Abstract
We are developing instrumentation for adaptive multimodality imaging, a form of non-linear optimization where imaging parameters are automatically adjusted in response to the object. We have designed and built an adaptive, helical-scan cone-beam x-ray CT system suitable for small-animal studies and are retrofitting one of our existing FastSPECT systems to add adaptive capabilities. We discuss the system designs and how adaptive strategies can provide an increase in performance on various medical imaging tasks relative to current imaging procedures.
I. MOTIVATION
X-ray CT systems are useful biomedical imaging tools, both as stand-alone instruments and in multimodality combinations with emission tomography (SPECT/CT PET/CT). Typically, imagers have fixed operating parameters and acquisition protocols that are selected prior to imaging a given subject. Using this imaging methodology, the configuration of the imaging system is unlikely to be optimized, in terms of performing a specified imaging task, for a given object or patient since there is a need to accommodate a wide range of objects. patients, and tasks to perform.
A simple example is how current CT systems deal with subjects with disparate sizes. Projections of smaller objects will not fill the detector so the system effectively wastes imaging resources, in this case pixels, that could be applied toward imaging that subject at a higher resolution. However, a system that is capable of adapting its configuration in response to the particular object being imaged should be able to acquire more optimal imaging data for that object [1]. In the simplest adaptive rules, the system measures the subject support (spatial dimensions) and reconfigures the magnification to make the best use of its detector space-bandwidth product. More promisingly, the system can use prior information, such as tissue density or approximate tracer distribution, gleaned from pre-scans (scout images) of the object to change its protocols and parameters to improve the statistics of the measurements.
II. REQUIREMENTS OF ADAPTIVE MEDICAL IMAGING SYSTEMS
The goal of an adaptive imaging system is to provide a wide range of imaging configurations so that, for all subjects and tasks presented to the system. the imager may select the best configuration it is capable of to conduct the study. Thus, a high degree of flexibility in operational settings is desirable. However, it is important to note that, although an infinite number of system states is ideal, in reality system states may need to be limited. For example, if one wishes to characterize and calibrate all of the possible configurations of the system, the number of system states must be physically reasonable.
Two design attributes stand out as essential for making adaptive systems practical: speed and repeatability. Speed is necessary for achieving scan times agreeable to the subject. Manual reconfigurations are out of the question. Repeatability of the different system states is required to make the system's behavior predictable and reliable.
For control, an adaptive system needs access to significant computing power. The system must autonomously analyze the scout data, use that analysis to decide upon the best system configuration for the situation, and then deploy to that configuration. Since the data sets involved in medical imaging can be quite large, the system needs high-bandwidth data-transport and processing capability.
The performance of adaptive imaging systems must be measured objectively to determine their effectiveness at various tasks. and for comparing them to other adaptive or conventional systems. This requires specifying a task and observer, and computing a figure-of-merit for the system's performance. Objective assessment provides feedback for improving system designs and adaptation strategies.
III. SYSTEMS AND CAPABILITIES
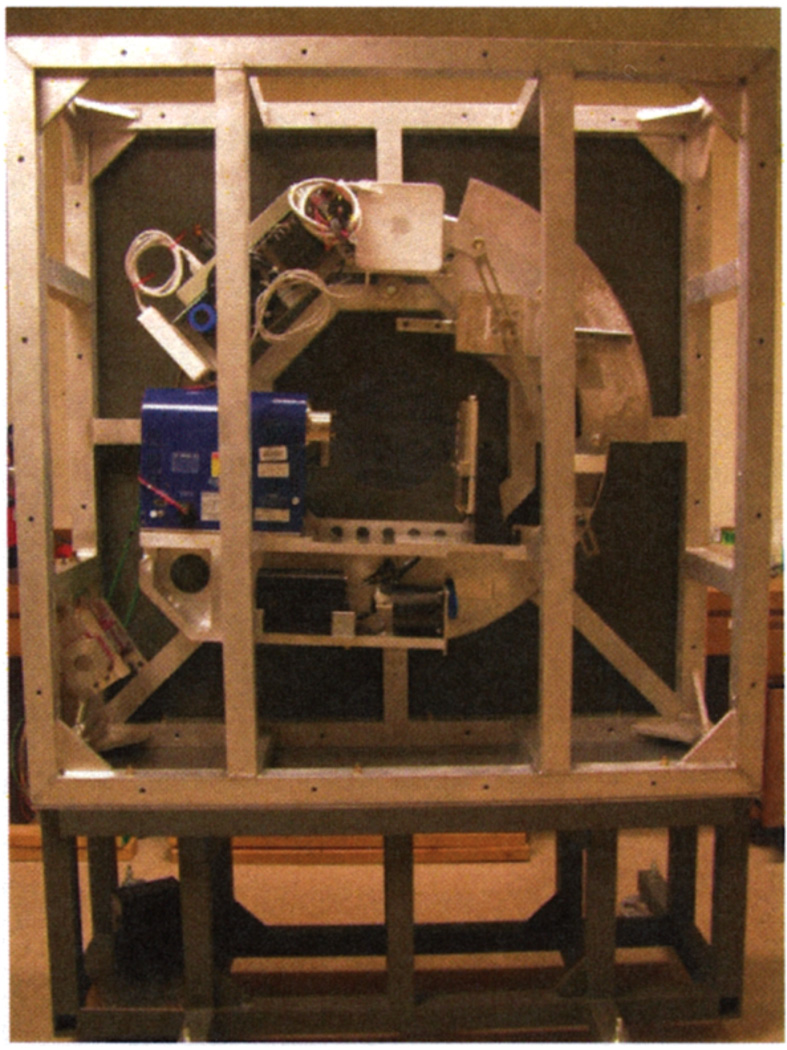
We have built an adaptive x-ray CT system for small animals, called FaCT, that is shown in Figure 1. FaCT has been designed to work in two roles: in stand-alone mode, and to augment the existing FastSPECT II SPECT imager (see [2]) to create a dual-modality SPECT/CT system.
Fig. 1.
The FaCT x-ray CT system shown with front and side covers removed.
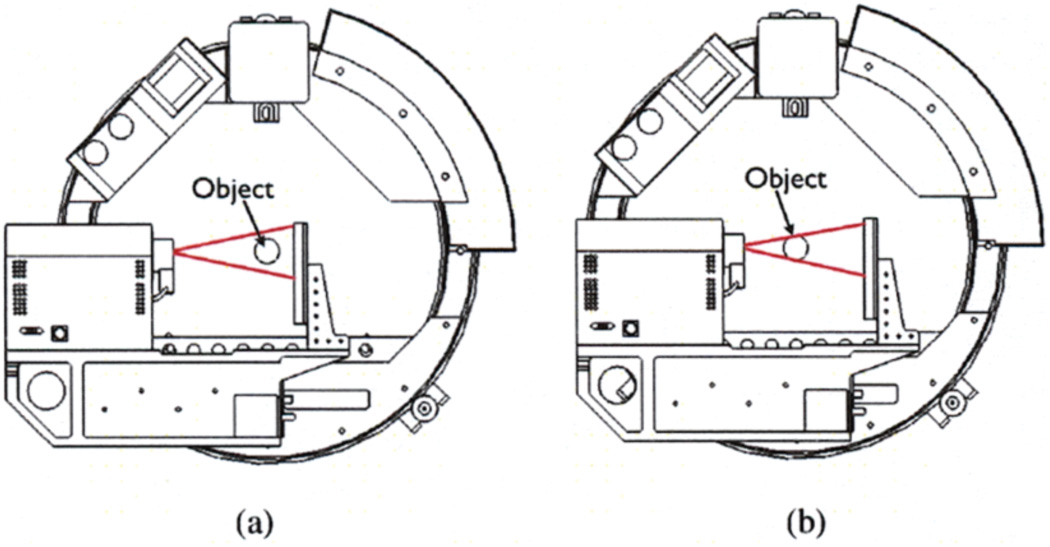
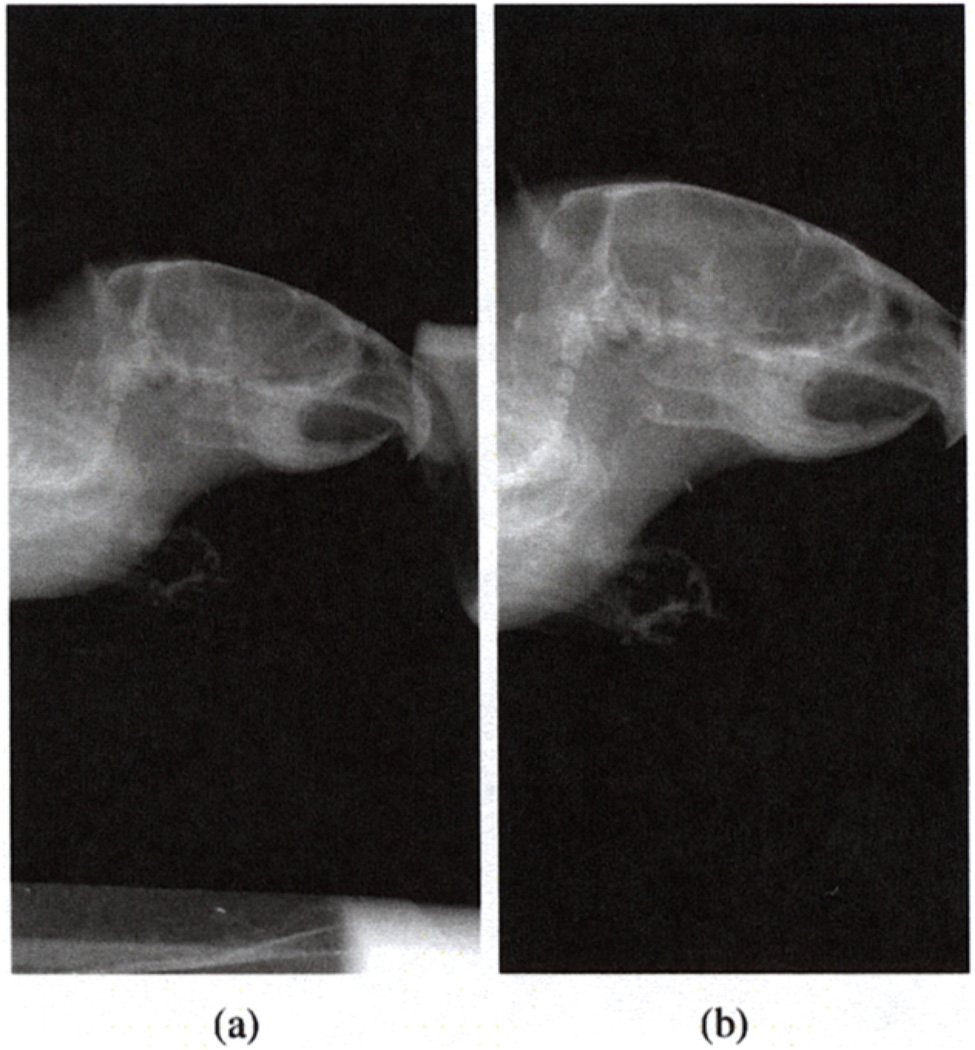
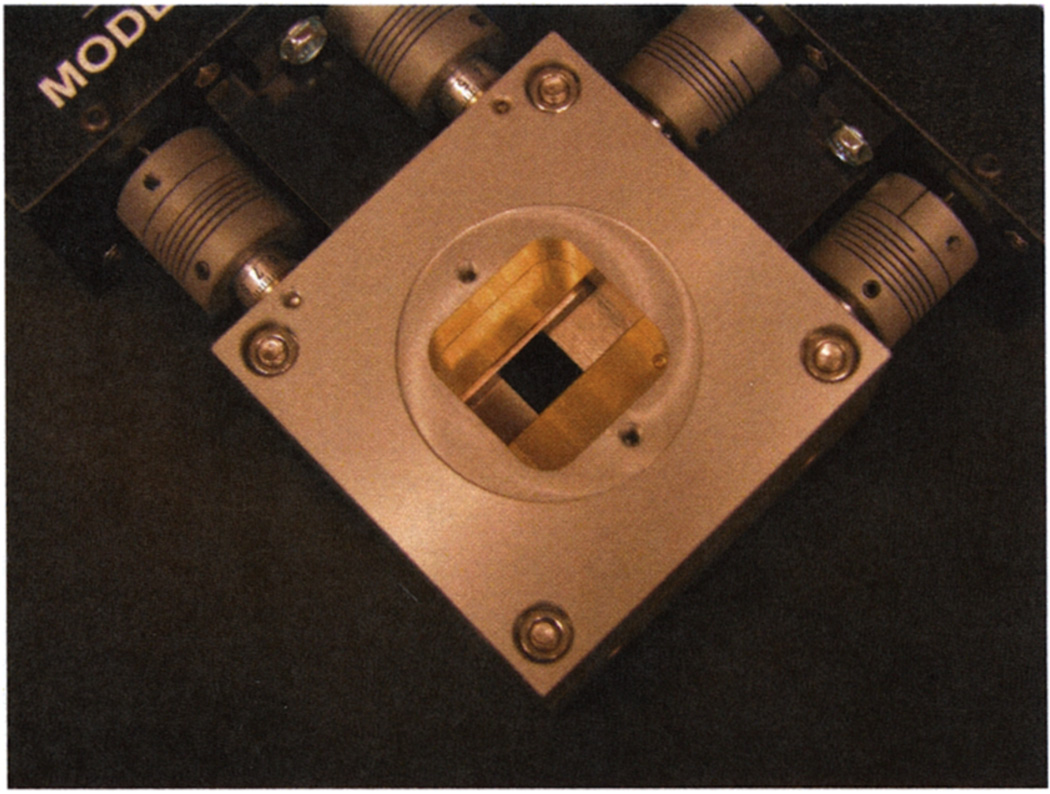
FaCT possesses several unique features that facilitate adaptive imaging procedures. First, the system can vary the source-to-object distance and object-to-detector distance (see Figure 2) in order to achieve different magnifications in the projections of the object being imaged, as shown in Figure 3. Second, the system has a motorized beam-shaping aperture, as shown in Figure 4, with four independently moveable slit blades that can dynamically shape the profile of the emitted cone beam. Furthermore, the beam energy, intensity, and exposure times are programmable, as is the overall acquisition trajectory.
Fig. 2.
(a) Low-magnification position; (b) High-magnification position.
Fig. 3.
Planar projection images of a mouse taken by FaCT at (a) 2.6X magnification level; (b) 3.3X magnification level.
Fig. 4.
Beam-shaping aperture with 4 independently moving slats attached to the x-ray source on FaCT.
Besides its adaptive elements, FaCT has severable notable design features. An on-rotating-gantry computer provides extensive functionality to the system such as control and decision making, data compression and/or reduction, and communication. Structurally, the system has been developed using finite-element analysis (FEA) to provide an ultra-rigid platform that ensures resolution targets are met. The various motions in the system are driven by fine-pitched power screws and micro-stepped electric motors to provide positioning repeatability. The system is also dynamically balanced for smooth rotation and to eliminate backlash.
Our existing SPECT system, FastSPECT II, must be switched among its available configurations manually, but is a proven research platform. It has 16 cameras that acquire SPECT projections simultaneously with a super-listmode data-acquisition architecture (all PMT signals and event time recorded). We are updating FastSPECT II to achieve an adaptive SPECT capability and complement our adaptive CT system.
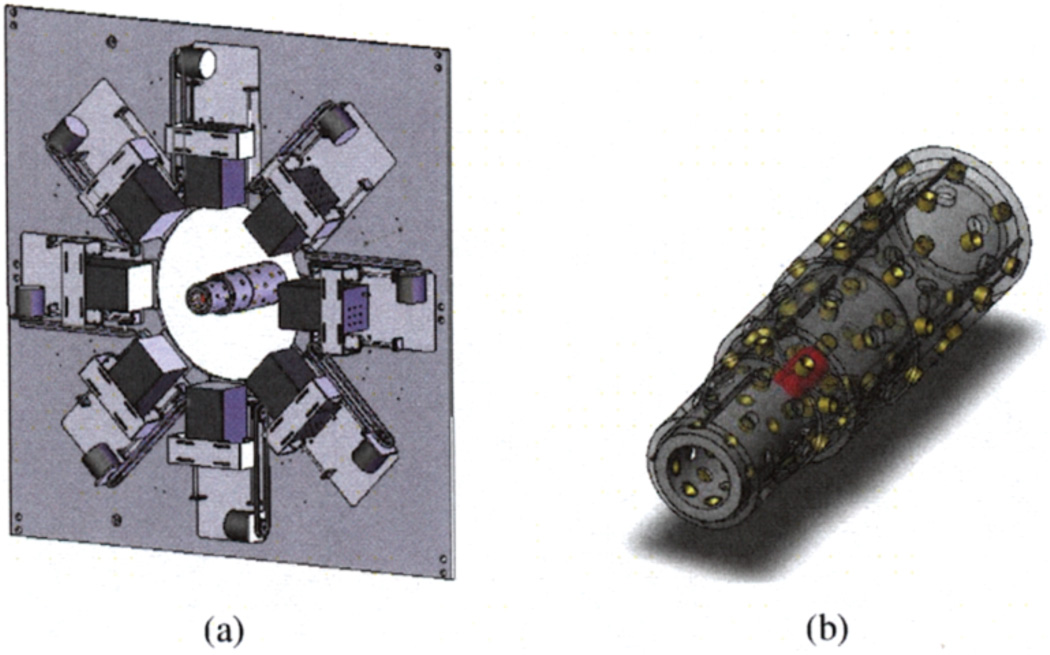
Experience with a prototype-adaptive system suggests that a finite number of settings that sample the resolution-sensitivity-field-of-view configuration space provides sufficient flexibility for adaptation [1]. We are motorizing the positioning of the SPECT cameras for 3 discrete magnification settings (see Figure 5a). In addition, we are installing an adaptive aperture that complements those magnification settings and also provides two pinhole configurations at each magnification setting through use of a coaxial shell design (see Figure 5b). This finite-configuration strategy permits us to continue to use fully-measured system imaging matrices (PSFs) for iterative reconstruction.
Fig. 5.
(a) Motorized cameras on interior frame of FastSPECT II with adaptive aperture; (b) Detail of adaptive aperture.
A key design feature of the adaptive SPECT upgrade is a custom-designed optical positioning system with feedback that guarantees camera position reproducibility to under 25 microns. Finite-element analysis has been employed to ensure the camera support structure remains rigid and our measured imaging matrices accurate. The adaptive aperture uses high-precision linear actuators and an encoded, motorized drive to achieve repeatability.
IV. ADAPTIVE IMAGING
In prior work, we have suggested a general framework and nomenclature for describing adaptive biomedical imaging systems [3]. FaCT, in stand-alone mode, may be classified as an isoscopic adaptive imaging system [3]. In this case, the adaptation is made to maximize task performance by imaging the same physical property, linear attenuation coefficient, in both scout and scientific scans. However, adaptation measures might also be used to meet other constraints, such as minimizing delivered radiation dose. A framework for evaluating adaptive systems, particularly adaptive SPECT, is presented in [4]. An example of an adaptive SPECT system used to improve task performance for detecting a necrotic tumor core can be found in this Conference Record by L. Caucci et al.
When a multimodality imaging system is used to image multiple physical properties, then it is classified as a polyscopic imaging system [3]. In adaptive polyscopic imaging, the two imaging chains need not be independent. For example, information derived from either raw projection data or a reconstructed image acquired with the CT can be used to alter the data-acquisition hardware or protocol of the SPECT system. Conversely, data acquired on the SPECT system (such as the location of focal tracer uptake) can be used to guide the CT acquisition. With an adaptive CT and adaptive SPECT system we will be able to pursue many variations of polyscopic adaptive imaging.
V. CONCLUSION
We have developed the hardware necessary to pursue adaptive imaging in both isoscopic and polyscopic imaging procedures. While adaptive systems pose challenges in terms of engineering design and computational requirements, their flexibility promises an eventual personalized solution for biomedical imaging studies.
Acknowledgments
This work was supported by the National Institutes of Health under NIBIB Grant P41-EB002035-5 (Center for Gamma-ray Imaging) and NCI Grant R24CA83148 (Southwest Animal Imaging Resource).
REFERENCES
- 1.Freed M, Kupinski MA, Furenlid R, Wilson DW, Barrett HH. A prototype instrument for single pinhole small animal adaptive spect imaging. Medical Physics. 2008;35(5):1912–1925. doi: 10.1118/1.2896072. [Online]. Available: http://link.aip.org/link/?MPH/35/l912/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furenlid LR, Wilson DW, Chen Y, Kim H, Pietraski P, Crawford M, Barrett HH. FastSPECT II: A Second-Generation High-Resolution Dynamic SPECT Imager. IEEE Transactions on Nuclear Science. 2004;51(3):631. doi: 10.1109/TNS.2004.830975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarkson E, Kupinski MA, Barrett HH, Furenlid LR. A task-based approach to adaptive and multimodality imaging. Proceedings of the IEEE. 2008;96(3):500–511. doi: 10.1109/JPROC.2007.913553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett HH, Furenlid LR, Freed M, Hesterman JY, Kupinski MA, Clarkson E, Whitaker MK. Adaptive SPECT. IEEE Transactions on Medical Imaging. 2008 Jun;27(6):775–788. doi: 10.1109/TMI.2007.913241. [DOI] [PMC free article] [PubMed] [Google Scholar]