Abstract
In the interests of food safety and public health, plants and their compounds are now re-emerging as an alternative approach to treat gastrointestinal diseases in chickens. Here, we studied the impact of the edible medicinal plant, B. pilosa, on growth performance, gut bacteria and coccidiosis in chickens. First, we found that B. pilosa significantly elevated body weight gain and lowered feed conversion ratio in chickens. Next, we showed that B. pilosa reduced cecal damage as evidenced by increased hemorrhage, villus destruction and decreased villus-to-crypt ratio in chicken ceca. We also performed pyrosequencing of the PCR ampilcons based on the 16S rRNA genes of gut bacteria in chickens. Metagenomic analysis indicated that the chicken gut bacteria belonged to 6 phyla, 6 classes, 6 orders, 9 families, and 8 genera. More importantly, we found that B. pilosa affected the composition of bacteria. This change in bacteria composition was correlated with body weight gain, feed conversion ratio and gut pathology in chickens. Collectively, this work suggests that B. pilosa has beneficial effects on growth performance and protozoan infection in chickens probably via modulation of gut bacteria.
Introduction
It is estimated that 50 billion chickens are raised in the world, reaching a global market value of 60 billion American dollars [1,2]. Chicken meat accounts for 30% of protein food consumed by humans [2].
Gut health determines growth performance and health in chickens because the gastrointestinal tract, the main digestive and absorption organ, can take in nutrients for growth and development, eliminate unwanted waste, and confer mucosal immunity against parasites [3]. A diverse microbiota is found throughout the digestive tract and is more profound in the cecum [3–6]. Gut microbiota affects nutrition, detoxification, growth performance, and protection against pathogens in chickens. Therefore, gut microbiota are important for gut health and diseases in chickens [7–9]. For instance, the absence of normal microbiota in the cecum has been considered a major factor in the susceptibility of chicks to bacterial infection [10]. Gut microbiota in chickens are gradually established from the mother and environment, including Streptococcus and enterobacteria within days, Escherichia coli and Bacteroides within a week [6], Lactobacillus, with a Bifidobacterium population, at an older age and finally, Clostridium, Salmonella, and Campylobacter. Avian coccidiosis is a bane to the poultry industry [11], causing high mortality, poor growth and high medical costs. The genus Eimeria (Coccidia subclass) is the causative protozoan parasite in chickens. Moreover, bacterial infections are frequently accompanied by coccidiosis [12–15].
Plants have been an extraordinary source of medicines for humans and animals since antiquity [16]. Edible plants and their compounds have become an alternative approach to treat intestinal parasites [17,18]. Further, this herbal approach can reduce or replace the abuse and misuse of antibiotics in chickens and help organic chicken production. B. pilosa (Asteraceae) is used as a medicinal plant for gastrointestinal disease, protozoan diseases and infections [16]. More recently, it was demonstrated to act against coccidiosis in chickens [19]. In this study, we extended our studies on the effect of B. pilosa on growth performance, gut microbiota and gut pathology in the presence or absence of E. tenella infection in chickens.
Materials and Methods
Preparation of chicken diets
Chicken diets were mixed with phosphate-buffered saline (PBS) vehicle or 0.5% B. pilosa (Chun-Yueh Biotech Company, Taiwan). Preparation of B. pilosa was processed as previously published [19]. Briefly, whole plant of B. pilosa was authenticated, processed and mixed with chicken feed.
Animal husbandry
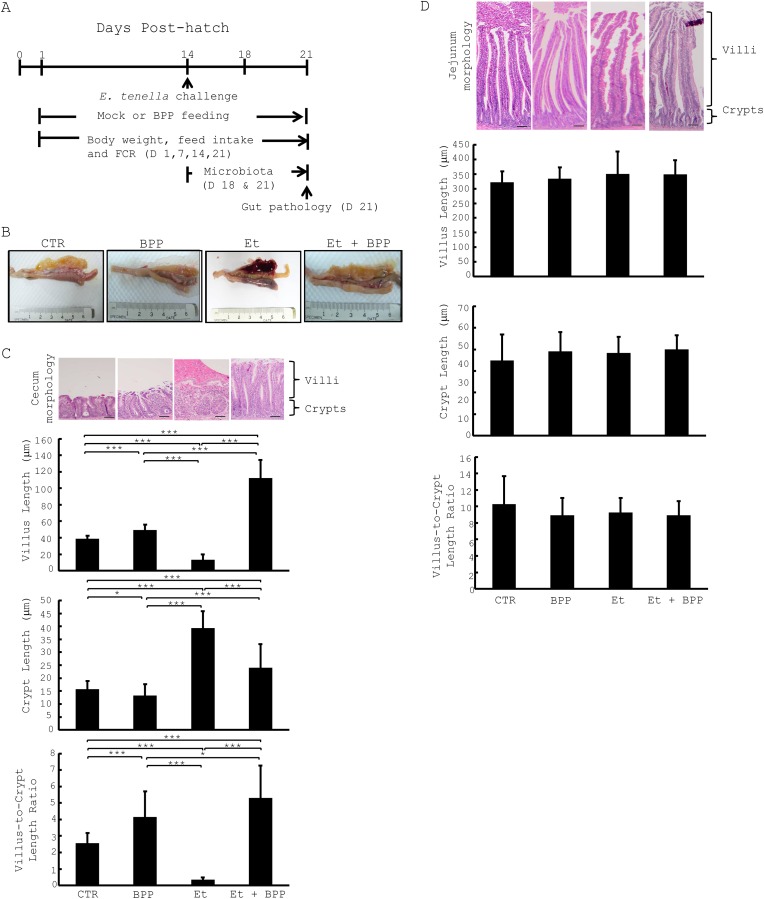
One-day-old disease-free 40 Lohmann layer chicks were purchased from a local hatchery in Taichung, Taiwan. The birds were randomly divided into 4 groups. Each group was housed in three cages: Group 1 (3, 3, 4 chicks), Group 2 (3, 3, 4 chicks), Group 3 (3, 3, 4 chicks), and Group 4 (3, 3, 4 chicks). The chicks in all the cages had free access to feed and water throughout the experiment. As shown in Fig 1A, chickens in Group 1 (uninfected untreated control chickens, CTR) and Group 2 (E. tenella-infected untreated chickens, Et) were fed with a standard diet whereas chickens in Group 3 (B. pilosa product-treated chickens, BPP) and Group 4 (E. tenella-infected B. pilosa product-treated chickens, Et + BPP) were fed with a standard diet containing 0.5% B. pilosa powder (5 g BPP/kg diet) from day 1 to day 21. On day 14, Groups 2 and 4 were infected with E. tenella. The birds were monitored once per day and raised in an institutional chicken house at 28–30°C and handled according to the guidelines of the National Chung-Hsing University Institutional Animal Care and Use Committee. The protocol was approved by the same committee (Permit Number: 103–34). Butorphenol at a dose of 1mg/kg was used to minimize suffering of the birds infected with E. tenella and all the birds were sacrificed using carbon dioxide euthanasia.
Fig 1. B. pilosa alleviates E. tenella-mediated gut pathology in chickens.
(A) Summary of experimental protocol for this study. (B) Representative images of the gut of 21-day-old chickens in each group are shown. (C-D) Hematoxylin and esosin (H&E) staining of the ceca (C) and jejuna (D) of the same chickens as (B).
Preparation and inoculation of E. tenella oocysts
E. tenella strain Et C1 was maintained, amplified and used throughout the experiment as previously described [19]. The oocysts were isolated from fresh feces of chickens, followed by sporulation with potassium dichromate. Four groups of birds, supplied with standard diets and standard diets containing 0.5% B. pilosa powder were tube-fed with 2 ml of sterile water (uninfected groups) or E. tenella sporulated oocysts (1 × 104, infected groups) [19].
Measurement of body weight, food conversion and gut pathology in animals
Each group of birds was individually weighed on a daily basis. Their diet consumption was monitored daily. Feed conversion ratio (FCR) was obtained by the normalization of diet consumed by body weight. To evaluate gut pathology, the ceca and intestines removed from each group of sacrificed chickens were fixed with formalin and embedded with paraffin. The gut slides were stained with hematoxylin and eosin (H&E) and examined under a microscope as described previously [19].
Pyrosequencing and data analysis
The gut bacterial DNA collected from the feces of the chickens in Groups 1 to 4 on day 18 or 21 (i.e., day 4 and 7 post infection) were purified and used as templates for PCR amplification with 16S rRNA primers. Following pyrosequencing (Roche 454), chimeric sequences of the 16S rRNA sequences were removed using Chimera Check [20]. The trimmed sequences over 300 bp were analyzed using RDPipeline as published [21]. Briefly, 16S rRNA gene sequence alignment (Aligner), 16S rRNA gene sequence clustering (Complete Linkage Clustering), α-diversity index (Shannon Index and Chao1 estimator), rarefaction curve, and phylogenetic analysis (RDP classifier) were conducted. Principle component analysis and clustering analysis for bacterial genera were performed using the prcomp, heatmap3 and ggplot2 functions in R (the R Foundation for Statistical Computing). The hierarchical multi-level pie charts of bacterial compositions of experimental samples based on phylogenetic classifications were constructed using KRONA software [22].
Statistical analysis
Data from 10 chickens in each group of chickens are presented as mean ± standard error (SE). Two-way ANOVA in the SPSS software was used to determine whether there were significant effects of BPP and E. tenella on chicken growth performance. Spearman's rank correlation coefficient (r) was computed using the cor. test function in R and used to test the association between microbiota and gut pathology and growth performance. For all statistical analyses, the P values less than 0.05 are considered to be statistically significant.
Results
B. pilosa improves growth performance and lowers FCR in control chickens and those infected with E. tenella
A previous publication showed that B. pliosa is a promising feed additive for coccidiosis control in chickens [19]. Here, we evaluated the benefits of this plant for growth performance in chickens. We first monitored the body weight gain and FCR of chickens fed with a standard diet containing vehicle and 0.5% BPP (Table 1). We found that chickens fed with B. pilosa had better body weight gain than those with a standard diet (Groups 1 and 3, Table 1). Consistently, B. pilosa significantly decreased FCR in chickens (Groups 1 and 3, Table 1). Next, we assessed the effect of B. pliosa on body weight gain and FCR in the chickens infected with E. tenella. We found that B. pilosa significantly augmented body weight gain and reduced FCR (Groups 2 and 4, Table 1). The data collectively demonstrated that B. pilosa promoted weight gain and diminished FCR in the presence or absence of E. tenella infection.
Table 1. Effects of B. pilosa on body weight of chickens before and after E. tenella challenge.
| Group1 | BPP | E. tenella | Day 14–21 | |
|---|---|---|---|---|
| (% of feed) | (oocysts) | Body weight gain (%)2 | FCR3 | |
| 1(CTR) | 0 | 0 | 48.8±3.2 | 3.3.14±0.27 |
| 2(Et) | 0 | 1×104 | 31.6±11.5 | 5.16±1.23 |
| 3(BPP) | 0.5 | 0 | 52.0±1.6 | 2.81±0.08 |
| 4(BPP+Et) | 0.5 | 1×104 | 45.5±5.8 | 3.71±0.64 |
| P value4 | BPP | 0.0046 | 0.0030 | |
| E. tenella | 0.0003 | <0.0001 | ||
| BPP × E. tenella | 0.0453 | 0.0481 |
1The chickens were given standard diet (Groups 1 and 2) and standard diet supplemented with 0.5% B. pilosa product (Groups 3 and 4) from days 1 to 21. On day 14, chickens in Groups 2 and 4 were orally inoculated with E. tenella at the dose of 1 × 104 sporulated oocysts per chicken.
2Body weight gain (%) was calculated based on the formula: 100% × (body weight on day 21 minus body weight on day 14)/body weight on day 14.
3FCR stands for feed conversion ratio and it was obtained by normalization of feed intake to body weight gain.
4The influence of BPP and E. tenella treatments on body weight gain (%) and FCR in chickens were analyzed by two-way ANVOA. The P values less than 0.05 are considered to be statistically significant.
Effect of B. pilosa on gut pathology associated with E. tenella infection
Next, we checked the effect of gut pathology in 4 groups of chickens. Gross examination showed that the ceca of the chickens fed with standard diet and the diet containing B. pilosa, without E. tenella infection, appeared to be similar (CTR and BPP, Fig 1B). Microscopy showed that B. pilosa seemed to have longer villus length, shorter crypt length and, in turn, higher villus-to-crypt ratio in chicken ceca (CTR and BPP, Fig 1C). However, no difference in structure of villi and crypts in the jejuna of chickens fed with and without B. pilosa was observed (CTR and BPP, Fig 1D). Further, we examined the gut pathology in the chickens infected with E. tenella. We found that the ceca of the chickens infected with E. tenella were damaged with hemorrhaging and loss of cecal villi, 7 days post Eimeria infection (Et, Fig 1B). Accordingly, microscopic examination indicated that E. tenella destroyed villi, increased crypt length and, in turn, reduced the villus-to-crypt ratio in chicken ceca (Et, Fig 1C), but not in chicken jejuna (Et, Fig 1D). In contrast, B. pilosa reversed the damage caused by E. tenella and, therefore, increased villus length, decreased crypt length and augmented the villus-to-crypt ratio in chicken ceca (Et + BPP, Fig 1C). Overall the data showed that B. pilosa reduced E. tenella-dependent damage in chickens via gut modulation.
Overview of chicken gut microbiota in 8 experimental settings
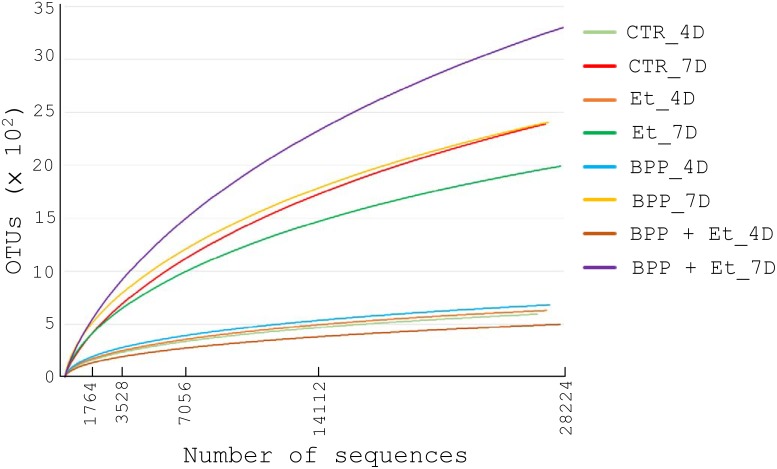
Next, we analyzed the effect of B. pilosa on gut bacteria in each group of chickens. Pyrosequencing-based metagenomic analysis was conducted to uncover the bacterial communities in the guts of chickens aged 18 (D4) or 21 days (D7). A total of 200, 250 16S rRNA gene sequences were produced from 8 experimental samples. The number of sequences, operational taxonomic units (OTUs) and diversity indices for each sample are summarized in Table 2. Rarefaction curves suggested that the number of sequences from 8 experimental samples were enough to uncover major OTUs (Fig 2). The gut microbiota of 21-day-old chickens (7D samples) are much more diverse than those of 18-day-old chickens (4D samples) as evidenced by Shannon and Chao1 diversity indices in Table 2 and curves in Fig 2.
Table 2. Number of sequences, OTUs, classification and diversity indexes for each sample.
| Sample DNA | Seq No. | OTUs | Phylum | Class | Order | Family | Genus | Shan | Chao1 |
|---|---|---|---|---|---|---|---|---|---|
| CTR_4D | 17048 | 595 | 4 | 9 | 9 | 14 | 23 | 4.27 | 823.4 |
| CTR_7D | 26031 | 2336 | 3 | 9 | 9 | 14 | 27 | 4.95 | 3837.2 |
| BPP_4D | 24305 | 682 | 4 | 8 | 9 | 13 | 21 | 4.20 | 927.8 |
| BPP_7D | 33008 | 2406 | 5 | 12 | 11 | 15 | 25 | 5.64 | 3563.7 |
| Et_4D | 22228 | 630 | 4 | 8 | 9 | 13 | 22 | 4.07 | 793.7 |
| Et_7D | 28806 | 1990 | 5 | 12 | 13 | 19 | 30 | 5.30 | 2874.7 |
| BPP + Et_4D | 20844 | 499 | 4 | 11 | 12 | 15 | 24 | 3.56 | 713.7 |
| BPP + Et_7D | 27980 | 3300 | 4 | 11 | 12 | 16 | 28 | 5.58 | 5077.8 |
OUT, Operational taxonomic unit; Shan, Shannon diversity index; Chao1, Chao1 diversity index
Fig 2. Rarefaction curves of bacterial OTUs in experimental samples from the guts of chickens fed with or without B. pilosa, infected with or without E. tenella.
Rarefaction curves of bacterial 16S rRNA sequences from the guts of 18-day-old (4D) and 21-day-old (7D) chickens fed with or without B. pilosa, infected with or without E. tenella, as in Fig 1B, were analyzed.
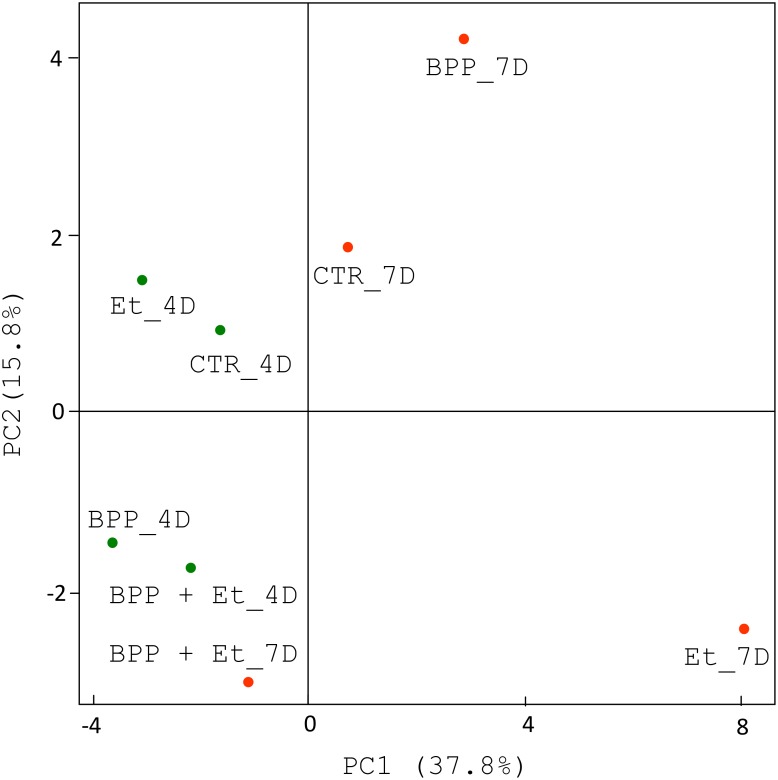
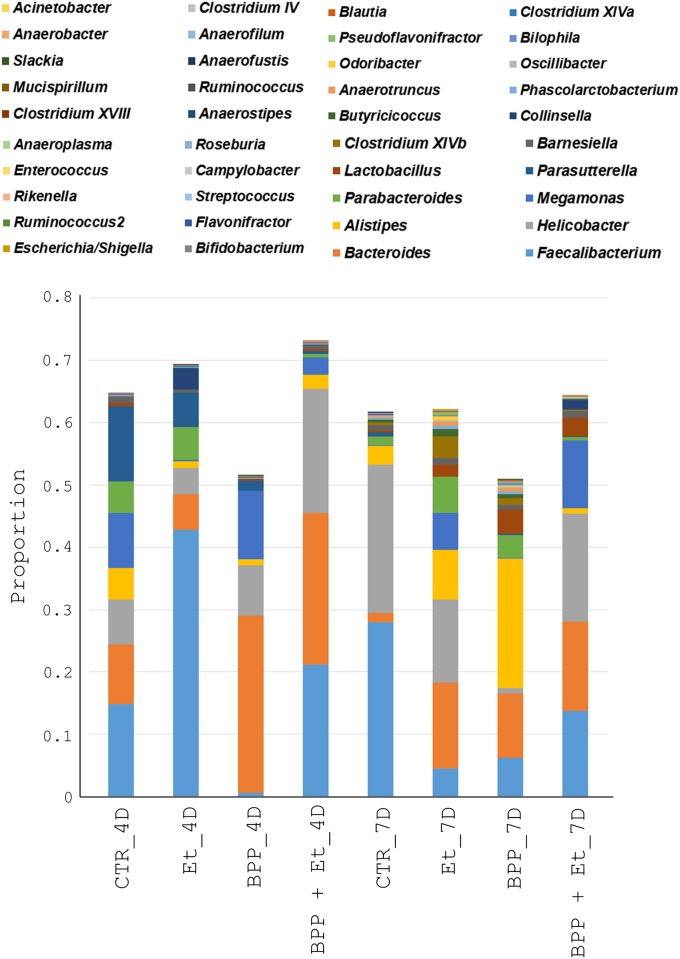
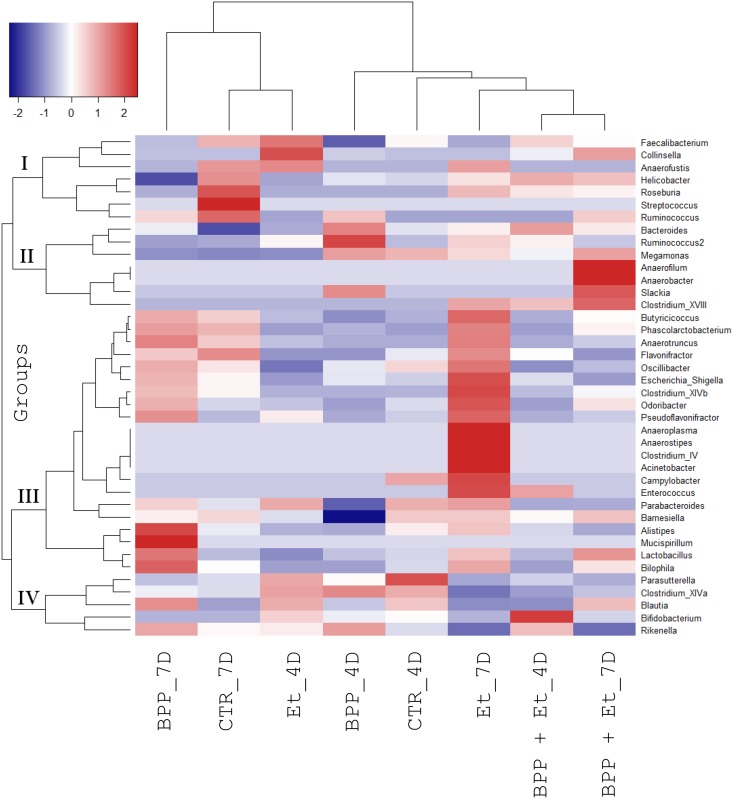
Sequence analysis using RDP classifier revealed 6 phyla, 13 classes, 15 orders, 25 families, and 42 genera of known bacteria were present in the samples. Overall, six phyla (Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Tenericutes and Deferribacteres), six classes (Clostridia, Bacteroidia, Epsilonproteobacteria, Negativicutes, Bacilli and Betaproteobacteria), six orders (Clostridiales, Bacteroidales, Campylobacterales, Selenomonadales, Lactobacillales and Burkholderiales), nine families (Ruminococcaceae, Helicobacteraceae, Bacteroidaceae, Lachnospiraceae, Rikenellaceae, Veillonellaceae, Porphyromonadaceae, Lactobacillaceae and Sutterellaceae) and eight genera (Faecalibacterium, Helicobacter, Bacteroides, Alistipes, Megamonas, Parabacteroides, Lactobacillus and Parasutterella) existed in all eight samples in different proportions. Principal component analysis indicated that the bacterial community compositions of the eight samples were diverse (Fig 3). The bacterial community composition at the genus level shown in Fig 4 further confirmed the data obtained from principal component analysis. Details about the bacterial community compositions in eight samples are shown in the supplementary data (S1 Fig). To identify the co-occurring bacterial genera groups among eight experimental samples, clustering analysis was performed. As shown in Fig 5, 40 bacterial genera were grouped into four clusters. Subsets of bacterial genera associated with growth performance and E. tenella infection in chickens were identified and described below.
Fig 3. Principal component analysis of the bacterial community compositions at the genus level in the guts of chickens.
Principal component analysis was conducted to compare the bacteria genera based on the 16S rRNA sequences in 8 samples as in Fig 2.
Fig 4. Proportion of the bacterial community compositions at the genus level in the guts of chickens.
Proportion of the bacterial genera in the guts of chickens (Fig 2) was determined.
Fig 5. Clustering analysis of the compositions of the bacterial genera in the guts of chickens.
Clustering analysis of the bacterial genera in chicken guts (Fig 2) was performed to correlate the proportion of the bacterial community with their genera.
Effect of B. pilosa on the change in gut microbiota
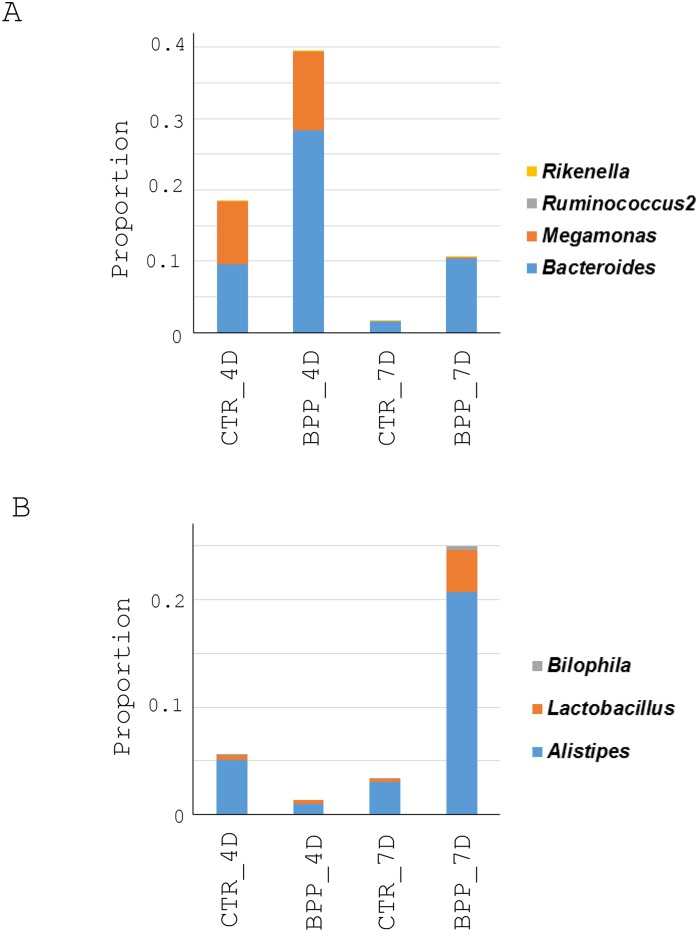
Gut microbiota have been documented to correlate to growth performance and gut health in chickens. We therefore, first investigated the correlation between microbiota and growth performance in chickens fed with B. pilosa. Two subsets of bacterial genera in Group II and III were found to exhibit higher proportions in the guts of the chickens fed with standard diet containing B. pilosa but lower proportions in those of the other groups (Fig 5). The first subset of bacterial genera, Bacteroides, Megamonas, Rikenella, and Ruminococcus2 was increased in B. pilosa-fed chickens aged 18 days (Fig 6A). Similarly, the second subset of bacterial genera, Alistipes, Bilophila and Lactobacillus, was elevated in B. pilosa-fed chickens aged 21 days (Fig 6B). All the above genera were reported to be beneficial microbiota. We failed to identify an elevation of these genera in the guts of chickens following E. tenella infection (data not shown) probably because the probiotics are easily disturbed by coccidiosis. Collectively, B. pilosa elevated a number of gut probiotics in chickens. Moreover, this elevation was inversely associated with FCR in chickens based on Spearman's rank correlation coefficient (r = −0.8 to −1).
Fig 6. Change in the proportion of the probiotic bacterial genera in the guts of chickens fed with or without B. pilosa.
The bacterial genera increased in proportion in the guts of the non-infected chickens by B. pilosa (Fig 2), were re-plotted into histograms. Only the data obtained from CTR_4D, BPP_4D, CTR_7D and BPP_7D are shown. Two patterns of change in the proportions of the bacterial genera are presented in Fig 6A and 6B. The genera in Fig 6A were increased in 18-day-old chickens whereas those in Fig 6B were increased in 21-day-old chickens.
Gut pathology-associated bacterial genera after E. tenella infection
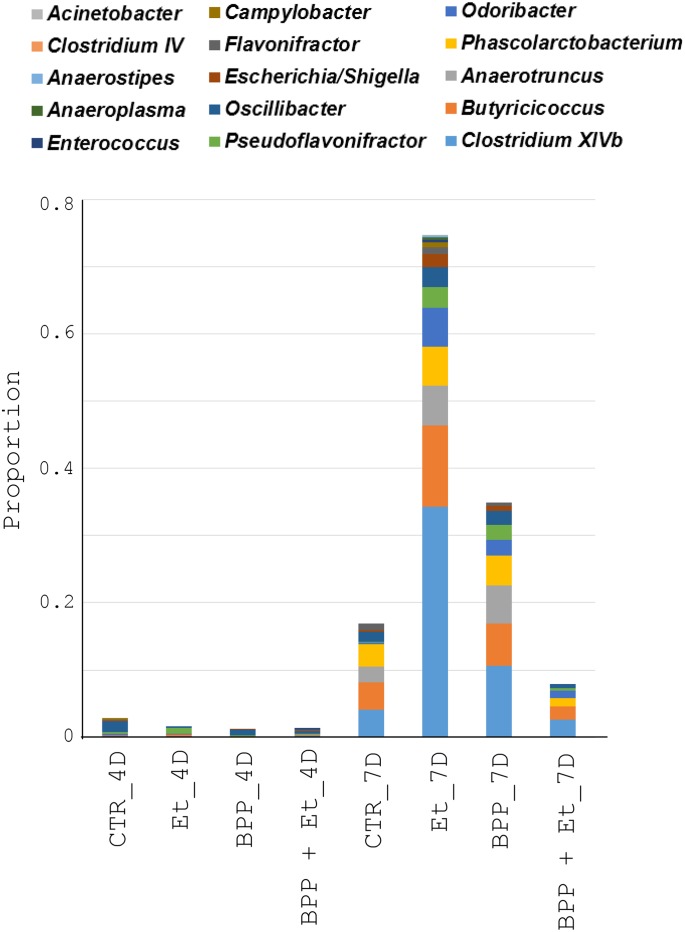
We also wanted to correlate microbiota with gut lesions in the guts of chickens, fed with PBS and B. pilosa, following E. tenella infection. Cluster analysis showed that one subset of 15 bacterial genera in Group III exhibited higher proportions in the guts of the E. tenella-infected chickens as they aged but lower proportions in the B. pilosa-fed E. tenella-infected chickens (Et_7D vs BPP + Et_7D, Fig 5). Change in the proportion of the 15 bacterial genera in the guts of chickens, aged 18 days, 4 days post-infection was not evident (Et_4D vs BPP + Et_4D, Fig 7). However, this change became evident in E. tenella-infected chickens, aged 21 days, suggesting that the 15 bacterial genera in chicken guts were associated with gut pathology (Et_7D vs BPP + Et_7D, Fig 7). These bacteria included Actinobacter, Clostridium IV, Anaerostipes, Anaeroplasma, Enterococcus, Campylobacteria, Flavonifractor, Escherichia/Shigella, Oscillibacter, PseodoFlavonifractor, Odoribacter, Phascolarctobacterium, Anaerotruncus, Butyricicoccus and Clostridium XIVb. Among them, Escherichia/Shigella, Campylobacter, Enterococcus, Clostridium and Acinetobacter are known as opportunistic pathogens of zoonotic origin, that not only affect the domestic animal industry but also cause public health problems in humans [23–29]. Of note, B. pilosa reduced the proportion of these opportunistic zoonotic pathogens in the guts of the chickens, suggesting this plant inhibited the pathogenic bacteria in the guts of chickens infected with E. tenella (Fig 7). Moreover, the decrease in the above 5 harmful genera was inversely correlated with villus length and the villus-to-crypt ratio, hallmarks of gut pathology, in chickens based on Spearman’s rank correlation coefficient (r = −0.8 to −1).
Fig 7. Change in the proportion of the zoonotic bacterial genera in the guts of chickens, fed with or without B. pilosa, following E. tenella infection.
The bacterial genera, which decreased in proportion in the guts of the E. tenella-infected chickens by B. pilosa (Fig 2), were re-plotted into histograms.
Discussion
Akin to human medicine, medicinal herbs and their compounds have been used as veterinary medicines in animals via multiple mechanisms of action. Their mechanisms include regulation of nutrient digestion and absorption, microbiota, immunity and ROS clearance, etc. Here, we showed that B. pilosa enhanced growth performance (Table 1), changed gut microbiota (Table 2 and Fig 5) and reduced E. tenella-implicated gut pathogenesis (Fig 1C and 1D). Besides, B. pilosa selectively increased probiotics and decreased harmful bacteria in the guts of chickens (Figs 5–7).
B. pilosa and its phytochemicals were reported to modulate gastrointestinal diseases, immunity and bacteria [16]. However, anti-coccidial compounds of B. pilosa need to be further ascertained. The mechanism by which B. pilosa ameliorates growth performance and coccidiosis in chickens is unknown. Our data collectively suggest that B. pilosa regulates a shift in gut microbiota in chickens. In fact, we found that B. pilosa did alter the proportion of gut microbiota in chickens, including an increase in 7 probiotic genera (Figs 5 and 6) and a decrease in 15 bacterial genera, including 5 harmful bacteria (Figs 5 and 7). As far as the 7 probiotics are concerned, Alistipes, Bacteroides, Lactobacillus, and Ruminococcus are known as probiotics for growth performance and weight gain in chickens [30,31]. Bacteroides and Megamonas were reported to be implicated in propionate production in chicken guts [32]. Megamonas and Ruminococcus were reported to be involved in polysaccharide degradation and utilization in chicken guts [32]. Bacteroides and Lactobacillus were shown to produce some essential vitamins (i.e., vitamin K, vitamin B12, and folic acid) and contribute to intestinal bile acid metabolism and recirculation [33]. Moreover, Lactobacillus has been used as a probiotic to control coccidiosis in chickens infected with Eimeria species [34,35]. Thus, gut microbiota play an important role in the clinical outcomes of coccidiosis in chickens [34,35]. In sharp contrast, the proportion of 15 bacterial genera in chicken guts was decreased by B. pilosa (Fig 7). Among them, Escherichia/Shigella, Campylobacter, Enterococcus, Clostridium and Acinetobacter, known as opportunistic pathogens of zoonotic origin, not only affect domestic animal industry but also cause public health problems in men [23–29]. Consistent with the function of B. pilosa in outgrowth of probiotics, this plant prevented growth of these opportunistic zoonotic pathogens in chicken guts (Fig 7). This study supports the notion that B. pilosa acts as a prebiotic to enhance the growth of probiotics in chicken guts to increase growth performance and reduce coccidiosis. It should be also noted that B. pilosa may be useful as a feed substituent and additive, as our results show that it can improve growth performance and coccidiosis (Table 1 and Fig 1). This application also lowers feed cost from crops and anti-coccidial agents, and risk of anti-coccidial contamination. This work also expands the medicinal utility of B. pilosa in animals [36,37], to target the balance of gut microbiota [38,39].
Conclusions
Here, we studied and demonstrated the beneficial effect of B. pilosa on growth performance (i.e., body weight gain and FCR), gut bacteria and E. tenella infection in chickens. Overall the data suggest that B. pilosa may have a novel function as a prebiotic, which elevates beneficial bacteria and reduces harmful bacteria in chicken guts. This work further illustrates the potential use of B. pilosa as a feed additive in organic chicken production.
Supporting Information
The chickens from Groups 1 to 4 were sacrificed on days 4 (D4) and 7 (D7) and the bacterial DNA samples of their guts (ceca and intestines) were pooled into 8 samples. Individual bacterial community composition was analyzed and listed in A (CTR_4D), B (BPP_4D), C (Et_4D), D (BPP + Et_4D), E (CTR_4D), F (BPP_4D), G (Et_4D) and H (BPP + Et_4D) from the guts of samples. B. pilosa alleviates E. tenella-mediated gut pathology in chickens.
(PDF)
Acknowledgments
We thank the members of W. C. Yang’s lab for constructive discussions and Miranda Loney for manuscript editing.
Abbreviations
- PBS
phosphate-buffered saline
- OTU
operational taxonomic unit
- CTR
uninfected untreated control
- Et
Eimeria tenella-infected untreated
- BPP
B. pilosa product-treated
- Et + BPP
Eimeria tenella-infected B. pilosa product-treated
- FCR
Feed conversion ratio
- H&E
hematoxylin and eosin
- OUT
Operational taxonomic unit
- Shan
Shannon diversity index
- Chao1
Chao1 diversity index
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Innovative Translational Agriculture Research (ITAR) grant of Academia Sinica, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sharman PA, Smith NC, Wallach MG, Katrib M (2010) Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol 32: 590–598. 10.1111/j.1365-3024.2010.01209.x [DOI] [PubMed] [Google Scholar]
- 2.Belova AV, Smutka L, Rosochatecká E (2012) World chicken meat market—Its development and current status. Acta Uni Agric Silvic Mendel Brun 4: 15–30. [Google Scholar]
- 3.Adil S, Magray SN (2012) Impact and manipulation of gut microflora in poultry: A review. J Ani Vet Adv 11: 873–877. [Google Scholar]
- 4.Barnes EM (1972) The avian intestinal flora with particular reference to the possible ecological significance of the cecal anaerobic bacteria. Am J Clin Nutr 25: 1475–1479. [DOI] [PubMed] [Google Scholar]
- 5.Barnes EM (1977) Ecological concepts of the anaerobic flora in the avian intestine. Am J Clin Nutr 30: 1793–1798. [DOI] [PubMed] [Google Scholar]
- 6.Barnes EM, Mead GC, Barnum DA, Harry EG (1972) The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria. Br Poult Sci 13: 311–326. [DOI] [PubMed] [Google Scholar]
- 7.Rantala M, Nurmi E (1973) Prevention of the growth of Salmonella infantis in chicks by the flora of the alimentary tract of chickens. Br Poult Sci 14: 627–630. [DOI] [PubMed] [Google Scholar]
- 8.van der Wielen PWJJ, Keuzenkamp DA, Lipman LJ, van Knapen F, Biesterveld S (2002) Spatial and temporal variation of the intestinal bacterial community in commercially raised broiler chickens during growth. Microb Ecol 44: 286–293. [DOI] [PubMed] [Google Scholar]
- 9.Vispo C, H. KW (1997) The interaction of avian gut microbes and their host: an elusive symbiosis Mackie RI, White BA, editors. London: Chapmann & Hall. [Google Scholar]
- 10.Barrow P (1992) Probiotics for chickens; Fuller R, editor. London: Chapman and Hallbook. [Google Scholar]
- 11.Williams RB (1998) Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int J Parasitol 28: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 12.Castanon JI (2007) History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci 86: 2466–2471. [DOI] [PubMed] [Google Scholar]
- 13.Serratosa J, Blass A, Rigau B, Mongrell B, Rigau T, Tortades M, et al. (2006) Residues from veterinary medicinal products, growth promoters and performance enhancers in food-producing animals: a European Union perspective. Rev Sci Tech 25: 637–653. [PubMed] [Google Scholar]
- 14.Williams RB, Gobbi L (2002) Comparison of an attenuated anticoccidial vaccine and an anticoccidial drug programme in commercial broiler chickens in Italy. Avian Pathol 31: 253–265. [DOI] [PubMed] [Google Scholar]
- 15.Orengo J, Buendia AJ, Ruiz-Ibanez MR, Madrid J, Del Rio L, Catala-Gregori P, et al. (2012) Evaluating the efficacy of cinnamaldehyde and Echinacea purpurea plant extract in broilers against Eimeria acervulina. Vet Parasitol 185: 158–163. 10.1016/j.vetpar.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 16.Bartolome AP, Villasenor IM, Yang WC (2013) Bidens pilosa L. (Asteraceae): Botanical Properties, Traditional Uses, Phytochemistry, and Pharmacology. Evid Based Complement Alternat Med 2013: 340215 10.1155/2013/340215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwale M, Masika PJ (2009) Ethno-veterinary control of parasites, management and role of village chickens in rural households of Centane district in the Eastern Cape, South Africa. Trop Anim Health Prod 41: 1685–1693. 10.1007/s11250-009-9366-z [DOI] [PubMed] [Google Scholar]
- 18.Sugiharto S (2015) Role of nutraceuticals in gut health and growth performance of poultry. J Saudi Soc Agric Sci: in press. [Google Scholar]
- 19.Yang WC, Tien YJ, Chung CY, Chen YC, Chiou WH, Hsu SY, et al. (2015) Effect of Bidens pilosa on infection and drug resistance of Eimeria in chickens. Res Vet Sci 98: 74–81. 10.1016/j.rvsc.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 20.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37: D141–145. 10.1093/nar/gkn879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ondov BD, Bergman NH, Phillippy AM (2011) Interactive metagenomic visualization in a Web browser. BMC Bioinformatics 12: 385 10.1186/1471-2105-12-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bittar F, Keita MB, Lagier JC, Peeters M, Delaporte E, Raoult D (2014) Gorilla gorilla gut: a potential reservoir of pathogenic bacteria as revealed using culturomics and molecular tools. Sci Rep 4: 7174 10.1038/srep07174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bublitz DC, Wright PC, Rasambainarivo FT, Arrigo-Nelson SJ, Bodager JR, Gillespie TR (2015) Pathogenic enterobacteria in lemurs associated with anthropogenic disturbance. Am J Primatol 77: 330–337. 10.1002/ajp.22348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemper N, Aschfalk A, Holler C (2006) Campylobacter spp., Enterococcus spp., Escherichia coli, Salmonella spp., Yersinia spp., and Cryptosporidium oocysts in semi-domesticated reindeer (Rangifer tarandus tarandus) in Northern Finland and Norway. Acta Vet Scand 48: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schilling AK, Hotzel H, Methner U, Sprague LD, Schmoock G, El-Adawy H, et al. (2012) Zoonotic agents in small ruminants kept on city farms in southern Germany. Appl Environ Microbiol 78: 3785–3793. 10.1128/AEM.07802-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warwick C, Corning S (2013) Managing patients for zoonotic disease in hospitals. JRSM Short Rep 4: 2042533313490287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weese JS, McCarthy L, Mossop M, Martin H, Lefebvre S (2007) Observation of practices at petting zoos and the potential impact on zoonotic disease transmission. Clin Infect Dis 45: 10–15. [DOI] [PubMed] [Google Scholar]
- 29.Zambrano LD, Levy K, Menezes NP, Freeman MC (2014) Human diarrhea infections associated with domestic animal husbandry: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg 108: 313–325. 10.1093/trstmh/tru056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angelakis E, Raoult D (2010) The increase of Lactobacillus species in the gut flora of newborn broiler chicks and ducks is associated with weight gain. PLoS One 5: e10463 10.1371/journal.pone.0010463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torok VA, Hughes RJ, Mikkelsen LL, Perez-Maldonado R, Balding K, MacAlpine R, et al. (2011) Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl Environ Microbiol 77: 5868–5878. 10.1128/AEM.00165-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Penn CW, Pallen MJ (2014) Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One 9: e91941 10.1371/journal.pone.0091941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo YH, Peng HW, Wright AD, Bai SP, Ding XM, Zeng QF et al. (2013) Broilers fed dietary vitamins harbor higher diversity of cecal bacteria and higher ratio of Clostridium, Faecalibacterium, and Lactobacillus than broilers with no dietary vitamins revealed by 16S rRNA gene clone libraries. Poult Sci 92: 2358–2366. 10.3382/ps.2012-02935 [DOI] [PubMed] [Google Scholar]
- 34.Giannenas I, Tsalie E, Triantafillou E, Hessenberger S, Teichmann K, Mohnl M, et al. (2014) Assessment of probiotics supplementation via feed or water on the growth performance, intestinal morphology and microflora of chickens after experimental infection with Eimeria acervulina, Eimeria maxima and Eimeria tenella. Avian Pathol 43: 209–216. 10.1080/03079457.2014.899430 [DOI] [PubMed] [Google Scholar]
- 35.Ritzi MM, Abdelrahman W, Mohnl M, Dalloul RA (2014) Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult Sci 93: 2772–2778. 10.3382/ps.2014-04207 [DOI] [PubMed] [Google Scholar]
- 36.Chang CL, Kuo HK, Chang SL, Chiang YM, Lee TH, Wu WM, et al. (2005) The distinct effects of a butanol fraction of Bidens pilosa plant extract on the development of Th1-mediated diabetes and Th2-mediated airway inflammation in mice. J Biomed Sci 12: 79–89. [DOI] [PubMed] [Google Scholar]
- 37.Chang SL, Chang CL, Chiang YM, Hsieh RH, Tzeng CR, Wu TK, et al. (2004) Polyacetylenic compounds and butanol fraction from Bidens pilosa can modulate the differentiation of helper T cells and prevent autoimmune diabetes in non-obese diabetic mice. Planta Med 70: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 38.Chang CL, Chang SL, Lee YM, Chiang YM, Chuang DY, Kuo HK, et al. (2007) Cytopiloyne, a polyacetylenic glucoside, prevents type 1 diabetes in nonobese diabetic mice. J Immunol 178: 6984–6993. [DOI] [PubMed] [Google Scholar]
- 39.Chang SL, Yeh HH, Lin YS, Chiang YM, Wu TK, Yang WC (2007) The effect of centaurein on interferon-gamma expression and Listeria infection in mice. Toxicol Appl Pharmacol 219: 54–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The chickens from Groups 1 to 4 were sacrificed on days 4 (D4) and 7 (D7) and the bacterial DNA samples of their guts (ceca and intestines) were pooled into 8 samples. Individual bacterial community composition was analyzed and listed in A (CTR_4D), B (BPP_4D), C (Et_4D), D (BPP + Et_4D), E (CTR_4D), F (BPP_4D), G (Et_4D) and H (BPP + Et_4D) from the guts of samples. B. pilosa alleviates E. tenella-mediated gut pathology in chickens.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.