Abstract
Glucocorticoid and epinephrine are important stress hormones secreted from the adrenal gland during critical illness. Adrenal glucocorticoid stimulates phenylethanolamine N-methyltransferase (PNMT) to convert norepinephrine to epinephrine in the adrenal medulla. Glucocorticoid is sometimes used in catecholamine-resistant septic shock in critically ill patients. By suppressing adrenal glucocorticoid production, glucocorticoid therapy might also reduce the secretion of epinephrine during stress. To investigate this, we used a mouse model subjected to glucocorticoid therapy under basal conditions (experiment 1) and during stress (experiment 2). In experiment 1, pellets containing 0% to 8% dexamethasone were implanted subcutaneously in mice for 4 weeks. In experiment 2, animals received 14 days of intraperitoneal injections of normal saline, low- or high-dose dexamethasone, followed by 2 h of restraint. We found that in experiment 1, adrenal corticosterone did not differ with dexamethasone treatment. Phenylethanolamine N-methyltransferase messenger RNA levels and adrenal catecholamines were highest in the 8% dexamethasone group. Compared with experiment 1, restrained control mice in experiment 2 had high adrenal corticosterone, which decreased with dexamethasone. Phenylethanolamine N-methyltransferase messenger RNA content doubled with restraint but decreased with dexamethasone treatment. As in experiment 1, adrenal catecholamine content increased significantly with dexamethasone treatment. We conclude that without stress, when adrenocorticotropic hormone is low, high doses of exogenous dexamethasone stimulate PNMT and catecholamine synthesis, likely independently of adrenal corticosterone concentration. After stress, adrenocorticotropic hormone levels are elevated, and exogenous dexamethasone suppresses endogenous corticosterone and PNMT production. Nonetheless, catecholamines increase, possibly due to direct neural stimulation, which may override the hormonal regulation of epinephrine synthesis during stress.
Keywords: PNMT, epinephrine, glucocorticoid, restraint stress, mouse, adrenal
INTRODUCTION
Many patients in the intensive care units worldwide die of septic shock. Because of the severe inflammation, corticosteroids have been used in the treatment of septic shock (1), especially when the blood pressure is dependent on or when the hypotension is resistant to catecholamine infusions. In sepsis, as in any other stressful situations, various neurohormonal pathways stimulate the hypothalamic-pituitary-adrenal axis to synthesize glucocorticoids—cortisol in humans and corticosterone in rodents. Corticotropin-releasing hormone is synthesized by the hypothalamus and stimulates the secretion of adrenocorticotropic hormone (ACTH) from the pituitary, which in turn stimulates the synthesis of corticosterone from the adrenal cortex. Corticosterone is carried from the cortex to the medulla by the adrenal portal system, where its concentration is much higher than that in the plasma. Epinephrine synthesis from norepinephrine is catalyzed by the enzyme phenylethanolamine N-methyltransferase (PNMT) that requires a very high intra-adrenal concentration of glucocorticoid for its activity (2). Glucocorticoids are thought to regulate PNMT at transcriptional, translational, and proteolytic levels (3).
In 1966, Wurtman (4) described a dose-response relationship between glucocorticoids and PNMT: the enzyme activity is suppressed at low glucocorticoid concentrations and is stimulated at very high concentrations of glucocorticoid in the adrenal medulla. Similarly, Wong et al. (5) in 1992 described the same U-shaped relationship between glucocorticoid and corticosterone in hypophysectomized rats injected with increasing doses of dexamethasone. Consistent with this, corticotropin-releasing hormone knockout mice, which are unable to raise adrenal or plasma corticosterone during stress, were shown to have depressed PNMT messenger RNA (mRNA) and enzymatic activity and epinephrine secretion during stress when compared with wild-type mice with normal corticosterone levels (6). Similarly, single or repeated restraint stress resulted in increased PNMT activity, which was abolished by hypophysectomy (7). Also, brief exposure to high doses of cortisol resulted in increased PNMT levels, an effect suppressed by the glucocorticoid receptor antagonist RU 38486 (8).
As a first step to understand whether the administration of exogenous glucocorticoid to critically ill patients might actually do harm by inhibiting the synthesis and secretion of adrenomedullary epinephrine, we wished to develop a mouse model to better study the dose-response relationship between glucocorticoid and PNMT, both in the basal, unstressed state and after an acute stress. We hypothesized that exogenous glucocorticoid would suppress the endogenous production of corticosterone, that adrenomedullary levels of glucocorticoid would fall, and that PNMT mRNA would be suppressed. Further, we asked whether still higher levels of exogenous glucocorticoid replacement would raise its medullary level high enough to stimulate PNMT synthesis. We used dexamethasone because we wished to create a model in mice analogous to patients who receive high pharmacological levels of steroids. In this manner, we wished to achieve an array of adrenal glucocorticoid concentrations ranging from low (due to suppression of endogenous glucocorticoid production) to high (reflecting the high level of administered steroid) to determine whether this range of exogenous glucocorticoid treatment is associated with low versus high secretion, respectively, of adrenomedullary epinephrine.
MATERIALS AND METHODS
Animals and procedures
C57B6 male littermates were maintained on 12-h light, 12-h dark cycle with lights on at 0700 h. All mice were given ad libitum access to food and drinking water and singly housed for 3 days before experimentation. We used up to four animals in each group. For experiment 1 (no stress), 3 days before the start time of the experiment, dexamethasone pellets were subcutaneously inserted under general anesthesia in the interscapular region. We chose dexamethasone because it would not interfere with the corticosterone assay. The pellets were made in molds as described by Meyer et al. (9) and Akana et al. (10) in concentrations of 0, 1%, 4%, and 8% dexamethasone (dexamethasone; Sigma, St Louis, Mo). For experiment 2 (stress), the mice received twice daily intraperitoneal injections of dexamethasone solution (dexamethasone sodium phosphate; American Pharmaceutical Partners, Inc, Schaumburg, Ill) or normal saline for control animals. The low-dose dexamethasone group received twice daily injection of 2.5 μg of dexamethasone for 2 weeks (approximately 0.2 mg/kg per day). The high-dose dexamethasone group received 2.5 μg of dexamethasone for 1 week followed by 100 μg twice daily for a week (approximately 8 mg/kg per day). We chose low-dose dexamethasone for a week followed by high-dose dexamethasone for a week rather than high-dose dexamethasone for 2 weeks because the mice would not tolerate high-dose dexamethasone for 2 weeks and would develop all the consequences of Cushing syndrome like hyperglycemia, weight gain, and hypertension. Control animals received normal saline. We decided to place pellets in the nonstress experiment so that we achieve high levels of circulating glucocorticoid without any manipulation that may stress the animals on a daily basis and because the nonstress experiment was 4 weeks long. We decided to do intraperitoneal injections in the stress experiment as extrapolated from the protocol of Wong et al. (5) to rapidly and consistently achieve high levels of circulating glucocorticoid. After the 2 weeks, the mice were subjected to restraint stress in ventilated 50-mL tubes for 2 h starting at 0800 h. The mice did not receive the glucocorticoid injection on the morning of the experiment to avoid the stress of the injection itself. The Animal Care and Use Committee of the Children’s Hospital Boston approved all animal experiments.
Sample collection
Blood sampling for corticosterone and catecholamine measurements was performed without anesthesia by retro-orbital sinus phlebotomy using heparinized capillary tubes. Majzoub and Krugger have shown that retro-orbital phlebotomy performed swiftly and with minimal handling did not result in corticosterone level elevation (catecholamines were not measured) as compared with anesthesia (unpublished data). For experiment 1, blood was collected at 1300 h at the end of the 4-week experiment. For experiment 2, blood for catecholamines was collected on day 14, 15 min after the start of the 2-h restraint period (at 0815 h). Catecholamine sampling was done early because they increase rapidly after the initiation for the stress, whereas blood was collected for corticosterone levels at the end of the 2 h of restraint (at 1000 h) because corticosterone does not rise immediately. Collected blood samples were kept on ice until plasma separation by centrifugation at 7,500 g at 4°C, and plasma was stored at −80°C until assayed. Animals were decapitated without anesthesia at the end of the experiments to collect adrenals glands for adrenal measurements of corticosterone, catecholamines, and PNMT mRNA content. Adrenal glands were immediately frozen on dry ice and stored at −80°C until homogenization or RNA extraction.
Hormone analysis
Immunoreactive corticosterone was measured by commercial RIA kit (MP Biomedicals, LLC, Orangeburg, NY) according to the manufacturer’s instructions. Adrenal content of corticosterone was measured using supernatants from adrenal glands homogenized in 100 volumes of 0.4 M perchloric acid (11). Catecholamine analysis on these same homogenates was performed using high-performance liquid chromatography with a C-18 reverse phase column in combination with an electrochemical detector (12).
Protein assay
Total protein concentration of adrenal glands was measured to normalize adrenal corticosterone and catecholamine content. Protein assay was performed based on the Lowry colorimetric assay using RC DC commercial reagents (Bio-Rad Laboratories, Inc, Hercules, Calif), with bovine serum albumin as a standard. Optical density was measured by spectrophotometry at 650 nm.
Quantitative reverse transcriptase polymerase chain reaction for adrenal PNMT mRNA
Quantitative reverse transcriptase polymerase chain reaction of adrenal PNMT mRNA was performed with total RNA purified from the adrenal glands as per manufacturers’ instructions using a commercial RNA extraction kit (Qiagen, Inc, Valencia, Calif). Reverse transcription was done using 1 μg of total RNA, with oligo-d(T)16 primer and iScript reverse transcription (Bio-Rad), yielding 20 μL of complementary DNA (cDNA). Real-time polymerase chain reaction using a Bio-Rad iCycler with Sybr Green (1× Sybr green master mix; Bio-Rad) was performed using 2 μL of cDNA. All reactions were performed in duplicates with a negative water control and a standard curve. Actin was used as the “house-keeping” gene. The primer sequences were derived from murine sequences obtained from the National Center for Biotechnology Information and were as follows: PNMT F, 5′-GTCGGGCCTTGGAAGCTGCG-3′ and PNMT R, 5′-CCAATATCAATGAGAACCCGTCCCGACACCTCA-3′; β-actin F, 5′-GGTCGTACCACAGGCATTGTGATG-3′ and β-actin R, 5′-GGAGAGCATAGCCCTCGTAGATGG-3′. Linear regression was used to quantify cDNA based on Ct values from the standard curve. PNMT mRNA was expressed as a ratio of PNMT/β-actin, corrected for the total amount of RNA per adrenal gland.
Data analysis
Comparisons among multiple groups were analyzed by one- or two-way ANOVA to assess for significant differences in treatment effect. The unpaired t test was used to compare mean differences between two different groups. A P value less than 0.05 was considered statistically significant. All data are presented as the mean ± SEM.
RESULTS
Experiment 1: adrenomedullary function under basal conditions
Irrespective of the level of exogenous dexamethasone supplementation, the concentration of intra-adrenal corticosterone measured in an unstressed state was very low and statistically invariant (Fig. 1A).
Fig. 1. Adrenal corticosterone and PNMT mRNA in unstressed mice receiving increasing concentrations of subcutaneous dexamethasone (Dex) pellets.
A, Adrenal corticosterone levels. There was no statistical difference between the adrenal corticosterone levels with increasing dose of pellet Dex (n = 2–4 animals/group). B, Adrenal PNMT/actin mRNA ratio normalized for total RNA content. There are significantly higher levels of PNMT mRNA with increasing dose of Dex supplementation compared with placebo (P = 0.02 by ANOVA for Dex dose, n = 2–4 animals/group).
We next asked whether increasing the amount of exogenous glucocorticoid supplementation affected the concentration of adrenomedullary PNMT mRNA (Fig. 1B). Because we had found no suppression of adrenal corticosterone with increasing dexamethasone, we did not expect to observe a fall in PNMT mRNA under these conditions. Indeed, PNMT mRNA levels were not suppressed and were significantly higher with exogenous dexamethasone supplementation (P = 0.02), potentially suggesting that these amounts of dexamethasone supplementation resulted in adrenal drug levels high enough to stimulate PNMT gene expression. This is consistent with the observed rise in intra-adrenal epinephrine (P = 0.003 in the 8% dexamethasone group; Fig. 2B), although the simultaneous rise in adrenal norepinephrine (P = 0.02; Fig. 2A) was not anticipated. The plasma levels of both catecholamines were high and not different among the four treatment groups, suggesting that the stressful manipulation of these animals during retro-orbital phlebotomy caused the release of preformed hormone that did not reflect recent intra-adrenal biosynthetic activity (Fig. 2, C and D).
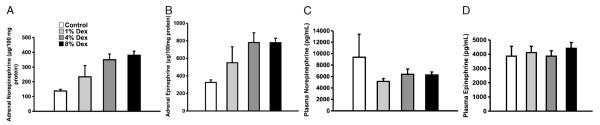
Fig. 2. Adrenal and plasma catecholamines in unstressed mice receiving increasing concentrations of subcutaneous Dex.
A, Adrenal norepinephrine level normalized to protein content. There was a statistically significant increase in adrenal norepinephrine levels with increasing dose of exogenous Dex (P = 0.02 by ANOVA for Dex dose, n = 3–4 animals/group). B, Adrenal epinephrine levels among the different groups. There was a tendency toward a rise in adrenal epinephrine concentration with increasing dose of exogenous Dex (n = 3–4 animals/group). C, Plasma concentration of norepinephrine among the different groups. There was no significant difference between the plasma levels of norepinephrine with increasing dose of exogenous Dex (n = 3–4 animals/group). D, Plasma concentration of epinephrine. There was no significant difference between the plasma epinephrine concentrations of the different groups with increasing dose of exogenous Dex (n = 3–4 animals/group).
Experiment 2: adrenomedullary function during stress
In this experiment, we asked whether a superimposed stressor could alter the effect of glucocorticoid on adrenomedullary PNMT expression and catecholamine synthesis. Under these conditions, a stressor-induced increase in ACTH would be expected to stimulate an increase in intra-adrenal corticosterone, which could be inhibited by exogenous glucocorticoid. Exogenous dexamethasone was given as an intermittent, twice daily injection in a low (0.2 mg/kg per day, approximating levels used in patients in the intensive care unit) or high (8 mg/kg per day) dose. With restraint stress, the adrenal corticosterone concentrations in the control group rose approximately 25-fold (Fig. 3A) compared with the nonstress levels (Fig. 1A). Low-dose dexamethasone treatment caused a 50% reduction in intra-adrenal corticosterone, and high-dose treatment suppressed endogenous adrenal corticosterone content down to a basal, unstressed concentration of 2.8 ± 0.5-μg/100-mg protein. It is not surprising that dexamethasone would have little effect on intra-adrenal corticosterone when ACTH is low (experiment 1), unlike its effect during restraint stress (Fig. 3A).
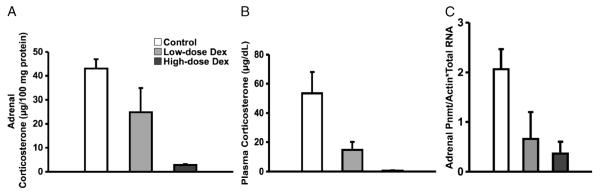
Fig. 3. Adrenal and plasma corticosterone and adrenal PNMT mRNA in restraint-stressed mice receiving increasing concentrations of intraperitoneal Dex.
A, Adrenal concentration of corticosterone. There was a statistically significant decrease in adrenal corticosterone levels with increasing exogenous dose of Dex (P = 0.04 by ANOVA for Dex dose, n = 2–4 animals/group). B, Plasma corticosterone levels in the three stressed groups. There was a statistically significant decrease in plasma corticosterone levels with increasing dose of exogenous Dex (P = 0.04 by ANOVA for Dex dose, n = 2–4 animals/group). C, Adrenal PNMT/actin mRNA ratio normalized for total RNA content. There was a tendency for decreasing levels of PNMT mRNA levels with increasing Dex dose (n = 2–3 animals/group).
After restraint stress, plasma corticosterone increased more than 25-fold (Fig. 2B) compared with the basal, unstressed state. A progressive increase in the dexamethasone dose was associated with a downward trend in plasma corticosterone in parallel with the effect of dexamethasone on adrenal corticosterone levels (Fig. 3A). With stress, the PNMT mRNA levels (Fig. 3C) were almost twice those measured under basal conditions (Fig. 1B) and tended to decrease with low-dose exogenous dexamethasone therapy. There was a significant decrease in PNMT mRNA content with the 8% exogenous dexamethasone dose (P < 0.05), consistent with the low adrenal corticosterone levels noted earlier (Fig. 3A).
Adrenal levels of catecholamines were not elevated in untreated, restraint-stressed mice (Fig. 4, A and B) compared with those under basal conditions (Fig. 2, A and B). They were, however, significantly more elevated in both dexamethasone treatment groups (Fig. 4, A and B), suggesting that the combination of the effect of stress plus exogenous dexamethasone in these groups caused increased synthesis of both norepinephrine and epinephrine despite a simultaneous fall in PNMT mRNA (Fig. 3C). The plasma catecholamine levels tended to increase with stress (Fig. 4, C and D) compared with during basal conditions (Fig. 2, C and D), and plasma epinephrine increased significantly with low-dose dexamethasone compared with basal, stressed, untreated levels (Fig. 4D). Both catecholamines had a tendency to fall with high-dose exogenous dexamethasone treatment.
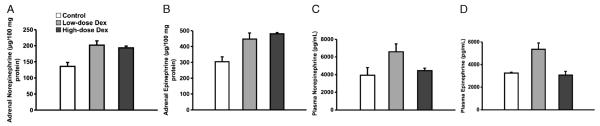
Fig. 4. Adrenal and plasma catecholamines in restraint-stressed mice receiving increasing concentrations of intraperitoneal Dex.
A, Adrenal levels of norepinephrine among the three stressed groups. There was a statistically significant increase of the adrenal norepinephrine levels with exogenous Dex administration (P = 0.012 by ANOVA for Dex dose, n = 2–4 animals/group). B, Adrenal levels of epinephrine in the three stressed groups. There was a statistically significant increase of the adrenal epinephrine levels with exogenous dexamethasone administration (P = 0.02 by ANOVA for Dex dose, n = 2–4 animals/group). C, Plasma norepinephrine levels in the three stressed groups. There was no significant difference in plasma levels of norepinephrine between the control and Dex groups (n = 2–4 animals/group). D, Plasma epinephrine levels in the three stressed groups. There is a significant increase in plasma epinephrine level between the sham and the low-dose Dex group (P < 0.05). Although there is a tendency for plasma epinephrine to fall with high-dose Dex, this was not significant (n = 2–4 animals/group).
DISCUSSION
PNMT gene expression requires an intact adrenocortical function (7, 13, 14). Glucocorticoids are thought to be important for the function of the adrenomedullary catecholaminergic system by regulating the synthesis and the activity of PNMT, the enzyme that catalyzes the synthesis of epinephrine from norepinephrine in the adrenal medulla (15). Because exogenous glucocorticoid supplementation suppresses endogenous glucocorticoid synthesis by negative feedback at the level of the hypothalamus and pituitary and others had shown that this inhibits the synthesis and activity of PNMT in vitro (16) and in vivo (17), we asked whether exogenous glucocorticoid could also inhibit the rise in epinephrine synthesis in the setting of stress. If this were also true in humans, it would raise the possibility that exogenous glucocorticoid treatment of critically ill patients might compromise their ability to respond to stress with adequate secretion of epinephrine. Although there is evidence that glucocorticoids increase the vascular response to catecholamines (18), there are no studies that examined the effect of glucocorticoid on catecholamine output, and there is no clinical data in humans supporting this point.
The results of the present study show that in the basal, unstressed state, exogenous glucocorticoids supplementation of mice does not suppress adrenal corticosterone levels, which are already low, and therefore has no major effect on epinephrine synthesis. In the basal, unstressed state, adrenal glucocorticoid should be low regardless of the amount of exogenous glucocorticoid. However, at very high doses of dexamethasone supplementation, the adrenal drug levels could be high enough to stimulate a rise in PNMT mRNA.
Plasma and adrenal corticosterone levels were very high during stress and fell markedly with exogenous glucocorticoid supplementation. We observed that the adrenal levels of epinephrine were significantly higher in the dexamethasone-suppressed animals in the setting of stress, despite the fact that PNMT mRNA content was lower. This suggests that PNMT activity during stress is regulated not only by glucocorticoid but also by stress-related inputs to the adrenal medulla, possibly of a neural nature. On the other hand, glucocorticoids are known to regulate PNMT enzyme activity independently of mRNA levels (2), suggesting that they might stimulate PNMT activity in the absence of a rise in PNMT mRNA content. We agree with Margolis et al. (19) that PNMT is not the sole determinant of epinephrine accumulation and secretion, especially during stress. This is supported by our finding that adrenal norepinephrine was also elevated during stress, suggesting that a PNMT-independent mechanism of catecholamine synthesis was operating during restraint stress. Although we observed suppression of PNMT mRNA at even the highest dose of dexamethasone used in experiment 2 (8 mg/kg per day, equivalent to 240 mg in a 30-kg child), it is possible that an even higher dose would have raised the concentration of adrenal dexamethasone enough to stimulate the enzyme. In experiment 1, we observed stimulation of adrenal PNMT mRNA with the 8% dexamethasone pellet (8% of a 100-mg pellet = 8 mg of dexamethasone in a mouse). This is equivalent to using 9.6 g of dexamethasone in a 30-kg child. It would be unlikely to ever achieve these high levels of dexamethasone treatment in human patients. We measured plasma catecholamine levels, but they did not correlate with adrenal catecholamine content or vary in a manner consistent with the physiological state of the mice. Perhaps the stress of blood collection (by the retro-orbital route) determined the level of secretion rather than the glucocorticoid treatment or presence or absence of restraint stress. General anesthesia may have avoided this problem (20) but would have precluded studying a psychological stressor like restraint.
In conclusion, in the absence of stress, extremely high doses of exogenous glucocorticoids, far beyond their therapeutic range, stimulate PNMT mRNA and adrenal levels of catecholamines in mice. A lower dose of glucocorticoid in the absence of stress would likely have had no suppressive effect because adrenal corticosterone would be low. With stress alone, adrenal corticosterone and PNMT mRNA rise compared with the unstressed state and fall during treatment with increasing amounts of dexamethasone supplementation. Despite this, adrenal catecholamine levels rise, possibly a direct consequence of the stress. If these findings are also true in humans, then during the stress of critical illness, treatment with even very high therapeutic amounts of glucocorticoid is unlikely to inhibit or to stimulate catecholamine synthesis and release.
ACKNOWLEDGMENTS
The authors thank Masato Asai, MD, PhD, for the helpful discussions regarding quantitative reverse transcriptase polymerase chain reaction and the generation of the cDNA plasmids and Edwin Lai for the catecholamine measurements. This work was funded in part by the Timothy Murphy Fund.
REFERENCES
- 1.Annane D, Cavaillon JM. Corticosteroids in sepsis: from bench to bedside? Shock. 2003;20:197–207. doi: 10.1097/01.shk.0000079423.72656.2f. [DOI] [PubMed] [Google Scholar]
- 2.Wurtman RJ, Axelrod J. Adrenaline synthesis: control by the pituitary gland and adrenal glucocorticoids. Science. 1965;150:1464–1465. doi: 10.1126/science.150.3702.1464. [DOI] [PubMed] [Google Scholar]
- 3.Wong DL, Siddall B, Wang W. Hormonal control of rat adrenal phenylethanolamine N-methyltransferase. Enzyme activity, the final critical pathway. Neuropsychopharmacology. 1995;13:223–234. doi: 10.1016/0893-133X(95)00066-M. [DOI] [PubMed] [Google Scholar]
- 4.Wurtman RJ. Control of epinephrine synthesis in the adrenal medulla by the adrenal cortex: hormonal specificity and dose-response characteristics. Endocrinology. 1966;79:608–614. doi: 10.1210/endo-79-3-608. [DOI] [PubMed] [Google Scholar]
- 5.Wong DL, Lesage A, Siddall B, Funder JW. Glucocorticoid regulation of phenylethanolamine N-methyltransferase in vivo. FASEB J. 1992;6:3310–3315. doi: 10.1096/fasebj.6.14.1426768. [DOI] [PubMed] [Google Scholar]
- 6.Jeong KH, Jacobson L, Pacak K, Widmaier EP, Goldstein DS, Majzoub JA. Impaired basal and restraint-induced epinephrine secretion in corticotropin-releasing hormone-deficient mice. Endocrinology. 2000;141:1142–1150. doi: 10.1210/endo.141.3.7370. [DOI] [PubMed] [Google Scholar]
- 7.Viskupic E, Kvetnansky R, Sabban EL, Fukuhara K, Weise VK, Kopin IJ, Schwartz JP. Increase in rat adrenal phenylethanolamine N-methyltransferase mRNA level caused by immobilization stress depends on intact pituitary-adrenocortical axis. J Neurochem. 1994;63:808–814. doi: 10.1046/j.1471-4159.1994.63030808.x. [DOI] [PubMed] [Google Scholar]
- 8.Betito K, Diorio J, Meaney MJ, Boksa P. Adrenal phenylethanolamine N-methyltransferase induction in relation to glucocorticoid receptor dynamics: evidence that acute exposure to high cortisol levels is sufficient to induce the enzyme. J Neurochem. 1992;58:1853–1862. doi: 10.1111/j.1471-4159.1992.tb10062.x. [DOI] [PubMed] [Google Scholar]
- 9.Meyer JS, Micco DJ, Stephenson BS, Krey LC, McEwen BS. Subcutaneous implantation method for chronic glucocorticoid replacement therapy. Physiol Behav. 1979;22:867–870. doi: 10.1016/0031-9384(79)90330-5. [DOI] [PubMed] [Google Scholar]
- 10.Akana SF, Cascio CS, Shinsako J, Dallman MF. Corticosterone: narrow range required for normal body and thymus weight and ACTH. Am J Physiol. 1985;249:R527–R532. doi: 10.1152/ajpregu.1985.249.5.R527. [DOI] [PubMed] [Google Scholar]
- 11.Berger GM, Jones JD. Perchloric acid extraction of adrenocorticosteroids from tissues. Steroids. 1971;18:473–476. doi: 10.1016/0039-128x(71)90060-2. [DOI] [PubMed] [Google Scholar]
- 12.Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: a test of Selye’s doctrine of nonspecificity. Am J Physiol. 1998;275:R1247–1255. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- 13.Kvetnansky R, Pacak K, Fukuhara K, Viskupic E, Hiremagalur B, Nankova B, Goldstein DS, Sabban EL, Kopin IJ. Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenocortical system. Ann N Y Acad Sci. 1995;771:131–158. doi: 10.1111/j.1749-6632.1995.tb44676.x. [DOI] [PubMed] [Google Scholar]
- 14.Jiang W, Uht R, Bohn MC. Regulation of phenylethanolamine N-methyltransferase (PNMT) mRNA in the rat adrenal medulla by corticosterone. Int J Dev Neurosci. 1989;7:513–520. doi: 10.1016/0736-5748(89)90010-5. [DOI] [PubMed] [Google Scholar]
- 15.Axelrod J. Purification and properties of phenylethanolamine-N-methyl transferase. J Biol Chem. 1962;237:1657–1660. [PubMed] [Google Scholar]
- 16.Kelner KL, Pollard HB. Glucocorticoid receptors and regulation of phenylethanolamine-N-methyltransferase activity in cultured chromaffin cells. J Neurosci. 1985;5:2161–2168. doi: 10.1523/JNEUROSCI.05-08-02161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wurtman RJ, Noble EP, Axelrod J. Inhibition of enzymatic synthesis of epinephrine by low doses of glucocorticoids. Endocrinology. 1967;8:825–828. doi: 10.1210/endo-80-5-825. [DOI] [PubMed] [Google Scholar]
- 18.Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol. 2004;2:1–12. doi: 10.2174/1570161043476483. [DOI] [PubMed] [Google Scholar]
- 19.Margolis FL, Roffi J, Jost A. Norepinephrine methylation in fetal rat adrenals. Science. 1966;154:275–276. doi: 10.1126/science.154.3746.275. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi K, Ota A, Togari A, Morita S, Mizuguchi T, Sawada H, Yamada K, Nagatsu I, Matsumoto S, Fujita K, et al. Alteration of catecholamine phenotype in transgenic mice influences expression of adrenergic receptor subtypes. J Neurochem. 1995;65:492–501. doi: 10.1046/j.1471-4159.1995.65020492.x. [DOI] [PubMed] [Google Scholar]