Abstract
The easily recognised genus Otidea is subjected to numerous problems in species identification. A number of old names have undergone various interpretations, materials from different continents have not been compared and misidentifications occur commonly. In this context, Otidea is monographed, based on our multiple gene phylogenies assessing species boundaries and comparative morphological characters (see Hansen & Olariaga 2015). All names combined in or synonymised with Otidea are dealt with. Thirty-three species are treated, with full descriptions and colour illustrations provided for 25 of these. Five new species are described, viz. O. borealis, O. brunneoparva, O. oregonensis, O. pseudoleporina and O. subformicarum. Otidea cantharella var. minor and O. onotica var. brevispora are elevated to species rank. Otideopsis kaushalii is combined in the genus Otidea. A key to the species of Otidea is given. An LSU dataset containing 167 sequences (with 44 newly generated in this study) is analysed to place collections and determine whether the named Otidea sequences in GenBank were identified correctly. Fourty-nine new ITS sequences were generated in this study. The ITS region is too variable to align across Otidea, but had low intraspecific variation and it aided in species identifications. Thirty type collections were studied, and ITS and LSU sequences are provided for 12 of these. A neotype is designated for O. cantharella and epitypes for O. concinna, O. leporina and O. onotica, along with several lectotypifications. The apothecial colour and shape, and spore characters are important for species identification. We conclude that to distinguish closely related or morphologically similar species, a combination of additional features are needed, i.e. the shape of the paraphyses, ectal excipulum structure, types of ectal excipulum resinous exudates and their reactions in Melzer’s reagent and KOH, tomentum and basal mycelium colours and exudates. The KOH reaction of excipular resinous exudates and basal mycelium are introduced as novel taxonomic characters.
Keywords: Flavoscypha, ITS, ITS1 minisatellites, LSU, Otideopsis, resinous exudates
INTRODUCTION
Species of Otidea produce typically ear-shaped apothecia that are unique within Pyronemataceae (Pezizomycetes). The genus is monophyletic based on multilocus phylogenetic analyses from a few, but broadly sampled, Otidea species (Hansen et al. 2013). Despite being distinct at the generic level, the species identification and nomenclature of Otidea are highly controversial. A few recent typifications have been proposed (Carbone 2009, 2010a), but many names are still subjected to different interpretations. Several new species were described in the last decades from Europe (Harmaja 1976, 2009a) and Asia (Cao et al. 1990, Zhuang & Yang 2008), with detailed descriptions and updated identification keys. However, often no illustrations were presented and colour photographs have rarely been published when describing new species. Many names of European species currently used in North America and Asia are misapplied. Multilocus phylogenetic analyses have not been previously implemented to critically address species delimitation issues and material from different continents has not been compared. A worldwide critical revision of Otidea to clarify species limits is highly needed. The aims of this study were: i) to undertake a nomenclatural and taxonomic revision of Otidea, to clarify misinterpretations and to propose pertinent typifications to stabilise the use of names; and ii) to provide detailed species descriptions and colour photographs of both macro- and microscopic structures, and a key for identification. Our multilocus phylogenies and robust hypotheses of species limits, employing genealogical concordance phylogenetic species recognition (GCPSR; Taylor et al. 2000), which is the basis for the present work, are given in Hansen & Olariaga (2015). In the present study we present an LSU rDNA phylogeny to place a larger number of collections for which we have been unable to obtain multiple genes, including several sequences from GenBank, many of which we here re-identify.
Taxonomic history
The first valid publication of Otidea is by Bonorden (1851), based on Peziza (unranked) Otidea Pers., although it has sometimes been attributed to Fuckel (Kanouse 1949, Nannfeldt 1966, Liu & Zhuang 2006, Smith & Healy 2009). Otidea species were treated in a broad heterogeneous genus Peziza by early authors. Persoon (1822) defined Peziza (unranked) Otidea as producing auriculate apothecia with a split, sometimes elongated on one side, and included 10 species. Fries (1822) referred to this group as Peziza (unranked) Cochleatae, but he included also taxa with non-split apothecia. Bonorden (1851) elevated Otidea to generic rank with split apothecia as the key feature, but also referred to Fries’ (1822) Cochleatae. He did not make any combinations or list any species. Fuckel (1870) refined the genus using microscopic details, namely uni- or biguttulate spores and filiform to subclaviform paraphyses, and included four species: O. abietina, O. cochleata O. leporina and O. onotica. Boudier (1885) notably contributed to disassemble the large genus Peziza into smaller and more natural genera. He placed in the genus Otidea species with entire or split apothecia, biguttulate spores and, importantly non-amyloid asci and curved paraphyses, which he was the first to introduce. He divided Otidea into two subgenera: Otidea with split apothecia and Pseudotis with entire apothecia. Interestingly, Boudier erected the genus Wynnella (Helvellaceae) to accommodate W. silvicola (as P. leporina / P. auricula), a species with distinctly ear-shaped apothecia, but differing from Otidea in the uniguttulate spores and tough consistency. In Boudier’s (1907) subsequent treatment, he elevated Pseudotis to genus rank and placed here species with entire apothecia, including O. daliensis (as P. apophysata) and O. propinquata (as P. abietina), both with biguttulate spores and hooked paraphyses. Saccardo listed O. onotica as an ‘exemplar’ species of Peziza subg. Otidea in his synopsis of the discomycete genera (1884; he listed in general only one species per genus / subgenus that appear to have been selected as typical for the genera) and it has since been accepted as the type species by most (Rifai 1968, Eckblad 1968, Korf 1972, Liu & Zhuang 2006, Parslow & Spooner 2013), Index Nominum Genericorum (Eckblad in Farr et al. 1979), NCU-3 (Greuter et al. 1993) and is prepared to be adopted on the List of Protected generic Names for fungi (Kirk et al. 2013). Clements & Shear (1931) listed O. cochleata as the type, but this name was not among the original species accepted by Persoon (1822) and has furthermore been considered an ambiguous name. Kanouse (1949) proposed O. leporina as the type species, but because of the confusions surrounding the identity of this species until now, it has been considered an inappropriate choice.
The genus Scodellina was described by Gray (1821) and involved species attributed today to Otidea, and also Aleuria, Peziza and Tarzetta. Seaver (1928) refined Scodellina to species with split to ear-shaped apothecia only and typified it with Peziza leporina (Seaver 1927), considering Otidea a later synonym. This was for a period followed by several American authors (e.g. Korf 1963, Kimbrough 1966). The typification by Seaver can however, be considered largely mechanical, taken as the first species listed by Gray, and be superseded under the ICN (Art. 10.5; McNeill et al. 2012). Also, even though Gray (1821) included several species with split apothecia, he did not mention this feature in the diagnosis of Scodellina, but emphasised “thallus … hemispherical, spreading” and coined the vernacular name “spread cup”. Therefore Rifai (1968) designated P. vesiculosa Bull.: Fr. as the type species of Scodellina, consequently making it a later synonym of the genus Peziza. Eckblad (1968) came to the same conclusion.
Kanouse (1949) broadened the concept of Otidea and described in detail a number of North American species. She included species with split apothecia and straight paraphyses with swollen apices. She also included Wynnella silvicola (as Otidea auricula), with straight paraphyses and uniguttulate spores. Nannfeldt (1966) delimited Otidea to species with non-amyloid asci; smooth, uninucleate, biguttulate spores; a medullary excipulum of textura intricata; and an ectal excipulum with isodiametric cells, covered by short chains of barrel-shaped cells. Nannfeldt’s concept more or less conforms to the genus Otidea as we recognise it today. He considered Wynnella so distant that it should be treated in a separate tribe of Pezizaceae. Korf (1963) reviewed the monotypic, sparassoid genus Ascosparassis, and subsequently (Korf 1973a) assigned A. shimizuensis to Otidea, based on the hooked paraphyses, small biguttulate spores and excipulum structure. Pfister (1979) however, considered Ascosparassis a distinct monotypic genus, based on “small asci and spores and peculiar growth habit”, and combined the older name Midotis heinricheri in Ascosparassis. Later Pfister collected A. heinricheri in South America, in north coastal mountains of Venezuela (Pfister & Halling 1989), extending its Asian distribution (China, Indonesia and Japan), still considering the species separate from Otidea.
A new genus Flavoscypha was erected for two species of Otidea, O. concinna (as Flavoscypha cantharella) and O. phlebophora, with strong emphasis on the ectal excipulum of textura prismatica (vs textura angularis in Otidea) (Harmaja 1974). Otidea was further emended to include a species with ornamented spores, O. unicisa, otherwise ‘fitting perfectly’ Otidea (Harmaja 1986). Otideopsis was published with Otideopsis yunnanensis as the type species, distinguished from Otidea by having ornamented spores and paraphyses fused at the apices (Liu & Cao 1987). Flavoscypha and Otideopsis are now considered synonyms of Otidea based on molecular phylogenetic analyses (Liu & Zhuang 2006, Hansen & Olariaga 2015).
Recently the circumscription of Otidea was further broadened when the first hypogeous species, O. subterranea, was discovered using ITS and LSU sequences (Smith & Healy 2009). All the characters proposed so far as diagnostic for Otidea have exceptions across the genus. Nevertheless, Otidea can be recognised by the non-amyloid asci, in combination with at least two of these characters (except O. subterranea): a) biguttulate spores; b) hooked or bent paraphyses; c) medium-large, split apothecia; and d) a medullary excipulum of textura intricata, and an ectal excipulum of textura angularis or textura prismatica.
Systematic position and relationships
Nannfeldt (1937, 1938, 1966) suggested a close relationship between Otidea (incl. Pseudotis), Tarzetta (as Pustularia) and Helvella (Pezizaceae, tribe Acetabuleae sensu Nannfeldt), based on similarities in asci, paraphyses and anatomical structures of the apothecia, i.e. a medullary excipulum of dense textura intricata, ectal excipulum of almost isodiametric large cells, and an outermost layer of shorter or longer chains of cells, possible forming distinct clusters or warts (and in Tarzetta prolonged to cylindrical, hyaline, wavy hairs). Le Gal (1947) similarly placed Otidea in the tribe Otideeae in her Aleuriaceae (i.e. a family including taxa with both amyloid and non-amyloid asci), together with Pseudotis and Tarzetta (as Pustularia), but placed Helvella in Helvellaceae. Following the ideas of Nannfeldt (1966), Eckblad (1968) erected the family Otideaceae as a small taxon of closely related genera that produce larger apothecia, most of which typically lack bright orange to red colours, including in it Tarzetta (as Pustulina) and Otidea, but also Ascosparassis, Geopyxis and Sowerbyella. Eckblad considered Helvella (Helvellaceae) to be distant, but having a possible shared origin with members of Otideaceae (and Morchellaceae and Rhizinaceae), due to the structure of the excipulum, spores and asci, especially of Tarzetta. At the same time, he expanded the concept of Pyronemataceae (to 21 genera) to taxa mostly characterised by the presence of carotenoid pigments, stating the inability to satisfactorily subdivide the family on the basis of common characters. Korf (1972, 1973b) placed Otidea in the tribe Otideeae (in the subfamily Otideoideae) in an even more encompassing Pyronemataceae (49 genera), together with Ascosparassis and Psilopezia. He followed the ideas of Arpin (1969), in excluding taxa with carotenoids from the Otideoideae, instead including taxa with prominent hairs such as Geopora, Humaria and Trichophaea. Otidea has generally been included in a broadly circumscribed Pyronemataceae in recent treatments (Dissing 2000, Hansen & Pfister 2006, Perry et al. 2007, Hansen et al. 2013). Multigene phylogenetic analyses of Pyronemataceae do not support a close relationship between Otidea and Geopyxis, Psilopezia, Sowerbyella or Tarzetta (Hansen et al. 2013). Surprisingly, the cleistothecial Warcupia and the highly reduced (gymnohymenial) Monascella are suggested as the closest relatives of Otidea. Otidea, Monascella and Warcupia are strongly supported as a distinct sister group to the rest of the Pyronemataceae in a strict sense (Hansen et al. 2013).
MATERIALS AND METHODS
Material and morphological methods
This study is based on a total of 450 specimens. One hundred and forty two of these were collected and studied fresh during this project and are deposited in S and a few in TUR-A. Specimens were studied from the following herbaria: AH, ARAN, BIO, C, FH, H, HKAS, K, MCVE, MICH, MIN, OSC, PC, PRM, S, TUR and UPS (Thiers 2014), GMFN (gruppo AMB di Fara Novarese, Italy), SEST (Sociedad de Ciencias Naturales de Sestao, Spain), and from the private herbaria of G. Corriol, GC; C. Lavorato, CL; N. Van Vooren, NV; and M. Tabarés, MT. Thirty type collections were examined, along with other original material. Colour codes are based on Kornerup & Wanscher (1961). For O. apophysata, O. borealis, O. daliensis, O. oregonensis, O. phlebophora, O. pseudoleporina, O. smithii and O. unicisa colour codes for fresh material were taken from photographs. Apothecial sections, i.e. the thickness of the apothecia including the hymenium, was measured midway between the apothecial margin and base. Smell and taste are listed when recorded. Only discharged, mature spores were measured from living material. To obtain and ensure mature spores from dried material, a square of c. 3 mm2 of an apothecium was revived in a drop of water on a slide, with the hymenium surface facing down and then removed after 1 hour. In this way mature spores deposited on top of the hymenium were recovered and measured. Spore measures exclude ornamentation. Spore statistics are based on measurements of 20 spores from each collection: Lm = mean length, Wm = mean width and Qm = Lm/Wm. The number of populations that the statistics are based on is indicated by ‘n’. Extreme values are given in parentheses. Hymenial elements were observed by teasing apart a small piece of hymenium with a needle. To observe the excipulum structure, sections of apothecia were made by hand prior to soaking the material in water. Basal mycelium was examined by mounting clumps of hyphae from the apothecial base (the tomentum) and from among the substrate particles. Only asci with mature spores were measured. All measurements were made in water: in living state whenever possible; only when not possible, measurements were made on rehydrated (over 2 hours in water) material. Melzer’s reagent (MLZ) and 10 % KOH were used to observe the reaction of resinous exudates and other pigmentation. Cotton Blue in lactic acid was used to observe spore ornamentation. Microanatomical terminology follows Korf (1973b). The notation ‘!’ indicates that type or other original material was examined by us.
If not otherwise indicated in the legends, the photographs presented in this paper were taken by K. Hansen and I. Olariaga.
DNA extraction, PCR amplification, sequencing and alignment
DNA was extracted from dried material, or from fresh material stored in 1 % SDS DNA extraction buffer. The extraction method follows Hansen et al. (1999), except dried material was ground in a Mini-BeadbeaterTM (Biospec Products, Bartlesville, OK, USA) and fresh material using a plastic pestle, in eppendorf tubes. The primer combination ITS5-ITS4, and in a few instances ITS1–ITS4, ITS5–5.8S and ITS3–ITS4, were used to PCR amplify the ITS region, and LR0R–LR5 the LSU region. For DNA extracted from fresh material (stored in SDS extraction buffer), the ITS and LSU regions were amplified in a single piece using the primers ITS1–LR5. The ITS2 region for O. integra (possible original material from 1892) was successfully amplified in three pieces, in combination with newly designed primers for the O. concinna clade: ITS3 – ConcITS2midR (5′-GCCTGTAAATTTTAAAGACGAA-3′); ConcITS2midF (5′-CCAGGGTTGCTTTGGTA-3′) –ConcITS4intR (5′-CACTGGGTAATTGGAGGTTT-3′); ConcITS2midF (5′-CCAGGGTTGCTTTGGTA-3′) – ITS4. PCR products were cleaned using ExoSAP-IT® (USB, Cleveland, OH, USA). The ITS was sequenced in both directions, using the primers ITS1 and ITS4 (and/or in a few instances ITS5, 5.8S and ITS3) and the LSU using LR0R and LR5. For O. integra, the same primers as used for PCR, were also used for sequencing. PCR and sequencing conditions follows Hansen & Olariaga (2015).
Sequences were edited and assembled using Sequencher v. 4.10 (Gene Codes Corporation Ann Arbor, Michigan, USA) and have been deposited in GenBank (Table 1). The sequences were aligned manually in Se-Al v. 2.0a11 Carbon (Rambaut 2002). An all taxa LSU dataset was prepared. Monascella botryosa and Warcupia terrestris were used as outgroup, based on a higher level phylogenetic study of Pyronemataceae (Hansen et al. 2013), which supports these as the closest sister group. To explore inter- and intraspecific variation of the new species O. borealis, O. subformicarum and their closest relatives, two smaller datasets were prepared using ITS and LSU rDNA sequences, based on a more species-inclusive, multi-gene phylogeny of Otidea (Hansen & Olariaga 2015). All three alignments are available from TreeBASE as accession no. S15887. The first dataset (the O. borealis dataset) contained O. borealis and related species in the O. concinna clade. The second dataset (the O. formicarum dataset) contained specimens of the O. formicarum clade. Otidea caeruleopruinosa and O. nannfeldtii were used as outgroup for each of these datasets. Nucleotide diversity (e.g. Nei 1987, equation 10.6), as the average number of nucleotide differences per sites between two ITS sequences, was calculated within O. brunneoparva and O. subformicarum. One insertion or deletion, despite the length, was calculated as only one basepair difference.
Table 1.
Collections used in the molecular phylogenetic analyses, with voucher information and GenBank accession numbers for ITS and LSU regions. Some GenBank sequences are re-identified by us and the names originally used in GenBank are listed after the taxon names (‘as’). For type specimens (in bold) the original names are kept regardless of synonymy. Numbers in parentheses following the species names indicate multiple collections of a species. The GenBank accessions of sequences generated in this study are in bold.
| Taxon | Voucher | Locality/year/collector | GenBank Accession no4 |
|
|---|---|---|---|---|
| ITS | LSU | |||
| Monascella botryosa | CBS 233.85 | Spain, 1985, J. Guarro | – | KC012688 |
| Otidea alutacea (1) | KH.09.170 (S) | Sweden, 2009, K. Hansen & I. Olariaga | KM0100591 | KC012691 |
| O. alutacea (2) | KH.10.193 (S) | Sweden, 2010, K. Hansen, K. Gillen & I. Olariaga | KM0100601 | KM8231881 |
| O. alutacea (3) | KH.07.46 (S) | Denmark, 2007, H. Knudsen | KM010061 | KM823457 |
| O. alutacea (4) | JS.08.81 (S) | Sweden, 2008, J. Santos | KM0100621 | KM8231871 |
| O. alutacea (5) | OSC 56747 | USA, 1996, E.T. Peterson | – | KM8231891 |
| O. alutacea (6) | OSC 56770 | USA, 1997, E.T. Peterson | – | AF072073 |
| O. alutacea (7) | OSC 56798 | USA, 1996, E.T. Peterson | – | AF086583 |
| O. alutacea (8) | OSC 56777 | USA, 1997, E.T. Peterson | – | AF086582 |
| O. alutacea (9) | JS.08.43 (S) | Sweden, 2008, J. Santos | KM010063 | KM823458 |
| O. alutacea (10) | KH.09.135 (S) | Norway, 2009, V. Kučera & I. Kautmanova | KM0100641 | KM8231901 |
| O. alutacea (11) | KH.10.198 (S) | Sweden, 2010, K. Hansen, K. Gillen & I. Olariaga | KM010065 | KM823459 |
| O. alutacea (12) | KH.09.178 (S) | Sweden, 2009, K. Hansen & I. Olariaga | KM0100661 | KM8231911 |
| O. alutacea (13) | KS-94-192 (C) | Denmark, 1994, K. Hansen & S.K. Sandal | KM010067 | KM823460 |
| O. alutacea (14) | C-F-48045 | Sweden, 1974, D. Paulsen & N. Tams | KM010068 | KM823461 |
| O. alutacea (15) | HMAS52742 | China | – | DQ443438 |
| O. alutacea (16) | HMAS57844 | China | – | DQ443439 |
| O. alutacea (17) | S-F257085 | Italy, 2010, M. Carbone | KM0100691 | KM8231921 |
| O. alutacea (18) | Moorefun19 (OSC) | USA, 2010, J. Moore | KM0100701 | KM8231941 |
| O. alutacea (19) | OSC 56758 | USA, 1996, E.T. Peterson | – | KM8231931 |
| O. alutacea (20) as O. umbrina | OSC 56813 | USA, 1997, E.T. Peterson | – | AF086584 |
| O. alutacea (21) as O. umbrina | OSC 56782 | USA, 2010, E.T. Peterson | – | AF086586 |
| O. alutacea (22) | KH.09.133 (S) | Norway, 2009, K. Hansen & I. Olariaga | KM0100711 | KM8231851 |
| O. alutacea (23) | ARAN A3023204 | Spain, 2009, J.I. López Amiano | KM0100721 | KM8231861 |
| O. alutacea (24) | GC 98092002 | France, 1998, G. Corriol | KM010073 | KM823462 |
| O. alutacea (25) | HMAS83560 | China, 2003, W.Y. Zhuang & Y. Nong | – | DQ443442 |
| O. alutacea (26) | HMAS83563 | China, 2003, W.Y. Zhuang & Y. Nong | – | DQ443440 |
| O. alutacea (27) | KS-94-111 (C) | Denmark, 1994, K. Hansen & S.K. Sandal | KM010074 | KM823463 |
| O. alutacea (28) | HMAS83559 | China, 2003, W.Y. Zhuang & Y. Nong | – | DQ443441 |
| O. alutacea (29) | S-F257084 | Italy, 2010, M. Carbone | KM010075 | KM823464 |
| O. alutacea (30) | KH.13.50 (S) | Sweden, 2013, K. Hansen & X. Wang | KM010076 | KM823465 |
| O. angusta | H6010804 | Finland, 1965, H. Harmaja | KF7175741 | KM8231951 |
| O. apophysata | S-F257062, dupl. private herb. Kasparek s.n. | Germany, 1999, F. Kasparek | KM0100771 | KM8231961 |
| O. borealis | S-F242694 | Finland, 2010, M. Carbone | KM0100231 | KM8231971 |
| O. brevispora as O. onotica var. brevispora | HKAS 43003 | China, 2003, Z.L. Yang | – | DQ443450 |
| O. brunneoparva (1) | KH.09.82 (S) | Sweden, 2009, K. Hansen & I. Olariaga | KM0100291 | KM8231981 |
| O. brunneoparva (2) | S-F249386 (Ex-H6017193) | Finland, 1978, H. Harmaja | KM010024 | KM823466 |
| O. brunneoparva (3) | S-F257086, dupl. TUR–A 198579 | Finland, 2009, M. Carbone | KM0100251 | KM8231991 |
| O. brunneoparva (4) | JS.08.66 (S) | Sweden, 2008, J. Santos | KM010028 | KM823467 |
| O. brunneoparva (5) | KH.08.107 (S) | Sweden, 2008, K. Hansen | KM0100261 | KM8232001 |
| O. brunneoparva (6) | TUR-A 198582 | Finland, 2011, M. Lahti | KM010027 | KM823468 |
| O. bufonia (1) | KH.09.172 (S) | Sweden, 2009, K. Hansen & I. Olariaga | JN942764 | JN941097 |
| O. bufonia (2) | JS.08.55 (S) | Sweden, 2008, J. Santos | KM010078 | KM823469 |
| O. bufonia (3) | KH.07.37 (S) | Denmark, 2007, K. Hansen | JN942767 | JN941098 |
| O. bufonia (4) | KH.09.248 (S) | Spain, 2009, J.L. Teres & P.M. Pasaban | JN942766 | JN941084 |
| O. bufonia (5) | KH.09.249 (S) | France, 2009, J.L. Teres | KM0100791 | KM8232011 |
| O. bufonia (6) | NV 2009.11.01 (S) | France, 2009, G. Moyne | JN942765 | JN941085 |
| O. bufonia (7) | C-F-94240 | Denmark, 2011, M. Sasa | KP119674 | – |
| O. caeruleopruinosa (1) | H6010805 | Finland, 1978, H. Harmaja | KF7175751 | KM8232021 |
| O. caeruleopruinosa (2) | MT 10082601 (SCM, dupl. S) | Spain, 2010, M. Tabarés & S. Santamaría | KM0100301 | KM8232031 |
| O. caeruleopruinosa (3) | KH.13.48 (S) | Sweden, 2013, I.-L. Walter | KM010081 | KM823470 |
| O. cantharella (1) | JS.08.18 (S) | Sweden, 2008, J. Santos | KM010082 | KM823471 |
| O. cantharella (2) | JS.08.47 (S) | Sweden, 2008, J. Santos | KM010083 | KM823472 |
| O. cantharella (3) | KH.09.125 (S) | Sweden, 2009, K. Hansen & I. Olariaga | KM0100841 | KM8232051 |
| O. cantharella (4) | NV 2008.09.16 (dupl. S) | France, 2008, J. Cavet | KM0100851 | KM8232041 |
| O. concinna (1) | JS.08.59 (S) | Sweden, 2008, B. Wasstorp | KM010031 | KM823473 |
| O. concinna (2) | KH.09.183 (S) | Sweden, 2009, K. Hansen & I. Olariaga | KM0100321 | JN941089 |
| O. concinna (3) | KH.09.250 (S) | Spain, 2009, F. Prieto & A. González | JN942775 | JN941095 |
| O. crassa | HMAS583571 | China, 2003, W.Y. Zhuang & Y. Nong | – | DQ443444 |
| O. daliensis (1) | HMAS57688 | China, 1988, S. Wang & W.Y. Zhuang | – | DQ443445 |
| O. daliensis (2) | SEST-06081702 | Spain, 2003, J.L. Pérez Butrón | KM0100861 | KM8232061 |
| O. flavidobrunneola (1) | H6010806 | Finland, 1978, H. Harmaja | KF7175761 | KM8232091 |
| O. flavidobrunneola (2) | H6010830 | Finland, 1987, P. Askola | KM0100871 | KM8232081 |
| O. flavidobrunneola (3) | KH.09.153 (S) | Norway, 2009, K. Hansen & I. Olariaga | KM0100881 | KM8232071 |
| O. formicarum (1) | H6003350 | Finland, 2005, U. Salo & P. Salo | KM010036 | KM823474 |
| O. formicarum (2) | JS.08.63 (S) | Sweden, 2008, J. Santos | KM0100351 | KM8232121 |
| O. formicarum (3) | H6003549 | Finland, 1970, L. Fagerström | KF7175771 | KM8232111 |
| O. formicarum (4) | S-F244372 (dupl. O) | Norway, 2009, J. Lorås | KM0100341 | KM8232101 |
| O. formicarum (5) | KH.11.104 (S) | Sweden, 2011, J.C. Zamora & I. Olariaga | KM010033 | KM823475 |
| O. fusconigra | GMFN 2293 | Italy, 2003, G. Jamoni | KM010037 | KM823476 |
| O. integra | S-F108342 | Italy, 1892, G. Bresadola | KP006504 | – |
| O. kauffmanii (1) | AH21147 (MICH) | USA, 1917, A.H. Smith & R.J. Porter | AF072095 | – |
| O. kauffmanii (2) | MICH14409 | USA, 1915, C.H. Kauffman | KF717579 | – |
| O. kaushalii | T. Læssøe 6236 (C, dupl. BORH) | Malaysia, 1999, T. Læssøe | KM0101191 | AF335111 |
| O. lactea | HMAS61359 (ex-MHSU 1803) | China, 1987, J. Z. Cao | – | DQ443447 |
| O. leporina (1) | HMAS83579 | China, 2003, W.Y. Zhuang & Y. Nong | – | DQ443448 |
| O. leporina (2) | HMAS83568 | China, 2003, W.Y. Zhuang & Y. Nong | – | DQ443449 |
| O. leporina (3) as O. smithii | − | − | – | AF0865732 |
| O. leporina (4) | OSC 56824 | USA, 1997, E.T. Peterson | – | KM8232161 |
| O. leporina (5) | OSC 56784 | USA, 1997, E.T. Peterson | – | KM8232151 |
| O. leporina (6) as O. sp. | HMAS583570 | China, 2003, W.Y. Zhuang & Y. Nong | – | DQ443443 |
| O. leporina (7) | JS.08.46 (S) | Sweden, 2008, J. Santos | KM010089 | KM823477 |
| O. leporina (8) | KH.09.93 (S) | Sweden, 2009, K. Hansen & I. Olariaga | KM0100901 | KM8232131 |
| O. leporina (9) | JS.08.92 (S) | Sweden, 2008, J. Santos | KM010091 | KM823478 |
| O. leporina (10) | NV 2008.09.28 (dupl. S) | France, 2008, N. Van Vooren | KM0100921 | KM8232141 |
| O. microspora | AH30502 (MICH) | USA, 1948, A.H. Smith | AF072094 | – |
| O. minor (1) | H6003841 | Finland, 2006, U. Salo & P. Salo | KM010040 | KM823479 |
| O. minor (2) | KH.10.311 (S) | Sweden, 2010, K. Hansen, K. Gillen & I. Olariaga | KM0100421 | KM8232181 |
| O. minor (3) | H6008618 | Finland, 1992, R. Saarenoksa | KM0100391 | KM8232191 |
| O. minor (4) | TL-Vorsø-0754 (C) | Denmark, 1982, T. Læssøe | KM010043 | KM823480 |
| O. minor (5) | CL 950914-01 (dupl. S) | Italy, 1995, C. Lavorato | KM0100441 | KM8232201 |
| O. minor (6) | KH.98.84 (C) | Denmark, 1998, K. Hansen | KM0100411 | KM8232171 |
| O. minor (7) | C-F-83445 | Denmark, 2007, T. Læssøe | KM010038 | KM823481 |
| O. mirabilis (1) | KH.09.188 (S) | Sweden, 2009, E. Bohus-Jensen, K. Hansen & I. Olariaga | JN942770 | JN941086 |
| O. mirabilis (2) | KH.10.285 (S) | Sweden, 2010, K. Hansen, K. Gillen & I. Olariaga | KM0100941 | KM8232211 |
| O. mirabilis (3) as O. umbrina | KH.01.09 (C) | Denmark, 2001, C. Lange | JN942769 | AY500540 |
| O. mirabilis (4) | S-F257083 | Finland, 2010, M. Carbone | KM010095 | KM823482 |
| O. mirabilis (5) | NV 2008.09.14 (dupl. S) | France, 2008, J. Cavet | JN942768 | JN941094 |
| O. mirabilis (6) | S-F256929 | Italy, 1999, D. Bolognini | KF717580 | KM823483 |
| O. myosotis | H6003548 | Finland, 1970, L. Fagerström | KF7175781 | KM8232221 |
| O. nannfeldtii (1) | CL 091116-17 (S) | Italy, 2009, C. Lavorato | KM010096 | KM823484 |
| O. nannfeldtii (2) | S-F257096 | Italy, 2009, B. De Ruvo | KM010097 | KM823485 |
| O. nannfeldtii (3) | CL 091207-01 (S) | Italy, 2009, C. Lavorato | KM010098 | KM823486 |
| O. nannfeldtii (4) (= O. lohjaënsis nom. prov. Harmaja) | S-F249387 (Ex-H6017194) | Finland, 1978, H. Harmaja | KM0100931 | KM8232251 |
| O. nannfeldtii (5) | JS.08.103 (S) | Sweden, 2008, J. Santos | KM0100451 | KM8232241 |
| O. nannfeldtii (6) | NV 2008.10.01 (dupl. S) | France, 2008, N. Van Vooren | KM0100991 | KM8232271 |
| O. nannfeldtii (7) | H6002902 | Finland, 1972, C.-A. Haeggström | KF7175811 | KM8232281 |
| O. nannfeldtii (8) | rh101310 (OSC) | USA, 2010, R. Helliwell | KM0101001 | KM8232261 |
| O. nannfeldtii (9) | KH.10.302 (S) | Sweden, 2010, K. Hansen, K. Gillen & I. Olariaga | KM0101011 | KM8232231 |
| O. onotica (1) | OSC 56801 | USA, 1997, E.T. Peterson | AF072067 | AF086578 |
| O. onotica (2) | OSC 56734 | USA, 1996, E.T. Peterson | AF072066 | AF086577 |
| O. onotica (3) | OSC 56759 | USA, 1996, E.T. Peterson | – | JN941088 |
| O. onotica (4) | C-F-89691 | Denmark, 2008, H. Knudsen | JN942773 | JN941090 |
| O. onotica (5) | JS.08.48 (S) | Sweden, 2008, J. Santos | KM010102 | KM823487 |
| O. onotica (6) | KH.10.284 (S) | Sweden, 2010, K. Hansen, K. Gillen & I. Olariaga | KP0065051 | KM8232291 |
| O. onotica (7) | KH.09.132 (S) | Norway, 2009, K. Hansen & I. Olariaga | KM010103 | KC012692 |
| O. onotica (8) | KH.09.136 (S) | Norway, 2009, K. Hansen & I. Olariaga | JN942772 | JN941096 |
| O. onotica (9) | MCVE 23277 | Italy, 2008, M. Carbone | KM010104 | KM823488 |
| O. onotica (10) | KH.98.107 (C) | Denmark, 1998, K. Hansen, T. Læssøe & C. Lange | – | AF335121 |
| O. oregonensis (1) | rh139 (S) | USA, 2010, R. Helliwell | KM010046 | KM823489 |
| O. oregonensis (2) | Moorefun 58 (OSC, S) | USA, 2010, J. Moore | KM0100481 | KM8232311 |
| O. oregonensis (3) | Moorefun 31 (S) | USA, 2010, J. Moore | KM0100471 | KM8232301 |
| O. oregonensis (4) as O. rainierensis | OSC 56829 | USA, 1997, M. Castellano | AF072087 | AF086597 |
| O. oregonensis (5) as O. rainierensis | NSW6354 (OSC) | USA, 1990, D. McKay | AF072088 | AF086598 |
| O. oregonensis (6) as O. rainierensis | OSC 56745 | USA, 1996, J. Trappe | AF072089 | KM8232321 |
| O. oregonensis (7) | EGS2179 (MICH) | USA, 1948, E.G. Simmons | AF072088 | – |
| O. papillata (1) | H6003547 | Finland, 1971, H. Harmaja | KF7175821 | KM8232341 |
| O. papillata (2) | TUR 102134 | Finland, 1990, T. Lindholm | KM0101051 | KM8232331 |
| O. papillata f. pallidefurfuracea | NV 2007.09.27 (S) | France, 2007, N. Van Vooren | KF7175841 | KM8232351 |
| O. phlebophora (1) | JV06-385 (C) | Denmark, 2006, L. & J. Vesterholt | KM0100491 | KM8232361 |
| O. phlebophora (2) | S-F108338 | Sweden, 1949, G. Haglund & R. Rydberg | KM010050 | KM823490 |
| O. phlebophora (3) | K(M)33068 | UK | EU784392 | – |
| O. platyspora (1) | KH.09.163 (S) | Sweden, 2009, K. Hansen & I. Olariaga | KM0101061 | KM8232381 |
| O. platyspora (2) | HK0846 (S) | Sweden, 2008, H. Kauffman | KM010107 | KM823491 |
| O. platyspora (3) | JV06-656 (C-F-75309) | Denmark, 2006, J. Vesterholt | KM0101081 | KM8232371 |
| O. propinquata (1) | KH.09.99 (S) | Sweden, 2009, K. Hansen & I. Olariaga | KM0101091 | KM8232391 |
| O. propinquata (2) | JS.08.67 (S) | Sweden, 2008, J. Santos | KM010110 | KM823492 |
| O. propinquata (3) | NV 2008.09.15 (dupl. S) | France, 2008, J. Cavet | KM0101111 | KM8232401 |
| O. pseudoleporina (1) as O. concinna | NSW7574 (OSC) | USA, N. S. Weber | AF072083 | AF086593 |
| O. pseudoleporina (2) as O. concinna | OSC 56749 | USA, 1996, E.T. Peterson | AF072082 | AF086592 |
| O. pseudoleporina (3) as O. concinna | OSC 56760 | USA, 1996, E.T. Peterson | AF072081 | KM8232441 |
| O. pseudoleporina (4) | rh101910 (OSC) | USA, 2010, R. Helliwell | KM0101121 | KM8232431 |
| O. pseudoleporina (5) | Moorefun 14 (S) | USA, 2010, J. Moore | KM0101131 | KM8232421 |
| O. pseudoleporina (6) | OSC 56809 | USA, 1997, J. Spatafora | AF072080 | KM8232411 |
| O. rainierensis | A.H. Smith 30553 (MICH) | USA, 1948, A.H. Smith | KF7175831 | KM8232451 |
| O. sinensis | HMAS61360 | China | – | DQ443451 |
| O. smithii (1) | OSC 56799 | USA, 1997, E.T. Peterson | AF072063 | JN941087 |
| O. smithii (2) | ecv3345 (S) | USA, 2005, E. Vellinga | JN942771 | JN941093 |
| O. smithii (3) | OSC 56753 | USA, 1996, E.T. Peterson | AF072062 | AF086574 |
| O. smithii (4) | OSC 56811 | USA, 1997, E.T. Peterson | AF072060 | AF086572 |
| O. subformicarum (1) | S-F242696 | Spain, 2012, J. Herranz & J.C. Campos | KM010054 | KM823495 |
| O. subformicarum (2) | S-F256979 | Spain, 2008, J. Fernández Vicente et al. | KM010051 | KM823494 |
| O. subformicarum (3) | CL 050928-30, dupl. S-F256978 | Italy, 2005, C. Lavorato | KM0100521 | KM8232471 |
| O. subformicarum (4) | Private herb. CMP 1179, RM 1095, dupl. S-F256980 | Spain, 2009, C. M. Pérez del Amo & R. Gil | KM0100531 | KM8232461 |
| O. aff. subformicarum (1) | FH301035 | Mexico, 2007, M. Hernández | KM0100551 | KM8232491 |
| O. aff. subformicarum (2) | FH301036 | Mexico, 2007, M.E. Smith | KM0100561 | KM8232481 |
| O. subterranea (1) | RH97 (FH) | USA, 1997, R. Healy | FJ404766 | FJ404766 |
| O. subterranea (2) | RH69 (FH) | USA, 1997, R. Healy | FJ404767 | FJ404767 |
| O. tuomikoskii (1) | JS.08.68 (S) | Sweden, 2008, J. Santos | KM010114 | KM823496 |
| O. tuomikoskii (2) | MK200065 (S) | Sweden, 2000, M. Karström | KM010115 | KM823497 |
| O. tuomikoskii (3) | H6002901 | Finland, 1972, R. Tuomikoski | KF7175851 | KM8232501 |
| O. tuomikoskii (4) | JS.08.100 (S) | Sweden, 2008, J. Santos | KM010116 | KM823498 |
| O. tuomikoskii (5) | NV 2008.09.08 (S) | France, 2008, N. Van Vooren | JN942777 | JN941091 |
| O. tuomikoskii (6) | KH.09.130 (S) | Norway, 2009, K. Hansen & I. Olariaga | JN942776 | JN941092 |
| O. tuomikoskii (7) | KH.11.77 (S) | Sweden, 2011, M. Prieto & I. Olariaga | KM010117 | KM823499 |
| O. tuomikoskii (8) as O. leporina | − | − | – | AF0865883 |
| O. tuomikoskii (9) | OSC 56756 | USA, 1996, E.T. Peterson | AF072084 | AF086594 |
| O. tuomikoskii (10) | OSC 56826 | USA, 1996, M. Madsen & R. Davis | AF072086 | AF086596 |
| O. tuomikoskii (11) | OSC 56761 | USA, 1996, E.T. Peterson | AF072085 | <KM8232511 |
| O. unicisa (1) | KH.06.06 (FH) | USA, 2006, L. Millman | KC012693 | |
| O. unicisa (2) as O. grandis | HMAS51684 | USA, Burdsall | – | DQ443446 |
| O. unicisa (3) as O. grandis | ZW Geo65-Clark (S) | USA, 2003, Z. Wang | KM010118 | AY789369 |
| O. yunnanensis | HMAS 82166 | China, 2003, Z.L. Yang | – | DQ443452 |
| O. sp. ‘a’ (1) | MK0942 (S) | Sweden, 2009, M. Karström | KM010057 | KM823500 |
| O. sp. ‘a’ (2) | MK1081 (S) | Sweden, 2010, M. Karström | KM010058 | KM823501 |
| O. sp. ‘b’ | KH.09.79 (S) | Sweden, 2009, K. Hansen & I. Olariaga | KM0101201 | KM8232521 |
| Warcupia terrestris | CBS 891.69 | Canada, 1966, J.W. Paden | – | DQ220467 |
1 Sequences from Hansen & Olariaga (2015).
2 The voucher specimen for AF086573 is mistakenly given as OSC 56823 in GenBank. This voucher (OSC 56823) is O. smithii based on morphological re-examination and the ITS sequence (AF072061) deposited by the same authors. The LSU sequence AF086573 is O. leporina (Fig. 1).
3 The voucher specimen for AF086588 is mistakenly given as OSC 56825 in GenBank. This voucher (OSC 56825) is O. leporina based on morphological re-examination and the ITS sequence (AF072078) deposited by the same authors. The LSU sequence AF086588 is O. tuomikoskii (Fig. 1).
4 ITS: Internal transcribed spacers (ITS1 and ITS2) and the 5.8S gene of the nrDNA; LSU: 28S large subunit of the nrRNA gene.
Phylogenetic analyses
Maximum Likelihood (ML) analyses of the all taxa LSU dataset were performed using the ‘RAxML HPC2 on XSEDE’ tool (Stamatakis 2006) via CIPRES Science Gateway (Miller et al. 2010), employing mixed models of evolution and starting from a random tree. For the two smaller O. borealis and O. formicarum datasets, ML analyses were conducted using RAxML v. 7.3.1 (Stamatakis 2006) on the Bioportal, University of Oslo (Kumar et al. 2009). A GTR-GAMMA model with four rate categories was assigned and all free model parameters were estimated by the program. For the ML bootstrap analyses (ML-BP) 1 000 rapid bootstrapping replicates from random starting trees were performed, followed by a subsequent ML search similarly using 1 000 replicates. As no strongly supported conflict was detected (ML-BP ≥ 75 %, PP ≥ 95 %), the ITS and LSU region were concatenated for the O. borealis and O. formicarum datasets. Each combined dataset was analysed using ML analyses under the same settings as specified above. Relationships were likewise constructed using Metropolis-coupled Markov Chain Monte Carlo (MCMCMC) and ‘model-jumping’ as implemented in MrBayes v. 3.2.1 (Ronquist et al. 2012). The substitution model was sampled across the GTR space by the MCMC analysis (Huelsenbeck et al. 2004). Four parallel searches, each with four chains, were run for ten and three million generations, respectively, for the all-inclusive LSU dataset and the O. borealis and O. formicarum datasets, initiated with random starting trees. The chains were sampled every 100 generations from the posterior distribution. The first 25 % of the trees sampled was discarded as the ‘burn-in’, and the remaining trees were used to calculate the posterior probabilities (PP) of the clades. For the combined ML and Bayesian analyses the ITS and LSU regions were specified as distinct partitions. ML bootstrap values ≥ 70 % and PP ≥ 95 % were considered to be significant.
RESULTS
Alignment and ITS minisatellites
Fourty-nine ITS and 44 LSU sequences were newly generated in this study (Table 1). In total 146 ITS sequences were utilised, including 34 obtained from GenBank and 63 from Hansen & Olariaga (2015). The ITS sequences were too variable to align across all of Otidea, due to a highly polymorphic part in ITS1 and large length variability (insertions and deletions), and therefore were not included in phylogenetic analyses of the entire genus. The ITS sequences were aligned among closely related species or species groups, and used as an aid to verify identifications. The ITS region was especially useful in cases were the protein-coding genes (RPB1, RPB2 and EF1) failed to amplify, due to poor quality DNA from old or poorly treated material. ITS sequences of the type specimens of O. mirabilis and O. kauffmanii that could not be amplified for the multiple genes are provided here. The all taxa LSU alignment consisted of 167 LSU sequences, including 57 from GenBank and 68 from Hansen & Olariaga (2015) (Table 1) and 956 bp including inserted gaps, of which 263 bp were parsimony informative characters.
The O. borealis dataset consisted of 31 taxa, represented by 24 complete ITS-LSU and six ITS sequences, and 1 555 bp including inserted gaps (ITS 614 bp; LSU 941 bp), of which 216 were parsimony informative characters. The O. formicarum dataset consisted of 14 sequences and 2 531 bp (ITS 1 695 bp; LSU 836 bp). Part of the ITS1 (805 bp) in the O. formicarum dataset was omitted from the analyses, due to a long insertion and tandem repeats in O. subformicarum and the two Mexican specimens, and the combined dataset thus included 1 726 bp, of which 145 were parsimony informative characters. The insertion was composed of four tandem repeats (minisatellites) in the four O. subformicarum sequences (212 bp in total), and in one of them (S-F256979) the repeat was present a fifth time (275 bp in total). In the Mexican FH301036 the insertion was extremely long (715 bp in total), of variable to random repeats. The tandem repeats were 43 or 63 nucleotides. They were composed of three parts (A-B-C) of 30, 20 and 13 nucleotides, respectively, which were duplicates of the preceding part of the ITS1 sequence. In the first two repeats the B part was missing, whereas in the third-fifth all parts were present. The C part was 100 % identical in all specimens and repeats; the A part was mostly identical, but showed 3.3–6.7 % variation in one repeat; and the B part showed 5–15 % variation in the repeats. To ascertain the correctness of the long insertion in the Mexican specimens, the ITS sequence of FH301036 was amplified and sequenced twice, using different sets of primers (in one piece using ITS1-ITS4 and in two pieces using ITS5-5.8S / ITS3-ITS4). The two sequences were found to be identical. Unfortunately, we were not able to sequence the complete insertion of FH301035 and the ITS1 was only sequenced in one direction; both the part of the insertion recovered and the ITS1 were highly different from FH301036.
All taxa LSU phylogeny
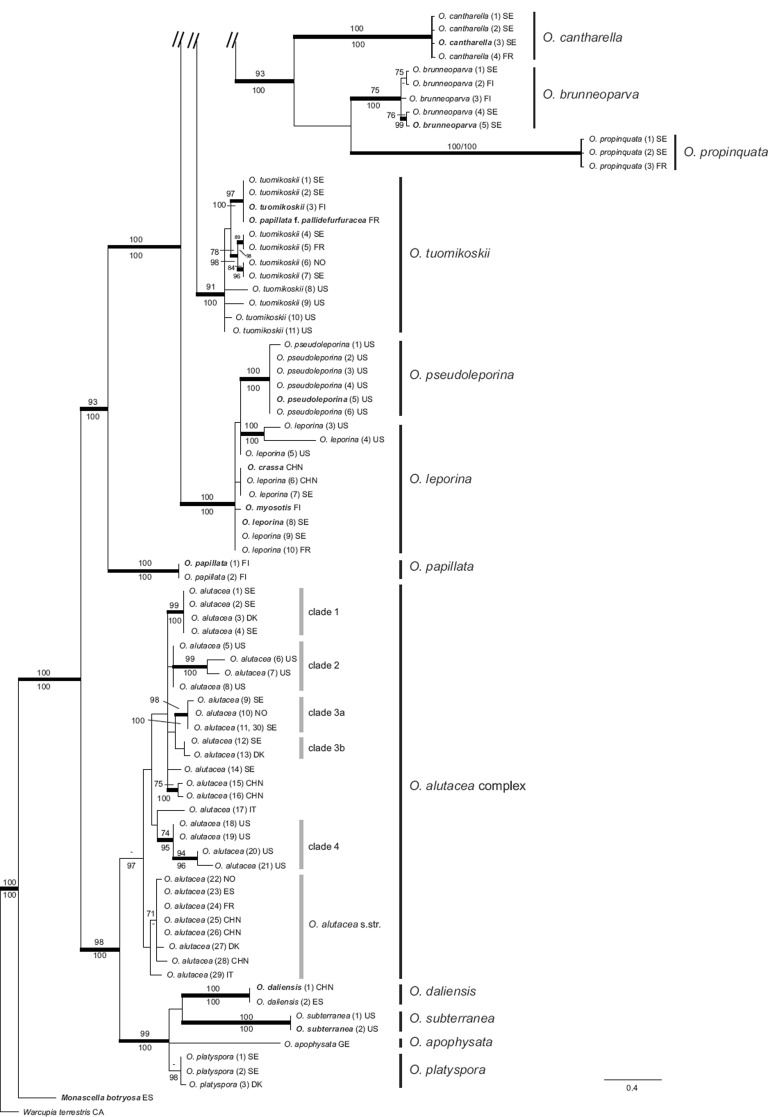
The ML analysis of the all taxa LSU dataset resulted in a single best ML tree of -lnL = 6511.68. Bayesian analyses reached an average standard deviation of split frequencies of 0.004 after 10 M generations. A majority rule consensus tree was constructed from the 300 000 trees sampled from the four runs, each consisting of 75 000 trees sampled from the stationary tree distribution (the first 25 % discarded as the burn-in) (Fig. 1). The ML and Bayesian tree topologies were congruent and recovered the same moderate to strongly supported clades (Fig. 1). The terminal clades that constitute species we recognise (Hansen & Olariaga 2015) have moderate to strong support in both analyses, except for O. leporina and O. mirabilis. A few synonymies inferred from sequences of type collections from which we were not able to obtain the protein-coding genes, and clear misidentifications of sequences deposited in GenBank are evidenced by the LSU phylogeny (see Table 1). Based on LSU sequences from GenBank: the holotype of O. crassa is nested within the O. leporina clade (= O. leporina and O. pseudoleporina); O. lactea is suggested to be a sister lineage to O. minor; O. sinensis is resolved as a sister species to O. caeruleopruinosa, but without support; O. yunnanensis forms a monophyletic group with O. kaushalii and O. unicisa; and the type of O. onotica var. brevispora (= O. brevispora) is supported as a sister lineage to O. onotica. The holotype of O. mirabilis is nested among other collections of O. mirabilis and the LSU sequence differs only in 1 bp from sequences of collections from Scandinavia and France.
Fig. 1.
Bayesian inference 50 % majority rule consensus phylogram of Otidea from LSU sequence data. Maximum Likelihood bootstrap values (ML-BP) ≥ 70 % and Bayesian posterior probabilities (PP) ≥ 95 % are shown above and below the branches, respectively. Thickened branches received support by both ML-BP ≥ 70 % and PP ≥ 95 %. Type collections are highlighted in bold. Country of origin for each collection is given using ISO country codes. Names of species recognised are indicated by the vertical bars.
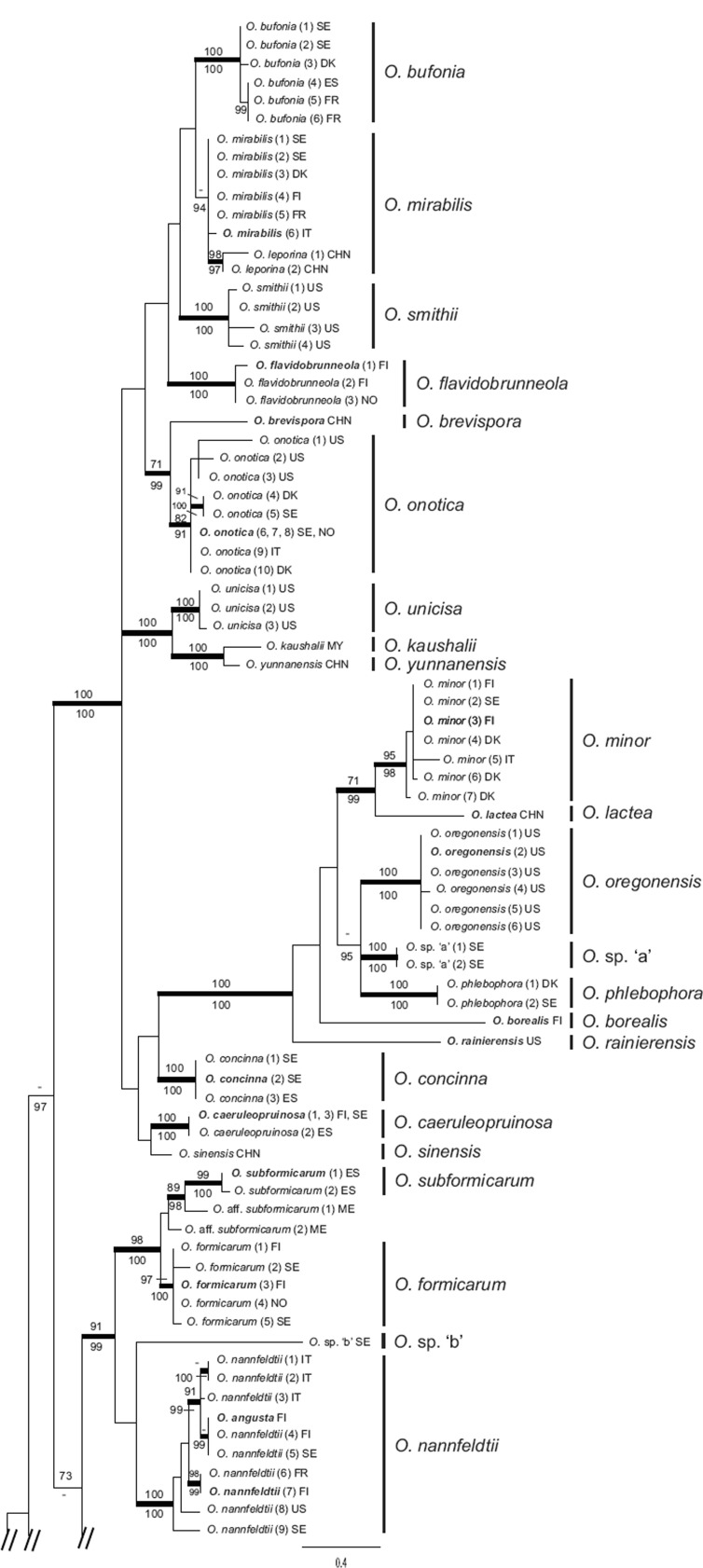
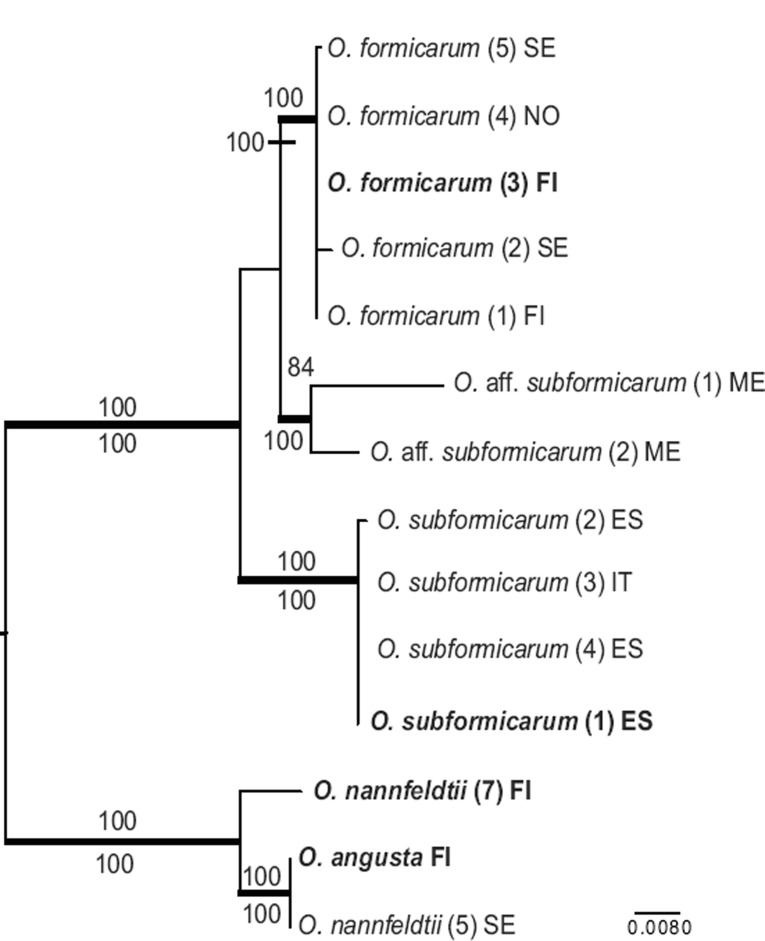
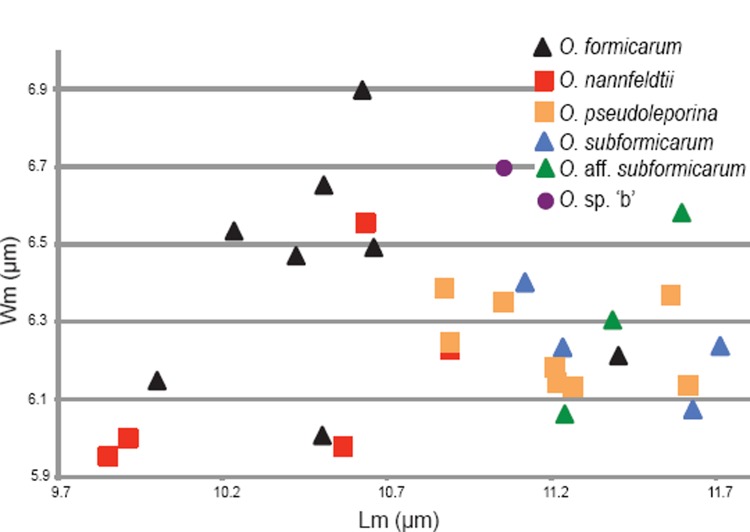
The O. formicarum and O. concinna clades in separate ITS-LSU phylogenies
The ML analysis of the O. formicarum dataset recovered a single tree of -lnL = 3579.93 (Fig. 2). Bayesian analyses reached an average standard deviation of split frequencies of 0.003 after 3 M generations. A majority rule consensus tree was constructed from the 90 004 trees sampled from the four runs, each consisting of 22 501 trees sampled from the stationary tree distribution (the first 25 % discarded as the burn-in). The four collections of O. subformicarum from Spain and Italy form a distinct, strongly supported monophyletic group (ML-BP and PP 100 %). Likewise, the five collections of O. formicarum from Fennoscandia, and the two collections of O. aff. subformicarum from Mexico, each form separate, strongly supported clades (ML-BP and PP 100 %; ML-BP 84 %, PP 100 %, respectively). Phylogenetic analyses of the combined ITS-LSU dataset fail however, to resolve relationships among these three clades with any certainty.
Fig. 2.
The single best tree resulting from the Maximum Likelihood analysis of the ITS-LSU regions of the O. formicarum clade. ML bootstrap values (ML-BP) are shown above nodes and Bayesian posterior probabilities (PP) below nodes. Thickened branches are nodes with high support (ML-BP ≥ 75; PP ≥ 95). Type collections are in bold.
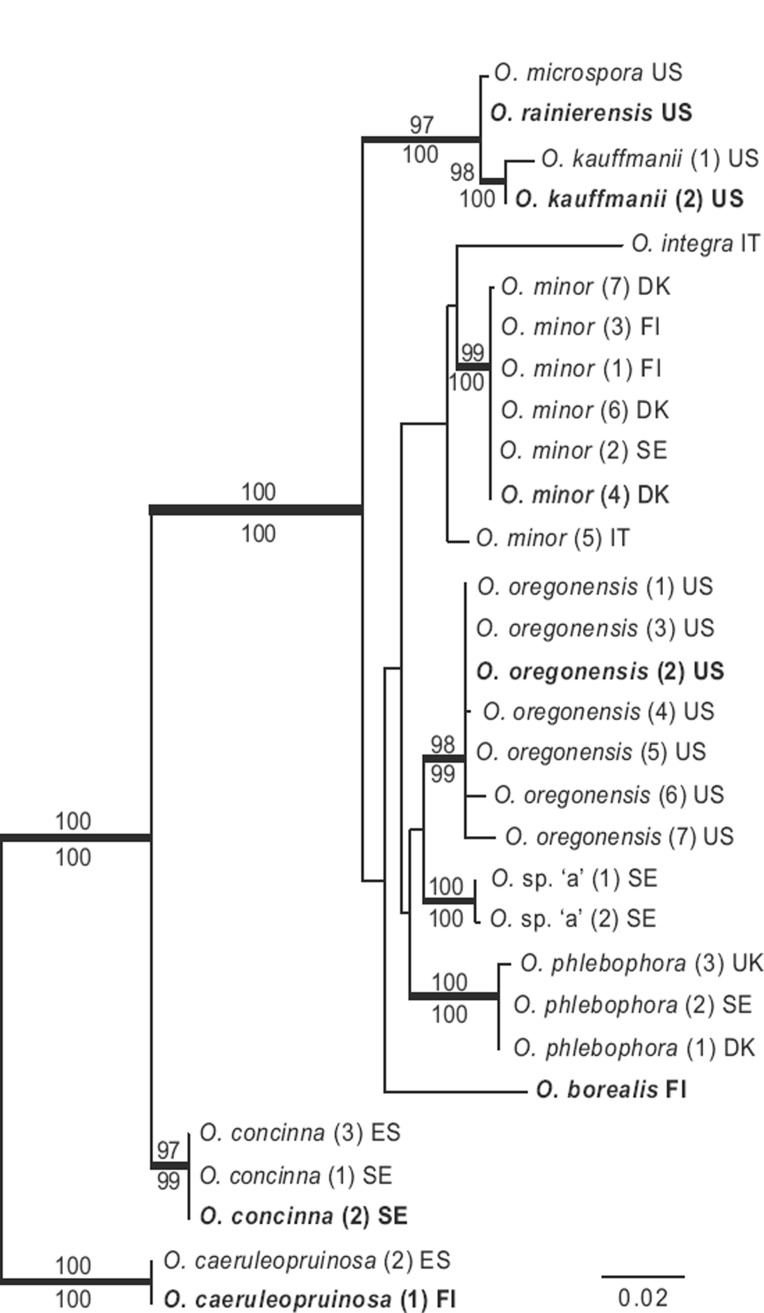
The combined ML analysis of the O. borealis dataset resulted in a single best ML tree of -lnL = 4230.82 (Fig. 3). Bayesian analyses reached an average standard deviation of split frequencies of 0.005 after 3 M generations. A majority rule consensus tree was constructed, as for the O. formicarum dataset (above). The supported topology (PP ≥ 95 %) did not differ from the supported topology recovered by ML analysis. The placement of the single O. borealis collection is unresolved, but the ITS-LSU phylogeny confirms it is genetically divergent from its sister species (Fig. 3). Other species with a yellow outer surface of the apothecia, O. concinna, O. minor, O. oregonensis and O. phlebophora, are each strongly supported as monophyletic (ML 97–99 %, PP 100 %). An exception is O. minor (5) from Italy that is resolved as a sister lineage to the rest of the O. minor collections and O. integra without support. The ITS and LSU sequences of O. minor (5) differ by 16 and 5 bp, respectively, from the rest of the sequences of O. minor, which are 100 % identical, except for the ITS sequence of O. minor (7) that differs in 1 bp. Otidea integra is represented only by the ITS2 region (281 bp). The holotype of O. rainierensis is forming a strongly supported clade with the holotype of O. kauffmanii and a paratype of O. microspora (ML 97 %, PP 100 %). The O. kauffmanii and O. microspora types are only represented by ITS and are therefore not included in any of our previous analyses. We conclude O. kauffmanii is a synonym of O. rainierensis and O. microspora a doubtful name (see further under Taxonomy). Otidea concinna is strongly supported as a sister group to the rest of the ingroup, but otherwise the relationships among the species are without support.
Fig. 3.
The single best tree resulting from the Maximum Likelihood analysis of the ITS-LSU regions of O. borealis and closely allied species. ML bootstrap values (ML-BP) are shown above nodes and Bayesian posterior probabilities (PP) below nodes. Thickened branches are nodes with high support (ML-BP ≥ 75 %; PP ≥ 95 %). Type collections are in bold.
The phylogenetic results, and the ITS sequence similarity and divergence (for species identification), will be further discussed where applicable in the descriptive notes below.
Morphological characters for species delimitation
All Otidea species recognised by concordance of our four genes phylogenies (Hansen & Olariaga 2015) can be recognised by a combination of morphological characters. We evaluated the characters in the context of the phylogeny and discovered several new characters. The apothecial shape, colours, and spore characters (size, shape, ornamentation) are important for species identification, but to distinguish closely related species (or otherwise morphologically similar species) additional characters are needed. These are the shape of the paraphyses, ectal excipulum structure, type of exudates on the medullary excipulum hyphae, resinous exudates on the outer surface of the ectal excipulum and on the mycelium at the base of the apothecia, and their possible reactions in MLZ and KOH (see further in Hansen & Olariaga 2015). Below we provide details on the resinous exudates, and their reactions in KOH and MLZ, because they largely have been overlooked.
Excipular resinous exudates and reactions in MLZ and KOH
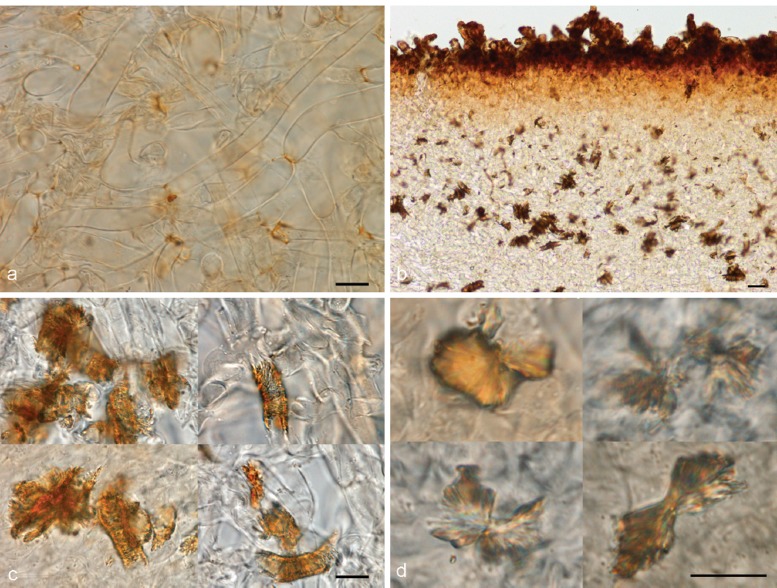
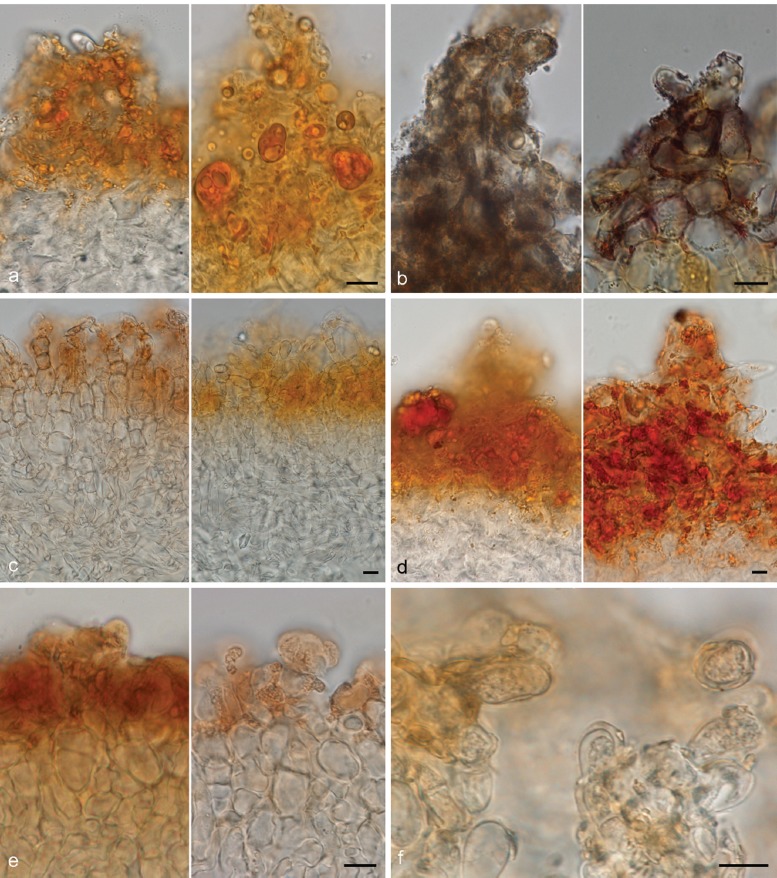
A resinous exudate is here used for a substance that is released from the cells and in many species is vulnerable to common mountants, but not water (following Huhtinen 1990). In Otidea the exudates are concentrated in the excipulum cells, and on the tomentum and mycelium at the base of the apothecia. The resinous exudates are deposited on the outside of the cell or hyphal walls. Harmaja (2009a) introduced the reaction of coloured resinous exudates on the outermost cells of the ectal excipulum in MLZ as a taxonomic character. Our study revealed in addition, different reaction patterns of exudates on the medullary excipulum cells and on the mycelium at the base of the apothecia (including the tomentum), extending out among the soil particles, which turned out to be diagnostic for some species (see under Mycelium at the base of the apothecia). In the medullary excipulum, most frequently scattered, golden brown, resinous exudates are present at septa, e.g. in O. alutacea, O. leporina, O. nannfeldtii (Fig. 4a). Otidea bufonia has unique exudates, wrapping some hyphae and appearing striate (referred to as ‘fingerprint-like’ by Korf & Zhuang (1991)), sometimes forming big crystal-like aggregates (Fig. 4b, c). In contrast, the sibling species, O. mirabilis, has only sometimes biflabellate crystal-like exudates in the medullary excipulum (Fig. 4d). Otidea papillata also possesses unique brown exudates, embedding some hyphae of the medullary excipulum and sometimes appearing rod-like.
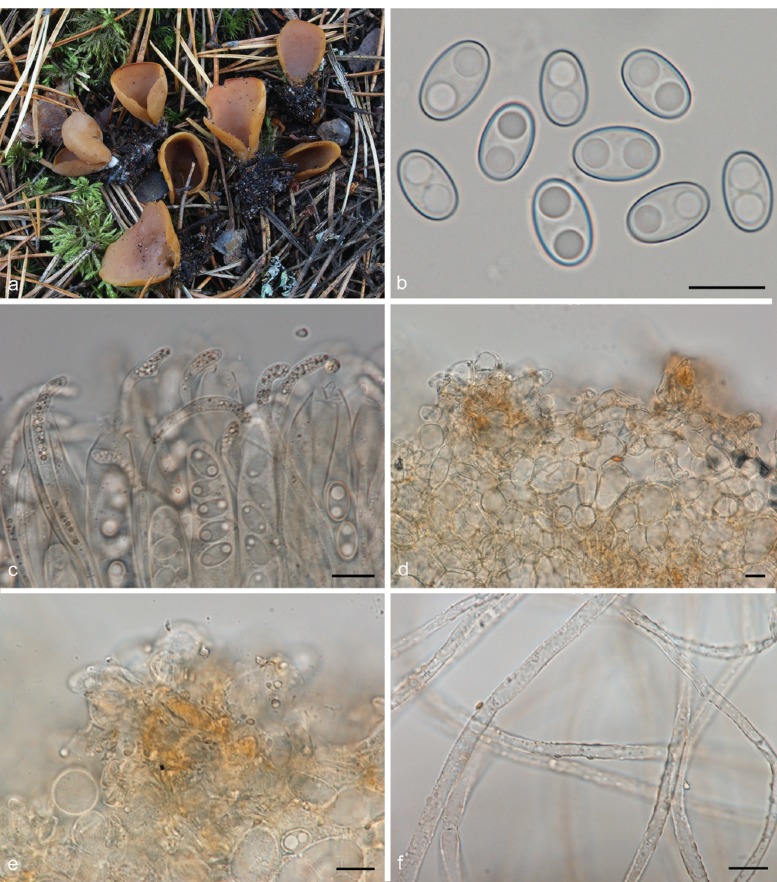
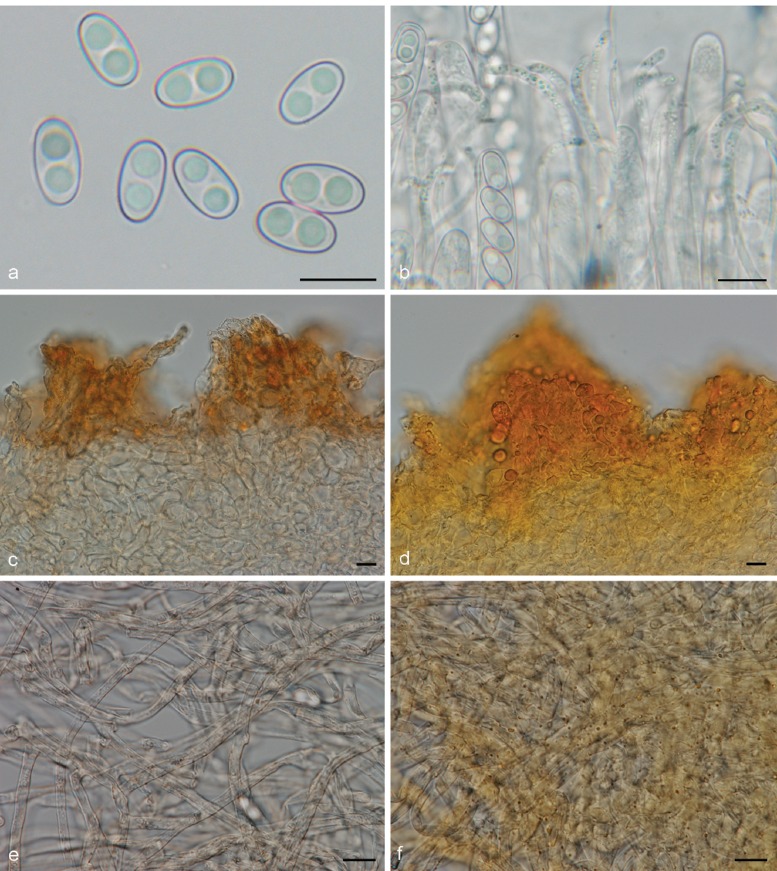
Fig. 4.
Medullary excipulum resinous exudates in Otidea. a. Hyphae with golden brown resinous exudates at septa in O. leporina (KH.11.02), in water*; b, c. brown crystal-like exudates in O. bufonia (KH.07.37) in water†: b. overview; c. close-up of hyphae wrapped in striate exudates; d. close-up of biflabellate crystal-like exudates in O. mirabilis (GMFN 1951, holotype), in water† — Scale bars = 10 μm; * = fresh material; † = dried material.
Small, resinous drops or amorphous matter are present in variable amounts on the outer surface of the apothecia of nearly all Otidea species. In most species they are abundant and easy to observe in water, but scarce and difficult to detect in a few (O. alutacea, O. formicarum). One species, O. kaushalii, has a unique type of exudate, i.e. crystal-like, oblate spheroid, striate bodies, with a constricted centre. The possible reaction of the exudates in MLZ and KOH is useful to separate certain species or groups. Using these characters requires experience. The reaction appears to vary depending on the amount of exudate and the concentration of MLZ. As Harmaja (2009a), we observed two types of reactions in MLZ: i) Resinous exudates dissolve and coalesce into spheroid drops, ‘amber drops’, that contain hyaline bubbles (Fig. 5a). The ‘amber drops’ are overlooked if the ectal excipulum is directly mounted in MLZ, since the exudates coalesce instantly and the drops can be washed away. The reaction is best observed if MLZ is added to a water mount. This reaction is present in many species. ii) Resinous exudates partly convert into small reddish particles (Fig. 5b). The reaction is often subtle and visible only in certain parts of a mount. This reaction is typical in O. bufonia, O. mirabilis and O. smithii. In some species the exudates do not react in MLZ or they simply dissolve.
Fig. 5.
Reactions of resinous exudates on the outermost ectal excipulum cells in Otidea. a. O. nannfeldtii† (H6010804, holotype of O. angusta), in water (left) and converting into amber drops in Melzer’s reagent (right); b. O. bufonia* (KH.09.171), in water (left) and converting into reddish particles in Melzer’s reagent (right); c. O. borealis† (S-F242694, holotype), in water (left) and turning bright yellow in KOH (right); d. O. nannfeldtii† (H6010804), in water (left) and turning reddish brown in KOH (right); e. O. pseudoleporina† (rh101910, holotype), in water (left) and converting into reddish grey drops in KOH (right); f. ectal excipulum cells showing a gelatinous sheath in O. formicarum* (KH.11.104). — Scale bars = 10 μm; * = fresh material; † = dried material.
In this study we detected three discriminative reactions of resinous exudates in 10 % KOH, which we propose as a novel taxonomic character: i) In water the resinous exudates range from yellow to dark reddish yellow (Fig. 5c, left) and in KOH these dissolve completely ± exuding a yellow pigment, or partly with the rest turning distinctly brighter yellow (Fig. 5c, right). This reaction occurs in O. concinna and closely related species, viz. O. borealis, O. caeruleopruinosa, O. flavidobrunneola, O. kaushalii, O. minor and O. oregonensis, and slightly less strikingly in O. unicisa. ii) The resinous exudates are yellow-brown in water (Fig. 5d, left) and turn reddish brown in KOH (Fig. 5d, right). This reaction has been observed in O. nannfeldtii and O. propinquata. iii) The resinous exudates, yellow brown in water (Fig. 5e, left), partly dissolve in KOH, and partly coalesce into heterogeneous, pale reddish grey drops, with bubbles inside (Fig. 5e, right). These drops are similar to the ‘amber drops’ observed in MLZ in many species, except for the pale red colour. This reaction has been observed in O. leporina and O. pseudoleporina. A number of species have the outermost cells of the ectal excipulum sometimes covered with a thin gelatinous sheath (Fig. 5f).
Mycelium at the base of the apothecia
All Otidea species studied showed a conspicuous tomentum covering the base of the apothecia and spreading out in the substrate. In the microscopic descriptions we refer to both as the basal mycelium. The hyphae are septate, straight and frequently branch and anastomose. No rhizomorphs have been observed, although slender hyphal threads are sometimes formed. Resinous exudates are often present on the surface, especially on the mycelia extending out in the substrate, and can appear like hyphal ornamentation.
Harmaja (2009a) proposed the colour of the basal tomentum as a taxonomic character, but microscopic features of the basal mycelium have been largely overlooked and have not been included in species descriptions. Two types of characters are useful for species identification: i) Resinous exudates occurring on the hyphal walls (Fig. 6a). These can be inconspicuous or nearly absent in some species, such as in O. alutacea s.l., in which only a few refractive drops or minute hyaline exudates are sometimes present. Resinous exudates are also scarce in some species of the O. concinna clade. Many other species show hyphae densely covered with resinous exudates, such as O. formicarum, O. propinquata and O. tuomikoskii. The shape of the exudates is variable, i.e. rod-shaped, hemispherical, conical or bipyramidal. They dissolve and completely disappear in MLZ (Fig. 6b). Sometimes doubts can arise about the nature of the differently shaped exudates, but the facts that they detach from the hyphal wall when the mount is squashed and dissolve in MLZ, show these are not true ornamentation, i.e. not part of the hyphal wall. ii) The hyphal wall turns yellow in KOH (Fig. 6d; see Fig. 6c in water). We have observed this reaction clearly in O. borealis and O. onotica. It can be observed in isolated hyphae, but is more conspicuous when a mass of hyphae is observed together. It can also be observed macroscopically.
Fig. 6.
Mycelium at the base of the apothecia and extending out in the substrate in Otidea†. a, b. O. flavidobrunneola (H6010806, holotype): a. resinous exudates on the hyphal walls in water; b. dissolved in Melzer’s reagent; c, d. O. borealis (S-F242694, holotype): c. pale yellow in water; d. turning bright yellow in KOH. — Scale bars = 10 μm; † = all dried material.
TAXONOMY
Otidea (Pers.) Bonord., Handb. Mykol.: 205. 1851
≡ Peziza (unranked) Otidea Pers., Mycol. Eur. 1: 220. 1822.
≡ Peziza (unranked) Cochleatae Fr., Syst. Mycol. 2: 46. 1822: Fr. loc. cit.
Type species. Otidea onotica (Pers. : Fr.) Fuckel, indicated by Saccardo, Bot. Centralbl. 18: 215. 1884 (‘P. onotica Pers.’).
= Flavoscypha Harmaja, Karstenia 14: 107. 1974.
Type species. Peziza phlebophora Berk. & Broome.
= Otideopsis B. Liu & J.Z. Cao, Shanxi Univ. J., Nat. Sci. Ed. 4: 70. 1987.
Type species. Otideopsis yunnanensis B. Liu & J.Z. Cao.
Apothecia small to large, 3–75 mm high, 4–80 mm wide, often in fascicles or caespitose, epigeous, cup- to ear-shaped and split to the base on one side, less often entire, stipitate or not; or hypogeous and enclosed. Hymenium white, yellow, ochre, brown, almost black, often with pink stains. Receptacle surface concolorous or with similar colours as hymenium, sometimes with purplish, greenish or bluish tones, with conical to broadly conical warts or pustules, less often smooth or furfuraceous, concolorous or darker than the background. Base of the apothecium tomentose, mycelium white, ochre, yellow, orange or brown, extending out in the substrate, base ribbed-veined in a few species. Spores uniseriate, ellipsoid, oblong or fusoid, typically with 2 guttules, sometimes with a few smaller granules, smooth (or verruculose in SEM), rarely spinose or with low ridges, with de Bary bubbles in MLZ and Cotton Blue when dried, thin-walled to slightly thick-walled, hyaline to very pale brown. Paraphyses typically curved to hooked, rarely straight, sometimes with notches or swollen at the apices, septate, typically containing refractive small guttules at the apices, fading in colour and collapsed when dried. Asci cylindrical, operculate, 8-spored, 116–275 × 8–19 μm, with pleurorhynchous base. Subhymenium c. 100–150 μm thick, of dense textura intricata, hyphae sometimes swollen, often with scattered pigmented exudates at septa. Medullary excipulum 400–1500 μm thick, of textura intricata, hyphae cylindrical to slightly swollen, thin-walled to thick-walled, hyaline to pale brown, often with pigmented resinous exudates at septa. Ectal excipulum 70–150 μm thick, of textura angularis, less often of textura prismatica. Surface with warts up to 180 μm high, formed by fasciculate, short hyphoid hairs of globose to elongated cells, or of textura globulosa-angularis with single hyphoid hairs. Resinous exudates often present on the surface, yellow to dark brown, sometimes dissolving in MLZ, turning reddish or into brownish yellow amber drops, sometimes changing colour in KOH. Basal mycelium of septate, straight hyphae, that frequently branch and anastomose, turning yellow or not in KOH, often covered with pigmented, small, resinous exudates.
Ecology & Distribution — see Hansen & Olariaga (2015).
Key to species of Otidea
We were not able to study and interpret the following Chinese species and these are therefore not treated nor included in the key: Otidea bicolor W.Y. Zhuang & Zhu L. Yang, O. kunmingensis W.Y. Zhuang, O. olivaceobrunnea Harmaja, O. sinensis J.Z. Cao & L. Fan, O. subpurpurea W.Y. Zhuang and O. tianshuiensis J.Z. Cao, L. Fan & B. Liu. For O. integra (Bres.) Harmaja see notes under O. phlebophora.
1. Ascomata hypogeous, globose to subglobose, truffle-like . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4. O. subterranea
1. Ascomata epigeous, cup-shaped to ear-shaped, split or entire . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
2. Spores ornamented . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2. Spores smooth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
3. Spores with fine warts ± ridges . . . . . . . . . . 17. O. unicisa
3. Spores spiny . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
4. Spores 14–17 × 7–9 μm . . . . . . . . . . . 16. O. kaushalii
4. Spores 16.5–20 × 7.6–10 μm . . . . . .18. O. yunnanensis
5. Spores Lm > 17 μm . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
5. Spores Lm < 17 μm . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
6. Apothecia broadly ear-shaped, split; typically ochraceous yellow to ochre orange; often associated with Cudonia in mossy Picea forests . . . . . . . . . . . . . 11. O. cantharella
6. Apothecia cup-shaped, split or entire; brown; under Picea or other trees . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
7. Apothecia entire; reddish brown; basal mycelium normally with abundant minute resinous exudates; ectal excipulum hyphoid hairs often with a gelatinous sheath (more easily seen in MLZ); with Picea . . . . . . . . . 12. O. propinquata
7. Apothecia split or entire; purple or ochre-brown; basal mycelium without or with sparse resinous exudates; ectal excipulum hyphoid hairs without a conspicuous sheath; with angiosperms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
8. Apothecia split, up to 75 mm wide; spores broadly ellipsoid to oblong, Qm = 1.7–1.8; associated with Fagaceae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3. O. platyspora
8. Apothecia entire or split, up to 16 mm diam; spores narrowly ellipsoid to fusoid, Qm = 1.9–2.1; most likely associated with Betulaceae and Salicaceae . . . . . . . . . . . . . . . 9
9. Apothecia deeply cup-shaped, split; ectal excipulum without resinous exudates . . . . . . . . . . . . . . 1. O. apophysata
9. Apothecia shallowly cup-shaped, usually entire; ectal excipulum surface with abundant resinous exudates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2. O. daliensis
10. Receptacle surface with bright citrine yellow tones in young apothecia . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
10. Receptacle surface without citrine yellow tones . . . . 15
11. Spores Qm = 1.7–2; apothecial base at most wrinkled, without high ribs or veins . . . . . . . . . . . . . . . . . 12
11. Spores Qm = 2–2.3; apothecial base with ribs or strongly veined at least in some apothecia . . . . . . . . . . . . . . . . 14
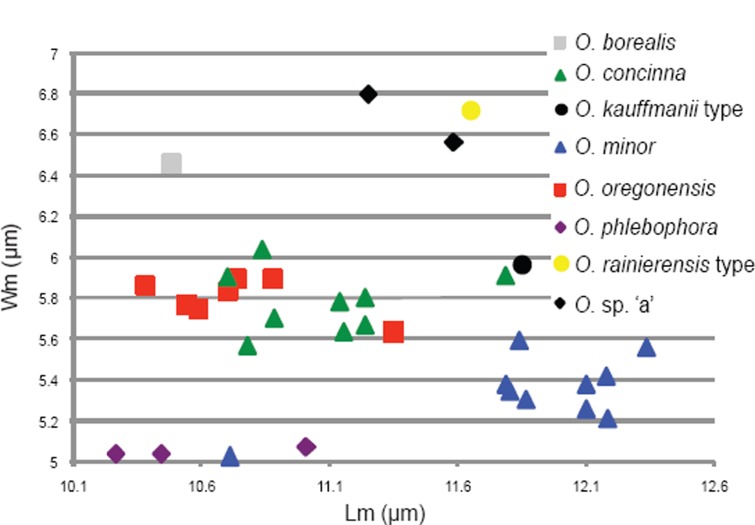
12. Receptacle surface ochraceous yellow; basal mycelium yellow in KOH; spores Wm = 6.5 μm, Qm = 1.7 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25. O. borealis
12. Receptacle surface citrine yellow; basal mycelium unchanged in KOH; spores Wm = 5.6–6 μm, Qm = 1.8–2 . . . . . 13
13. Apothecia sometimes entire, sometimes with blunt ribs at the base; North America . . . . . . . . . . 31. O. oregonensis
13. Apothecia split, without ribs at the base; Europe . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27. O. concinna
14. Apothecia mostly entire, base strongly ribbed-veined and anastomosing in all the apothecia . . . 32. O. phlebophora
14. Apothecia mostly split, base with a few ribs-veins, smooth in some apothecia . . . . . . . . . . . . . . . . . . . . 30. O. minor
15. At least some paraphyses straight or curved, claviform to almost capitate at apices; and/or resinous exudates of the ectal excipulum yellow or reddish yellow, turning bright yellow in KOH; apothecia split or not . . . . . . . . . . . . . 16
15. Paraphyses not as such; resinous exudates of the ectal excipulum, when present, not turning bright yellow in KOH; apothecia always split . . . . . . . . . . . . . . . . . . . . . 20
16. Apothecia shallowly cup-shaped and irregular, entire . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29. O. lactea
16. Apothecia ear-shaped or deeply cup-shaped, split . . . 17
17. Paraphyses often claviform or capitate at apices, 3–9 μm broad; spores Qm = 1.6–1.8 . . . . . . . . . . . . . . . . . . . . 18
17. Paraphyses at most slightly swollen at apices, 2–5 μm broad; spores Qm = 1.9–2 . . . . . . . . . . . . . . . . . . . . . 19
18. Basal mycelium turning yellow in KOH; Europe . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (see under O. borealis) O. sp. ‘a’
18. Basal mycelium not yellow in KOH; North America . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33. O. rainierensis
19. Spores Lm = 11.2–11.8 μm; sometimes receptacle with a bluish hue, ochraceous grey when dried; basal tomentum light ochre in dried specimens . . 26. O. caeruleopruinosa
19. Spores Lm = 10–10.6 μm; receptacle without bluish hue when fresh, reddish brown when dried; basal tomentum orange-ochre in dried specimens 28. O. flavidobrunneola
20. Spores Lm < 12 μm . . . . . . . . . . . . . . . . . . . . . 21
20. Spores Lm > 12 μm . . . . . . . . . . . . . . . . . . . . . 28
21. Apothecia dark brown with lilaceous tones; Asia . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23. O. purpurea
21. Apothecia not dark brown, without lilaceous tones; Asia, Europe or North America . . . . . . . . . . . . . . . . . . . . . 22
22. Medullary excipulum with reddish brown resinous exudates scattered among and covering some hyphae; ectal excipulum of textura prismatica to textura intricata; receptacle surface with contrasting brown warts . . . . . . . 6. O. papillata
22. Medullary excipulum sometimes with yellowish brown resinous exudates at septa, not covering hyphae; ectal excipulum of textura angularis; receptacle surface with contrasting warts or not . . . . . . . . . . . . . . . . . . . . . 23
23. At least some warts higher than 85 μm; basal tomentum orange-ochre when dried; apothecial sections often yellow in KOH; without resinous exudates at septa in the medullary excipulum . . . . . . . . . . . . . . . . . . . . . . . 9. O. tuomikoskii
23. Warts up to 85 μm; basal tomentum pale ochre or yellow when dried; apothecial sections not yellow in KOH; sometimes with resinous exudates at septa in the medullary excipulum . . . . . . . . . . . . . . . . . . . . . . . . 24
24. Hymenium with distinct yellow or orange tones, ochre-yellow to pinkish orange; resinous exudates on the ectal excipulum partly dissolving into reddish grey, heterogeneous drops in KOH or basal mycelium yellow in KOH . 25
24. Hymenium without or with weak orange tones; resinous exudates sometimes turning reddish brown but not dissolving into drops in KOH; basal mycelium not turning yellow in KOH . . . . . . . . . . . . . . . . . . . . . . . . 26
25. Resinous exudates on the ectal excipulum partly dissolving into reddish grey heterogeneous drops in KOH; basal mycelium not turning yellow in KOH; North America . . . . . . . . . . . . . . . . . . . . . . . . 8. O. pseudoleporina
25. Resinous exudates on the ectal excipulum not dissolving into reddish grey heterogeneous drops in KOH; basal mycelium turning yellow in KOH; Asia . . . . . 19. O. brevispora
26. Hymenium sometimes with pink tones; apothecia yellowish ochre to brown; narrowly ear-shaped in the beginning . . . . . . . . . . . . . . . . . . . . . . . . . . . 14. O. nannfeldtii
26. Hymenium without pink tones; apothecia reddish brown to orange-brown; broadly ear-shaped . . . . . . . . . . . . 27
27. Spores Lm = 10–10.7 μm; Qm = 1.6–1.7 . . . . . . . . . . . . . . . . . . . . . . . . . . . 13. O. formicarum
27. Spores Lm = 11.1–11.7 μm; Qm = 1.7–1.9 . . . . . . . . . . . . . . . . . . . . . . . . 15. O. subformicarum
28. Spores Qm < 1.8; ear-shaped . . . . . . . . . . . . . . . . . . . . . 29
28. Spores Qm > 1.8; ear- or cup-shaped . . . . . . . . . . . . . 30
29. Apothecia dark brown, sometimes with olivaceous tint; paraphyses mostly with distinct notches . 10. O. brunneoparva
29. Apothecia cinnamon brown, without olivaceous tint; paraphyses not or slightly notched . . . . . . . . . . 7. O. leporina
30. Apothecia ochraceous yellow, hymenium often with pink tones; basal mycelium turning yellow in KOH . . . . . . . . . . . . . . . . . . . . . . . . 22. O. onotica
30. Apothecia pale or dark brown, without pink tones; basal mycelium not turning yellow in KOH . . . . . . . . . . . . . . . 31
31. Receptacle medium brown; ectal excipulum resinous exudates absent or scarce, light yellowish brown; basal mycelium without dark brown resinous exudates . . . . . . . . . . . . . . . . . . . . . . . . 5. O. alutacea s.l.
31. Receptacle dark purple brown; ectal excipulum resinous exudates abundant, dark brown; basal mycelium with dark brown resinous exudates . . . . . . . . . . . . . . . . . . 32
32. Spores Qm < 2, ellipsoid; apothecia mostly ear-shaped; North America . . . . . . . . . . . . . . . . . . 24. O. smithii
32. Spores Qm > 2, ellipsoid-fusoid; apothecia mostly cup-shaped, split; Eurasia and North America . . . . . . . . . . 33
33. Receptacle strikingly purple-violaceous (fresh); medullary excipulum without, or rarely with flabellate crystal-like exudates, forming cross-like aggregates; under conifers on calcareous ground . . . . . . . . . . . . . . . . . . 21. O. mirabilis
33. Receptacle mostly without purple tones; medullary excipulum with striate exudates covering some hyphae, sometimes forming crystal-like aggregates; often under deciduous trees or on acidic ground . . . . . . . . . . 20. O. bufonia
Based on genealogical concordance phylogenetic species recognition (GCPSR: Taylor et al. 2000), using the four loci, RPB1, RPB2, EF1 and LSU rDNA, we delimited 25 species within Otidea (see Hansen & Olariaga 2015). In addition eight species were recognised by genetic divergence from their sisters. Twenty-eight of these are treated and discussed below, along with O. brevispora, O. lactea, O. subterranea and O. yunnanensis included in our LSU phylogeny, and O. purpurea that has only been studied morphologically. The species are presented following their phylogenetic relationships, inferred from our combined three- and four-gene analyses (f. 3 in Hansen & Olariaga 2015).
Otidea platyspora clade
Apothecia disc-shaped, cup-shaped and split, or globose and hypogeous, brown. Spores large, exceeding 20 μm, except 14–16.5 μm if hypogeous. Basal mycelium smooth or with very sparse resinous exudates.
Species — Otidea apophysata, O. daliensis, O. platyspora, O. subterranea.
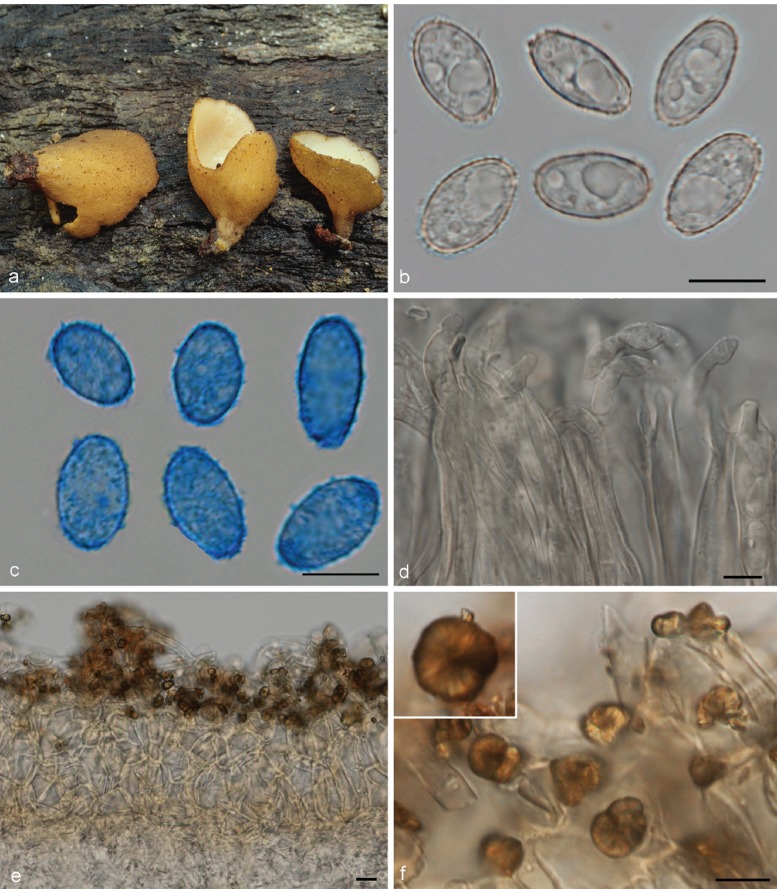
1. Otidea apophysata (Cooke & W. Phillips) Sacc., Syll. Fung. 8: 96. 1889
Basionym. Peziza apophysata Cooke & W. Phillips in Cooke, Grevillea 5: 60. 1876.
≡ Pseudotis apophysata (Cooke & W. Phillips) Boud., Hist. Classific. Discomyc. Europe: 52. 1907.
Holotype. ENGLAND, Shrewsbury, in a damp ditch, 1876, W. Phillips (K(M) 30410 ex Herb. Phillips). Isotype (K(M) 167215 ex Herb. Cooke) !
Misapplied names
– Otidea felina sensu Boudier, Icon. Mycol. livr. 29: n°. 512, pl. 331. 1910 (preliminary text with ‘circulaires’).
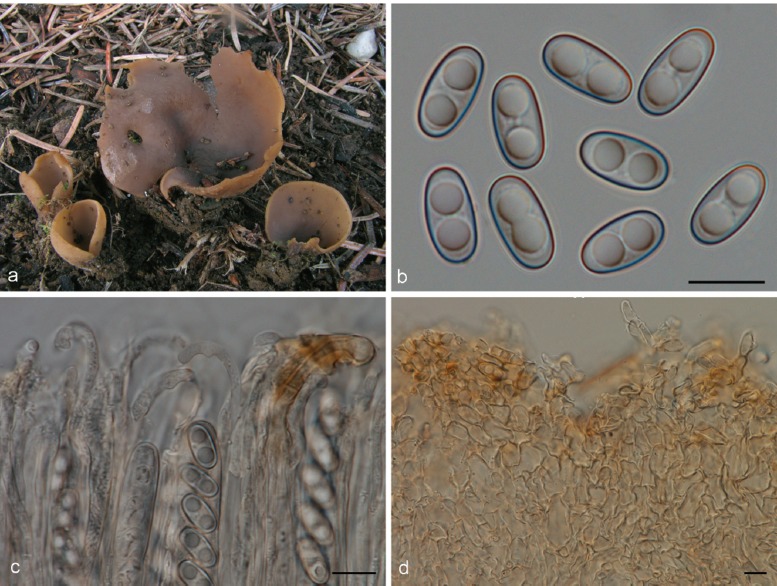
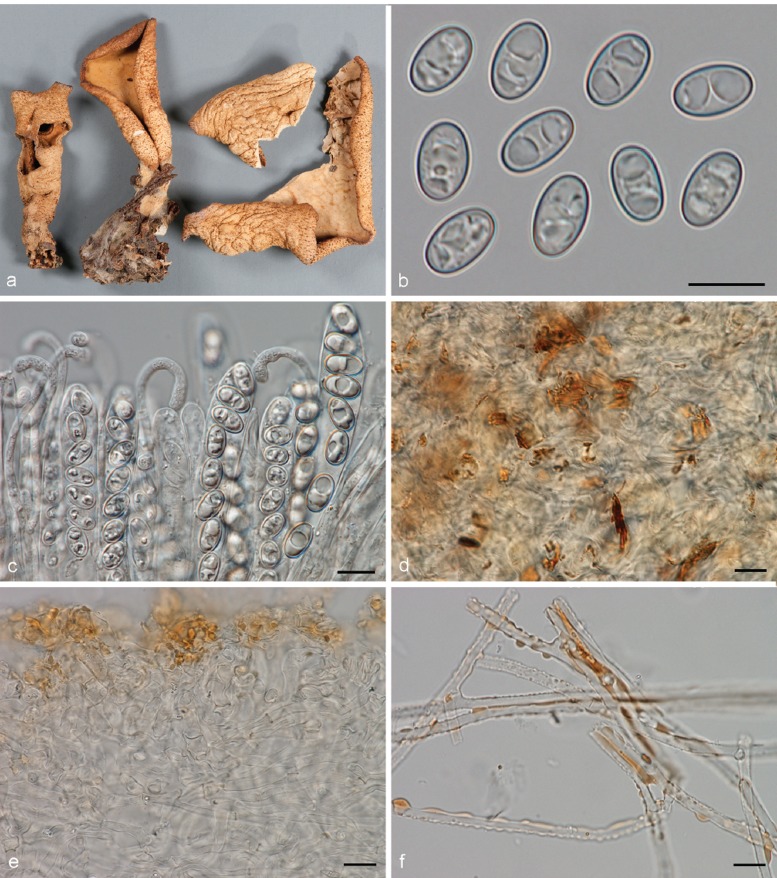
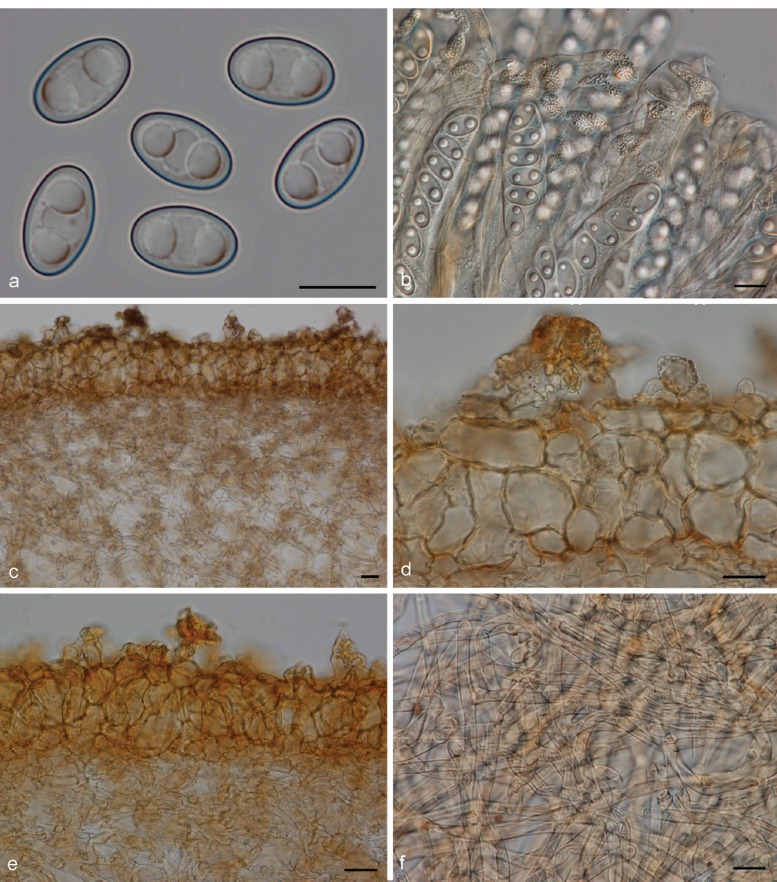
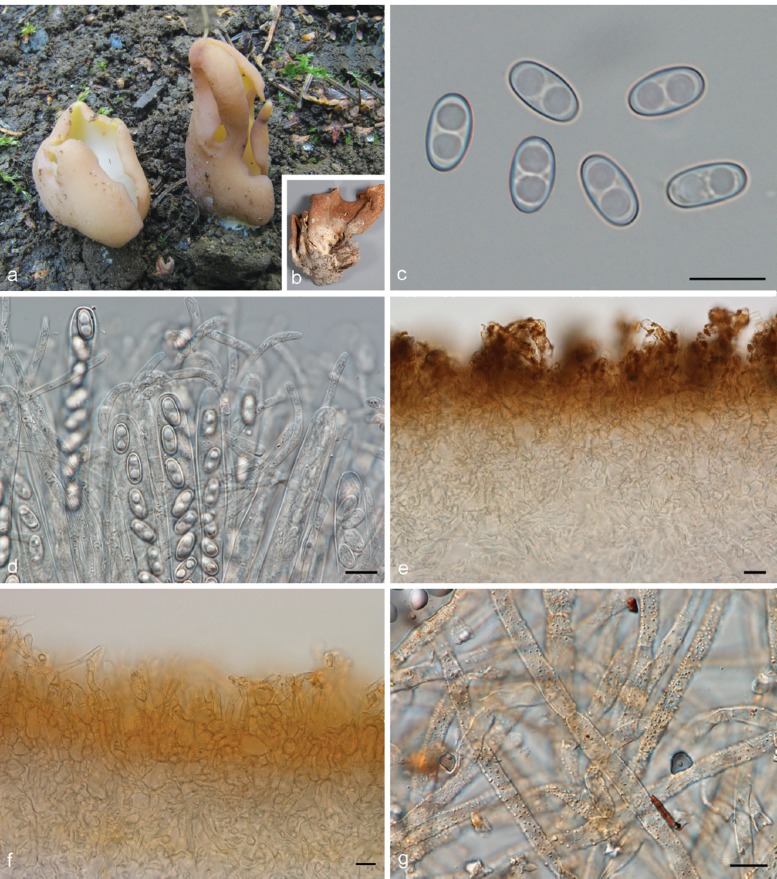
Apothecia solitary to caespitose, 8–30 mm high, up to 15 mm wide, initially ear-shaped, then soon expanding and becoming cup-shaped, split, sessile or stipitate. Hymenium purple brown (6D3, 6D4), when dried dark orange brown (6E7, 6F7). Receptacle surface pale greyish orange (6C5), purple brown (6D4), when dried dark orange brown (6E7, 6F7), furfuraceous. Warts scarce to absent, low. Stipe absent. Basal tomentum and mycelium whitish to pale orange grey (5B2). Spores narrowly ellipsoid to fusoid, narrowing toward the poles, sometimes inequilateral, with two large guttules, and often with several smaller guttules, smooth, hyaline, 20–24.5 × 9–11 μm (Lm = 21.6 μm, Wm = 10 μm, Qm = 2.1; n = 1). Paraphyses curved to hooked, seldom straight, slightly enlarged at apices, 3–4(–5) μm wide, sometimes with a sinuous underside or with 1–2 notches, frequently branching, entangled and interconnected, when dried containing small, refractive, hyaline guttules. Asci 172–197 × 12–13 μm. Apothecial section 700–850 μm thick. Medullary excipulum of textura intricata, 400–500 μm thick, hyphae slightly thick-walled, 5–9 μm wide, pale brown. Ectal excipulum of textura angularis, 70–110 μm, cells thin-walled, yellowish brown, 18–33 × 13–22 μm. Surface with low warts, up to 40 μm high, cells ovoid to globose, constricted at septa, 8–13 μm wide. Resinous exudates absent. Basal mycelium of 4–5 μm broad, pale brown hyphae, sometimes with oily refractive drops on the surface.
Specimens examined. GERMANY, Nordrhein, Herten, 1 Sept. 1999, F. Kasparek, private herb. Kasparek s.n. (dupl. S-F257062).
Notes — Otidea apophysata is characterised by deeply cupshaped, split, brown apothecia, and large, ellipsoid to fusoid spores. Otidea daliensis is a closely related species, distinguished by darker brown, shallowly cup-shaped, usually entire apothecia, and abundant dark brown resinous exudates on the outermost cells of the ectal excipulum. Otidea platyspora has also brown apothecia and large spores, but it differs from O. apophysata in the larger apothecia, partly buried in the substrate, broadly ellipsoid to oblong spores, and non-entangled paraphyses without notches.
The name O. apophysata has been misapplied twice for O. daliensis (Boudier 1909b, Pérez-Butrón & Fernández-Vicente 2008). The type material of O. apophysata lacks resinous exudates on the ectal excipulum, which clearly distinguishes it from O. daliensis. An original painting by W. Phillips, based on the type material of O. apophysata and preserved at RBG Kew (reproduced in Parslow & Spooner 2013), shows typical brown, split, deeply cup-shaped apothecia. The illustration by Cooke (1878, f. 350), based on drawings and (likely dried) specimens communicated by W. Phillips, shows slightly darker apothecia than typical.
Otidea apophysata is only known from very few reports from France (Boudier 1910 as O. felina), Germany (Häffner & Winterhoff 1989, Kasparek 2000), Belgium and Spain (Van Vooren 2011a). In Mid to South Britain it is widely distributed, with collections from fourteen different localities (Parslow & Spooner 2013). Unlike most Otidea species, O. apophysata shows preference for damp habitats, and might be associated with Alnus and Populus (Häffner & Winterhoff 1989, Parslow & Spooner 2013).
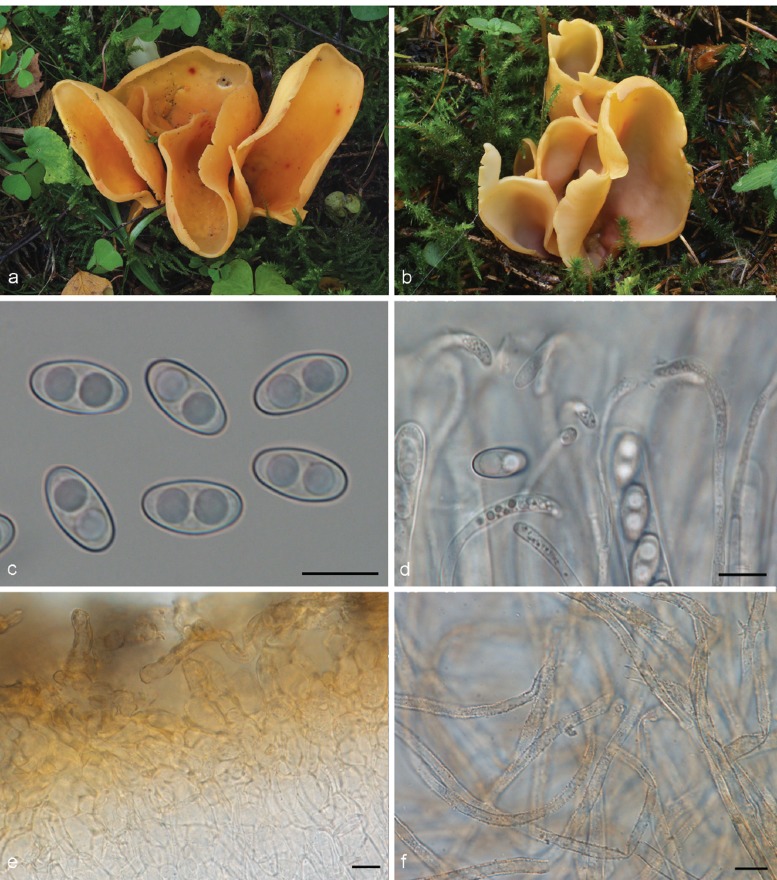
2. Otidea daliensis W.Y. Zhuang & Korf, Mycotaxon 35: 300. 1989
Holotype. CHINA, Yunnan, Dali, Hudiequan Park, alt. 2100 m, on bare soil under seedlings of Plantago major, 5 Nov. 1988, R.P. Korf, L.S. Wang & W.Y. Zhuang (HMAS 57688). Isotype (CUP-CH 2532).
Misapplied names
–Pseudotis apophysata sensu Boudier, Icon. Mycol. livr. 24: n°. 471, pl. 332. 1909 (preliminary text with ‘circulaires’).
–Otidea apophysata sensu Pérez-Butrón & Fernández-Vicente, Errotari 5: 37. 2008.
Apothecia gregarious, up to 9 mm high, 3–16 mm wide, initially cup-shaped, sometimes split, then becoming shallowly cup-shaped, sessile or stipitate. Hymenium when dried dark purple brown (7F5, 7F6) to dark brown (6F5). Receptacle surface dark purple brown (7F5, 7F6), when dried dark brown (6F5), furfuraceous. Warts absent or very low. Stipe if present very short. Basal tomentum and mycelium whitish to pale orange grey (5B2). Spores broadly ellipsoid to ellipsoid and narrowing toward the poles, sometimes inequilateral, with two large guttules, and often with several smaller granules, smooth, hyaline, (19.5–)20.5–23 × 10.5–12(–13) μm (Lm = 21.2–21.5 μm, Wm = 10.7–11 μm, Qm = 1.9–2.1; n = 2). Paraphyses curved to hooked, sometimes slightly enlarged at apices, 2.5–3.5(–5) μm wide, sometimes with slightly swollen areas, apices sometimes embedded in a brown matter, when dried containing small, refractive, brown guttules. Asci 199–212 × 15–17 μm. Apothecial section 600–850 μm thick. Subhymenium c. 90–110 μm thick, of dense textura intricata, visible as a darker zone, cells cylindrical to swollen, with scattered brown resinous exudates at septa. Medullary excipulum of textura intricata, 300–400 μm thick, hyphae 4–6.5 μm wide, slightly thick-walled, pale brown, with brown resinous exudates at septa. Ectal excipulum of textura angularis, 90–120 μm thick, cells thin-walled, yellowish brown, 18–28 × 11–28 μm. Surface with broadly conical warts. Non-warted parts with 2–5-celled hyphoid hairs, with claviform uppermost cell, more rarely cylindrical, constricted at septa, 6–9 μm wide. Resinous exudates abundant, dark brown, partly dissolving in MLZ. Basal mycelium of 3.5–4 μm wide, hyaline hyphae, with yellowish brown, small, resinous exudates.
Specimens examined. FRANCE, 1869, L. Quélet (UPS F-629790). – SPAIN, Basque Country, Bizkaia, Galdames, Presa de Aguas Juntas, sandy soil under Populus nigra, 11 Aug. 2003, J.L. Pérez Butrón, SEST-03071103; 17 Aug. 2006, SEST-06081702.
Notes — Otidea daliensis is recognised by small, usually entire, dark purple brown, shallowly cup-shaped apothecia, large ellipsoid spores often narrowing toward the poles, and abundant brown resinous exudates on the ectal excipulum. This species has been confused with O. apophysata (see O. apophysata).
The first known report of O. daliensis was by Boudier (1909b), as Pseudotis apophysata. The plate 332 (n°. 471) shows the typical dark purple, shallowly cup-shaped apothecia, in contrast to O. apophysata, depicted in plate 331 (n°. 512) as O. felina (Boudier 1910). Mornand & Courtecuisse (2005) proposed a provisional name, O. boudieri, for the Boudier P. apophysata plate (= O. daliensis, n°. 332). Zhuang & Korf (1989) described O. daliensis without comparing it to O. apophysata. Material with small, shallowly cup-shaped apothecia and darker colour was reported from the Iberian Peninsula as O. apophysata (Pérez-Butrón & Fernández-Vicente 2008), and thus had similarities with O. daliensis and Boudier’s plate 332. Van Vooren (2011a) considered the Iberian O. apophysata material to represent O. daliensis. After restudying the Iberian material and comparing it to O. apophysata, we agree with that statement. LSU sequences obtained from the Iberian material and from the Chinese holotype of O. daliensis are identical.
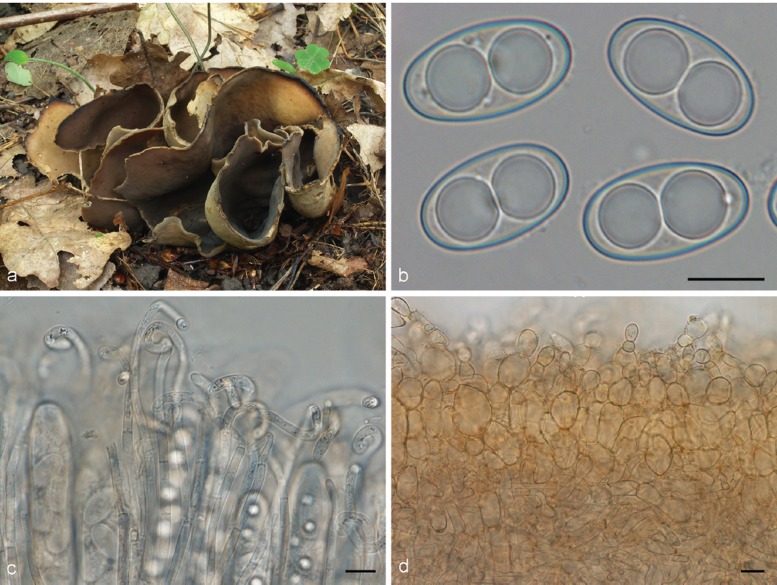
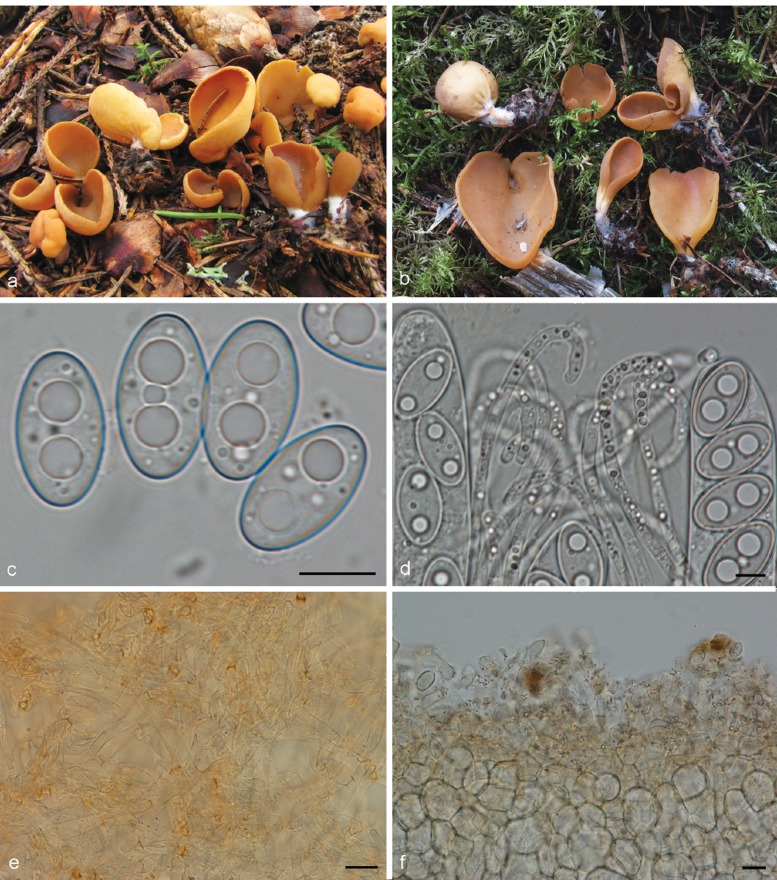
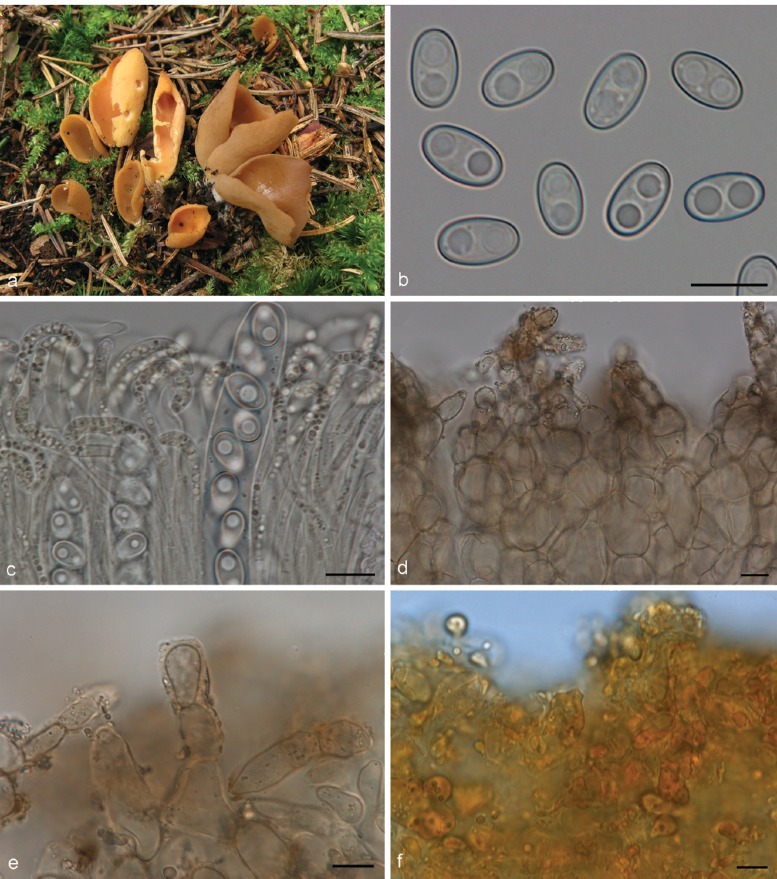
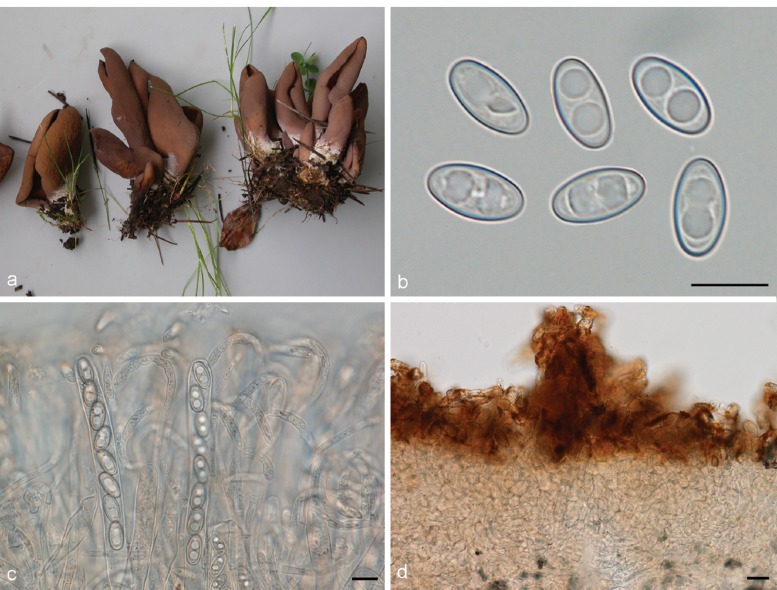
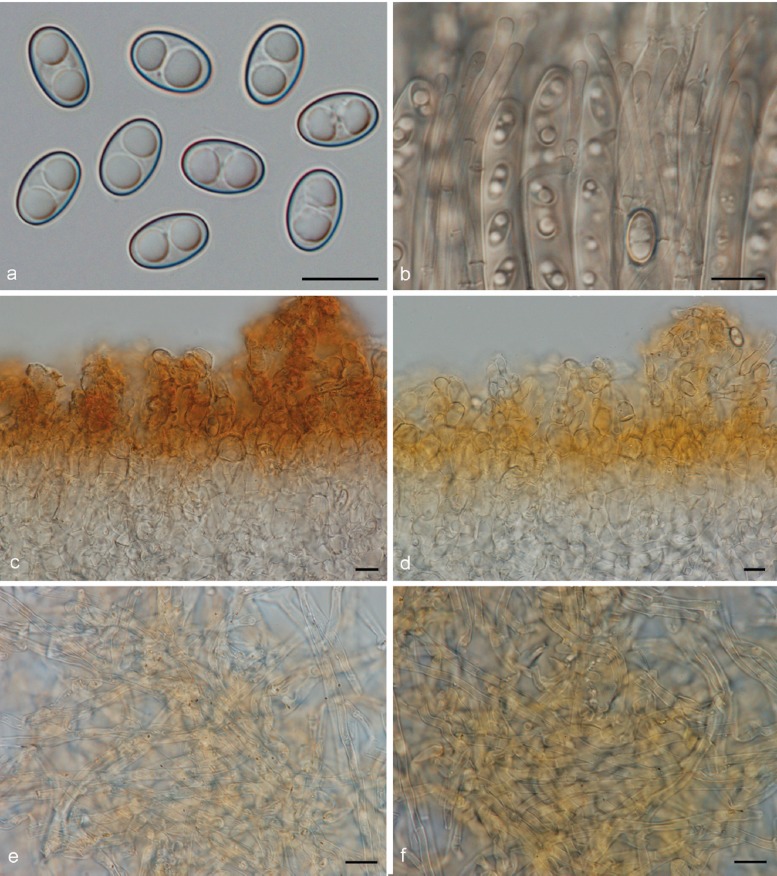
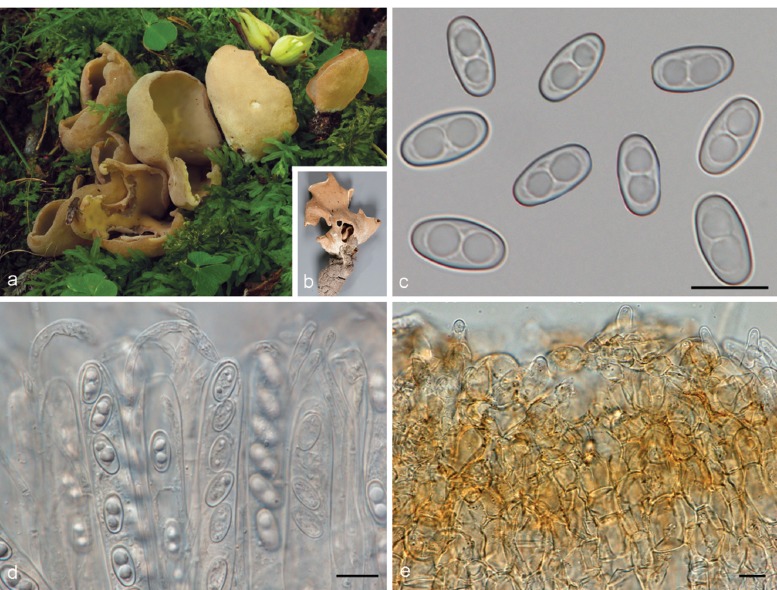
3. Otidea platyspora Nannf., Ann. Bot. Fenn. 3: 317. 1966. — Fig. 7
Fig. 7.
Otidea platyspora*. a. Apothecia; b. spores; c. paraphyses; d. ectal excipulum (a: KH.10.183; b–d: KH.09.163). — Scale bars = 10 μm; * = all fresh material.
Holotype. SWEDEN, Uppland, Djursholm, Oct. 1951, A. Zander, Fungi Exs. Suec. 3284 (UPS F-005428). Isotype (S-F88395) !
Misapplied names
– Otidea cochleata sensu Boudier, Icon. Mycol. livr. 21: n°. 461, pl. 329. 1908 (preliminary text with ‘circulaires’).
Apothecia caespitose, 60–70 mm high, 40–75 mm wide, initially ear-shaped, then soon expanding and becoming deeply cup-shaped, split, sessile or shortly stipitate. Hymenium initially yellowish brown (5C6, 5C7), pale greyish brown (5C3, 5C4) to dark brown (6F3–6F6), when bruised margin blackish, when dried brownish ochre (5B4, 5B5). Receptacle surface dark ochre brown (5D7, 5D8), slightly hygrophanous, in drying pale ochre brown (5B6), when dried yellowish brown (5C6, 5C7), sometimes wrinkled at the base, finely furfuraceous in the margin. Warts absent. Stipe not well developed. Basal tomentum and mycelium whitish to pale brown (5A3). Spores broadly ellipsoid to oblong, rarely slightly inequilateral, with two large guttules, often with several smaller guttules, smooth, hyaline, 18–22 × (9.5–)10.5–12 μm (Lm = 19.8–20.7 μm, Wm = 10.9–11.6 μm, Qm = 1.7–1.8; n = 6). Paraphyses curved to hooked, of the same width or slightly enlarged at apices, 2.5–3.5(–5) μm wide, without notches, rarely with a slightly swollen area on the underside, when fresh containing small, refractive, light brownish yellow guttules; when dried tiny, light yellow granules. Asci 168–213 × 14–19 μm. Apothecial section 700–1700 μm thick. Subhymenium 80–100 μm thick, visible as a darker zone, cells cylindrical, densely arranged, with scarce yellowish brown resinous exudates at septa. Medullary excipulum of textura intricata, 550–1100 μm thick, hyphae 3.5–10 μm wide, sometimes slightly swollen, thin-walled, light yellowish brown. Ectal excipulum of textura angularis, 70–90 μm, cells thin-walled, pale brown, 13–37 × 12–22 μm. Surface with hyphoid hairs, 33–70 μm long, of 4–7 ovoid to subglobose cells, constricted at septa, 6–9 μm wide, sometimes with a light brown matter. Resinous exudates absent to scarce, pale brown, dissolving in MLZ. Basal mycelium of 3.5–4.5 μm wide, hyaline to very pale brown hyphae, sometimes with oily refractive drops on the surface, sometimes with minute bipyramidal resinous exudates.
Specimens examined. AZERBAIJAN, Montes Talysh, in silva mixta, 14 Oct. 1962, E. Parmasto (UPS F-629452). – DENMARK, East Jylland, Kammerherrens Ege, Moesgård Skov, S of Århus, under Fagus and Quercus, 11 Sept. 2006, J. Vesterholt, JV06-656 (C). – FRANCE, Ain, commune de Saint-Benoit, forêt d′Évieu, under Quercus robur, Carpinus betulus, Corylus avellana and Alnus glutinosa, 15 July 2011, F. Armada, NV 2011.07.04 (dupl. S). – SWEDEN, Uppland, Stockholm, Drottningsholm, under Quercus robur, 13 Sept. 2009, K. Hansen & I. Olariaga, KH.09.163 (S); Uppland, Stockholm, Solna, Karlbergsparken, garden with broadleaf trees, 29 July 2006, H.-G. Toresson (S-F248339); Uppland, Stockholm, Sånga, Svartsjö slott, under Quercus, Fagus and Corylus, 31 Aug. 2008, H. Kauffman, HK08046 (S); Uppland, Uppsala, mixed forest with Pinus sylvestris, Picea abies, Betula pendula and Quercus robur, 9 Oct. 2011, J.C. Zamora (BIO-Fungi 16391); Uppland, Uppsala, Hågadalen-Nåsten Nature Reserve, Predikstolen, under Corylus, Populus, Quercus and Picea, on rich ground, 6 Sept. 2010, K. Hansen & I. Olariaga, KH.10.183 (S).
Notes — Otidea platyspora is characterised by large, caespitose, brown apothecia, blackening in bruised margins and large spores. Macroscopically it resembles members of the O. alutacea complex, but these differ in the smaller spores. Otidea apophysata and O. daliensis have likewise brown apothecia and large spores, but can be distinguished by having even larger spores and smaller (up to 30 mm) apothecia (see further under those species).
Otidea platyspora is a striking species with scarce records. It was described from Sweden and has been reported from France (Boudier 1908, Nannfeldt 1966, Van Vooren & Armada 2011), the Netherlands (Maas Geesteranus 1967) and recently from Britain and Denmark (Parslow & Spooner 2013). We report it as new from Azerbaijan and give additional records from Denmark and Sweden. Otidea platyspora appears to have been overlooked and is more widespread than thought previously.
4. Otidea subterranea Healy & M.E. Sm. in Smith & Healy, Mycol. Res. 113: 860. 2009
Holotype. USA, Iowa, Ledges State Park, shallowly hypogeous, erumpent on soil, 30 Aug. 1997, R. Healy RH69 (FH).
Notes — Otidea subterranea is the only known hypogeous species of Otidea. The pustules (c. 50–100 μm high) on the outer surface of the ptychothecia and the incrusted tomentum are typical for Otidea. The asci are cylindrical, with 8 uniseriate spores, placed in a defined hymenium. Unlike most Otidea species the spores are uniguttulate. As an adaptation to a hypogeous habit active spore discharge has been lost. Probably for the same reason, the paraphyses are aggregated and fused subapically to form an epithecium of brown thick-walled cells, and are reminiscent of the frequently branching, entangled and interconnected paraphyses in O. apophysata. There are no other morphological features that support the exact placement of O. subterranea in the O. platyspora clade. The receptacle surface of O. subterranea ascomata is whitish to cream in young and peach-cream to buff with tan-brown areas in older specimens. The gleba is of dark brown fertile veins lined with a thin, light yellowish brown hypothecium, but the sterile veins are of whitish hyphae (Smith & Healy 2009).
Otidea alutacea clade
Apothecia cup-shaped and split, brown. Spores typically ellipsoid, with almost parallel sides. Outermost ectal excipulum and basal mycelium smooth or with very sparse resinous exudates.
Species — Otidea alutacea s.l.
5. Otidea alutacea (Pers.) Massee, Brit. Fungus-Fl. 4: 446. 1895. — Fig. 8
Fig. 8.
Otidea alutacea s.str. (KH.09.133). a. Apothecia; b. spores in water†; c. paraphyses in water†; d. ectal excipulum in KOH†. — Scale bars = 10 μm; † = dried material.
Basionym. Peziza alutacea Pers., Observ. Mycol. 2: 78. 1799; non Peziza alutacea Schumach., Enum. Pl. 2: 431. 1803 (homonym).
≡ Scodellina alutacea (Pers.) Gray, Nat. Arr. Brit. Pl. 1: 668. 1821.
≡ Peziza cochleata var. alutacea (Pers.) Fr., Syst. Mycol. 2: 50. 1822: Fr. loc. cit. (‘ß alutacea’).
≡ Plicaria alutacea (Pers.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 327. 1870.
≡ Aleuria alutacea (Pers.) Gillet, Champ. France Discom. 1: 42. 1879.
Lectotype. Bull., Hist. Champ. France 1: t. 154, f. b., designated by Carbone (2010a). Epitype. (L0111551, Herb. Persoon), designated by Carbone (2010a).
1Apothecia gregarious, rarely caespitose, 15–75 mm high, 8–48 mm wide, initially ear-shaped, soon expanding, becoming shallowly to deeply cup-shaped, split, sessile or stipitate. Hymenium initially brown (5D6), then yellowish brown (5D4, 5D5) to dark reddish brown (7D7, 7E7), when dried purple brown (6D6, 6D7). Receptacle surface slightly hygrophanous, in drying yellowish brown (5D5, 5D6), or sometimes with purplish brown (7D5) tones, when dried light ochre (5A4, 5B4) brownish ochre (5B5, 5B6), finely furfuraceous to slightly warty in the margin. Warts flattened, gregarious, concolorous, darker when the outside in drying. Stipe 3–6 × 3–4 mm. Basal tomentum and mycelium white to very light ochre (5A2). Spores ellipsoid to broadly ellipsoid, oblong ellipsoid, slightly inequilateral, with two large guttules, often with several smaller granules, smooth, hyaline, (13.5–)14.5–16.5(–17.5) × 6.5–7.5(–8) μm (Lm = 14.6–16.1 μm, Wm = 6.6–7.5 μm, Qm = 2.1–2.2; n = 5). Paraphyses curved to hooked, only few straight, of the same width or slightly enlarged at apices, 2.5–4.5 μm wide, without notches, sometimes embedded in a brown matter at apices, when fresh containing small, refractive, light brownish yellow guttules; when dried brownish yellow. Asci 140–187 × 11–13 μm. Apothecial section 750–1050 μm thick. Subhymenium c. 80–100 μm thick, visible as a darker zone, cells cylindrical to swollen, densely arranged, with scattered yellowish brown resinous exudates at the septa. Medullary excipulum of textura intricata, 400–650 μm thick, hyphae thin-walled to slightly thick-walled, 4–9 μm wide, hyaline to light brown, sometimes with yellowish brown resinous exudates at septa. Ectal excipulum of textura angularis of 80–100 μm, cells thin-walled, pale brown, 9–21 × 9–17 μm. Surface with broad conical warts, 35–57 μm high, formed by short, fasciculate, hyphoid hairs, of 6–7 globose to subglobose cells, constricted at septa, 7–10 μm wide. Resinous exudates absent to scarce, yellowish brown, dissolving in MLZ. Basal mycelium of 3–4.5 μm wide, hyaline hyphae, with oily refractive drops on the surface, sometimes with minute resinous exudates.
Specimens examined. O. alutacea s.str. — DENMARK, S Sjælland, Møn, Store Klinteskov, by Svantestenen, on calcareous soil (pH 7.0) along forest road, under deciduous trees, together with Humaria hemisphaerica and Trichophaea woolhopeia, 11 Sept. 1994, K. Hansen & S.K. Sandal, KS-94-111 (C). – FRANCE, Puy-de-Dôme, Auvergne, Nadayat, sous feuillus en terrain neutrocline, 20 Sept. 1998, G. Corriol, GC 98092002 (dupl. S). – ITALY, Piemonte, Vignole Borbera (AL), Fraz. Variano superiore, under Quercus pubescens and Castanea sativa, 19 Oct. 2010, M. Carbone (S-F257084). – NORWAY, Nord-Trøndelag, Leksvik, Gjøråsvika, on rich, bare ground, under Corylus and Picea, on a steep slope, 3 Sept. 2009, K. Hansen & I. Olariaga, KH.09.133 (S). – SPAIN, Gipuzkoa, Tolosa, Elosegi markesaren lorategiak, under broadleaf trees in a garden, 29 May 2009, J.I. López-Amiano, JLA 2009052902 (ARAN-Fungi A3023204). – SWEDEN, Gotland, Ollajvs Nature Reserve, close to Ljugarn, under Picea and Pinus on calcareous ground, 27 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.278 (S). Clade 1 — DENMARK, Eastern Falster, Korselitze-forests, 5 Oct. 2007, H. Knudsen, KH.07.46 (S). – SWEDEN, Uppland, Stockholm, Norra Järvafältet, Hansta Nature Reserve, on rich ground under Corylus and Quercus, 8 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.193 (S); Uppland, Stockholm, N Djurgården, Stora skuggan, on soil in grazed open oak forest, 12 Sept. 2008, J. Santos, JS.08.81 (S); Uppland, Uppsala, Hågadalen-Nåsten Nature Reserve, Predikstolen, under Quercus robur, Picea abies, Corylus and Salix, on rich bare ground, 17 Sept. 2009, K. Hansen & I. Olariaga, KH.09.170 (S). Clade 2 — USA, Oregon, Lincoln Co., Devil’s Punchbowl State Park, 13 Mar. 1997, E.T. Peterson (OSC 56770); Washington, Pierce Co., Mt Rainier National Park, Lower Tahoma Creek, under Pseudotsuga, Tsuga, Picea and Calocedrus, 29 Oct. 1996, E.T. Peterson (OSC 56747); ibid., 30 Oct. 1996 (OSC 56754); ibid., 18 Oct. 1997 (OSC 56798); Washington, Snohomish Co., Sloan Creek trail, 24 Sept. 1997, E.T. Peterson (OSC 56777). Clade 3a — NORWAY, Nord-Trøndelag, Leksvik, Gjøråsvika, on slope under Corylus and Picea, on rich ground, 3 Sept. 2009, V. Kučera & I. Kautmanová, KH.09.135 (S). – SWEDEN, Södermanland, Nynäshamn, Herrhamra, on soil under Fagus, in narrow forest area along the road, 19 Sept. 2013, K. Hansen & X.H. Wang, KH.13.50 (S); Uppland, Norrtälje, Länna, under Corylus, 26 Aug. 2008, J. Santos, JS.08.43 (S); Uppland, Uppsala, Hågadalen-Nåsten Nature Reserve, Predikstolen, under Quercus robur, Picea abies, Corylus and Salix, on rich, bare ground, 6 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.198 (S). Clade 3b — DENMARK, S Sjælland, Møn, Store Klinteskov, Vestre Ulvemose, on calcareous soil in deciduous forest, 26 Sept. 1994, K. Hansen & S.K. Sandal, KS-94-192 (C). – SWEDEN, Uppland, Uppsala, Hågadalen-Nåsten Nature Reserve, Predikstolen, under Corylus, Populus and Picea, on rich ground, 19 Sept. 2009, K. Hansen & I. Olariaga, KH.09.178 (S). Clade 4 — USA, Oregon, Benton Co., Corvallis, McDonald-Dunn Research Forest, 10 Oct. 1997, E.T. Peterson (OSC 56782); ibid., 18 Nov. 1996 (OSC 56758); Oregon, Benton Co., Corvallis, west side of NW Beechwood Place, scattered to clustered on rotting bark mulch and in thin grass under Pseudotsuga menziesii, 17 Nov. 2010, N.S. Weber, NSW10200 (OSC 150345); Oregon, Corvallis, Witham Hill, 25 Nov. 1997, E.T. Peterson (OSC 56813); Oregon, Douglas Co., 20 Oct. 2010, J. Moore, Moorefun 19 (OSC); Oregon, Douglas Co., Bear Gulch, under Pseudotsuga menziesii, 13 Jan. 1999, R. Davidson (OSC 67524); Oregon, Douglas Co., Slimewater, under Pseudotsuga menziesii, Quercus garryana, Abies grandis, etc., 12 Sept. 1999, Frymire (OSC 72978); Oregon, Douglas Co., Umpqua, under Pseudotsuga menziesii, Abies concolor, Calocedrus decurrens, Corylus cornuta, etc., 23 Mar. 2000, E. Stewart (OSC 72979); under Pseudotsuga menziesii, Pinus ponderosa, Calocedrus decurrens, Pinus lambertiana, Abies concolor, etc., 15 Dec. 1999, C. Rusch (OSC 72176); Oregon, Lane Co., Willamette National Forest, Middle Fork Ranger District, under Tsuga heterophylla, Thuja plicata and Abies grandis, 18 Nov. 2002, Smith (OSC 119567).
Additional material of O. alutacea s.l. DENMARK, Sjælland, Hareskoven, N of Copenhagen, 2 Aug. 1961, H. Dissing (C-F-48301). – FRANCE, Orliénas, sous Quercus et Cedrus atlantica, 13 Nov. 2008, B. Rivoire & N. Van Vooren, NV 2008.11.01 (dupl. S-F256976); Rhône, Bron, Parc de Parilly, 24 Sept. 2008, J. Cavet, NV 2008.09.32 (dupl. S-F256974); Rhône, Courzieu, hameau des Verchères, sous Pseudotsuga menziesii, 26 Oct. 2008, D. Carbonnel, NV 2008.10.02 (dupl. S-F256975). – ITALY, Puglia, Mesagne (BR), Bosco Lucci, in soil, mainly under Quercus ilex, 12 Oct. 2010, M. Carbone (S-F257085). – NORWAY, Nord-Trøndelag, Leksvik, Gjøråsvika, mixed forest on rich ground, 3 Sept. 2009, K. Hansen & I. Olariaga, KH.09.137 (S); ibid., R. Braathen, KH.09.139 (S); Nordland, Rana, Rausandaksla, in salico-betuletum, on limestone, 21 Sept. 1974, S. Sivertsen (C-F-60697). – SPAIN, Madrid, Arboreto de ETSI Montes, under Quercus suber, Pinus pinea and Nerium oleander, 21 Nov. 2006, L. Rubio Casas (AH42204). – SWEDEN, Gotland, near Visby, Värnhem, on rich ground under Fagus and Quercus, with Hepatica nobilis, 22 Sept. 2009, E. Bohus-Jensen, K. Hansen & I. Olariaga, KH.09.187 (S); Gästrikland, Hofors, Sibbersbovägen, on rich ground under Corylus, 1 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.154 (S); Jämtland, Ändsjön Nature Reserve, in rich Picea forest, 26 Aug. 2009, H. Lindström, KH.09.97 (S); Närke, Ekeby, Kvarntorp, under a big Quercus by the road, 10 Sept. 2008, J. Santos & K. Hansen, JS.08.57 (S); Närke, Tysslinge, Latorpsbruk, Grytsätterskogen, grassland with Quercus, 13 Sept. 2008, JS.08.76 (S); Närke, Örebro, Hästhagen, by Svartån, mixed forest, 13 Sept. 2008, H. Kauffman, JS.08.74 (S); Skåne, Helsingborg, Fredriksdals Friluftmuseum, 16 Sept. 2010, G. Hamilton, KH.10.206 (S); Skåne, Kjugekull Nature Reserve, on bare ground under Quercus rubra, Corylus and Fagus, 24 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.262 (S); Skåne, Maltesholm, forest close to the castle, on the ground under Fagus, close to Alnus, 25 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.265 (S); Torne Lappmark, Abisko, 12 Aug. 1974, M.D. Paulsen & N. Tams (C-F-48045); Uppland, Stockholm, Enebyberg, Rinkebyskogen, on bare soil in a ditch, under Picea, Betula and Populus, 28 Aug. 2008, J. Santos, JS.08.50 (S); ibid., in deciduous forest under Corylus, but also Tilia, Quercus and Betula, 1 Sept. 2008, J. Santos, JS.08.56 (S); Uppland, Stockholm, Norra Järvafältet, Hansta Nature Reserve, on naked soil among leaves, under large Corylus, also Quercus, 8 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.189 (S); Uppland, Uppsala, Hågadalen-Nåsten Nature Reserve, Predikstolen, on rich ground under Quercus, Corylus, Populus tremula and Picea, 17 Sept. 2009, K. Hansen & I. Olariaga, KH.09.173 (S).
Notes — We consider O. alutacea s.l. to comprise a species complex. It is recognised by the medium brown, cup-shaped, split apothecia, an ectal excipulum with only sparse resinous exudates if any, and predominantly oblong spores. Although sometimes treated as a well-delimited species (Harmaja 2009a), spore sizes of O. alutacea provided by different authors vary considerably, e.g. 14–16 × 7–9 μm (Dissing 2000) or 12.5–14.5 × 6.2–7.3 μm (Harmaja 2009a). In fact, O. cochleata has been separated from O. alutacea on account of larger spores, 16–18 × 7–8 μm (Dissing 2000), or darker apothecia (Mornand & Courtecuisse 2005, Liu & Zhuang 2006, Zhuang 2006). Meanwhile, two taxa of the O. alutacea species complex have been separated in North America, based on apothecia colour, and spore size and shape (Peterson 1998). Our LSU phylogeny resolved several clades within O. alutacea s.l. (Fig. 1), which are strongly supported in our multigene phylogeny (Hansen & Olariaga 2015). It appears that the spore sizes within each clade have a fairly narrow range, but overlap exists between the clades. Patterns of continental speciation are suggested as well; two clades have North American specimens (clade 2, 4), and the rest contain samples from Europe and Asia.
Carbone (2010a) selected a lectotype and an epitype for O. alutacea. A spore range of 15.5–17 × 7 μm was given for the epitype specimen (Carbone 2010a), and based on this we assign O. alutacea s.str. to the clade inferred from the phylogenetic analyses encompassing this spore size. The description above is based solely on the specimens of that clade, which are characterised by the initially shallowly cup-shaped apothecia, with rather light ochraceous brown hymenium, which later becomes purple brown and more deeply cup-shaped. The lectotypification of O. alutacea proposed by Parslow & Spooner (2013) is superfluous.
Among the Eurasian clades, clade 1 contains North European specimens characterised by small spores (12–13.5 × 5.5–7 μm, Lm = 12.2–12.9 μm, Wm = 5.8–6.6 μm, Qm = 1.9–2.2), non-overlapping with O. alutacea s.str. and clade 3. Clade 1 should be compared to O. kunmingensis, a taxon belonging to the O. alutacea complex characterised by short spores (Zhuang & Yang 2008). Clade 3a encompasses three specimens with spores sizes 13.5–15 × 6.5–8 μm (Lm = 14.5–14.6 μm, Wm = 6.7–7.3 μm, Qm = 2–2.2) overlapping with those of O. alutacea s.str. as described here. The apothecia differ slightly macroscopically from O. alutacea s.str. in being deeply cup-shaped in the beginning, reddish brown when young, later pale ochre-brown. Clade 3b is composed of two collections with larger spores, 15.5–17.5 × 7.5–8 μm (Lm = 16.1–17.4 μm, Wm = 7.7–8 μm, Qm = 2–2.3), but slightly overlapping with O. alutacea s.str. and clade 3a. Clade 3b may correspond to O. cochleata sensu Dissing (2000). The two North American clades (2 and 4) comprise specimens with clearly non-overlapping spore sizes, 15–18 × 7–8 μm (Lm = 15.5–16.9 μm, Wm = 7.3–7.9 μm, Qm = 2–2.2) and 12–14.5 × 6.5–8.5 μm (Lm = 12.5–14 μm, Wm = 6.6–7.5 μm, Qm = 1.8–2), respectively. These two clades correspond to the two species distinguished by Peterson (1998) in Western North America, as O. alutacea (clade 2) and O. umbrina (clade 4). These species were said to differ in colour of fresh apothecia. Clades 2 and 4 have spores that overlap with European clades, and it is so far problematic to distinguish them using only morphology and disregarding the geographical origin.
To be able to fully clarify species boundaries within the O. alutacea complex, sampling of additional collections for molecular study is needed. Distinguishing morphological and ecological characters should be sought, especially through studying fresh material. Several names that belong to this complex, such as O. alba, O. cinerascens, O. cochleata, O. felina and O. kunmingensis should be considered as this study is undertaken.
Otidea papillata clade
Apothecia cup-shaped, split. Receptacle surface with contrasting warts. Spores small, 9.5–11 μm. Medullary excipulum with brown resinous exudates embedding some hyphae. Ectal excipulum poorly differentiated, of textura prismatica to textura intricata. Resinous exudates on the ectal excipulum not dissolving in MLZ.
Species — Otidea papillata.
6. Otidea papillata Harmaja, Karstenia 15: 31. 1976 — Fig. 9
Fig. 9.
Otidea papillata†. a. Apothecia; b. spores in water; c. paraphyses; d. resinous exudates in the medullary excipulum in water; e. ectal excipulum in KOH; f. basal mycelium in KOH (a–d, f: H6003547, holotype; e: TUR 102134). — Scale bars = 10 μm; † = all dried material. — Photos: a. J. Kearey.
Holotype. FINLAND, Kainuu, Paltamo, Melalahti, Myllymäki, predominantly coniferous grass-herb forest on distinctly calcareous soil, in litter mainly composed of spruce needles, 23 Sept. 1971, H. Harmaja (H6003547) !
Apothecia 23–30 mm high, 7–33 mm wide, initially broadly ear-shaped, with upper margin rounded, then becoming cup-shaped, split, stipitate or sessile. Hymenium ochre (5B5, 5B6) to yellowish ochre (4A5) when dried. Receptacle surface yellowish brown (5C6, 5C7) when dried, warty. Warts conical, angular or rounded, gregarious, distinctly darker than the background, dark ochre brown to brown. Stipe 7–10 × 3–4 mm. Basal tomentum and mycelium abundant, pale brownish ochre (5A3) to orange-ochre (6A3). Spores broadly ellipsoid, seldom very slightly inequilateral, with two large guttules, smooth, hyaline, 9.5–11(–11.5) × 5.5–6.5 μm (Lm = 10–10.7 μm, Wm = 6.1–6.3 μm, Qm = 1.6–1.7; n = 2). Paraphyses curved to tightly hooked, usually enlarged at apices, 3–4 μm wide, sometimes with 1–2 shallow notches, sometimes truncate or forked at apices, when dried containing small, refractive, hyaline granules. Asci 116–165 × 9–10.5 μm. Apothecial section 900–1200 μm thick. Subhymenium 70–90 μm thick, of dense textura intricata, visible as a pale brown zone. Medullary excipulum of textura intricata, 600–800 μm thick, hyphae 5.5–10.5 μm wide, thin-walled to slightly thick-walled, hyaline to very pale yellow, with brown resinous exudates scattered among and covering some hyphae, sometimes rod-shaped, paler and partially dissolving in KOH. Ectal excipulum of textura prismatica to textura intricata, 70–100 μm, cells thin-walled, hyaline, 13–35 × 9–18 μm. Surface with conical to rounded warts, 65–100 μm high, formed by short, fasciculate, hyphoid hairs, sometimes with a gelatinous sheath. Resinous exudates abundant, yellowish brown to reddish brown, paler in KOH, not dissolving in MLZ. Basal mycelium of 2.5–4.5(–6) μm wide, septate, hyaline to very pale yellow hyphae, unchanged in KOH, smooth or with regularly arranged, spheroid, yellow, resinous exudates, sometimes embedded in a yellowish matter, dissolving in MLZ, partially and more slowly in KOH.
Specimen examined. FINLAND, Varsinais-Suomi, Parainen, Petteby, Stornäset (Paltbacken), in coniferous forest among mosses, 30 Sept. 1990, T. Lindholm (TUR 102134).
Notes — Otidea papillata is only known from two Finnish collections and its apothecial colours in fresh state are still unknown. It is a distinct species, characterised by cup-shaped apothecia with conspicuous warts on the outside, and small spores (Harmaja 1976). Two diagnostic characters have been observed in the two collections examined: 1) a very poorly differentiated ectal excipulum of textura prismatica to textura intricata, which is unique within the Otidea species studied by us; and 2) scattered brown resinous exudates on the hyphae of the medullary excipulum, that are somewhat reminiscent of those in O. bufonia.
Harmaja (1976) emphasised the high warts on the outside of the apothecia as a unique character for O. papillata, but two additional Otidea species, O. tuomikoskii and O. nannfeldtii, have as high or higher warts. These two species have in fact been confused with O. papillata (Lundell et al. 1985, Van Vooren et al. 2008). Otidea tuomikoskii is distinguished from O. papillata by narrowly ear-shaped apothecia, a yellow reaction of the excipulum in KOH, an ectal excipulum of textura angularis, and by lacking pigmented exudates on the hyphae of the medullary excipulum. Highly warted apothecia of O. nannfeldtii probably resemble O. papillata more. However, O. nannfeldtii possesses pigmented resinous exudates at the septa in the medullary excipulum, resinous exudates of the ectal excipulum that convert into amber drops, and most importantly, an ectal excipulum of textura angularis.
Otidea leporina clade
Apothecia ear-shaped, yellowish ochre to brown. Resinous exudates on the ectal excipulum converting into reddish grey drops in KOH. Associated with conifers.
Species — Otidea leporina, O. pseudoleporina.
7. Otidea leporina (Batsch) Fuckel, Jahrb. Nassauischen Ver-eins Naturk. 23–24: 330. 1870 ‘1869–1870’ — Fig. 4a, 10
Fig. 10.
Otidea leporina*. a, b. Apothecia; c. spores; d. paraphyses; e. ectal excipulum with resinous exudates, insert shows close-up of amber drops on the ectal excipulum in Melzer’s reagent; f. basal mycelium pale brown, with very small, regularly arranged, resinous exudates (a: KH.09.93, epitype; b: KH.09.102; c–g: KH.11.02). — Scale bars = 10 μm; * = all fresh material.
Basionym. Peziza leporina Batsch, Elench. Fung. 1: 117. 1783: Fr., Syst. Mycol. 2: 47. 1822.
≡ Scodellina leporina (Batsch) Gray, Nat. Arr. Brit. Pl. 1: 668. 1821.
≡ Helvella leporina (Batsch) Franchi, L. Lami & M. Marchetti, Rivista Micol. 1: 63. 1999.
≡ Helvella auricula Schaeff., Fung. Bavar. Palat. Nasc. 4: 103. 1774 (‘Elvela’).
≡ Wynnella auricula (Schaeff.) Boud., Icon. Mycol. list prél. 600 sp.: (2). 1904.
≡ Otidea auricula (Schaeff.) Sacc., Syll. Fung. 8: 95. 1889.
Lectotype designated here: Schaeffer, Fung. Bavar. Palat. Nasc. 2: t. 156. 1763 (‘Elvela decima tertia’). Epitype designated here: SWEDEN, Jämtland, Östersund, Andersön Nature Reserve, under Picea abies on rich ground, 28 Aug. 2009, K. Hansen & I. Olariaga, KH.09.93 (S); MycoBank MBT178082.
= Otidea leporina f. minor Rehm, Ber. Naturhist. Vereins Augsburg 26: 63: 1881.
≡ Otidea leporina var. minor (Rehm) Sacc., Syll. Fung. 8: 94. 1889.
Lectotype designated here: GERMANY, Leipzig, in der Harth, in spruce forest, Aug. 1873, G. Winter, Rehm Ascomyceten no. 251 (S-F88382) ! Isolectotype (UPS F-641412) !; MycoBank MBT178088.
= Otidea leporina f. major Rehm, Hedwigia 3–4: 2. 1883.
= Otidea leporina var. rubescens Velen., Monograph. Discom. Bohemiae 1: 354. 1934.
Lectotype designated here: Czech Republic, Kosoř near Prague, Sept. 1920, F. Fechtner (PRM 614790) !; MycoBank MBT200087.
= Otidea myosotis Harmaja, Karstenia 15: 32. 1976.
Holotype. FINLAND, Etelä-Karjala, Hamina, Vehkalahti, Pyhältö, mixed forest, 3 Oct. 1970, L. Fagerström (H6003548) !
= Otidea crassa W.Y. Zhuang, Mycotaxon 94: 366. 2006 (‘2005’).
= Otidea fuckelii M. Carbone & Van Vooren, Rivista Micol. 52: 322. 2010 (‘2009’).
Holotype. AUSTRIA, Nassau, in pinetis umbrosis, Fungi Rhen. Exs. no. 1233 (G00110768). Isotype (S-F114092) !
Misapplied names
– Non Wynnella auricula sensu Boudier, Icon. Mycol. livr. 26: n°. 535, pl. 250. 1909 (preliminary text with ‘circulaires’) (= Wynnella silvicola (Beck) Nannf.).
Apothecia gregarious or caespitose, 17–52 mm high, 4–25 mm wide, narrowly to broadly ear-shaped, split, stipitate or sessile. Hymenium yellowish brown (5C6), cinnamon brown (5D6), pale ochre brown (5A5, 5B6) to orange brown (6C7, 6D6), sometimes dark brown (5D8, 5F8, 6E8) when young, seldom with pale pink stains (6A2), bruised margin orange brown (6D8), when dried cinnamon brown (5A5, 5B5) to rusty brown (6D7, 6D8). Receptacle surface ochre brown (5B6), hygrophanous, in drying paler ochre brown (4A4, 4A5, 4B6), when dried yellowish brown (5C7, 5C8), furfuraceous to finely warty, seldom wrinkled at the base. Warts conical to flattened, gregarious, concolorous, sometimes darker than the background, golden brown. Stipe 4–15 × 2–8 mm. Taste slightly bitter. Basal tomentum and mycelium abundant, white to cream white (5A3), sometimes very pale brown when dried. Spores broadly ellipsoid, sometimes inequilateral, with two large guttules, smooth, hyaline, (12–)12.5–14(–15) × 7–8.5 μm (Lm = 12.8–13.8 μm, Wm = 7.5–8.2 μm, Qm = 1.6–1.8; n = 16). Paraphyses curved to hooked, of the same width or slightly enlarged at apices, 2.5–4 μm wide, without notches or with 1–3 low notches, seldom forked at apices, when fresh containing small, refractive, light yellow guttules; when dried small, refractive, hyaline granules. Asci 170–215 × 9–10.5 μm. Apothecial section 700–900 μm thick. Subhymenium c. 80–100 μm thick, of dense textura intricata, visible as an orange-brown darker zone, cells cylindrical to swollen. Medullary excipulum of textura intricata, 300–550 μm thick, sometimes differentiated into two parts: a) textura angularis underneath the subhymenium, 40–50 μm thick, cells 6–12 μm broad; b) textura intricata, hyphae 5–13 μm wide, sometimes slightly swollen, thin-walled to slightly thick-walled, hyaline to very pale brown, sometimes with yellow-brown resinous exudates at septa. Ectal excipulum of textura angularis, 85–110 μm thick, cells thin-walled, pale brown, 15–48 × 12–31 μm. Surface with broadly conical warts, 50–85 μm high, formed by short, fasciculate, hyphoid hairs, of 2–4 subglobose to elongated cells, constricted at septa, 10–16 μm wide, sometimes with a gelatinous sheath. Resinous exudates abundant, yellow brown, sometimes dissolving in part into amber drops or converting into reddish particles in MLZ, dissolving into yellowish reddish grey heterogeneous drops in KOH. Basal mycelium of 3–4.5(–6) μm wide, hyaline to pale brown hyphae, unchanged in KOH, smooth or with very small, regularly arranged, spheroid, pale brown, resinous exudates, dissolving in MLZ, and partially in KOH.
Specimens examined. CANADA, Québec, Le Verendrye Park, on ground under spruce, 16 Sept. 1965, M.E. Elliot 65-123 (UPS F-629640). – CZECH REPUBLIC, Central Bohemian region, Zdice, Aug. 1924, F. Fechtner (PRM 614787, as O. felina); in piceto ad aciculos, Sept. 1925 (PRM 148836); Prague-West district, Kosoř near Prague, Sept. 1920, J. Velenovský (PRM 614792, as O. umbrina). – DENMARK, Bornholm, Rø Plantage, coniferous forest, 29 Sept. 1985, W. Rummel (C-F-47633); N Jylland, Langdal Plantage (near Tranum), under Juniperus, near Picea, 13 Aug. 2009, T. Læssøe, TL-13769 (C); N Jylland, Rønhøj Plantage, 7 Oct. 1962, A. Hauerbach (C-F-86691); Jylland, Virklund, Silkeborg Sønderskov, in moss, coniferous forest, 26 Sept. 1964, H. Dissing (C-F-48298); Sjælland, Bromme Plantage, N of Sorø, under Picea, 9 Oct. 1965, H. Dissing (C-F-48299). – FINLAND, Etelä-Häme, Mustiala, in pineto, 29 Aug. 1866, P.A. Karsten (UPS F-146429); Perä-Pohjanmaa, Rovaniemi, Kaittiainen, acidic Picea forest, 11 Sept. 2011, T. Kekki, TK407 (TUR); Perä-Pohjanmaa, Rovaniemi, Pisajärvi, old Picea forest, 2 Sept. 2011, T. Kekki, TK304 (TUR); Perä-Pohjanmaa, Rovaniemi, Välljoki, calciferous Picea forest, 25 Aug. 2011, T. Kekki, TK231 (TUR). – FRANCE, Loire, La Chamba, au sol dans la litière d’aiguilles d’épicéa, 27 Sept. 2008, N. Van Vooren, NV 2008.09.28 (dupl. S). – GERMANY, Thüringen, in silvis abiegnis, Kl. & Op. (UPS F-629404, Klotzsch, Herb. Viv. Mycol. 143). – NORWAY, Nord-Trøndelag, Kvam, Noem Northeast, under Picea, among Rhytidiadelphus squarrosus, 2 Sept. 2009, H. Lindström, KH.09.131 (S); Nord-Trøndelag, Namdalseid, Flåbekkåsen Nature Reserve, Picea and Pinus old-growth forest, on acidic soil, among mosses, 4 Sept. 2009, K. Hansen & I. Olariaga, KH.09.141 (S); Nord-Trøndelag, Steinkjer, Skrattåsen, in rich Picea abies forest, 5 Sept. 2009, K. Hansen & I. Olariaga, KH.09.145 (S); ibid., KH.09.147 (S). – SWEDEN, Härjedalen, Torkilstöten, Ljungdalen, on an active anthill in Picea forest, 19 Aug. 2011, J.C. Zamora & I. Olariaga, KH.11.02 (S); Jämtland, in the surroundings of Sällsjö, in young stand of Picea abies, with Betula and Salix, 29 Aug. 2009, K. Hansen & I. Olariaga, KH.09.100 (S); ibid., KH.09.102 (S); Jämtland, SW of Mörsil, Sandtjärndalen Nature Reserve, under Picea abies on rich ground, 7 Sept. 2009, K. Hansen & I. Olariaga, KH.09.156 (S); Lappland, Ekopark Vuollerim, stream from Brännmyran, Picea forest, 28 Aug. 2008, M. Karström, MK0828 (S); Lappland, Jokkmokk, ‘Nornaskogen’ by Ållojaur, mossy Picea forest, on rich ground, 29 Aug. 2011, K. Hansen & I. Olariaga, KH.11.12 (S); ibid., KH.11.14 (S); Lappland, Jokkmokk, Ultevis Fjällurskog Nature Reserve, Sitoätno, near a Picea, 31 Aug. 2011, K. Hansen & I. Olariaga, KH.11.33 (S); ibid., KH.11.36 (S); Lappland, Kuouka, 15 km SE Messaure, herb-rich Picea forest on rich ground, among mosses, 3 Sept. 2011, K. Hansen & I. Olariaga, KH.11.67 (S); Lappland, S of Kvikkjobb-Kabla FUR Nature Reserve, by Kassavare Mt, Köpenhamn, under Picea, mossy place on acidic ground, 1 Sept. 2011, K. Hansen & I. Olariaga, KH.11.76 (S); Närke, Knista, Lekhyttan, Kungshall, under Picea on calcareous ground, among mosses and litter, 12 Sept. 2008, J. Santos, JS.08.065 (S); ibid., coniferous forest, with old Picea and Pinus, K. Hansen, KH.08.108 (S); Skåne, Loshult, Lilla Loshult, Picea forest, 5 Sept. 1998, S.-Å. Hanson, SÅH 105838 (C); Uppland, Stockholm, Enebyberg, Rinkebyskogen, under Picea and Betula on acidic ground, 2 Sept. 2009, K. Hansen & I. Olariaga, KH.09.169 (S); Uppland, Täby, Rönninge, close to parking place by Arninge, under Picea on thick litter layer, 21 Sept. 2008, J. Santos, JS.08.92 (S); Uppland, Uppsala, Ersta Nature Reserve, on soil under Picea in young plantation, 23 Sept. 2008, J. Santos & K. Hansen, JS.08.99 (S); Uppland, Uppsala, Sävja, Norra Lunsen Nature Reserve, under Picea, 28 Aug. 2008, J. Santos, JS.08.46 (S); Värmland, Gustav Adolf, Hagfors, Malmbackarna, on moss, under Picea and Betula, 10 Aug. 2009, F. Turander s.n. (S). – USA, California, Del Norte Co., Lake Earl Wildlife Area, 14 Dec. 1997, E.T. Peterson (OSC 56824); ibid., 15 Dec. 1997 (OSC 56825); ibid., under Picea sitchensis, Pinus contorta, Abies concolor, 26 Nov. 2001, M. Castellano & E. Cazares (OSC 108820); California, Humboldt Co., Big Lagoon Park, 14 Dec. 1956, A.H. Smith 56668 (UPS F-629302); ibid., under Picea, 16 Dec. 1956, A.H. Smith 56799 (UPS F-629304); ibid., 23 Dec. 1956, A.H. Smith 56954 (UPS F-629305); Colorado, Tolland, on ground in coniferous woods, 28 Aug. 1920, F.B. Cotner (UPS F-629390); Oregon, Lincoln Co., Fogarty Creek State Park, 15 Oct. 1997, E.T. Peterson (OSC 56784); Washington, Okanogan National Forest, Pasayten Wilderness, under Picea engelmannii, Pseudotsuga menziesii, Abies lasiocarpa, Pinus contorta, 16 Sept. 1999, R. Davis (OSC 108856).
Notes — Otidea leporina is probably the most common Otidea species in boreal coniferous forests of Europe. In the Alps it often occurs together with Cudonia circinans, an association not seen in Fennoscandia. It is characterised by ear-shaped, brown apothecia, together with relatively broad spores that are almost unique within Otidea. Otidea brunneoparva shares similar spores, but differs from O. leporina in the darker brown apothecia and strongly notched paraphyses. Other species of Otidea that macroscopically resemble O. leporina are distinguished by different spore size and shape.
Our morphological and molecular study of the holotype of O. myosotis shows it is a synonym of O. leporina (Hansen & Olariaga 2015). The original description of O. myosotis (Harmaja 1976) pointed out the apothecial shape and colours, and paraphyses as diagnostic characters, all of which agree with our concept of O. leporina. Recently, Harmaja (2009a) stated that the excipular resinous exudates in O. leporina convert in MLZ into reddish particles and show no reaction in O. myosotis. In the material of O. leporina examined by us, the resinous exudates dissolve in part, can appear unchanged or can convert into reddish particles. Sometimes, small amber-drops have also been observed, though not as strikingly as in other species. Therefore, it seems that the reaction of the exudates in MLZ is variable within O. leporina, and cannot be used to separate O. myosotis from O. leporina. Otidea crassa is a synonym based primarily on the GenBank LSU sequence of the type collection (DQ443444).
Nomenclatural notes — When Batsch (1783) described Peziza leporina, he referred to Schaeffer’s plate (1763), being unaware or disregarding Schaeffer’s later description of ‘Elvela’ auricula Schaeff. (1774) based on the same plate. Since Fries (1822) sanctioned Batsch’s name, referring to H. auricula Schaeff. (as P. auricula Schaeff.) as a synonym, P. leporina has priority. While most authors have interpreted the plate by Schaeffer (1763, t. 156) as a species that belongs to Otidea (e.g. Fuckel 1870, Rehm 1883, Bresadola 1898, Seaver 1904), others have considered it to represent the monotypic Wynnella (Gonnermann & Rabenhorst 1869, Quélet 1886). Recently, Franchi et al. (1999) stated that the Schaeffer plate represents W. silvicola and as they consider Wynnella to be part of the genus Helvella, they made the combination Helvella leporina. Carbone & Van Vooren (2010) expressed doubts about how to interpret the protologue by Batsch (1783) and Schaeffer’s plate, and concluded the name is ambiguous and recommended it not be used. Instead they introduced the new name O. fuckelii for the Otidea species treated here. Based on our phylogenetic and morphological studies (see also Hansen & Olariaga 2015) this new name is, however, superfluous, since O. myosotis and O. crassa are shown to be synonyms of O. leporina.
The original Schaeffer plate shows several, more or less evenly coloured, light ochraceus brown apothecia, conforming to O. leporina, and not bi-coloured apothecia (dark reddish brown with a white base) as in W. silvicola. In our opinion, it leaves little doubt it shows a species of Otidea. To settle the use of the name O. leporina, and at the same time preserve the use of the well-established name, W. silvicola (Beck) Nannf. (Nannfeldt 1966), we propose a modern epitype for Peziza leporina Batsch: Fr. (Fig. 10a), which represents the Otidea species for which the name has most often been used. The epitype is from Sweden where Fries saw and studied living material, as indicated by the abbreviation ‘v. v.’ (vidi vivam, seen living).
8. Otidea pseudoleporina Olariaga & K. Hansen, sp. nov. — MycoBank MB808972; ITS barcode GenBank: KM010112; Fig. 5e, 11
Fig. 11.
Otidea pseudoleporina. a, b. Apothecia; c. apothecia†; d. spores in water†; e. paraphyses in water†; f. ectal excipulum in water† (a, d–f: rh101910, holotype; b, c: Moorefun 14). — Scale bars = 10 μm; † = dried material. — Photos: a. R. Helliwell; b. c. J. Moore.
Etymology. From ancient Greek ψɛνδo-, which means ‘false, fake’, referring to a close relationship with O. leporina.
Holotype. USA, Oregon, Douglas Co., E of Mill Creek, under Pseudotsuga menziesii, Abies concolor, Pinus lambertiana, 19 Oct. 2010, R. Helliwell, rh101910 (OSC).
Misapplied names
– Otidea cantharella var. minor sensu Kanouse, Mycologia 41: 667. 1949.
Apothecia gregarious, 10–30(–50) mm high, 8–22(–31) mm wide, initially narrowly to broadly ear-shaped, margin rounded, then expanding and sometimes becoming irregularly cup-shaped, split, stipitate or sessile. Hymenium ochre-orange (4A6, 5A7) to pinkish orange (5A6, 5A7), sometimes with pink spots or stains (6A4), when dried orange-ochre (5A5, 5B5) to reddish brown (6D7). Receptacle surface ochre-brown (5B5), hygrophanous, in drying yellowish ochre (4A4, 4A5), when dried yellowish ochre (5A5) to brownish ochre (5B5), furfuraceous to finely warty, sometimes wrinkled at the base. Warts conical to rounded, gregarious, concolorous, sometimes distinctly darker than the background, reddish brown. Stipe 4–11 × 3–5 mm. Basal tomentum and mycelium abundant, white to pale yellow (4A2) or ochre (5A2). Spores ellipsoid, sometimes slightly inequilateral, with two large guttules, sometimes with up to 4 smaller guttules, smooth, hyaline, (9.5–)10–12(–12.5) × 5.5–6.5 μm (Lm = 10.2–11.6 μm, Wm = 5.7–6.4 μm, Qm = 1.7–1.9; n = 6). Paraphyses curved to hooked, of the same width or slightly enlarged at apices, 2.5–4.5 μm wide, sometimes with 1–3 notches, apices seldom forked and rarely covered with a hyaline coating, when dried containing small, refractive, yellow granules. Asci 155–231 × 9–10 μm. Apothecial section 900–1200 μm thick. Subhymenium c. 80–100 μm thick, of dense textura intricata, visible as an orange-brown darker zone, of cylindrical to swollen cells. Medullary excipulum 500–750 μm thick, differentiated into two parts: a) textura angularis underneath the subhymenium, 150–200 μm thick, hyphae 8–18 μm broad; b) textura intricata, hyphae 5–10(–18) μm wide, sometimes slightly swollen, thin-walled to slightly thick-walled, very pale yellow, sometimes with yellow-brown resinous exudates at septa. Ectal excipulum of textura angularis, sometimes of a textura prismatica, 80–110(–150) μm thick, cells thin-walled to slightly thick-walled, pale yellow-brown, 13–37 × 8–27 μm. Surface with broadly conical warts, 77–115 μm high, formed by short, fasciculate, hyphoid hairs, of 2–3 subglobose to elongated cells, constricted at septa, 6–12 μm wide, sometimes with a gelatinous sheath. Resinous exudates abundant, yellow-brown, dissolving into amber drops in MLZ, partially to entirely dissolving into reddish grey heterogeneous drops in KOH. Basal mycelium of 3–5 μm wide, very pale yellow hyphae, unchanged in KOH, with very small, yellow resinous exudates, regularly arranged, spheroid, dissolving in MLZ, partially and more slowly dissolving in KOH.
Specimens examined. USA, California, Trinidad, under spruce, 30 Nov. 1956, A.H. Smith 56168 (UPS F-629690); Idaho, Idaho Co., Rickliff Creek Public Camp, on the ground in Thuja-Tsuga woods, 10 Oct. 1947, W.B. Cooke 21227 (UPS F-629388); Idaho, Papoose Creek, Seven Devils Mts, on the ground in Douglas fir association, 3 Sept. 1954, A.H. Smith & H.E. Bigelow, 47346 (UPS F-629331); Oregon, Bear Springs, Mt Hood National Forest, 18 Oct. 1947, A.H. Smith 27946 (UPS F-629430); Oregon, Benton Co., Corvallis, west side of NW Beechwood Place, scattered to clustered on rotting bark mulch and in thin grass under Pseudotsuga menziesii, 14 Nov. 2010, N.S. Weber, NSW 10202 (OSC 150347); ibid., 17 Nov. 2010, N.S. Weber, NSW 10200 (OSC 150345); ibid., 27 Nov. 2010, NSW 10201 (OSC 150346); Oregon, Douglas Co., E of Mill Creek, under Pseudotsuga menziesii, Abies concolor, Pinus lambertiana, 22 Oct. 2010, J. Moore, Moorefun 24 (OSC); Oregon, Douglas Co., Mill Creek, under conifers, 19 Oct. 2010, J. Moore, Moorefun 14 (S); Oregon, Douglas Co., Roseburg District Bureau of Land Management, under Pseudotsuga menziesii, Arbutus menziesii, Castanopsis chrysophylla, 11 May 1997, J. Klein (OSC 66261); Oregon, Douglas Co., south of Lemolo Lake, under conifers, 5 Nov. 2010, R. Heliwell, rh 179 (S); Oregon, Douglas Co., Thorn Unit I, under Pseudotsuga menziesii, Tsuga heterophylla, 21 Oct. 2010, C. Durbecq, Durbecq 16 (OSC); Oregon, Jackson Co., Medford Bureau of Land Management, Ashland Resource Area, Beaver Creek, under Pseudotsuga menziesii, Arbutus menziesii, Toxicodendron diversilobum, Berberis piperiana, 18 Dec. 2000, R. Brock (OSC 119311); Oregon, Jackson Co., Medford District Bureau of Land Management, Butte Falls Resource Area, under Pseudotsuga menziesii, Calocedrus decurrens, Pinus lambertiana, Pinus ponderosa, Quercus kellogii, Rhus diversiloba, Berberis piperiana, Fragaria vesca, Moehringia macrophylla, grasses, 8 Mar. 2000, M. Wineteer (OSC 72956); Oregon, Lane Co., Willamette National Forest, Blue River Ranger District, under Pseudotsuga menziesii, 18 Nov. 1999 (OSC 72296); Oregon, Marion Co., Breitenbuch Hot Springs Community, near Detroit Reservoir, in woods, 8 Nov. 1997, J.W. Spatafora (OSC 56809); Oregon, Mt Hood, among moss under conifers, 15 Oct. 1922, L.E. Wehmeyer (UPS F-629375); Oregon, Warm Springs R., Mt Hood National Forest, Skyline Trail, 29 Sept. 1947, A.H. Smith & W.B. Gruber, 27064 (UPS F-629689); Washington, Clallam Co., Olympic National Park, Whiskey Bend trailhead, 26 Nov. 1996, E.T. Peterson (OSC 56760); Washington, Fish Creek Region, Mt Rainier National Park, 25 Aug. 1948, E.G. Simmons 2067 (UPS F-629332); Washington, lower slopes of Rampart Ridge, W. of Longmire, Mt Rainier National Park, 4 Sept. 1948, E.G. Simmons 2172 (UPS F-629386); Washington, Park Creek, Mt Baker National Forest, 9 Sept. 1941, A.H. Smith 16755 (UPS F-629820); Washington, Pierce Co., Mt Rainier National Park, Lower Tahoma Creek, 29 Oct. 1996, E.T. Peterson (OSC 56749).
Notes — Otidea pseudoleporina is recognised by the broadly ear-shaped apothecia, ochre-orange to pinkish orange hymenium and small spores. Our multi-gene phylogenetic analyses (Hansen & Olariaga 2015) suggest O. pseudoleporina is the sister species of O. leporina. They share ear-shaped apothecia and resinous exudates on the outer excipulum that partly convert into heterogeneous reddish drops in KOH. Otidea leporina differs in the brown apothecia and larger, broadly ellipsoid spores. Otidea pseudoleporina resembles O. nannfeldtii and O. formicarum in the general apothecial shape and small spores. Otidea nannfeldtii is distinguished by most often lacking orange tones, having narrowly ear-shaped young apothecia, and ectal excipular resinous exudates turning reddish brown in KOH. Otidea formicarum is distinguished by having apothecia devoid of orange tones, and spores with a lower Qm (1.6–1.7) than O. pseudoleporina (1.7–1.9).
The material cited by Kanouse (1949) under O. cantharella var. minor most likely represents O. pseudoleporina. Otidea cantharella var. minor as described by Boudier (1909a) has a pale ochre or grey hymenium, citrine yellow outside, and veins at the apothecial base, and represents a different species (see under O. minor). Peterson (1998) treated under O. concinna material that we refer to O. pseudoleporina and stated that Harmaja (1974) used the name O. cantharella for the same species. Otidea concinna is a well-known species in Europe, clearly distinct from O. pseudoleporina (see O. concinna). As for the name O. cantharella, the protologue describes a fungus with the colour of Cantharellus cibarius (Fries 1822), and we typify it with material of the large-spored species sometimes called O. caligata (see O. cantharella). So far O. pseudoleporina is only known from Western North America.
Otidea tuomikoskii clade
Apothecia ear-shaped. Receptacle surface with warts often more than 100 μm high. Basal tomentum light ochre to orange ochre. Spores small, 10–11 μm long. Sections of apothecia turning yellow in KOH, especially the subhymenium and ectal excipulum. Associated with conifers.
Species — Otidea tuomikoskii.
9. Otidea tuomikoskii Harmaja, Karstenia 15: 30. 1976 — Fig. 12a
Fig. 12.
Otidea tuomikoskii*. a, b. Apothecia; c. spores; d. paraphyses; e. ectal excipulum; f. basal mycelium (a: JS.08.68; b–f: KH.11.77). — Scale bars = 10 μm; * = all fresh material. — Photos: a. J. Santos.
Holotype. FINLAND, Etelä-Häme, Lammi, Pappilankylä, Koiransuolenoja, in needles of Picea abies on an anthill, 9 Sept. 1972, R. Tuomikoski (H6002901) !
= Otidea papillata f. pallidefurfuracea Van Vooren & Hairaud, Bull. Mycol. Bot. Dauphiné-Savoie 188: 56. 2008.
Holotype. FRANCE, Jura, Les Rousses, tourbière près du lac des Rousses, au sol dans la litière d’aiguilles, sous épicéas (Picea abies), 19 Sept. 2007, N. Van Vooren, NV 2007.09.27 (PC). Isotype (S) !
Misapplied names
– Otidea papillata sensu Lundell, Nannfeldt & Holm, Fungi Exs. Suec. 66: 3282. 1985.
Apothecia gregarious to caespitose, 17–60 mm high, 7–30 mm wide, long and narrowly ear-shaped, split, stipitate or sessile. Hymenium pale whitish ochre (4A2, 4A3) to ochre yellow (4A4–4A6) rarely with pink stains, when dried light ochre yellow (4A4) to ochre (5B7, 5B8). Receptacle surface brownish ochre (4B7, 4C7) to yellow brown (5B6–5D6), hygrophanous, ochre yellow (4A5, 4A6, 4B6) in drying, when dried yellowish brown (5C8, 5D8), warty, rarely wrinkled at the base. Warts conical, gregarious, brown, distinctly darker than the background or rarely lighter. Stipe 3–6 × 2–3 mm. Smell faintly aromatic. Basal tomentum and mycelium abundant, light ochre (5A2) to orange-ochre (5A4). Spores ellipsoid, slightly inequilateral, with two large guttules, and sometimes with 1–4 smaller granules, smooth, hyaline, (9.5–)10–11(–12) × 5.5–6.5(–7) μm (Lm = 10.3–11.4 μm, Wm = (5–)5.5–6.5(–7.5) μm, Qm = 1.7–1.9; n = 15). Paraphyses curved to hooked, often broader at apices, 2.5–5(–6) μm wide, sometimes with up to two shallow notches or forked at apices, when fresh containing small to large, refractive, hyaline to pale yellow guttules; when dried pale yellow. Asci 113–199 × 9–11.5 μm. Apothecial section 600–700(–1200) μm thick, pale to bright yellow in KOH. Subhymenium c. 50–90 μm thick, of dense textura intricata, visible as a yellowish brown zone. Medullary excipulum of textura intricata, 300–500(–850) μm thick, hyphae 3–13 μm wide, thin-walled to slightly thick-walled, hyaline to very pale yellow, without resinous exudates at septa. Ectal excipulum of textura angularis, 80–120 μm, cells thin-walled, hyaline to light yellow, 13–40 × 8–25 μm. Surface with conical warts, 55–177 μm high, formed by fasciculate, short, hyphoid hairs, of 3–8 globose to elongated cells, constricted at septa, 7–15 μm wide, sometimes with a gelatinous sheath. Resinous exudates abundant, yellow-orange to yellowish brown, dissolving into amber drops in MLZ, unchanged in KOH. Basal mycelium of 3.5–6(–7.5) μm wide, often thick-walled, septate, hyaline to very pale yellow hyphae, unchanged in KOH, with regularly arranged, spheroid, yellow to orange resinous exudates, dissolving in MLZ, partially and more slowly in KOH.
Specimens examined. DENMARK, NW Jylland, Klim Bjerg, soil along forest road, S.A. Elborne & K. Hansen, 16 Sept. 1998, KH.98.92 (C-F-53155). – ESTONIA, Põlvamaa, ad terram in piceto humida, 11 Aug. 1960, A. Elango (UPS F-629392). – FINLAND, Perä-Pohjanmaa, Rovaniemi, Pisajärvi, old Picea forest, 2 Sept. 2011, T. Kekki, TK305 (TUR). – FRANCE, Charente-Maritime, île de Ré, près du camping ‘La Bonne Étoile’, under Pinus maritima and Quercus ilex, on leaf litter, 26 Nov. 2006, M. Hairaud, NV 2006.11.05 (dupl. S); Rhône, Les Halles, col de Croix-Régis, 25 Oct. 2006, J. Cavet, NV 2006.10.33 (dupl. S); Rhône, Saint-Nizier-d’Azergues, forêt de Pramenoux, sous Picea abies, 20 Sept. 2008, N. Van Vooren, NV 2008.09.08 (dupl. S). – GERMANY, Lower Saxony, Lüneburg, Boitze, Pinus and Abies, Oct. 2010, M. Vega private herb. s.n. (dupl. S-F256977). – NORWAY, Nord-Trøndelag, Snåsa, Bergsåsen Nature Reserve, under Picea and Pinus, 2 Sept. 2009, K. Hansen & I. Olariaga, KH.09.130 (S). – SPAIN, Navarre, Orokieta, Loiandi, Picea abies plantation, 17 Oct. 2008, J.M. Lekuona (ARAN-Fungi A5041195). – SWEDEN, Lappland, 3 miles NW Vuollerim, Bombmurkleskogen, along the Stora Luleälven, herb-rich Picea forest, 19 Aug. 2000, M. Karström, MK200065 (S); Lappland, Norrbotten, Messaure, Kaltisbäcken Nature Reserve, herb rich Picea forest, 3 Sept. 2011, M. Karström, KH.11.60 (S); Medelpad, Södra Sillre, Hussborg, on wood, 22 Aug. 1998, K. Olofsson (S-F256896); Närke, Hidinge, Lekhyttan, Katte Majaskogen, coniferous forest on lime rich soil, B. Wasstorp, 13 Sept. 2008, JS.08.77 (S); Närke, Snavlunda, Ö Snavlunda Nature Reserve, under Picea in a mixed forest, 12 Sept. 2008, L.G. Hellsten & A. Stridvall, JS.08.68 (S); Närke, Vintrosa, Kanterboda skans Nature Reserve, on soil under conifers, 10 Sept. 2008, A.B. Nilsson, JS.08.60 (S); Skåne, Loshult, Lilla Loshult, Picea forest with a few broadleaf trees (Betula, Quercus), 5 Sept. 1998, S.-Å. Hanson, SÅH 105768 (C); Södermanland, Nacka, Kvarnhagen by Söderbysjön, on soil in shadow, moist area, under Picea, 26 Sept. 2008, J. Santos, JS.08.100 (S); Uppland, Björklinge, Drälinge, amongst needles and mosses under pine in coniferous woods, 10 Sept. 1936, H.G. Bruun & H. Smith (S-F92983, Fungi Exs. Suec. 3282); Uppland, Trehörningsskogen Nature Reserve, under Picea on rich ground, on needle litter and decayed wood, 1 Sept. 2011, M. Prieto & I. Olariaga, KH.11.77 (S). – USA, California, Del Norte Co., Earl Lake State Park, access by Sand Hill Road, 15 Dec. 1997, M. Madsen & R. Davis (OSC 56826); California, Humboldt Co., Trinidad, Nov. 1931, H.E. Parks 3749 (UPS F-629376); Oregon, Benton Co., Corvallis, McDonald-Dunn Research Forest, under conifers, 23 Oct. 1996, E.T. Peterson (OSC 56761); Oregon, Benton Co., Corvallis, west side of NW Beechwood Place, scattered clusters of apothecia on duff and adjacent to rotting wood under Pseudotsuga menziesii, 19 Nov. 1997, N.S. Weber, NSW 8553 (OSC 150344); Oregon, Douglas Co., Bureau of Land Management, Roseburg District, Swiftwater Resource Area, under Tsuga heterophylla, Pseudotsuga menziesii, Polystichum munitum, Berberis nervosa and Holodiscus tricolor, 8 Nov. 2000, R. Furriel (OSC 105550); Oregon, Marion Co., Salem District Bureau of Land Management, Cascades Resource Area, under Pseudotsuga menziesii, Tsuga heterophylla, Gautheria shallon, Polystichum munitum, Berberis nervosa, Oxalis oregana, Acer circinatum, Alnus rubra and Rhododendron macrophyllum, 12 Nov. 1997, K. Dougan (OSC 66350); Washington, Bremerton, 26 Oct. 1942, J.B. Flett (UPS F-629383); Washington, Eatonville, 18 Oct. 1954, A.H. Smith 49143 (UPS F-629385); Washington, Lower Nisqually R., Mt Rainier National Park, 2 Sept. 1948, A.H. Smith 30888 (UPS F-629384).
Notes — Otidea tuomikoskii is characterised by the narrowly ear-shaped apothecia, with high warts on the outside, small spores, and the excipulum almost always turning yellow in KOH, together with the ochre to orange-ochre basal tomentum in dried specimens. Otidea nannfeldtii is probably the species that resembles O. tuomikoskii most, but O. nannfeldtii has lower warts, lacks orange tones in the basal tomentum, has paraphyses only rarely with slightly swollen areas, and resinous exudates on the outer excipulum that turn reddish in KOH. Otidea papillata shares with O. tuomikoskii conspicuous dark warts on the outside of the apothecia (see under O. papillata).
The yellow KOH reaction of the excipulum, especially strong in the subhymenium and ectal excipulum, has been observed to be constant, although weak in some collections. The KOH reaction is stronger in recent collections, and can also be macroscopically observed in fresh apothecia. The holotype of O. tuomikoskii is from an anthill (Harmaja 1976), but O. tuomikoskii most often produces apothecia among needle litter or even on very decayed wood, in coniferous forests. It is widespread in Europe, where it occurs in coniferous plantations, and in Western North America (Peterson 1998), and has been found in Asia (Cao et al. 1990).
Otidea cantharella clade
Apothecia ear-shaped, or cup-shaped and entire, usually clearly stipitate. Spores exceeding 20 μm, biguttulate and with several additional small guttules (except in O. brunneoparva). Paraphyses often strongly notched. Associated with Picea.
Species — Otidea brunneoparva, O. cantharella, O. propinquata.
10. Otidea brunneoparva K. Hansen, M. Carbone, Olariaga & Van Vooren, sp. nov. — MycoBank MB537590; ITS barcode GenBank: KM010026; Fig. 13, 14
Fig. 13.
Otidea brunneoparva apothecia. a. KH.08.107, holotype; b. JS.08.66; c. TUR-A 198579; d. TUR-A 198580. — Photos: b. J. Santos; c, d. M. Carbone.
Fig. 14.
Otidea brunneoparva (KH.08.107, holotype). a. Spores in water†; b. paraphyses*; c. medullary and ectal excipulum in water†; d. wart from the ectal excipulum in water†; e. ectal excipulum in Melzer’s reagent†; f. basal mycelium in water†. — Scale bars = 10 μm; * = fresh material; † = dried material.
Etymology. Harmaja (2009a) used the epithet brunneoparva to provisionally name this species. The name is validated here and refers to the small size and brown colour of the apothecia.
Holotype. SWEDEN, Närke, Knista, Lekhyttan, Kungshall, calcareous oldgrowth forest, in thick litter layer, with Picea and Pinus, 12 Sept. 2008, K. Hansen, KH.08.107 (S). Isotype (C).
Apothecia gregarious, 12–35 mm high, 7–25 mm wide, initially ear-shaped, apex subacute, broadly ear-shaped in the end, seldom almost cup-shaped, split, stipitate. Hymenium dark brown (6F3–6F7), sometimes olivaceous brown (5D7, 5E7, 5E8) or reddish brown (7F3–7F5), sometimes paler in the margin, purplish ochre (5B3, 5B4, 6D7), when dried olivaceous brown (5F4, 5F5, 5F7, 5E7) or dark brown (6E6, 7F7, 7F8). Receptacle surface concolorous or slightly lighter, dark brown (5F6, 6F7, 7F8), slightly hygrophanous, in drying golden brown (5D6, 5D7), when dried dark brown (6E7, 7E7) to cinnamon brown (5D7, 6D7, 6E7), furfuraceous, sometimes finely warty at the base, often longitudinally wrinkled at the base, sometimes almost reaching the margin. Warts hemispherical, gregarious, concolorous, sometimes darker or paler brown than the background. Stipe 4–7 × 2–3 mm. Basal tomentum and mycelium white to very pale brown, when dried ochre brown. Spores ellipsoid to broadly ellipsoid, sometimes very slightly inequilateral, with two large guttules, seldom with 1–2 additional smaller granules, smooth, hyaline, (11–)11.5–14(–15) × 6.5–8.5 μm (Lm?=?11.7?13.8 ?m, W = 11.7–13.8 μm, Wm = 7.1–8.3 μm, Qm = 1.6–1.7; n = 6). Paraphyses hooked, often inrolled, of the same width or slightly enlarged at the apices to 3–5.5 μm wide, often with clear notches or forked at apices, when fresh containing refractive, pale yellowish brown guttules, restricted to the uppermost part of the paraphyses; when dried pale yellow. Asci 131–195 × 8–10 μm. Apothecial section 700–800 μm thick. Subhymenium c. 100–120 μm thick, visible as a darker brown zone, cells cylindrical to swollen, densely arranged, with scattered brown resinous exudates at septa. Medullary excipulum of loosely woven textura intricata, 300–500 μm thick, hyphae cylindrical to slightly swollen, thick-walled, 5–11 μm wide, hyaline to very pale brown, sometimes with brown resinous exudates at septa. Ectal excipulum of textura angularis, 80–100 μm, cells thick-walled, yellowish brown, 20–47 × 12–25 μm. Surface with broadly conical warts, 25–65 μm high, composed of fasciculate, short hyphoid hairs. Non-warted parts with scattered hyphoid hairs, of 2–3 subglobose to elongated cells, 7.5–10.5 μm wide, slightly constricted at septa, sometimes with a thin gelatinous sheath. Resinous exudates abundant, yellowish to reddish brown, dissolving into amber drops in MLZ. Basal mycelium of 3–4.5 μm wide, very pale brown hyphae, unchanged in KOH, smooth or with very small, resinous exudates, dissolving in MLZ.
Specimens examined. FINLAND, Etelä-Häme, Hämeen, Lammi, Evo, Kotinen virgin forest, mesic forest of the Myrtillus type, in needle litter of Pinus sylvestris and Picea abies in basal part of an active anthill, 8 Sept. 1978, H. Harmaja (S-F249386); Kainuu, Paltamo, Saukkovaara, under Picea abies in moist spring-fed site, nearly in water, 24 Aug. 2011, M. Lahti (TUR-A 198582); Koillismaa, Kuusamo, Oulanka National Park, first part of the Kiutaköngäs trail, on rich soil among Picea and Betula leaves, 25 Aug. 2008, M. Carbone (TUR-A 198581); ibid., 16 Aug. 2009 (S-F257086, dupl. TUR-A 198579); ibid., 14 Aug. 2010 (TUR-A 198580). – SWEDEN, Jämtland, Östersund, Ändsjön Nature Reserve, on an abandoned anthill in rich Picea forest, 26 Aug. 2009, K. Hansen & I. Olariaga, KH.09.82 (S); Närke, Hidinge, Lekhyttan, Katte Majaskogen, coniferous lime-rich forest, in litter, 13 Sept. 2008, B. Wasstorp, JS.08.73 (S); Närke, Knista, Lekhyttan, Kungshall, calcareous oldgrowth forest, in thick litter layer, with Picea and Pinus, 12 Sept. 2008, J. Santos, JS.08.66 (S); ibid., JS.08.69 (S).
Notes — Otidea brunneoparva is morphologically and genetically a clearly distinct species. It is macroscopically characterised by stipitate, broadly ear-shaped apothecia with dark brown colours and sometimes olivaceous shades. Microscopically, the spore shape and size are diagnostic (Fig. 14a), although with wide variation. The strongly inrolled and notched apices of the paraphyses are very characteristic (Fig. 14b) and otherwise only found in a few species like O. propinquata or O. daliensis. The thick-walled, yellowish brown, angular cells forming the outer excipulum are diagnostic too (Fig. 14c, d); such thick-walled cells in the outer excipulum are only otherwise found in O. propinquata. The six ITS sequences of O. brunneoparva, from five different localities in Finland and Sweden, are identical or with 3–8 bp differences. The ITS nucleotide diversity within O. brunneoparva, as sampled here, is 0.74 % per site. Phylogenetic analyses of four gene-regions (Hansen & Olariaga 2015) show O. brunneoparva forms a distinct monophyletic group with O. propinquata and O. cantharella. These three species are nevertheless, easily distinguished both macro- and microscopically.
The distinctive spores of O. brunneoparva are only otherwise found in O. leporina within Otidea. Otidea brunneoparva is clearly distinguished from O. leporina by the darker coloured apothecia and notched paraphyses. Otidea bufonia, O. mirabilis and O. smithii share dark brown colours with O. brunneoparva, but clearly differ in apothecial shape and stature (O. brunneoparva being more delicate), narrower spores (Lm = 6.3–7.3 μm, Qm = 1.9–2.5) of different shape (fusoid in O. bufonia and O. mirabilis) and the resinous exudates on the ectal excipulum not dissolving into amber drops. Otidea fusconigra was published as a provisional name too. It also resembles O. brunneoparva in the dark brown apothecia, and spore size and shape (Jamoni 2004). Nevertheless, the paler hymenium colour (‘grey caffelatte’), the paraphyses without notches and the habitat among alpine dwarf Salix, suggests O. fusconigra is a different species. ITS and LSU sequences of O. fusconigra (collection GMFN 2293), obtained by us, confirms O. fusconigra is not conspecific, but a sister taxon to O. smithii.
Following Cao et al. (1990), O. brunneoparva keys out as O. olivacea J.Z. Cao & L. Fan. The cup-shaped, dark brown apothecia, with olivaceous tinge, suggest these may be closely related. However, the spores of O. olivacea were described as considerably longer (14–17 × 8–8.5 μm), almost non-overlapping with those of O. brunneoparva. Unfortunately, we were not able to get the type specimen of O. olivacea on loan for study. It should be noted that O. olivacea is a later homonym of O. olivacea Bucholtz. Therefore Harmaja (2009b) published the new name O. olivaceobrunnea for the illegitimate O. olivacea.
Otidea pusilla Rahm might be conspecific with O. brunneoparva, but the name is not validly published since no type was indicated (Art. 40.1 ICN) and more than one gathering was cited (Art. 40.2 ICN) (McNeill et al. 2012), ‘collected over three weeks in the same site’. The description of O. pusilla agrees with O. brunneoparva in the dark brown, cup-shaped apothecia and the relatively broad spores. The spore size given in the protologue was ‘15/6–9 μ’ (Rahm 1958), which we find difficult to interpret due to the unusually broad width range. Unfortunately, no material could be traced in ZH (pers. comm. R. Berndt).
A collection named by Harmaja as O. brunneoparva (H6017193) was found to be morphologically identical to our material. Also ITS and LSU sequences confirm that Harmaja′s and our material are conspecific. Therefore the name O. brunneoparva is here adopted and validated as a new species.
Otidea brunneoparva appears to be rather widespread in the Scandinavian Picea forests. While at least two of our collections, as indicated by Harmaja (2009a) grew on anthills, the rest were found in places with abundant Picea litter, often with presence of Betula. Two localities, including the type locality, were calcareous suggesting O. brunneoparva is calciphilous.
11. Otidea cantharella (Fr.) Quél., Enchir. Fung.: 275. 1886 — Fig. 15
Fig. 15.
Otidea cantharella*. a, b. Apothecia; c. spores; d. paraphyses; e. medullary excipulum showing resinous exudates at septa; f. ectal excipulum (a: KH.09.125, neotype; b: KH.11.69; c, d: KH.10.152; e: KH.09.144; f: KH.09.155). — Scale bars = 10 μm; * = all fresh material.
Basionym. Peziza cantharella Fr., Syst. Mycol. 2: 48. 1822 : Fr., loc. cit.
≡ Flavoscypha cantharella (Fr.) Harmaja, Karstenia 14: 107. 1974.
Neotype designated here: SWEDEN, Jämtland, Östersund, Ändsjön Nature Reserve, in rich Picea forest, with Hepatica nobilis and Oxalis acetosella, 31 Aug. 2009, K. Hansen & I. Olariaga, KH.09.125 (S); MycoBank MBT178085.
= Peziza caligata Nyl., Ex Not. Sällsk. Fauna Fl. Fenn. Förh. 10: 8. 1868 ‘1869’.
≡ Otidea caligata (Nyl.) Sacc., Syll. Fung. 8: 95. 1889.
≡ Acetabula caligata (Nyl.) Boud., Hist. Classific. Discomyc. Europe: 41. 1907.
Holotype. FINLAND, Uusimaa, Helsinki, 1850, W. Nylander (H009215) !
Misapplied names
– Otidea abietina sensu Breitenbach & Kränzlin, Fung. Switzerland 1: 82. 1984.
– Peziza propinquata sensu Nannfeldt, Ann. Bot. Fenn. 3: 313. 1966.
Apothecia gregarious or caespitose, 23–68 mm high, 13–43 mm wide, initially broadly ear-shaped, in the end broadly ear-shaped to almost cup-shaped, split, stipitate. Hymenium initially light brown (5B5, 5B6, 5C6), then ochraceous yellow (4A5, 4A6, 5A5), ochre-orange (4A7, 5B7), sometimes with pinkish areas or red dots (6B8), when wounded pinkish and margin brownish red (6B8), when dried orange brown (6D7, 6D8) to reddish brown (6C6, 6D6). Receptacle surface concolorous, ochre brown (4B7, 5C7, 5B6), slightly hygrophanous, in drying yellowish ochre (4A6, 4A7), sometimes with pale brown stains, when dried orange brown (6D7, 6D8), furfuraceous to finely warty, sometimes wrinkled at the base. Warts hemispherical, gregarious, concolorous, sometimes darker than the background, brown. Stipe 4–25 × 3–9 mm. Basal tomentum and mycelium abundant, white to very pale brown (5A3), very pale brown when dried. Spores ellipsoid and often narrowing toward poles, sometimes very slightly inequilateral, with two large and several smaller guttules, smooth, hyaline, (17–)18–21 × (9–)10–11.5(–12) μm (Lm = 17.7–20 μm, Wm = 10.4–11.4 μm, Qm = 1.7–1.8; n = 13). Paraphyses hooked, of the same width or slightly enlarged at apices, 2.5–3.5(–5.5) μm wide, without or with a low notch, seldom forked at apices, when fresh containing small, refractive, pale yellow guttules; when dried small, refractive granules. Asci 191–217 × 11–12 μm. Apothecial section 900–1200 μm thick. Subhymenium c. 70–100 μm thick, visible as a darker yellow zone, of cylindrical to swollen cells, densely arranged. Medullary excipulum of textura intricata, (500–)700–900 μm thick, hyphae cylindrical to slightly swollen, thick-walled, 3–12 μm wide, very pale yellow, sometimes with yellow-brown resinous exudates at septa. Ectal excipulum of textura angularis 90–120 μm, cells thin-walled, pale yellow, 23–55 × 12–25(–30) μm. Surface with conical to broadly conical warts, 55–80 μm high, formed by fasciculate, parallel, short hyphoid hairs, of 3–4 ovoid cells, constricted at septa, 5–8 μm wide. Non-warted parts with single hyphoid hairs, of 2–4 subglobose to elongated cells, slightly constricted at septa, 7–10 μm wide, sometimes with a gelatinous sheath. Resinous exudates abundant, yellow to yellow-brown, dissolving into amber drops in MLZ. Basal mycelium of 3.5–4.5(–6) μm wide, hyaline to very pale yellowish brown hyphae, unchanged in KOH, smooth or with very small, regularly arranged, spheroid, resinous exudates, dissolving in MLZ and partially in KOH.
Specimens examined. FINLAND, Etelä-Häme, Hattula, Parola, Alppilankallio, Vaccinium myrtillus-type forest, under Picea abies, 25 Sept. 1967, P. Uotila 618 (H); Etelä-Häme, Janakkala, Tervakoski, 6 Sept. 1970, P. Uotila 6195 (H); Etelä-Häme, Mustiala, 5 Sept. 1895, J. Lindroth (H6010923); ibid., in pineto, 24 Aug. 1866, P.A. Karsten (H6010922); Etelä-Häme, Mustiala, Tammela, 2 Sept. 1882, P.A. Karsten (H6010829); Perä-Pohjanmaa, Rovaniemi, Kalkkinulkki, near old limestone quarry, under Picea, 23 Aug. 2011, T. Kekki, TK211 (TUR); Perä-Pohjanmaa, Rovaniemi, Ounasvaara, Picea forest, 29 Aug. 2011, T. Kekki, TK279 (TUR); Perä-Pohjanmaa, Rovaniemi, Välijoki, calciferous Picea forest, 25 Aug. 2011, T. Kekki, TK236 (TUR); Perä-Pohjanmaa, Tervola, Peura, old calciferous Picea forest, 5 Sept. 2011, T. Kekki, TK177 (TUR); Perä-Pohjanmaa, Ylitornio, Kuusikkorommas, calciferous Picea and Pinus forest, 2 Sept. 2011, T. Kekki, TK301 (TUR); Varsinais-Suomi, Vihti, Nummela, Metsäkulma, on soil, 16 Sept. 1979, H. Kotiranta (H6010925). – FRANCE, Isère, Villard-de-Lans, Bois Barbu, under Picea abies, 20 Sept. 2008, J. Cavet, NV 2008.09.16 (dupl. S). – HUNGARY, Hohe Tatra bei Unter-Schmecks, Oct. 1884, Linhart (UPS F-629314, Rehm Ascomyceten 251b, as O. leporina f. minor). – ITALY, Trentino-Alto Adige, Cavelonte, in silvis coniferis, semper socia Cudoniae confusae, Aug. 1898, G. Bresadola (UPS F-629361). – NORWAY, Nord-Trøndelag, Steinkjer, Noem, under Picea, 2 Sept. 2009, K. Hansen & I. Olariaga, KH.09.129 (S); Nord-Trøndelag, Steinkjer, Strattåsen, in rich Picea forest, 5 Sept. 2009, K. Hansen & I. Olariaga, KH.09.144 (S). – SWEDEN, Blekinge, Rödeby, Spjutsbygd, c. 2 km NW from the train station, on needle litter under a Picea in coniferous forest, 10 Sept. 1946, S. Lundell & S. Wikland (UPS F-146484); Gästrikland, close to Bergby, mossy Picea forest on acidic ground, 31 Aug. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.152 (S); Hälsingland, Kårböle, St Olofs, 26 Aug. 2001, H.-G. Toresson s.n. (S); Hälsingland, south to crossing between roads E45 and 310, under Picea abies, among leaf litter, 21 Aug. 2011, J.C. Zamora & I. Olariaga, KH.11.109 (S); Härjedalen, Linsell, Djursvallen, montane coniferous forest with birch, on very rotten log, 13 Aug. 2000, B. Gahne (UPS F-125589); Härjedalen, Torkilstöten, under Picea abies among leaf litter, on acidic ground, 20 Aug. 2011, J.C. Zamora & I. Olariaga, KH.11.106 (S); Jämtland, Hammarstrand, Picea mossy forest, 26 Aug. 2009, H. Lindström, KH.09.83 (S); ibid., KH.09.84 (S); Jämtland, SW of Mörsil, Sandtjärndalen Nature Reserve, under Picea abies on rich ground, 7 Sept. 2009, K. Hansen & I. Olariaga, KH.09.155 (S); Jämtland, Sällsjö surroundings, in young stand of Picea abies, on rich ground with Betula and Salix, 29 Aug. 2009, K. Hansen & I. Olariaga, KH.09.104 (S); Jämtland, Östersund, Andersön Nature Reserve, Picea forest on rich ground, 28 Aug. 2009, K. Hansen & I. Olariaga, KH.09.95 (S); ibid., under Picea and Pinus, KH.09.96 (S); ibid., KH.09.101 (S); Jämtland, Östersund, Fillstabäcken Nature Reserve, on old anthill under Picea, K. Hansen & I. Olariaga, 30 Aug. 2009, KH.09.111 (S); ibid., KH.09.117 (S); ibid., K. Hansen & X.H. Wang, 5 Sept. 2012, KH.12.99 (S); Jämtland, Östersund, Ändsjön Nature Reserve, in rich Picea forest, with Hepatica nobilis and Oxalis acetosella, 26 Aug. 2009, K. Hansen & I. Olariaga, KH.09.78 (S); ibid., KH.09.80 (S); ibid., KH.09.98 (S); Jämtland, Åre, Kall, along Stor-Grundsviken, Kallsjön, on old anthill in lime rich Picea forest, 15 Aug. 2008, J. Santos & K. Hansen, JS.08.18 (S); Lappland, Jokkmokk, SE of Vuollerim, Andersviksravinerna (Natura 2000 area), mixed forest, 31 Aug. 2011, A. Stridvall, KH.11.111 (S); ibid., KH.11.112 (S); Lappland, Jokkmokk, by Kassavare mountain, Köpenhamn, under Picea abies, 1 Sept. 2011, K. Hansen & I. Olariaga, KH.11.110 (S); Lappland, Jokkmokk, ‘Nornaskogen’ by Ållojaur, mossy Picea forest on rich ground, 29 Aug. 2011, K. Hansen & I. Olariaga, KH.11.16 (S); Lappland, Kuouka, 15 km SE Messaure, herb-rich Picea forest on rich ground, among mosses, 3 Sept. 2011, K. Hansen & I. Olariaga, KH.11.68 (S); ibid., KH.11.69 (S); Lappland, 5 km SE Vuollerim, part of Andersviksravinerna, Rävabacken Nature Reserve, herb-rich Picea forest, 21 Aug. 2000, M. Karström, MK200061 (S); ibid., under Picea on mossy ground, together with Cudonia confusa, 2 Sept. 2011, K. Wiking, KH.11.59 (S); Småland, Ryssby, Gärdsholmen, Björnö, coniferous forest, 6 Sept. 1930, H.G. Bruun (UPS F-146485); ibid., on deep moss in coniferous forest (UPS F-146486); Uppland, Uppsala, Sävja, Norra Lunsen Nature Reserve, Picea forest, on soil, thick layer of litter and mosses, 28 Aug. 2008, J. Santos, JS.08.47 (S). – SWITZERLAND, Wallis Canton, Liddes, Palazuit, in side of brook under Picea abies, 17 Aug. 2008, M. Carbone (MCVE 24217).
Notes — Otidea cantharella can be recognised by broadly ear-shaped apothecia, with yellow to orange tones, often with a well-developed stipe, and large spores. No other species of Otidea have the spore size of O. cantharella, in combination with those macroscopic characters.
Otidea cantharella is associated with Picea abies, and often produces apothecia on anthills or thick needle layers. Furthermore, we have often found apothecia of O. cantharella and Cudonia confusa together (Fig. 15a), and once with Spathularia rufa, both species belonging to the Rhytismatales. An apparent closer association in the same fairy ring has also been observed once. Interestingly, Bresadola noted the association with C. confusa in one of his collections (UPS F-629361) studied by us. The presence of C. confusa in the same collecting spot may give a first plausible field identification of O. cantharella. Moreover, it suggests a possible biotrophic association between O. cantharella and C. confusa.
Nomenclatural notes — This species has been referred to as O. caligata (Nyl.) Sacc. (Nannfeldt 1966, Dissing 2000). Nevertheless, Harmaja (2009a) came to the conclusion that the name O. cantharella must refer to the large-spored species treated here, with which some authors have later disagreed (Carbone 2010b, Van Vooren 2011b). Harmaja stressed the following characters from the protologue supporting the usage of the name as presented here: ear-shaped, stipitate apothecia, the yellow colour of Cantharellus cibarius and the occurrence in Picea forests in southern Sweden. We have studied material from Småland (UPS F-146485, UPS F-146486), from where Fries described O. cantharella. It is therefore very likely that Fries had in mind the species described here when he coined the name O. cantharella, although he had only seen dried material, as indicated by the abbreviation ‘v. s.’ (vidi siccam, seen dried). Since no original material is known to exist, we propose a neotype that will attach the name O. cantharella to the Otidea species described here. This stabilises the interpretation proposed by Harmaja (2009a), also used by Mornand & Courtecuisse (2005), and long before adopted by Bresadola (1900: 102), and should serve to settle the interpretation of O. cantharella. Our ITS-LSU sequences of four O. cantharella collections from Sweden and France are identical.
12. Otidea propinquata (P. Karst.) Harmaja, Karstenia 15: 32.
1976 — Fig. 16
Fig. 16.
Otidea propinquata. a. Apothecia; b. spores in water†; c. paraphyses in KOH†; d. wart of the ectal excipulum in water†; e. ectal excipulum in KOH†; f. basal mycelium in water† (a: KH.11.21; b–f: KH.09.99). — Scale bars = 10 μm; † = dried material.
Basionym. Peziza propinquata P. Karst., Not. Sallsk. Fauna Fl. Fenn. Forh. 10: 110. 1869.
Lectotype. FINLAND, Tavastland, Messuby, 7 Oct. 1860, P.A. Karsten (H6010807) !, selected by Nannfeldt (1966).
= Otidea abietina f. nigra Rick, Oesterr. Bot. Z. 48: 62. 1898.
≡ Otidea abietina var. nigra (Rick) Sacc., Syll. Fung. 14: 746. 1899.
Lectotype. AUSTRIA, Vorarlberg, an der Gamp, im Nadelwald, 1700 m, Sept. 1897, Rick (S-F9962) !, indicated by Nannfeldt (1966).
= Otidea indivisa Velen., Monograph. Discom. Bohemiae 1: 355. 1934.
Lectotype. CZECH REPUBLIC, Karlštejn N, Oct. 1922, J. Fechtner (PRM 149147) !, selected by Nannfeldt (1966).
Misapplied names
–Pseudotis abietina sensu Boudier, Icon. Mycol. livr. 7: nº. 131, pl. 333. 1906 (preliminary text with ‘circulaires’).
–Otidea cochleata sensu Breitenbach & Kränzlin, Fung. Switzerland 1: 84. 1984.
Apothecia gregarious or caespitose, 7–20 mm high, 15–35 mm wide, obconical to broadly cup-shaped, entire, very rarely split, regular or sometimes undulate in the margin, stipitate. Hymenium ochre brown (5C7, 6D5, 6D6) to dark reddish brown (6F6, 6F7, 7F7, 7F8), when dried ochre brown (6B7, 6C7). Receptacle surface concolorous, orange brown (6F6, 6F7), slightly hygrophanous, in drying yellowish brown (5C6, 5C7), when dried orange brown (6D6, 6D7, 6E7), furfuraceous to warty, sometimes wrinkled at the base. Warts hemispherical, gregarious, concolorous, sometimes darker than the background, brown. Stipe 7–16 × 2–7 mm. Basal tomentum and mycelium abundant, white to very pale brown (5A3), very pale brown when dried. Spores ellipsoid and often narrowing toward the poles, sometimes very slightly inequilateral, with two large and several smaller guttules, smooth, hyaline, (18–)19–21 × 10–12.5 μm (Lm = 19.3–20 μm, Wm = 10.9–11.6 μm, Qm = 1.6–1.7; n = 4). Paraphyses hooked, of the same width or often enlarged at apices, 3–5 μm wide, often with 1–3 notches or forked at apices, when fresh containing small, refractive, light yellow guttules; when dried hyaline. Asci 231–275 × 12–16 μm. Apothecial section 1100–1500 μm thick. Subhymenium c. 100–120 μm thick, of dense textura intricata, visible as a darker brown zone. Medullary excipulum of textura intricata, 600–800 μm thick, hyphae 4–9 μm wide, sometimes slightly swollen, thin- to thick-walled, very pale brown, sometimes with brown resinous exudates at septa. Ectal excipulum of textura angularis 100–130 μm, cells rather thick-walled, pale brown, 17–50 × 10–40 μm. Surface with conical to broadly conical warts, 50–75 μm high, formed by short, fasciculate hyphoid hairs, of 2–3 subglobose to elongated cells, constricted at septa or not, 8–11 μm wide, sometimes with a pale brown thick gelatinous sheath. Resinous exudates abundant, yellow-brown to brown, turning brownish red in KOH, dissolving in MLZ. Basal mycelium of 3–6 μm wide, hyaline to pale brown hyphae, unchanged in KOH, smooth or normally with regularly arranged, spheroid to rod-shaped, resinous exudates, dissolving in MLZ and partially in KOH.
Specimens examined. CZECH REPUBLIC, Praha, Karlštejn, 9 Oct. 1922, F. Fechtner (UPS F-629369, syntype of O. indivisa); Sept. 1924 (UPS F-629367, syntype of O. indivisa). – DENMARK, NE Sjælland, Asserbo Plantage, under Pinus and Picea among needles, 7 Oct. 1975, H. Knudsen (C-F-87203). – FRANCE, Isère, Lans-en-Vercors, on the ground, on Picea leaf litter, 17 Sept. 2008, J. Cavet, NV 2008.09.15 (dupl. S). – ITALY, Trentino-Alto Adige, Sopramonte, ad acus abiegnos in sylvis coniferis, Aug. 1898, G. Bresadola (UPS F-629357). – SWEDEN, Jämtland, Sällsjö surroundings, in young stand of Picea abies, on rich ground, 28 Aug. 2009, K. Hansen & I. Olariaga, KH.09.99 (S); Jämtland, Östersund, Andersön Nature Reserve, under Pinus and Picea, on rich ground, among mosses, 28 Aug. 2009, K. Hansen & I. Olariaga, KH.09.94 (S); ibid., KH.09.103 (S); Jämtland, Östersund, Ändsjön Nature Reserve, in rich Picea forest, 26 Aug. 2009, K. Hansen & I. Olariaga, KH.09.81 (S); ibid., 31 Aug. 2009, KH.09.123 (S); Lappland, Jokkmokk, ‘Nornaskogen’ by Ållojaur, mossy Picea forest on rich ground, 28 Aug. 2011, K. Hansen & I. Olariaga, KH.11.21 (S); Lappland, Jokkmokk, Ultevis Fjällurskog Nature Reserve, Sitoätno, Picea mossy forest on rich ground, among leaf litter, 31 Aug. 2011, K. Hansen & I. Olariaga, KH.11.30 (S); ibid., KH.11.35 (S); Lappland, S of Kvikkjobb-Kabla FUR Nature Reserve, by Kassavare mountain, Köpenhamn, young Picea stand by a road, 1 Sept. 2011, K. Hansen & I. Olariaga, KH.11.53 (S); Lappland, Messaure, herb-rich Picea forest, 29 Aug. 2008, M. Karström, MK0834 (S); Lappland, 17 km WSW of Vuollerim, Slubbojaureskogen, herb-rich Picea forest, 8 Aug. 2002, M. Karström, MK0222 (S); Närke, Knista, Lekhyttan, under Picea, in thick litter layer, in lime rich forest, 12 Sept. 2008, J. Santos, JS.08.67 (S); Uppland, Uppsala, Sätra Nature Reserve, on litter on lime rich soil, under Picea, 23 Sept. 2008, J. Santos & K. Hansen, JS.08.98 (S). – USA, Washington, Lake Crescent, under fir, 28 Oct. 1935, A.H. Smith (UPS F-629351).
Notes — Otidea propinquata is easily recognised by the stipitate, entire, brown apothecia, large spores, and notched or forked paraphyses. Otidea daliensis shares brown, entire apothecia with O. propinquata, but differs in having sessile apothecia, with purplish brown tones, and basal mycelium that lacks abundant resinous exudates.
There has been uncertainty surrounding the correct name of this taxon. A number of early authors used the epithet abietina to refer to O. propinquata (Boudier 1906, Bresadola 1933). In agreement with Harmaja (2009a) and Carbone (2010c), we consider Peziza abietina a nomen confusum. Nannfeldt (1966) referred to O. propinquata as O. indivisa and argued that the type of O. propinquata was conspecific with O. cantharella (as caligata). Harmaja (1976) examined the lectotype of O. propinquata and found it to be clearly distinct from O. cantharella and O. indivisa, a later synonym. This is confirmed by our study of the type of O. propinquata. Otidea propinquata occurs in Picea forests on calcareous ground. It is widespread in Northern Fennoscandia and present in central Europe.
Otidea formicarum clade
Apothecia ear- to cup-shaped, split, ochre to reddish brown. Spores small, 9.5–12 μm long. Basal mycelium with abundant yellow resinous exudates. Associated with conifers.
Species — Otidea formicarum, O. nannfeldtii, O. subformicarum, O. aff. subformicarum, Otidea sp. ‘b’.
13. Otidea formicarum Harmaja, Karstenia 15: 31. 1976 — Fig. 5f, 17
Fig. 17.
Otidea formicarum (KH.11.104)*. a. Apothecia; b. spores; c. paraphyses; d. ectal excipulum; e. resinous exudates of the ectal excipulum; f. basal mycelium. — Scale bars = 10 μm; * = all fresh material.
Holotype. FINLAND, Etelä-Karjala, Miehikkäla, Savanjärvi, on anthill in spruce forest, 26 Sept. 1970, L. Fagerström (H6003549) !
Apothecia gregarious to caespitose, 8–22 mm high, 5–20 mm wide, broadly ear-shaped, upper margin rounded, then expanding and sometimes in the end becoming cup-shaped and flattened, split, stipitate or sessile. Hymenium yellowish brown (5C7, 5C8) to reddish brown (6C7, 6C8, 6D8), when dried ochre (4A5, 4A6) to brownish ochre (5B7). Receptacle surface yellowish brown (5C6) to reddish brown (6C6), hygrophanous, in drying ochre (5B7), when dried ochre (4A5, 4A6), finely warty, smooth at the base. Warts broadly conical to rounded, gregarious, concolorous, sometimes slightly darker than the background, brown. Stipe 3–6 × 2–4 mm. Basal tomentum and mycelium abundant, light yellow-ochre (5A2). Spores ellipsoid, seldom very slightly inequilateral, with two large guttules, smooth, hyaline, 9.5–11(–11.5) × (5.5–)6–7 μm (Lm = 10–10.7 μm, Wm = 6–6.9 μm, Qm = 1.6–1.7; n = 7). Paraphyses curved to hooked, of the same width or slightly enlarged at apices, 3–4 μm wide, with up to two slightly swollen areas, occasionally notched, sometimes when fresh containing small, refractive, light yellow or green guttules; when dried small, refractive, hyaline to light yellow granules. Asci 160–232 × 10.5–13 μm. Apothecial section 700–800 μm thick. Subhymenium c. 80–100 μm thick, of dense textura intricata, visible as a yellowish brown zone, cells cylindrical to swollen, densely arranged. Medullary excipulum 400–500 μm thick, differentiated into two parts: a) textura angularis underneath the subhymenium, 120–200 μm thick, cells 6–15 μm broad; b) textura intricata, hyphae sometimes slightly swollen, thin-walled to thick-walled, 3–11 μm wide, hyaline to very pale yellow, sometimes with pale yellow resinous exudates at septa. Ectal excipulum of textura angularis, 70–120 μm, cells thin-walled, light yellow, 17–40 × 11–25 μm. Surface with broadly conical warts, 45–60 μm high, formed by short, fasciculate, hyphoid hairs, of 2–3 elongated cells, 7–12 μm wide, not or slightly constricted at septa, sometimes with a gelatinous sheath. Resinous exudates abundant, brownish yellow, dissolving into amber drops in MLZ. Basal mycelium of 3–5 μm wide, hyaline to very light yellow hyphae, unchanged in KOH, with regularly arranged, very small, spheroid resinous exudates, dissolving in MLZ, partially and more slowly in KOH.
Specimens examined. FINLAND, Etelä-Häme, Lammi, Evo, Vahtervehmas, Kotinen virgin forest, 6 Sept. 1988, H. Harmaja (H6003551); Etelä-Häme, Loppi, Topeno, Piimästennummi, on a huge anthill, under Picea, 16 Sept. 2011, S. Huhtinen 11/65 (TUR); Perä-Pohjanmaa, Rovaniemi, Välijoki, calciferous Picea forest, on anthill, 25 Aug. 2011, T. Kekki, TK223 (TUR); Uusimaa, Elimäki, Villikkala, Lääksynmäki, mesic heath spruce forest, on anthill, 22 Oct. 2005, U. Nummela-Salo & P. Salo (H6003550); Varsinais-Suomi, Koski Tl., Hongisto, on old anthill under Picea abies, with Betula sp., Salix caprea and Pinus sylvestris, 8 Aug. 1998, M.-L. & P. Heinonen (TUR 124728); Varsinais-Suomi, Lieto, Suopohja, SE of Päivärinne, along the road by the house Esko-Ukura, on old anthill under Picea abies, 2 Oct. 2009, K. Ruottinen (TUR-A 183242). – FRANCE, Haute Savoie, Thorens-Glières, plateau des Glières, under Picea, on the ground, 22 Sept. 2006, L. Francini, NV 2006.09.11 (S). – NORWAY, Nordland, Grane, Holmvassdalen Nature Reserve, on old anthill under Picea, 29 Oct. 2009, J. Lorås (S-F244372). – SWEDEN, Dalarna, Stora Tuna, between Falubäcken and Övre Morbygge fäbod, abundant in the lower part of an active anthill, 22 Sept. 1963, R. Morander (UPS F-146725); Närke, Askersund, Orkarebäckens Nature Reserve, calcareous forest, on an active anthill under Picea, 11 Sept. 2008, J. Santos, JS.08.63 (S); ibid., under Picea in litter, JS.08.62 (S); Uppland, Bondkyrka, Gottsundabergen, 1 Oct. 1927, J.A. Nannfeldt (UPS F-146706); Uppland, Bondkyrka, Nåsten, S of Läbyvad station, among coniferous needles, 16 Sept. 1932, J.A. Nannfeldt (UPS F-146709); Uppland, Älvkarleby, Billuddens Nature Reserve, under Pinus and Picea on sandy ground, at the base of a Pinus, 15 Sept. 2011, J.C. Zamora & I. Olariaga, KH.11.104 (S); Ångermanland, Ullanger, Håll, in the eastern slope of Mt Moberget, c. 0.5 km SW of p. 35,29, on the ground, 14 Sept. 1974, R. Moberg (UPS F-146735).
Notes — Otidea formicarum is characterised by relatively small, reddish brown, broadly ear-shaped apothecia, small spores, and by its habitat, often occurring on anthills. For a comparison with the sister species O. subformicarum see under that species below. Otidea nannfeldtii resembles O. formicarum, but differs in often having more narrowly ear-shaped and paler coloured apothecia, sometimes with pink tones in the hymenium. Dried apothecia of O. nannfeldtii have, on the contrary, darker colours than O. formicarum. Otidea pseudoleporina and O. formicarum share apothecial shape, but O. pseudoleporina can be distinguished by the ochre-orange colour, and comparatively narrower spores (Qm = 1.7–1.9).
The original description of O. formicarum was based on several collections made on anthills in Finland (Harmaja 1976), and more recent material has been cited from anthills (Harmaja 2009a, b), mainly associated with Picea. Most of the material examined by us was also from anthills, but at least one of our Swedish finds (KH.11.104) was from Pinus needle litter, which shows the habitat of O. formicarum partly overlaps with the habitat of O. subformicarum.
14. Otidea nannfeldtii Harmaja, Karstenia 15: 31. 1976 — Fig. 5a, d, 18
Fig. 18.
Otidea nannfeldtii. a. Apothecia; b. spores*; c. paraphyses*; d. ectal excipulum*; e. hyphoid hairs with gelatinous sheath*; f. amber drops on the outermost ectal excipulum cells in Melzer’s reagent† (a–e: KH.10.302; f: S-F249387). — Scale bars = 10 μm; * = fresh material; † = dried material.
Holotype. FINLAND, Ahvenanmaa, Lemland, Nåtö, spruce forest near Övergård, 17 Sept. 1972, C.-A. Haeggström (H6002902) !
= Otidea angusta Harmaja, Karstenia 48: 35. 2009.
Holotype. FINLAND, Varsinais-Suomi, Lohja, Jalassaari, Ahtiala, E of Heimo house, mixed somewhat moist rich woods with Picea, Betula, Corylus etc. on somewhat calcareous soils, 23 Aug. 1965, H. Harmaja (H6010804) !
Misapplied names
– Otidea papillata sensu Van Vooren et al., Bull. Mycol. Bot. Dauphiné-Savoie 188: 52. 2008.
Apothecia gregarious to caespitose, 8–35 mm high, 5–15 mm wide, initially long, narrowly ear-shaped, sometimes expanding and becoming broadly ear-shaped, split, stipitate or sessile. Hymenium ochre (4A5), orangish ochre (5B4, 5C4) or pale brown (6D6, 6D7), sometimes with pink tones or entirely pinkish (6A4), when dried orange-ochre (5A5) to reddish brown (6C6). Receptacle surface brown orange (5B4, 5C4) to pale brown (6D6–7), slightly hygrophanous, in drying ochre (4A5), when dried reddish brown (7D7, 7E7), finely warty, smooth at the base. Warts conical, gregarious, concolorous, sometimes distinctly darker than the background, brown. Stipe 4–8 × 2–3 mm. Basal tomentum and mycelium abundant, white to light yellow (5A2) or ochre (5A3). Spores ellipsoid, sometimes slightly inequilateral, with two large guttules, smooth, hyaline, (9–)9.5–10.5(–11.5) × 5.5–6.5(–7) μm (Lm = 9.8–10.9 μm, Wm = 5.5–6.6 μm, Qm = 1.6–1.9; n = 10). Paraphyses curved to hooked, of the same width or slightly broader at apices, 2.5–5 μm wide, without notches, rarely with up to two slightly swollen areas or forked at apices, when fresh containing small, refractive, light yellow guttules; when dried hyaline to light yellow. Asci 137–190 × 8–10 μm. Apothecial section 650–900 μm thick. Subhymenium c. 80–100 μm thick, visible as a yellowish brown zone, of cylindrical to swollen cells, densely arranged. Medullary excipulum of textura intricata, 400–600 μm thick, differentiated into two parts: a) textura angularis underneath the subhymenium, 100–150 μm thick, cells 13–32 × 12–21 μm; b) textura intricata, 400–450 μm thick, hyphae 4–9 μm wide, thin-walled to slightly thick-walled, hyaline to very pale yellow, sometimes with pale yellow resinous exudates at septa. Ectal excipulum of textura angularis, sometimes of a textura prismatica, 80–120 μm, cells thin-walled, hyaline to light brown, 16–40 × 11–22 μm. Outer part of conical to broadly conical warts, 45–85 μm high, formed by short, fasciculate, hyphoid hairs, of 2–3(–4) subglobose to elongated cells, constricted at septa, 5–9 μm wide, sometimes with a gelatinous sheath. Resinous exudates abundant, yellow brown, dissolving into amber drops in MLZ, reddish brown in KOH. Basal mycelium of 3–5.5 μm wide, septate, hyaline to very light yellow hyphae, unchanged in KOH, with very small, regularly arranged, spheroid, yellow resinous exudates, dissolving in MLZ, partially and more slowly in KOH.
Specimens examined. FINLAND, Varsinais-Suomi, Lohja, Jalassaari, Ahtiala, Alho, very close to the Ahtiala Nature Reserve, below a spruce tree in mixed forest on fairly calcareous soil, 29 Sept. 1978, H. Harmaja (S-F249387, ex H6017194 as O. lohjaënsis nom. prov.). – FRANCE, Hautes Alpes, Provence-Alpes-Côte-d’Azur, La Bâtie-Montsaléon, Les Chariots du Buech, au sol dans la litière d’aiguilles de pins (P. sylvestris), 26 Oct. 2008, N. Van Vooren, NV 2008.10.01 (dupl. S). – ITALY, Abruzzo, Pietracamela (TE), Prati di Tivo, on soil in mixed forest, mainly with Larix but also Picea and Pinus, 2 Oct. 2009, B. De Ruvo (S-F257096); Calabria, Celico (CS), Contrada Colamauci, under Pinus and Picea abies, 16 Sept. 2009, C. Lavorato, CL 091116-17 (dupl. S); Calabria, Morano Calabro (CS), Campotenese, under Pinus sylvestris, 7 Dec. 2009, C. Lavorato, CL 091207-01 (dupl. S). – SWEDEN, Gotland, Ollajvs Nature Reserve, close to Ljugarn, under Picea and Pinus on calcareous ground, 31 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.302 (S); ibid., under Picea and Pinus on rich calcareous soil, 27 Sept. 2011, H. Tuovila & S. Huhtinen, KH.11.115 (S); Lappland, Jokkmokk, ‘Nornaskogen’ by Ållojaur, under Picea among mosses, K. Olofsson, 1 Sept. 2011, KH.11.45 (S); Lappland, Messaure, 3 miles NW Vuollerim, herb rich Picea forest, 1 Sept. 2005, M. Karström, MK0536 (S); Lappland, Västra Tjetnekbäcken, 9 km E of Jokkmokk, under Picea abies, K. Olofsson, 1 Sept. 2011, KH.11.112 (S); Uppland, Stockholm, Enebyberg, Rinkebyskogen, on soil under Picea and deciduous trees in side of footpath, 29 Sept. 2008, J. Santos, JS.08.103 (S). – USA, Oregon, Douglas Co., Thielsen Creek, under conifers, 13 Oct. 2010, R. Helliwell, rh101310 (OSC).
Otidea cf. nannfeldtii — DENMARK, Løvenholm Skov, Langsø, 25 km W of Grenaa, 30 Sept. 1968, H. Folkmar (C-F-48295), (C-F-48296), (C-F-48297).
Notes — Otidea nannfeldtii is characterised by ochre to light brown, narrowly ear-shaped apothecia, small spores and resinous exudates on the ectal excipulum turning reddish brown in KOH. It resembles other species with ear-shaped apothecia and small spores, such as O. formicarum, O. papillata, O. pseudoleporina and O. tuomikoskii. The most similar species is O. formicarum (see under O. formicarum). Otidea tuomikoskii is separated from O. nannfeldtii by higher and more densely placed warts on the apothecial outer surface, along with the yellow reaction of the excipulum in KOH. Otidea pseudoleporina has a brighter ochre-orange hymenium, and the resinous exudates of the outer excipulum only partly convert into reddish grey heterogeneous drops in KOH.
Otidea nannfeldtii, as treated here, shows some phylogenetic structure (Fig. 1; but see Hansen & Olariaga 2015). Recently O. angusta was described as distinct from O. nannfeldtii, based on a few subtle characters, i.e. taller and slightly thicker fleshed apothecia, with very faintly brownish basal mycelium, paraphyses with shorter and thinner apical cells, and maybe smaller spores (Harmaja 2009a). At that time only a single collection of each species was known. Based on studies of additional collections, we did not find correlations between any morphological characters and the subgroups supported by our multiple molecular phylogenies. Therefore, we propose O. angusta be placed in synonymy with O. nannfeldtii. Supporting this, our study of the holotype of O. angusta revealed larger spores (9.5–11 × 5–5.8 μm) than cited in the protologue (8–9.8 × 4.5–5.2 μm), and broader paraphyses (2–3 μm) with longer terminal cells (up to 67 μm). We have also observed both tall, narrow and rounded apothecia of O. nannfeldtii in the same spots, and we consider the apothecial shape to vary during development. Otidea lohjaënsis, proposed by Harmaja (2009a) as a provisional name, is also suggested to be conspecific with O. nannfeldtii. A collection identified by Harmaja as O. lohjaënsis (S-F249387, ex-H6017194) showed excipular resinous exudates dissolving into amber drops in MLZ (Fig. 18f), contrary to the key character given for O. lohjaënsis (i.e. not responding to MLZ). The variation in the ITS region within O. nannfeldtii as recognised here is high, but displays no length variation (alignment 790 bp long). The ITS sequences of the Finnish holotypes of O. angusta and O. nannfeldtii show 26 bp differences. At the same time, however, the ITS sequence of the Swedish KH.10.302 shows 33 and 32 bp differences from the O. nannfeldtii and O. angusta holotypes, respectively. The North American rh101310 shows 14 and 20 bp differences from the two holotypes, O. nannfeldtii and O. angusta, respectively. The collections showing no LSU sequence variation (Fig. 1), show also no, or only 1–2 bp differences in the ITS region. No variation was found between the holotype of O. angusta and the Finnish S-F249387 (identified as the provisional O. loh-jaënsis by Harmaja).
Otidea nannfeldtii was previously known only from South West Finland (Harmaja 1976, 2009a). Here we report it from other areas in Europe, and for the first time from North America.
15. Otidea subformicarum Olariaga, Van Vooren, M. Carbone & K. Hansen, sp. nov. — MycoBank MB809252; ITS barcode GenBank: KM010054; Fig. 19, 21
Fig. 19.
Otidea subformicarum apothecia. a. S-F242696, holotype; b. S-F256980; c. S-F256979; d. CL 050928-30. — Photos: b. C. Pérez del Amo; c. J. Fernández Vicente; d. C. Lavorato.
Fig. 21.
Otidea subformicarum (S-F242696, holotype). a. Spores*; b. paraphyses and asci*; c. ectal excipulum in water showing reddish brown resinous exudates†; d. ectal excipulum in Melzer’s reagent showing amber drops†; e. basal mycelium in water†; f. basal mycelium in Melzer’s reagent†. — Scale bars = 10 μm; * = fresh material; † = dried material.
Etymology. Referring to its similarity to O. formicarum.
Holotype. SPAIN, Huesca, Bielsa, Ermita de Nª Señora de Pineta, 1270 m, 42.638039, 0.180532 (decimal format), under Pinus sylvestris and Abies alba on calcareous ground, 13 Oct. 2012, J.C. Campos & J. Herranz (S-F242696).
Apothecia gregarious to caespitose, 13–32 mm high, 10–20 mm wide, broadly ear-shaped, soon becoming deeply cup-shaped, split, margin sometimes lobate, sessile or stipitate. Hymenium orange brown (6C6, 6C8) to dark reddish brown (6D7, 6D8), slightly hygrophanous, when dried orange brown (7A8). Receptacle surface orange brown (6B7, 6C7) to reddish brown (6D8), hygrophanous, in drying orange ochre (6A6, 6A7), when dried ochre brown (5B6), brown (6C8) or reddish brown (7D8), finely furfuraceous, smooth at the base. Warts present near the margin, flat and rounded, concolorous, brown. Stipe 3–4 × 2–3 mm. Basal tomentum and mycelium pale yellow (4A3). Spores ellipsoid, slightly inequilateral, with two large guttules, rarely with one to several smaller granules, smooth, hyaline, 10.5–12 × 6–6.5 μm (Lm = 11.1–11.7 μm, Wm = 6.1–6.7 μm, Qm = 1.7–1.9; n = 4). Paraphyses broadly hooked, sometimes curved, sometimes enlarged at the apices to 2.5–3 μm wide, without notches, sometimes with a brown matter covering the apices, when fresh containing small, pale yellow guttules; when dried heterogeneous, pale yellow. Asci 184–237 × 11–11.5 μm. Apothecial section 800–1200 μm thick. Subhymenium c. 100–150 μm thick, of dense textura intricata, with tendency to textura angularis towards the medullary excipulum, visible as a darker orange brown zone, cells 2.5–6(–15) μm wide, with scattered brown resinous exudates. Medullary excipulum 300–700 μm thick, of textura intricata, hyphae thin-walled to slightly thick-walled, 3–12 μm wide, very pale yellow to pale brownish yellow, sometimes with brown resinous exudates at septa. Ectal excipulum of textura angularis, (85–)100–120 μm, cells thin-walled, very pale brown, (9–)17–22(–35) × (6–)11.5–23 μm. Surface with narrowly to broadly conical warts, 45–65 μm high, formed by short, fasciculate, hyphoid hairs, of 2–3 cylindrical to ovoid cells, 7–10 μm wide, not or slightly constricted at septa. Non-warted areas with single hyphoid hairs, of 2–3 cylindrical to ovoid cells, (5–)7–12 μm wide, not or slightly constricted at septa, sometimes with a gelatinous sheath. Resinous exudates abundant, pale yellowish brown, dissolving into amber drops in MLZ, turning slightly darker and partly dissolving in KOH. Basal mycelium of 2–5.5 μm wide, hyaline to very light yellow hyphae, unchanged in KOH, with refractive, pale yellow drops on the surface, dissolving in MLZ.
Specimens examined. ITALY, Cosenza, Calabria, Colamauci, Celico, under Pseudotsuga menziesii, C. Lavorato, 28 Sept. 2005, CL 050928-30 (dupl. S-F256978). – SPAIN, Canary Islands, La Palma, under Pinus canariensis, 26 Nov. 2008, J. Fernández Vicente, P. Iglesias, F. Hidalgo, J.R. Undagoitia, S. Lequerica & R. Martínez (S-F256979); La Rioja, Clavijo, under Pinus sylvestris, on the ground, C.M. Pérez del Amo & R. Gil, 3 Jan. 2009, private herb. CMP 1179, RM 1095 (dupl. S-F256980); Madrid, Bustarviejo-Canencia, Puerto de Canencia, 3 Oct. 1979, E. Álvarez (AH44526).
Other specimens examined. Otidea aff. subformicarum — MEXICO, Salazar, Parque Nacional Miguel Hidalgo, Abies forest, 23 Sept. 2007, M. Hernández (FH301035); Veracruz, Cofre de Perote, Camino de los Conejos, Los Lescados, montane forest of Pinus teocote, P. montezumae, Arbutus jalapensis, 18 Sept. 2007, M.E. Smith (FH301036).
Notes — Otidea subformicarum is closely related to O. formicarum based on both morphological and molecular characters. Both have broadly ear- to cup-shaped apothecia and small spores, and are associated with conifers. Diagnostic features of O. subformicarum are the orange-brown to reddish brown apothecial colours, and especially the long and narrow spores compared to related species. Otidea formicarum has shorter and comparatively more rounded spores (Fig. 20). All O. subformicarum apothecia were collected under Pinus, Pseudotsuga menziesii or Abies. The ecology of O. formicarum partly overlaps (see further under that species). Otidea subformicarum appears to have a southern European distribution, whereas O. formicarum is known only from Fennoscandia and the Alps. Otidea subformicarum forms a strongly supported clade in the ITS-LSU phylogeny (Fig. 2). The four ITS sequences of O. subformicarum, from Spain and Italy, are nearly identical (only S-F256979 differs in 1 bp), except for a small variation in the minisatellites. The ITS nucleotide diversity is 0.71 % in the four minisatellites unique to O. subformicarum. The five ITS sequences of O. formicarum, from Finland, Norway and Sweden, are likewise almost identical (only JS.08.63 differs in 2 bp). The ITS sequences of O. subformicarum and O. formicarum show many nucleotide differences (40 / 747 bp). Thus the interspecific ITS nucleotide diversity is much higher (5.35 %) than the intraspecific diversity (in O. subformicarum 0.07 % without the minisatellites and in O. formicarum 0.10 %).
Fig. 20.
Mean spore length and width in collections of species in the O. formicarum clade and O. pseudoleporina, based on 20 spores from each collection.
Two collections from Mexico, FH301035 and FH301036 (here referred to as O. aff. subformicarum), belong to the O. formicarum clade based on our molecular phylogenetic analyses (Fig. 1, 2). Although analyses of the combined ITS-LSU dataset did not resolve the relationships among these three lineages (Fig. 2) and the LSU phylogeny place FH301035 as a strongly supported sister taxon to two collections of O. subformicarum, our three- and four-gene phylogenies (Hansen & Olariaga 2015) suggest the two European species share a most recent common ancestor, and the Mexican collections diverged earlier. The spore size and shape of the Mexican collections conform to those of O. subformicarum (Fig. 20), but the apothecial colours differ in these collections. Fresh material has not been available to us, but the photo of fresh apothecia of FH301035 shows egg-yellow apothecia, clearly distinct from O. subformicarum. Photos of apothecia of FH301036 have a dark purplish brown colour, which has not been observed in O. subformicarum. Based on these colour differences and molecular characters, we suggest that those collections belong to an additional undescribed species, possibly endemic to North America. Further collections are needed to get insights into the variation and delimitation of this taxon for its formal description.
Outside the O. formicarum clade, a few species have similar spores to O. subformicarum. These differ by a combination of other features: Otidea tuomikoskii by narrowly ear-shaped apothecia, a receptacle surface with high warts (55–177 μm high), an ectal excipulum that turns yellow in KOH and typically orange ochre basal tomentum; and O. nannfeldtii by apothecia often having yellow tones, sometimes pink stains, and above all by the shorter spores (Lm = 10–10.7 μm).
Otidea unicisa clade
Apothecia with ochre yellow tones. Basal tomentum with ochre tones. Spores ellipsoid, with warts ± ridges or spinose. Resinous exudates on the outermost ectal excipulum cells dissolve and exude bright yellow pigment in KOH.
Species — Otidea kaushalii, O. unicisa, O. yunnanensis.
16. Otidea kaushalii (J. Moravec) K. Hansen & Olariaga, comb. nov. — MycoBank MB810994; Fig. 22
Fig. 22.
Otidea kaushalii. a. Apothecia; b. spores in water†; c. spores in Cotton Blue†; d. paraphyses in KOH†; e. ectal excipulum and warts with abundant crystal-like, resinous exudates in water†; f. close-up of crystal-like, oblate spheroid exudates on outermost ectal excipulum cells in water† (a–d, insertion on f: T. Læssøe 6236; e, f: PAN 18169, isotype). — Scale bars = 10 μm; † = dried material. — Photos: a. T. Læssøe.
Basionym. Sowerbyella kaushalii J. Moravec, Mycol. Helv. 2: 94. 1986.
≡ Aleurina kaushalii (J. Moravec) W.Y. Zhuang & Korf, Mycotaxon 29: 312. 1987.
≡ Otideopsis kaushalii (J. Moravec) J. Moravec, Mycol. Helv. 3: 138. 1988.
Holotype. INDIA, West Bengal, Darjeeling, Batasi, on soil and decayed wood in angiosperm forest, alt. 7600 f, 6 Sept. 1979, R. Kaushal, PAN 18169. Isotypes herb. J. Moravec (CUP 61814, C-F-60847 !).
Apothecia 16–65 mm high, 12–62 mm wide, broadly ear-shaped, split, stipitate. Hymenium dirty grey to very faintly incarnate, greyish yellow, orange-ochre (5A6) when dried. Receptacle surface dark orange brown (6E7, 6E8) when dried, densely warty. Warts conical, acute or blunt, densely gregarious, darker than the background, dark reddish brown. Stipe 3–32 mm long, 3–10 mm wide. Basal tomentum and mycelium ochre (5A4). Spores ellipsoid to slightly subfusoid, inequilateral, with one or two large and/or a few small guttules, with thin, often curved, spines, up to 1(–1.5) μm high, denser at the poles, sometimes united in short ridges, hyaline, 14–17 × 7–9 μm (Lm = 14.9–15.7 μm, Wm = 7.7–7.9 μm, Qm = 1.9–2; n = 2). Paraphyses curved to hooked, sometimes enlarged at apices, 2.5–5 μm wide, when dried containing small, yellowish refractive granules. Apothecial section 700–800 μm thick. Asci 188–213 × 11–13 μm. Medullary excipulum of textura intricata, hyphae thick-walled, hyaline to very pale yellow. Ectal excipulum of textura globulosa-angularis, 70–90 μm thick, of 3–4 cell layers, cells thin-walled, pale yellowish brown, 12–46 × 13–33 μm. Surface with conical warts, densely placed, 40–140 μm high, 50–137 μm wide, formed by globose to elongated cells, 8–11.5 μm broad. Resinous exudates abundant, reddish brown, amorphous, and/or many yellowish brown, crystal-like, oblate spheroid, 6–8 × 7.5–11 μm, striate bodies, with a constricted centre, not dissolving in MLZ, dissolving and exuding bright yellow pigment in KOH. Basal mycelium of 4.5–6 μm wide, thin-walled to slightly thick-walled, very pale yellow hyphae, unchanged in KOH, with regularly arranged, spheroid, yellow resinous exudates, dissolving in MLZ, unchanged in KOH.
Specimens examined. MALAYSIA, Sabah Kinabalu, on rotten wood, 2 Mar. 1999, T. Læssøe 6236 (C, dupl. BORH).
Notes — Otidea kaushalii is closely related to O. yunnanensis based on our analyses of the LSU (Fig. 1) and morphology. Already Moravec (1988) stated this, when he combined O. kaushalii in Otideopsis. The distinctly spiny spore ornamentation in these two species appears identical; see SEM photographs of the spores of the holotype of O. kaushalii in Moravec (1986) and of O. yunnanensis in Liu & Zhuang (2006). Both species have a receptacle surface with densely placed, high, dark brown warts and a lighter coloured hymenium, i.e. greyish yellow, cream to yellow. Otidea kaushalii is distinguished by the smaller spores, and possibly by the ectal excipulum of large, yellowish brown globose to angular cells (Fig. 22e). We report here for the first time a so far unique type of crystal-like, oblate spheroid, striate exudates on the outermost ectal excipulum cells of O. kaushalii (Fig. 22e, f) that might be confined to O. kaushalii within Otidea. These are abundant in both the isotype and the Malaysian material. A third collection of O. kaushalii has been reported by Zhuang & Korf (1987) from Xizang, China. The Malaysian and Chinese collections have smaller apothecia with shorter or almost no stipe (apothecia up to 19 × 18 mm; stipe 3 × 3 mm in the Malaysian material, Fig. 22a) as compared to the holotype (apothecia up to 65 × 62 mm; stipe 32 × 10 mm). The apothecia of O. yunnanensis have also been described with varying stipe length (6–25 × 4–6 mm).
17. Otidea unicisa (Peck) Harmaja, Karstenia 26: 44. 1986. — Fig. 23
Fig. 23.
Otidea unicisa. a. Apothecium; b. apothecia†; c. spores in water†; d. spores in Cotton Blue†; e. paraphyses in water†; f. ectal excipulum in water† (a: JK12082101; b: H7003343; c–f: KH.06.06). — Scale bars = 10 μm; † = dried material. — Photos: a. J. Karakehian.
Basionym. Peziza unicisa Peck, Rep. (Annual) New York State Mus. Nat. Hist. 26: 81. 1874.
≡ Sowerbyella unicisa (Peck) J. Moravec, Czech Mycol. 47: 266. 1994.
Holotype. USA, New York, Lewis County, Croghan, Felt House, ground in woods, Sept. (NYSf 3283).
Misapplied names
– Otidea grandis sensu Kanouse, Mycologia 41: 672. 1949; Liu & Zhuang, Fung. Diversity 23: 188. 2006.
Apothecia gregarious to caespitose, 12–25 mm high, 12–35 mm wide, initially broadly ear-shaped, then becoming cup-shaped, split, stipitate or sessile. Hymenium ochraceous yellow, sometimes with pink tinges, yellowish ochre (5A5, 5B5), orange-ochre (5A6) when dried. Receptacle surface ochraceous yellow, when dried dark orange brown (6E7, 6F7), ochre-brown (5A4) towards the base, warty, occasionally with shallow ribs at the base. Warts rounded, gregarious, concolorous or slightly darker than the background, reddish brown. Stipe 4–8 × 2–4 mm. Basal tomentum and mycelium abundant, yellowish ochre (5A4). Spores ellipsoid to slightly fusoid, inequilateral, with two large guttules, often with a few smaller guttules, with small, low warts, often irregular ridges, denser at the poles, hyaline, (13–)14–15.5(–16.5) × 6.5–8.5 μm (Lm = 14.6–15.2 μm, Wm = 7.1–8 μm, Qm = 1.8–2.1; n = 3). Paraphyses curved to hooked, sometimes enlarged at apices, 2.5–4 μm wide, sometimes with 1–2 low notches, when dried containing refractive, pale yellow granules. Asci 181–197 × 8–11 μm. Apothecial section 750–900 μm thick. Subhymenium c. 70–120 μm thick, of cylindrical cells, densely arranged. Medullary excipulum of textura intricata, 500–700 μm thick, hyphae thin-walled, 3.5–9 μm wide, hyaline to very pale yellow, without resinous exudates at septa. Ectal excipulum of textura angularis, 70–95 μm thick, cells thin-walled, hyaline to light yellow, 11–30 × 6.5–18 μm. Surface with conical warts, 35–85 μm high, formed by fasciculate, short, hyphoid hairs, of globose to elongated cells, constricted at septa, 6–11 μm wide. Resinous exudates abundant, dark yellowish to reddish brown, dissolving in part and converting into reddish particles in MLZ, turning brighter yellow in KOH. Basal mycelium of 4.5–6 μm wide, thin-walled to slightly thick-walled, hyaline to very pale yellow hyphae, unchanged in KOH, with regularly arranged, spheroid, yellow resinous exudates, dissolving in MLZ, unchanged in KOH.
Specimens examined. USA, Massachusetts, Carlisle, Great Farm, on woody debris, 8 July 2006, L. Millman, KH.06.06 (FH); Massachusetts, Carlisle, Towle Conservation Land, 15 July 2006, L. Millman (FH301030); Massachusetts, Purgatory Charm, 9 July 2003, Z. Wang, ZW Geo 65-Clark (S); Michigan, Cut R., Mackinac Co., in frondose woods, 10 Aug. 1949, A.H. Smith 33020 (UPS F-629380); Michigan, N of Hessel, Mackinac Co., in frondose woods, 15 Aug. 1949, H. Imshaug 3458 (UPS F-629377); Michigan, Luce Co., Tahquamenon Falls State Park, 21 Aug. 1951, A.H. Smith 39070 (UPS F-629382); Michigan, W of Detour on M134, Chippewa Co., in beech-maple woods, 14 Aug. 1949, H. Imshaug 3348 (UPS F-629379); New Hampshire, Shelburne (UPS F-629427); North Carolina, Macon Co., near summit of Standing Indian Mountain, on duff and buried wood of Betula, 1 Aug. 1969, H.H. Burdsall 2605 (dupl. H7003343); West Virginia, on light loam and leaf mold, 15 July 1896, L.W. Nuttall, Ellis 868 (UPS F-630023); West Virginia, Monongahela National Forest, Dolly Sods Wilderness, Wildlife Trail (TR 560), on soil among leaf litter under Fagus grandifolia, Acer pennsylvanicum, and Betula sp., 21 Aug. 2012, J. Karakehian, JK12082101 (FH, dupl. S).
Notes — Otidea unicisa is easily recognised by the spore or-namentation of low, delicate warts and short ridges. The dried specimens are typically bicoloured, with dark brown outside and orange-ochre hymenium.
The spore ornamentation of O. unicisa was for a long time over-looked (Harmaja 1986). The ornamentation is clearly visible at 1 000′, especially at the poles where it is more prominent. We agree with Harmaja (2009a) that what Kanouse (1949) described under the name O. grandis is O. unicisa. The bicoloured apothecia with brown outside and orange hymenium, along with the ornamented spores, and spore sizes in the range of O. unicisa, support this view. Liu & Zhuang (2006) also gave a collection with the typical spore ornament of O. unicisa under the name O. grandis (for SEM of the ornament see their f. 8). Both studies mention the Boudier plate 328 (n°. 134, 1905; as O. grandis), which shows bicoloured apothecia. Otidea unicisa has not been recorded outside Eastern North America, and in our opinion the Boudier plate 328 shows typical O. bufonia apothecia. Corroborating this, two Boudier specimens labelled O. grandis (UPS F-629342 and likely the collection used for plate 328: PC0093644), studied by us, are in fact O. bufonia. For further comments on O. grandis see Excluded, dubious and imperfectly known taxa.
18. Otidea yunnanensis (B. Liu & J.Z. Cao) W.Y. Zhuang & C.Y. Liu, Fung. Diversity 23: 188. 2006
Basionym. Otideopsis yunnanensis B. Liu & J.Z. Cao, Shanxi Univ. J., Nat. Sci. Ed. 4: 70. 1987.
Holotype. CHINA, Yunnan, Dulong River, Lang Tuan, on ground in forest, 30 Aug. 1982, D.C. Zhang (HKAS 12150).
Otidea yunnanensis was described when the monotypic genus Otideopsis was erected. It was separated from Otidea because of the ornamented spores and paraphyses with fused apical portion (Liu & Cao 1987). Upon re-examination of the type material, Liu & Zhuang (2006) discovered that the paraphyses had free apices. They also concluded that the spores were with two large and several small guttules, and not multi-guttulate as originally described by Liu & Cao (1987). Our LSU phylogeny confirms that O. yunnanensis is deeply nested within Otidea (Fig. 1). The species is easily recognised within Otidea by the large spores with fine, curved spines. Different spore sizes have however, been reported from the holotype: 18–20 × 8–10 μm (including ornament, Liu & Cao 1987) and 15–21 × 9.7–10.5 μm (excluding ornament, Moravec 1988), and including one additional collection: 16.5–20 × 7.6–10 μm (Liu & Zhuang 2006). The very wide spore measurements given by Moravec overlap somewhat with the spore length of the closely related O. kaushalii (for further comparisons see under O. kaushalii).
Otidea bufonia-onotica clade
Apothecia dark brown or ochre yellow. Basal tomentum with brown or yellow tones, especially when dried. Spores fusoid or ellipsoid, smooth. Resinous exudates on the ectal excipulum converting into reddish particles or melting into amber drops.
Species — Otidea brevispora, O. bufonia, O. fusconigra ad interim, O. mirabilis, O. onotica, O. purpurea, O. smithii.
19. Otidea brevispora (W.Y. Zhuang) Olariaga & K. Hansen, comb. & stat. nov. — MycoBank MB808974
Basionym. Otidea onotica var. brevispora W.Y. Zhuang, Mycotaxon 94: 368. 2006 ‘2005’.
Holotype. CHINA, Yunnan, Baoshan, 24 July 2003, Z.L. Yang (HKAS 43003) !
Otidea onotica var. brevispora was distinguished from O. onotica based on shorter spores (Zhuang 2006). Our study of the O. brevispora holotype confirmed the smaller spores (9.5–10.5 × 5.5–6 μm; Lm = 9.9 μm, Wm = 5.6 μm, Qm = 1.75). Otherwise O. brevispora shares all macro- and microscopic features with O. onotica, including the yellow reaction of the basal mycelium in KOH. Our ML analyses, with an LSU GenBank sequence of the Chinese holotype, show O. onotica var. brevispora is a sister taxon to a clade of O. onotica specimens (Fig. 1). Based on this and the smaller spores we consider it to be a distinct species.
20. Otidea bufonia (Pers.) Boud., Hist. Classif. Discomyc. Europe: 52. 1907. — Fig. 4b, c, 5b, 24
Fig. 24.
Otidea bufonia*. a, b. Apothecia; c. spores; d. paraphyses; e. ectal excipulum; f. basal mycelium (a: KH.09.172; b: JS.08.55; c–f: KH.09.171) — Scale bars = 10 μm; * = all fresh material. — Photos: b. J. Santos.
Basionym. Peziza bufonia Pers., Mycol. Eur. 1: 225. 1822: Fr., Syst. Mycol. 2: 54. 1822.
≡ Geopyxis bufonia (Pers.) Sacc., Syll. Fung. 8: 73. 1889.
Lectotype designated here: FRANCE, in sylvula Vincennes, Aug. 1816 (L 0116690 / 911.81.97, Persoon herbarium) !; MycoBank MBT178089.
= Peziza umbrina Pers., Observ. Mycol. 2: 77. 1799.
≡ Scodellina umbrina (Pers.) Gray, Nat. Arr. Brit. Pl. 1: 668. 1821.
≡ Peziza cochleata var. umbrina (Pers.) Fr., Syst. Mycol. 2: 50. 1822: Fr. loc. cit (‘a umbrina’).
≡ Otidea umbrina (Pers.) Bres., Fungi Trident. Ser. 2, fasc. 11–13: 68. 1898.
Lectotype designated here: Sowerby, Col. Fig. Engl. Fung. 1: t. 5. 1797 (as Peziza cochleata); MycoBank MBT200088.
= Peziza pseudobadia Cooke, Mycographia part 4: 176. 1877.
≡ Aleuria pseudobadia (Cooke) Gillet, Champ. France Discomycetes: 38. 1879 (‘pseudo-badia’).
≡ Geopyxis pseudobadia (Cooke) Sacc., Syll. Fung. 8: 69. 1889 (‘pseudo-badia’).
Holotype. FRANCE, Mérignac, sur un mur, 1814 (K(M) 195314, ex herb. Cooke).
= Otidea pedunculata Velen., Monogr. Discomyc. Bohemiae 1: 354. 1934.
Lectotype designated here: CZECH REPUBLIC, Hrusice, Aug. 1924, J. Velenovský (PRM 147622) !; MycoBank MBT200086.
Misapplied names
– Otidea grandis sensu Boudier, Icon. Mycol. livr. 6: n°. 134, pl. 328. 1905 (preliminary text with ‘circulaires’).
– Peziza cochleata sensu Sowerby, Col. Fig. Engl. Fung.1: t. 5. 1797.
Apothecia gregarious, rarely caespitose, 15–45 mm high, 17–32 mm wide, initially ear-shaped, then soon expanding and becoming deeply cup-shaped, split, stipitate or sessile. Hymenium initially orange brown (6C6) sometimes olivaceous brown (4D6), then dark orange brown (6F6, 7E8), when dried greyish brown (5E3, 5E4), slightly purple. Receptacle surface dark brown (6E4–6E7), sometimes pale rusty brown (6D8, 6E8) or purplish brown (6E3) or with olivaceous tones, slightly hygrophanous, in drying slightly paler, when dried dark orange brown (6E5, 6F4), warty, seldom slightly wrinkled at the base. Warts conical to flattened, gregarious, dark brown, sometimes distinctly darker than the background. Stipe 5–14 × 7–10 mm, often hollow and felty inside. Smell weak; taste mild. Basal tomentum and mycelium abundant, brownish white to light brown (6B3). Spores narrowly fusoid, rarely ovoid, inequilateral, with two large guttules, very rarely with a third small guttule, smooth, hyaline to pale yellowish, (12–)13–16.5(–18) × 6–7.5(–8) μm (Lm = 12.4–16.1 μm, Wm = 6.3–7.3 μm, Qm = 1.9–2.5; n = 8). Paraphyses hooked, a few curved, of the same width or slightly enlarged at apices, 3.5–5(–7) μm wide, without notches or rarely with a notch on the underside, when fresh containing small, refractive, light brownish yellow guttules; when dried light brownish yellow. Asci 143–172 × 10–12 μm. Apothecial section 1000–1300 μm thick. Subhymenium c. 70–100 μm thick, visible as a darker zone, composed of cylindrical to swollen cells, densely arranged, with scattered brownish resinous exudates at septa. Medullary excipulum of textura intricata, 500–700 μm thick, hyphae thick-walled, 3.5–9 μm wide, hyaline to light brown, some covered with strikingly dark brown, striate resinous exudates. Big crystal-like aggregates sometimes present among the hyphae, dissolving in KOH, visible in MLZ. Ectal excipulum of textura angularis, 80–100 μm thick, cells thin-walled, brownish, 16–31 × 11–25(–30) μm. Surface with broadly conical warts, 40–80 μm high, formed by short, fasiculate hyphoid hairs, of 3–4 subglobose to elongated cells, constricted at septa, 6–11 μm wide, sometimes with a gelatinous sheath. Resinous exudates abundant, dark brown, partly dissolving and converting into small reddish particles in MLZ, partly dissolving in KOH. Basal mycelium of 3.5–4.5 μm wide, hyaline to brown hyphae, with oily, light brown drops on the surface, sometimes crystalloid and rod-shaped.
Specimens examined. CZECH REPUBLIC, Prague-East district, infra Klokočná, in carpineto, 5 Oct. 1931, J. Velenovský (PRM 150074, syntype of O. pedunculata). – DENMARK, Fyn, Juelsberg Skov, N of Nyborg, along roadside in Fagus forest, 28 Sept. 1986, D. Boertman (C-F-47790); E Jylland, Løvenholm Skovene, Eldrup Skov, in grass along road, 23 Sept. 1979, E. Andersen (C-F-86688); E Jylland, Vejle Nørreskov, at roadside on rich soil under Populus tremula, Alnus, Corylus, Betula, Fraxinus, Fagus, etc., 13 Sept. 1993, J. Vesterholt (C-F-20453); NE Jylland, Rubjerg Knude, under Abies, 18 Sept. 1989, C. Lange & J. Vesterholt (C-F-25955); NW Jylland, Nystrup Plantage near Thisted, 1 Sept. 1972, K. Toft (C-F-48018); W Jylland, Bordrup Plantage, in Picea and Pinus plantation along forest road on sandy ground, 17 Sept. 2011, M. Sasa (C-F-94240); Lolland, Favrsted Skov, among mosses, on calcareous soil, by forest-road under Fagus, 4 Oct. 2007, K. Hansen & B. Kullman, KH.07.37 (S); Sjælland, Feldskoven near Sorø, 14 Aug. 1974, M. Lange (C-F-47995); N Sjælland, Gribskov, under Fagus in the forest, 19 Sept. 1971, H. Dissing (C-F-48019); ibid., 8 Oct. 1973, P.M. Pedersen (C-F-48015); Sjælland, Horserød Hegn, 6 km W of Helsingør, along roadside, 17 Sept. 1964, H. Dissing (C-F-48000). – FINLAND, Varsinais-suomi, Lohja, Virkkala, Pähkinäniemi, herb-rich deciduous forest on calcareous, mull soil, with Corylus avellana, Betula pendula, Populus tremula, Picea abies and Pinus sylvestris, 28 Aug. 1997, J. Vauras 12503F (TUR-A, dupl. S). – FRANCE, Fontainebleau, on the ground under Quercus and Abies, Oct. 1876, E. Boudier (PC0093644, as O. grandis); Forêt de Châtellerault (L 0111782, Herb. Persoon, as Peziza umbrina); Landes, Contis, under Pinus pinaster on sandy soil, 6 Dec. 2009, J.L. Teres, KH.09.249 (S); Loire-Atlantique, Bourgneuf-en-Retz, 5 Nov. 2009, G. Moyne, NV 2009.11.01 (S); Picardie, Compiègne, Oct. 1892, E. Boudier (UPS F-629342, as O. grandis); Saône-et-Loire, Robin, on the ground under Corylus and Quercus, J.-P. Dechaume, NV 2008.09.12 (dupl. S); Vendée, Jard-sur-Mer, in dune forest with dominance of Pinus pinaster and Quercus ilex, G. Ouvrard, NV 2006.11.07 (S); Vosges, Rambervillers, forêt de Saint-Hélène, under Alnus in a peatbog, 5 Oct. 2006, M. Hurtu, NV 2006.10.12 (S). – ITALY, Caorle (VE), Brussa, Vallevecchia, under Pinus pinea close to the sea, 4 Nov. 2001, E. Campo (S-F257089); Ceva (CN), 9 km from the city on the road to Viola, on the side of a track under Quercus pubescens, Castanea sativa and Corylus avellanea, 16 Sept. 2005, M. Carbone (S-F257088); Deiva Marina (SP), under Quercus ilex, 5 Nov. 2008, M. Carbone (S-F257090); Piemonte, Vignole Borbera (AL), Fraz. Variano superiore, in soil under Quercus pubescens and Castanea sativa, 19 Oct. 2010, M. Carbone (S-F257087). – SPAIN, Barcelona, entre Sant Feliu de Codines y Castellterçol, forest with Quercus ilex and Pinus sylvestris, on calcareous ground, 14 Oct. 1976, C. Montoliu (AH44527); Huesca, Javierregay, under Quercus rotundifolia and Q. humilis humus, 5 Dec. 2009, F. Prieto & A. González s.n. (S); Navarre, Arbizu, in mixed forest, 14 Nov. 2009, J. Martín (ARAN-Fungi A5048005); Navarre, Etxauri mendatea, under Quercus ilex, 2 Dec. 2006, J.M. Lekuona (ARAN-Fungi A5053019); Navarre, Lete, under Quercus faginea and Q. rotundifolia on calcareous ground, 19 Dec. 2009, J.L. Teres & P.M. Pasaban, KH.09.248 (S); Valladolid, Tudela de Duero, Puente Hierro, under Quercus ilex, J. Santos (S-F22110). – SWEDEN, Gotland, near Visby, Värnhem, under Fagus and Quercus robur on rich ground, with Hepatica nobilis, 22 Sept. 2009, E. Bohus-Jensen, K. Hansen & I. Olariaga, KH.09.189 (S); Skåne, Degeberga, Mörkavad, broadleaf forest, 27 Sept. 2001, S.-Å. Hanson, SÅH 2001-253 (C); Skåne, Helsinborg, Gyhult, broadleaf forest dominated by Fagus and Quercus, on the ground, 13 Oct. 1994, S.-Å. Hanson, SÅH 16457 (C); Uppland, Stockholm, Enebyberg, Rinkebyskogen, on rich soil in deciduous forest, under Corylus, but also Tilia, Quercus and Betula, 1 Sept. 2008, J. Santos, JS.08.55 (S); ibid., 17 Sept. 2008, JS.08.79 (S); Uppland, Uppsala, Hågadalen-Nåsten Nature Reserve, Predikstolen, under Quercus robur, Picea abies, Corylus and Salix, on rich bare ground, 17 Sept. 2009, K. Hansen & I. Olariaga, KH.09.171 (S); ibid., KH.09.172 (S); Uppland, Vaksala, near Törnby, Ekbacken, grassy ground with bare patches of soil, close to a Quercus robur tree, 13 Sept. 2010, K. Gillen & I. Olariaga, KH.10.199 (S); Öland, Algustrum, Hönstorp, 500 m SO of the village, grazed mixed deciduous forest, 19 Sept. 1993, T. Knutsson, TK93-209 (S); Öland, Långlöt, Åstad, c. 75 m from Björkerumsvägen, c. 550 S of the T-crossing in Åstad, under Corylus, 6 Sept. 1998, T. Knutsson, TK98-208 (S). – uSA, Michigan, Cheboybogan Co., Colonial Pt, Burt L., in beech-maple woods, 9 Aug. 1951, A.H. Smith 37560 (UPS F-629510); Michigan, Emmet Co., Pellston, in beech-maple woods, 11 Aug. 1951, A.H. Smith 37654 (UPS F-629511); Minnesota, Itasca State Park, on soil in mesic deciduous woods of red oak, bur oak, birch, hazelnut, Ostrya, 28 July 2010, R.A. Healy, RH1218 (MIN 933332); Minnesota, Lake Alexander SNA, on soil in mixed woods with canopy or red oak, Ostrya, white birch, 16 Aug. 2011, R.A. Healy, RH1393 (MIN 9333323). – WITHOUT LOCALITY (L 0111780, Herb. Persoon, as Peziza umbrina); (L 0111781, Herb. Persoon, as Peziza umbrina).
Notes — Otidea bufonia is macroscopically characterised by the cup-shaped apothecia, with a dark brown outside and a brown basal tomentum, especially in dried specimens. Microscopically, the narrowly fusoid spores and the presence of hyphae with striate resinous exudates in the medullary excipulum are important diagnostic characters. Otidea mirabilis is very similar to O. bufonia (see under O. mirabilis).
The hymenium colour shows a high variability in O. bufonia (Fig. 24a, b); we have observed orange brown, olivaceous or dark brown tones within a single collection. Also the colour of the receptacle surface varies and can be with rusty to purplish brown or olivaceous tones. If purplish tones are present it may be confused with O. mirabilis. The pigmented, striate, crystal-like exudates on the hyphae of the medullary excipulum are considered a unique feature for O. bufonia (Korf & Zhuang 1991, Harmaja 2009a, Parslow & Spooner 2013), and have been used to separate O. bufonia from O. mirabilis (Carbone et al. 2010, Van Vooren 2010). Our results strengthen this view. We have observed striate exudates in all the material examined (Fig. 4b, c). The size and abundance of the striate exudates are variable, and often restricted to the outermost part of the medullary excipulum (Fig. 4b). In O. mirabilis we have only observed biflabellate, crystal-like exudates (Fig. 4d).
An exceptional O. bufonia specimen with deviant spore characters was discovered (NV 2009.11.01), in which the spores were ovoid and considerably shorter than in the rest of the material. This collection is nested within the O. bufonia clade, along with typical collections.
Nomenclatural notes — A specimen from the Persoon herbarium (911.81.97, L0116690) labelled as O. bufonia by Persoon can be considered type material (Harmaja 2009a, b), being collected prior to the publication of O. bufonia. We designate it here as the lectotype to stabilise the name. It includes a single dark brown apothecium, with uniseriate, smooth, subfusoid spores, 14–16 × 5.8–7 μm, curved paraphyses, numerous dark brown, striate exudates on the hyphae of the medullary excipulum, and outermost ectal excipular cells with dark brown resinous exudates.
Otidea umbrina has been recognised as a separate species (Medardi 1995, Dissing 2000), based mainly on different hymenium colour. The original description of O. umbrina and the Sowerby plate to which Persoon (1799) referred, agree with our concept of O. bufonia. Confirming Harmaja (2009a), three of the four collections of O. umbrina studied by us and kept in Persoon′s herbarium in L belong to O. bufonia. Thus we regard O. umbrina as a synonym of O. bufonia in accordance with other authors (Dennis 1978). The name O. bufonia is sanctioned at specific rank and thus has priority over O. umbrina, which is sanctioned only at variety rank. The name O. grandis has been used for O. bufonia collections with bicoloured hymenium, i.e. orange-brown or olivaceous hymenium in contrast to a dark brown outside (Boudier 1905, Van Vooren 2010). For the interpretation of O. grandis see Excluded, dubious and imperfectly known taxa. We synonymise O. pedunculata with O. bufonia based on the study of two syntypes, and lectotypify it with PRM 147622. The lectotype shows dark brown, split apothecia, fusoid spores (13.5–15.5 × 5.5–6.5 μm, from 8 spores) and importantly striate exudates on some of the hyphae of the medullary excipulum. Harmaja (2009b) studied the holotype of P. pseudobadia and found it is a later synonym of O. bufonia. Only one collection of P. pseudobadia could be located in Kew (K(M) 195314); it is from Mérignac as given in the protologue, but from a wall, whereas Cooke wrote ‘on the ground’. In any case, one of the apothecia corresponds well to the illustration in the protologue, and we consider this the holotype (and the material studied by Harmaja, although no annotation by him could be found; pers. comm. B. Aguirre-Hudson).
21. Otidea mirabilis Bolognini & Jamoni in Jamoni, Funghi e Ambiente 85–86: 56. 2001. — Fig. 4d, 25
Fig. 25.
Otidea mirabilis*. a, b. Apothecia; c. spores; d. paraphyses; e. ectal excipulum; f. ectal excipulum wart in Melzer’s reagent, showing reddish reaction of resinous exudates (a: KH.09.188; b: KH.10.294; c–f: KH.10.308). — Scale bars = 10 μm; * = all fresh material.
Holotype. ITALY, Piemonte, Alagna valsesia (VC), Val d’Otro, on soil under Picea and Larix, alt. c. 1500 m, 3 Sept. 1999, D. Bolognini, GMFN 1951. Isotype (S-F256929) !
Misapplied names
– Otidea leporina sensu Zhuang pro parte, Mycotaxon 96: 367. 2005.
Apothecia gregarious or caespitose, 18–62 mm high, 9–52 mm wide, initially ear-shaped, short or elongated, soon expanding and becoming deeply cup-shaped, split, stipitate or sessile. Hymenium initially reddish ochre (5B7, 6B7) or sometimes olivaceous brown (5F5, 5F6), then dark reddish ochraceous brown (5D8) to dark purple brown (6F4–6F7), when dried ochraceous brown (5D6) to purplish brown (6E5, 6E6). Receptacle surface dark purple brown with bluish lilaceous shades (7F2, 7F3), fading away with time to ochraceous dark-brown (5D7), then dark purple brown (6F4–6F7), sometimes with greyish ochre (4A3, 4B3) patches in unexposed parts, hygrophanous, in drying light purple brown (5D2, 6D2) to ochre brown (5B4), when dried purple brown (6D4, 6F3, 6F4), warty, often wrinkled at the base. Warts broadly conical to hemispherical, obtuse, gregarious, dark brown, sometimes distinctly darker than the background. Stipe 7–17 × 3–7 mm. Smell weak, vaguely resin-like; taste mild, slightly bitter in the end. Basal tomentum and mycelium abundant, whitish to light brown (5A3). Spores narrowly fusoid, inequilateral, with two large guttules, very rarely with additional 1–3 small guttules, smooth, hyaline to pale yellowish, (13–)13.5–16(–17) × 6–7(–7.5) μm (Lm = 14.1–15.4 μm, Wm = 6.3–6.9 μm, Qm = 2.1–2.3; n = 9). Paraphyses curved to hooked, of the same width to slightly enlarged at apices, 2.5–5 μm wide, without or occasionally with a few low notches towards the apex, when fresh containing small, refractive, light brownish yellow guttules; when dried refractive, hyaline guttules. Asci 178–204 × 9–11 μm. Apothecial section 1000–1300 μm thick. Subhymenium c. 100–150 μm thick, visible as a darker zone, composed of irregularly, densely arranged, globose cells, with scattered brownish resinous exudates at septa. Medullary excipulum of textura intricata, 600–900 μm thick, hyphae 4–11(–15) μm wide, sometimes slightly swollen, thin to thick-walled, hyaline to light brown, seldom with brown resinous exudates at septa or biflabellate exudates, sometimes forming cross-like aggregates. Ectal excipulum of textura angularis, 80–100 μm thick, cells thin-walled to slightly thick-walled, brownish, 8–30 × 8–18 μm. Surface with broadly conical warts, 35–55 μm high, formed by short, fasciculate, hyphoid hairs, of 2–4 subglobose to elongated cells, constricted at septa, 6.5–10(–17) μm wide, sometimes with a gelatinous sheath. Resinous exudates abundant, dark brown, partially dissolving and turning reddish in MLZ. Basal mycelium of 3–6 μm wide, light yellow to light brown hyphae, seldom with oily brownish drops on the surface, with abundant small dark brown resinous exudates, rounded to irregular.
Specimens examined. DENMARK, Bornholm, Rø Plantage, on needle layer under Picea, 30 Sept. 2001, C. Lange, KH.01.09 (C). – FINLAND, Koillismaa, Kuusamo, Oulanka National Park, Ampumavaara, south of the main road to Liikanen, 14 Aug. 2010, M. Carbone (S-F257083). – FRANCE, Isère, Lans-en-Vercors, 1300 m, on the ground, on litter, under Larix, 20 Sept. 2008, J. Cavet, NV 2008.09.14 (dupl. S). – INDIA, Uttarakhand, Kalika, Nainital, on loose humus soil, 22 Sept. 1973, S. Chandes (UPS F-630072). – ITALY, Friuli Venezia Giulia, Tarvisio (UD), Val Saisera-Valbruna, on calcareous soil under Picea abies, Larix decidua and Fagus sylvatica, 27 Sept. 2010, G. Dose (S-F257092). – NORWAY, Rana, St. Alteren, 7 km W of Mo i Rana, 4 Sept. 1972, H. Dissing (C-F-87187). – SWEDEN, Gotland, Ala, Näsmyr, under Picea and Pinus sylvestris on calcareous ground, 30 Sept. 2010, E. Bohus-Jensen, K. Hansen, K. Gillen & I. Olariaga, KH.10.294 (S); Gotland, Ljugarn, forest close to Kaupungs Fridhem, under Picea abies on calcareous ground, under a cliff, 27 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.279 (S); Gotland, Lojsta hed, Russpark, grazed forest with Pinus sylvestris, on calcareous ground, 2 Oct. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.308 (S); Gotland, near Visby, Rävhagen, under Pinus sylvestris, with small Quercus robur, Helianthemum sp., Melampyrum pratense, Gallium verum, on sandy and acidified soil, 22 Sept. 2009, E. Bohus-Jensen, K. Hansen & I. Olariaga, KH.09.188 (S); Gotland, Tofta, Smågårde naturskog Nature Reserve, Tofta strand, under Pinus sylvestris on calcareous ground, 28 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.285 (S); ibid., KH.10.288 (S).
Otidea cf. mirabilis — SWEDEN, Öland, Gräsgård, Solberga, Stora Alvaret, 1300 m S of the village, 17 Oct. 1993, T. Knutsson, TK93-276 (S).
Notes — Otidea mirabilis is characterised by dark brown apothecia, purple to lilaceous-bluish shades in the receptacle surface of especially young apothecia, fusoid spores, and when present, biflabellate, crystal-like exudates in the medullary excipulum. Otidea mirabilis and O. bufonia are strongly supported as sister species in our multigene phylogeny (Hansen & Olariaga 2015). Thorough morphological comparisons show that O. bufonia differs from O. mirabilis in lacking lilaceous-bluish shades, and having brown striate exudates on some hyphae of the medullary excipulum. Otidea smithii is distinguished from O. mirabilis by the shorter spores with lower Qm (1.9–2.0).
Otidea mirabilis was described with emphasis on the lilaceous tones of the outside, in contrast to the paler ochre to olivaceous hymenium (Jamoni 2001). Part of our material shows this colour pattern, but collections with dark brown hymenium have also been observed. Some authors have noted the absence of pigmented resinous exudates in the medullary excipulum of O. mirabilis (Carbone et al. 2010, Van Vooren 2010). We have, however, observed brown crystal-like exudates in KH.10.308 and the holotype. They are flabellate and forming cross-like aggregates (Fig. 4d), clearly different from those present in O. bufonia (Fig. 4b, c).
Coniferous forests on calcareous ground are the typical habitat of O. mirabilis. Two Chinese collections assigned to O. leporina by Zhuang (2006) and sequenced by Liu & Zhuang (2006) are resolved in the O. mirabilis clade in our LSU tree (Fig. 1). Based on morphology we report it also from India (UPS F-630072).
22. Otidea onotica (Pers.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 330. 1870. — Fig. 26
Fig. 26.
Otidea onotica*. a, b. Apothecia; c. spores; d. paraphyses; e. ectal excipulum; f. basal mycelium (a: KH.10.284, epitype; b: KH.09.132; c–f: KH.09.165). — Scale bars = 10 μm; * = all fresh material.
Basionym. Peziza onotica Pers., Syn. Meth. Fung.: 637. 1801: Fr., Syst. Mycol. 2: 48. 1822.
≡ Scodellina onotica (Pers.) Gray, Nat. Arr. Brit. Pl. 1: 668. 1821.
≡ Peziza leporina var. onotica (Pers.) P. Karst., Bidrag Kannedom Finlands Natur Folk 19: 41. 1871 (‘P. leporina * P. onotica’).
Lectotype designated here: Sowerby, Col. Fig. Engl. Fung. 1: t. 79. 1797 (as Peziza leporina). Epitype designated here: SWEDEN, Gotland, Ollajvs Nature Reserve, close to Ljugarn, calcareous ground in mossy Picea forest, 27 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.284 (S); MycoBank MBT178083.
= Peziza rosea Schumach., Enum. Pl. 2: 416. 1803.
Misapplied names
– Otidea concinna sensu Boudier, Icon. Mycol. livr. 26: n°. 552, pl. 325. 1909 (preliminary text with ‘circulaires’).
– Peziza leporina sensu Sowerby, Col. Fig. Engl. Fung.1: t. 79. 1797.
Apothecia gregarious to caespitose, 25–100 mm high, 14–80 mm wide, initially long and narrowly ear-shaped, then soon expanding, and becoming deeply cup-shaped, split, stipitate or sessile. Hymenium initially light yellow (4A3–4A5), then ochraceous yellow (4A6, 4A7, 5A6, 5A7, 5B6, 5B7), in some parts light orange (5A3, 5A4), often with pink tones or entirely pinkish (6A4), sometimes with red dots, when dried orange-ochre (5A5, 5A6) to reddish ochre (6B7, 6B8). Receptacle surface ochraceous yellow (4A6, 4A7), slightly hygrophanous, in drying slightly paler, when bruised sometimes brownish ochre (5B7) in the margin, when dried brownish ochre with orange tinge (5B7, 5B8), slightly warty, sometimes wrinkled at the base when old. Warts broadly conical, gregarious, concolorous or sometimes distinctly darker than the background, brown. Stipe 13–27 × 4–14 mm. Smell weak; taste mild. Basal tomentum and mycelium abundant, whitish to light yellow (4A2, 5A2). Spores ellipsoid to broadly ellipsoid, inequilateral, with two large guttules, smooth, hyaline, (11–)12–13.5(–14) × (5.5–)6–7 μm (Lm = 12.1–13.3 μm, Wm = 6.2–6.8 μm, Qm = 1.8–2; n = 10). Paraphyses curved to hooked, of the same width or slightly broader at apices, 2.5–4.5 μm wide, without notches or rarely with a slightly swollen area on the underside, when fresh containing small, refractive, light yellow guttules; when dried hyaline to pale yellow guttules. Asci 138–233 × 9.5–12 μm. Apothecial section 850–1400 μm thick. Subhymenium c. 100–120 μm thick, of dense textura intricata, visible as a darker zone, cells cylindrical to swollen, densely arranged. Medullary excipulum of textura intricata, 400–600 μm thick, hyphae 5–7(–12) μm wide, thick-walled, hyaline. Ectal excipulum of textura angularis, 80–110 μm thick, cells 13–55 × 11–28 μm, thin-walled, light yellow. Surface with conical warts, 85–105 μm high, formed by short, fasciculate hyphoid hairs, of 2–3 subglobose to elongated cells, constricted at septa, 11–14 μm wide. Resinous exudates abundant, yellow, dissolving into amber drops in MLZ. Basal mycelium of 3.5–6 μm wide, hyaline to very light yellow hyphae, turning yellow in KOH, with very small, regularly arranged, spheroid, resinous exudates on the surface, dissolving in MLZ, partially and more slowly in KOH.
Specimens examined. CZECH REPUBLIC, South Bohemia, Netolice, Sept. 1922, Hampl (PRM 148341). – DENMARK, Amager, Kongelunden, soil along forest path, 21 July 1998, K. Hansen, T. Læssøe & C. Lange, KH.98.107 (C); Møn, Fanefjord Skov, under conifers on calcareous influenced soil, 30 Sept. 2008, H. Knudsen (C-F-89691); Sjælland, Geelskov, 12 km N of Copenhagen, under Fagus, 18 Sept. 1963, L. Hansen & A. Kjøller (C-F-47985). – FRANCE, Landes, Contis phare, under Pinus pinaster on sandy soil, 9 Sept. 2009, J.L. Teres (ARAN-Fungi A8200131C). – ITALY, Piemonte, Vinadio (CN), San Bernolfo, Picea abies and Abies alba forest, with presence of Fagus sylvatica, 29 Sept. 2008, M. Carbone (MCVE 23277). – NORWAY, Nord-Trøndelag, Gjøråsvika, Leksvik, under Picea on rich ground, among mosses, 3 Sept. 2009, K. Hansen & I. Olariaga, KH.09.132 (S); under Corylus and Picea on rich ground, KH.09.136 (S). – SPAIN, Gipuzkoa, Aia, Amezketalardiko bidegurutzea, under Abies alba, 3 Oct. 2009, J.L. Teres (ARAN-Fungi A3033701A); Madrid, Sierra de Guadarrama, deciduous forest, among Quercus pyrenaica leaves, 4 Oct. 1981, G. Moreno (AH2528); Navarre, Arbizu, mixed forest, 14 Nov. 2009, J. Martín (ARAN-Fungi A5048003); Navarre, Orokieta, Loiandi, in Picea abies plantation, 10 Oct. 2009, Aranzadi ZE (ARAN-Fungi A5041174-2). – SWEDEN, Skåne, Vittskövle, Segesholm, Herremöllan, broadleaf forest, 27 Sept. 2001, S.-Å. Hanson, SÅH 2001-266 (C); Uppland, Stockholm, Norra Järvafältet, Hansta Nature Reserve, on rich ground under Picea abies with Corylus avellana, 15 Sept. 2009, K. Hansen & I. Olariaga, KH.09.164 (S); ibid., KH.09.165 (S), KH.09.166 (S); Uppland, Uppsala, forest-covered hill NNE of Naturicum, coniferous forest with Quercus and Corylus, 4 Oct. 2011, R. Sundin, KH.11.108 (S); Uppland, Uppsala, Norra Lunsen Nature Reserve, Lunsentorpet, on rich organic soil, under dead standing Picea, 28 Aug. 2008, J. Santos, JS.08.48 (S); Uppland, Uppsala, Vänge, Fiby urskog Nature Reserve, old Picea forest, in the middle of a path, 17 Sept. 2009, K. Hansen & I. Olariaga, KH.09.175 (S). – USA, Minnesota, Cedar Creek ESR, on sandy soil in oak savannah, canopy of pin oak, hazelnut, 18 July 2011, R.A. Healy, RH1121 (MIN 933307); Minnesota, Lake Alexander SNA, on soil, mixed woods, canopy of red oak, paper birch, Ostrya, 29 July 2010, R.A. Healy, RH1222 (MIN 933311); Minnesota, Wild River State Park Many, on moist, sandy soil in mesic deciduous woods with oak, 23 July 2010, R.A. Healy, RH1199 (MIN 933309); Oregon, Benton Co., Philomath, Wood Creek Road, 18 Nov. 1996, E.T. Peterson (OSC 56759); Washington, Lower Tahoma Creek, Mt Rainier National Park, 27 Aug. 1948, A.H. Smith 30764 (UPS F-629424); Washington, Nisqually R., Mt Rainier National Park, 30 Aug. 1948, H.A. Imshaug 2116 (UPS F-629438).
Notes — Otidea onotica is one of the most common and wellknown species of the genus. It is characterised by rather large, ochraceous yellow apothecia, often with a pinkish tinge, dots or stains in the hymenium, and spores of unusual size within Otidea. The presence of pinkish tones varies considerably. Apothecia ranging from entirely pink to completely devoid of pinkish tones have been observed in the same locality, in KH.09.132 and KH.09.136, respectively. These two collections have identical ITS sequences (GenBank accessions KM010103 and JN942772). A consistent character, here reported for the first time, is the yellow reaction of the basal mycelium in KOH. Otidea unicisa resembles O. onotica macroscopically, but is distinguished by ornamented spores.
Nomenclatural notes — Carbone (2009) revised the nomenclature of O. onotica. He considered the plate by Sowerby (1797, as Peziza leporina) the only element seen by Persoon, and thus, the holotype. However, Persoon did indirectly refer to two elements by giving the habitat in the protologue as beech forests (faginetis) and citing Sowerby’s plate, which shows an oak leaf. Thus, the plate is not the holotype; i.e. the one element on which the author based the name (Art. 9.1 ICN; McNeill et al. 2012). The use of the term holotype could be corrected to lectotype according to Art. 9.9, but this article is not applicable since Carbone’s holotype indication does not fulfil Art. 7.10 of the ICN (it does not include the phrase ‘designated here’ or equivalent) (McNeill et al. 2012). Carbone (2009) selected in addition an epitype from Persoon′s herbarium. But as an epitype must refer to the type it interprets and there was no validly selected type in 2009, the epitypification by Carbone is also not valid. We therefore typify O. onotica here, by designating as lectotype the Sowerby plate, supported by a newly collected epitype with multiple gene sequences and colour photographs. The selection of an epitype from Sweden is justified by the name being sanctioned (Fries saw and studied living material as indicated by ‘v. v.’), and our ITS sequences of O. onotica (4, 5, 6, 7, 8, 9) from Denmark, Italy, Norway and Sweden (including the epitype) being identical. The LSU sequences of O. onotica (4, 5) from Denmark and Sweden differ only 2 bp from the other collections.
23. Otidea purpurea (M. Zang) Korf & W.Y. Zhuang, Mycotaxon 22: 507. 1985
Basionym. Acetabula purpurea M. Zang, Acta Bot. Yunnan. 1: 101. 1979.
Holotype. CHINA, Tibet, Zayu, on the ground in forest with Pinus yunnanensis, 1 Sept. 1976, M. Zang 670 (HKAS 5670) !
Notes — Otidea purpurea is probably closely related to O. mirabilis. It is also characterised by dark brown apothecia with lilaceous tones, but it clearly differs in the smaller spores (8.8–10 × 4.5–5.2 μm). Two additional taxa, O. subpurpurea and O. bicolor, should be compared with O. purpurea. Otidea subpurpurea likewise has violaceous tones on the outside, but has larger spores and asci (Zhuang & Yang 2008). Otidea bicolor has dark brown apothecia and small spores (Zhuang 2010), and is probably also closely related or conspecific with O. purpurea, based on the spore size. We treat here O. purpurea in the O. bufonia clade, because of the dark brown apothecia and brown basal tomentum.
24. Otidea smithii Kanouse, Pap. Michigan Acad. Sci., Part 1. 24: 28. 1939 ‘1938’ — Fig. 27
Fig. 27.
Otidea smithii (ECV3345). a. Apothecia; b. spores in water†; c. paraphyses in water†; d. ectal excipulum in water†. — Scale bars = 10 μm; † = dried material. — Photos: a. E. Vellinga.
Holotype. USA, California, Crescent City, 18 Nov. 1937, A.H. Smith 8843 (MICH 14408) !
Apothecia gregarious or caespitose, 32–70 mm high, 13–40 mm wide, initially narrowly ear-shaped, short or elongated, finally expanding, at late stages becoming deeply cup-shaped, split, normally shortly stipitate. Hymenium purple-brown (6D5) to dark purple-brown (7E5), then ochraceous brown (5D8), when dried very pale purplish brown (5A2). Receptacle surface dark purple-brown (7E5), sometimes with lilaceous shades or lighter ochre-brown (6C5) patches in unexposed parts, slightly hygrophanous, in drying dark ochraceous brown (6D7), when dried reddish brown (6E5, 6E6), warty, sometimes wrinkled at the base. Warts conical, obtuse or acute, gregarious, dark brown, sometimes slightly darker than the background. Stipe 12–26 × 5–12 mm. Basal tomentum and mycelium abundant, whitish to brown (5B2, 5C2). Spores ellipsoid, sometimes fusoid, inequilateral, with two large guttules, smooth, hyaline, 12–14(–14.5) × 6–7.5 μm (Lm = 12.5–13.6 μm, Wm = 6.4–7.1 μm, Qm = 1.9–2.0; n = 5). Paraphyses curved to hooked, of the same width to slightly enlarged at apices, 3–5 μm wide, sometimes with 1–3 notches or with a low notch near the apex, when dried containing refractive, pale yellow guttules. Asci 175–251 × 9–11 μm. Apothecial section 800–1300 μm thick. Subhymenium c. 140–180 μm thick, of dense textura intricata, visible as a darker zone, hyphae intermixed with a few subglobose cells, with scattered brownish resinous exudates at septa. Medullary excipulum of textura intricata, 700–950 μm thick, hyphae 4–14(–21) μm wide, sometimes slightly swollen, thin-walled to slightly thick-walled, light brown, seldom with light brown resinous exudates at septa. Ectal excipulum of textura angularis, 70–100 μm thick, cells thin-walled to slightly thick-walled, pale brown, 13–30 × 8–15 μm. Surface with conical warts, 34–71 (–102) μm high, formed by short, fasciculate, hyphoid hairs, of 2–5 subglobose to elongated cells, constricted at septa, 6–14 μm wide, sometimes with a gelatinous sheath. Resinous exudates rather abundant on the outside, dark brown, partly dissolving and turning reddish in MLZ. Basal mycelium of 3–5 μm wide, light to darker brown hyphae, sometimes with pale brown drops on the surface, with abundant small, rod-shaped to irregular, brown resinous exudates.
Specimens examined. CANADA, British Columbia, Goldstream Park, Victoria, on decayed wood under mature Douglas fir, 21 Sept. 1968, J. Ginns 1212 (UPS F-629486). – USA, California, Alameda Co., Berkeley, Le Conte Avenue, in lawn under Betula and Cedrus, 25 Oct. 2005, E. Vellinga, ECV3345 (S); California, Del Norte Co., California State Park, Lake Earl Wildlife Area, access by Sand Hill Road, with Abies grandis, Picea sitchensis, Polystichum munitum, Rubus spectabilis, Vaccinum ovatum, 15 Dec. 1997, M. Madsen & R. Davis (OSC 56823); Oregon, Benton Co., Philomath, Woods Creek Road, 15 Nov. 1997, E.T. Peterson (OSC 56811); Washington, Pierce Co., Mt Rainier National Park, Lower Tahoma Creek, 30 Oct. 1996, E.T. Peterson (OSC 56753); ibid., 18 Oct. 1997 (OSC 56799); Washington, Lewis Co., Gifford-Pinchot National Forest, Camp Creek Falls Trail, on litter with Acer circinatum, A. macrophyllum, Tsuga heterophylla, Berberis nervosa, Polystichum munitum, Linnaea borealis, Rubus sp., Goodyera oblongifolia, 6 Nov. 1997, E. Hathaway & E. Millian (OSC 56830).
Notes — Otidea smithii is closely related to O. bufonia and O. mirabilis, as shown by morphological and molecular characters. These three species share dark brown apothecia, brown basal tomentum and dark brown resinous exudates in the ectal excipulum. Otidea smithii is distinguished by shorter spores with a lower Qm value, and typically narrower, ear-shaped apothecia. The unique, biflabellate or striate exudates in the medullary excipulum, as seen in O. bufonia and O. mirabilis (Fig. 4c, d), respectively, are not present in O. smithii. Only pigmented resinous exudates at the hyphal septa in the medullary excipulum are rarely found in O. smithii (as in Fig. 4a). Otidea smithii is so far only known from Western North America (Kanouse 1949, Peterson 1998). Our ITS sequence of O. smithii (JN942771) is identical to the ITS sequence of the holotype (AF072065).
Otidea concinna clade
Apothecia cup-shaped, split or sometimes entire, often with bright yellow tones. Spores small, 10–12.5 μm long, smooth. Para-physes typically straight and claviform at apices. Basal mycelium with scattered reddish or yellow resinous exudates.
Species — Otidea borealis, O. caeruleopruinosa, O. concinna, O. flavidobrunneola, O. lactea, O. minor, O. oregonensis, O. phlebophora, O. rainierensis, O. sinensis, O. tianshuiensis, O. sp. ‘a’.
25. Otidea borealis M. Carbone, Olariaga, K. Hansen & Van Vooren, sp. nov. — MycoBank MB809250; ITS barcode GenBank KM010023; Fig. 28, 30
Fig. 28.
Otidea borealis (S-F242694, holotype). a, b. Apothecia; c. close-up of apothecia showing a warty external receptacle and dentate margin; d. Young narrowly cup-shaped apothecium. — Photos: M. Carbone.
Fig. 30.
Otidea borealis (S-F242694, holotype)†. a. Spores in water; b. paraphyses and asci in KOH; c. ectal excipulum in water, with resinous exudates on the outside; d. ectal excipulum turning bright yellow in KOH, resinous exudates partly dissolving; e. basal mycelium in water; f. basal mycelium in KOH. — Scale bars = 10 μm; † = all dried material.
Etymology. Referring to a supposedly boreal distribution.
Holotype. FINLAND, Koillismaa, Kuusamo, Juuma, Jäkälävuoma, western part of Jäkälävuoma gorges, many apothecia in moist soil among mosses at brookside, under Picea abies, 16 Aug. 2010, M. Carbone (S-F242694). Isotype (TUR-A 198578).
Apothecia gregarious to caespitose, 12–22 mm high, 8–15 mm wide, initially narrowly ear-shaped, upper margin subacute to rounded, then broadly ear-shaped, split, stipitate. Hymenium pale yellowish ochre (4A2, 5A2), when dried greyish ochre (4A2, 5A2). Receptacle surface ochraceous yellow (4A5, 5A5), yellowish brown (5B5, 5B6) in some parts, sometimes brown (5D7) in the margin, hygrophanous, when dried reddish brown (6D5, 6D6), warty, seldom slightly wrinkled at the base. Margin sometimes finely dentate and darker. Warts broadly conical, gregarious, light to dark brown. Stipe well developed, 3–12 × 2–6 mm. Basal tomentum and mycelium white to very pale yellow (3A2), when dried very pale yellow (3A2). Spores broadly ellipsoid, symmetrical, with two large guttules, smooth, hyaline, (10–)10.5–11.5 × 6–7 μm (Lm = 10.5 μm, Wm = 6.5 μm, Qm = 1.7; n = 1). Paraphyses straight to curved, very few hooked, 2–2.5 μm wide, at apices up to 3–4.5(–5) μm wide, without notches, terminal segment 33–47 μm long, narrowly claviform to claviform, when dried containing slightly refractive, hyaline granules. Asci 167–198 × 9–10.5 μm. Apothecial section 1 000 μm thick. Subhymenium c. 100 μm thick, of dense textura intricata, cells 2–4 μm thick. Medullary excipulum 600 μm thick, of textura intricata, hyphae thin-walled to slightly thick-walled, 4–7 μm wide, hyaline, without resinous exudates. Ectal excipulum of textura prismatica, 80 μm thick, cells thin-walled, hyaline, unchanged to very pale yellow in KOH, 10–21 × 8.5–11 μm. Surface with broadly conical warts, 63–90 μm high, formed by fasciculate, short, hyphoid hairs, of 3–6 subglobose to ovoid cells, constricted at septa, 6–12 μm wide. Non-warted parts with single, 2–5-celled hyphoid hairs, with cylindrical to claviform upper cell, 45–73 × 8–10 μm. Resinous exudates golden brown, partly dissolving in MLZ (amber drops not observed), bright yellow and partly dissolving in KOH. Basal mycelium of 3–4.5 μm wide, very pale yellow hyphae, bright yellow in KOH, with spheroid to rod-shaped, reddish yellow resinous exudates, dissolving in MLZ, partially dissolving and turning bright yellow in KOH.
Other specimens examined. Otidea sp. ‘a’ — SWEDEN, Lappland, Arvidsjaurs, Lillån Allmänningreservat, 8 km SV Järvträsk, herb-rich Picea forest on calcareous ground, 12 Sept. 2009, M. Karström, MK0942 (S); ibid., 10 Sept. 2010, MK1081 (S).
Notes — Within the O. concinna clade, O. borealis is distinguished by broadly ear-shaped apothecia, with a pale yellowish ochre hymenium and a darker ochre yellow outer surface with small brown warts. Microscopically, the spores are proportionally broader than in closely related species, and the mycelium at the base of the apothecia turns bright yellow in KOH (tested in dried specimen only; Fig. 30f).
At least four species of the O. concinna clade have bright yellow outer apothecial colours, similar to O. borealis. Otidea concinna appears morphologically most similar, but besides the citrine-yellow outer receptacle, it differs in the narrower spores (Fig. 29) with higher Qm value. Otidea phlebophora and O. minor are distinguished by the often entire apothecia with ribs or anastomosing veins at the base and distinctly narrower spores. Otidea oregonensis differs from O. borealis in typically having obconical apothecia, sometimes with strongly rugulose base, citrine-yellow colour in the outer receptacle when young, narrower spores (Fig. 29), and the mycelium at the base of the apothecia does not turn yellow in KOH. The North American O. rainierensis (= O. kauffmanii) appears to have some yellowish apothecial pigment (see further under O. rainierensis). Never-theless, the spores of O. rainierensis are longer than the spores of O. borealis (Fig. 29). Furthermore, our ITS-LSU phylogenies (Fig. 3) suggest that these species are distinct from O. borealis.
Fig. 29.
Mean spore length and width in collections of species in the core of the O. concinna clade, based on 20 spores from each collection.
Two Swedish collections, MK0942 and MK1081, constitute an-other undescribed species (Otidea sp. ‘a’) nested within the O. concinna group. It resembles O. borealis in spore shape (only slightly longer in Otidea sp. ‘a’: 11–12(–13) × 6–7 μm; Lm = 11.2–11.6 μm, Wm = 6.6–6.8 μm, Qm = 1.6–1.7; n = 2) and yellow reaction of the basal mycelium in KOH. The two Otidea sp. ‘a’ collections differ from O. borealis in the brownish ochre receptacle surface, without yellow tones, and ochre to pinkish ochre hymenium. Also our analyses of the ITS-LSU regions support O. sp. ‘a’ and O. borealis as distinct species (Fig. 3). Since only dried material has been available to us, we postpone a formal description of O. sp. ‘a’ until fresh and more abundant material, with good colour photographs, becomes available.
26. Otidea caeruleopruinosa Harmaja, Karstenia 48: 37. 2009 — Fig. 31
Fig. 31.
Otidea caeruleopruinosa. a. Apothecia; b. apothecium showing basal tomentum†; c. spores in water†; d. paraphyses in water†; e. ectal excipulum in water† (a: MT 11080205; b–e: H6010805, holotype). — Scale bars = 10 μm; † = dried material. — Photos: a. M. Tabarés; b. J. Kearey.
Holotype. FINLAND, Varsinais-Suomi, Lohja, Jalassaari, 20 Sept. 1978, H. Harmaja (H6010805) !
Apothecia gregarious to caespitose, 31–60 mm high, 17–50 mm wide, initially ear-shaped, upper margin rounded, then cup-shaped, split, margin sometimes lobulate, stipitate or sessile. Hymenium yellowish brown (5C6), when dried yellowish brown with a faint olivaceous tint (4D6) or orange-ochre (6B6, 6C6). Receptacle surface upper half concolorous with hymenium or slightly duller (5C5, 5C6), lower half more whitish (pubescent pruinose), slightly hygrophanous, when dried concolorous with hymenium towards the margin, below cream, or pale reddish brown (6C6), furfuraceous to very finely warty, seldom slightly ribbed at the base. Warts broadly conical, gregarious, paler or bluish. Stipe not well developed, rooting, up to 11 × 5 mm. Basal tomentum and mycelium abundant, when fresh ochraceous white, when dried light ochre (5A2). Spores narrowly ellipsoid to ellipsoid, often inequilateral, with two large guttules, smooth, hyaline, (10.5–)11–12.5 × 5.5–6.5 μm (Lm = 11.2–11.8 μm, Wm = 5.9–6.1 μm, Qm = 1.9; n = 3). Paraphyses straight or curved, or broadly hooked, of the same width or broader at apices, 2–4(–5) μm wide, without notches, terminal segment 26–78 μm long, when fresh containing few, small, weakly refractive, pale guttules; when dried refractive, hyaline granules. Asci 144–200 × 9–11 μm. Apothecial section 700–950 μm thick. Subhymenium c. 70–80 μm thick, of 2.5–5 μm wide cylindrical cells, with scattered swollen, 12–18 μm wide cells, densely arranged. Medullary excipulum of textura intricata, 550–700 μm thick, hyphae thin to thick-walled, 3–9 μm wide, intercalated with swollen cells, 12–18 μm diam, hyaline, without resinous exu-dates. Ectal excipulum of textura prismatica-angularis, 100–120 μm, cells thin-walled, pale brown, sometimes yellow in KOH, 10–32 × 10–22 μm. Surface with conical to broadly conical warts, 60–80 μm high, formed by short, fasciculate hyphoid hairs, of 2–4 ovoid to cylindrical cells, 6–10 μm wide, not constricted at septa. Non-warted parts with single 2–7-celled hyphoid hairs, of globose to cylindrical cells, 20–33 × 9–17 μm. Resinous exudates abundant, yellow to reddish brown, dissolving in MLZ, bright yellow and partly dissolving in KOH and the outer excipulum turning bright yellow. Basal mycelium of 3.5–6(–8) μm wide hyphae, with spheroid to rod-shaped, reddish yellow resinous exudates, dissolving in MLZ, more slowly and partially dissolving in KOH, turning yellow.
Specimens examined. SPAIN, Girona, Ripollés, Setcases, under Corylus avellana, Betula verrucosa and Buxus sempervirens, on calcareous soil, 26 Aug. 2010, M. Tabarés & S. Santamaría, MT 10082601 (dupl. S). – SWEDEN, Södermanland, Nynäshamn, Herrhamra, on soil under Fagus, in narrow forest area along the road, 18 Sept. 2013, I.-L. Walter, KH.13.48 (S); Uppland, Älvkarleby, V from the train station, 24 Sept. 1950, G. Fåhraeus & G. Stenlid (UPS F-146664).
Notes — The main diagnostic characters of O. caeruleopruinosa are cup-shaped, split apothecia, whitish to pale cream hymenium, grey outside and rather small spores. Harmaja (2009a) described O. caeruleopruinosa with strong emphasis on the bluish shades of the warts on the apothecial outside, a character that has not been observed in our Swedish, or the Iberian material (Van Vooren et al. 2011). A third gathering from the Iberian site does not show bluish tones either (M. Tabarés, pers. comm.).
Otidea caeruleopruinosa belongs to the O. concinna clade, and it shares several characters with the other members of the clade, such as cup-shaped apothecia, small spores, and an ectal excipulum of textura prismatica to textura angularis. It is morphologically most similar to O. flavidobrunneola (see Notes under that species).
27. Otidea concinna (Pers.) Sacc., Syll. Fung. 8: 96. 1889 —Fig. 32
Fig. 32.
Otidea concinna*. a, b. Apothecia; c. spores; d. paraphyses; e. ectal excipulum; f. basal mycelium (a, c, e, f: KH.09.183, epitype; b: KH.09.217; d: KH.09.176). — Scale bars = 10 μm; * = all fresh material.
Basionym. Peziza concinna Pers., Mycol. Eur. 1: 221. 1822: Fr., Syst. Mycol. 2: 49. 1822.
≡ Helvella scutellata Schaeff., Fung. Bavar. Palat. Nasc. 4: 101. 1774 (‘Elvela’).
Lectotype designated here: Schaeffer, Fung. Bavar. Palat. Nasc. 2: t. 150, f. 1. 1763 (‘Elvela tertia’). Epitype designated here: SWEDEN, Uppland, Stockholm, Naturhistoriska riksmuseet, in front of the Botany building, under a big Quercus robur tree, by a row of Populus, 20 Sept. 2009, K. Hansen & I. Olariaga, KH.09.183 (S); MycoBank MBT178084.
= Helvella pyxidata Schaeff., Fung. Bavar. Palat. Nasc. 4: 111. 1774 (‘Elvela’).
≡ Peziza marsupium var. pyxidata (Schaeff.) Pers., Syn. Meth. Fung. 2: 640. 1801 (‘ß pyxidata’).
≡ Scodellina pyxidata (Schaeff.) Gray, Nat. Arr. Brit. Pl. 1: 669. 1821.
Misapplied names
– Flavoscypha cantharella sensu Harmaja, Karstenia 14: 107. 1974.
Apothecia gregarious to caespitose, 17–71 mm high, 12–66 mm wide, initially broadly ear-shaped, then soon expanding and be-coming deeply cup-shaped, split, often broader above, very rarely entire when young, stipitate or broadly sessile. Hymenium pale yellowish ochre (3A3, 4A5) to pale greyish ochre (5A2), sometimes with rose stains or spots, when dried light yellowish grey (2A2) to very pale yellowish ochre (3A2, 3A3). Receptacle surface bright citrine yellow (3A5–3A7), sometimes with a greenish hue (2A6), slightly hygrophanous, in drying slightly paler, fading to brownish ochre (4B5) in age, when dried yellow (3A7), furfuraceous, sometimes warty in young apothecia, sometimes shallowly wrinkled at the base. Warts flat to broadly conical, leaving a reticulum among them, concolorous. Stipe not well developed, rooting, 7–8 × 4–5 mm. Smell sweet, fruity, weak; taste mild. Basal tomentum and mycelium abundant, white to very pale cream (4A2), sometimes with very pale purplish tones, when dried white to very pale yellow (4A2). Spores narrowly ellipsoid to ellipsoid, inequilateral, with two large guttules, smooth, hyaline, (10–)10.5–12 × (5–)5.5–6.5 μm (Lm = 10.8–11.2 μm, Wm = 5.6–6 μm, Qm = 1.8–2; n = 8). Paraphyses straight to bent, seldom broadly hooked, at apices claviform to abruptly capitate, 2.5–6(–8.5) μm wide, without notches, terminal segment 19–66 μm long, when fresh content hyaline homogenous or of small, refractive, hyaline to pale yellow granules; when dried refractive, hyaline granules. Asci 176–196 × 10–11.5 μm. Apothecial section 1200–2100 μm thick. Subhymenium c. 60–90 μm thick, composed of cylindrical cells, 2–4 μm wide, with scattered swollen cells, 9–13 μm wide, densely arranged. Medullary excipulum 600–1000(–1500) μm thick, of textura intricata, hyphae cylindrical to slightly swollen, 3–11(–15) μm wide, thin to thick-walled, hyaline, without resinous exudates. Ectal excipulum of textura prismatica-angularis, 80–100 μm thick, cells thin-walled, hyaline to very pale yellow, sometimes light reddish in KOH, 12–35 × 6–22 μm. Surface with low broadly conical warts, 60–70 μm high, formed by fasciculate, parallel, short, hyphoid hairs, of 4–6 ovoid cells, constricted at septa, 5–9.5 μm wide. Non-warted parts with single, 2–6-celled, hyphoid hairs, uppermost cell narrowly claviform to subcapitate, 20–45 × 4.5–7 μm, sometimes with a gelatinous sheath. Resinous exudates abundant, yellow or reddish, dissolving into amber drops in MLZ, turning bright yellow in KOH. Basal mycelium of cylindrical to slightly swollen, hyaline to very pale yellow hyphae, 3–4.5(–8.5) μm wide, yellow in KOH, with scattered, spheroid to rod-shaped, yellow or reddish resinous exudates, dissolving in MLZ, partially in KOH.
Specimens examined. DENMARK, NE Sjælland, Jægersborg Dyrehave, 8 Sept. 1979, H. Knudsen (C-F-87186); NW Jylland, Nystrup Plantage, ‘Kridtstien’, on calcareous soil with Abies, 14 Sept. 1985, T. Læssøe (C-F-81617). – ESTONIA, Saaremaa, Abruka, ad terram, 16 Sept. 1966, K. Kalamees (UPS F-629562). – FINLAND, Varsinais-Suomi, Turku, Ispoinen Katariinanlaakso, W part of the reserve, in rich, essentially deciduous woods with Corylus, Quercus, Tilia, etc. on bare clayey mull soil, 22 Aug. 1977, H. Harmaja (S-F249360, ex-H6015773). – FRANCE, Saône-et-Loire, La Grande-Verrière, Senavelle, on the ground under Pseudotsuga menziesii, 12 Sept. 2008, J.-P. Dechaume, NV 2008.09.13 (dupl. S). – SPAIN, Huesca, Javierregay, humus of Quercus rotun-difolia and Q. humilis, 5 Dec. 2009, F. Prieto & A. González, KH.09.250 (S); Huesca, Sallent de Gállego, Lanuza, 14 Oct. 2006, I. Olariaga (BIO-Fungi 13002); Huesca, Yebra de Basa, close to Sta Orosia chapel, in Fagus sylvatica forest, 11 Oct. 2009, P. Siljeström (ARAN-Fungi A8700091). – SWEDEN, Gotland, Ala, Stenstugu, near a big Quercus robur tree in meadow, on rich ground, among grass, 26 Sept. 2009, E. Bohus-Jensen, K. Hansen & I. Olariaga, KH.09.217 (S); Gotland, Lojsta, Lojsta slot, under Corylus on a chalk-rich slope, 19 Sept. 2000, T. Knutsson, TK2000-078 (S); Närke, Havsta, Bruntorpskärret, under conifers, 10 Aug. 2008, B. Wasstorp, JS.08.59 (S); Skåne, Degeberga, Segesholm, on a steep slope on bare ground, under Fagus and Ulmus, 23 Sept. 2010, K. Hansen, K. Gillen, I. Olariaga, KH.10.256 (S); Skåne, Fjälkstad, Balsberget, on rich ground among leaf litter under Fagus, 20 Sept. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.212 (S); Skåne, Kristianstad, N. Lingenäset, Storskogen, on ground in broadleaf forest, 18 Sept. 1996, S.-Å. Hanson s.n. (C); Uppland, Uppsala, Carolinaparken, under Corylus avellana, 7 Sept. 2010, S. Ryman, K. Hansen, K. Gillen & I. Olariaga, KH.10.180 (S); Uppland, Uppsala, Hågadalen-Nåsten Nature Reserve, Predikstolen, under Quercus robur, Ulmus glabra, Sorbus aucuparia, with Hepatica nobilis, Geum urbanum, Convallaria majalis, on rich ground, 19 Sept. 2009, K. Hansen & I. Olariaga, KH.09.176 (S); ibid., 6 Oct. 2010, KH.10.182 (S).
Notes — Otidea concinna is characterised by cup-shaped, split apothecia, citrine yellow receptacle surface, along with paraphyses predominantly straight and claviform at apices. Otidea minor and O. phlebophora share with O. concinna a yellow receptacle surface, but they have anastomosing ribs at the apothecial base and narrower spores (Fig. 29). Otidea oregonensis is distinguished from O. concinna by the often obconical apothecia, sometimes with blunt ribs at the base.
The bright yellow receptacle surface is one of the main diagnostic characters of O. concinna, but we have observed the yellow colour can disappear with age, and the outer surface becomes brownish ochre (Fig. 32b) thus making it difficult to recognise the species. Otidea rainierensis is a North American taxon that shows an apothecial shape similar to O. concinna. Nevertheless, bright citrine yellow tones are absent in O. rainierensis, and our molecular data support it as distinct from O. concinna (Fig. 1,3). For further comments see under O. rainierensis.
Nomenclatural notes — No authentic material has been located in Persoon’s herbarium in L. We therefore select one element in Schaeffer’s plate (t. 150, f. 1) as the lectotype, because it most closely resemble Persoon’s description, showing two or more large, convolute, caespitose, sessile, cup-shaped apothecia with a citrine (although light) outer surface. Persoon (1822) collected the species in ‘sylvula Vincennes prope Parisios’ and Schaeffer’s plate is from Bavaria, Germany. Fries sanctioned the name, but did not see material (‘v. ic.’, seen from icones). Nevertheless, we select a newly collected epitype from Sweden (Fig. 32a, c, e, f), associated with Quercus (the habitat described by Persoon) and backed by sequences of multiple gene regions (ITS, LSU, PRB1, RPB2 and EF1) of O. concinna collections from southern Europe (Spain) and from the epitype being identical (Hansen & Olariaga 2015), acknowledging the species is widely distributed in Europe.
28. Otidea flavidobrunneola Harmaja, Karstenia 48: 38. 2009 — Fig. 33
Fig. 33.
Otidea flavidobrunneola. a. Apothecia; b. apothecium showing basal tomentum†; c. spores in water†; d. paraphyses in water†; e. ectal excipulum in water†; f. ectal excipulum in KOH†; g. basal mycelium in water† (a, c: KH.09.153; b, d–g: H6010806, holotype). — Scale bars = 10 μm; † = dried material. — Photos: b. J. Kearey.
Holotype. FINLAND, Varsinais-Suomi, Lohja, Jalassaari, Ahtiala, Alho, Ahtiala Nature Reserve, rich, predominantly deciduous (Quercus, Corylus etc.) woods on calcareous soils, 20 Sept. 1978, H. Harmaja (H6010806) !
Apothecia gregarious to caespitose, 15–70 mm high, 8–50 mm wide, initially narrowly to broadly ear-shaped, then cup-shaped, split, margin sometimes lobulate, shortly stipitate or sessile. Hymenium cream or pale yellowish (4A2, 4A3), when dried orange-ochre (5B6). Receptacle surface pale brownish ochre (5A3, 5A4), slightly hygrophanous, when dried dark reddish brown (5E5), furfuraceous to very finely warty, seldom shallowly wrinkled at the base. Warts flattened, gregarious, concolorous. Stipe not well developed. Basal tomentum and mycelium abundant, white to pale cream (4A2) when fresh, ochre (5A4) to orange-ochre (6A4) when dried. Spores narrowly ellipsoid to ellipsoid, inequilateral, with two large guttules, very rarely with a third small guttule, smooth, hyaline, 9.5–11 × (4.5–)5–6 μm (Lm = 10–10.6 μm, Wm = 5.1–5.5 μm, Qm = 1.9–2; n = 5). Paraphyses curved, a few straight or broadly hooked, of the same width or slightly broader at apices, 3–4 μm wide, without notches or slightly sinuous underside, uppermost cell 40–76 μm long, when fresh containing yellow guttules; when dried hyaline guttules or granules. Asci 127–170 × 9–10 μm. Apothecial section 900–1900 μm thick. Subhymenium c. 100 μm thick, of dense textura intricata, visible as a yellowish zone, cells cylindrical to slightly swollen. Medullary excipulum 600–1300 μm thick, of textura intricata, hyphae 5–13 μm wide, thick-walled, sometimes with a thinner outer wall, hyaline, without resinous exudates. Ectal excipulum of textura prismatica, sometimes of a textura angularis, 100–120 μm, cells thin-walled, yellowish, distinctly citrine yellow in KOH, 18–38 × 11–13 μm. Surface with broadly conical warts, 40–70 μm high, formed by fasciculate, short hyphoid hairs, of 3–4 ovoid to elongated cells, constricted at septa, 5–9 μm wide, sometimes with a gelatinous sheath. Non-warted parts with 2–3-celled hyphoid hairs, with claviform uppermost cell, more rarely cylindrical, 33–55 × 8–13 μm. Resinous exudates abundant, yellowish brown, partially dissolving and turning slightly reddish in MLZ, partly dissolving, bright yellow and appearing gelatinous in KOH. Basal mycelium of 4–6 μm wide, hyaline to very pale yellow hyphae, with scattered swollen septa, with rounded to rod-shaped, yellow resinous exudates, slowly dissolving in KOH, quickly in MLZ.
Specimens examined. FINLAND, Uusimaa, Nurmijärvi, parish centre, under Quercus, 16 Sept. 1987, P. Askola 2220 (H); Uusimaa, Nurmijärvi, parish centre, clayey soil under Quercus, 11 Aug. 1988, P. Askola 2334 (H); Uusimaa, Nurmijärvi, parish centre, the margin of the park by the vicarage, under Quercus, 18 Aug. 1988, P. Askola 2360 (H). – NORWAY, Nord-Trøndelag, Leksvik, Gjøråsvika, on rich ground, on slope under Corylus and Picea, 3 Sept. 2009, K. Hansen & I. Olariaga, KH.09.153 (S). – SWEDEN, Uppland, Bondkyrka, Vårdsätra naturpark, 17 Aug. 1927, H. Svensson (UPS F-146554); Uppland, Uppsala, in front of the prison, on bare ground under deciduous trees, 14 Sept. 1938, R. Gustafsson (UPS F-146718).
Notes — Otidea flavidobrunneola is macroscopically characterised by cup-shaped, split apothecia, especially with age very pale yellowish brown outside and cream-white to pale yellow hymenium. Microscopically, the small spores and the yellow ectal excipulum turning brighter yellow in KOH are diagnostic. The apothecia and basal tomentum become characteristically darker upon drying: the outside turns dark brown, the hymenium and the basal tomentum orange ochre (Fig. 33b). Otidea caeruleopruinosa and O. flavidobrunneola are morphologically very similar, but differ in the spore sizes (Lm = 11.2–11.8 μm vs 10.1–10.6 μm) and colours of the basal tomentum in dried specimens. According to Harmaja (2009a), O. flavidobrunneola is reminiscent of O. bufonia, but the latter has a darker brown basal tomentum and larger, narrowly fusoid spores. We report here the first finds outside Finland.
29. Otidea lactea J.Z. Cao & L. Fan in Cao et al., Mycologia 82: 735. 1990
Holotype. CHINA, Heilongjiang province, Yichun City, on ground (rotten wood?) under broadleaf trees, 6 Sept. 1987, J.Z. Cao (HMAS 61359, ex-MHSU 1803).
Notes — We were not able to get the material of O. lactea on loan, but it appears to be a distinct species based on the entire, cup-shaped, cream white apothecia and paraphyses with broadly clavate to subglobose apices (Cao et al. 1990). Also, in our LSU phylogeny (including a GenBank sequence of the holotype, DQ443447 from Liu & Zhuang (2006)), O. lactea forms a distinct sister lineage (ML 71 %, PP 99 %) to a clade of seven collections of O. minor from Europe (ML 95 %, PP 98 %, Fig. 1). Otidea minor differs from O. lactea in the (most often) split apothecia, with a yellow outer surface and often more narrow, straight to bent, subclaviform to claviform paraphyses apices. Otidea lactea has the characteristic broad apices of the paraphyses of the O. concinna clade, which however, in O. lactea becomes hooked with age (Cao et al. 1990). The holotype was originally deposited in MHSU (Cao et al. 1990), but later transferred to HMAS (confirmed by Hong-Mei Lu, HMAS).
30. Otidea minor (Boud.) Olariaga & K. Hansen, comb. & stat. nov. — MycoBank MB808975; Fig. 34
Fig. 34.
Otidea minor (KH.10.311)*. a. Apothecia; b. apothecium showing veined outer receptacle; c. spores; d. paraphyses showing a crystallized body; e. ectal excipulum; f. ectal excipulum in Melzer’s reagent, resinous exudates mostly washed away, showing free hypoid hairs. — Scale bars = 10 μm; * = fresh material.
Basionym. Otidea cantharella var. minor Boud., Icon. Mycol. livr. 23: n°. 411. 1909 (preliminary text with ‘circulaires’).
≡ Flavoscypha cantharella var. minor (Boud.) Häffner, Rheinland Pfäl. Pilzj. 4: 36. 1994.
Lectotype designated here: Boud., Icon. Mycol. livr. 23: n°. 411, pl. 326. 1909; MycoBank MBT178086.
Misapplied names
– Otidea cantharella sensu Lundell & Nannfeldt, Fungi Exs. Suec. 1–2: 93. 1934.
– Flavoscypha cantharella sensu Dennis, Brit. Ascomyc.: pl. 8 D. 1978.
Apothecia gregarious to caespitose, 8–33 mm high, 4–41 mm wide, broadly ear-shaped or shallowly cup-shaped, often elongated on one side, normally split, often stipitate. Hymenium light ochre (3A2, 3A3) to yellowish grey (4A3), sometimes with weak rose stains or spots, when dried yellowish ochre (4A4, 4A5) to ochre (5A4). Receptacle surface citrine yellow (2A6, 2A7), slightly hygrophanous, in drying slightly paler, fading to brownish ochre (4B5, 4B6) in age, when dried reddish ochre (6B6–6D6) to reddish brown (5B4, 5B5), slightly furfuraceous to slightly warty, some apothecia with low ridges coming from the stipe, restricted to the base or reaching ½– ⅓ of the apothecium, sometimes interconnected by low veins. Warts minute, conical, concolorous to brownish. Stipe often well developed, cylindrical, 2–12 × 2–5 mm. Basal tomentum and mycelium abundant, white, seldom with purplish tones, very pale yellow (4A2) when dried. Spores narrowly ellipsoid, often inequilateral, with two large guttules, smooth, hyaline, (10–)10.5–12.5(–13) × (4.5–)5–5.5(–6.2) μm (Lm = 10.7–12.3 μm, Wm = 5–5.6 μm, Qm = 2.1–2.3; n = 10). Paraphyses straight to bent, seldom curved, subclaviform to claviform at apices, 3–8 μm wide, without notches, uppermost cell 20–71 μm long, when fresh with homogeneous content or seldom containing slightly refractive, light yellow granules at apices, often with a yellow refractive body (Fig. 34d); when dried small, slightly refractive, hyaline granules. Asci 153–169 × 9–10.5 μm. Apothecial section 750–1000 μm thick. Subhymenium c. 100 μm thick, of dense textura intricata, hyphae 3–6 μm wide. Medullary excipulum of textura intricata, 500–700 μm thick, hyphae 4–9(–13) μm wide, sometimes slightly swollen, thin to thick-walled, hyaline to very pale yellow, without resinous exudates. Ectal excipulum of textura prismatica-angularis, 80–150 μm, cells thin-walled, very pale yellow, unchanged in KOH, 10–33 × 6–15 μm. Surface with low flattened warts, 35–53 μm high, formed by short, fasciculate, hyphoid hairs, of 2–3 ovoid cells, constricted at septa, 6–9 μm wide. Non-warted parts with 2–4-celled hyphoid hairs, with subclaviform to claviform uppermost cell, 43–58 × 7.5–13 μm. Resinous exudates abundant, yellow or reddish to brownish yellow, dissolving into amber drops in MLZ. Basal mycelium of 3–7 μm wide, very pale yellow, slightly swollen hyphae, not changing in KOH, with very scattered amorphous or rod-shaped, yellow resinous exudates, dissolving in MLZ.
Specimens examined. DENMARK, Jylland, Nystrup Plantage, Kridtstien, on calcareous soil under Abies, 1 Nov. 2007, T. Læssøe, TL-13332 (C); N Jylland, Rold Skov, Buderupholm Bjergskov, by fence of Cypripedium, calcareous soil along roadside, deciduous forest, 14 Sept. 1998, K. Hansen, KH.98.84 (C); Jylland, Vorsø, under Salix caprea, 25 Sept. 1981, T. Læssøe, TL-0684 (C); ibid., on base of Salix caprea, 8 Sept. 1982, T. Læssøe, TL-0754 (C); Sjælland, Geelskov, 10 km N of Copenhagen, 3 Aug. 1950, M. Lange (C-F-47967). – FINLAND, Helsinki, Toukola, Koreankatu, Acer, Betula, Populus tremula, Salix caprea, Sambucus, Sorbus, Aegopodium podagraria, Urtica dioica, 8 Sept. 1992, R. Saarenoksa 24592 (H); Varsinais-Suomi, Lohja, Pähkinäniemi, very rich, somewhat dry grass-herb forest with calcareous ground, 1 Aug. 1997, U. Nummela-Salo & P. Salo 4051 (H); Varsinais-Suomi, Lohja, Virkkala, NE-slope of Pähkinäniemi, very rich, somewhat dry grass-herb forest with calcareous soil, nearby Corylus avellana, Populus tremula, Betula pendula, 12 Sept. 2006, U. Nummela-Salo & P. Salo 10724 (H). – ITALY, Calabria, Acri (CS), Croce di Greca, 14 Sept. 1995, C. Lavorato, CL 950914-01 (dupl. S). – SWEDEN, Gotland, Fårö, Avanäset, under Pinus sylvestris, on humus, sandy soil, 27 Sept. 2011, J.C. Zamora, KH.11.103 (S); Gotland, Lojsta hed, Russpark, grazed forest with Pinus sylvestris, on calcareous ground, 2 Oct. 2010, K. Hansen, K. Gillen & I. Olariaga, KH.10.311 (S); Småland, Stenbrohult, Stockanäs SSV of Stenbrohults kyrka, under Pyrus, Salix and Prunus domestica, 9 Aug. 2011, G. Aronsson (UPS F-548414); Södermanland, Södertalje, Mörkö, Oaxen, in rich soil in deciduous forest, Corylus avellana, Salix caprea, with Lactarius citriolens, 10 Sept. 1994, P. Höljer (H7003652); Uppland, Uppsala, the park in front of the prison, on bare soil, among needles etc. under Abies, 16 Aug. 1932, S. Lundell (S-F108335, Fungi Exs. Suec. 93); Öland, Högby, Horns kungsgårds Nature Reserve, under Corylus, 6 Aug. 2000, T. Knutsson, TK2000-057 (S).
Notes — Otidea minor is recognised macroscopically by apothecia with a yellow outside, and shallow ribs and veins at the base of at least some apothecia. Otidea concinna and O. oregonensis can be distinguished by the broader spores with a lower Qm (Fig. 29). In fresh material of O. minor, some paraphyses had a strikingly, yellowish refractive body in the upper part, a so far unique feature within Otidea. To assess its taxonomic value, this feature has to be checked in additional fresh material and in closely related species.
Otidea minor has been confused with O. phlebophora due to the presence of ribs or veins at the base of the apothecia (Lundell & Nannfeldt 1934, Dennis 1978). Harmaja (2009a) proposed the provisional name O. subconcinna for O. minor as circumscribed here. After examining two collections annotated by him, we consider the Finnish material to be conspecific with our Swedish finds, based on both morphological and molecular data. Although Harmaja (2009a) did not directly compare O. minor (as O. subconcinna) and O. phlebophora, he stated in the key that O. phlebophora has ‘other tinges of yellow’ and mostly straight paraphyses. We could not confirm these differences in the material we examined. Instead, O. minor can be distinguished from O. phlebophora by the presence of at least some apothecia without ribs, and ribs when present shallower and less anastomosing, as well as normally split apothecia. In spite of their morphological similarity, O. minor and O. phlebophora are not sister species in our four-gene phylogeny, but both are deeply nested within the O. concinna clade (Hansen & Olariaga 2015; see also Fig. 3). Boudier′s plate under Otidea cantharella var. minor shows shallow ribs at the base of some apothecia, agreeing with our material, and as considered by Harmaja (2009a). No original material could be located in PC, and we therefore designate Boudier’s plate as the lectotype.
31. Otidea oregonensis K. Hansen & Olariaga, sp. nov. — MycoBank MB808973; ITS barcode GenBank KM010048; Fig. 35
Fig. 35.
Otidea oregonensis. a. Apothecia; b. apothecia showing faded colour in the outer receptacle; c. spores in water†; d. paraphyses in water†; e. ectal excipulum in water†; f. ectal excipulum in KOH† (a, c–f: Moorefun 58, holotype; b: rh139). — Scale bars = 10 μm; † = dried material. — Photos: a. J. Moore; b. R. Helliwell.
Etymology. Named after Oregon, the area where most of the specimens of this species have been collected.
Holotype. USA, Oregon, Douglas Co., Umpqua National Forest, Diamond Lake Ranger District, under Pseudotsuga menziesii and Abies concolor, 7 Nov. 2010, J. Moore, Moorefun 58 (OSC). Isotype (S).
Apothecia gregarious to caespitose, 12–48 mm high, 23–80 mm wide, shallowly to deeply cup-shaped, sometimes elongated on one side or obconical, split, seldom entire, sometimes stipitate. Hymenium greyish white (2A2) to pale ochre (4A2–4A4), sometimes with rose stains or spots, when dried pale ochre (4A2–4A4). Receptacle surface bright citrine yellow (2A6–2A8), slightly hygrophanous, fading to brownish ochre (4B5, 4B6) in age, when dried brownish ochre (4A4, 4B4, 5B4), furfuraceous, sometimes some apothecia wrinkled-veined at the base, seldom with short ribs reaching up ⅓ to the margin, partly covered by white tomentum from the base. Warts sometimes present toward the base, minute, flat, concolorous. Stipe sometimes well developed, cylindrical, 3–18 × 3–11 mm. Smell mild. Basal tomentum and mycelium abundant, white, when dried very pale yellow (4A2). Spores narrowly ellipsoid to ellipsoid, sometimes inequilateral, with two large guttules, rarely with a few small granules, smooth, hyaline, 10–11.5 × 5.5–6(–6.5) μm (Lm = 10.4–11.3 μm, Wm = 5.6–5.9 μm, Qm = 1.8–2; n = 7). Paraphyses bent to curved, sometimes straight, subclaviform to capitate at apices, 3–6(–8) μm wide, without notches, uppermost cell 28–70 μm long, when dried containing small, weakly refractive, hyaline granules. Asci 171–203 × 9–10.5 μm. Apothecial section 1000–1400(–2000) μm thick. Subhymenium 100–140 μm thick, of dense textura intricata, hyphae 2–4.5 μm wide, with scattered swollen, up to 12 μm wide cells. Medullary excipulum 650–850(–1400) μm thick, of textura intricata, hyphae 5–9(–17) μm wide, sometimes swollen, thin- to thick-walled, hyaline, without resinous exudates. Ectal excipulum of textura prismatica to textura angularis, 70–100 μm thick, cells thin-walled, very pale yellow, brighter yellow in KOH, 16–37 × 8–14 μm. Surface with low warts, 35–70 μm high, formed by short, fasciculate hyphoid hairs, of 2–4 ovoid cells, constricted at septa, 5–9 μm wide. Non-warted parts with 2–4-celled, hyphoid hairs, with claviform uppermost cell, 33–55 × 7–12 μm. Resinous exudates often abundant, yellow, dissolving into amber drops in MLZ, brighter yellow in KOH. Basal mycelium of 3–5(–8) μm wide, sometimes slightly swollen, very pale yellow hyphae, not changing in KOH, with very scattered, spheroid to rod-shaped, yellow resinous exudates, dissolving in MLZ.
Specimens examined. USA, Oregon, Jackson Co., Rogue River National Forest, under Pseudotsuga menziesii, Abies concolor, Pinus ponderosa, 2 Dec. 1999, B. Schroeter (OSC 72950); ibid., 27 Oct. 1990, D. McKay, NSW6354 (OSC 132740, dupl. S); Oregon, Josephine Co., Bureau of Land Management, Medford District, Grants Pass Resource Area, Little Pickett Creek, under Pseudotsuga menziesii, Calocedrus decurrens, Lithocarpus densiflorus, Quercus chrysolepis, 19 Dec. 2000, R. Meyer (OSC 108041); Oregon, Marion Co., near Breitenbush Hot Springs Community, 27 Oct. 1996, J. Trappe (OSC 56745); Oregon, Umpqua NF, Diamond Lake RD, under Pseudotsuga menziesii, 2 Nov. 2010, J. Moore, Moorefun 31 (S); ibid., old growth forest with Abies concolor, Pseudotsuga menziesii, 2 Nov. 2010, R. Helliwell, rh139 (OSC); Washington, Lewis Co., Gifford Pinchot National Forest, Camp Creeks Falls Trail, 6 Nov. 1997, M. Castellano (OSC 56829).
Notes — Otidea oregonensis is characterised by a citrine yellow receptacle surface, often a wrinkled to veined, or shallowly ribbed apothecial base, and relatively broad spores. The apothecia are typically obconical cup-shaped, with a narrow base. Otidea oregonensis was treated as O. rainierensis Kanouse by Peterson (1998), primarily based on the presence of swollen apices of the paraphyses. Also, one of the paratypes of O. rainierensis (EGS2179), sequenced by Peterson (1998), is nested within the O. oregonensis lineage in our ITS-LSU phylogeny (Fig. 3). Nevertheless, our sequences of the holotype of O. rainieriensis, from four different gene regions, show it belongs to another lineage, well separated from O. oregonensis (Hansen & Olariaga 2015). Kanouse′s concept of O. rainierensis was therefore mixed. The ITS-LSU phylogeny, including a larger sampling of the O. concinna clade, likewise resolves O. oregonensis as a distinct species.
Otidea phlebophora and O. minor, so far only known from Europe, resemble O. oregonensis in the cup-shaped apothecia with yellow outside and swollen paraphyses. Otidea phlebophora differs macroscopically from O. oregonensis in predominantly entire apothecia, with always a strongly veined-ribbed base reaching up ½ to the margin, while only small veins are present in some apothecia of O. oregonensis. Based on the material examined here, the slightly narrower spores in O. phlebophora (Qm = 2–2.1 vs Qm = 1.8–2 in O. oregonensis) are a constant and reliable character to separate these species (Fig. 29). Otidea minor tends to have smaller, shallower and more broadly cup-shaped apothecia than O. oregonensis. The spores of O. minor have a higher Qm value (2.1–2.3) that does not overlap with the Qm of O. oregonensis.
32. Otidea phlebophora (Berk. & Broome) Sacc., Syll. Fung. 8: 97. 1889 — Fig. 36
Fig. 36.
Otidea phlebophora (JV06-385). a. Apothecia; b. spores in water†; c. paraphyses in water†; d. ectal excipulum in water†. — Scale bars = 10 μm; † = dried material. — Photos: a. J. Vesterholt.
Basionym. Peziza phlebophora Berk. & Broome, Ann. Mag. Nat. Hist., ser. III, 18: 122. 1866.
≡ Flavoscypha phlebophora (Berk. & Broome) Harmaja, Karstenia 14: 107. 1974.
Lectotype designated here: ENGLAND, North Somerset, Brislington, 16 Sept. 1853 (K(M) 144045, ex Herb. C.E. Broome) !; MycoBank MBT178087.
Apothecia gregarious, 8–12 mm high, 3–40 mm wide, shallowly to deeply cup-shaped, sometimes elongated on one side, entire, seldom split, often stipitate. Hymenium pale ochre (4A2, 4A3), sometimes with rose stains or spots, when dried yellowish ochre (4B4, 5B6, 5C6) to reddish brown (6C5, 6C6). Receptacle surface bright citrine yellow (3A7, 2A7), sometimes with a greenish hue (2A6), slightly hygrophanous, fading to brownish ochre (4B5) in age, when dried yellowish brown (5B6, 5C6) to reddish brown (6C6, 6D6), furfuraceous, with high ridges in the basal part of all apothecia reaching up ½– ⅓ to the margin, interconnected by veins, partly covered by white basal tomentum. Warts minute, flat to rounded, appressed, concolorous. Stipe often well developed, cylindrical, 1–10 × 1.5–3 mm. Basal tomentum and mycelium abundant, white, very pale ochre (5A2) when dried. Spores narrowly ellipsoid, sometimes inequilateral, with two large guttules, smooth, hyaline, (9.5–)10–11.5(–12) × (4.5–)5–5.5(–6.2) μm (Lm = 10.2–11 μm, Wm = 4.9–5.1 μm, Qm = 2–2.1; n = 5). Paraphyses straight to bent, cylindrical to claviform at apices, 2–3.5 μm wide, without notches, uppermost cell 23–46 μm long, when dried containing small, rather refractive, hyaline guttules. Asci 130–153 × 8–9 μm. Apothecial section 650–1000 μm thick. Subhymenium 80–100 μm thick, of dense textura intricata, hyphae 2–3 μm wide. Medullary excipulum of textura intricata, 450–650 μm thick, hyphae 4–7(–14) μm wide, sometimes swollen, thick-walled, hyaline to very pale yellow, without resinous exudates. Ectal excipulum of textura prismatica-angularis, 80–100 μm thick, cells thin-walled, hyaline to very pale yellow, unchanged in KOH, 15–21 × 6–11 μm. Surface with low flattened warts, 40–60 μm high, formed by short, fasciculate hyphoid hairs, of 3–4 ovoid cells, constricted at septa, 8–12 μm wide. Non-warted parts with 2–3-celled hyphoid hairs, with claviform uppermost cell, 30–60 × 7–13 μm. Resinous exudates abundant, yellow, dissolving into amber drops in MLZ. Basal mycelium of 3.5–7 μm wide, slightly swollen, very pale yellow hyphae, not changing in KOH, with very scattered, spheroid to rod-shaped, yellow resinous exudates, dissolving in MLZ.
Specimens examined. DENMARK, NE Jylland, Rubjerg Knude Plantage, under Abies in large fairy ring, 28 Aug. 2006, J. Vesterholt & L. Vesterholt, JV06-385 (C); NE Sjælland, Ravnsholts Hegn, under Picea abies, 30 Aug. 1999, B.W. Pedersen (C-F-71506). – FINLAND, Varsinais-Suomi, Lohja, Jalassaari, Alho, by the Ahtiala manor, in rich forest with Quercus robur, Corylus avellana, Prunus padus, cultivated Larix sibirica and Betula, 30 Aug. 1967, H. Harmaja (H6010675). – SWEDEN, Dalarna, Husby, Husby värdhus, on lawn under Quercus, Tilia, Larix and Acer, 31 Aug. 1958, R. Morander (UPS F-144691); Skåne, Helsinborg, Jordbodalen by Harlyckan, on sandy ground in deciduous forest under Quercus, 13 July 1995, S.-Å Hanson, SÅH 30601 (C); Uppland, Djurö, Runmarö, Södersunda, in the city, on the ground under Syringa, 18 Sept. 1949, G. Haglund & R. Rydberg (S-F108338, UPS F-144689); Uppland, Uppsala, Sunnersta, Almlund, calcareous, humus-rich clay, 23 Aug. 1986, J. Nitare (UPS F-119845). – UK, Northampshire, King′s Cliffe, on soil, 1853, M.J. Berkeley (K(M) 144046, syntype of P. phlebophora); North Somerset, Brislington, The Beeches, Sept. 1853, C.E. Broome (K(M) 194582, syntype of P. phlebophora).
Other specimen examined. Otidea integra — ITALY, Sopramonte, 1892, G. Bresadola (S-F108342). Locality not specified, in silvis mixtis, 1892, G. Bre-sadola (PC 124965).
Notes — Otidea phlebophora is primarily characterised by high anastomosing ribs and veins towards the apothecial base. Diagnostic characters are in addition, the predominantly entire apothecia, with a citrine yellow outside. For a comparison with O. concinna, O. minor and O. oregonensis see Notes under those species. Harmaja (1986) elevated O. integra to species rank based on smaller apothecia and broader, curved paraphyses. The likely original specimen of O. integra collected in 1892 and kept in Bresadola’s herbarium (S-F108342), shows curved paraphyses up to 5 μm broad, the same as Harmaja (1986, 2009a) gave for O. phlebophora. The paraphyses in the O. phlebophora material examined by us were straight to bent, and otherwise similar to the original material of O. integra. In spite of the morphological similarity, the ITS2 sequence of O. integra (281 bp obtained) is different from the ITS sequences of the material assigned to O. phlebophora by us, and we preliminary accept O. integra as a separate species. The position of O. integra is without support in our ITS-LSU phylogeny (Fig. 3).
Nomenclatural notes — Harmaja (1974: 107) indicated a lectotype of O. phlebophora at K, but he gave no collection number and the typification was not achieved. We have studied three of four syntypes at Kew and select here the richest collection containing ten apothecia as the lectotype. It conforms to the current interpretation of the name, with several entire apothecia (i.e. without a split), a ribbed-veined base seen on two of the apothecia (the others with the base glued to the cardboard), spores in the range 9.5–11.7 × 4.7–5.9 μm (Lm = 10.6 μm, Wm = 5.1 μm), paraphyses straight and enlarged at apices, and abundant yellow resinous exudates in the ectal excipulum that dissolve into amber drops in MLZ.
33. Otidea rainierensis Kanouse, Mycologia 41: 674. 1949
Holotype. USA, Washington, Pierce County, Lower Tahoma Creek, Mt Rainier National Park, 23 Aug. 1948, A.H. Smith 30553 (MICH 14410) !
= Otidea kauffmanii Kanouse, Mycologia 41, 6: 673. 1949.
Holotype. USA, Michigan, Lakeland, 18 July 1915, C.H. Kauffman (MICH 14409) !
Kanouse (1949) only had dried material of O. rainierensis and described the outside of the apothecia as ‘ochraceous buff’, ‘cinnamon buff’ to ‘wood brown’ and the hymenium as ‘avellaneous’, ‘vinaceous buff’ to ‘drab grey’. For O. kauffmanii she had notes on fresh material and she separated it from O. rainierensis based on the presence of yellow tones in the apothecia (outside ‘chamois’ to ‘ochraceous’, hymenium ‘cream buff’). We believe O. rainierensis does have yellow apothecial tones, as we observed small yellow resinous exudates (in water) in the type collection of O. rainierensis, and as observed in closely allied species the yellow colour can fade and almost disappear with age (Fig. 32b). Different spore sizes were also used to distinguish the two species. The spores of the holotype of O. kauffmanii are however, larger than noted in the protologue, 11.5–12.5 × 5.5–6.5 μm, Lm = 11.9 μm, Wm = 6 μm, Qm = 2 (spores 8–10(–12) × 5–6(–7) μm in the protologue), and thus overlapping with the spores of the holotype of O. rainierensis, 11–12 × 6.5–7 μm, Lm = 11.7, Wm = 6.7 μm, Qm = 1.7. We suggest O. kauffmanii and O. rainierensis constitute a single species, based on molecular and morphological study of the type material (Fig. 3). Our ITS sequences of the holotypes differ by 4 bp. See also comments on O. microspora under Excluded, dubious and imperfectly known taxa. Otidea rainierensis is characterised by a smooth apothecial base, long and relatively broad spores, compared to the rest of the species in the O. con-cinna clade, and by paraphyses with abruptly enlarged, broadly clavate to globose apices.
EXCLUDED, DUBIOUS AND IMPERFECTLY KNOWN TAXA
Cochlearia (Cooke) Lambotte, Mém. Soc. Roy. Sci. Liege, ser. 2. 14: 323. 1888
Nom. illegit. Art. 53.1, non Cochlearia L., Sp. Pl. 2: 647. 1753 (Cruciferae).
Basionym. Peziza subg. Cochlearia Cooke, Mycographia part 6: 252 (‘Index Systematicus’). 1879.
Notes — Eckblad (1968) selected Peziza cochleata as the type species for the genus Cochlearia considering it a synonym of Otidea. However, Rifai (1968) had already selected Peziza aurantia as the type species, herewith making Cochlearia a synonym of Aleuria. He felt too much doubt had surrounded the identity of P. cochleata and it better not be selected as the type species.
Otidea abietina (Pers.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 330. 1870 ‘1869–1870’
Basionym. Peziza abietina Pers., Neues Mag. Bot. 1: 113. 1794: Fr., Syst. Mycol. 2: 47. 1822.
≡ Discina abietina (Pers.) Rehm, Rabenh. Krypt.-Fl., ed. 2, 3: 977. 1896.
≡ Pseudotis abietina (Pers.) Boud., Icon. Mycol. list prél. 600 sp.: 3 (unnumbered page). 1904.
Notes — Nannfeldt (1966) stated that the original material belongs to Peziza badia, but Harmaja (2009a) and Carbone (2010c) reported two collections in Persoon’s herbarium under O. abietina representing O. propinquata and a third collection O. bufonia. If typified with elements belonging to either O. propinquata or O. bufonia, the name O. abietina would take priority over any of those. Otidea abietina is the type species of the genus Pseudotis (Boud.) Boud. The identity of Pseudotis will thus remain open until O. abietina is clarified or typified. Another way to typify O. abietina would be to select an element belonging to Peziza badia. However, this choice should be studied more thoroughly, since it would make Pseudotis available as a genus name for the Peziza depressa-Ruhlandiella lineage, if Peziza is split into smaller genera in the future (Hansen et al. 2005). For the time being we regard O. abietina as a nomen ambiguum, as several others (Harmaja 2009a, Carbone 2010c, Parslow & Spooner 2013).
Otidea alba Velen., Monograph. Discom. Bohemiae 1: 354. 1934
Holotype. CZECH REPUBLIC, Karlštejn, Sept. 1924, Fechtner (PRM 149788) !
Notes — The holotype specimen has the typical oblong spores of the O. alutacea complex, (13.5–)14.5–16.5(–17.5) × 6.5–7.5 μm (Lm = 14.9 μm, Wm = 6.9, Qm = 2.1). Judging from the spore size, O. alba might represent either O. alutacea s.str. or the O. alutacea clade 3b. It should be considered in future studies of the complex.
Otidea aurantia (Pers.) Massee, Brit. Fungus-Fl. 4: 448. 1895
Basionym. Peziza aurantia Pers., Observ. Mycol. 2: 76. 1800: Fr., Syst. Mycol. 2: 49. 1822.
Notes — This is the type species of Aleuria, A. aurantia (Pers.: Fr.) Fuckel.
Otidea aurantia var. atromarginata (W. Phillips & Plowr.) Massee, Brit. Fungus-Fl. 4: 449. 1895
Basionym. Peziza aurantia var. atromarginata W. Phillips & Plowr., Gard. Chron. 17: 191. 1882.
Notes — The blood red disc with short, obtuse, dark brown, 3–4 septate hairs, giving the margin a dark appearance suggest this may be a species of Melastiza. The spores are tuberculate with thread-like appendages.
Otidea aurantia var. stipitata (W. Phillips) Massee, Brit. Fungus-Fl. 4: 448. 1895
Basionym. Peziza aurantia var. stipitata W. Phillips, Man. Brit. Discom.: 57. 1887.
Notes — The small, bright scarlet apothecia with a ‘stem equalling the height of the cup, 4 mm’ and the ornamented spores suggest this is a species of Aleuria or Sowerbyella. It was described as a variety of Aleuria aurantia (as Peziza aurantia Oed.). Ramsbottom (1914) cited O. aurantia var. stipitata as a synonym of Sowerbyella rhenana (as Aleuria rhenana Fuckel), but the type material is presumably lost (Spooner & Yao 1995) and no modern interpretation can be provided.
Otidea auriculariiformis Henn., Hedwigia 36: 232. 1897
Holotype. BRAZIL, A. Glaziou no. 20181 (S-F9965, ex Herb. Sydow) !
Notes — This species belongs to the genus Phillipsia, Sarcoscyphaceae. The large spores, (30.5–)31.5–36.5(–37) × 12.5–14.0 μm (Lm = 33.2 μm, Wm = 13.1 μm, from 13 spores), are ellipsoid, inequilateral in profile view, smooth or with faint cyanophobic, parallel, longitudinal ridges. Asci seem thick-walled, with an internal eccentric thickened apical pad. The medullary excipulum is of interwoven hyphae, running mostly parallel with the outer surface and the ectal excipulum is a narrow band of textura prismatica, with the long axes of the cells parallel to the exterior. The tropical distribution and the substrate, suggested in the diagnosis to be wood, are typical for Phillipsia.
Otidea cinerascens Velen., Novit. Mycol.: 152 .1947
Holotype. CZECH REPUBLIC, Moravia, Žarošice, Aug. 1940, V. Vacek (PRM 151779).
Notes — The grey-ochraceous apothecia and spores with parallel sides suggest O. cinerascens belongs to the O. alutacea complex. Type not studied by us, but annotated in 2009 by B. Spooner as O. alutacea (a photograph of the collection and annotation provided by Jan Holec, PRM).
Otidea cochleata (L.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 329. 1870. ‘1869–1870’
Basionym. Peziza cochleata L., Sp. Pl. 4: 183. 1753: Fr., Syst. Mycol., Index: 129. 1832.
≡ Cochlearia cochleata (L.) Lambotte, Fl. Mycol. Belgique 1: 323. 1880.
Notes — The interpretation of the original description is difficult, but O. cochleata has been treated as a taxon in the O. alutacea group (e.g. Lundell & Nannfeldt 1938, Dissing 2000, Mornand & Courtecuisse 2005, Zhuang 2006), probably following Bulliard (1791: plate 154 as Peziza cochleata). Part of the Bulliard plate (f. b) has now been selected as the lectotype for O. alutacea (Carbone 2010a). The name O. cochleata should be considered in future revisions of the O. alutacea complex. For a review of the nomenclatural history of O. cochleata see Carbone (2010a) and Parslow & Spooner (2013).
Otidea darjeelensis (Berk.) Sacc., Syll. Fung. 10: 4. 1892
Basionym. Peziza darjeelensis Berk., Hooker’s J. Bot. Kew Gard. Misc. 3: 202. 1851.
Notes — Rifai (1968) stated that the type specimen of P. darjeelensis at Kew has iodine positive asci and echinulate spores, and does not belong to Otidea. Two collections of P. darjeelensis are present in Kew that may represent original material: INDIA, Sikkim, J.D. Hooker (K(M) 177412, ex Herb. Berkeley); and INDIA, Sikkim (K(M) 177413, ex Herb. Cooke). No annotation label by Rifai was found (B. Aguirre-Hudson, pers. comm. ), but he most likely studied the collection from Berkeley’s herbarium, because he listed J.D. Hooker as the collector. The collection K(M) 177413 might be a part of K(M) 177412, because Cooke (1876, f. 215) illustrated P. darjeelensis from specimens in Berkeley’s herbarium. Both collections should be studied and a lectotype selected.
Otidea dochmia (Berk. & M.A. Curtis) Sacc., Syll. Fung. 8: 95. 1889
Basionym. Peziza dochmia Berk. & M.A. Curtis, J. Linn. Soc. Bot. 10: 364. 1869.
≡ Phillipsia dochmia (Berk. & M.A. Curtis) Seaver, N. Amer. Cup-fungi, Operc.: 184. 1928.
≡ Aurophora dochmia (Berk. & M.A. Curtis) Rifai, Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk., sect. 2, 57: 52. 1968.
Notes — This is the type species of the genus Aurophora. Rifai (1968) distinguished Aurophora from Phillipsia by its fan-shaped apothecia and the presence of a gelatinous matrix in the medullary excipulum.
Otidea domingensis (Berk.) Sacc., Syll. Fung. 8: 97. 1889
Basionym. Peziza domingensis Berk., Ann. Mag. Nat. Hist., ser. II, 9: 201. 1852.
Notes — This is the type species of the genus Phillipsia, P. domingensis (Berk.) Berk. (see Hansen et al. 1999).
Otidea doratophora (Ellis & Everh.) Sacc., Syll. Fung. 8: 96. 1888
Basionym. Peziza doratophora Ellis & Everh., J. Mycol. 1: 90. 1885.
Notes — The small spores and asci, along with the pointed paraphyses, suggest this taxon does not belong to Otidea. Cash (1953) proposed O. doratophora is a synonym of Ionomidotis irregularis (Schwein.) E.J. Durand (as Midotis irregularis).
Otidea euplecta (Cooke) Sacc., Syll. Fung. 8: 97. 1889
Basionym. Peziza euplecta Cooke, Mycographia part 3: 125. 1876.
Holotype. USA, Alabama, Peters 4560 (K(M) 161851, ex Herb. Berkeley as Peziza phlebophora var.) !
Notes — The type is in a poor condition: one apothecium immature; the other infected. But the species likely belongs to Sarcoscypha, Sarcoscyphaceae. The asci are inamyloid, thick-walled and with an eccentrically placed, thickened operculum, spores are ellipsoid, slightly inequilateral, 19–21 × 10.5–11 μm, smooth, and paraphyses straight, filiform, branching above. The excipulum is composed of interwoven hyphae that give rise on the outside to shallow pustules.
Otidea felina (Pers.) Bres., Fungi Trident. ser. 2, fasc. 14: 103. 1900
Basionym. Peziza felina Pers., Mycol. Eur. 1: 223. 1822.
Holotype. FRANCE, prope Pariseos, sylvula Meudon (L0116774, Herb. Persoon).
Notes — Van Vooren & Carbone (2012) revised the holotype and demonstrated that it belongs to the O. alutacea group. Parslow & Spooner (2013) considered O. felina a synonym of O. alutacea. Further studies on the O. alutacea group should consider the name O. felina, which might be epitypified for an unequivocal interpretation.
Otidea fibrillosa Massee, Brit. Fungus-Fl. 4: 449. 1895
≡ Pseudaleuria fibrillosa (Massee) J. Moravec (‘Pseudoaleuria’), Acta Mus. Morav. Sci. Biol. 88: 51. 2003.
Notes — According to Moravec (2005) this is a species of Pseudaleuria, P. fibrillosa.
Otidea grandis (Pers.) Boud., Bull. Soc. Mycol. France 9: 10. 1893
Basionym. Peziza grandis Pers., Ann. Bot. Usteri 15: 27. 1795.
≡ Peziza abietina var. grandis (Pers.) Pers., Mycol. Eur. 1: 233. 1822.
≡ Aleuria grandis (Pers.) Gillet, Champ. France Discomyc.: 42. 1879.
≡ Scodellina grandis (Pers.) Seaver, N. Amer. Cup-fung., Operc.: 186. 1928.
Notes — The original sense of O. grandis corresponds most likely to a species of the Peziza depressa-Ruhlandiella lineage (Hansen et al. 2005) due to the lack of a split. No original material of O. grandis seems to be kept in Persoon′s herbarium (L). The name O. grandis has been used for O. bufonia (Boudier 1905; specimen PC0093644 is O. bufonia) or O. unicisa (Kanouse 1949, Liu & Zhuang 2006). We regard it here as a nomen dubium and confusum in agreement with Harmaja (2009a).
Otidea grandis var. scheremetjeffii Henn., Hedwigia 42, 3: (116). 1903
Notes — Hennings (1903) described this taxon based on specimens kept in formalin and the colours provided in the protologue are probably imprecise. The spore size can fit O. bufonia or O. onotica, but there is no type material extant in B or S, and a precise interpretation cannot be proposed here.
Otidea harperiana Rehm, Ann. Mycol. 2: 34. 1904
Holotype. USA, Ohio, Blue Mountains, on ground, 6 June 1903, Harper 333 (S-F9961, ex Herb. Rehm, ‘Herb. R.A. and A.M. Harper 333’) !
Notes — This species is closely related or conspecific with Peziza phyllogena Cooke. The asci are strongly amyloid in MLZ with a general bluing over the apex and the spores are ornamented with irregular, low, separate warts that are higher and more densely placed at the poles (forming ‘pole caps’). Our spore measurements from the holotype, 18–19.5 × 8–9 μm (Lm = 18.6 μm, Wm = 8.5 μm, from 15 spores) are larger than those given in the protologue, 15–17 × 5–7 μm.
Otidea hirneoloides (Berk.) Sacc., Syll. Fung. 8: 96. 1889
Basionym. Peziza hirneoloides Berk. in Berkeley & Curtis, J. Linn. Soc. Bot. 10: 365. 1869.
≡ Phillipsia hirneoloides (Berk.) Berk., J. Linn. Soc. Bot. 18: 388. 1881.
Notes — The wood-inhabiting, ear-shaped apothecia, and especially, the cymbiform spores suggest this name is to be referred to Phillipsia. Hansen et al. (1999) suggested P. hirneoloides belongs to the Phillipsia domingensis complex.
Otidea lechria (Berk. & Broome) Sacc., Syll. Fung. 8: 97. 1889
Basionym. Peziza lechria Berk. & Broome, J. Linn. Soc. Bot. 14: 103. 1875.
Holotype. SRI LANKA, on rotten wood, Nov. 1867, G.H.K. Thwaites (K(M)161847, ex Herb. Berkeley).
Notes — B. Spooner annotated the holotype in 2008, and noted it has amyloid asci and belongs to Peziza.
Otidea lilacina R. Heim & L. Remy, Bull. Soc. Mycol. France 48: 65. 1932
Notes — The ornamented, multi-guttulate spores and straight paraphyses suggest that this taxon does not belong to Otidea. No original material could be traced in PC (B. Buyck, pers. comm.).
Otidea lobata Rodway, Pap. & Proc. Roy. Soc. Tasmania: 116. 1925 ‘1924’
Notes — Rifai (1968) stated that the type specimen appears to represent the inoperculate genus Discinella.
Otidea luculenta (Cooke) Massee, Brit. Fungus-Fl., 4: 450. 1895 (‘leuculenta’)
Basionym. Peziza luculenta Cooke, Mycographia part 3: 121. 1876.
Notes — The entire, orange apothecia and straight paraphyses with orange granules suggest this name does not belong to Otidea. Nannfeldt (1966) noted that O. luculenta has ‘other affinities’ than Otidea, but did not provide a generic placement.
Otidea luteonitens (Berk. & Broome) Massee, Brit. Fungus-Fl. 4: 449. 1895
Basionym. Peziza luteonitens Berk. & Broome, Ann. Mag. Nat. Hist., ser. II, 7: 180. 1851.
Notes — This name is currently placed in Aleuria as Aleuria luteonitens (Berk. & Broome) Gillet.
Otidea micropus (Pers.) Sacc., Syll. Fung. 8: 98. 1889
Basionym. Peziza micropus Pers., Icon. Desc. Fung. 2: 30. 1800: Fr., Syst. Mycol. 2: 54. 1822.
Notes — This name is a synonym of Peziza varia (Hedw.: Fr.) Fr. sensu Hansen et al. (2002).
Otidea microspora (Kanouse) Harmaja, Karstenia 15: 32. 1976
Basionym. Otidea alutacea var. microspora Kanouse, Mycologia 41: 668. 1949.
Notes — Kanouse (1949) described this taxon as a variety of O. alutacea. She indicated two different collections as the type; A.H. Smith 9351 after the diagnosis and A.H. Smith 17699 in the material examined. We have studied A.H. Smith 9351 (MICH 14406, dupl. UPS F-629985 !) and it has far larger spores than stated in the protologue (13–15.5 × 7–8 μm vs 9–10 × 5.5–6.5 μm). The oblong spores, an ectal excipulum of textura angularis, and the absence of yellow pigment in KOH indicate it belongs to the O. alutacea complex. As for A.H. Smith 17699 (UPS F-629996 !), the spores match the original description. The apothecial shape is similar to that of O. rainierensis. A Gen-Bank ITS sequence of a paratype of O. microspora (A.H. Smith 30502) differs only 1 bp from the ITS sequence of the holotype of O. rainierensis (Fig. 3), but we prefer not to select a lectotype until all the original material has been examined. We therefore treat the name as doubtful for the time being.
Otidea neglecta Massee, Grevillea 22: 66. 1894
Notes — This name was erected as a new name for O. auricula in the sense of Rehm (1883), Saccardo (1889) and Bresadola (1884, as Peziza). The species that these three authors treated under the epithet auricula is Wynnella silvicola (Beck) Nannf. in its current sense.
Otidea obtecta (Schwein.) Sacc., Syll. Fung. 8: 98. 1889
Basionym. Peziza obtecta Schwein., Trans. Amer. Philos. Soc. ser. 2, 4: 170. 1832 ‘1834’.
Notes — The original description gives stipitate, 1–1.5 cm wide, cinnamon-coloured apothecia with a split, growing among leaves. Seaver (1928) stated that the identity of O. obtecta is uncertain. No original material could be located in PH and the identification of this species cannot be inferred.
Otidea olivacea Bucholtz, Bull. Soc. Imp. Naturalists Moscou 2: 325. 1897
Notes — The curved, uniguttulate spores and the occurrence on a rotten trunk suggest that this taxon does not refer to a species of Otidea. The Bucholtz herbarium was bought by the FH, but no authentic material of O. olivacea could be located there.
Otidea onotica var. ochracea (Fr.) Sacc., Syll. Fung. 8: 95. 1889
Basionym. Peziza onotica var. ochracea Fr., Syst. Mycol. 2: 48. 1822: Fr. loc. cit. (‘ß ochracea’).
≡ Peziza ochracea (Fr.) P. Karst., Not. Sallsk. Fauna Fl. Fenn. Forh. 10: 110. 1869.
≡ Otidea ochracea (Fr.) Seaver, Bull. Lab. Nat. Hist. Iowa State Univ. 5: 45. 1904.
Notes — Fries (1822) stated that this variety is close to O. onotica. It was elevated to species rank by Karsten (1869), who later considered it a synonym of O. onotica (Karsten 1871). As no original material exists and the original description is meagre, we are not able to provide a good interpretation of this taxon.
Otidea pleurota (W. Phillips) Sacc., Syll. Fung. 8: 97. 1889
Basionym. Peziza pleurota W. Phillips in Cooke, Mycographia part 5: 208. 1878.
≡ Iotidea pleurota (W. Phillips) Clem., Gen. Fungi: 175. 1909.
Holotype. ENGLAND, 1877, W. Phillips (K(M)29973).
Notes — Eckblad (1968) observed strongly amyloid asci in the type and concluded it belongs to Peziza. B. Spooner annotated the type as ‘Peziza badiofusca ?’.
Otidea radiculata (Sowerby) Bres., Fungi Trident. ser. 2, fasc. 11–13: 72. 1898
Basionym. Peziza radiculata Sowerby, Col. Fig. Engl. Fung. 1: 46 (unnumbered page), t. 114. 1797: Fr., Syst. Mycol. 2: 81. 1822.
Notes — This name is placed in Sowerbyella, as S. radiculata (Sowerby) Nannf. Yao & Spooner (2006) examined the type at K and confirmed its placement.
Otidea reisneri Velen., České Houby 4–5: 872. 1922
Notes — Svrček (1976) studied the type material and concluded it is a synonym of Sowerbyella radiculata (Sowerby: Fr.) Nannf.
Otidea schulzeri Quél. in Schulzer, Hedwigia 24, 4: 150. 1885
Notes — The apothecia of O. schulzeri were described as elongated on one side, split, pale yellow-grey and pseudostipitate. However, the very thick flesh (3–4 mm), the straight paraphyses and the spore size (20–28 μm) suggest this taxon does not represent Otidea. Also Nannfeldt (1966) stated that O. schulzeri could hardly belong to Otidea.
Otidea silvicola Beck in Sacc., Syll. Fung. 8: 97. 1889
Notes — This is Wynnella silvicola. The name was created for Peziza atrofusca Beck, a later homonym for P. atrofusca Berk. & M.A. Curtis.
Otidea sparassis Quél., Rev. Mycol. (Toulouse) 54: 65. 1892
Notes — The uniguttulate spores do not suggest an Otidea. This taxon might refer to a sparassoid Helvella species.
Otidea subonotica Henn., Hedwigia 36: 232. 1897
Notes — The original description does not provide any discordant feature for Otidea, except it was reported from Brazil. No type material of O. subonotica exists in Hennings herbarium in B (Carbone 2009) or in S, and its correct placement cannot be inferred with certainty here.
Otidea succosa (Berk.) Thüm., Mycoth. Univ. 15: no. 1411. 1879
Basionym. Peziza succosa Berk., Ann. Mag. Nat. Hist., ser. I, 6: 358. 1841.
Notes — This is currently considered to be a species of Peziza s.l. (Hansen et al. 2005).
Otidea tasmanica Rodway, Pap. & Proc. Roy. Soc. Tasmania: 116. 1925 ‘1924’
Notes — Rifai (1968) studied the type specimen of O. tasmanica in Kew and synonymised it with Peziza praetervisa Bres. (sensu Dennis, as Rifai depicted ornamented spores from the type specimen). Thus, O. tasmanica might be close to, or a synonym of, Peziza subviolacea Svrček.
Otidea violacea A.L. Sm. & Ramsb., Trans. Brit. Mycol. Soc. 5: 237. 1916
Holotype. ENGLAND, Warwickshire, W.B. Grove 1915 (K(M) 30407, ex Herb. W.B. Grove).
Notes — Parslow & Spooner (2013) examined the holotype and concluded it is a species of Peziza (cf. azureoides Donadini).
Acknowledgments
This monograph became more comprehensive thanks to the great efforts of numerous mycologists who provided us with interesting material. All our colleagues who contributed collections, discussed issues or helped us in other ways are warmly thanked. Especially we thank Nancy S. Weber, Rosanne Healy, Mats Karström and Tapio Kekki for putting at our disposal valuable material accompanied by colour photographs. We also thank the curators and staff in the herbaria mentioned in the taxonomic part, H.J.M. Sipman (B), M. Herrera and I. Salcedo (BIO), H. Knudsen (C), P.G. Jamoni and D. Bolognini (GMFN), D.H. Pfister and G. Lewis-Gentry (FH), P. Salo and S. Stenroos (H), E.D. Liu (HKAS), Hong-Mei Lu (HMAS), B. Aguirre-Hudson, B. Dentinger and B. Spooner (K), R. Rabeler and P. Rogers (MICH), D. McLaughlin (MIN), A.B. Mujic and J. Spatafora (OSC), B. Buyck (PC), A. Freire-Fierro (PH), J. Holec (PRM), S. Huhtinen (TUR, TUR-A) and R. Berndt (ZH), for searching material and arranging loans. We appreciate Kerstin Gillen′s assistance during fieldwork in 2010. Juan Santos and Xiang-Hua Wang collected part of the material during their participation in the Swedish Pezizomycetes project. We are indebted to Xiang-Hua Wang for sequencing a few important Otidea collections, for discussions and help in editing the manuscript, and Seppo Huhtinen for instructive discussions on resinous exudates. We thank D.H. Hawksworth and L.A. Parra for nomenclatural advice. Donald H. Pfister and Trond Schumacher reviewed the manuscript and we are grateful for their valuable comments and corrections. Funding for this research was provided by a grant from the Swedish Taxonomy Initiative to K.H. (grant no. 143/07 1.4).
Footnotes
Description based only on specimens of O. alutacea s.str.
INDEX to species, varieties and forms
Accepted names treated in the monograph are in bolditalic.
abietina 166, 167, 193, 195, 197, 224
abietina var. grandis 225
abietina f. / var. nigra 195
alba 185, 224
alutacea 175–177, 179, 181, 182, 224, 225
alutacea var. microspora 226
angusta 176, 198, 200
apophysata 167, 168, 178, 179, 180–182
atrofusca 226
aurantia 223, 224
aurantia var. atromarginata 224
aurantia var. stipitata 224
auricula 167, 185, 187, 226
auriculariiformis 224
azureoides 227
badiofusca 226
bicolor 178, 211
borealis 168, 171, 174, 176–178, 212, 213
boudieri 180
brevispora 174, 179, 205
brunneoparva 168, 179, 187, 191
bufonia 175, 176, 179, 185, 192, 205, 206, 209, 211, 213, 219, 224, 225
caeruleopruinosa 168, 174, 177, 179, 213,
215, 219
caligata 189, 193, 195, 197
cantharella 167, 178, 189, 191, 192, 193, 197, 216, 219
cantharella var. minor 188, 189, 219, 220
cinerascens 185, 224
cochleata 166, 167, 180, 183–185, 195, 206, 207, 223, 224
cochleata var. alutacea 182
cochleata var. umbrina 206, 175
concinna 167, 168, 174, 175, 177, 178, 189,210, 213, 216, 219, 223
crassa 174, 185, 187
daliensis 167, 168, 178–180, 181, 192, 197
darjeelensis 224, 225
dochmia 224
domingensis 225
doratophora 225
euplecta 225
felina 179, 180, 185, 187, 225
fibrillosa 225
flavidobrunneola 177, 179, 213, 216, 217
formicarum 168, 171, 174, 176, 177, 179, 189,197, 200, 201
fuckelii 185, 187
fusconigra 192, 205
grandis 205, 207, 208, 209, 225
grandis var. scheremetjeffii 225
harperiana 225
hirneoloides 225
indivisa 195–197
integra 168, 174, 178, 223
kauffmanii 171, 175, 213, 214, 223
kaushalii 174, 176, 177, 178, 202, 205
kunmingensis 178, 184, 185
lactea 174, 178, 179, 213, 219
lechria 225
leporina 166, 167, 171, 174, 175, 177, 179, 185, 188, 189, 192, 209–211
leporina f. major 185
leporina f. / var. minor 185, 195
leporina var. onotica 209
leporina var. rubescens 185
lilacina 225
lobata 225
lohjaënsis 200
luculenta 226
luteonitens 226
marsupium var. pyxidata 216
micropus 226
microspora 226
minor 174, 177, 178, 189, 213, 217, 219, 222
mirabilis 171, 174–176, 179, 192, 205, 207, 209, 211, 213
myosotis 185, 187
nannfeldtii 168, 175–177, 179, 185, 189, 191, 197, 198, 201, 202
neglecta 226
obtecta 226
ochracea 226
olivacea 192, 226
olivaceobrunnea 178, 192
onotica 166, 167, 174, 177–179, 205, 209, 225, 226
onotica var. brevispora 174, 205, 206
onotica var. ochracea 226
oregonensis 168, 174, 177, 178, 213, 217, 219, 220, 223
papillata 175, 179, 185, 189, 191, 198, 200
papillata f. pallidefurfuracea 189
pedunculata 206, 207, 209
phlebophora 167, 168, 174, 178, 213, 217, 219, 220, 222, 225
phyllogena 225
platyspora 178, 179, 180, 182
pleurota 226
praetervisa 227
propinquata 167, 177, 178, 191–193, 195, 224
pseudobadia 206, 209
pseudoleporina 168, 174, 176, 177, 179, 185, 188, 197, 200, 201
purpurea 179, 205, 211
pusilla 192
pyxidata 216
radiculata 226
rainierensis 174, 175, 179, 213, 214, 217, 222, 223, 226
reisneri 226
rhenana 224
rosea 209
schulzeri 226
scutellata 216
shimizuensis 167
silvicola 167, 185, 187, 226
sinensis 174, 178, 213
smithii 168, 171, 176, 179, 192, 205, 209, 211
sparassis 226
subconcinna 219
subformicarum 168, 171, 174, 179, 197, 198, 200
subonotica 226
subpurpurea 178, 211
subterranea 167, 178, 179, 181
subviolacea 227
succosa 227
tasmanica 227
tianshuiensis 178, 213
tuomikoskii 171, 177, 179, 185, 189, 200, 202
umbrina 184, 187, 206–208
unicisa 167, 168, 174, 177, 178, 202, 205, 211, 225
varia 226
violacea 227
yunnanensis 167, 174, 178, 179, 202, 204, 205
REFERENCES
- Arpin N. 1969. Les caroténoïdes des Discomycètes: essai chimotaxinomique. Bulletin Mensuel de la Société Linnéenne de Lyon 38 (suppl.): 1–169. [Google Scholar]
- Batsch AJGK. 1783. Elenchus Fungorum. Halle, Germany. [Google Scholar]
- Bonorden HF. 1851. Handbuch der allgemeinen mykologie als anleitung zum studium derselben, nebst speciellen beiträgen zur vervolkommung dieses zweiges der naturkunde. Schweizerbart, Stuttgart, Germany. [Google Scholar]
- Boudier JLÉ. 1885. Nouvelle classification naturelle des Discomycètes charnus connus généralement sous le nom de Pezizes. Bulletin de la Société Mycologique de France 1: 91–120. [Google Scholar]
- Boudier JLÉ. 1905. Icones Mycologicae, livr. 6. Klincksieck, Paris, France. [Google Scholar]
- Boudier JLÉ. 1906. Icones Mycologicae, livr. 7. Klincksieck, Paris, France. [Google Scholar]
- Boudier JLÉ. 1907. Histoire et classification des Discomycètes d’Europe. Klincksieck, Paris, France. [Google Scholar]
- Boudier JLÉ. 1908. Icones Mycologicae, livr. 21. Klincksieck, Paris, France. [Google Scholar]
- Boudier JLÉ. 1909a. Icones Mycologicae, livr. 23. Klincksieck, Paris, France. [Google Scholar]
- Boudier JLÉ. 1909b. Icones Mycologicae, livr. 24. Klincksieck, Paris, France. [Google Scholar]
- Boudier JLÉ. 1910. Icones Mycologicae, livr. 29. Klincksieck, Paris, France. [Google Scholar]
- Bresadola G. 1884. Fungi Tridentini novi, vel nondum delineati, descripti, et iconibus illustrati. Ser. 1, fasc. 4–5: 43–70, pl. 46–75. Monauni, Trento, Italy. [Google Scholar]
- Bresadola G. 1898. Fungi Tridentini novi, vel nondum delineati, descripti, et iconibus illustrati. Ser. 2, fasc. 11–13: 47–81, pl. 151–195. Zippel, Trento, Italy [Google Scholar]
- Bresadola G. 1900. Fungi Tridentini novi, vel nondum delineati, descripti, et iconibus illustrati. Ser. 2, fasc. 14: 83–118, pl. 196–217. Zippel, Trento, Italy. [Google Scholar]
- Bresadola G. 1933. Iconographia mycologica. Vol. XXV. Trento, Italy. [Google Scholar]
- Bulliard JBF. 1791. Herbier de la France. Tome 11; Paris, France. [Google Scholar]
- Cao JZ, Fan L, Liu B. 1990. Some species of Otidea from China. Mycologia 82: 734–741. [Google Scholar]
- Carbone M. 2009. Il genere Otidea I. Sull’identità di Peziza onotica. Rivista di Micologia 52: 11–28. [Google Scholar]
- Carbone M. 2010a. Il genere Otidea III. Identità e tipifizione di Peziza alutacea. Bollettino dell’Associazione Micologica ed Ecologica Romana 80–81: 22–38. [Google Scholar]
- Carbone M. 2010b. Il genere Otidea IV. Prima parte. Otidea caligata, l’attuale nome di Otidea abietina sensu Breitenbach & Kränzlin, con discussione sull’abbandono dell’epiteto abietina. Schweizerische Zeitschrift für Pilzkunde 88: 14–17. [Google Scholar]
- Carbone M. 2010c. Il genere Otidea IV. Seconda parte. Otidea caligata, l’attuale nome di Otidea abietina sensu Breitenbach & Kränzlin, con discussione sull’abbandono dell’epiteto abietina. Schweizerische Zeitschrift für Pilzkunde 88: 64–66. [Google Scholar]
- Carbone M, Campo E, Vauras J. 2010. Records on Otidea mirabilis and O. tuomikoskii from Finland. Karstenia 50: 25–34. [Google Scholar]
- Carbone M, Van Vooren N. 2010 ‘2009’. Il genere Otidea – II. Otidea fuckelii, una nuova specie pubblicata per chiarire le differenti interpretazioni di O. leporina. Rivista di Micologia 52: 313–330. [Google Scholar]
- Cash EK. 1953. A record of the fungi named by J.B. Ellis. Part. II. The division of mycology and disease survey, special publication 2: 167–345. [Google Scholar]
- Clements FE, Shear CL. 1931. The genera of fungi. Wilson, New York, USA. [Google Scholar]
- Cooke MC. 1876. Mycographia seu Icones Fungorum. Part. 3. Williams & Norgate, London, Great Britain. [Google Scholar]
- Cooke MC. 1878. Mycographia seu Icones Fungorum. Part. 5. Williams & Norgate, London, Great Britain. [Google Scholar]
- Dennis RWG. 1978. British Ascomycetes. 2nd edn Cramer, Vaduz, Germany. [Google Scholar]
- Dissing H. 2000. Pezizales Bessey. In: Hansen L, Knudsen H. (eds), Nordic Macromycetes. Vol. 1. Ascomycetes: 55–127. Nordsvamp, Copenhagen, Denmark. [Google Scholar]
- Eckblad F-E. 1968. The genera of operculate Discomycetes. A reevaluation of their taxonomy, phylogeny and nomenclature. Norwegian Journal of Botany 15: 1–191. [Google Scholar]
- Farr ML, Leeusink JA, Stafleu FA. 1979. Index Nominum Genericorum (Plantarum). Regnum Vegetabile vols. 100–102. Bohn, Scheltema &; Holkema, Utrecht. [Google Scholar]
- Franchi P, Lami L, Marchetti M. 1999. Helvella leporina, nome corretto per Helvella silvicola. Rivista di Micologia 42: 63–72. [Google Scholar]
- Fries EM. 1822. Systema mycologicum. II. Officina Berlingiana, Sweden. [Google Scholar]
- Fuckel L. 1870. ‘1869–1870’. Symbolae mycologicae. Jahrbücher des Nassauischen Vereins für Naturkunde 23–24: 1–459. [Google Scholar]
- Gal M le. 1947. Recherches sur les ornamentations sporales des Discomycètes operculés. Bibliotheca Mycologica 28: 73–297. Reprint 1970. Cramer, New York, USA. [Google Scholar]
- Gonnermann W, Rabenhorst L. 1869. Mycologia Europaea. Abbildungen sämmtlicher Schwämme Europas. 3. Dresden, Germany. [Google Scholar]
- Gray SF. 1821. A natural arrangement of British plants. Baldwin, Cradock & Joy, London, United Kingdom. [Google Scholar]
- Greuter W, Brummitt RK, Farr E, et al. (eds). 1993. Names in current use for extant plant genera. Regnum Vegetabile vol. 129. Koeltz Scientific Books, Koenigstein. [Google Scholar]
- Häffner J, Winterhoff W. 1989. Rezente Ascomycetenfunde VI. Otidea apophysata (Cooke & Phill.) Sacc. ein extrem seltener Öhrling. Beiträge zur Kenntnis der Pilze Mitteleuropas 5: 175–184. [Google Scholar]
- Hansen K, Læssøe T, Pfister DH. 2002. Phylogenetic diversity in the core group of Peziza inferred from ITS sequences and morphology. Mycological Research 106: 879–902. [Google Scholar]
- Hansen K, LoBuglio KF, Pfister DH. 2005. Evolutionary relationships of the cup-fungus genus Peziza and Pezizaceae inferred from multiple nuclear genes: RPB2, ß -tubulin, and LSU rDNA. Molecular Phylogenetics and Evolution 36: 1–23. [DOI] [PubMed] [Google Scholar]
- Hansen K, Olariaga I. 2015. Species limits and relationships within Otidea inferred from multiple gene phylogenies. Persoonia 35: 148–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K, Perry BA, Dranginis AW, et al. 2013. A phylogeny of the highly diverse cup-fungus family Pyronemataceae (Pezizomycetes, Ascomycota) clarifies relationships and evolution of selected life history traits. Molecular Phylogenetics and Evolution 67: 311–335. [DOI] [PubMed] [Google Scholar]
- Hansen K, Pfister DH. 2006. Systematics of the Pezizomycetes - the operculate discomycetes. Mycologia 98: 1029–1040. [DOI] [PubMed] [Google Scholar]
- Hansen K, Pfister DH, Hibbett DS. 1999. Phylogenetic relationships among species of Phillipsia inferred from molecular and morphological data. Mycologia 91: 299–314. [Google Scholar]
- Harmaja H. 1974. Flavoscypha, a new genus of the Pezizales for Otidea cantharella and O. phlebophora; Karstenia: 14: 105–108. [Google Scholar]
- Harmaja H. 1976. New species and combinations in the genera Gyromitra, Helvella and Otidea. Karstenia 15: 29–32. [Google Scholar]
- Harmaja H. 1986. Studies on Pezizales. Karstenia 26: 41–48. [Google Scholar]
- Harmaja H. 2009a. Studies in Otidea (Pezizales). Karstenia 48: 33–48. [Google Scholar]
- Harmaja H. 2009b. A note on Otidea (Pezizales, Fungi). Phytotaxa 2: 49–50. [Google Scholar]
- Hennings P. 1903. Beiträge zur Pilzflora Südamerikas. II. Hedwigia 36: 190–246. [Google Scholar]
- Huelsenbeck JP, Larget B, Alfaro ME. 2004 Bayesian phylogenetic model selection using reversible jump Markov chain Monte Carlo. Molecular Biology and Evolution 21: 1123–1133. [DOI] [PubMed] [Google Scholar]
- Huhtinen S. 1990‘1989’. A monograph of Hyaloscypha and allied genera. Karstenia 29: 45–252. [Google Scholar]
- Jamoni PG. 2001. Validazione di nuovi taxa. Funghi e Ambiente 85–86: 56. [Google Scholar]
- Jamoni PG. 2004. I funghi dell’ambiente alpino – XVIII. Funghi e Ambiente 94–95: 5–19. [Google Scholar]
- Kanouse B. 1949 Studies in the genus Otidea. Mycologia 41: 660–677. [Google Scholar]
- Karsten PA. 1869 Monographia Pezizarum fennicarum. Notiser ur Sallskapets pro Fauna et Flora Fennica Forhandlingar 10: 99–206. [Google Scholar]
- Karsten PA. 1871 Mycologia Fennica. Pars prima. Discomycetes. Bidrag till Kannedom of Finlands Natur och Folk 19: 1–263. [Google Scholar]
- Kasparek F. 2000. Über einige bemerkenswerte Schlauchpilze. Der Tintling 19: 7–15. [Google Scholar]
- Kimbrough JW. 1966 Studies in the Pseudoascoboleae. Canadian Journal of Botany 44: 685–704. [Google Scholar]
- Kirk PM, Stalpers JA, Braun U, et al. 2013 A without-prejudice list of generic names of fungi for protection under the International Code of Nomenclature for algae, fungi, and plants. IMA Fungus 4: 381–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf RP. 1963. Discomycete flora of Asia, precursor II: A revision of the genera Acervus and Ascosparassis and their new positions in the Pezizales. Lloydia 26: 21–26. [Google Scholar]
- Korf RP. 1972. Synoptic key to the genera of the Pezizales. Mycologia 64: 937–994. [Google Scholar]
- Korf RP. 1973a. Sparassoid ascocarps in the Pezizales and Tuberales. Reports of the Tottori Mycological Institute 10: 389–403. [Google Scholar]
- Korf RP. 1973b. Discomycetes and Tuberales. In: Ainsworth GC, Sparrow FK, Sussman S. (eds), The fungi: An advanced treatise. Vol. 4A: 249–319. Academic Press, New York, USA. [Google Scholar]
- Korf RP, Zhuang WY. 1991 A preliminary discomycete flora of Macaronesia: part 15, Terfeziaceae, and Otideaceae, Otideoideae. Mycotaxon 40: 413–433. [Google Scholar]
- Kornerup A, Wanscher JH. 1961 Farver i farver. Politikens forlag, København, 1st edn. [Google Scholar]
- Kumar S, Skjæveland Å, Orr RJS, et al. 2009 AIR: A batch-oriented web program package for construction of supermatrices ready for phylogenomic analyses. BMC Bioinformatics 10: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Cao JZ. 1987 Otideopsis yunnanensis gen. et sp. nov. of Pezizales from China and its position in Pezizales system. Journal of Shanxi University, Natural Science edition 4: 70–73. [Google Scholar]
- Liu CY, Zhuang WY. 2006 Relationships among some members of the genus Otidea (Pezizales, Pyronemataceae). Fungal Diversity 23: 181–192. [Google Scholar]
- Lundell S, Nannfeldt JA. 1934. Fungi Exsiccati Suecici, praesertim upsalienses Fasc. 1–2. Almqvist & Wiksells Boktryckeri, Sweden. [Google Scholar]
- Lundell S, Nannfeldt JA. 1938. Fungi Exsiccati Suecici, praesertim upsalienses. Fasc. 11–12. Almqvist & Wiksells Boktryckeri, Sweden. [Google Scholar]
- Lundell S, Nannfeldt JA, Holm L. 1985 Fungi Exsiccati Suecici, prasertim upsalienses. Fasc. 66 (No 3251–3300).Publications from the Herbarium, University of Uppsala, Sweden: 18: 1–18. [Google Scholar]
- Maas Geesteranus RA. 1967. De fungi van Nederland 2a. Pezizales – deel 1, Discinaceae, Helvellaceae, Morchellaceae, Pezizaceae, Rhizinaceae. Wetenschappelijke mededelingen van de Koninklijke Nederlandse Natuurhistorische Vereniging 69: 1–72. [Google Scholar]
- McNeill J, Barrie FR, Buck WR, et al. (eds). 2012. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. [Regnum Vegetabile No. 154.] Koeltz Scientific Books, Königstein, Germany. [Google Scholar]
- Medardi G. 1995 Considerazioni sul genere Otidea Fuck., 1870. Bolletino del Circolo Micologico ‘G. Carini’ 29–30: 23–32. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010 Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov.: 1–8. New Orleans, LA, USA. [Google Scholar]
- Moravec J. 1986. A new species and two new combinations in the genus Sowerbyella. Mycologia Helvetica 2: 93–102. [Google Scholar]
- Moravec J. 1988. Sowerbyella angustispora spec. nov. and Otideopsis kaushalii comb. nov. (Discomycetes, Pezizales, Pyronemataceae). Mycologia Helvetica 3: 135–142. [Google Scholar]
- Moravec J. 2005. A world monograph of the genus Cheilymenia (Discomycetes, Pezizales, Pyronemataceae). Libri Botanici 21: 3–256. [Google Scholar]
- Mornand J, Courtecuisse R. 2005. Le genre Otidea et espèces affines en France. Bulletin mensuel de la Société linnéenne de Lyon 74, numero spécial: 65–84. [Google Scholar]
- Nannfeldt JA. 1937. Contributions to the mycoflora of Sweden. 4. On some species of Helvella, together with a discussion of the natural affinities within the Helvellaceae and Pezizaceae trib. Acetabuleae. Svensk Botanisk Tidskrift 31: 47–66. [Google Scholar]
- Nannfeldt JA. 1938. Contributions to the mycoflora of Sweden. 5. On Peziza catinus Holmskj. ex Fr. and P. radiculata Sow. ex Fr. with a discussion of the genera Pustularia Fuckel emend. Boud. and Sowerbyella Nannf. n. gen. Svensk Botanisk Tidskrift 32: 108–120. [Google Scholar]
- Nannfeldt JA. 1966. On Otidea caligata, O. indivisa and O. platyspora (Discomycetes, Operculatae). Annales Botanici Fennici 3: 309–318. [Google Scholar]
- Nei M. 1987 Molecular evolutionary genetics.Columbia University Press, USA. [Google Scholar]
- Parslow M, Spooner B. 2013 The British species of Otidea: overview and the large spored species. Mycosystema 32: 347–365. [Google Scholar]
- Pérez-Butrón JL, Fernández-Vicente J. 2008 Otidea apophysata, en la Península Ibérica. Errotari 5: 36–43. [Google Scholar]
- Perry BA, Hansen K, Pfister DH. 2007 A phylogenetic overview of the family Pyronemataceae (Ascomycota, Pezizales). Mycological Research 111: 549–571. [DOI] [PubMed] [Google Scholar]
- Persoon CH. 1799. Observationes Mycologicae. Pars secunda; Leipzig & Luzern. [Google Scholar]
- Persoon CH. 1822. Mycologia europaea. I. Erlangae, Germany. [Google Scholar]
- Peterson ET. 1998 Systematics of the genus Otidea in the Pacific Northwest. Unpublished thesis; Oregon State University. [Google Scholar]
- Pfister DH. 1979. A monograph of the genus Wynnea (Pezizales, Sarcoscyphacea). Mycologia 71: 144–159. [Google Scholar]
- Pfister DH, Halling RE. 1989 Ascosparassis heinricheri from Venezuela: an extended distribution. Mycotaxon 35: 283–285. [Google Scholar]
- Quélet L. 1886 Enchiridion fungorum in Europa media et praesertim in Gallia vigentium. Douin, Paris, France. [Google Scholar]
- Rahm E. 1958. Otidea pusilla nov. spec. Zwerg-Öhrling. Schweizerische Zeit-schrift für Pilzkunde 36: 33–35. [Google Scholar]
- Rambaut A. 2002 Se-Al. Sequence Alignment Editor. Version 2.0 alpha 11. University of Oxford, Oxford: From http://tree.bio.ed.ac.uk/software/seal/. [Google Scholar]
- Ramsbottom J. 1914. A list of the British species of Discomycetes arranged according to Boudier’s system, with a key to the genera. Transactions of the British Mycological Society 4: 343–381. [Google Scholar]
- Rehm H. 1883 Ascomyceten XIV. Hedwigia 22: 1–18 (reprint). [Google Scholar]
- Rifai MA. 1968 The Australasian Pezizales in the herbarium of the Royal Botanic Gardens Kew. Verhandelingen der Koninklijke Nederlandse Akademie van Wetenschappen, Afd; Natuurkunde, Tweede Reeks: 57: 1–295. [Google Scholar]
- Ronquist F, Teslenko M, Mark P van der, et al. 2012 MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo PA. 1884. Conspectus generum discomycetum hucusque cognitorum. Botanisches Centralblatt 18: 213–256. [Google Scholar]
- Saccardo PA. 1889. Sylloge Fungorum omnium hucusque cognitorum 8. Patavii. [Google Scholar]
- Schaeffer JC. 1763. Fungorum qui in Bavaria et Palatinatu circa Ratisbonam nascuntur icons natives coloribus expressae. Tomus II. Regensburg, Ger-many. [Google Scholar]
- Schaeffer JC. 1774. Fungorum qui in Bavaria et Palatinatu circa Ratisbonam nascuntur icons natives coloribus expressae. Tomus IV. Regensburg, Ger-many. [Google Scholar]
- Seaver FJ. 1904. The discomycetes of eastern Iowa. Bulletin from the laboratories of natural history of the State University of Iowa 5: 230–297. [Google Scholar]
- Seaver FJ. 1927. A tentative scheme for the treatment of the genera of the Pezizaceae. Mycologia 19: 86–89. [Google Scholar]
- Seaver FJ. 1928. The North American cup-fungi (operculates). Reprint 1961. Hafner Publishing Company, New York, USA. [Google Scholar]
- Smith ME, Healy RA. 2009 Otidea subterranea sp. nov.: Otidea goes below ground. Mycological Research 113: 858–866. [DOI] [PubMed] [Google Scholar]
- Sowerby J. 1797 Coloured figures of English fungi or mushrooms. Vol. I. London, England. [Google Scholar]
- Spooner BM, Yao Y-J. 1995. Notes on British taxa referred to Aleuria. Mycological Research 99: 1515–1518. [Google Scholar]
- Stamatakis A. 2006 Raxml-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Svrček M. 1976 A taxonomic revision of Velenoský’s types of operculate discomycetes (Pezizales) preserved in national museum, Prague. Sborník národního muzea v Praze. Acta Musei Nationalis Pragae 32 B: 115–194. [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, et al. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32. [DOI] [PubMed] [Google Scholar]
- Thiers B. (ed). 2014. Index Herbariorum. A global directory of public herbaria and associated staff. New York Botanical Garden′s Virtual Herbarium. http://sweetgum.nybg.org/ih/. [Google Scholar]
- Van Vooren N. 2010 Note sur Otidea mirabilis (Pezizales). Ascomycete.org 2: 33–35. [Google Scholar]
- Van Vooren N. 2011a Le genre Otidea V. Otidea apophysata et ses interprétations. Bulletin mycologique et botanique Dauphiné-Savoie: 165–174. [Google Scholar]
- Van Vooren N. 2011b Premiers signalements d’Otidea caligata (Nyl.) Sacc. (Ascomycota, Pezizales) en France. Ascomycete.org 3: 61–64. [Google Scholar]
- Van Vooren N, Armada F. 2011 Redécouverte d’Otidea platyspora Nannf. (Ascomycota, Pezizales) en France. Bulletin mycologique et botanique Dauphiné-Savoie 203: 57–62. [Google Scholar]
- Van Vooren N, Carbone M. 2012 The genus Otidea. VI. Otidea felina and its interpretations. Ascomycete.org 4: 29–34. [Google Scholar]
- Van Vooren N, Hairaud M, Jindřich O. 2008. Otidea tuomikoskii, Otidea papillata et Otidea papillata f. pallidefurfuracea f. nov. trois taxons remarquables appartenant au genre Otidea (Pezizales, Pyronemataceae). Bulletin mycologique et botanique Dauphiné-Savoie 188: 47–57. [Google Scholar]
- Van Vooren N, Olariaga I, Tabarés M. 2011 First record of Otidea caeruleopruinosa Harmaja (Ascomycota, Pezizales) in the Iberian Peninsula. Ascomycete.org 3: 43–46. [Google Scholar]
- Yao Y-J, Spooner BM. 2006 Species of Sowerbyella in the British Isles, with validation of Pseudoombrophila sect. Nannfeldtiella (Pezizales). Fungal Diversity 22: 267–279. [Google Scholar]
- Zhuang WY. 2006 ‘2005’. Notes on Otidea from Xinjiang, China. Mycotaxon 94: 365–370. [Google Scholar]
- Zhuang WY. 2010 Taxonomic assessment of some pyronemataceous fungi from China. Mycotaxon 112: 31–46. [Google Scholar]
- Zhuang WY, Korf RP. 1987. Some new species and new records of discomycetes in China. II. Mycotaxon 29: 309–314. [Google Scholar]
- Zhuang WY, Korf RP. 1989. Some new species and new records of discomycetes in China. III. Mycotaxon 35: 297–312. [Google Scholar]
- Zhuang WY, Yang ZL. 2008. ‘2007’. Some pezizalean fungi from alpine areas of southwestern China. Mycologia Montenegrina 10: 235–249. [Google Scholar]