Abstract
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract disease with high morbidity and mortality in young infants and children. Despite numerous efforts, a licensed vaccine against RSV remains elusive. Since young infants form the primary target group of RSV disease, maternal immunization to boost the protection in neonates is an attractive strategy. In this study we tested the efficacy of maternal immunization with a chimpanzee adenovirus expressing codon-optimized RSV fusion protein (AdC7-Fsyn) to protect infants against RSV infection. Single intranasal immunization of mice by AdC7-Fsyn induced robust anti-RSV systemic and mucosal immunity that protected against RSV without causing vaccine-enhanced RSV disease. RSV humoral immunity was transferred to pups born to immunized mothers that provided protection against RSV. Immunization with AdC7-Fsyn was effective even in the presence of Ad5 preimmunity. The maternally derived immunity was durable with the half-life of 14.63 days that reduced the viral replication up to 15 weeks of age. Notably, the passively immunized mice could be actively re-immunized with AdC7-Fsyn to boost and extend the protection. This substantiates maternal immunization with an AdC7-based vaccine expressing RSV F as feasible approach to protect against RSV early in life.
Keywords: Respiratory syncytial virus, maternal immunization, chimpanzee adenoviral vector, RSV vaccine
Introduction
Lower respiratory tract infections with respiratory syncytial virus (RSV) are the most common cause for hospitalization of infants and children [1]. It is estimated that globally, RSV causes more than 3 million hospitalizations and up to 200,000 deaths in children under the age of 5 years [2]. More than 70% of the children in the first year of life, and by the age of 2 years almost all children have been infected at least once with RSV and continue to be re-infected throughout life [3]. Premature infants, especially those with chronic lung disease or congenital heart disease, as well as the elderly are most susceptible to severe disease [4]. Early RSV infections are also associated with the later development of asthma [5]. No safe and effective RSV vaccine has yet been licensed. Only immunoprophylaxis with the neutralizing monoclonal anti-RSV-F antibody palivizumab can reduce hospitalization rates due to RSV [6]. The high costs associated with monthly doses of palivizumab remains a challenge for its universal use and for developing countries.
The hurdles for the development of a safe RSV vaccine for vulnerable infants include (1) the immature immune system of infants may not respond well to vaccination; (2) the presence of variable levels of maternal anti-RSV antibodies in infants; (3) the inability of wild-type RSV infection to induce protective immunity; and (4) most importantly, the risk of vaccine-enhanced RSV disease that initially occurred following vaccination with a formalin-inactivated vaccine [7]. A strong mucosal inflammatory and Th2-biased immune response seem to be critical components of this complex pulmonary response triggered by RSV [8]. Adenovirus (Ad)-vectored vaccines are known to favor Th1-biased transgene-specific immune response and do not result in vaccine-enhanced disease [9-13], are not adversely affected by maternal anti-RSV antibodies and can be used for mucosal immunization [14]. Although the human serotype 5 Ad (Ad5) has been an effective vaccine platform against a variety of pathogens [15], use of Ad5-based vaccines is limited by widespread pre-existing immunity [16]. Non-human primate-derived Ad vaccine vectors such as chimpanzee Ad serotype 7 (AdC7) have been developed to overcome pre-existing immunity to common human Ad serotypes and to broaden the repertoire of Ad vaccines for booster immunizations. We have previously shown that AdC7 vector induces superior mucosal and protective immunity compared to human Ad5-based vectors that is further enhanced when AdC7 is administered directly to the respiratory tract [17, 18].
Neutralizing antibody responses correlate with protection against RSV infections [19, 20] and maternally transferred neutralizing antibodies effectively protect RSV-naïve infants against RSV-associated illness [21-23]. Considering that the infants constitute the primary risk group, maternal immunization could be a feasible approach to protect infants during their first months of life [24].
In this study we investigated the efficacy of maternal immunization with AdC7 vector expressing codon-optimized RSV Fusion protein (AdC7-Fsyn) to protect the infants against RSV infection. Immunization of mice with AdC7-Fsyn effectively induced protective neutralizing antibodies that were maternally transferred, even in the presence of Ad5-preimmunity, to protect infant mice from RSV.
Material and methods
Ad vectors
The recombinant Ad vectors used in this study are replication-defective E1-, E3-deleted Ad vectors based on the chimpanzee AdC7 or human Ad5 genome [25, 26]. An expression cassette carrying codon-optimized RSV Fusion gene (Fsyn) under the CMV promoter with tetracyclin operator 2 (TetO2) sequences (CMV/TetO2) and SV40 poly (A) signal was inserted into the E1 region of AdC7 or Ad5 [11] backbone. The AdC7-Fsyn was propagated in T-REx HEK293 cells (Invitrogen) that constitutively express a tetracycline repressor protein. Tet-Off system was used to control expression and potential inhibitory effects of Fsyn during propagation. AdC7-Null and Ad5-Null, vectors with no transgene, were used as controls. The vectors were used on the basis of equal number of particle units (pu) and were propagated and purified as described previously [27].
RSV
The RSV strain A2 (VR-1540; ATCC) used for protection experiments was propagated, purified and quantified as described previously [9]. Formalin-inactivated RSV (FI-RSV) was prepared [9] as positive control for vaccine-enhanced RSV disease.
Mice
Female BALB/c mice were obtained from Taconic Farms (Hudson, NY). The animals were housed under specific pathogen–free conditions and were used at 6–8 weeks of age. Mice were immunized by intranasal inoculation of 50 μl of the Ad vectors AdC7-Fsyn, Ad5-Fsyn or AdC7-Null at a dose of 1010 pu/animal or FI-RSV (105 pfu equivalent) diluted in PBS. To evaluate maternal immunization, mice were immunized 2 weeks prior to breeding. All animal studies were conducted in accordance to the protocols reviewed and approved by the institutional Animal Care and Use Committee.
Western analysis
To evaluate the expression of the RSV Fsyn, lysate of HEK293 cells infected with AdC7-Fsyn was separated by SDS-PAGE under non-reducing conditions. Following transfer to a PVDF membrane, it was probed with RSV Fusion protein monoclonal antibody (18F12) (1:500) and detected using peroxidase-conjugated goat anti-mouse antibody (1:1000) and chemiluminescent peroxidase substrate-1 (Sigma-Aldrich).
Anti-RSV humoral immune response
Mice were immunized intranasally with AdC7-Fsyn, Ad5-Fsyn, AdC7-Null or FI-RSV and serum was collected at 2, 4 and 8 wk following administration. Lung bronchoalveolar lavage (BAL) fluid was collected by intratracheal instillation and aspiration with 0.5 ml PBS. Anti-RSV IgGs were assessed by ELISA as previously described [9]. Pulmonary anti-RSV IgA titers were analyzed using mouse typer isotyping panel (Bio-Rad laboratories).
To induce anti-Ad5 preexisting immunity, mice were inoculated intranasally with Ad5-Null (1011 pu/mouse) four weeks prior to immunization. For maternal immunization, female BALB/c mice were intranasally inoculated with AdC7-Fsyn, Ad5-Fsyn or AdC7-Null, 2 weeks prior to breeding. Serum was collected from newborn mice at 2 weeks intervals starting from 3 weeks till 15 weeks of age. Anti-RSV IgG titers were evaluated as described above. Half-life (t½) of the maternal-derived anti-RSV antibodies transferred to the pups was calculated by the formula – t½ = t * ln(2)/ln(N0/Nt), where t = time elapsed, N0 = antibody titer 3 weeks of age, and Nt= antibody titer at 15 weeks of age.
Serum anti-RSV neutralizing antibody titers were evaluated as previously described [9].
Protection of Mice from Intranasal Challenge with RSV
To evaluate protection against RSV pulmonary infection, immunized mice were challenged with RSV (106 pfu) by intranasal inoculation. Four days later the mice were sacrificed and total RNA from lung homogenates was isolated with TRIzol reagent (Invitrogen). RNA was converted to cDNA using random hexamers and the Taqman Reverse Transcription Reagents (Applied Biosystems). Hundred ng of cDNA was processed for quantitative RT-PCR (qRT-PCR) using RSV G-specific FAM-labeled taqman MGB probe (5′-AAGCCCACCACAAAAC-3′) and primers (Forward: 5′-AAGACCAAAAACACAACAACAACTC-3′, Reverse: 5′-AGTGAAAATCATTATTGGGTTTGCTTGGTG-3′). Endogenous 18S rRNA was used for normalization. The qRT-PCR was performed using the ABI PRISM 7000 Sequence detection system (Applied Biosystems) under the conditions - 45 cycles of denaturation (95 °C for 15 s) and annealing/extension (60 °C for 1 min). The relative quantity of RSV genomes was calculated by ΔΔCt method and expressed in relation to RSV genomes detected in mock-vaccinated mice.
Vaccine-enhanced RSV Disease
Mice were intranasally immunized by with AdC7-Fsyn, Ad5-Fsyn or AdC7-Null or FI-RSV and challenged with RSV (106 pfu) at 5 wk post-immunization. Lung histopathology and BAL differential cell counts were evaluated as previously described [9].
Virus cross-neutralization assay
Serum from mice that were intramuscularly inoculated with AdC7-Null or Ad5-Null (1010 pu/mouse; n=5 per group) were collected after 4 weeks. Cross-neutralizing antibodies against AdC7-Null and Ad5-Null in each serum sample was evaluated as described earlier [28]. Briefly, two fold serial dilutions of each serum sample in 96-well plates were reacted with 100 pfu of AdC7-Null and Ad5-Null for 1 h at 37 °C followed by the addition of 5000 HEK293 cells in each well. The plates were incubated at 37 °C for 5–7 days for the development of cytopathic effect. The virus-neutralizing antibody titer was the reciprocal of the highest serum dilution that completely prevented the development of cytopathic effect.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using Two-Way ANOVA and statistical significance was determined at p<0.05.
Results
Generation of chimpanzee adenovirus vector expressing RSV fusion protein
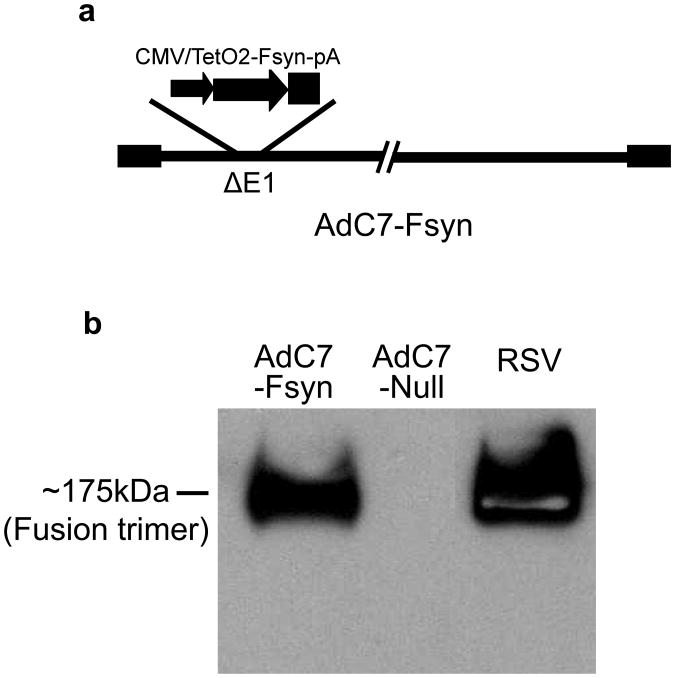
The AdC7 vector carrying the cDNA encoding codon-optimized fusion protein (Fsyn) under the control of CMV/TetO2 promoter (Fig. 1a) was generated and propagated in T-Rex HEK293 cell line. The expression of Fsyn in T-Rex HEK293 remain suppressed (data not shown) that facilitated the generation of higher titers of AdC7-Fsyn. Codon-optimization overcomes the poor expression of wild-type RSV fusion protein because of premature polyadenylation in eukaryotic cells [11]. Expression of the Fsyn trimer was detected in AdC7-Fsyn infected HEK293 cells by Western analysis at equal size compared to RSV (Fig. 1b). No signal was detected with control AdC7-Null infected cell lysates.
Figure 1. Adenoviral vector and expression of RSV F protein.
(a) Replication-deficient AdC7 vector carrying the codon-optimized RSV F (Fsyn) protein under the control of CMV/TetO2 promoter. (b) The expression of RSV F protein was confirmed by Western blot. Lysates of HEK293 cells infected with AdC7-Fsyn or AdC7-Null vectors were probed with RSV F-specific monoclonal antibody (18F12). Purified RSV was run in parallel as positive control.
Immunization with AdC7-Fsyn induces anti-RSV systemic and mucosal protective humoral immunity
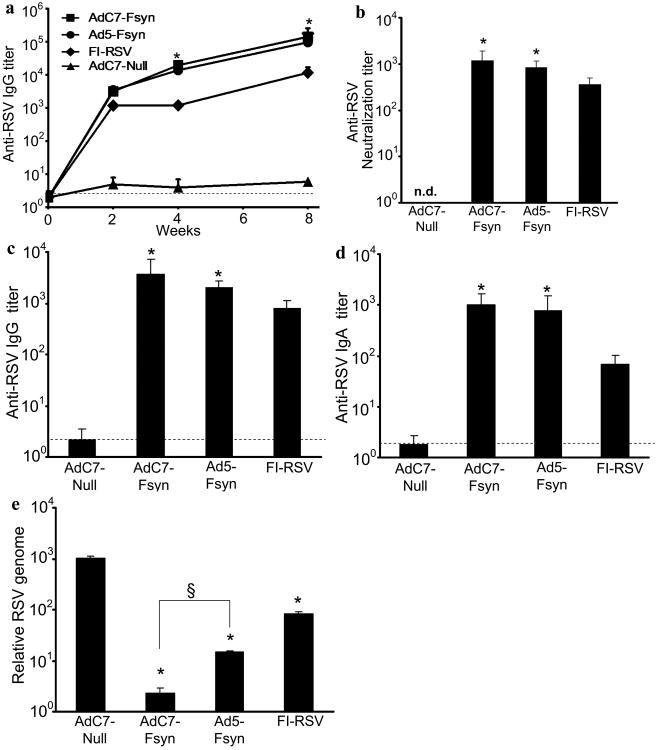
To assess the anti-RSV humoral response induced by mucosal immunization with AdC7-Fsyn, mice were immunized intranasally with AdC7-Fsyn, Ad5-Fsyn, AdC7-Null or FI-RSV. Anti-RSV IgG was detected after 2 wk and continued to rise until 8 wk following immunization with AdC7-Fsyn and Ad5-Fsyn (Fig. 2a). Titers were similar in the AdC7-Fsyn or Ad5-Fsyn immunized mice (p>0.05), and were higher compared to immunization with FI-RSV (p<0.05). Similarly, AdC7-Fsyn and Ad5-Fsyn immunized mice had higher RSV neutralizing antibody levels compared to FI-RSV (p<0.05) (Fig. 2b). To assess the mucosal humoral immune response, RSV IgG and IgA were assessed in BAL at 8wk. Both, immunization with AdC7-Fsyn and Ad5-Fsyn, elicited comparable anti-RSV IgG (Fig. 2c) and IgA (Fig. 2d) (p>0.05) that were again higher compared to immunization with FI-RSV (p<0.05; all comparisons). To evaluate if the elicited immune responses would be protective against pulmonary infection with RSV, immunized mice were challenged with RSV 8 wk after immunization and viral RNA quantified by qRT-PCR. Though qRT-PCR for viral RNA is not absolute quantification of viral replication, it provides a reliable assay for assessment of protective immunity [11, 12]. Mice immunized with AdC7-Fsyn showed strongest reduction in RSV lung titers compared to Ad5-Fsyn or FI-RSV (p<0.05; all comparisons; Fig. 2e). These data indicate that mucosal immunization with AdC7-Fsyn induces strong humoral and protective anti-RSV immunity.
Figure 2. Immunization with AdC7-Fsyn induces anti-RSV systemic and mucosal protective humoral immunity.
BALB/c mice were immunized via the intranasal route with AdC7-Fsyn, Ad5-Fsyn, AdC7-Null (all 1010 pu/mouse) or FI-RSV (a) Anti-RSV IgG antibodies in serum were analyzed at 2, 4 and 8 weeks by ELISA. (b) Anti-RSV virus neutralization titers were evaluated at 8 weeks. Anti-RSV IgG (c) or IgA (d) antibodies in BAL fluid were analyzed at 8 weeks by ELISA. Data are shown as the mean ± SEM of 5 mice/group. Limit of detection is indicated by the dashed line. * denotes p<0.05 vs Ad-Null and FI-RSV. (e) Eight weeks after immunization mice were challenged intranasally with RSV (106 pfu/mouse). RSV titers in the lung homogenate were evaluated after 4 days by qRT-PCR for viral genome. Data are shown as the mean ± SEM of 5 mice/group. * denotes p<0.05 versus AdC7-Null. § denotes p<0.05.
Absence of RSV-induced vaccine-enhanced disease following immunization with AdC7-Fsyn
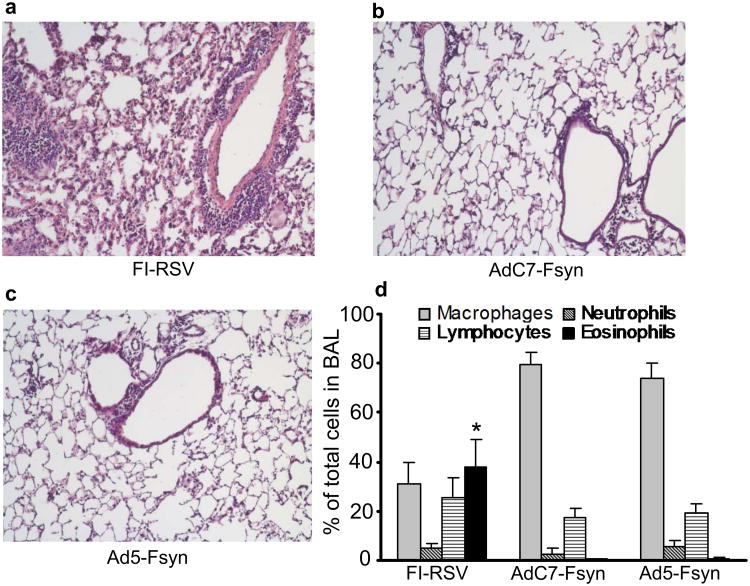
To evaluate if immunization with AdC7-Fsyn induces enhanced RSV lung disease, mice were immunized intranasally with AdC7-Fsyn, Ad5-Fsyn, AdC7-Null or FI-RSV and then challenged with RSV at 5 wk post-immunization. Vaccine-enhanced RSV disease was assessed by analyzing lung histology and evaluating the cellular composition of the BAL following RSV infection. Consistent with vaccine-enhanced disease, pronounced inflammation was seen in the lungs of mice immunized with FI-RSV (Fig. 3a). In contrast, no significant inflammatory changes were observed in the mice immunized with AdC7-Fsyn (Fig. 3b) or Ad5-Fsyn (Fig. 3c). Likewise, the cellular composition of the BAL showed an increase in eosinophils only in the mice that were immunized with FI-RSV (p<0.05; Fig. 3d), no eosinophils were seen in the AdC7-Fsyn or Ad5-Fsyn group. These data shows that immunization with AdC7-Fsyn does not trigger enhanced RSV pulmonary disease.
Figure 3. Absence of RSV-induced inflammatory responses in lung and BAL following immunization with AdC7-Fsyn.
BALB/c mice were immunized intranasally with AdC7-Fsyn, Ad5-Fsyn (all 1010 pu/mouse) or FI-RSV. Five wk later the mice were challenged intranasally with RSV (106 pfu) and lungs were harvested after 7 days (histopathology) or 4 days (differential cell count). (a-c). Lung histology (H&E stain): (a) FI-RSV, (b) AdC7-Fsyn, (c) Ad5-Fsyn, (d) Differential quantification of cells in BAL. Data for (d) is shown as mean ± SEM of 5 mice/group. * denotes p<0.05 vs AdC7-Fsyn and Ad5-Fsyn.
Maternal immunization with AdC7-Fsyn protects offspring against RSV infection
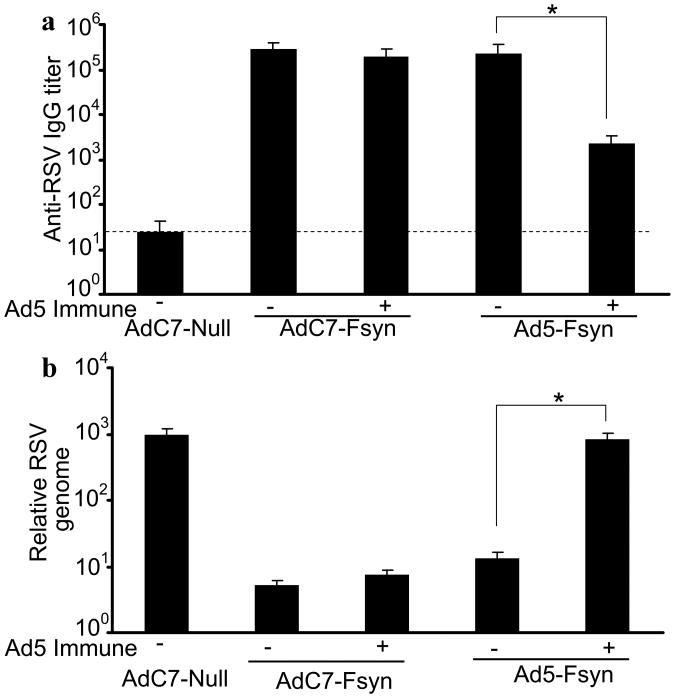
To evaluate if protective immunity can be transferred from AdC7-Fsyn immunized mothers to their offspring, female mice were immunized with AdC7-Fsyn or Ad5-Fsyn, bred and anti-RSV immunity evaluated in the pups at 3 wk of age. Since preexisting immunity against Ad5 can potentially hamper the efficacy of Ad-based vaccines, we also investigated the effect of Ad5 preimmunity on maternal immunization with AdC7-Fsyn or Ad5-Fsyn. We observed only minimal cross-neutralization by antibodies against AdC7 and Ad5 vectors (Table 1), favoring the application of AdC7 vector even in presence of Ad5-immunity. To mimic the Ad5 preimmunity, one group of female mice was inoculated with Ad5-Null 4 weeks prior to the immunization. Pups born to the naïve mothers immunized with AdC7-Fsyn or Ad5-Fsyn showed comparable anti-RSV titers (p>0.05) (Fig. 4a). Notably, pups born to the Ad5-primed mothers that were immunized with AdC7-Fsyn have high anti-RSV titers that were comparable to those in pups born to the naïve AdC7-Fsyn immunized mothers (p>0.05). However, as expected, only low levels of anti-RSV immunity was transferred to pups by Ad5-Fsyn immunization of mothers in the presence of Ad5 preimmunity (p<0.05) (Fig. 4a). Consistent with the anti-RSV titers, maternal immunization with AdC7-Fsyn protected pups against pulmonary RSV challenge with RSV, irrespective of the preimmune status of the mothers (Fig. 4b). However, Ad5 pre-existing immunity adversely affected the efficacy of maternal immunization with Ad5-Fsyn (Fig. 4b). These data suggests that maternal immunization with AdC7-Fsyn can protects pups against RSV infection and AdC7-Fsyn immunization is effective even in the presence of anti-Ad5 immunity.
Table 1.
Cross-neutralizing serum antibody titers from mice inoculated with Ad5-Null or AdC7-Null vectors. Data are shown as the mean ± SEM of 5 mice/group.
| Anti-Serum | Anti-Ad cross-neutralization titer | |
|---|---|---|
| Ad5 | AdC7 | |
| Ad5 | 1792±701 | 32±10 |
| AdC7 | 24±8 | 2048±701 |
Figure 4. Maternal immunization with AdC7-Fsyn protects offspring against RSV infection.
Female BALB/c mice, with or without Ad5 preimmunity, were immunized intranasally with AdC7-Fsyn, Ad5-Fsyn or AdC7-Null and bred 2 wks after immunization. (a) Anti-RSV IgG antibodies in serum of the 3 week old mice born to immunized mothers were analyzed by ELISA. (b) At 3 weeks of age mice born to immunized mothers were challenged intranasally with RSV (106 pfu/mouse). RSV titers in the lung homogenate were performed after 4 days by qRT-PCR for viral genome. Data are shown as the mean ± SEM of 5 mice/group. Limit of detection is indicated by the dashed line. * denotes p<0.05.
Kinetics of anti-RSV titers
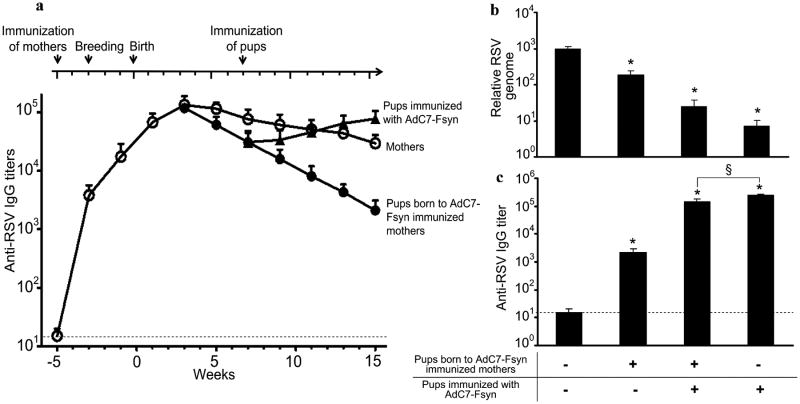
Duration and kinetics of anti-RSV titers was estimated in serum from AdC7-Fsyn immunized mother and their pups. Following immunization, the anti-RSV titers in mothers increases gradually, peaking at around 8 weeks of immunization (Fig. 5a). At the weaning age of 3 wks, pups have anti-RSV titers equivalent to those of mothers, which gradually decrease by up to 2 logs by 15 wks of age (Fig. 5a). The half-life of maternally transferred anti-RSV antibodies in the pups was 14.63 days. On pulmonary RSV challenge at 15 weeks, anti-RSV antibodies could reduce the pulmonary RSV load by 8-fold (Fig. 5b).
Figure 5. a) Serum anti-RSV IgG titers in AdC7-Fsyn immunized mothers and their pups.
Serum from immunized mothers (○) and their pups (●) was collected at 2 weeks intervals and analyzed for anti-RSV IgG by ELISA. Mice born to immunized mothers were reimmunized with AdC7-Fsyn at 7 weeks of age and analyzed for serum anti-RSV titers ▲. (b and c) Mice born to immunized mothers can be actively immunized with AdC7-Fsyn. Mice born to AdC7-Fsyn immunized or naïve mothers were reimmunized with AdC7-Fsyn at 7 weeks of age. 8 weeks after immunization (b) protection against RSV challenge and (c) anti-RSV IgG titers in serum were evaluated. Data are shown as the mean ± SEM of 5 mice/group. Limit of detection is indicated by the dashed line. * denotes p<0.05 versus AdC7-Null. § denotes p<0.05.
Mice born to immunized mothers can be actively immunized with AdC7-Fsyn
Although the maternal immunization and passive transfer of anti-RSV immunity effectively protects the newborn mice against RSV, the gradually declining levels of anti-RSV antibodies could make the young mice again susceptible to RSV infection. Therefore, we evaluated the efficacy of active immunization with AdC7-Fsyn to boost the anti-RSV antibody titers in the young mice. Mice born to the AdC7-Fsyn-immunized mothers were intranasally immunized with AdC7-Fsyn at 7 weeks of age and evaluated for anti-RSV immunity. A strong boost in the levels of anti-RSV IgG titers was achieved in the mice born to AdC7-Fsyn-immunized mothers, though the titers were significantly lower compared to those born to naïve mothers (p<0.05; Fig. 5c). However, on pulmonary challenge with RSV, no significant difference in protection of the mice born to AdC7-Fsyn or naïve mothers was observed (p>0.05; Fig. 5b). These results suggest that passively immunized mice can be revaccinated with AdC7-Fsyn to extend the protective efficacy against RSV infections.
Discussion
Despite the extensive efforts, realization of a safe and effective RSV vaccine has remained a challenge. Given the evidence that naturally derived maternal antibodies are effective in protecting very young infants against RSV, boosting maternal anti-RSV immunity resulting in the placental transfer of RSV neutralizing antibodies represents a strategy to protect newborns and young infants. Here we demonstrate that immunization with AdC7-based vector expressing codon-optimized RSV fusion protein induces potent systemic and mucosal protective RSV immunity without predisposing to vaccine-enhanced RSV lung disease. Protective immunity induced by AdC7-Fsyn was transferred from immunized mothers to their offspring even in the presence of Ad5-preimmunity and could be further extended by active immunization.
AdC7-Fsyn as RSV vaccine
Due to their ability to induce potent transgene product-specific immune responses, replication-defective Ad vectors have been explored as vaccine platforms against a variety of pathogens [15, 29]. Because of the high prevalence of Ad infections in humans, it is commonly believed that pre-existing Ad-neutralizing antibodies negatively impacts the anti-pathogen immunity that can be elicited with human Ad (HAd) vectors [16, 30, 31]. With the anticipation that anti-HAd preimmunity may not cross-neutralize non-HAd, a number of Ad-based vaccine vectors derived from various animal species have been developed [16, 32-34]. Nonhuman primate-derived Ad vectors such as AdC7 share many structural and biological characteristics with human Ad vectors including the capacity to act as a vaccine carrier [33, 35]. We showed that anti-Ad5 neutralizing antibodies do not cross-neutralize AdC7 (Table 1), thus favoring their successful application in the presence of Ad5 immunity.
We have previously demonstrated that AdC7-based vaccines targeted to the P. aeruginosa OprF protein induce superior mucosal and protective immunity compared to human Ad5-based vectors, in particular following intranasal administration [17, 18]. Others have also reported the induction of stronger immune responses by Ad vectors with intranasal immunization compared to intramuscular or subcutaneous routes [11, 13, 36]. Single intranasal immunization with AdC7-Fsyn induced robust systemic and mucosal neutralizing antibody responses that protected against RSV. Although the anti-RSV immunity induced by AdC7-Fsyn was comparable to that of Ad5-Fsyn, the reduction in RSV load in lungs was stronger following AdC7-Fsyn immunization. The superior efficacy of AdC7 over Ad5 vector on mucosal immunization is consistent with our previous observations [17, 18]. Importantly, unlike FI-RSV, AdC7-Fsyn immunization did not induce vaccine-enhanced RSV disease. Given the fact that Ad vectors are known potent inducers of Th1-biased transgene-specific immunity, more recently, other groups have also investigated human [11-13, 36] or nonhuman [10] Ad vectors for RSV vaccine. However, none of these studies evaluated the efficacy of maternal immunization.
The F-glycoprotein is highly conserved among both A and B groups of RSV, an attractive feature for a vaccine antigen. An F protein epitope is also the target of the prophylactic monoclonal antibody palivizumab. The codon-optimization of F (Fsyn) enhances its expression in eukaryotic cells compared to wild-type RSV-F that is impaired by premature polyadenylation [11]. Higher in vitro expression was achieved with AdC7-Fsyn compared to AdC7-F (wild-type) (data not shown). RSV F-protein is known to cause cell fusions; nevertheless, we did not notice any significant fusion-associated lung pathology in mice.
Maternal immunization with AdC7-Fsyn
One of the challenges of RSV vaccine development is protecting young infants at 2-3 months of age when the disease is most severe [2, 37]. Active immunization at this age is challenging due to immaturity of parts of the immune system, characterized by Th2-biased immune responses, poor antigen presentation and affinity maturation [38]. Maternal antibodies in infants can also block the efficacy of RSV vaccines [39]. Maternal immunization to boost the transfer of anti-RSV neutralizing antibodies to the neonates can potentially address these challenges [24].
RSV neutralizing antibody responses strongly correlates with protection against RSV disease [19, 20]. The experience with palivizumab [6, 40], as well as protection of young infants against RSV by maternally derived antibodies [21-23] provides the basis to aim for induction of neutralizing antibodies for a successful vaccine. This is further supported by the observation that neutralizing antibodies have not been associated with severe disease following RSV reinfections [40, 41]. However, once the RSV infection is established, cellular immunity is required to completely eliminate RSV. Since only the humoral and not the cellular immunity is transferred from mother to the fetus, we investigated only the humoral immunity induced by AdC7-Fsyn.
Vaccine-enhanced disease in RSV-naïve infants is the most feared unwanted outcome of any RSV candidate vaccine. We ([9], this study) and others [10-13, 36] have demonstrated that Ad-vectored RSV vaccine do not cause severe RSV disease following reinfection. Fortunately, since neutralizing antibodies have not been associated with severe disease following RSV reinfections [40, 41], maternal immunization alleviates the risk of enhanced RSV disease in infants. It has been recently demonstrated that maternal immunization with FI-RSV (known inducer of enhanced disease) did not lead to vaccine-enhanced disease in infants [42]. Moreover, since adults have been exposed to RSV multiple times, they are not considered at risk for vaccine-enhanced disease. Thus, the multiple check points in our approach of maternal immunization with AdC7-Fsyn ensure safety against vaccine-enhanced disease.
We demonstrated that following the maternal immunization with single intranasal inoculation of AdC7-Fsyn, protective antibodies can be transferred to the infant mice. The anti-RSV titer in pups at 3 weeks of age mirrors that of their mothers and then gradually decline with the half-life of about 2 weeks. Protective immunity against RSV pulmonary infection was observed up to 15 weeks. As expected, the preexisting immunity against Ad5 in dams did not reduce the efficacy of maternal immunization by AdC7-Fsyn. Because of the gradual drop in passively transfer protective antibodies, maternal immunization is a classic but only a short-term approach to prevent infant RSV infections. A dual immunization approach that combined the passively acquired and actively elicited antibody to provide long-term protection after birth has been suggested [43]. Accordingly, we demonstrated that the waning titers of maternal antibodies could be further boosted by active immunization with AdC7-Fsyn to extend the potency and duration of protection in passively immunized mice. Hence, maternal immunization and then additional infant immunization with AdC7-Fsyn presents an effective way to confer long-term prophylaxis to the susceptible infants.
Immunization during pregnancy is a proven approach to protect newborns against diseases such as tetanus and influenza [44]. In a clinical trial on pregnant women, a subunit RSV vaccine was shown to be safe and tolerable, though only modest neutralizing antibody responses were observed in vaccine recipients [45]. Vaccines based on Ad vectors are strongly immunogenic and capable of inducing stronger neutralizing antibody responses. Although the replication-defective Ad vectors have been extensively tested in preclinical and clinical studies for their safety [46-48] (Journal of Gene Medicine, http://www.wiley.co.uk/genmed/clinical/), live vaccines administered to a pregnant woman pose a theoretical risk to the fetus; therefore, in the absence of substantial clinical data, live vaccines generally are contraindicated during pregnancy (CDC). However, benefits of vaccinating pregnant women usually outweigh potential risks when the likelihood of disease exposure is high, for example during the influenza pandemic in 2009 experts recommended the live-attenuated H1N1 vaccine for pregnant women. No unusual pregnancy complications or fetal outcomes were observed following immunization with live-attenuated influenza vaccine [49]. Additional safety data on use of replication-deficient Ad vectors in pregnant women is required for approval. Although mice are only semi-permissible to RSV infection, mice model for RSV has been well established and frequently been used to establish the preclinical features and immunogenicity of numerous vaccine candidates [9, 11, 36, 42, 50]. Further preclinical advancement of this vaccine requires confirmation in another animal model, most likely cotton rats.
Taken together, we present a first proof-of-concept study utilizing an adenoviral vector for maternal immunization against RSV disease. We demonstrated that AdC7-based vaccine expressing codon-optimized RSV fusion protein is safe and effective for maternal immunization to protect infants against RSV infections early in life.
Highlights.
We evaluated AdC7 vector expressing codon-optimized fusion protein (Fsyn) for RSV vaccine
Maternal immunization of mice with AdC7-Fsyn protected pups against RSV
Immunization with AdC7-Fsyn was effective even in the presence of Ad5 preimmunity
The maternally immunized mice can be actively reimmunized with AdC7-Fsyn to extend protection
Acknowledgments
These studies were supported by NIH RO1 grant (AI059228-01). A.S. is supported by the Lili Foo Hing Fund.
Abbreviations
- RSV
Respiratory syncytial virus
- Ad
Adenovirus
- Ad5
Human adenovirus serotype 5
- AdC7
Chimpanzee adenovirus serotype 7
- F protein
Fusion protein
- Fsyn
codon optimized fusion protein
- qRT-PCR
quantitative reverse-transcriptase polymerase chain reaction
- FI-RSV
Formalin-inactivated respiratory syncytial virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- 1.Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979-1997. J Infect Dis. 2001;183(1):16–22. doi: 10.1086/317655. [DOI] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162(1-2):80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommer C, Resch B, Simoes EA. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J. 2011;5:144–54. doi: 10.2174/1874285801105010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalina WV, Gershwin LJ. Progress in defining the role of RSV in allergy and asthma: from clinical observations to animal models. Clin Dev Immunol. 2004;11(2):113–9. doi: 10.1080/10446670410001722131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102(3 Pt 1):531–7. [PubMed] [Google Scholar]
- 7.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 8.Habibi MS, Openshaw PJ. Benefit and harm from immunity to respiratory syncytial virus: implications for treatment. Curr Opin Infect Dis. 2012;25(6):687–94. doi: 10.1097/QCO.0b013e32835a1d92. [DOI] [PubMed] [Google Scholar]
- 9.Krause A, Xu Y, Ross S, Wu W, Joh J, Worgall S. Absence of vaccine-enhanced RSV disease and changes in pulmonary dendritic cells with adenovirus-based RSV vaccine. Virol J. 2011;8:375. doi: 10.1186/1743-422X-8-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson TR, Rangel D, Graham BS, Brough DE, Gall JG. Genetic Vaccine for Respiratory Syncytial Virus Provides Protection Without Disease Potentiation. Mol Ther. 2014;22(1):196–205. doi: 10.1038/mt.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohlmann R, Schwannecke S, Tippler B, Ternette N, Temchura VV, Tenbusch M, et al. Protective efficacy and immunogenicity of an adenoviral vector vaccine encoding the codon-optimized F protein of respiratory syncytial virus. J Virol. 2009;83(23):12601–10. doi: 10.1128/JVI.01036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunwald T, Tenbusch M, Schulte R, Raue K, Wolf H, Hannaman D, et al. Novel vaccine regimen elicits strong airway immune responses and control of respiratory syncytial virus in nonhuman primates. J Virol. 2014;88(8):3997–4007. doi: 10.1128/JVI.02736-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim E, Okada K, Beeler JA, Crim RL, Piedra PA, Gilbert BE, et al. Development of an Adenoviral-based RSV Vaccine: Preclinical Evaluation of Efficacy, Immunogenicity, and Enhanced Disease in a Cotton Rat Model. J Virol. 2014;88(9):5100–08. doi: 10.1128/JVI.03194-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santosuosso M, McCormick S, Xing Z. Adenoviral vectors for mucosal vaccination against infectious diseases. Viral Immunol. 2005;18(2):283–91. doi: 10.1089/vim.2005.18.283. [DOI] [PubMed] [Google Scholar]
- 15.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10(4):616–29. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahi YS, Bangari DS, Mittal SK. Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther. 2011;11(4):307–20. doi: 10.2174/156652311796150372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause A, Whu WZ, Qiu J, Wafadari D, Hackett NR, Sharma A, et al. RGD capsid modification enhances mucosal protective immunity of a non-human primate adenovirus vector expressing Pseudomonas aeruginosa OprF. Clin Exp Immunol. 2013;173(2):230–41. doi: 10.1111/cei.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krause A, Whu WZ, Xu Y, Joh J, Crystal RG, Worgall S. Protective anti-Pseudomonas aeruginosa humoral and cellular mucosal immunity by AdC7-mediated expression of the P. aeruginosa protein OprF. Vaccine. 2011;29(11):2131–9. doi: 10.1016/j.vaccine.2010.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–8. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 20.Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine. 2003;21(24):3479–82. doi: 10.1016/s0264-410x(03)00355-4. [DOI] [PubMed] [Google Scholar]
- 21.Ogilvie MM, Vathenen AS, Radford M, Codd J, Key S. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol. 1981;7(4):263–71. doi: 10.1002/jmv.1890070403. [DOI] [PubMed] [Google Scholar]
- 22.Piedra PA. Clinical experience with respiratory syncytial virus vaccines. Pediatr Infect Dis J. 2003;22(2 Suppl):S94–9. doi: 10.1097/01.inf.0000053893.15894.ff. [DOI] [PubMed] [Google Scholar]
- 23.Stensballe LG, Ravn H, Kristensen K, Meakins T, Aaby P, Simoes EA. Seasonal variation of maternally derived respiratory syncytial virus antibodies and association with infant hospitalizations for respiratory syncytial virus. J Pediatr. 2009;154(2):296–8. doi: 10.1016/j.jpeds.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 24.Kaaijk P, Luytjes W, Rots NY. Vaccination against RSV: is maternal vaccination a good alternative to other approaches? Hum Vaccin Immunother. 2013;9(6):1263–7. doi: 10.4161/hv.24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reyes-Sandoval A, Fitzgerald JC, Grant R, Roy S, Xiang ZQ, Li Y, et al. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J Virol. 2004;78(14):7392–9. doi: 10.1128/JVI.78.14.7392-7399.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashimoto M, Boyer JL, Hackett NR, Wilson JM, Crystal RG. Induction of protective immunity to anthrax lethal toxin with a nonhuman primate adenovirus-based vaccine in the presence of preexisting anti-human adenovirus immunity. Infect Immun. 2005;73(10):6885–91. doi: 10.1128/IAI.73.10.6885-6891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70(11):7498–509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma A, Tandon M, Ahi YS, Bangari DS, Vemulapalli R, Mittal SK. Evaluation of cross-reactive cell-mediated immune responses among human, bovine and porcine adenoviruses. Gene Ther. 2010;17(5):634–42. doi: 10.1038/gt.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira TB, Alves PM, Aunins JG, Carrondo MJ. Use of adenoviral vectors as veterinary vaccines. Gene Ther. 2005;12(Suppl 1):S73–83. doi: 10.1038/sj.gt.3302618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6(9):1574–83. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 31.Hackett NR, Kaminsky SM, Sondhi D, Crystal RG. Antivector and antitransgene host responses in gene therapy. Curr Opin Mol Ther. 2000;2(4):376–82. [PubMed] [Google Scholar]
- 32.Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 2006;24(7):849–62. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4(115):115ra2. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma A, Bangari DS, Tandon M, Pandey A, HogenEsch H, Mittal SK. Comparative analysis of vector biodistribution, persistence and gene expression following intravenous delivery of bovine, porcine and human adenoviral vectors in a mouse model. Virology. 2009;386(1):44–54. doi: 10.1016/j.virol.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatsis N, Tesema L, Robinson ER, Giles-Davis W, McCoy K, Gao GP, et al. Chimpanzee-origin adenovirus vectors as vaccine carriers. Gene Ther. 2006;13(5):421–9. doi: 10.1038/sj.gt.3302675. [DOI] [PubMed] [Google Scholar]
- 36.Fu YH, He JS, Qiao W, Jiao YY, Hua Y, Zhang Y, et al. Intranasal immunization with a helper-dependent adenoviral vector expressing the codon-optimized fusion glycoprotein of human respiratory syncytial virus elicits protective immunity in BALB/c mice. Virol J. 2013;10:183. doi: 10.1186/1743-422X-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States,1997-2006. Pediatr Infect Dis J. 2012;31(1):5–9. doi: 10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 38.Philbin VJ, Levy O. Developmental biology of the innate immune response:implications for neonatal and infant vaccine development. Pediatr Res. 2009;65(5 Pt 2):98R–105R. doi: 10.1203/PDR.0b013e31819f195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crowe JE, Jr, Firestone CY, Murphy BR. Passively acquired antibodies suppress humoral but not cell-mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J Immunol. 2001;167(7):3910–8. doi: 10.4049/jimmunol.167.7.3910. [DOI] [PubMed] [Google Scholar]
- 40.Gimenez HB, Chisholm S, Dornan J, Cash P. Neutralizing and enhancing activities of human respiratory syncytial virus-specific antibodies. Clin Diagn Lab Immunol. 1996;3(3):280–6. doi: 10.1128/cdli.3.3.280-286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham BS. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev. 2010;239(1):149–66. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon YM, Hwang HS, Lee JS, Ko EJ, Yoo SE, Kim MC, et al. Maternal antibodies by passive immunization with formalin inactivated respiratory syncytial virus confer protection without vaccine-enhanced disease. Antiviral Res. 2014;104:1–6. doi: 10.1016/j.antiviral.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw CA, Ciarlet M, Cooper BW, Dionigi L, Keith P, O'Brien KB, et al. The path to an RSV vaccine. Curr Opin Virol. 2013;3(3):332–42. doi: 10.1016/j.coviro.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Healy CM. Vaccines in pregnant women and research initiatives. Clin Obstet Gynecol. 2012;55(2):474–86. doi: 10.1097/GRF.0b013e31824f3acb. [DOI] [PubMed] [Google Scholar]
- 45.Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21(24):3465–7. doi: 10.1016/s0264-410x(03)00352-9. [DOI] [PubMed] [Google Scholar]
- 46.O'Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD, et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J Infect Dis. 2012;205(5):772–81. doi: 10.1093/infdis/jir850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29(2):304–13. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 48.Capone S, D'Alise AM, Ammendola V, Colloca S, Cortese R, Nicosia A, et al. Development of chimpanzee adenoviruses as vaccine vectors: challenges and successes emerging from clinical trials. Expert Rev Vaccines. 2013;12(4):379–93. doi: 10.1586/erv.13.15. [DOI] [PubMed] [Google Scholar]
- 49.Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204(2):146 e1–7. doi: 10.1016/j.ajog.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 50.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342(6158):592–8. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]