Abstract
Although Clostridium difficile infection (CDI) is a common disease in swine, there is a lack of prevention strategies. The objectives of this study were to evaluate: i) the effectiveness of Lactobacillus spp. and ii) non-toxigenic C. difficile (NTCD) as prevention for the development of CDI in piglets. Cesarean-derived piglets (N = 150) were randomly assigned to 6 groups: GROUP 1 — negative control (n = 10); GROUP 2 — NTCD only (n = 13); GROUP 3 — Lactobacillus spp. only (n = 14); GROUP 4 — positive control (challenged with toxigenic C. difficile strain) (n = 35); GROUP 5 — NTCD and challenged with the toxigenic C. difficile strain (n = 34); and GROUP 6 — Lactobacillus spp. and challenged with the toxigenic C. difficile strain (n = 44). Piglets which received NTCD showed lower prevalence of toxin-positive feces, mesocolonic edema, and microscopic lesions compared with positive control piglets. Administration of Lactobacillus spp. did not reveal clear benefits.
Résumé
Probiotiques bactériens pour faciliter le contrôle de la maladie à Clostridium difficile chez les porcelets néonataux. Même si l’infection par Clostridium difficile (ICD) est une maladie commune chez les porcs, il existe une absence de stratégies de prévention. Les objectifs de cette étude consistaient à évaluer: i) l’efficacité de Lactobacillus sp. et de ii) C. difficile non toxinogène (CDNT) comme méthode de prévention contre le développement de l’ICD chez les porcelets. Les porcelets délivrés par césarienne (N = 150) ont été assignés au hasard à 6 groupes: GROUPE 1 — groupe témoin négatif (n = 10); GROUPE 2 — CDNT seulement (n = 13); GROUPE 3 — Lactobacillus sp. seulement (n = 14); GROUPE 4 — groupe témoin positif (avec épreuve pour la souche toxinogène de C. difficile) (n = 35); GROUPE 5 — CDNT et avec épreuve pour la souche toxinogène de C. difficile (n = 34); et GROUPE 6 — Lactobacillus sp. et avec épreuve pour la souche toxinogène de C. difficile (n = 44). Les porcelets ayant reçu CDNT ont affiché une prévalence inférieure de fèces positives pour les toxines, de l’œdème du mésocôlon et de lésions microscopiques comparativement aux porcelets du groupe témoin positif. L’administration de Lactobacillus sp. n’a pas révélé de bienfaits évidents.
(Traduit par Isabelle Vallières)
Introduction
Clostridium difficile is a Gram-positive, anaerobic, spore-forming bacterium and one of the most important enteric pathogens in pigs within the first week of life (1). Within C. difficile-affected herds up to 2/3 of the litters can be diseased, and within-litter morbidity can be as high as 97% to 100% (1,2). Mortality rates vary significantly; however, mortality as high as 16% has been documented (2). Also, growth retardation and lower weaning weights in surviving pigs have been reported (1).
The newborn piglet is born with a virtually sterile gastrointestinal tract, but colonization by mixed populations of bacteria occurs within hours of birth. Colonizing microbes are mechanically acquired by the piglets via oral contact within the dam’s vaginal canal, perineum, teats, exposure to feces, and skin contact (3). Several factors play a role in the dynamic succession of organisms that make up the microflora. During the piglet’s life, several microbes compete for places in microbial niches in a process of succession that eventually establishes the flora, consisting of well over 500 distinct species of bacteria in the mature gastrointestinal tract (4). Intestinal colonization with C. difficile occurs within the first hours of life in the neonatal pig, and nearly 100% of piglets in some commercial herds are colonized within 48 h of birth (5).
Although CDI is a common disease in the swine industry, there is a lack of sound prevention strategies. Therefore, the objectives of this study were to evaluate the use of Lactobacillus spp. and a non-toxigenic C. difficile strain (NTCD) as ingested microorganism (probiotic) alternatives to prevent the development of CDI in piglets.
Materials and methods
Animals
Sixteen pregnant, second and third parity, cross-bred sows from a commercial herd with no history of C. difficile disease were purchased and delivered to Iowa State University (ISU) approximately 1 wk prior to the expected farrowing date. Four sows were used per replicate and all replicates were performed within a 4-month period. On day 113 of gestation, cesarian surgeries were performed on sows and the neonatal piglets were manually provided 10 mL of pooled colostrum and an iron injection. All 150 piglets in this study were processed at birth with navels clamped, cut, and sprayed with 5% iodine solution (Durvet, Missouri, USA). Piglets were kept in a BSL-2 animal facility for the duration of the experiment.
Sera from all neonatal piglets were negative for porcine reproductive and respiratory virus (PRRSV) nucleic acid by polymerase chain reaction (PCR). Serum was analyzed for PRRSV nucleic acids using a licensed real-time PCR assay (Applied Biosystems, Foster City, California, USA).
Housing
Piglets were individually housed in new 18-gallon plastic containers at a room temperature of 29°C; heat lamps were placed above the containers with the objective to increase the microenvironmental temperature of piglets to approximately 35°C. All piglets receiving toxigenic C. difficile were housed in the same room and airspace. Negative control, Lactobacillus spp. only and NTCD only piglets were housed in separate rooms. Piglet housing and daily care have been described by Arruda et al (6). Piglets were fed milk replacer (Esbilac; Pet-Ag, Hampshire, Illinois, USA) 3 times daily (7 am, 12 pm, and 7 pm) by oral-gastric lavage using an 8 French catheter (Sovereign; Tyco/Healthcare, Mansfield, Massachusetts, USA). At feeding time, piglets were monitored for clinical signs associated with C. difficile disease.
Experimental design
The study design is summarized in Table 1. Briefly, the study contained 6 groups of piglets as follows: GROUP 1 — negative control (n = 10); GROUP 2 — NTCD only (n = 13); GROUP 3 — Lactobacillus spp. only (n = 14); GROUP 4 — positive control (challenged with a toxigenic C. difficile strain) (n = 35); GROUP 5 — NTCD and challenged with the toxigenic C. difficile strain (n = 34); and GROUP 6 — Lactobacillus spp. (Probiotic Complex) and challenged with the toxigenic C. difficile strain (n = 44). Four replicates of the study were performed totaling 150 piglets for the study; the number of piglets per replicate ranged between 35 and 40. Two potentially preventative treatments were used: i) commercially available Lactobacillus spp. (GNC, General Nutrition Corporation, Pittsburg, Pennsylvania, USA); and ii) an NTCD strain. In each experiment, pigs were randomly allocated into 1 of the 6 groups using several random number iterations in Excel (Microsoft, Redmond, Washington, USA). The experimental protocol was approved by the ISU Institutional Animal Care and Use Committee, protocol number 10-12-7445.
Table 1.
Experimental design for 1-day-old piglets administered 2 bacterial probiotics and subsequently challenged with toxigenic C. difficile isolate ISU-15454-1, ribotype 078
| Group | na | Treatmentb | Challenge dose |
|---|---|---|---|
| 1 | 10 | — | — |
| 2 | 13 | Non-toxigenic C. difficile spores @ 2 × 106 |
— |
| 3 | 14 | Lactobacillus spp./Yogurt | — |
| 4 | 35 | — | C. difficile spores @ 2 × 106 |
| 5 | 34 | Non-toxigenic C. difficile spores @ 2 × 106 |
C. difficile spores @ 2 × 106 |
| 6 | 44 | Lactobacillus spp./Yogurt | C. difficile spores @ 2 × 106 |
Number of cesarean derived piglets per group.
Treatment was administered at 4 h after parturition.
Preventive treatments were administrated intragastrically, according to experimental design, approximately 4 h after birth. Piglets from GROUPS 2 and 5 received 2 × 106 heat-shocked NTCD spores, and piglets from GROUPS 3 and 6 received 2 × 106 Lactobacillus spp. in a yogurt suspension. Sixteen hours following probiotic administration, piglets in GROUPS 4, 5, and 6 were challenged with 2 × 106 heat-shocked toxigenic C. difficile spores (Table 1). Piglets were euthanized 72 h after challenge.
Inoculum
The toxigenic bacterial isolate (C. difficile isolate ISU-15454-1) was obtained from a field case of piglet diarrhea received at the ISU Veterinary Diagnostic Laboratory (ISU-VDL). High levels of toxin (4+) were detected by enzyme-linked immunosorbent assay (ELISA) (C. DIFFICILE TOX A/B IITM, Blacksburg, Virginia, USA) from the clinically affected piglet. Isolate 15454-1 is ribotype 078, toxinotype V, and contains both toxin A and toxin B gene sequences (7). Isolate spores were stored in chopped meat broth at 3°C to 5°C until experimental use.
Procedures involving C. difficile isolation, growth, spore harvest and titration, and heat shock activation prior to challenge were as previously described (8).
Non-toxigenic Clostridium difficile
Non-toxigenic strain JGS653 was obtained from a clinically normal piglet in North Carolina. Polymerase chain reaction assays for tcdA and tcdB were negative and toxin production was not detected in 7-day dialysis bag cultures in brain heart infusion (9).
Lactobacillus spp
The Lactobacillus spp. inoculum was prepared as follows: 75 × 109 colony forming units (CFUs) of Probiotic Complex (GNC, General Nutrition Corporation) were used to fortify the amount of Lactobacillus spp. in yogurt. One capsule containing 75 × 109 CFUs of probiotic was thoroughly mixed with 190 mL of yogurt.
Inoculation
Approximately 4 h after birth, piglets in GROUPS 2, 3, 5, and 6 received either NTCD or Lactobacillus spp. intragastrically using an 8 French catheter as an oral-gastric tube (Sovereign; Tyco/Healthcare). At 20 h after birth (16 h post-prevention intervention), all challenged pigs received a 1.25 mL of inoculum preparation containing heat-shocked toxigenic C. difficile spore solution intragastrically as previously described (6).
Necropsy
Piglets were monitored for 72 h post-challenge and then euthanized by an intravenous overdose of pentobarbital. Gross observations at necropsy included i) body condition (normal, thin, emaciated); ii) hydration status (normal, mild, moderate, severe dehydration); iii) perineal fecal staining (none, mild, moderate, severe); iv) consistency of colonic contents (firm/pelleted, normal, pudding-like, watery); v) mesocolonic edema (mild = 1 mm separation between loops, moderate = 2 to 3 mm separation between loops, severe > 3 mm separation between loops); and vi) the presence of visible colonic luminal necrosis. Piglets were scored independently in a blinded fashion as previously described (6,8,10). Necropsies, clinical sign scores, and gross lesion scores were completed by the same 2 individuals for all replicates (PHEA and DMM).
Sample collection
Rectal swabs were taken from all pigs prior to inoculation. At necropsy, fresh and formalin-fixed tissues were collected with instruments disinfected between animal necropsies. Samples included: ileum, jejunum, descending colon, cecum, and a cross section of spiral colon containing 4 to 5 loops. Colonic and cecal contents were collected and stored in sterile plastic cups. An ileal swab (Dacron Fiber Tipped; Fisher, Loughborough, Leicestershire, United Kingdom) was also taken at necropsy.
Bacterial culture
Ileal swabs collected at necropsy were examined by routine aerobic and anaerobic culture for the presence of Escherichia coli, Clostridium perfringens, and Salmonella spp. The Salmonella spp. culture protocol has been previously described (11). Isolates of E. coli and C. perfringens were further characterized by PCR genotype (12,13).
Toxin detection
Rectal swabs collected prior to inoculation and pooled colon and cecal contents (from the same pig) collected at necropsy were assayed for C. difficile toxins. Swabs and intestinal pooled content were frozen and stored at −80°C until completion of the study. Pooled colon and cecal contents were processed simultaneously in accordance with the manufacturer’s instructions and analyzed on a microplate reader (Molecular Device; IDEXX Corp, Lake Forest, Illinois, USA). A commercial toxin ELISA kit (C. DIFFICILE TOX A/B II) was used to semi-quantitatively measure the amounts of toxin from 0 (no toxin detection) to 4+ (marked toxin detection) as indicated by the manufacturer.
Microscopic evaluation
Tissue sections were collected in 10% neutral buffered formalin and submitted for routine sectioning followed by paraffin embedding and staining with hematoxylin and eosin (H&E). All tissues were examined by a veterinary pathologist (PHEA) who was blinded to animal group designation. Large intestinal sections were assessed for goblet cell loss, neutrophilic aggregates within the lamina propria, and mucosal epithelial defects as previously described (6,8).
Scoring
Four categories of scores were compared: i) clinical signs; ii) ELISA results; iii) mesocolonic edema; and iv) microscopic lesions. Summing the scores for body condition, hydration status, and perineal staining was done to create the clinical sign scores. Enzyme-linked immunosorbent assays were performed on fecal and colon contents at the beginning and end of the experiment. At necropsy, pathologists, who were blinded to the treatment group, assigned the mesocolonic edema score. Microscopic lesion score was the sum of scores for all histopathology categories. The scoring system has been previously described (6,8).
Statistical methods
Scores for clinical signs, gross and microscopic lesions were analyzed by a non-parametric test. Kruskal-Wallis test was used to determine if there was an overall difference among study groups. Pair-wise comparison was performed using Wilcoxon rank-sum tests; P-values were then adjusted using Bonferroni correction. Correlations between microscopic lesions, mesocolonic edema, and ELISA results were assessed by Spearman’s rank correlation coefficient, a non-parametric test. Statistical software (JMP version 9; Cary, North Carolina, USA) was used to perform analyses.
Results
Bacterial culture
Clostridium perfringens and E. coli were isolated from most pigs regardless of treatment group. Nine C. perfringens isolates were randomly selected for PCR genotype (12) and all were type A. No hemolytic E. coli were isolated; PCR genotype (13) was performed on 9 randomly selected isolates. One isolate was positive for STb toxin gene; however, all were negative for pilus antigen and other associated toxin genes. Salmonella spp. was not isolated from any intestinal swab.
Clinical signs
Clinical scores were recorded at necropsy for all pigs. Pigs that received the toxigenic isolate had slightly higher clinical scores than pigs which did not receive the isolate, but differences were not statistically significant (P > 0.05).
At necropsy, most pigs from GROUPS 1, 2, and 3 had normal body condition and hydration status: 90%, 85%, and 93%, respectively. Among pigs that received the toxigenic isolate (GROUPS 4, 5, and 6) there was normal body condition and hydration status in 66%, 61%, and 61%, respectively, but this difference was not statistically significant (P > 0.05). Twenty-three percent of pigs that received the toxigenic strain had some level of dehydration and loss of body condition, while approximately 6% of pigs from GROUPS 1, 2, and 3 showed similar levels of dehydration.
Gross lesions
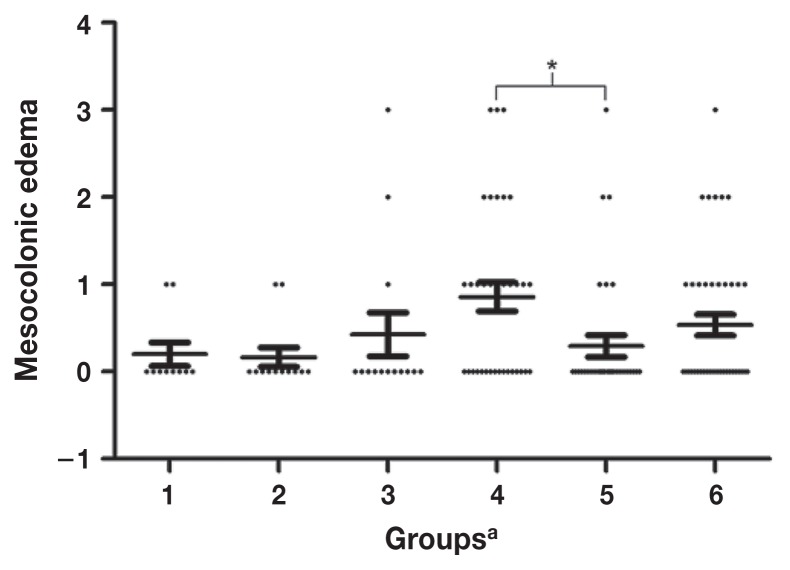
Pigs in GROUP 5 had significantly lower scores for mesocolonic edema compared with pigs in GROUPS 4 and 6 (P = 0.01). Scores for other groups were not significantly different. Grossly visible mucosal necrosis was not observed within the cecum or spiral colon of individual piglets in any experiment. Figure 1 illustrates the individual data points and medians across the study groups.
Figure 1.
Mesocolonic edema scores according to study groups involving 1-day-old piglets administered 2 bacterial probiotics and subsequently challenged with toxigenic C. difficile isolate ISU-15454-1, ribotype 078.
a GROUP 1 negative control: GROUP 2 — non-toxigenic C. difficile (NTCD) only; GROUP 3 — Lactobacillus spp. only; GROUP 4 — toxigenic C. difficile strain only; GROUP 5 — NTCD and challenged with the toxigenic C. difficile strain; GROUP 6 — Lactobacillus spp. and challenged with the toxigenic C. difficile strain. Each bar represents the mean ± SEM of the mesocolonic edema scores. * denotes a significant difference in the mesocolonic edema scores (P = 0.01).
All animals were C. difficile toxin ELISA negative at the beginning of the experiment. At necropsy (72 h post-inoculation), ELISA showed that pigs in GROUP 5 had lower levels of toxin compared with pigs in GROUPS 3, 4, and 6, but these results were not significantly different (P = 0.12).
Microscopic evaluation
Microscopic examination revealed classic C. difficile lesions characterized by variable numbers of neutrophils within lamina propria, loss of goblet cells, and single to multiple sites of epithelial erosion or ulceration which were occasionally covered by moderate amounts of cellular and karyorrhectic debris and fibrin; lesions were only observed in the colon and cecum. Pigs in GROUP 4 had higher scores compared with pigs in other groups and pigs in GROUP 5 had lower microscopic scores compared with pigs in GROUPS 3, 4, and 6; scores for pigs in GROUP 4 were similar to those for pigs in GROUPS 1 and 2. Although there were numerical differences, pair comparisons did not yield significant results (P > 0.05).
Correlations
Presence of mesocolonic edema was correlated with histologic scores and ELISA results with a similar P-value of < 0.001 and with respective Spearman coefficients of 0.4064 and 0.3442. Histologic scores and ELISA results were correlated with P < 0.001 and Spearman coefficient of 0.3254.
Discussion
Despite the significant health and economic impact of disease in humans and other species, there is no commercial vaccine against CDI. In this study, we used a well-established C. difficile piglet model (6,8) to evaluate 2 probiotics as potential alternatives to control and prevent CDI in piglets. A non-toxigenic strain of C. difficile and a commercial Lactobacillus sp. product were investigated.
Probiotics are “living organisms, which when administrated in adequate amounts, confer a health benefit to the host” (14). Three studies have shown that colonization with a NTCD can prevent CDI in a hamster model (15–17). Another study reported that 2 human patients with recurrent CDI showed significant improvement after treatment with NTCD (17). Songer et al (9) showed that piglets, in farm settings, exposed to NTCD had lower levels of fecal toxin compared with controls. To our knowledge this is the first experiment, in controlled settings, to investigate the benefits of such NTCD in piglets.
Four replicates were included in this study; however, data were combined and analyzed together as differences between replicates were not found. The use of replicates allowed for a greater number of piglets and the control of extraneous variables such as room variation, air quality, staff involved in animal care, potential differences in timing of events and biological and genetic differences across individual pigs. Our results are in agreement with a previous report (10) in which clinical signs such as diarrhea or peri-anal staining were not statistically associated with C. difficile disease in piglets. In summary, clinical signs are inconsistently observed in cases of C. difficile disease in piglets and therefore should not be used as an indicator of disease.
Mesocolonic edema, although not pathognomonic, is still the major and often only macroscopic lesion associated with clinical cases of CDI in piglets. This study showed that piglets challenged with toxigenic C. difficile are more likely to have mesocolonic edema compared with control piglets. This result is in accordance with a previous report (18). These results indicate a possible benefit of NTCD in the prevention of macroscopic lesions associated with CDI. Toxins A and B are the main virulence factors of C. difficile; the pathophysiology and mechanisms of CDI has been described in different studies (19,20). The prevalence of pigs testing positive for toxins was lower among piglets receiving NTCD prior to challenge compared with positive control piglets as well as challenged piglets which also received Lactobacillus spp. as a probiotic. Only 5.8% of the pigs from GROUP 5 tested positive while toxins were detected in approximately 30% of pigs from GROUPS 3, 4, and 6.
Microscopic examination is the most objective and accurate method to characterize and diagnose CDI in piglets. Yaeger et al (10) showed that constipation is commonly observed in pigs with CDI and diarrhea is an inconsistent finding. Although toxin levels, mesocolonic edema and microscopic lesions were positively correlated in the present study; evaluation of field cases of CDI showed that a large proportion of healthy piglets (79%) were positive for C. difficile toxins (10).
Multiple studies have investigated the efficacy of probiotic bacteria in the prevention of CDI; however, there is great controversy among researchers. Recently, 1 meta-analysis (21) and 2 systematic reviews (22,23) on this topic have shown enough evidence to support the benefit of using probiotics to prevent CDI in humans (14). Probiotics in humans are administrated at multiple time points, as these bacteria often do not permanently colonize the intestines and will disappear in about 5 to 7 days (24). In the present study, probiotic was administrated as a single dose at study initiation to mimic potential administration of the product in a field setting. Benefits of this single administration were not readily apparent; however, repeated administration of the probiotic product may have had a more favorable outcome as that described in humans.
Single administration of NTDC did not produce side effects and was well-tolerated by the piglets. Reduction in toxin levels, and macroscopic and microscopic lesions were observed in piglets administered NTCD prior to challenge; however, only macroscopic scores were significantly reduced (P < 0.01). Although the objective of this study was not to elucidate the mechanism by which NTDC prevents or reduces CDI, we hypothesize that competitive exclusion occurs, wherein non-toxigenic strains colonize the same niche as toxigenic strains and decrease the amount of bacterial colonization and consequently reduce disease. Other factors including competition for nutrients, modulation of immune response, and cross-talk among bacteria via quorum sensing are also possibly involved. Development of new equipment with self-feed capabilities to neonatal pigs might allow the use of daily administration of such a product. Likewise, spray application of NTCD spores on sow’s teats at parturition is also considered a potential alternative; however, a larger field study is necessary to evaluate the efficacy of such technique.
The true prevalence of pigs naturally colonized with NTCD has not been investigated. The prevalence of C. difficile carriage in humans is estimated to be between 4% and 7.6% and 42% to 50% of the isolates are non-toxigenic (25). Metagenomic studies investigating how the NTCD strain potentially modulates and alters the dynamic process of bacterial intestinal colonization in the neonate might shed light on the mechanism of protection conferred by this technique.
This study showed that administration of NTCD decreased prevalence of toxin-positive piglets, reduced mesocolonic edema and microscopic lesions, suggesting a benefit to administration of NTCD as a competitive exclusion technique to prevent CDI in piglets. The study does not, however, support the use of a single administration of Lactobacillus spp. as an alternative to prevent the development of disease.
Acknowledgments
We thank Iowa Pork Producers Association for funding this project, Iowa State Veterinary Diagnostic Laboratory for the services, LAR personnel, and numerous ISU veterinary students for assistance. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Songer JG. The emergence of Clostridium difficile as a pathogen of food animals. Anim Health Res Rev. 2004;5:321–326. doi: 10.1079/ahr200492. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MA, Songer JG. Evaluation of two enzyme immunoassays for detection of Clostridium difficile toxins A and B in swine. Vet Microbiol. 2008;1–2:204–206. doi: 10.1016/j.vetmic.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 4.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nature Immunol. 2008;8:411. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 5.Hopman NE, Keessen EC, Harmanus C, et al. Acquisition of Clostridium difficile by piglets. Vet Microbiol. 2011;149:186–192. doi: 10.1016/j.vetmic.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Arruda PH, Madson DM, Ramirez A, Rowe E, Lizer JT, Songer JG. Effect of age, dose and antibiotic therapy on the development of Clostridium difficile infection in neonatal piglets. Anaerobe. 2013;22:104–110. doi: 10.1016/j.anaerobe.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Rupnik M, Avesani V, Janc M, von Eichel-Streiber C, Delmee M. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J Clin Microbiol. 1998;36:2240–2247. doi: 10.1128/jcm.36.8.2240-2247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lizer JT, Madson DM, Hank Harris DL, Bosworth BT, Kinyon JM, Ramirez A. Experimental infection of conventional neonatal pigs with Clostridium difficile: A new model. J Swine Health Prod. 2013;21:22–29. [Google Scholar]
- 9.Songer JG, Jones R, Anderson MA, Barbara AJ, Post KW, Trinh HT. Prevention of porcine Clostridium difficile-associated disease by competitive exclusion with nontoxigenic organisms. Vet Microbiol. 2007;124:358–361. doi: 10.1016/j.vetmic.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Yaeger MJ, Kinyon JM, Songer JG. A prospective, case control study evaluating the association between Clostridium difficile toxins in the colon of neonatal swine and gross and microscopic lesions. J Vet Diagn Invest. 2007;19:52–59. doi: 10.1177/104063870701900108. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Wang C, McKean J, et al. Salmonella enterica in swine production: Assessing the association between amplified fragment length polymorphism and epidemiological units of concern. J Appl Environ Microbiol. 2011;77:8080–8087. doi: 10.1128/AEM.00064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meer RR, Songer JG. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am J Vet Res. 1997;58:702–705. [PubMed] [Google Scholar]
- 13.Casey TA, Bosworth BT. Design and evaluation of a multiplex polymerase chain reaction assay for the simultaneous identification of genes for nine different virulence factors associated with Escherichia coli that cause diarrhea and edema disease in swine. J Vet Diagn Invest. 2009;21:25–30. doi: 10.1177/104063870902100104. [DOI] [PubMed] [Google Scholar]
- 14.Parkes GC, Sanderson JD, Whelan K. The mechanisms and efficacy of probiotics in the prevention of Clostridium difficile-associated diarrhoea. Lancet Infect Dis. 2009;9:237–244. doi: 10.1016/S1473-3099(09)70059-3. [DOI] [PubMed] [Google Scholar]
- 15.Borriello SP, Barclay FE. Protection of hamsters against Clostridium difficile ileocaecitis by prior colonisation with non-pathogenic strains. J Med Microbiol. 1985;19:339–350. doi: 10.1099/00222615-19-3-339. [DOI] [PubMed] [Google Scholar]
- 16.Sambol SP, Merrigan MM, Tang JK, Johnson S, Gerding DN. Colonization for the prevention of Clostridium difficile disease in hamsters. J Infect Dis. 2002;186:1781–1789. doi: 10.1086/345676. [DOI] [PubMed] [Google Scholar]
- 17.Seal D, Borriello SP, Barclay F, Welch A, Piper M, Bonnycastle M. Treatment of relapsing Clostridium difficile diarrhoea by administration of a non-toxigenic strain. Eur J Clin Microbiol. 1987;6:51–53. doi: 10.1007/BF02097191. [DOI] [PubMed] [Google Scholar]
- 18.Songer J, Post K, Larson D, Jost B, Glock R. Infection of neonatal swine with Clostridium difficile. J Swine Health Prod. 2000;8:185–189. [Google Scholar]
- 19.Davies AH, Roberts AK, Shone CC, Acharya KR. Super toxins from a super bug: Structure and function of Clostridium difficile toxins. Biochem J. 2011;436:517–526. doi: 10.1042/BJ20110106. [DOI] [PubMed] [Google Scholar]
- 20.Chumbler NM, Farrow MA, Lapierre LA, et al. Clostridium difficile Toxin B causes epithelial cell necrosis through an autoprocessing-independent mechanism. PLoS Pathog. 2012;8:e1003072. doi: 10.1371/journal.ppat.1003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 22.Dendukuri N, Costa V, McGregor M, Brophy JM. Probiotic therapy for the prevention and treatment of Clostridium difficile-associated diarrhea: A systematic review. CMAJ. 2005;173:167–170. doi: 10.1503/cmaj.050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pillai A, Nelson R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008:CD004611. doi: 10.1002/14651858.CD004611.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Mecenier A, Muller-Alouf H, Grangette C. Lactic acid bacteria as live vaccines. Curr Issues Mol Biol. 2000;2:17–25. [PubMed] [Google Scholar]
- 25.Natarajan M, Walk ST, Young VB, Aronoff DM. A clinical and epidemiological review of non-toxigenic Clostridium difficile. Anaerobe. 2013;22:1–5. doi: 10.1016/j.anaerobe.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]