Abstract
Purpose
To evaluate the effects of idiopathic intracranial hypertension (IIH) on rod-, cone-, and melanopsin-mediated pupillary light reflexes (PLRs).
Methods
Pupillary light reflexes elicited by full-field, brief-flash stimuli were recorded in 13 IIH patients and 13 normal controls. Subjects were dark-adapted for 10 minutes and the PLR was recorded in response to short-wavelength flashes (0.001 cd/m2: rod condition; 450 cd/m2: melanopsin condition). Subjects were then exposed to a rod-suppressing field and 10 cd/m2 long-wavelength flashes were presented (cone condition). Pupillary light reflexes were quantified as the maximum transient constriction (rod and cone conditions) and the post-illumination pupil constriction (melanopsin condition), relative to the baseline pupil size. Diagnostic power was evaluated using receiver operating characteristic (ROC) analysis.
Results
The IIH patients had significantly smaller PLRs under the melanopsin (P < 0.001) and rod (P = 0.04) paradigms; a trend for reduced cone-mediated PLRs was also found (P = 0.08). Receiver operating characteristic analysis indicated areas under the curves (AUC) of 0.83 (melanopsin-meditated; P = 0.001), 0.71 (rod-mediated; P = 0.07), and 0.77 (cone-mediated; P = 0.02). The AUC (0.90, P < 0.001), sensitivity (85%), and specificity (85%) were high for ROC analysis performed on the mean of the rod, cone, and melanopsin PLRs.
Conclusions
Pupillary light reflex reductions in IIH patients indicate compromised RGC function. PLR measurement, particularly under rod- and melanopsin-mediated conditions, may be a useful adjunct to standard clinical measures of visual function in IIH.
Keywords: pupil, idiopathic intracranial hypertension (IIH), melanopsin, rod, cone
Idiopathic intracranial hypertension (IIH) is a condition of elevated intracranial pressure (ICP) for which a cause cannot be determined. Loss of visual function is the primary morbidity for most IIH patients, with approximately 10% of patients progressing to bilateral blindness.1,2 As reviewed elsewhere,3 visual dysfunction in IIH appears to be due to a series of events initiated by increased ICP. Elevation in ICP likely produces increased pressure around the distal optic nerve, which, in turn, results in axoplasmic flow stasis.4–6 Reduced axoplasmic transport produces intra-axonal edema,7 which is likely followed by venule compression, ischemia, and loss of visual function. Because visual function within the central visual field is typically normal, or nearly normal, until the late stages of the disease8,9 vision loss in IIH is most commonly characterized by standard automated perimetry to measure peripheral visual field sensitivity.1,8–10 However, not all IIH patients have marked visual field abnormalities, and ganglion cell dysfunction may precede measurable reductions assessed by perimetry.11 Moreover, perimetry is an inherently subjective test and it is vulnerable to patient error. Objective assessments of visual pathway function in IIH may provide new insight into vision loss associated with the disease and could have the potential to provide additional data upon which clinical management decisions can be based.
Objective measures of RGC function have been performed in patients with IIH using electrophysiological techniques. For example, previous work has shown that the amplitude of the pattern electroretinogram (pERG) can be reduced in patients with IIH.12 However, standard pERG measurements are limited in that they primarily assess function within the central visual field. More recently, it was shown that the photopic negative response (PhNR), a late negative component of the full-field photopic single-flash ERG that originates largely from RGCs,13,14 can also be abnormal in patients with IIH.11
Pupillometry is an additional objective technique that can assess inner retina, outer retina, and subcortical function, which may have application to patients with IIH. The response of the pupil to a flash of light (the pupillary light reflex; PLR) is a complex response with contributions from more than one photoreceptor type. However, by altering the adaptation conditions and stimulus characteristics, contributions of the rod pathway, cone pathway, and intrinsically photosensitive RGC (ipRGC) pathway, which contains the photopigment melanopsin,15,16 can be assessed.17–19 Thus, the PLR is a powerful tool because it provides insight into ipRGC function, as well as rod and cone inputs into the ipRGCs. The PLR has been useful for understanding diseases of the inner retina and optic nerve, such as in hereditary optic neuropathies,20–22 glaucoma,23–26 and ischemic optic neuropathy.27 To date, the PLR has not been reported in patients with IIH.
Pupillometry is a promising approach for functional assessment in IIH because it shares many of the advantages of PhNR measurement, in that it is noninvasive, objective, performed quickly with minimal patient demands, does not require refraction or steady fixation, and can be measured using full-field stimuli. Additionally, pupillometry does not require pupil dilation and can provide measures of rod, cone, and melanopsin RGC function. The generators of the PLR likely differ from those of the PhNR, as pupillometry selectively targets ipRGCs. Although IIH is not typically associated with outer retina abnormalities, a recent report has shown cone receptor density loss in a patient with IIH28 and an earlier report showed light adapted flicker ERG deficits in patients with chronic papilledema.29 Thus, pupillometry may provide new insight into outer retina function in patients with IIH.
The goal of the present study was to evaluate rod-, cone-, and melanopsin-mediated PLRs in patients with IIH. These pupil responses were assessed using a previously published paradigm,19 with slight modification, in patients with IIH and in visually-normal control subjects. The patients' PLRs were compared with other measures of visual function including Humphrey visual field mean deviation (HVF MD) scores and PhNR amplitude. The results of the present study are intended to determine the extent to which pupillometry can be used as a clinical tool to assess retinal dysfunction in patients with IIH.
Methods
Subjects
Thirteen subjects who have IIH and current or prior papilledema were recruited from the neuro-ophthalmology service at the University of Illinois at Chicago (age 33.2 ± 8.2 years, 12 females). The diagnosis of IIH was based on lumbar puncture with opening pressure greater than or equal to 25 cm H2O, normal cerebrospinal fluid constituents, and unremarkable brain imaging results.10 No patient had neurologic or ophthalmic disease other than IIH, refractive error greater than 6 diopters (D), or distance visual acuity worse than 20/20. HVF MD (24-2 Swedish Interactive Testing Algorithm [SITA] Humphrey Field Analyzer; Carl Zeiss Meditec, Jena, Thuringia, Germany) was normal (<2 dB loss) in three subjects, mildly abnormal (2–5 dB loss) in four subjects, and moderately to severely abnormal (>5 dB loss) in six subjects. Optic nerve appearance was Frisen papilledema grade (FPG)30 two or less in nine subjects, three or more in two subjects, and atrophic in two subjects. Three subjects with IIH were untreated at the time of testing, whereas the other 10 subjects had received medical intervention consisting of acetazolamide combined with weight loss, ventriculoperitoneal shunt, or ventriculoperitoneal stent. Of the 10 treated subjects, five had resolved symptoms at the time of testing (no headaches and FPG grade of 0, 1 [due to residual disk elevation], or atrophy), whereas the other five continued to have symptoms. Data were also obtained from 13 visually-normal individuals (age 31.8 ± 9.5 years, 8 females) without history of ophthalmic or neurologic disease. The mean ages of the controls and IIH subjects did not differ significantly (t = 0.36, P = 0.73).
The research followed the tenets of the Declaration of Helsinki and was approved by a University of Illinois at Chicago institutional review board. Written informed consent was obtained from all subjects prior to testing.
Apparatus and Stimuli
A light-emitting diode (LED)-driven ganzfeld system was used for stimulus generation and display (Espion V6, ColorDome desktop ganzfeld; Diagnosys LLC, Lowell, MA, USA). The stimuli were presented to one eye and the pupil responses were recorded from the same eye using a ViewPoint EyeTrack infrared camera system (Arrington Research, Scottsdale, AZ, USA), with the fellow eye patched. This system allows for real-time pupillometry with high spatial resolution (>0.03 mm) at a 60-Hz sampling rate. During the pupil recordings, the subject's head was stabilized with a chin rest. Stimuli consisted of short-wavelength (“blue;” dominant wavelength of 465 nm) and long-wavelength (“red;” dominant wavelength of 642 nm) pulses of light that were 1 second in duration. The field of view was approximately 90° (horizontal diameter) by 60° (vertical diameter). Stimulus wavelength and luminance were verified with a spectroradiometer (SpectraScan 740; Photo Research, Chatsworth, CA, USA).
Procedure
Pupillometry was performed on the worse seeing eye of each patient, assessed by HVD MD; the right eye was tested in each visually normal control. Test protocols intended to target the rod, cone, and melanopsin pathways were performed, as described in detail elsewhere.19 Subjects were first dark-adapted for 10 minutes and three pupil protocols were performed in the following order: (1) under the rod pathway protocol, a low luminance (0.001 cd/m2) blue flash was presented in the dark, (2) under the melanopsin protocol, a high luminance (450 cd/m2) blue flash was presented in the dark, and (3) under the cone pathway protocol, the subject was first light adapted for 2 minutes to a uniform 6 cd/m2 rod-suppressing blue field and a red flash (10 cd/m2) was presented against the blue adapting field. Of note, the luminance of the red flash used for the cone pathway protocol (10 cd/m2) was lower than that used in the standard cone paradigm reported previously (450 cd/m2).19 Preliminary results indicated that the lower luminance flash does not drive the pupil response to saturation (maximum constriction), which increases sensitivity of the cone pathway measurement. For all conditions, each stimulus was presented a minimum of two times and the data shown in the figures below are based on the mean response (the two responses were highly similar, with a mean difference between the two of 4%, averaged across all subjects and conditions).
Data Analysis
Data were analyzed offline using custom scripts programmed in MATLAB (MathWorks, Inc., Natick, MA, USA), which allowed for semiautomated analysis as follows: first, a median filter with a 300-ms time window was applied to remove eye blinks. Long eye blinks (or eye closure) could not be removed by the filter, and these artifacts were removed manually. The filtered pupil responses were then normalized by the median pupil size during the 1 second prior to each stimulus onset (prestimulus baseline pupil size). The relative pupillary light reflex (PLR) was defined as the ratio of the absolute pupil size (mm) to the baseline pupil size (mm), consistent with previous definitions.19 The relative transient PLR was defined as the difference between the normalized baseline and the minimum relative PLR after stimulus onset, whereas the relative sustained PLR was defined as the difference between the normalized baseline and the median relative PLR measured over a 5 to 7 seconds time range following stimulus offset. The normalization was used to reduce the effects of the small, but statistically significant, difference in the dark-adapted baseline pupil diameter between the IIH patients and controls (mean difference of 1.16 mm; t = 2.76, P = 0.01). There was also a small difference in baseline pupil size between the IIH patients and controls for the light-adapted condition (mean difference of 0.86 mm; t = 2.89, P = 0.01).
Results
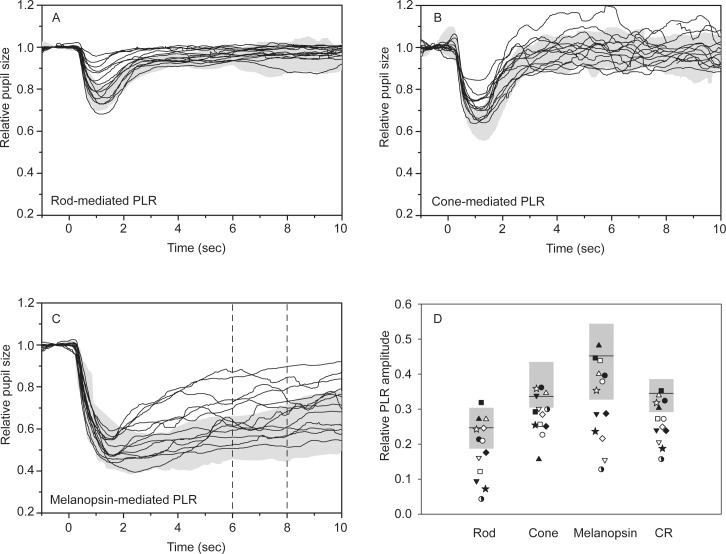
Figure 1 shows the IIH patients' PLRs for the rod- (A), cone- (B), and melanopsin-mediated (C) conditions. In each panel, the 5th to 95th percentile of the normal control range is represented by the gray region. For the rod and cone conditions, the normal PLR is characterized by a transient constriction (peak latency < 2 seconds) followed by a relatively rapid return to the baseline. Although the PLRs of the IIH patients generally followed the same pattern, the transient responses of some patients were abnormally small, falling outside of the normal range. The normal melanopsin-mediated response is characterized by a prolonged constriction following the offset of the stimulus that lasts for several seconds (Fig. 1C). The two vertical dashed lines in this panel indicate the time range over which the sustained response was measured. Similar to the rod- and cone-mediated responses, the IIH patients' melanopsin-mediated PLRs were variable, with some falling into the normal range and others showing substantial abnormalities. Note, however, that measurable PLRs were obtained for all subjects under all conditions. The relative PLR amplitude for each subject under each condition was measured and is plotted in Figure 1D. This figure shows the PLR amplitude corresponding to the 5th to 95th percentile of the normal control range (gray box, the mean value is marked by the horizontal line) and for the IIH patients. There was substantial variation among the relative PLRs for the IIH patients under each condition, as expected from the pupil traces shown in Figures 1A through 1C. The variation among the relative PLRs for the IIH patients was greater for the rod- and melanopsin-mediated PLRs, compared with the cone-mediated PLR. The variation in PLR values for the controls was also greatest under the melanopsin-mediated condition, with somewhat less variation among the controls under the rod- and cone-mediated conditions. For the rod and melanopsin conditions, 6 of 13 IIH patients had a PLR below the lower limit of the normal range, whereas 9 of 13 were below the normal range for cone condition.
Figure 1.
Waveforms obtained under the rod (A), cone (B), and melanopsin (C) paradigms. Each trace represents a different IIH patient and the gray regions are the normal ranges. (D) The relative PLR values for each patient and the normal range for each paradigm. The relative PLR (D) is defined as 1 − the normalized pupil response.
The effects of IIH are likely to be greatest on inner retina function, affecting RGCs, including the ipRGCs. Because the rod and cone PLRs are largely mediated through inputs to the ipRGCs,31 it might be expected that all three responses would be affected in IIH. Consequently, combining the PLR amplitudes obtained under the three conditions into a single metric may increase our ability to identify pathology. The combined response (CR) was defined as the mean pupil response for the three conditions; the CR for each subject was computed and is plotted in Figure 1D (rightmost data points). The CR for 8 of 13 patients was below the normal range. Of note, the melanopsin-mediated PLR amplitudes were larger than the rod- and cone-mediated PLRs, which resulted in a slight weighting of the melanopsin response in the mean. Although this weighting could be minimized by normalizing the PLRs (e.g., converting to z-scores before averaging), our results indicated that the weighting had negligible effects on the results and, for simplicity, we only present the mean.
A 2-way ANOVA was performed with subject group (control and IIH) and condition (rod, cone, and melanopsin) as main effects. The ANOVA showed significant differences in subject group (F = 23.47, P < 0.001) and condition (36.24, P < 0.001). There was no significant group by condition interaction (F = 2.29, P = 0.11). Holm-Sidak follow-up comparisons indicated significant differences between the IIH patients and the controls for the rod (t = 2.09, P = 0.04, group difference: 6%) and melanopsin conditions (t = 4.53, P < 0.001, group difference: 13%). A trend for smaller cone-mediated PLRs for the IIH patients compared with the controls was also found (t = 1.77, P = 0.08, group difference: 5%).
Receiver operating characteristic (ROC) curves were constructed as an additional approach to separate the control and patient groups based on PLR amplitude under the three conditions. These ROC curves are shown in Figure 2, which plots the proportion of the IIH patients classified as abnormal (sensitivity) as a function of the proportion of the normal controls classified as abnormal (1-specificity; false positives). The areas under the ROC curves (AUC) ranged from 0.71 to 0.90, with CR showing the largest area. Importantly, CR achieved high specificity (0.85) without compromising sensitivity (0.85). The optimal cutoff, sensitivity, specificity, AUC, standard error (SE), and corresponding P value are shown for each condition in the Table.
Figure 2.
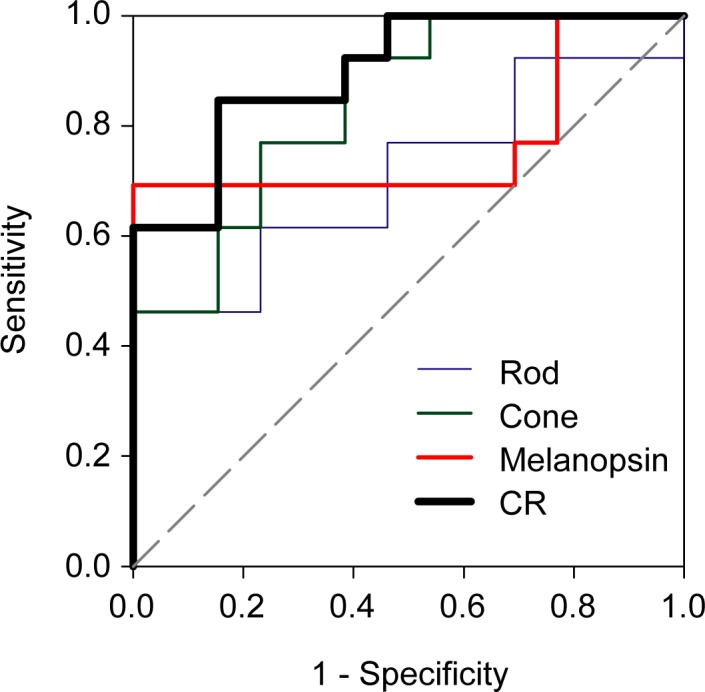
Receiver operating characteristic curves for the rod (blue), cone (green), melanopsin (red), and combined PLR (black). The proportion of the IIH patients classified as abnormal (sensitivity) is plotted as a function of the proportion of the controls classified as abnormal (1-specificity; false positives). The PLR cutoff values, sensitivity, specificity, AUC, SE, and corresponding P value are given in the Table.
Table.
Results of the ROC Analysis
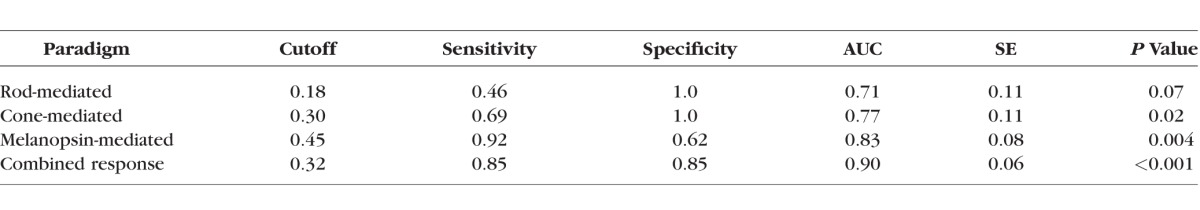
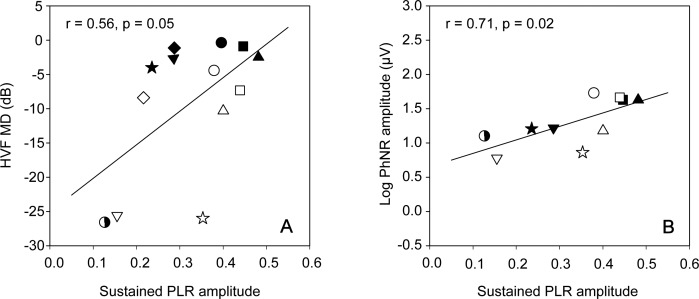
Figure 3A shows the relationship between the patients' HVF MD value and the melanopsin-mediated sustained PLR amplitude. The solid line is a linear regression line fit to the data. There was a modest relationship between the degree of visual field abnormality and the melanopsin-mediated PLR (r = 0.56, P = 0.05). A subset of the IIH patients (N = 10) participated in a previous study in which the PhNR of the full-field ERG was measured.11 The PhNR amplitude data obtained from that study are plotted as a function of the melanopsin-mediated PLR obtained in the present study (Fig. 3B). Note that the y-axes in both panels span 3.5 log units and the x-axes are identical; the solid line is a linear regression fit to the data. The relationship between the log PhNR amplitude and the melanopsin-mediated PLR was stronger (r = 0.71, P = 0.02) than that observed between the HVF MD and the melanopsin-mediated PLR (Fig. 3A). This may be expected, as both the PhNR and the melanopsin-mediated PLR target RGC function and both are full-field measures of function.
Figure 3.
Humphrey visual field MD is plotted as a function of the sustained (melanopsin-mediated) PLR amplitude (A). (B) Log PhNR amplitude as a function of the sustained PLR amplitude. Each symbol represents a different patient (N = 13 in [A] and N = 10 in [B]). The solid lines represent linear regression fits to the data.
Discussion
The goal of the present study was to evaluate rod-, cone-, and melanopsin-mediated PLRs in patients with IIH. As a group, the patients with IIH had PLR reductions under each of the three conditions. Although the pattern of deficit was not identical for all patients, the patients can be subdivided into those with abnormal (N = 6) and those with normal (N = 7) melanopsin-mediated responses. Of the patients with abnormal melanopsin-mediated responses, all had abnormal rod and/or cone mediated PLRs, which is the expected result of ipRGC damage. That is, in patients with ipRGC dysfunction, the rod- and cone-mediated PLRs would also likely be abnormal, as these pathways feed into the ipRGCs. In contrast, patients with normal melanopsin-mediated PLRs had more varied patterns of outer retina PLR dysfunction: three had normal rod- and cone-mediated PLRs (i.e., all responses were normal, indicating that current or past elevated ICP did not affect the pupil response). Three other patients had reductions only in the cone-mediated response and one patient had reduced cone- and rod-mediated responses. These four patients with rod- and/or cone-mediated PLR abnormalities, but normal melanopsin-mediated PLRs, are of interest because they suggest a relatively greater outer retina abnormality compared with inner retina abnormality, at least as assessed by pupillometry. Although IIH is not commonly associated with outer-retinal changes, a previous adaptive optics optical coherence tomography (OCT) study showed reduced cone density in a patient with IIH.28
The explanation for the heterogeneity among the patients is uncertain. However, there are at least two possible explanations: first, increased ICP may result in different pathophysiologic processes in different people. Alternatively, the different subgroups may represent different stages of pupil abnormality along a continuum. For example, PLR abnormalities mediated by the outer retina may precede PLR abnormalities mediated by the inner retina. This speculation is based on the observation that patients with normal melanopsin-mediated PLRs could have abnormal outer retina–mediated PLRs, whereas no patient with an abnormal melanopsin-mediated PLR had normal rod-and cone-mediated PLRs. Although this is highly speculative, and a time-course of abnormality cannot be determined from our data, it may help guide hypotheses for future work.
As noted above, the mean PLR was reduced for the IIH patients compared with normal under the rod-, cone, and melanopsin-mediated conditions, but there were subtle differences among the results obtained under the three paradigms. For example, under the rod-mediated condition, the patients' PLRs ranged from slightly super-normal to nearly extinguished. In comparison, the cone-mediated PLRs were abnormal for nine patients, but four of nine were only slightly subnormal. The differences in the magnitude of dysfunction under the three conditions are reflected in the correlation coefficients among them: there was a significant correlation between the melanopsin- and rod-mediated PLR (r = 0.63, P = 0.02), but not between the melanopsin- and cone-mediated PLR (r = −0.21, P = 0.49) or between the rod- and cone-mediated PLR (r = −0.03, P = 0.93). This suggests that the cone-mediated PLR may provide information regarding the pupil response that differs from that of the rod- and melanopsin-mediated PLRs. This may be explained by the unique spatial summation characteristics of the cone-mediated PLR, which is dominated by contributions from the central visual field32 that is typically less affected in IIH. In contrast, spatial summation for the rod- and melanopsin-mediated PLRs occurs the throughout the entire visual field,32 including the periphery that is affected in IIH. Thus, the generally small cone-mediated PLR abnormalities for the IIH patients may be attributed to a combination of their relative central visual field preservation and the minimal spatial summation of the cone-mediated PLR.
In addition to the abnormal PLRs recorded in the patients with IIH, the patients had, on average, reduced baseline pupil sizes in the dark and in the light. However, the baseline pupil size reductions were generally small and there was substantial overlap between the control and patient distributions. Although the explanation for reduced baseline pupil sizes is unclear, changes in sympathetic innervation may play a role as sympathetic innervation is thought to largely control the resting (baseline) pupil size.33,34 We cannot unequivocally exclude the effects of mild efferent pathway defects arising from cortical (or subcortical) sources. Cognitive changes are becoming better recognized in high ICP states35,36 and pain due to headache could affect pupil size (although pain generally results in pupil dilation).37 However, we note no significant difference in baseline pupil size between the five patients who had undergone treatment with resolution of symptoms and the eight patients with active symptoms, suggesting that headache did not significantly affect the baseline pupil size in our subjects.
As discussed above, there was substantial variation among the patients in the extent of the PLR abnormalities. In addition, there was also substantial variation in visual function assessed by HVF MD, which ranged from normal to substantially impaired. Given the differences between pupillometry and perimetry, such as the region of the visual field stimulated, threshold versus super-threshold response measures, and adaptation state, it can be difficult to compare the two measures meaningfully. Nevertheless, previous studies in patients with hereditary optic neuropathy22 and glaucoma19 have found significant linear correlations between the PLR and HVF MD. In our patients with IIH, was a moderate correlation between HVF MD and melanopsin-mediated PLR amplitude. The lack of a strong correlation suggests that the information provided by PLR assessment is not entirely captured by the HVF MD value. As such, PLR assessment may be a useful adjunct measure for targeting inner retina function in patients with IIH.
The abnormal melanopsin-mediated PLR in our IIH patients is of interest in light of recent reports indicating that ipRGCs may be selectively resistant to disease.38–40 The reports of selective resistance to disease are based on a relative preservation of the pupil response in patients with optic atrophy38,40 and in animal models of autosomal dominant optic atrophy,41 ocular hypertension,42 and N-methyl-D-aspartic excitotoxicity.39 However, other studies in human subjects have shown significantly reduced melanopsin-mediated PLRs in glaucoma,23–26 hereditary optic neuropathies,20–22 anterior ischemic optic neuropathy,27 and in diabetic retinopathy.43 The results of the current study are consistent with these latter reports, suggesting abnormal ipRGC function in patients with IIH.
In conclusion, we provide evidence of abnormal rod-, cone-, and melanopsin-mediated PLRs in patients with IIH. Given that the rod- and melanopsin-mediated PLRs appear to be well suited for capturing dysfunction throughout the entire visual field, these measures may be particularly useful for identifying pathologic changes in IIH patients. Additional work is needed to determine the suitability of the PLR as a clinical monitoring tool in IIH and to determine the extent to which it may be a useful measure in future clinical trials.
Acknowledgments
Supported by National Institutes of Health Grants R00EY019510, K12EY021475, K23EY024345, and P30EY01792 (Bethesda, MD, USA); an Illinois Society for the Prevention of Blindness Research Grant (Chicago, IL, USA); Sybil B. Harrington RPB Special Scholar Award (New York, NY, USA); and an unrestricted departmental grant from Research to Prevent Blindness (New York, NY, USA).
Disclosure: J.C. Park, None; H.E. Moss, None; J.J. McAnany, None
References
- 1. Corbett JJ,, Savino PJ,, Thompson HS,, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982; 39: 461–474. [DOI] [PubMed] [Google Scholar]
- 2. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 1991; 9: 73–95. [PubMed] [Google Scholar]
- 3. Lee AG,, Wall M. Papilledema: are we any nearer to a consensus on pathogenesis and treatment? Curr Neurol Neurosci Rep. 2012; 12: 334–339. [DOI] [PubMed] [Google Scholar]
- 4. Hayreh SS. Pathogenesis of optic disc oedema in raised intracranial pressure. Trans Ophthalmol Soc U K. 1976; 96: 404–407. [PubMed] [Google Scholar]
- 5. Hayreh SS. Optic disc edema in raised intracranial pressure. V. Pathogenesis. Arch Ophthalmol. 1977; 95: 1553–1565. [DOI] [PubMed] [Google Scholar]
- 6. Hayreh SS,, March W,, Anderson DR. Pathogenesis of block of rapid orthograde axonal transport by elevated intraocular pressure. Exp Eye Res. 1979; 28: 515–523. [DOI] [PubMed] [Google Scholar]
- 7. Tso MO,, Hayreh SS. Optic disc edema in raised intracranial pressure. IV. Axoplasmic transport in experimental papilledema. Arch Ophthalmol. 1977; 95: 1458–1462. [DOI] [PubMed] [Google Scholar]
- 8. Rowe FJ,, Sarkies NJ. Assessment of visual function in idiopathic intracranial hypertension: a prospective study. Eye (Lond). 1998; 12 (pt 1): 111–118. [DOI] [PubMed] [Google Scholar]
- 9. Wall M,, George D. Visual loss in pseudotumor cerebri. Incidence and defects related to visual field strategy. Arch Neurol. 1987; 44: 170–175. [DOI] [PubMed] [Google Scholar]
- 10. Friedman DI,, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002; 59: 1492–1495. [DOI] [PubMed] [Google Scholar]
- 11. Moss HE,, Park JC,, McAnany JJ. The photopic negative response in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci. 2015; 56: 3709–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falsini B,, Tamburrelli C,, Porciatti V,, et al. Pattern electroretinograms and visual evoked potentials in idiopathic intracranial hypertension. Ophthalmologica. 1992; 205: 194–203. [DOI] [PubMed] [Google Scholar]
- 13. Rangaswamy NV,, Shirato S,, Kaneko M,, et al. Effects of spectral characteristics of ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci. 2007; 48: 4818–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viswanathan S,, Frishman LJ,, Robson JG,, Walters JW. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001; 42: 514–522. [PubMed] [Google Scholar]
- 15. Dacey DM,, Liao HW,, Peterson BB,, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005; 433: 749–754. [DOI] [PubMed] [Google Scholar]
- 16. Provencio I,, Rodriguez IR,, Jiang G,, et al. A novel human opsin in the inner retina. J Neurosci. 2000; 20: 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDougal DH,, Gamlin PD. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010; 50: 72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrionuevo PA,, Nicandro N,, McAnany JJ,, et al. Assessing rod, cone, and melanopsin contributions to human pupil flicker responses. Invest Ophthalmol Vis Sci. 2014; 55: 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park JC,, Moura AL,, Raza AS,, et al. Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci. 2011; 52: 6624–6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moura AL,, Nagy BV,, La Morgia C,, et al. The pupil light reflex in Leber's hereditary optic neuropathy: evidence for preservation of melanopsin-expressing retinal ganglion cells. Invest Ophthalmol Vis Sci. 2013; 54: 4471–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawasaki A,, Herbst K,, Sander B,, Milea D. Selective wavelength pupillometry in Leber hereditary optic neuropathy. Clin Experiment Ophthalmol. 2010; 38: 322–324. [DOI] [PubMed] [Google Scholar]
- 22. Kawasaki A,, Collomb S,, Leon L,, Munch M. Pupil responses derived from outer and inner retinal photoreception are normal in patients with hereditary optic neuropathy. Exp Eye Res. 2014; 120: 161–166. [DOI] [PubMed] [Google Scholar]
- 23. Gracitelli CP,, Duque-Chica GL,, Roizenblatt M,, et al. Intrinsically photosensitive retinal ganglion cell activity is associated with decreased sleep quality in patients with glaucoma. Ophthalmology. 2015; 122: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 24. Rukmini AV,, Milea D,, Baskaran M,, et al. Pupillary responses to high-irradiance blue light correlate with glaucoma severity. Ophthalmology. 2015; 122: 1777–1785. [DOI] [PubMed] [Google Scholar]
- 25. Kankipati L,, Girkin CA,, Gamlin PD. The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci. 2011; 52: 2287–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feigl B,, Mattes D,, Thomas R,, Zele AJ. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Invest Ophthalmol Vis Sci. 2011; 52: 4362–4367. [DOI] [PubMed] [Google Scholar]
- 27. Tsika C,, Crippa SV,, Kawasaki A. Differential monocular vs. binocular pupil responses from melanopsin-based photoreception in patients with anterior ischemic optic neuropathy. Sci Rep. 2015; 5: 10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Werner JS,, Keltner JL,, Zawadzki RJ,, Choi SS. Outer retinal abnormalities associated with inner retinal pathology in nonglaucomatous and glaucomatous optic neuropathies. Eye (Lond). 2011; 25: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kirkham TH,, Coupland SG. Abnormal electroretinograms and visual evoked potentials in chronic papilledema using time-difference analysis. Can J Neurol Sci. 1981; 8: 243–248. [DOI] [PubMed] [Google Scholar]
- 30. Frisen L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982; 45: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guler AD,, Ecker JL,, Lall GS,, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008; 453: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park JC,, McAnany JJ. Effect of stimulus size and luminance on the rod- cone-, and melanopsin-mediated pupillary light reflex. J Vis. 2015; 15: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferrari GL,, Marques JL,, Gandhi RA,, et al. Using dynamic pupillometry as a simple screening tool to detect autonomic neuropathy in patients with diabetes: a pilot study. Biomed Eng Online. 2010; 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith SA,, Dewhirst RR. A simple diagnostic test for pupillary abnormality in diabetic autonomic neuropathy. Diabet Med. 1986; 3: 38–41. [DOI] [PubMed] [Google Scholar]
- 35. Yri HM,, Fagerlund B,, Forchhammer HB,, Jensen RH. Cognitive function in idiopathic intracranial hypertension: a prospective case-control study. BMJ Open. 2014; 4: e004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zur D,, Naftaliev E,, Kesler A. Evidence of multidomain mild cognitive impairment in idiopathic intracranial hypertension. J Neuroophthalmol. 2015; 35: 26–30. [DOI] [PubMed] [Google Scholar]
- 37. Szabadi E. Modulation of physiological reflexes by pain: role of the locus coeruleus. Front Integr Neurosci. 2012; 6: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bose S,, Dhillon N,, Ross-Cisneros FN,, Carelli V. Relative post-mortem sparing of afferent pupil fibers in a patient with 3460 Leber's hereditary optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2005; 243: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 39. DeParis S,, Caprara C,, Grimm C. Intrinsically photosensitive retinal ganglion cells are resistant to N-methyl-D-aspartic acid excitotoxicity. Mol Vis. 2012; 18: 2814–2827. [PMC free article] [PubMed] [Google Scholar]
- 40. La Morgia C,, Ross-Cisneros FN,, Sadun AA,, et al. Melanopsin retinal ganglion cells are resistant to neurodegeneration in mitochondrial optic neuropathies. Brain. 2010; 133: 2426–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perganta G,, Barnard AR,, Katti C,, et al. Non-image-forming light driven functions are preserved in a mouse model of autosomal dominant optic atrophy. PLoS One. 2013; 8: e56350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li RS,, Chen BY,, Tay DK,, et al. Melanopsin-expressing retinal ganglion cells are more injury-resistant in a chronic ocular hypertension model. Invest Ophthalmol Vis Sci. 2006; 47: 2951–2958. [DOI] [PubMed] [Google Scholar]
- 43. Feigl B,, Zele AJ,, Fader SM,, et al. The post-illumination pupil response of melanopsin-expressing intrinsically photosensitive retinal ganglion cells in diabetes. Acta Ophthalmol. 2012; 90: e230–e234. [DOI] [PubMed] [Google Scholar]