Abstract
Studies have reported an increased risk of developing diabetes in subjects receiving statins versus placebo. Our purpose was to compare the effects of maximal doses of rosuvastatin and atorvastatin on plasma levels of the insulin, glycated albumin (GA), adiponectin (ADN), and C reactive protein (CRP) versus baseline in hyperlipidemic patients. We studied 252 hyperlipidemic men and women who were randomized to receive atorvastatin 80 mg/day or rosuvastatin 40 mg/day over a 6-week period. Atorvastatin and rosuvastatin were both highly effective in lowering low density lipoprotein cholesterol (LDL-C) and triglyceride (TG) levels, with rosuvastatin being more effective than atorvastatin in raising high density lipoprotein cholesterol (HDL-C). Atorvastatin and rosuvastatin at maximum dosage both significantly (p<0.05) raised median insulin levels by 5.2% and 8.7% respectively from baseline. However, only atorvastatin increased GA levels from baseline (+0.8% for atorvastatin vs −0.7% for rosuvastatin, p=0.002). Both atorvastatin and rosuvastatin caused significant (p<0.001) and similar median reductions in CRP of −40% and −26% as compared to baseline values respectively. However, there was no statistical significant difference between the two groups in ADN changes from baseline (−1.5% vs −4.9%, p=0.15). In conclusion, our data indicated that maximum dosage of atorvastatin or rosuvastatin therapy significantly lower CRP levels, but also moderately increase insulin levels.
Keywords: Statins, hyperlipidemia, insulin, glycated albumin, adiponectin, C reactive protein
Despite the beneficial effects of atorvastatin and rosuvastatin on lipoprotein cholesterol1–6 and C reactive protein (CRP) levels, there are concerns regarding the effects of these statins on glucose homeostasis. In a substudy of the PROVE-IT (Pravastatin or Atorvastatin Evaluation in Myocardial infarction), patients with baseline HbA1c <6% who received atorvastatin (80 mg/day) had a higher risk of developing HbA1c >6% than patients who received pravastatin (40 mg/day).7 In the JUPITER study (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) a 25% higher incidence of physician-reported diabetes was noted in subjects receiving rosuvastatin (20 mg/day) as compared to those on placebo. An increase in HbA1c was also observed in those on rosuvastatin.8 More recently a meta-analysis by Sattar and colleagues confirmed that statins do indeed increase the risk of developing type 2 diabetes.9 The current study compares the effects of maximal doses of atorvastatin and rosuvastatin on insulin, glycated albumin (GA), and adiponectin (ADN) levels, measures of glucose homeostasis, as well as CRP levels using serum samples from the STELLAR study. This study was carried out to determine whether maximal statin therapy has an effect on glucose homeostasis in light of data from large statin trials reporting an increased risk of developing diabetes mellitus in those placed on statins versus placebo.7–9
Methods
The details of the design and conduct of the STELLAR study and of the patient population have been published.1,2 It was an open-label; randomized, parallel group study in hypercholesterolemic patients conducted in 182 US centers. The primary objective was to compare the efficacy of rosuvastatin in the reduction of LDL-C with other statins across dose ranges. Secondary objectives included a comparison of the effects of the statins on other lipoprotein parameters such as HDL-C, apolipoprotein (apo) A-I and B, and lipid ratios.1 Men and non-pregnant women (adults aged 18 or more) with hypercholesterolemia were asked to follow a National Cholesterol Education Program step 1 diet for 6 weeks. Those who were compliant with the diet and had fasting calculated LDL-C levels ≥160 mg/dl (4.1 mmol/l) and <250 mg/dl (6.5 mmol/l) and triglyceride (TG) <400 mg/dl (4.5 mmol/l) were randomized to the different statin doses as described. Blood samples were collected on at least 3 occasions before randomization and after 4 and 6 weeks treatment and sent to a central lab (Medical Research International (MRI), Highland Heights, KY) for the measurement of lipid and lipoprotein parameters that included total cholesterol (TC), TG, calculated LDL-C, HDL-C, apo A-I and apo B measurements as described.1,2 Serum samples were stored at −80°C at MRI. All subjects provided informed consent to participate in the study and to have their blood samples used for analysis of lipoproteins and other cardiovascular risk markers. The protocol was approved at multiple human investigational review boards.
For this sub-study, available serum samples that had never been thawed and had been frozen at −80°C, corresponding to the baseline (week 0) and the 6-week time points of the atorvastatin 80 mg/day and rosuvastatin 40 mg/day arms of the main study were sent on dry ice to the Lipid Metabolism Laboratory, Tufts University, in Boston, MA. In the rosuvastatin 40 mg/day and atorvastatin 80 mg/day groups of the STELLAR study, 158 and 167 patients, respectively were randomized and 152 and 160 patients had data recorded at baseline and after 6 weeks treatment. Archived serum samples corresponding to the randomization and 6-week time points were available for this study in 135 (89%) and 137 (86%) of these patients. These were serum samples that had been frozen at −80° C, and were obtained after an overnight fast at baseline of medication and after 6 weeks of therapy with either atorvastatin 80 mg/day or rosuvastatin 40 mg/day.
Direct LDL-C and HDL-C levels were measured using kits obtained from Roche Diagnostics (Indianapolis), and small dense LDL-C were measured using kits provided by Denka Seiken Corp. (Tokyo, Japan) as previously described.3,4,10–12 Our laboratory participates in the Centers for Disease Control National Heart Lung and Blood Institutes lipid standardization program (Atlanta, GA). Glycated albumin was measured using kits obtained from Asahi-Kasei Pharma Corporation, Tokyo Japan as previously described.13,14 Adiponectin was measured using a latex particle-enhanced immunoturbidometric assay, and insulin was measured using a latex immunoassay (both assays were obtained from the Otsuka Pharmaceutical Corporation, Tokyo Japan). The characteristics of these assays have been previously described.15–17 CRP was measured using a high sensitivity immunoassay obtained from Wako Diagnostics Inc., Richmond, VA. All assays had between run and within run coefficients of variation of <5%.
The statistical analysis was done using SPSS for Windows software (SPSS, Cary, NC). All continuous variables were checked for their distributions. Results were expressed as means ± SD if they were normally distributed or as medians and interquartile ranges if they were nonlinearly distributed. Changes of all parameters from baseline and by treatment were compared using Student t test, Wilcoxon Signed Ranks tests or Mann-Whitney U test according to their distributions. A p values of <0.05 were considered statistically significant. Thirteen patients (7 in rosuvastatin and 6 in atorvastatin) who had high glycated albumin level (GA>16.5%, consistent with diabetes) were excluded from the analysis of glucose homeostasis and inflammatory markers as shown in table 2 (change from baseline) because of possible interference in insulin level interpretation and higher prevalence of diabetic patients in rosuvastatin group at baseline. We calculated that 68 subjects/group would provide 80% power with α = 0.05 for detecting a 25% difference in insulin between the two treatment groups.18
Table 2.
Glucose homeostasis and inflammatory markers after treatments of subjects who had glycated albumin <16.5%
| Variable | Atorvastatin 80 mg/day
|
Rosuvastatin 40 mg/day
|
||
|---|---|---|---|---|
| 6 weeks | P values* | 6 weeks | P values* | |
| Insulin (μIU/ml)a | 7.5 [2.6 to 35.8] | 0.007 | 8.6 [2.5 to 25.5] | 0.026 |
| Adiponectin (μg/ml)a | 11.0 [4.0 to 35.9] | 0.262 | 9.8 [2.4 to 60.2] | 0.005 |
| Glycated albumin (%)b | 13.4 [10.6 to 18.7] | 0.53 | 13.1 [10.1 to 16.4] | 0.0017 |
| C reactive protein (mg/l)b | 1.1 [0.1 to 33.5] | <0.001 | 1.6 [0.1 to 25.7] | <0.001 |
Values are expressed as median [Interquartile range];
P values are based on an analysis comparing changes from baseline;
the numbers of subjects analyzed were 72 for both atorvastatin and rosuvastatin;
the numbers of subjects analyzed were 129 for atorvastatin and 130 for rosuvastatin
Results
Subject characteristics and lipids and other biochemical levels at baseline are shown in Table 1. The two groups of patients were well-matched according to gender, age, and disease characteristics, except that those placed on rosuvastatin had a higher prevalence of diabetes. The two groups were also well-matched with regard to baseline levels of lipoproteins, except that subjects randomized to the rosuvastatin group had significantly higher levels of non HDL-C, calculated LDL-C, direct LDL-C, and small dense LDL-C, although these differences were not large between groups at baseline. The sample sizes presented in the tables represent the number of patients who both completed treatment and had serum samples available for the measurements (135 in atorvastatin and 137 in rosuvastatin group). However, 76 samples in both atorvastatin and rosuvastatin groups were available for insulin and ADN measurement. Samples not measured had insufficient serum available for the analysis. There was no statistical difference between mean age and baseline median GA levels between those with missing data and those who had samples available for the measurements in both treatment groups (Table 1).
Table 1.
Patient characteristics at baseline and mean baseline levels of measured variables
| Variable | Atorvastatin 80 mg
|
Rosuvastatin 40 mg
|
||
|---|---|---|---|---|
| (n= 135)a | (n=76)b | (n= 137)a | (n=76)b | |
| Men/Women | 67/68 | 38/38 | 71/66 | 39/37 |
| Age (years) | 58.6 ± 11.0 | 59.6 ± 10.1 | 55.9 ± 12.7 | 55.9 ± 13.5 |
| Body mass index (kg/m2) | 29.4 ± 6.2 | - | 29.5 ± 12.6 | - |
| Coronary heart disease | 20.4% | - | 19.6% | - |
| Diabetes mellitus | 5.4% | 5.3% | 9.5%* | 5.3% |
| Total cholesterol (mg/dl) | 278.3 ± 30.7 | 275.9 ± 32.2 | 283.2 ± 26.7 | 284.7 ± 26.8 |
| Triglycerides (mg/dl) | 178.1 ± 75.1 | 168.7 ± 76.2 | 183.2 ± 70.8 | 175.1 ± 69.7 |
| HDL-C (mg/dl) | 52.3 ± 14.1 | 53.1 ± 13.7 | 50.1 ± 12.6 | 52.9 ± 11.7 |
| TC/HDL-C ratio | 5.7 ± 1.5 | 5.5 ± 1.3 | 6.0 ± 1.4 | 6.0 ± 1.1 |
| Non HDL-C (mg/dl) | 226.0 ± 31.4 | 223.1 ± 31.0 | 233.2 ± 25.5* | 231.8 ± 23.9* |
| Calculated LDL-C (mg/dl) | 190.7 ± 25.7 | 189.4 ± 26.7 | 196.7 ± 24.0* | 196.8 ± 25.4 |
| Direct LDL-C (mg/dl) | 197.8 ± 27.2 | 198.1 ± 27.5 | 204.4 ± 27.1* | 206.1 ± 27.5 |
| Small dense LDL-C (mg/dl) | 63.2 ± 25.4 | 62.5 ± 23.4 | 71.6 ± 29.9* | 72.9 ± 28.8* |
| C reactive protein (mg/l) | 1.8 [0.2 to 23.8] | 1.9 [0.2 to 23.8] | 2.3 [0.2 to 60.6] | 2.3 [0.2 to 10.4] |
| Glycated albumin (%) | 13.4 [7.2 to 22.6] | 13.4 [10.4 to 22.6] | 13.3 [9.8 to 18.1] | 13.2 [9.8 to 17.4] |
| Insulin (μIU/ml) | 7.1 [0.9 to 22.3] | 7.9 [2.3 to 32.0] | ||
| Adiponectin (μg/ml) | 11.9 [3.9 to 40.4] | 11.0 [2.5 to 64.8] | ||
Values are expressed as mean ± SD, as median [Interquartile range] for non-normally distributed variables, or as percentages;
characteristics of all subjects in this study;
characteristics of subjects who had samples available for insulin and adiponectin analysis
significant difference between the two drugs (p<0.05);
- data not available; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TC, total cholesterol
Table 2 shows that both statins significantly increased insulin levels from baseline. Rosuvastatin slightly decreased GA from baseline, with no significant change observed with atorvastatin treatment. ADN levels decreased from baseline with both treatments with a significant change in rosuvastatin group. Both drugs decreased CRP significantly from baseline. Analysis was also performed in all subjects including those with high GA level (GA>16.5%), but statistical remained significant in all parameters mentioned above.
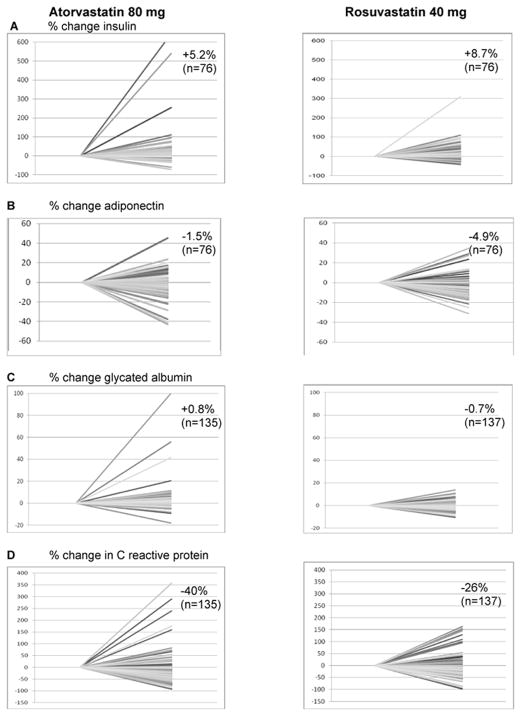
In table 3 it is evident that both atorvastatin and rosuvastatin caused significant and similar decreases in TC and TG, but the rosuvastatin 40 mg/day group had greater reductions in the TC/HDL ratio, non HDL-C, LDL-C, and small dense LDL-C levels. In addition, rosuvastatin was also more effective in raising HDL-C. Both statins caused similar reductions in CRP. Although both statins raised insulin level significantly from baseline, there was no significant difference between the two groups. GA levels increased with atorvastatin treatment, but not with rosuvastatin treatment. There was no significant difference between the two groups in ADN changes from baseline. The variability in responses to the statins (% change from baseline) in terms of changes in insulin, ADN, GA, and CRP are shown in figure 1 (composite figure). As can clearly be seen, there was a very wide variability in response, especially for statin induced changes in insulin and CRP.
Table 3.
Mean differences and percent changes of variables after treatment versus baseline
| Variable | Atorvastatin 80 mg (n= 135)
|
Rosuvastatin 40 mg (n= 137)
|
P treatment† | ||
|---|---|---|---|---|---|
| Differences | % change | Differences | % change | ||
| Total cholesterol (mg/dl) | −109.7 ± 36.1 | −39 | −116.3 ± 37.1 | −41 | 0.137 |
| Triglyceride (mg/dl) | −54 [−329 to 243] | −29 | −44 [−267 to 60] | −28 | 0.774 |
| HDL-C (mg/dl) | +0.72 ± 6.8 | +2.4 | +4.2 ± 6.8 | +9.9 | <0.001 |
| TC/HDL-C ratio | −2.3 ± 1.1 | −40 | −2.8 ± 1.0 | −46 | 0.001 |
| Non HDL-C (mg/dl) | −110.3 ± 36.3 | −48 | −120.5 ± 35.8 | −51 | 0.021 |
| Calculated LDL-C (mg/dl) | −99.0 ± 31.0 | −51 | −109.9 ± 33.6 | −55 | 0.006 |
| Direct LDL-C (mg/dl) | −100.5 ± 32.9 | −50 | −107.8 ± 36.1 | −52 | 0.013 |
| Small dense LDL-C (mg/dl) | −31.4 ± 19.0 | −45 | −43.1 ± 24.4 | −53 | <0.001 |
| C reactive protein (mg/l) | −0.5 [−11.3 to 14.04] | −40 | −0.6 [−59 to 15.6] | −26 | 0.259 |
| Glycated albumin (%) | +0.1 [−2.6 to 9.3] | +0.8 | −0.1 [−1.6 to 1.9] | −0.7 | 0.002 |
| Insulin (μIU/ml)** | +0.5 [−10.7 to 30.2] | +5.2 | +0.6 [−5.8 to 13.9] | +8.7 | 0.711 |
| Adiponectin (μg/ml)** | −0.2 [−6.8 to 5.0] | −1.5 | −0.4 [−5.0 to 5.1] | −4.9 | 0.150 |
Values are expressed as mean ± SD, median [Interquartile range] or percentage;
p value for the differences between two treatments;
samples available for analysis in 76 subjects;
HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TC, total cholesterol
Figure 1.
The individual responses and median percent changes from baseline after treatments of insulin (A), adiponectin (B), glycated albumin (C), and C reactive protein (D)
Discussion
In this study, atorvastatin and rosuvastatin at maximal doses were very effective in improving lipid profiles, while rosuvastatin had additional benefits on raising HDL-C as well. A meta-analysis of all large prospective randomized placebo controlled statin trials indicated that regardless of baseline LDL-C levels, for every 1.0 mmol/L or 38.5 mg/dl of LDL-C reduction, statin treatments over 5 years significantly reduced all-cause mortality by 12%, coronary heart disease (CHD) mortality by 19%, myocardial infarction and CHD death by 23%, coronary bypass surgery by 24%, fatal or non-fatal stroke by 17%, and combined endpoints by 21%.19 Moreover it has been reported in a meta-analysis that intensive statin therapy causes an additional 16% reduction in CHD risk as compared to standard therapy.20
Atorvastatin and rosuvastatin also have been shown to reduce circulating inflammatory markers along with triglyceride lowering effects, but the effects of these two drugs on glucose homeostasis remain controversial. Large scale clinical trials demonstrated worsening of insulin and glycated hemoglobin (HbA1c) with atorvastatin and rosuvastatin treatment.7,8 Data also suggested dose-dependent effects of atorvastatin and rosuvastatin on insulin levels.18,21 Meta-analysis of non-diabetic subjects from 16 randomized controlled trial showed that pravastatin improved insulin sensitivity while atorvastatin and rosuvastatin showed a trend toward worsening in insulin sensitivity.22 Moreover, the same investigators also showed that when atorvastatin, rosuvastatin and simvastatin were combined, there was a significant increased risk of developing diabetes.23 While data suggested differential effects of various statins on glucose homeostasis markers, head to head comparison trials comparing different statin treatments are limited and most studies have small sample sizes. Our study is the first study that has compared atorvastatin and rosuvastatin at maximal doses on parameters of glucose homeostasis and inflammation. From our data, both drugs reduced CRP significantly from baseline with median reductions of 40% for atorvastatin 80 mg/day and 26% for rosuvastatin 40 mg/day, with no significant differences between the two drugs. Both drugs also caused significant and similar increases in insulin levels after treatments with median increases of 5.2% and 8.7%, respectively. Serum glycated albumin reflects glycemic control for the 2 – 3 week period preceding the assay. Recently, it was proposed to be an alternative marker for glycemic control in hemodialysis patients with diabetes and patients with hemoglobinopathy because it is not affected by erythrocyte survival time. We used GA as a marker of glucose control of subjects in this study because of its 6 week duration, and we found that these two drugs had differential effects on GA levels. While atorvastatin at maximal dose increased GA levels by 0.8% from baseline, rosuvastatin slightly decreased GA level by 0.7%. ADN levels decreased with both treatments although statistical difference was found only for the rosuvastatin group. Therefore, the difference in GA levels between both drugs cannot be explained by ADN. Her et al. compared the 8-week effects of 3 different statin regimens with comparable LDL lowering efficacy and found that atorvastatin 20 mg/day significantly increased mean percentage changes of HbA1c from baseline by 3%. However atorvastatin/ezetimibe 5 mg/5mg decreased HbA1c by 0.4% and rosuvastatin 10 mg/day increased HbA1c by 1.2% but the changes did not reach statistical significant difference. The authors stated that their findings might partly be explained by the dose-dependent effect of statins on glucose metabolism.24
The JUPITER study found that rosuvastatin 20 mg/day caused a small but significant increase in HbA1c in normolipidemic subjects with high CRP.8 However, our study found that maximal dose of rosuvastatin decreased GA level after 6 weeks treatment in hyperlipidemic subjects. The reasons for this discrepancy are unclear, but the difference in rosuvastatin dosage and patient characteristic may contribute to these differences. Moreover our sample size was much smaller.
The mechanisms by which statins have differential effects on glucose homeostasis remain unclear. Statins are known to decrease metabolites such as isoprenoids, faresylpyrophosphate, geranylgeranyl pyrophosphate and ubiquinone (CoQ10), which are produced via the cholesterol synthetic pathway. Such effects may cause worsening of glycemic control and increase insulin resistance. Isoprenoids upregulate glucose transporter 4 (GLUT4) resulting in enhanced glucose uptake. CoQ10 depletion results in delayed ATP production, thereby impairing insulin secretion from beta cells. Lipophilic statins such as atorvastatin and simvastatin enter extra-hepatocytic cells more easily thereby inhibiting isoprenoids synthesis. Atorvastatin was found to attenuate the expression of GLUT4 in adipocytes and impair glucose tolerance.25 Differences in lipophilicity between the two statins may play a role in the different results on GA levels.
Our study has some limitations. Our sample size was relatively small and not all subjects had samples available for adiponectin and insulin measurements, although baseline characteristics did not differ between the whole group and those with available sample. Further large clinical studies are essential to address this issue. In addition further basic research is required to fully understand the mechanisms whereby statins may increase insulin resistance. In conclusion, our data indicate that maximal atorvastatin or rosuvastatin therapy significantly lower CRP levels, but also moderately increases insulin levels. Larger clinical study should be conducted to confirm the results.
Acknowledgments
Dr. Thongtang was supported by a fellowship from Siriraj hospital, Mahidol University, Bangkok, Thailand. Ms. Otokozawa was supported by a fellowship from the Kyowa Medex Corporation, Tokyo, Japan, and Dr. Ai was supported by a fellowship from the Denka-Seiken Corporation, Tokyo, Japan. The research was supported by grants HL-60935, HL-74753 and PO50HL083813 from the National Institutes of Health and contract 53-3K06-05-10 from the U.S. Department of Agriculture. Dr. Schaefer is a consultant to Asahi-Kasei, AsztraZeneca, Denka-Seiken, and Otsuka. The original study by Dr. Jones, Dr. Stein and others were funded by AstraZeneca Inc, Wilmington, DE.
Abbreviations
- ADN
Adiponectin
- CRP
C reactive protein
- GA
glycated albumin
- HDL-C
high density lipoprotein cholesterol
- TC
total cholesterol
- TG
triglyceride
- LDL-C
low density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, Cain VA, Blasetto JW. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial) Am J Cardiol. 2003;92:152–160. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 2.Jones PH, Hunninghake DB, Ferdinand KC, Stein EA, Gold A, Caplan RJ, Blasetto JW. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin Ther. 2004;26:1388–1399. doi: 10.1016/j.clinthera.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Asztalos BF, Le Maulf F, Dallal GE, Stein E, Jones PH, Horvath KV, McTaggart F, Schaefer EJ. Comparison of the effects of high doses of rosuvastatin versus atorvastatin on the subpopulations of high-density lipoproteins. Am J Cardiol. 2007;99:681–685. doi: 10.1016/j.amjcard.2006.09.117. [DOI] [PubMed] [Google Scholar]
- 4.Ai M, Otokozawa S, Asztalos BF, Nakajima K, Stein E, Jones PH, Schaefer EJ. Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low-density lipoprotein cholesterol levels. Am J Cardiol. 2008;101:315–318. doi: 10.1016/j.amjcard.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 5.Otokozawa S, Ai M, Van Himbergen T, Asztalos BF, Tanaka A, Stein EA, Jones PH, Schaefer EJ. Effects of intensive atorvastatin and rosuvastatin treatment on apolipoprotein B-48 and remnant lipoprotein cholesterol levels. Atherosclerosis. 2009;205:197–201. doi: 10.1016/j.atherosclerosis.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Himbergen TM, Matthan NR, Resteghini NA, Otokozawa S, Ai M, Stein EA, Jones PH, Schaefer EJ. Comparison of the effects of maximal dose atorvastatin and rosuvastatin therapy on cholesterol synthesis and absorption markers. J Lipid Res. 2009;50:730–739. doi: 10.1194/jlr.P800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabatine MSWS, Morrow DA, McCabe CH, Cannon CP. High-dose atorvastatin associated with worse glycemic control: a PROVE-IT TIMI 22 substudy. Circulation. 2004;110:III–834. [Google Scholar]
- 8.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 9.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 10.Okada M, Matsui H, Ito Y, Fujiwara A, Inano K. Low-density lipoprotein cholesterol can be chemically measured: a new superior method. J Lab Clin Med. 1998;132:195–201. doi: 10.1016/s0022-2143(98)90168-8. [DOI] [PubMed] [Google Scholar]
- 11.Sugiuchi H, Uji Y, Okabe H, Irie T, Uekama K, Kayahara N, Miyauchi K. Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated alpha-cyclodextrin. Clin Chem. 1995;41:717–723. [PubMed] [Google Scholar]
- 12.Ai M, Otokozawa S, Asztalos BF, Ito Y, Nakajima K, White CC, Cupples LA, Wilson PW, Schaefer EJ. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem. 56:967–976. doi: 10.1373/clinchem.2009.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta. 2002;324:61–71. doi: 10.1016/s0009-8981(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 14.Kouzuma T, Uemastu Y, Usami T, Imamura S. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta. 2004;346:135–143. doi: 10.1016/j.cccn.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura A, Sawai T. Determination of adiponectin in serum using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta. 2006;371:163–168. doi: 10.1016/j.cca.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Ai M, Otokozawa S, Schaefer EJ, Asztalos BF, Nakajima K, Shrader P, Kathiresan S, Meigs JB, Williams G, Nathan DM. Glycated albumin and direct low density lipoprotein cholesterol levels in type 2 diabetes mellitus. Clin Chim Acta. 2009;406:71–74. doi: 10.1016/j.cca.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura AST. Evaluation and clinical significance of latex immunoassay (LIA) of insulin using the routine biochemical autoanalyzer. Jap J Clin Chem. 2006;35:48–53. [Google Scholar]
- 18.Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol. 55:1209–1216. doi: 10.1016/j.jacc.2009.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 20.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 21.Kostapanos MS, Milionis HJ, Agouridis AD, Rizos CV, Elisaf MS. Rosuvastatin treatment is associated with an increase in insulin resistance in hyperlipidaemic patients with impaired fasting glucose. Int J Clin Pract. 2009;63:1308–1313. doi: 10.1111/j.1742-1241.2009.02101.x. [DOI] [PubMed] [Google Scholar]
- 22.Baker WL, Talati R, White CM, Coleman CI. Differing effect of statins on insulin sensitivity in non-diabetics: a systematic review and meta-analysis. Diabetes Res Clin Pract. 87:98–107. doi: 10.1016/j.diabres.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Coleman CI, Reinhart K, Kluger J, White CM. The effect of statins on the development of new-onset type 2 diabetes: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 2008;24:1359–1362. doi: 10.1185/030079908x292029. [DOI] [PubMed] [Google Scholar]
- 24.Her AY, Kim JY, Kang SM, Choi D, Jang Y, Chung N, Manabe I, Lee SH. Effects of atorvastatin 20 mg, rosuvastatin 10 mg, and atorvastatin/ezetimibe 5 mg/5 mg on lipoproteins and glucose metabolism. J Cardiovasc Pharmacol Ther. 2010;15:167–174. doi: 10.1177/1074248409357922. [DOI] [PubMed] [Google Scholar]
- 25.Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia. 2006;49:1881–1892. doi: 10.1007/s00125-006-0269-5. [DOI] [PubMed] [Google Scholar]