HSR of 7.1% (42/588) among treated dogs. There is 1 reported death attributed to an acute HSR to L-asparaginase in a dog (21). However, numerous other publications report no HSRs among dogs receiving L-asparaginase (22–25) so the overall frequency of HSR is less than 7.1%. Review of the veterinary literature reveals only 1 cat that experienced an HSR to L-asparaginase and it was administered IP (11). Despite the relatively low frequency of serious HSR, some authors advocate against repeated dosing of L-asparaginase, at least in dogs, because of perceived risk (9,10). Others continue to use the drug based on clinical observation of repeated tumor response and benefit (26,27).
Table 1.
Previous reports of L-asparaginase (L-asp) hypersensitivity reactions in dogs and cats
| Reference | Number and species | Protocol | Type and route | Dose | Number of L-asp prescribed | Number and type of HSR | Percent reaction |
|---|---|---|---|---|---|---|---|
| 20 | 1 dog | Adria/DTIC, L-asp, vaccine | Native IP | 20 000 IU/m2 | 3 | 1 total: death | Case report |
| 10 | 62 dogs | L-asp maintenance | PEG IM vs native IP | 400 IU/kg BW | Until relapse | 12 total: all native IP; 9 shock, 3 angioedema | 19.3% |
| 15 | 69 dogs | L-asp maintenance | PEG IM vs native IM | 400 IU/kg BW | Until relapse | 3 total: 1 PEG: urticaria, 2 native: 1 limb edema & 1 shock, seizures | 4.3% |
| 16 | 68 dogs | ACOPA ll | Native IM | 10 000 IU/m2 Max 10 000 IU |
4 | 4 total: 3 collapse, 1 angioedema | 5.8% |
| 17 | 98 dogs | VELCAP-L | Native IM | 10 000 IU/m2 | 5 | 5 total | 5.1% |
| 18 | 82 dogs | VELCAP-S | Native IM | 10 000 IU/m2 Max 10 000 IU |
5 | 6 total | 7.3% |
| 19 | 93 dogs | VELCAP-SC | Native SQ | 10 000 IU/m2 Max 10 000 IU |
2 | 4 total | 4.3% |
| 8 | 48 dogs | Lomustine, L-asp, pred rescue | Native IM or SQ | 400 IU/kg BW | Until relapse | 5 total: all SQ; 3 grade l, 2 grade 2, vomiting, facial swelling, urticaria | 10.4% |
| 6 | 66 dogs | L-CHOP +/− CCNU, MOPP | Native SQ | 400 IU/kg BW | 2 | 2 total | 3.0% |
| 9 | 36 cats | L-asp rescue | Native IV, then IP | 400 IU/kg BW | Until relapse | 1 total | 2.7% |
BW — body weight.
No study to date has examined the risk of hypersensitivity to L-asparaginase in cats or the risk in dogs or cats after multiple doses of the drug have been given. Given the conflicting information and opinions in the veterinary literature, the purpose of this study was to retrospectively evaluate the frequency of hypersensitivity to L-asparaginase given by IM injection to dogs and cats with lymphoid malignancies in both front-line and rescue settings.
Materials and methods
Case selection
The medical records of all dogs and cats treated with at least 1 dose of L-asparaginase chemotherapy at the Animal Specialty Group from June 2007 to June 2012 were reviewed. Data pertaining to HSRs were extracted from the medical records to include historical information from the owners, physical examination findings, laboratory findings, and treatment administered. Other data extracted from the medical records included patient signalment, weight, the dose of L-asparaginase administered, whether the dose was capped, concurrent medications, whether the patient received L-asparaginase in a front-line or rescue setting, the type of lymphoid malignancy being treated, and the total number of doses of L-asparaginase administered.
Treatment
During the study period, a 6-month CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy protocol was recommended as front-line therapy for dogs with lymphoma (22) and a 12-month CHOP chemotherapy protocol was recommended as front-line therapy for cats (28). L-asparaginase was not routinely included in the induction phase of CHOP chemotherapy for all dogs and cats, but was used in unstable or systemically ill animals as well as those that were neutropenic due to myelophthesis. When L-asparaginase was used during induction, no other chemotherapeutics except corticosteroids were administered concurrently.
The recommended rescue protocol during the study period consisted of L-asparaginase IM in week 1, lomustine (NextSource Biotechnology, Miami, Florida, USA), 50 to 70 mg/m2, PO in dogs and 35 to 40 mg/m2 PO in cats in week 2, and L-asparaginase IM in week 3. If a partial or complete response was seen, dogs and cats were continued on every 2-week therapy alternating L-asparaginase IM and lomustine PO. When progressive disease occurred after 1 of the drugs, that drug was removed from the protocol and the other drug was continued until relapse.
All animals received L-asparaginase (Elspar, Lundbeck) at a dosage of 400 IU/kg body weight (BW), IM. Each 10 000 IU vial was reconstituted with 2 mL of sterile water. Diphenhydramine (Qualitest Pharmaceuticals, Huntsville, Alabama, USA) was given at 2 mg/kg BW, IM in a single location 10 min prior to administration of L-asparaginase in the dogs only. Total doses of L-asparaginase were capped at a single vial (10 000 IU) if owners expressed financial constraints and the prescribed dose was not greater than 15 000 IU; this approach was based on previous publications reporting the use of a maximum dose (17,19,20,29). Animals being treated as stable outpatients were monitored in the hospital for 30 min after injection and then discharged to the care of their owners.
An HSR was said to have occurred if the hospital staff or owner noticed any of the following signs within an arbitrarily defined time frame of 24 h of administration of L-asparaginase: pruritus, urticaria, flushing, diffuse erythema, angioedema, hypotension, tachypnea, tachycardia, hypoxemia, respiratory distress, facial swelling, or repeated vomiting. Owners were told to return to the hospital immediately if any of these signs were observed. Hypersensitivity reactions were graded based on the Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events (VCOG-CTCAE; v.1.0) specifically addressing allergic/immunologic events (30) (Table 2).
Table 2.
Veterinary Cooperative Oncology Group criteria for grading adverse allergic and hypersensitivity events
| Grade | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Clinical signs | Transient urticaria | Rash; urticaria; dyspnea | Symptomatic hypotension, with or without urticaria; parenteral medications necessary; edema | Anaphylaxis requiring parenteral medications | Death |
Results
A total of 142 dogs and 68 cats with a histologic or cytologic diagnosis of lymphoid malignancy that received at least 1 dose of L-asparaginase chemotherapy as first-line or rescue chemotherapy were identified for further study. The mean age of the dogs was 9.7 y (range: 3 to 15 y). The mean age of the cats was 11.7 y (range: 1 to 16 y). Of the dogs treated, 78 were males, 66 of which were castrated, and 64 were female, 60 of which were spayed. There were 50 (35%) mixed breed dogs and 92 (65%) purebred dogs. Of the cats treated, 46 were neutered males and 22 were spayed females. There were 11 purebred cats and the remaining were domestic long or short-haired cats. Fifty-eight cats were tested for feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV). Three cats were positive for FeLV only, and 8 cats were positive for FIV only. Two cats tested positive for both viruses.
Of the 142 dogs, 79% were treated for intermediate to high grade multicentric lymphoma, 9% for epitheliotropic cutaneous T cell lymphoma, 3% for gastrointestinal lymphoma, 6% for low-grade lymphoma or leukemia, and 4% for ALL. Of the 68 cats, 81% were treated for intermediate- to high-grade lymphoma, 18% for low-grade lymphoma (6 gastrointestinal, 3 hepatic, 2 multicentric, 1 tarsal), and 2% for chronic lymphocytic leukemia.
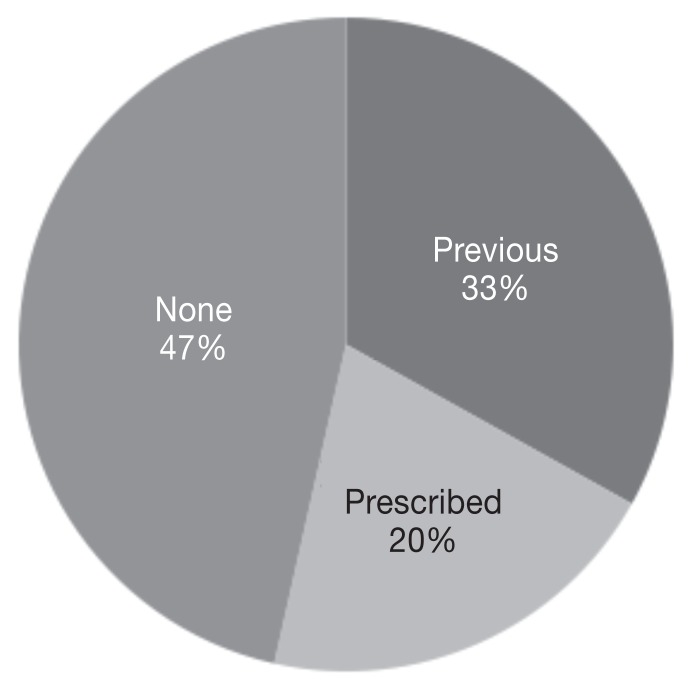
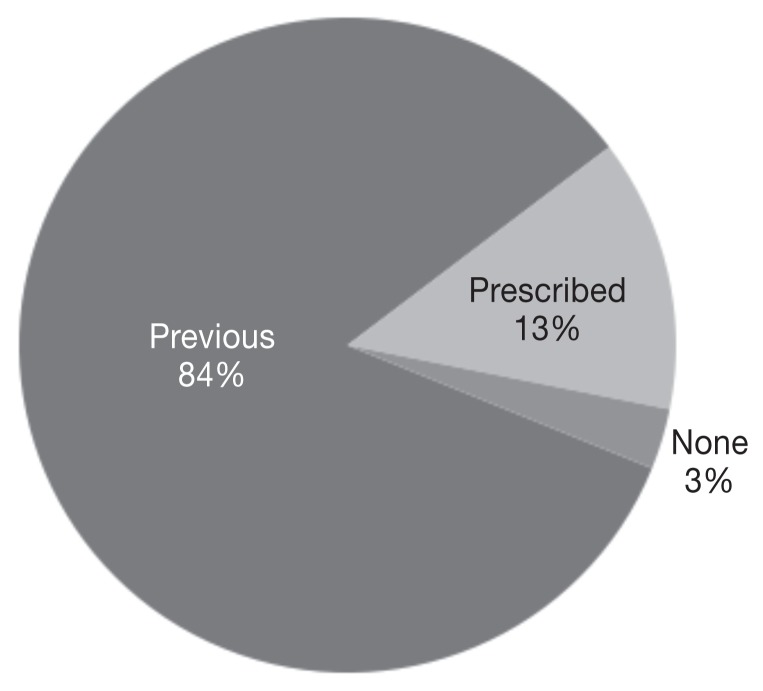
Forty-four percent of dogs (63/142) received L-asparaginase during front-line chemotherapy, 69% (98/142) received L-asparaginase during rescue chemotherapy, and 13% (19/142) received L-asparaginase as part of both front-line and rescue chemotherapy. Of the 68 cats, 65% received L-asparaginase during front-line chemotherapy, 45% received L-asparaginase during rescue chemotherapy, and 10% received L-asparaginase as part of both front-line and rescue chemotherapy. L-asparaginase was administered in a lomustine-containing protocol in 66% (94/142) of dogs and 49% of cats (33/68). There were 79 dogs (55%) weighing more than 25 kg whose calculated dose was greater than a single vial of L-asparaginase. Of these 79 dogs, 58 received a capped dose of 10 000 IU of L-asparaginase (5) while the remaining 21 dogs received the full calculated dose. Corticosteroids were variably administered to both dogs and cats in this study, depending on treatment protocol and clinician preference (Figures 1, 2).
Figure 1.
Percentage of dogs i) previously prescribed prednisone and currently on prednisone at the time of L-asparaginase administration (previous), ii) prescribed prednisone at the time of L-asparaginase administration (prescribed), or iii) not on concurrent prednisone at the time of L-asparaginase administration (none), n = 142 dogs.
Figure 2.
Percentage of cats i) previously prescribed corticosteroids and currently on corticosteroids at the time of L-asparaginase administration (previous), ii) prescribed corticosteroids at the time of L-asparaginase administration (prescribed), or iii) not on concurrent corticosteroids at the time of L-asparaginase administration (none), n = 68 cats.
A total of 370 doses of L-asparaginase were administered to 142 dogs for a mean of 2.6 doses (range: 1 to 11 doses) and a median of 2 doses. A single dose was administered to 54 dogs (38%). The 88 dogs receiving multiple doses of L-asparaginase had a mean of 3.6 doses and a median of 3 doses. The following numbers of dogs had multiple doses: 2 doses (n = 34), 3 doses (n = 19), 4 doses (n = 14), 5 doses (n = 11), 6 doses (n = 3), 7 doses (n = 1), 8 doses (n = 3), 9 doses (n = 1), and 11 doses (n = 2).
A total of 197 doses of L-asparaginase were administered to 68 cats for a mean of 2.9 doses (range: 1 to 20 doses) and a median of 1 dose. A single dose was administered to 35 cats (51%). The 33 cats receiving multiple doses of L-asparaginase had a mean of 4.9 doses and a median of 4 doses. The following numbers of cats had multiple doses: 2 doses (n = 12), 3 doses (n = 4), 4 doses (n = 6), 5 doses (n = 2), and 6 doses (n = 3). One cat each received 8, 10, 11, 12, 13, and 20 doses of L-asparaginase.
There were 6 HSRs in 6 different dogs in the 5-year period (Table 3) for an overall frequency of 4.2% (6/142) of dogs and 1.6% (6/370) of doses. For dogs receiving multiple doses, the frequency was 5.7% (5/88) of dogs and 1.6% (5/316) of doses. There were 35 dogs that received ≥ 4 doses of L-asparaginase and 1 HSR was seen after the fourth dose. In the subset of 35 patients receiving 4 doses or more, the frequency of allergic reaction was 2.9% (1/35) of dogs and 0.5% (1/191) of doses. Based on the VCOG-CTCAE, 3 of the dogs had a grade 4 adverse event, 1 dog had a grade 3 adverse event, and 2 dogs had a grade 2 adverse event (30). Only Dog 3 was receiving concurrent prednisone at the time of its HSR to L-asparaginase. None of the cats experienced an HSR to L-asparaginase chemotherapy.
Table 3.
Summary data for 6 dogs experiencing an HSR to L-asparaginase
| Dog | Signalment | LSAa grade | Concurrent chemotherapy | Dose (IM, per kg BW) | L-asp number @ reaction | L-asp number post HSR | Clinical signs | VCOG grade |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.5 y FS Shar Pei | High | L-asp/CCNU | 295 IU | 3 | 6 | Pruritus, anxiety, trembling | 2 |
| 2 | 7 y MC Mixed | Intermedb to high | L-asp/CCNU | 400 IU | 2 | 0 | Head-shaking, vomiting, diarrhea, fever, tachycardia | 3 |
| 3 | 6 y FS Mixed | Intermed | CHOP | 400 IU | 1 | 0 | Skin rash | 2 |
| 4 | 6.5 y FS Pit bull | Intermed | L-asp/CCNU rescue | 385 IU | 2 | 0 | Ptyalism, lethargy, generalized erythema, urticaria, hypotension. Recurrent urticaria required repeat therapy | 4 |
| 5 | 6.5 y FS Mixed | High | L-asp/CCNU rescue | 400 IU | 2 | 0 | Kyphosis, lethargy, anorexia, vomiting, colitis, tachycardia, urticaria, facial edema, swollen paws. Recurrent urticaria and vomiting required repeat therapy | 4 |
| 6 | 7 y MC Airedale | High | L-asp/CCNU | 400 IU | 3 | 0 | Vomiting, lethargy, tachycardia, hypotension | 4 |
LSA — Lymphoma.
Intermed — Intermediate.
BW — body weight.
Dog 1 Treatment: IM and PO diphenhydramine (PO diphenhydramine continued, PO prednisone prescribed at the 7th dose and continued).
Dog 2 Treatment: IV fluids; IM dexamethasone and diphenhydramine; SQ metaclopromide; PO famotidine and sucralfate.
Dog 3 Treatment: One dose PO diphenhydramine.
Dog 4 Treatment: IV fluids, epinephrine, dexamethasone, and ondansetron; IM diphenhydramine; PO diphenhydramine and prednisone.
Dog 5 Treatment: IV epinephrine, dexamethasone, and ondansetron; IM diphenhydramine; PO prednisone and diphenhydramine.
Dog 6 Treatment: IV fluids, dexamethasone, ondansetron, and famotidine; IM diphenhydramine; SQ epinephrine and maropitant.
Discussion
The results of the current study suggest that HSRs occur relatively infrequently among dogs receiving 1 or more doses of L-asparaginase for the treatment of lymphoid malignancies, and they appear even more uncommon in cats. In veterinary oncology, the risk of any adverse effect to chemotherapy requiring hospitalization is < 5% and the risk of a treatment-associated fatality is < 1% (29,31). Thus, the frequency of L-asparaginase-related HSRs appears similar to the accepted risk of more common side effects of chemotherapy such as gastrointestinal complications and neutropenic sepsis. It is also noteworthy that all 6 of the dogs that experienced an HSR responded well to treatment, eventually recovering completely. Only a single fatality associated with an L-asparaginase-related HSR is reported in the veterinary literature (21), and no deaths occurred in the current study.
The indications for L-asparaginase in dogs and cats are controversial, and have evolved over time because of changes in best chemotherapy practice as well as intermittent issues with drug availability. Older papers suggest that L-asparaginase is less effective in cats with lymphoma (9,11), although more recent work reveals an overall response rate to L-asparaginase of 30% in drug-naive feline lymphomas (8). Some investigators have recommended that L-asparaginase be removed from front-line therapy for canine lymphoma based on work that suggests no improvement in the efficacy of induction chemotherapy, as well as long-term survival that did not appear significantly prolonged (22). Others advocate against the repeated use of L-asparaginase in the rescue setting because of perceived risk of HSR (10,24). The authors of 1 study found that L-asparaginase increased the cost and toxicity of their lomustine-based rescue protocol for canine lymphoma without improving disease control, and concluded that administration of more than 2 doses of L-asparaginase was not warranted (10). However, the authors of the present study continue to use L-asparaginase in front-line therapy for dogs and cats with lymphoid malignancy because of a number of significant advantages, including ease of administration, relatively mild myelosuppressive effects, lack of overlapping toxicity, lack of cross resistance, and efficacy in acute leukemia. In addition, alternating L-asparaginase and lomustine as described in this study is still used as rescue therapy for lymphoma in both dogs and cats, since there is a subset of animals that either continue to respond or maintain their remission while receiving repeated cycles of therapy. The results of this study provide valuable support for the safety of L-asparaginase in both single and multiple dose scenarios for dogs and cats with lymphoid neoplasia. However, owners should be advised that every dose of L-asparaginase has the potential to cause an HSR (14), and vomiting may indicate a more serious reaction (21). Fortunately, L-asparaginase HSRs are amenable to therapy, especially if recognized early and treated aggressively.
Although it would be useful to be able to identify animals at increased risk for developing L-asparaginase-induced HSRs, the retrospective design of this study makes identification of potential risk factors difficult. There is no known breed or gender predisposition to L-asparaginase-induced HSRs. Furthermore, because L-asparaginase is used almost exclusively for the treatment of lymphoid malignancy in dogs and cats, the signalment of the animals herein is likely to reflect their risk of having these diseases as much as the risk of an HSR. Five of the 6 dogs that experienced an HSR were 6 years old at the time of reaction and the remaining dog was 7 years old; this is 4 y younger than the 10-year median age of all dogs in the study. However, age is not reported to affect the rate of HSR to L-asparaginase in humans (3) and it is unlikely that this observation is significant.
Other potential risk factors for developing L-asparaginase-induced HSRs are nonspecific histamine release as well as the development of antibodies, both of which may contribute to the anaphylactoid side effects observed in L-asparaginase-induced HSRs (3,14). Allergic reactions have been observed in humans who do not have detectable antibodies (3). Nonspecific reactions to chemotherapy that are documented in humans and in dogs are typically not immunologic reactions and can be prevented with pre-medication or slower infusion (32). The 2 dogs that experienced grade 2 adverse events both had histories of skin allergies. It is possible that their allergies predisposed them to have nonspecific release of histamine and vasoactive substances from mast cells. One of these dogs was subsequently treated with concurrent antihistamines and eventually prednisone, and received 6 additional doses of L-asparaginase without reaction. In a previous report, a dog which experienced an L-asparaginase-induced HSR received 3 additional doses without reaction after pretreatment with diphenhydramine and dexamethasone (19). More commonly, however, L-asparaginase-induced HSRs are immunologic reactions, eliciting the production of antibodies (3). Most humans produce antibodies to E. coli derived L-asparaginase (15), more than other formulations of L-asparaginase (3), and antibody development has also been shown to occur in dogs (33).
It is intuitive that the risk of HSR might vary with the dose of L-asparaginase administered. Two of the 6 dogs in the present study that had an HSR to L-asparaginase received a capped dose and these HSRs were the mildest seen: trembling, pruritus, and anxiety in 1 dog and a skin rash in the other. Higher doses are reported to increase the risk of HSR to L-asparaginase in humans (2) and the only reported death in veterinary medicine secondary to presumed L-asparaginase HSR was observed after a significantly higher dose of 20 000 IU/m2 (21). However, of the dogs reported in the literature that had an HSR to L-asparaginase (Table 1), a maximum dose of 10 000 IU was reported in 3 of 9 publications involving 243 dogs with an observed HSR frequency of 5.8%. This is not different than the 5.7% frequency of HSR seen when all the studies reporting an HSR to L-asparaginase administered IM or SQ are combined. It is possible that lowering the L-asparaginase dose by techniques such as capping at a maximum dose of 10 000 IU may decrease the risk of HSR in dogs, but it is not possible to draw this conclusion based on the existing literature or the results of the current study. In addition, the potential benefit of decreased HSR risk would have to be weighed against the potential for decreased drug efficacy. A randomized prospective trial utilizing a standardized method of dose reduction would be the best way to further investigate the potential relationship between L-asparaginase dose and risk of HSR in dogs and cats.
Another factor that has been proposed to affect the risk of HSR is the number of L-asparaginase treatments administered, with individuals receiving a greater number of doses having progressively increased risk of reaction (2,9). In the current study, only 1 dog had an HSR after a single dose of L-asparaginase, potentially supporting an increased risk of reaction with multiple doses (2,9). Most reactions (3/6) occurred after the second dose, similar to previous reports (Table 4). However, dogs that received greater than 4 doses of L-asparaginase did not appear to have an increased risk of an HSR, contrary to an increased risk reported in humans after 4 doses (14). The number and timing of L-asparaginase doses administered to the dogs in the current study was also unavoidably impacted by choice of treatment protocol. In humans, post-induction patients are more likely to have a reaction compared with those who receive the drug during induction (3). Continuous dosing and shorter time intervals between doses may decrease the risk of HSR, while re-instituting L-asparaginase after a treatment break may increase the risk of reaction in humans (3). Five of the 6 dogs in this study that had an HSR to L-asparaginase received the drug for the first time in the rescue setting. Only 1 dog had an HSR during induction and this was after the first dose and was the mildest reaction seen. Unfortunately, the small number of HSRs observed in this study, in combination with the variable treatment protocols used, makes drawing firm conclusions with respect to the impact of treatment protocol difficult.
Table 4.
Number of L-asparaginase doses prior to developing an HSR
The route of L-asparaginase administration has been suggested by some authors to influence the risk of HSR (2,9,14,16). We chose the IM route of administration based on a previous report that suggested it resulted in a faster response to chemotherapy and longer remission and survival times in dogs with multicentric lymphoma compared to the SQ route (23). As well, other authors have stated that IM administration of L-asparaginase is a safer route (9) compared to IP based on a lower risk of HSRs (16). Since all of the animals in this study received L-asparaginase by IM injection, it is not possible to compare the risk of HSR associated with other routes of administration.
A final factor that could reasonably be expected to impact the risk of L-asparaginase-induced HSR is the immunosuppressive effects of concomitant medications (1,2,9,14,34). All dogs in this study were pretreated with diphenhydramine, but this may not have had an impact on the number of HSRs since similar rates of HSR have been documented in dogs that did not receive diphenhydramine (18). Five of the 6 dogs that experienced an HSR in this study were not on concurrent prednisone. Most of the cats in the current study (82%) were on concurrent prednisolone at the time of L-asparaginase administration and none experienced an HSR. In humans, clinical hypersensitivity is most common when L-asparaginase is administered without corticosteroids (1,2,3,14).
There are several limitations to this study, many of which are related to its retrospective design. Only 6 dogs were observed to have an L-asparaginase-induced HSR and each received a different treatment, so it is not possible to make definitive recommendations for optimal or standardized therapy of suspected reactions based on this small group of animals. The same dose of L-asparaginase was not administered to all dogs, and the technique used to alter dose (capping at a maximum of 10 000 IU) was not applied in a consistent way. Variability in treatment protocol had a significant impact on the timing, sequence, and number of doses of L-asparaginase administered, and any or all of these factors could have affected the observed frequency of HSR. Another limitation was the inconsistent use of prednisone during the treatment period, something that has been shown to influence the risk of HSRs in humans. Finally, the short period of expert observation (30 min) following L-asparaginase administration may have resulted in subtle or delayed signs of hypersensitivity being missed. One hour of observation has been recommended previously (31) and 1 author (GEM) recommends daytime monitoring for animals that have previously received multiple doses of L-asparaginase.
In conclusion, this study provides valuable information for veterinarians using L-asparaginase as part of frontline or rescue therapy for lymphoid malignancies in dogs and cats. There is a risk of HSR associated with the use of L-asparaginase, but the frequency of this complication does not appear substantially different than that of other commonly accepted side effects of chemotherapy, even with repeated dosing, at least when animals are pretreated with antihistamines or corticosteroids. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Avramis VI, Panosyan EH. Pharmocokinectic/pharmacodynamic relationships of asparaginase formulations. Clin Pharmacokinet. 2005;44:367–393. doi: 10.2165/00003088-200544040-00003. [DOI] [PubMed] [Google Scholar]
- 2.Chabner BA, Sallan SE. L-asparaginase. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy. 4th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2006. pp. 476–483. [Google Scholar]
- 3.Raetz EA, Salzer WL. Tolerabilty and efficacy of L-asparaginase therapy in pediatric patients with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32:554–563. doi: 10.1097/MPH.0b013e3181e6f003. [DOI] [PubMed] [Google Scholar]
- 4.Matus R, Leifer C, MacEwen EG. Acute lymphoblastic leukemia in the dog: A review of 30 cases. J Am Vet Med Assoc. 1983;183:859–862. [PubMed] [Google Scholar]
- 5.Matus RE. Chemotherapy of lymphoma and leukemia. In: Kirk RW, Bonagura JD, editors. Kirk’s Current Veterinary Therapy X. Philadelphia, Pennsylvania: WB Saunders; 1989. pp. 482–488. [Google Scholar]
- 6.Fan TM, Kitchell BE. Asparaginase. Compend Contin Educ Pract Vet. 2000;9:834–837. [Google Scholar]
- 7.Rassnick KM, Bailey DB, Malone EK, et al. Comparison between L-CHOP and an L-CHOP protocol with interposed treatments of CCNU and MOPP (L-CHOP-CCNU-MOPP) for lymphoma in dogs. Vet Comp Oncol. 2010;8:243–253. doi: 10.1111/j.1476-5829.2010.00224.x. [DOI] [PubMed] [Google Scholar]
- 8.LeBlanc AK, Cox SK, Kirk CA, Newman SJ, Bartges JW, Legendre AM. Effects of L-asparaginase on plasma amino acid profiles and tumor burden in cats with lymphoma. J Vet Intern Med. 2007;21:760–763. doi: 10.1892/0891-6640(2007)21[760:eolopa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Rogers KS. L-asparaginase for treatment of lymphoid neoplasia in dogs. J Am Vet Med Assoc. 1989;194:1626–1630. [PubMed] [Google Scholar]
- 10.Saba CF, Hafeman SD, Vail DM, Thamm DH. Combination chemotherapy with continuous L-asparaginase, lomustine, and prednisone for relapsed canine lymphoma. J Vet Intern Med. 2009;23:1058–1063. doi: 10.1111/j.1939-1676.2009.0357.x. [DOI] [PubMed] [Google Scholar]
- 11.Jeglum KA, Whereat A, Young K. Chemotherapy of lymphoma in 75 cats. J Am Vet Med Assoc. 1987;190:174–178. [PubMed] [Google Scholar]
- 12.Teske E, Rutteman GR, van Heerde P, Misdorp W. Polyethylene glycolL-asparaginase versus native L-asparaginase in canine non-Hodgkin’s lymphoma. Eur J Cancer. 1990;26:891–89. doi: 10.1016/0277-5379(90)90193-w. [DOI] [PubMed] [Google Scholar]
- 13.Viau AT, Abuchoski, McCoy JR, Kazo GM, Davis FF. Toxicologic studies of a conjugate of asparaginase and polyethylene glycol in mice, rats, and dogs. Am J Vet Res. 1986;47:1398–1401. [PubMed] [Google Scholar]
- 14.Weiss RB. Hypersensitivity reactions. Semin Oncol. 1992;19:458–477. [PubMed] [Google Scholar]
- 15.Oettgen HF, Stephenson PA, Schwartz MK, et al. Toxicity of E. coli L-asparaginase in man. Cancer. 1970;25:253–278. doi: 10.1002/1097-0142(197002)25:2<253::aid-cncr2820250204>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.MacEwen EG, Rosenthal RC, Fox LE, Loar AS, Kurzman ID. Evaluation of L-asparaginase: Polyethylene glycol conjugate versus native L-asparaginase combined with chemotherapy. J Vet Intern Med. 1992;6:230–234. doi: 10.1111/j.1939-1676.1992.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 17.Myers NC, Moore AS, Rand WM, Gliatto J, Cotter SM. Evaluation of a multidrug chemotherapy protocol (ACOPA II) in dogs with lymphoma. J Vet Intern Med. 1997;11:333–339. doi: 10.1111/j.1939-1676.1997.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 18.Zemann BI, Moore AS, Rand WM, et al. A combination chemotherapy protocol (VELCAP-L) for dogs with lymphoma. J Vet Intern Med. 1998;12:465–470. doi: 10.1111/j.1939-1676.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 19.Moore AS, Cotter SM, Rand WM, et al. Evaluation of discontinuous treatment protocol (VELCAP-S) for canine lymphoma. J Vet Intern Med. 2001;15:348–354. [PubMed] [Google Scholar]
- 20.Morrison-Collister KE, Rassnick KM, Northrup NC, et al. A combination chemotherapy protocol with MOPP and CCNU consolidation (Tufts VELCAP-SC) for the treatment of canine lymphoma. Vet Comp Oncol. 2003;1:180–190. doi: 10.1111/j.1476-5810.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 21.Hansen JF, Carpenter RH. Fatal acute systemic anaphylaxis and hemorrhagic pancreatitis following asparaginase treatment in a dog. J Am Anim Hosp Assoc. 1983;19:977–980. [Google Scholar]
- 22.McDonald VS, Thamm DH, Kurzman ID, Turek MM, Vail DM. Does L-asparaginase influence efficacy or toxicity when added to a standard CHOP protocol for dogs with lymphoma? J Vet Intern Med. 2005;19:732–736. doi: 10.1892/0891-6640(2005)19[732:dlieot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Valerius KD, Ogilvie GK, Fettman MJ, et al. Comparison of the effects of asparaginase administered subcutaneously versus intramuscularly for treatment of multicentric lymphoma in dogs receiving doxorubicin. J Am Vet Med Assoc. 1999;214:353–356. [PubMed] [Google Scholar]
- 24.Piek CJ, Rutteman GR, Teske E. Evaluation of the results of a L-asparaginase-based continuous chemotherapy protocol versus a short doxorubicin-based induction chemotherapy protocol in dogs with malignant lymphoma. Vet Q. 1999;21:44–49. doi: 10.1080/01652176.1999.9694990. [DOI] [PubMed] [Google Scholar]
- 25.Ogilvie GK, Atwater SW, Ciekot PA, Bergman PJ, Henkel S, Walters LM. Prevalence of anaphylaxis associated with the intramuscular administration of L-asparaginase to 81 dogs with cancer: 1989–1991. J Am Anim Hosp Assoc. 1994;30:62–65. [Google Scholar]
- 26.Sorenmo K, Overley B, Krick E, Ferrara T, LaBlanc A, Shofer F. Outcome and toxicity associated with a dose-intensified, maintenance- free CHOP-based chemotherapy protocol in canine lymphoma: 130 cases. Vet Comp Oncol. 2010;8:196–208. doi: 10.1111/j.1476-5829.2010.00222.x. [DOI] [PubMed] [Google Scholar]
- 27.Daters AT, Mauldin GE, Mauldin GN, Brodsky EM, Post GS. Evaluation of a multidrug chemotherapy protocol with mitoxantrone based maintenance (CHOP-MA) for the treatment of canine lymphoma. Vet Comp Oncol. 2009;8:11–22. doi: 10.1111/j.1476-5829.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 28.Zwahlen CH, Lucroy MD, Kraegel SA, Madewell BR. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993–1997) J Am Vet Med Assoc. 1998;213:1144–1149. [PubMed] [Google Scholar]
- 29.Chun R, Garrett L, Vail D. Cancer chemotherapy. In: Withrow SJ, Vail DM, editors. Withrow and MacEwen’s Small Animal Clinical Oncology. 4th ed. Vol. 174. St. Louis, Missouri: Saunders Elsevier; 2007. p. 185. [Google Scholar]
- 30.Veterinary Co-operative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol. 2004;2:194–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 31.Thamm DH, Vail DM. Aftershocks of cancer chemotherapy: Managing adverse effects. J Am Anim Hosp Assoc. 2007;43:1–7. doi: 10.5326/0430001. [DOI] [PubMed] [Google Scholar]
- 32.Eschalier A, Lavarenne J, Burtin C, Renoux M, Chapuy E, Rodriguez M. Study of histamine release induced by acute administration of anti-tumor agents in dogs. Cancer Chemother Pharmacol. 1988;21:246–250. doi: 10.1007/BF00262779. [DOI] [PubMed] [Google Scholar]
- 33.Kidd J, Ross P, Buntzman A, Hess P. Development of an ELISA to detect circulating anti-asparaginase antibodies in dogs with lymphoid neoplasia treated with E. coli L-asparaginase. Vet Comp Oncol. 2015;13:77–88. doi: 10.1111/vco.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez CA, Smith C, Karol SE, et al. Effect of premedications in murine model of asparaginase hypersensitivity. J Pharmacol Exp Ther. 2015;352:541–551. doi: 10.1124/jpet.114.220780. [DOI] [PMC free article] [PubMed] [Google Scholar]