Abstract
The global expansion of Aedes albopictus together with the absence of vaccines for most of the arboviruses transmitted by this mosquito has stimulated the development of sterile-male strategies aiming at controlling disease transmission through the suppression of natural vector populations. In this context, two environmentally friendly control strategies, namely the Sterile Insect Technique (SIT) and the Wolbachia-based Incompatible Insect Technique (IIT) are currently being developed in several laboratories worldwide. So far however, there is a lack of comparative assessment of these strategies under the same controlled conditions. Here, we compared the mating capacities, i.e. insemination capacity, sterilization capacity and mating competitiveness of irradiated (35 Gy) and incompatible Ae. albopictus males at different ages and ratios under laboratory controlled conditions. Our data show that there was no significant difference in insemination capacity of irradiated and incompatible males, both male types showing lower capacities than untreated males at 1 day but recovering full capacity within 5 days following emergence. Regarding mating competitiveness trials, a global observed trend is that incompatible males tend to induce a lower hatching rate than irradiated males in cage controlled confrontations. More specifically, incompatible males were found more competitive than irradiated males in 5:1 ratio regardless of age, while irradiated males were only found more competitive than incompatible males in the 1:1 ratio at 10 days old. Overall, under the tested conditions, IIT seemed to be slightly more effective than SIT. However, considering that a single strategy will likely not be adapted to all environments, our data stimulates the need for comparative assessments of distinct strategies in up-scaled conditions in order to identify the most suitable and safe sterilizing technology to be implemented in a specific environmental setting and to identify the parameters requiring fine tuning in order to reach optimal release conditions.
Introduction
The Asian tiger mosquito Aedes albopictus has emerged as a mosquito species of major medical concern following its global expansion over the past 30–40 years [1–3] and its recent involvement in several arboviral epidemic outbreaks. Aedes albopictus is a proven competent vector, in laboratory tests, for over 20 arboviruses including Dengue and Chikungunya viruses [4] and has been shown to be the main vector in a Chikungunya epidemic that hit La Réunion Island in 2005–2006 [5] and Italy in 2007 [6]. This aggressive day-biting mosquito has spread worldwide from its native range in South-East Asia [7, 8] probably mainly disseminating through the international trade of used tires [9]. Its ecological plasticity in different traits including egg diapause, the ability to use natural or urban larval breeding sites [5] and an opportunistic feeding behavior [10, 11] may have favored dispersal and adaptation to newly colonized environments with widely distinct climatic conditions ranging from tropical to temperate [12]. Given the absence of effective vaccines against most of these arboviruses, prevention of epidemics primarily relies on vector control measures. Considerable efforts have been made in order to control wild mosquito populations notably through the use of insecticides. However, their use is increasingly impaired by negative effects on non-targeted organisms and on the environment together with the rapid selection of resistance in insect natural populations [13–15], therefore stimulating the development of innovative control methods.
Among these methods, sterile-male systems aiming at suppressing pest populations using modified males able to introduce sterility in target populations are of particular interest as they are species-specific and environmentally friendly [16–18]. Mating of released sterile males with native wild females may lead to a decrease in the females’ reproductive potential and ultimately to the local elimination or suppression of the pest population if inundative numbers of males are released over a sufficient period of time. The Sterile Insect Technique (SIT), using ionizing radiation to induce random chromosomal rearrangements thus conferring males’ sterility, was the first developed sterile-male system [19]. Several SIT programs have been ever since successfully implemented against a number of agricultural insect pests including the New World screwworm fly or fruit flies, as well as insects of medical importance such as tsetse flies [20, 21] and mosquitoes [22–25]. Several phase 1 studies have demonstrated the potential of SIT in the control of mosquito populations (see [26, 27] for reviews) and field releases of gamma-irradiated Ae. albopictus males undertaken from 2005 to 2009 in three small Italian towns have shown significant induced sterility in the local mosquito populations [22]. Different techniques have also been developed providing alternative methods to irradiation-based sterilization. These include transgenesis such as the dominant lethal genetic system (RIDL) [28–32] or the use of the endosymbiotic Wolbachia [33–38].
Wolbachia are maternally inherited Alphaproteobacteria commonly found in arthropods [39], notably in mosquito species of medical importance such as the common house mosquito Culex pipiens [40–42] and Ae. albopictus [36]. In addition, Wolbachia infections can be achieved artificially through microinjections as performed in several mosquito species including Ae. albopictus [43–45], Aedes aegypti [46, 47], Anopheles stephensi [48] and Culex tarsalis [49]. In mosquitoes, Wolbachia induce a form of embryonic death called cytoplasmic incompatibility (CI) [50] resulting from sperm-egg incompatibility occurring when Wolbachia-infected males mate with uninfected females or females infected with an incompatible Wolbachia strain. CI can be either bidirectional when the death of embryos is observed in both reciprocal crosses, or unidirectional when one cross is incompatible while the reciprocal cross is viable. CI can thus be exploited as a source of sterility through a strategy called the Incompatible Insect Technique (IIT) [51], which was first deployed in 1967 in a promising pilot trial carried out in Burma against the filariasis vector Culex quinquefasciatus [52]. More recently, encouraging results were also reported under laboratory [33, 35], semi-field [34, 36, 38] and field conditions [37].
In the present study, we compared mating capacities of irradiated (SIT) and CI-inducing (IIT) Ae. albopictus males in the presence of males and females from La Réunion, a remote oceanic island lying 700 km East of Madagascar that has experienced a major Chikungunya epidemics in 2005–2006 [53]. The magnitude of the epidemics, which infected a third of the Island population, led health authorities to dramatically strengthen vector control measures mainly through the use of larvicides (temephos, then Bacillus thuringiensis var israelensis or Bti) and adulticides (fenitrothion, then deltamethrine). Following a restriction on organophosphates, the vector control unit was left with pyrethroids and Bti as the only available insecticides. This challenging situation has stimulated research programs aiming at assessing the feasibility of environmental-friendly strategies such as SIT, developed in an area-wide integrated pest management (AW-IPM) program targeting Ae. albopictus [25, 54, 55], and IIT [35, 38]. As both SIT and IIT are currently being developed in La Réunion, we took advantage of this unique opportunity to compare the competitiveness of irradiated and incompatible Ae. albopictus males with the goal of pinpointing the potential strength and drawbacks of each strategy.
One of the key parameters needed for the evaluation of a sterile-male system is the actual mating competitiveness of released males since these must compete with wild males in seeking and inseminating wild females. Male mating competitiveness is dependent on several parameters such as survival rate, mating capacity and sterilizing properties of the inseminated sperm, all of which can be affected by irradiation treatment or by Wolbachia infections. Irradiation was shown to affect competitiveness of Ae. albopictus males when performed at a dose inducing nearly full sterility (40 Gy) [56, 57], while the artificial infection with wPip Wolbachia was not found to decrease male competitiveness even if inducing full sterility [36]. Here, we examined the (i) insemination capacity, (ii) sterilizing capacity and (iii) mating competitiveness of irradiated and incompatible Ae. albopictus males under laboratory-controlled conditions. Results presented herein provide important insights on the relative effectiveness of SIT and IIT for the control of Ae. albopictus natural populations, and potential drawbacks and associated improvements of each technique are discussed.
Materials and Methods
Mosquito collections
Two Ae. albopictus lines were used in the experiments: the wild type LR line naturally co-infected with two Wolbachia strains (wAlbA and wAlbB) and established from approximately 1000 eggs sampled in three localities of La Réunion Island in 2012 (Saint Denis, Sainte Suzanne and Saint Benoît); and the ARwPIT line previously constructed using embryonic microinjections of eggs’ cytoplasm (including Wolbachia wPip infections) from Cx. pipiens [43]. As the ARwPIT line was generated with Ae. albopictus mosquitoes from Italy and in order to limit the influence of the nuclear genome of Italian origin, the cytoplasm (including wPip infections) of the ARwPIT line was introduced into the nuclear background of the LR line through 4 consecutive backcrosses (100 virgin ARwPIT females crossed with 100 LR 2 weeks aged males) expected to restore ~ 90% of LR nuclear genes. In fact, ARwPIT females have been reported to be partially compatible (about 20% fertile) when mated with wild-type males aged more than two weeks, due to a mean reduction in wAlbA Wolbachia titer [58]. Thus, the ARwPLR line carrying most of the LR nuclear genome together with a wPip infection was obtained and further used in the experiments. Wolbachia infections were controlled in the LR and ARwPLR lines through a PCR genotyping procedure using the ankyrin domains ank2 gene, which is specific to wPip infections [42, 59]. Therefore, no PCR amplification was observed in samples from the LR line whilst a PCR amplified fragment of 511 bp was observed using samples from the ARwPLR line.
Mosquitoes were reared in the laboratory under standard conditions at 27 ± 2°C and 75 ± 2% relative humidity (RH) and an LD 12:12 h photoperiod. Larvae were fed ad libitum with a mixture of rabbit and fish-food. Adults were maintained in cages (30×30×30 cm) and fed ad libitum with 10% sucrose solution. Females were fed on sheep blood and collected eggs were stored at room temperature. Experiments were performed with G30 and G4 generations for LR and ARwPLR lines, respectively.
Ethics statement
No specific permissions are required for the field activities as they do not involve endangered or protected species and field sites where eggs were sampled are not privately-owned. In addition, blood meals were carried out using artificial systems containing sheep blood that was purchased from a qualified supplier (Prolab, Saint-Pierre, Réunion Island), thus avoiding the need for any vertebrate as blood-feeding source.
Irradiation procedure
Batches of 20 to 24 hours old male pupae from the LR line were maintained in 4 cm wide cups filled with dechlorinated water and further exposed to gamma rays emitted by a cesium-137 irradiator (IBL 437, Cis Bio International, Germany). The chosen irradiation dose was 35 Gray (Gy) delivered at 2.35 Gy/min, consistent with previous investigations [24, 25]. This sterilizing condition doesn’t induce full sterility but was shown to have limited adverse effects on Ae. albopictus males competitiveness [24, 25]. Hence, all data presented herein were produced using specimens from the LR line irradiated with 35 Gy and thereafter named LRi.
Insemination and sterilizing capacities
Following emergence of adults, different batches of twenty virgin LR females aged 3–5 days were mixed with 20 LR, LRi or ARwPLR males in 30×30×30 cm cages. Tests were performed using either 1, 5 or 10 day-old virgin males, with the day of emergence being considered as day 0. Three replicates were performed for each type of cross thus requiring a total of 27 cages: 9 cages with 1 day-old males, 9 cages with 5 day-old males and 9 cages with 10 day-old males. Males and females were allowed to mate for 24 h. Males were then removed from cages using a mouth aspirator and wing size was measured. Females were blood fed two days after the removal of males and laid eggs were collected, counted, dried for four days under laboratory conditions and then allowed to hatch. A second blood meal was given to females 10 days following the first one and eggs were subsequently collected and treated under the same conditions as for the first batch.
Male size was assessed using the left wing of individuals dissected under a binocular microscope (Leica MZ 6). The wing length was measured by considering the distance from the distal edge of the alula to the end of the radius vein (excluding fringe scales). A digital image of the wing was captured using a ScopeTek DCM310 camera mounted on the binocular microscope and the measures were performed using a custom-written MATLAB-based user interface. Scales were determined capturing an image of a micrometer.
To assess male insemination capacity, surviving females were collected after oviposition and dissected on a slide under a binocular microscope (Leica MZ 6) with 80X magnification. Spermathecal capsules were isolated, torn gently with micropins and checked for the presence of spermatozoa. Two parameters were then recorded: (a) the number of inseminated females (i.e. found with at least one filled spermathecal capsule), and (b) the number of females with 0, 1, 2 or 3 filled spermathecal capsules amongst inseminated females.
Sterilizing capacity of males was examined by comparing egg-hatching rates in sterile crosses (i.e. between ♂LRi×♀LR or ♂ARwPLR×♀LR) to that of the fertile control cross (i.e. between ♂LR×♀LR).
Mating competitiveness
Confrontations were carried out by mixing 20 virgin LR females with different ratios of sterile to LR males’ in cages of 30×30×30 cm. Two ratios were tested: 1:1 (20♂LRi+20♂LR or 20♂ARwPLR+20♂LR) and 5:1 (100♂LRi+20♂LR or 100♂ARwPLR+20♂LR). All females were 3 to 5 day-old, whilst virgin males of 1, 5 and 10 day-old were used in the different confrontations. Three replicates were performed for each type of confrontation, thus requiring a total of 36 cages: 18 trials for the 1:1 ratio (6 with 1 day-old males, 6 with 5 day-old males and 6 with 10 day-old males) and 18 trials for the 5:1 ratio (6 with 1 day-old males, 6 with 5 day-old males and 6 with 10 day-old males). Males were first released in cages followed by females and mosquitoes were allowed to mate for 24 h. Thereafter, males were removed from cages using a mouth aspirator and females were blood fed in the laboratory two days after the removal of males. Eggs were collected, counted, dried for 4 days under laboratory conditions and then allowed to hatch in order to measure hatching rates. A second blood meal was given to females 10 days after the first one to enable further oviposition. This second batch of eggs was treated in the same way as the first one.
Data analysis
Male wing size was analyzed using the GLM Size = Type + ε, where Size is the quantitative response variable and Type a three-level factor corresponding to LR, LRi and ARwPLR males. ε represents the error term, following a binomial distribution. Normality of the model residuals was tested using a Shapiro-Wilk test (Shapiro).
Insemination capacity was analyzed using the GLM Fi = Type + Age + Type:Age + ε, where Fi, the response variable, corresponds to the proportion of inseminated females, Type a three-level factor corresponding to LR, LRi and ARwPLR males, and Age a three-level factor corresponding to the number of days between emergence and the assay (1, 5 or 10 days). ε represents the error term, following a binomial distribution. The ":" represents the interaction between the two factors. The distributions of the number of filled spermathecae (among inseminated females) were compared between the different types of males at each age using Fisher exact test.
Hatching rate (egg fertility) was calculated for each individual cage by dividing the number of hatched eggs by the total number of laid eggs. It was analyzed using the GLM Hr = Type + Age + Type:Age + ε, where Hr, the response variable, corresponds to the proportion of hatching eggs, Type a three-level factor corresponding to LR, LRi and ARwPLR males, Age a three-level factor corresponding to the number of days between emergence and the assay (1, 5 or 10 days). ε represents the error term, following a binomial distribution. The ":" represents the interaction between the two factors.
The competitive index, ‘C’, defined by Fried [60] was calculated for each type of confrontation using hatch rates from the fertile control (Hn, i.e. between ♂LR×♀LR), sterile control (Hs, i.e. between ♂LRi×♀LR or ♂ARwPLR×♀LR) and the confrontation cages (Ho, i.e. ♂LRi×♂LR×♀LR or ♂ARwPLR×♂LR×♀LR) as follows: C = N/S × Hn-Ho/Ho-Hs, where N is the number of wild type LR males and S is the number of sterile males (irradiated LRi males or incompatible ARwPLR males). Values around 1 indicate equivalent mating performance between sterile and wild type males. To evaluate the effects of sterile male releases on the cage confrontations’ resulting fertility, the induced sterility (IS) was calculated as 100% minus the residual fertility value, which was obtained by dividing the observed hatch rate (Ho) by the control hatch rate (Hn).
All computations were performed using the R free software (v.3.1.1, http://www.r-project.org). GLM were simplified as follows: significance of the different terms was tested starting from the higher-order terms using likelihood ratio test (LRT). Non-significant terms (P> 0.05) were removed [61]. Factor levels of qualitative variables that were not significantly different were grouped (LRT, [61]).
Results
Irradiated and incompatible males have lower insemination capacities at emergence
The insemination rates of wild type LR females caged for 24 h with wild type LR males, irradiated LRi males or incompatible ARwPLR males were examined by assessing the percentage of inseminated females and the percentage of females with 0, 1, 2 or 3 filled spermathecal capsules.
There was no interaction effect between males’ age and type on the percentage of inseminated females (Male:Type, LRT, X = 6.3, Δdf = 4, P = 0.35). However, LR males were more successful in inseminating females (LRT, X = 21.1, Δdf = 2, P< 0.001, no significant difference between LRi and ARwPLR males, LRT, X = 2.02, Δdf = 1, P = 0.15), but only at day 1 (LRT, X = 117.7, Δdf = 2, P< 0.001, no significant difference between day 5 and 10, LRT, X = 0.2, Δdf = 1, P = 0.64; Table 1). We then analyzed the number of filled spermathecae in inseminated females at day 1. It appeared that ARwPLR males filled significantly less spermathecae that the other males (Fisher exact test, P< 0.001; Table 1), since they mostly filled only one spermathecae while LR and LRi males usually filled two (no significant difference between LR and LRi distributions, Fisher exact test, P = 0.44).
Table 1. Insemination capacity of irradiated and incompatible males caged with females for 24 h.
| Male | Age at release | Number of females | Percentage of inseminated females (N) | Percentage of filled spermathecae (N) | |||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| LR | 1 day-old | 57 | 74% (42) | 26% (15) | 12% (7) | 58% (33) | 4% (2) |
| LRi | 1 day-old | 54 | 46% (25) | 54% (29) | 4% (2) | 43% (23) | 0% (0) |
| ARwPLR | 1 day-old | 56 | 34% (19) | 66% (36) | 21% (12) | 13% (7) | 0% (0) |
| LR | 5 day-old | 47 | 100% (47) | 0% (0) | 0% (0) | 89% (42) | 11% (5) |
| LRi | 5 day-old | 57 | 91% (52) | 9% (5) | 2% (1) | 86% (49) | 4% (2) |
| ARwPLR | 5 day-old | 54 | 91% (49) | 9% (5) | 9% (5) | 80% (43) | 2% (1) |
| LR | 10 day-old | 56 | 93% (52) | 7% (4) | 0% (0) | 88% (49) | 5% (3) |
| LRi | 10 day-old | 56 | 95% (53) | 5% (3) | 4% (2) | 89% (50) | 2% (1) |
| ARwPLR | 10 day-old | 52 | 90% (47) | 10% (5) | 6% (3) | 83% (43) | 2% (1) |
Three types of males (i.e. LR = wild type, LRi = wild type irradiated and ARwPLR = incompatible) aged of 1, 5 or 10 days were compared.
Incompatible and irradiated males display distinct sterilizing capacities over their lifespan
We measured the strength of induced sterility through crosses involving LR females and LRi or ARwPLR males. The fertility of LR females in control crosses with LR males of 1, 5 and 10 day-old was 77.0%, 56.1% and 83.3%, respectively (Table 2). Irradiated males showed a residual fertility when crossed with LR females; the hatching rates were 6.1%, 4.6% and 8.1% with males aged of 1, 5 and 10 days, respectively (Table 2; significant age effect: LRT, X = 30.8, Δdf = 2, P< 0.001). These hatching rates were significantly higher than those measured in crosses with ARwPLR males (LRT, X = 665.4, Δdf = 1, P< 0.001) where total sterility (i.e. hatching rate = 0%) was indeed recorded regardless males’ age (Table 2). In addition, there was no significant variation in the hatching rates between the first and second batch of eggs (i.e after a second blood meal; LRT, X = 1.9, Δdf = 1, P = 0.17).
Table 2. Sterilizing capacity of irradiated and incompatible males.
| Crosses | 1 day-old | 5 day-old | 10 day-old | ||||
|---|---|---|---|---|---|---|---|
| (males × females) | N. eggs | Hatching rate (95% CI) | N. eggs | Hatching rate (95% CI) | N. eggs | Hatching rate 95% CI | |
| Sterile | LRi × LR | 1772 | 0.061 (0.051–0.073) | 2600 | 0.046 (0.038–0.055) | 3243 | 0.081 (0.072–0.091) |
| ARwPLR × LR | 1271 | 0 (-) | 3799 | 0 (-) | 2693 | 0 (-) | |
| Fertile | LR × LR | 2365 | 0.770 (0.752–0.787) | 2972 | 0.561 (0.543–0.579) | 2606 | 0.833 (0.818–0.847) |
Males (N = 20) were allowed to mate with females (N = 20) for 24 h. Three replications were performed for each type of cross and two series of eggs were collected. LR = wild type, LRi = wild type irradiated, ARwPLR = incompatible. The hatching rates are indicated with their 95% confidence intervals (95% CI). Note that the two batches were pooled as there was no significant difference (see text).
Variability in mating competitiveness of irradiated and incompatible males according to age and ratios
Experiments were carried out to examine the hatching rate and the competitiveness index (C) of LRi and ARwPLR males in competition with LR males for inseminating LR females. Males were released at 1, 5 and 10-day old in a 1:1 or a 5:1 ratio.
Hatching rate analysis revealed strong interactions between males’ age, males type and the ratios of sterilizing males implemented during the trial (Type:Age:Ratio interaction, LRT, X = 550.9, Δdf = 2, P< 0.001). Interactions between males’ type and age remained significant even when analyzing the ratios independently (Type:Age interaction, LRT, X = 465.1, Δdf = 2, P< 0.001 for the 1:1 ratio; X = 202.2, Δdf = 2, P< 0.001 for the 5:1 ratio). Nevertheless, a global observed trend is that ARwPLR males tend to induce a lower hatching rate than LRi males (Table 3).
Table 3. Mating competitiveness of irradiated (LRi) and incompatible (ARwPLR) Ae. albopictus males in cages containing either a 1:1 or a 5:1 ratio with respect to wild-type males (LR).
| Ratio | Male age | Crosses | Number of eggs | Number of hatched eggs | Hatching rate (95% CI) | Fried index C (95% CI) | Induced sterility (IS) |
|---|---|---|---|---|---|---|---|
| 1:1 | 1-day-old | ♂LRi×♂LR×♀LR | 2329 | 1846 | 0.793 (0.775–0.809) | -0.031 (0.073–0.078) | -0.029 |
| ♂ARwPLR×♂LR×♀LR | 1907 | 960 | 0.503 (0.481–0.526) | 0.530 (0.154–0.181) | 0.346 | ||
| 5-day-old | ♂LRi×♂LR×♀LR | 2091 | 687 | 0.327 (0.309–0.349) | 0.824 (0.299–0.397) | 0.415 | |
| ♂ARwPLR×♂LR×♀LR | 3644 | 1188 | 0.326 (0.311–0.341) | 0.723 (0.251–0.311) | 0.419 | ||
| 10-day-old | ♂LRi×♂LR×♀LR | 3833 | 1456 | 0.380 (0.365–0.395) | 1.516 (0.369–0.514) | 0.544 | |
| ♂ARwPLR×♂LR×♀LR | 2028 | 1033 | 0.509 (0.488–0.531) | 0.635 (0.156–0.185) | 0.388 | ||
| 5:1 | 1-day-old | ♂LRi×♂LR×♀LR | 2444 | 1269 | 0.519 (0.499–0.539) | 0.109 (0.034–0.041) | 0.326 |
| ♂ARwPLR×♂LR×♀LR | 2664 | 989 | 0.371 (0.353–0.390) | 0.215 (0.051–0.063) | 0.518 | ||
| 5-day-old | ♂LRi×♂LR×♀LR | 3173 | 592 | 0.187 (0.173–0.201) | 0.533 (0.173–0.303) | 0.668 | |
| ♂ARwPLR×♂LR×♀LR | 4048 | 546 | 0.135 (0.125–0.146) | 0.633 (0.179–0.268) | 0.760 | ||
| 10-day-old | ♂LRi×♂LR×♀LR | 2413 | 1218 | 0.505 (0.485–0.525) | 0.155 (0.040–0.050) | 0.394 | |
| ♂ARwPLR×♂LR×♀LR | 2564 | 440 | 0.172 (0.158–0.187) | 0.770 (0.177–0.255) | 0.794 |
The hatching rates and the values of the Fried index’s (C) are indicated with their 95% confidence intervals (95% CI).
In the 1:1 ratio, the competitiveness index (C) for LRi males were: -0.031, 0.824 and 1.516; and the induced sterility (IS) was: -2.9%, 41.5% and 54.4% for 1, 5 and 10 day-old males, respectively (Table 3). The estimated C values for ARwPLR males were: 0.530, 0.723 and 0.635; and the IS values were: 34.6%, 41.9% and 38.8% for 1, 5 and 10 day-old males, respectively (Table 3). The comparison of mating competitiveness of LRi and ARwPLR males in the 1:1 ratio showed a better competitiveness of the former at 10 days and of the latter at 1 day, respectively; while the difference between the two types of male was not significant at 5 days. When the 5:1 ratio was implemented, the estimated C values were: 0.109, 0.533 and 0.155; and IS values were: 32.6%, 66.8% and 39.4% for 1, 5 and 10 day-old LRi males, respectively (Table 3). As for ARwPLR males, the estimated C values were: 0.215, 0.633 and 0.770; and the IS values were 51.8%, 76% and 79.4% for 1, 5 and 10 day-old males, respectively (Table 3). Thus, the comparison of mating competitiveness of LRi and ARwPLR males in the 5:1 ratio showed a better competitiveness of ARwPLR males regardless of age.
Finally, as male mating competitiveness could be affected by size we measured male wings’ size as a proxy of adult size for all male types. As shown on Fig 1, irradiation of LR pupae did not affect wing size of emerging adults as there was no significant difference between untreated males and their irradiated counterparts (LRT, F = 2.66, P = 0.11). However, ARwPLR males were significantly larger than the other males (LRT, F = 101.4, P< 0.001, Fig 1).
Fig 1. Wing size of males.
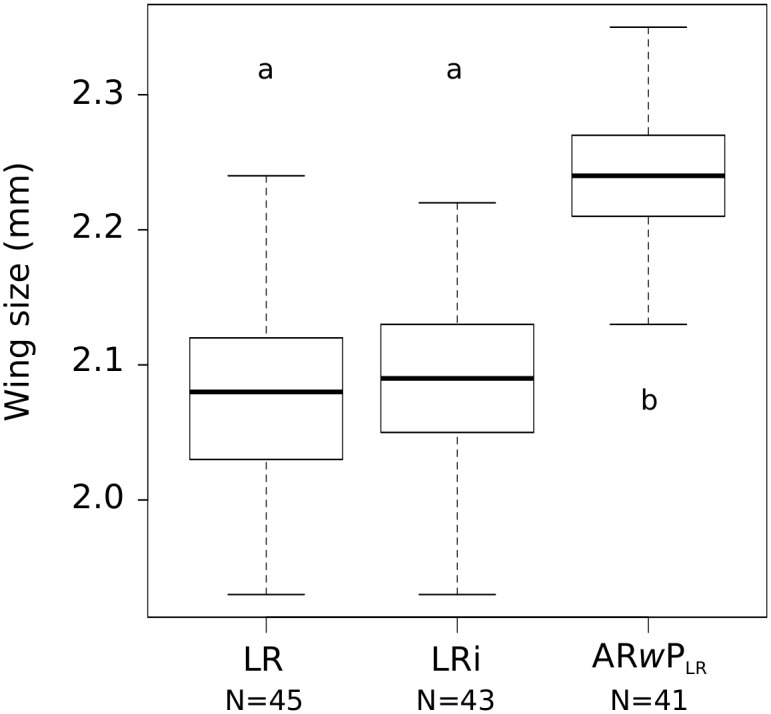
LR = wild type males, LRi = wild type irradiated males and ARwPLR = incompatible males. N = number of mosquitoes measured. a and b represent statistical group (LRT).
Discussion
Several environmental friendly vector control strategies willing at reducing disease transmission through the control of vector populations are currently under development. Among these, sterile-male release strategies are being implemented at pilot scales in several countries [18, 27]. Although laborious, as they require the consistent mass production of sterile males, sterile-male strategies provide the benefits of strong species specificity while implementation may be stopped at any time. In mosquitoes, males’ sterility can be achieved through irradiation, Wolbachia-based CI or transgenesis. Yet no attempt has been made to directly compare these approaches in order to provide insight into their relative efficacy in the same experimental framework. To address this gap, we conducted a series of laboratory experiments aiming at comparing mating capacities of irradiated (LRi) and incompatible (ARwPLR) males.
Crosses between ARwPLR males and wild type females showed total sterility regardless of male age and number of blood meals offered to the females; while 5–8% of hatched eggs were observed in crosses involving LRi males, in keeping with previous investigations using the irradiation dose of 35 Gy [24, 25]. Several studies have addressed the effect of increasing irradiation on induced sterility and other life history traits. An increased dose will lead to full sterility of the treated males but may also affect life expectancy and mating competitiveness and, as a result, the capacity of irradiated males to reduce egg hatch when confronted to wild males [56, 57]. Indeed, Bellini et al. [56] showed that irradiation doses of 30 and 40 Gy were both considered as the best compromise since the reduction in male sterility was overcompensated by the increased competitiveness of partially fertile males. So, it is in the interest of SIT programs to choose radiation doses representing the best compromise between sterilization and mating competitiveness, as in the case reported for the fly Anastrepha obliqua [62].
The insemination capacities of LRi and ARwPLR males were similar, both male types showing lower capacities at 1 day as compared to wild type males, but recovering full capacities 5 days after emergence. Hatching rate measured in cages confrontations revealed strong interactions between the types and ages of males and the ratios of sterilizing males used in the trial. In the 1:1 ratio, hatching rates from cages with LRi males at 5 and 10 days were lower than that recorded in cages with ARwPLR males; whereas in the 5:1 ratio, all hatching observed in cages with ARwPLR males were lower than that observed in cages where LRi males were introduced. However, generally, ARwPLR males tended to induce a lower hatching rate (and thus superior sterility, Table 3) than LRi males under the tested conditions. The difference in mating competitiveness of LRi and ARwPLR males according to the ratio could be explained by the density of mosquitoes within experimental cages. Indeed, all confrontations were performed in identical cages of 30×30×30 cm, and 40 males (20 LRi or 20 ARwPLR + 20 LR) were released in cages with the 1:1 ratio whereas 120 males (100 LRi or 100 ARwPLR + 20 LR) were released in cages with the 5:1 ratio. The effect of mosquito density on hatching has been previously described by Madakacherry et al. [24] who observed higher hatched eggs in small laboratory cages compared to large laboratory cages containing the same number of mosquitoes. So, in competition trials, mating and inseminating capacities may depend on insect densities. The C values for LRi and ARwPLR males were lower than 1 in general, suggesting a lower mating competitiveness of both male types as compared to wild-type males. A lower competitiveness of ARwPLR males may result from insufficient number of backcrosses. Indeed, although the cytoplasm of ARwPIT was introgressed into a LR nuclear background through four successive backcrosses, we cannot exclude a nuclear effect of the remaining (theoretically 10%) ARwPIT nuclear genome that may somehow reduce mating capacities of ARwPLR males with LR females. Thus, in future investigations, a greater number of backcrosses between transinfected and wild type mosquitoes will be performed in order to avoid side effect of nuclear genes on mating capacities of incompatible males. Surprisingly, the C value of LRi males at 1 day was negative due to the higher hatching rate in competition trials as compared to fertile control (see Tables 2 and 3). While the reason for this higher hatch is not clear, human error during egg maturation and counting or problems with the blood cannot be ruled out.
We found an effect of male age in mating competitiveness. Generally, the fertility of LR females was significantly lower in cage confrontations where 5 and 10 day-old LRi and ARwPLR males were released, as compared to cages with 1 day-old males. Several factors including magnitude and persistence of induced sterility as well as delayed sexual maturation may account for this transitory mating competitiveness. We measured both strength and persistence of induced sterility in LRi and ARwPLR males. We observed a residual fertility in matings with LRi males in agreement with previous investigations showing the existence of residual fertility in crosses between Ae. albopictus females and 35 Gy irradiated males [24, 25]. Although there was a significant difference in hatching rates between age classes, this difference was not consistent with a mating competitiveness increasing with age. As for Wolbachia, it has been shown that Wolbachia-induced sterility can change with male age as observed in some arthropod species [63–66] including Ae. albopictus [58, 67–69]. Crosses between ARwPLR males and wild type females led to full sterility, thus confirming that CI properties of ARwPIT males, previously shown to induce total embryonic mortality in crosses with wild type females [36, 43, 69], was not altered by the nuclear background of the LR line. Importantly, the levels of sterility remained constant in 1, 5 and 10 day-old males, hence allowing rejecting the hypothesis of any change of Wolbachia-induced sterility with males’ age. Lastly, we addressed the effect of male age on insemination capacity. Irradiated LRi and ARwPLR were less successful in inseminating females at 1 day as compared to LR, and no significant difference was noted between the three male types at days 5 and 10. To become sexually mature, males must complete several physical changes including complete rigidity of antennal fibrillar hairs and a 180° rotation of the terminalia part of the genitalia after emergence (see [70] for review). The time required to complete terminalia rotation varies between mosquito species. For instance, 18 to 24 h was reported for A. aegypti [71] and 11 to 25 h for A. albopictus [72]. Oliva et al. [72] showed that genitalia rotation was slightly delayed in irradiated males although they did not observe statistical difference in insemination rates according to age. However, comparing our data to this previous study is not straightforward since we allowed females to mate for 24 h while Oliva and co-workers allowed females to mate for 48 h, which must have significantly increased the insemination rate as previously shown for Anopheles coluzzii [23]. As far as Wolbachia is concerned, there is no study describing any delay in sexual maturation for artificially infected Ae. albopictus lines, so future investigations comparing sexual maturation of irradiated and incompatible males should investigate this point of interest. Although we cannot clearly demonstrate the correlation between insemination capacity and mating competitiveness, this factor should definitely be considered for the implementation of SIT or IIT in the field. The effect of male life history and mating behavior has been investigated theoretically for sterile-male release programs [73]. The results suggest that if male mating capacity increases over the first week of life and if released males suffer a mortality cost, older males should be released due to their increased mating capacity [73]. So, in the context of an operational implementation of SIT and/or IIT, irradiated or incompatible males should be released in the field at their maximum competitiveness, while devices allowing the emergence of sterile males in natura should be avoided.
Although SIT and IIT are vector control techniques based on a common strategy, i.e. the inundative release of sterilizing males, each method has its pros and cons. Due to residual fertility observed with irradiated (35 Gy) males, an operational implementation of SIT using this radiation dose may require a greater number of irradiated males to be released than IIT. On the other hand, although both techniques require selective release of males only, IIT is extremely sensitive to accidental release of females, especially when repeated releases have suppressed local mosquito populations making any female release favorable to population replacement. Nevertheless, bidirectional CI occurring between incompatible and wild mosquitoes (including mosquitoes immigrating into the treated area) is expected to significantly lower the population replacement risk. Overall, our data reveal that incompatible males were slightly superior to irradiated males under the tested conditions. However, this study may highlight a possible complementarity of both techniques. Indeed, application of low dose of irradiation with the aim of sterilizing females without affecting the quality of the released males could lower the risk of accidental releases of incompatible females [74–76]. We propose an alternative approach: IIT implementation requires a surveillance of Wolbachia infection in treated populations all over releases, and the appearance of any sign of population replacement may be overcome by the release of irradiated males. Indeed, our data suggest that incompatible males are more competitive in the presence of high densities of mosquitoes and irradiated males in low densities. Given that the accidental release of incompatible females is most risky in the presence of low densities of mosquitoes, SIT and IIT may be used successively based on an evaluation of the density of mosquito populations in targeted sites. Of course, the availability of an irradiator together with the cost of such combination need to be taken into consideration. Altogether, our results call for additional experiments comparing life history traits and mating competitiveness of irradiated and incompatible males under semi-field conditions since discrepancies between laboratory and field cage experiments have been previously reported [32].
Conclusion
In this study, we compared mating capacities of irradiated and incompatible males. Both males showed a better competitiveness with ageing, highlighting the need for accurate analyses of male life history traits and mating behavior for sterile-male release strategies in order to increase the effectiveness of SIT and IIT control programs. Our data suggest that both techniques would benefit from a release of males a few days after emergence. Altogether, the analysis of mating competitiveness revealed that incompatible males were slightly superior to irradiated males. However, since the possible fate of ARwPLR females, eventually co-released in the field, was still not ascertained by a rigorous risk assessment protocol, our comparative data highlight that both techniques are likely complementary and may be indeed implemented alternatively or in combination within a same treated area. Comprehensive investigations in semi-field conditions will allow selecting for the most relevant strategy at a specific site and fine-tuning the releasing conditions with the aim of maximizing the success of these appealing environmental-friendly strategies.
Acknowledgments
We are grateful to Frederic Jean and Gilbert Legoff for technical assistance; David Damien for helpful comments on the manuscript. We appreciate the collaboration with the Etablissement Français du Sang (EFS) of Saint Denis, Réunion Island, for the use of their radiation source.
Data Availability
All relevant data are within the paper.
Funding Statement
Celestine M. Atyame Post-Doctoral position was funded by the European Union's Seventh Framework Programme ([FP7/2007–2013]) under grant agreement n°263958 (RUN-Emerge project). This investigation had received the joint financial supports from the French Ministry of Health and the European Regional Development Fund (FEDER-Reunion) under the Convergence 2007–2013 Programme.
References
- 1.Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004; 18: 215–227. [DOI] [PubMed] [Google Scholar]
- 2.Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007; 7: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caminade C, Medlock JM, Ducheyne E, McIntyre KM, Leach S, Baylis M, et al. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface. 2012; 9: 2708–2717. 10.1098/rsif.2012.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009; 11: 1177–1185. 10.1016/j.micinf.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 5.Delatte H, Dehecq JS, Thiria J, Domerg C, Paupy C, Fontenille D. Geographic distribution and developmental sites of Aedes albopictus (Diptera: Culicidae) during a Chikungunya epidemic event. Vector Borne Zoonotic Dis. 2008; 8: 25–34. 10.1089/vbz.2007.0649 [DOI] [PubMed] [Google Scholar]
- 6.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007; 370: 1840–1846. [DOI] [PubMed] [Google Scholar]
- 7.Smith CEG. The history of dengue in tropical Asia and its probable relationship to the mosquito Aedes aegypti. J Trop Med Hyg. 1956; 59: 243–251. [PubMed] [Google Scholar]
- 8.Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc. 1988; 4: 1–40. [PubMed] [Google Scholar]
- 9.Reiter P, Sprenger D. The used tire trade: A mechanism for the worldwide dispersal of container breeding mosquitoes. J Am Mosq Control Assoc. 1987; 3: 494–501. [PubMed] [Google Scholar]
- 10.Delatte H, Desvars A, Bouetard A, Bord S, Gimonneau G, Vourc'h G, et al. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Reunion. Vector Borne Zoonotic Dis. 2010; 10: 249–258. 10.1089/vbz.2009.0026 [DOI] [PubMed] [Google Scholar]
- 11.Kamgang B, Nchoutpouen E, Simard F, Paupy C. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasit Vectors. 2012; 5: 57 10.1186/1756-3305-5-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013; 29: 460–468. 10.1016/j.pt.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000; 45: 371–391. [DOI] [PubMed] [Google Scholar]
- 14.Tantely ML, Tortosa P, Alout H, Berticat C, Berthomieu A, Rutee A, et al. Insecticide resistance in Culex pipiens quinquefasciatus and Aedes albopictus mosquitoes from La Reunion Island. Insect Biochem Mol Biol. 2010; 40: 317–324. 10.1016/j.ibmb.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 15.Labbe P, Lenormand T, Raymond M. On the worldwide spread of an insecticide resistance gene: a role for local selection. J Evol Biol. 2005; 18: 1471–1484. [DOI] [PubMed] [Google Scholar]
- 16.Alphey L. Genetic control of mosquitoes. Annu Rev Entomol. 2014; 59: 205–224. 10.1146/annurev-ento-011613-162002 [DOI] [PubMed] [Google Scholar]
- 17.Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, Moreira LA, et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014; 132 Suppl: S150–63. 10.1016/j.actatropica.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 18.Lees SR, Gilles JRL, Hendrichs J, Vreysen MJB, Bourtzis K. Back to the future: the sterile insect technique against mosquito disease vectors. Curr Opin Insect Sci. 2015; 10: 156–162. [DOI] [PubMed] [Google Scholar]
- 19.Knipling EF. Possibilities of insect control or eradication through the use of sexually-sterile males. J Econ Entomol. 1955; 48: 459–462. [Google Scholar]
- 20.Krafsur E. Sterile insect technique for suppressing and eradicating insect populations: 55 years and counting. J Agric Entomol. 1998; 15: 303–317. [Google Scholar]
- 21.Dyck VA, Hendrichs J, Robinson AS. Sterile Insect Technique: Principle sand practice in Area-Wide Integrated Pest Management. Springer; Dordrecht; 2005. [Google Scholar]
- 22.Bellini R, Medici A, Puggioli A, Balestrino F, Carrieri M. Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J Med Entomol. 2013; 50: 317–325. [DOI] [PubMed] [Google Scholar]
- 23.Maiga H, Damiens D, Niang A, Sawadogo SP, Fatherhaman O, Lees RS, et al. Mating competitiveness of sterile male Anopheles coluzzii in large cages. Malar J. 2014; 13: 460 10.1186/1475-2875-13-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madakacherry O, Lees RS, Gilles JR. Aedes albopictus (Skuse) males in laboratory and semi-field cages: release ratios and mating competitiveness. Acta Trop. 2014; 132: S124–129. 10.1016/j.actatropica.2013.11.020 [DOI] [PubMed] [Google Scholar]
- 25.Oliva CF, Jacquet M, Gilles J, Lemperiere G, Maquart PO, Quilici S, et al. The sterile insect technique for controlling populations of Aedes albopictus (Diptera: Culicidae) on Reunion Island: mating vigour of sterilized males. PLoS One. 2012; 7(11): e49414 10.1371/journal.pone.0049414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dame DA, Curtis CF, Benedict MQ, Robinson AS, Knols BG. Historical applications of induced sterilisation in field populations of mosquitoes. Malar J. 2009; 8: S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliva CF, Vreysen MJ, Dupe S, Lees RS, Gilles JR, Gouagna LC, et al. Current status and future challenges for controlling malaria with the sterile insect technique: technical and social perspectives. Acta Trop. 2014; 132: S130–139. 10.1016/j.actatropica.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 28.Thomas DD, Donnelly CA, Wood RJ, Alphey LS. Insect population control using a dominant, repressible, lethal genetic system. Science. 2000; 287: 2474–2476. [DOI] [PubMed] [Google Scholar]
- 29.Fu G, Lees RS, Nimmo D, Aw D, Jin L, Gray P. et al. Female-specific flightless phenotype for mosquito control. Proc Natl Acad Sci USA. 2010; 107: 4550–4554. 10.1073/pnas.1000251107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wise de Valdez MR, Nimmo D, Betz J, Gong HF, James AA, Alphey L, et al. Genetic elimination of dengue vector mosquitoes. Proc Natl Acad Sci USA. 2011; 108: 4772–4775. 10.1073/pnas.1019295108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labbe GM, Scaife S, Morgan SA, Curtis ZH, Alphey L. Female-specific flightless (fsRIDL) phenotype for control of Aedes albopictus. PLoS Negl Trop Dis. 2012; 6(7): e1724 10.1371/journal.pntd.0001724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Facchinelli L, Valerio L, Ramsey JM, Gould F, Walsh RK, Bond G, et al. Field cage studies and progressive evaluation of genetically-engineered mosquitoes. PLoS Negl Trop Dis. 2013; 7(1): e2001 10.1371/journal.pntd.0002001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brelsfoard CL, Sechan Y, Dobson SL. Interspecific hybridization yields strategy for South Pacific filariasis vector elimination. PLoS Negl Trop Dis. 2008; 2(1): e129 10.1371/journal.pntd.0000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chambers EW, Hapairai L, Peel BA, Bossin H, Dobson SL. Male mating competitiveness of a Wolbachia-introgressed Aedes polynesiensis strain under semi-field conditions. PLoS Negl Trop Dis. 2011; 5(8): e1271 10.1371/journal.pntd.0001271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atyame CM, Pasteur N, Dumas E, Tortosa P, Tantely M, Pocquet N, et al. Cytoplasmic incompatibility as a means of controlling Culex pipiens quinquefasciatus mosquito in the islands of the south-western Indian Ocean. PLoS Negl Trop Dis. 2011; 5(12): e1440 10.1371/journal.pntd.0001440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moretti R, Calvitti M. Male mating performance and cytoplasmic incompatibility in a wPip Wolbachia trans-infected line of Aedes albopictus (Stegomyia albopicta). Med Vet Entomol. 2012; 274(4): 377–386. [DOI] [PubMed] [Google Scholar]
- 37.O'Connor L, Plichart C, Cheong Sang A, Brelsfoard CL, Bossin HC, Dobson SL. Open release of male mosquitoes infected with a Wolbachia biopesticide: field performance and infection containment. PLoS Negl Trop Dis. 2012; 6(11): e1797 10.1371/journal.pntd.0001797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atyame CM, Cattel J, Lebon C, Flores O, Dehecq JS, Weill M, et al. Wolbachia-based population control strategy targeting Culex quinquefasciatus mosquitoes proves efficient under semi-field conditions. PLoS One. 2015; 10(3): e0119288 10.1371/journal.pone.0119288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008; 6(10): 741–751. 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 40.Rasgon JL, Scott TW. Wolbachia and cytoplasmic incompatibility in the California Culex pipiens mosquito species complex: parameter estimates and infection dynamics in natural populations. Genetics. 2003; 165(4): 2029–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duron O, Fort P, Weill M. Hypervariable prophage WO sequences describe an unexpected high number of Wolbachia variants in the mosquito Culex pipiens. Proc R Soc Lond Ser B. 2006; 273(1585): 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atyame CM, Delsuc F, Pasteur N, Weill M, Duron O. Diversification of Wolbachia endosymbiont in the Culex pipiens mosquito. Mol Biol Evol. 2011; 28(10): 2761–72. 10.1093/molbev/msr083 [DOI] [PubMed] [Google Scholar]
- 43.Calvitti M, Moretti R, Lampazzi E, Bellini R, Dobson SL. Characterization of a new Aedes albopictus (Diptera: Culicidae)-Wolbachia pipientis (Rickettsiales: Rickettsiaceae) symbiotic association generated by artificial transfer of the wPip strain from Culex pipiens (Diptera: Culicidae). J Med Entomol. 2010; 47(2): 179–187. [DOI] [PubMed] [Google Scholar]
- 44.Fu Y, Gavotte L, Mercer DR, Dobson SL. Artificial triple Wolbachia infection in Aedes albopictus yields a new pattern of unidirectional cytoplasmic incompatibility. Appl Environ Microbiol. 2010; 76(17): 5887–5891. 10.1128/AEM.00218-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blagrove MS, Arias-Goeta C, Failloux AB, Sinkins SP. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci USA. 2012; 109(1): 255–260. 10.1073/pnas.1112021108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu GJ, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009; 139(7): 1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 47.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks Dengue and invades caged Aedes aegypti populations. Nature. 2011; 476(7361): 450–453. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- 48.Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X, et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013; 340(6133): 748–751. 10.1126/science.1236192 [DOI] [PubMed] [Google Scholar]
- 49.Dodson BL, Hughes GL, Paul O, Matacchiero AC, Kramer LD, Rasgon JL. Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl Trop Dis. 2014; 8(7): e2965 10.1371/journal.pntd.0002965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffmann AA. Entomology: incompatible mosquitoes. Nature. 2005; 436(7048): 189 [DOI] [PubMed] [Google Scholar]
- 51.Zabalou S, Riegler M, Theodorakopoulou M, Stauffer C, Savakis C, Bourtzis K. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc Natl Acad Sci USA. 2004; 101(42): 15042–15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967; 216: 383–384. [DOI] [PubMed] [Google Scholar]
- 53.Gerardin P, Guernier V, Perrau J, Fianu A, Le Roux K, Grivard P, et al. Estimating Chikungunya prevalence in La Reunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis. 2008; 8: 99 10.1186/1471-2334-8-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliva CF, Maier MJ, Gilles J, Jacquet M, Lemperiere G, Quilici S, et al. Effects of irradiation, presence of females, and sugar supply on the longevity of sterile males Aedes albopictus (Skuse) under semi-field conditions on Reunion Island. Acta Trop. 2013; 125(3): 287–293. 10.1016/j.actatropica.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 55.Boyer S, Gilles J, Merancienne D, Lemperiere G, Fontenille D. Sexual performance of male mosquito Aedes albopictus. Med Vet Entomol. 2011; 25(4): 454–459. 10.1111/j.1365-2915.2011.00962.x [DOI] [PubMed] [Google Scholar]
- 56.Bellini R, Balestrino F, Medici A, Gentile G, Veronesi R, Carrieri M. Mating competitiveness of Aedes albopictus radio-sterilized males in large enclosures exposed to natural conditions. J Med Entomol. 2013; 50(1): 94–102. [DOI] [PubMed] [Google Scholar]
- 57.Yamada H, Parker AG, Oliva CF, Balestrino F, Gilles JRL. X-ray-induced sterility in Aedes albopictus (Diptera: Culicidae) and male longevity following irradiation. J Med Entomol. 2014; 51(4): 811–816. [DOI] [PubMed] [Google Scholar]
- 58.Calvitti M, Marini F, Desiderio A, Puggioli A, Moretti R. Wolbachia density and cytoplasmic incompatibility in Aedes albopictus: concerns with using artificial Wolbachia infection as a vector suppression tool. PLoS One. 2015; 10(3): e0121813 10.1371/journal.pone.0121813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duron O, Boureux A, Echaubard P, Berthomieu A, Berticat C, Fort P, et al. Variability and expression of ankyrin domain genes in Wolbachia variants infecting the mosquito Culex pipiens. J Bacteriol. 2007; 189(12): 4442–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fried M. Determination of sterile-insect competitiveness. J Econ Entomol. 1971; 64: 869–872. [Google Scholar]
- 61.Crawley M. The R Book. John Wiley & Sons Ltd, Chichester, UK; 2007. [Google Scholar]
- 62.Toledo J, Rull J, Oropeza A, Hernández E, Liedo P. Irradiation of Anastrepha obliqua (Diptera: Tephritidae) revisited: optimizing sterility induction. J Econ Entomol. 2004; 97(2): 383–389. [DOI] [PubMed] [Google Scholar]
- 63.Turelli M, Hoffmann AA. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics. 1995; 140(4): 1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jamnongluk W, Kittayapong P, Baimai V, O'Neill SL. Wolbachia infection and expression of cytoplasmic incompatibility in Armigeres subalbatus (Diptera: Culicidae). J Med Entomol. 2000; 37(1): 53–57. [DOI] [PubMed] [Google Scholar]
- 65.Noda H, Miyoshi T, Zhang Q, Watanabe K, Deng K, Hoshizaki S. Wolbachia infection shared among planthoppers (Homoptera: Delphacidae) and their endoparasite (Strepsiptera: Elenchidae): a probable case of interspecies transmission. Mol Ecol. 2001; 10(8): 2101–2106. [DOI] [PubMed] [Google Scholar]
- 66.Reynolds KT, Hoffmann AA. Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet Res. 2002; 80(2): 79–87. [DOI] [PubMed] [Google Scholar]
- 67.Kittayapong P, Mongkalangoon P, Baimai V, O'Neill SL. Host age effect and expression of cytoplasmic incompatibility in field populations of Wolbachia-superinfected Aedes albopictus. Heredity. 2002; 88(4): 270–274. [DOI] [PubMed] [Google Scholar]
- 68.Tortosa P, Charlat S, Labbe P, Dehecq JS, Barre H, Weill M. Wolbachia age-sex-specific density in Aedes albopictus: a host evolutionary response to cytoplasmic incompatibility? PLoS One. 2010; 5(3): e9700 10.1371/journal.pone.0009700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calvitti M, Moretti R, Skidmore AR, Dobson SL. Wolbachia strain wPip yields a pattern of cytoplasmic incompatibility enhancing a Wolbachia-based suppression strategy against the disease vector Aedes albopictus. Parasit Vectors. 2012; 5: 254 10.1186/1756-3305-5-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oliva CF, Damiens D, Benedict MQ. Male reproductive biology of Aedes mosquitoes. Acta Trop. 2014; 132: S12–19. 10.1016/j.actatropica.2013.11.021 [DOI] [PubMed] [Google Scholar]
- 71.Roth LM. Study of mosquito behavior. An experimental laboratory study of the sexual behavior of Aedes aegypti (Linnaeus). Am Midl Nat. 1948; 40: 265–352. [Google Scholar]
- 72.Oliva CF, Damiens D, Vreysen MJ, Lemperiere G, Gilles J. Reproductive strategies of Aedes albopictus (Diptera: Culicidae) and implications for the sterile insect technique. PLoS One. 2013; 8(11): e78884 10.1371/journal.pone.0078884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stone CM. Transient population dynamics of mosquitoes during sterile male releases: modelling mating behaviour and perturbations of life history parameters. PLoS One. 2013; 8(9): e76228 10.1371/journal.pone.0076228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brelsfoard CL, St Clair W, Dobson SL. Integration of irradiation with cytoplasmic incompatibility to facilitate a lymphatic filariasis vector elimination approach. Parasit Vectors. 2009; 2(1): 38 10.1186/1756-3305-2-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang D, Zheng X, Xi Z, Bourtzis K, Gilles JR. Combining the sterile insect technique with the incompatible insect technique: I-impact of Wolbachia infection on the fitness of triple- and double-infected strains of Aedes albopictus. PLoS One. 2015; 10(4): e0121126 10.1371/journal.pone.0121126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang D, Lees RS, Xi Z, Gilles JR, Bourtzis K. Combining the sterile insect technique with Wolbachia-based approaches: II- a safer approach to Aedes albopictus population suppression programmes, designed to minimize the consequences of inadvertent female release. PLoS One. 2015; 10(8): e0135194 10.1371/journal.pone.0135194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


