Abstract
Diaporthe (syn. Phomopsis) species are well-known saprobes, endophytes or pathogens on a range of plants. Several species have wide host ranges and multiple species may sometimes colonise the same host species. This study describes eight novel Diaporthe species isolated from live and/or dead tissue from the broad acre crops lupin, maize, mungbean, soybean and sunflower, and associated weed species in Queensland and New South Wales, as well as the environmental weed bitou bush (Chrysanthemoides monilifera subsp. rotundata) in eastern Australia. The new taxa are differentiated on the basis of morphology and DNA sequence analyses based on the nuclear ribosomal internal transcribed spacer region, and part of the translation elongation factor-1α and ß-tubulin genes. The possible agricultural significance of live weeds and crop residues (‘green bridges’) as well as dead weeds and crop residues (‘brown bridges’) in aiding survival of the newly described Diaporthe species is discussed.
Keywords: alternate weed hosts, multi-locus, Phomopsis, phylogeny, taxonomy
INTRODUCTION
Diaporthe (syn. Phomopsis) species have been recorded on a wide range of hosts. Species in this genus are well-known in the plant pathology literature as the cause of many significant plant diseases worldwide, including stem cankers, leaf and pod blights, and seed decay (Rehner & Uecker 1994, Santos et al. 2011, Udayanga et al. 2011). Further, Diaporthe species have been recorded as opportunistic saprobes on decaying leaves, twigs and stem residues, as well as endophytes on healthy leaves, stems, seeds and roots (Muralli et al. 2006, Gomes et al. 2013).
The recent use of DNA sequence-based methods and the application of the Genealogical Concordance Phylogenetic Species Recognition (GCPSR) criteria have resulted in a rapid increase in the discovery of cryptic species in several large genera of plant pathogenic fungi, such as Colletotrichum (Damm et al. 2012a, b, Weir et al. 2012), Diaporthe (Shivas & Cai 2012, Udayanga et al. 2014) and Fusarium (O’Donnell et al. 2009, 2012). This approach also provides a more stable taxonomy for Diaporthe, from which a clearer understanding about the host range of particular species is emerging. It is known that many species of Diaporthe have wide host ranges (Mengistu et al. 2007, Santos et al. 2011, Udayanga et al. 2011, Gomes et al. 2013) and multiple species can colonise the same host (Farr et al. 2002, Crous & Groenewald 2005, van Niekerk et al. 2005, Thompson et al. 2011).
It is well documented in plant pathology literature that live weeds and volunteer crop plants serve as alternative hosts for a range of pathogens, including Diaporthe species, by providing a ‘green bridge’ that facilitates pathogen survival between crop phases. Following the first outbreaks of Diaporthe helianthi (syn. Phomopsis helianthi) on sunflower in the former Yugoslavia (now Serbia), Mihaljcevic & Muntañola-Cvetković (1985) recovered Diaporthe species from 15 plant species, including the weeds Xanthium italicum and X. strumarium. Subsequent studies by Vrandečić et al. (2010) confirmed Arctium lappa, X. italicum and X. strumarium as weed hosts for D. helianthi.
Alternative weed hosts have been suspected to play a role in the epidemiology of three species, D. gulyae, D. kochmanii and D. kongii, recently found associated with sunflower stem canker in Australia (Thompson et al. 2011). During recent investigations to identify alternative hosts of the Diaporthe species that cause sunflower canker in eastern Australia, eight novel species were identified based on GCPSR criteria, from both live crop and weed hosts as well as crop stubble and weed residues in Queensland (Qld) and New South Wales (NSW). Dead standing weeds and residues are common amongst crop stubble in Australian broad acre and low tillage cropping systems, where herbicides are often used for weed control. Additionally, one of the new Diaporthe species was also identified from a study into the cause of dieback of the coastal environmental weed Chrysanthemoides monilifera subsp. rotundata (bitou bush) in northern NSW. All eight species of Diaporthe are described and illustrated here.
MATERIALS AND METHODS
Isolates
Isolates from broad acre cropping regions
Plant material was collected from a range of summer crops including lupin, maize, mungbean, soybean and sunflower, as well as major weed species and plant residues on the soil surface across the broad acre cropping regions of Qld and NSW (Table 1). The material included necrotic lesions or visible pycnidia on stems, leaves, petioles, heads and seeds from live plants and/or dead plants. Specimens from plant residues were only selected from material for which the inflorescence was present so that the plant species could be identified.
Table 1.
Diaporthe spp., and the outgroup taxon Diaporthella corylina, included in the phylogenetic analysis of this study. Newly described taxa and deposited sequences are in bold.
| Species | Isolate no.a | Host | Localityb | GenBank accession no.c |
||
|---|---|---|---|---|---|---|
| ITS | TEF | BT | ||||
| Diaporthe ambigua | CBS 114015* | Pyrus communis | South Africa | AF230767 | GQ250299 | KC343978 |
| Diaporthe anacardii | CBS 720.97* | Anacardium occidentale | East Africa | KC343024 | KC343750 | KC343992 |
| Diaporthe batatas | CBS 122.21 | Ipomea batatas | USA | KC343040 | KC343766 | KC344008 |
| Diaporthe beilharziae | BRIP 54792* | Indigofera australis | NSW, Australia | JX862529 | JX862535 | KF170921 |
| Diaporthe charlesworthii | BRIP 54884m* | Rapistrum rugostrum | Qld, Australia | KJ197288 | KJ197250 | KJ197268 |
| Diaporthe cinerascens | CBS 719.96 | Ficus carica | Bulgaria | KC343050 | KC343776 | KC344018 |
| Diaporthe cuppatea | CBS 117499* | Aspalathus linearis | South Africa | AY339322 | AY339354 | KC344025 |
| Diaporthe elaeagni | CBS 504.72 | Elaeagnus sp. | Netherlands | KC343064 | KC343790 | KC344032 |
| Diaporthe endophytica | CBS 133811* | Schinus terebinthifolius | Brazil | KC343065 | KC343791 | KC344033 |
| Diaporthe foeniculacea | CBS 123208* | Foeniculum vulgare | Portugal | KC343104 | KC343830 | KC344072 |
| Diaporthe goulteri | BRIP 55657a* | Helianthus annuus | Qld, Australia | KJ197289 | KJ197252 | KJ197270 |
| Diaporthe gulyae | BRIP 54025* | Helianthus annuus | Qld, Australia | JF431299 | JN645803 | KJ197271 |
| Diaporthe helianthi | CBS 592.81* | Helianthus annuus | Serbia | KC343115 | GQ250308 | KC343841 |
| Diaporthe hordei | CBS 481.92 | Hordeum vulgare | Norway | KC343120 | KC343846 | KC344088 |
| Diaporthe infecunda | CBS 133812 | Schinus terebinthifolius | Brazil | KC343126 | KC343852 | KC344094 |
| Diaporthe kongii | BRIP 54031* | Helianthus annuus | Qld, Australia | JF431301 | JN645797 | KJ197272 |
| Diaporthe macinthoshii | BRIP 55064a* | Rapistrum rugostrum | Qld, Australia | KJ197290 | KJ197251 | KJ197269 |
| Diaporthe masirevicii | BRIP 57330 | Chrysanthemoides monilifera subsp. rotundata | NSW, Australia | KJ197275 | KJ197237 | KJ197255 |
| BRIP 54256 | Glycine max | Qld, Australia | KJ197276 | KJ197238 | KJ197256 | |
| BRIP 57892a* | Helianthus annuus | Qld, Australia | KJ197277 | KJ197239 | KJ197257 | |
| BRIP 54120c | Zea mays | Qld, Australia | KJ197278 | KJ197240 | KJ197258 | |
| Diaporthe melonis | CBS 507.78* | Cucumis melo | USA | KC343141 | KC343867 | KC344109 |
| Diaporthe middletonii | BRIP 57329 | Chrysanthemoides monilifera subsp. rotundata | NSW, Australia | KJ197285 | KJ197247 | KJ197265 |
| BRIP 54884e* | Rapistrum rugostrum | Qld, Australia | KJ197286 | KJ197248 | KJ197266 | |
| Diaporthe miriciae | BRIP 55662c | Glycine max | Qld, Australia | KJ197283 | KJ197245 | KJ197263 |
| BRIP 54736j* | Helianthus annuus | NSW, Australia | KJ197282 | KJ197244 | KJ197262 | |
| BRIP 56918a | Vigna radiata | Qld, Australia | KJ197284 | KJ197246 | KJ197264 | |
| Diaporthe neoarctii | CBS 109490* | Ambrosia trifida | USA | KC343145 | KC343871 | KC344113 |
| Diaporthe phaseolorum | CBS 116019 | Caperonia palustris | USA | KC343175 | KC343901 | KC344143 |
| Diaporthe raonikayaporum | CBS 133182* | Spondias mombin | Brazil | KC343188 | KC343914 | KC344156 |
| Diaporthe sackstonii | BRIP 54669b* | Helianthus annuus | Qld, Australia | KJ197287 | KJ197249 | KJ197267 |
| Diaporthe serafiniae | BRIP 55665a* | Helianthus annuus | Qld, Australia | KJ197274 | KJ197236 | KJ197254 |
| BRIP 54136 | Lupinus albus ‘Rosetta’ | NSW, Australia | KJ197273 | KJ197235 | KJ197253 | |
| Diaporthe sojae | CBS 180.55 | Glycine soja | KC343200 | KC343926 | KC344168 | |
| Diaporthe stitica | CBS 370.54 | Buxus sempervirens | Italy | KC343212 | KC343938 | KC344180 |
| Diaporthella corylina | CBS 121124 | Corylus sp. | China | KC343004 | KC343730 | KC343972 |
a BRIP: Plant Pathology Herbarium, Dutton Park, Queensland, Australia; CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
b NSW, New South Wales; Qld, Queensland; USA, United States of America.
c Other than those in bold, all sequences were downloaded from GenBank and published in van Rensberg et al. (2006), Santos et al. (2010), Udayanga et al. (2011, 2012), Gomes et al. (2013) and Tan et al. (2013).
*Ex-type or ex-epitype culture.
Small pieces (10–30 mm) of tissue or entire seeds were surface sterilised in 1 % sodium hypochlorite solution for 3 min, then rinsed with sterile distilled water. The surface sterilised tissue was placed onto 9 cm diam Petri plates containing water agar amended with 100 μg/mL streptomycin sulphate (WAS), and incubated at 23–25 °C under ambient light. After 2–21 d, conidial ooze from individual pycnidia characteristic of Diaporthe species was streaked onto potato dextrose agar (PDA) (Oxoid) amended with 100 μg/mL streptomycin sulphate (PDAS) and incubated as above. After 12–24 h, single germinating conidia or hyphal tips were removed aseptically with a fine needle and placed on the surface of fresh plates of PDAS and incubated as above.
Isolates from bitou bush
Stems of live bitou bush plants affected by dieback were collected from Bongil Bongil National Park and Bellingen Head State Park in northern NSW (Table 1). Pieces of stem tips with necrotic symptoms were cut in 1–2 cm long sections, including the margin between healthy and dead tissue, immersed in 70 % ethanol for 30 s followed by 2 % sodium hypochlorite for 2–4 min. Tissue pieces were rinsed three times in sterile distilled water, blotted dry with paper towel, then cut longitudinally with a sterile scalpel and placed on ½ strength PDA amended with 200 μg/mL streptomycin sulphate (½ PDAS) or on acidified PDA (one drop of 25 % lactic acid added per plate when pouring) in 9 cm diam Petri dishes. Plates were incubated at room temperature under 24 h fluorescent lights.
The bark of stem pieces (c. 1–3 cm diam and 12–15 cm long), cut near the base of wilting bitou bush plants was removed, using a sharp, surface-sterilised knife, over more than half of the circumference of the pieces and for c. 7–8 cm long in the middle of the pieces. A surface-sterilised wood chisel was then used to remove thin slices (up to c. 30, each c. 1–3 cm long) from the wood (xylem) of each of the stem pieces. Small pieces (c. 0.5 cm2) were cut from each slice of wood tissue and surface sterilised either by: i) immersing in 2 % NaOCl for 1 min, followed by 1 min in 70 % ETOH, then rinsed three times in sterile distilled water; or ii) by spraying 70 % ETOH onto the pieces surface, and then blotting dry with paper towel and plating onto ½ PDAS. Plates were incubated as above.
Pieces of hyphae at the margin of colonies that grew from the bitou bush pieces were transferred onto fresh ½ PDAS and PDA plates, and incubated as above. Plates were examined for pycnidia characteristic of Diaporthe species at weekly intervals over a 2 mo period with a stereoscopic microscope. Single-conidium isolates were produced as described above for isolates from broad acre cropping regions and grown on ½ PDAS under the same conditions as above. All isolates recovered were deposited in the Plant Pathology Herbarium (BRIP), Brisbane, Australia.
Morphology
To determine morphological characteristics, isolates were grown on water agar with pieces of sterilised wheat stems placed on the surface (WSA) and incubated under a 12 h photoperiod with near ultraviolet light (NUV) (Smith 2002) at 23 °C. Fungal structures were mounted on glass slides in lactic acid (100 % v/v) for microscopic examination after 28 d of incubation. At least 20 measurements of selected structures were made and, means and standard deviations (SD) calculated. Ranges were expressed as (min–) mean-SD – mean+SD (–max) with values rounded to 0.5 μm. Images were captured with a Leica DFC 500 camera attached to a Leica DM5500B compound microscope with Nomarski differential interference contrast.
For colony morphology, 3-d-old cultures on 9 cm diam plates of PDA and oatmeal agar (OMA) (Oxoid) that had been grown in the dark at 23 °C were grown for a further 7 d under 12 h photoperiod with NUV light at 23 °C (Thompson et al. 2011). Colony colours (surface and reverse) were described according to the colour charts of Rayner (1970). Nomenclatural novelties were deposited in MycoBank (Crous et al. 2004) (www.mycobank.org).
DNA isolation, amplification and analyses
For isolates from broad acre cropping regions, mycelia were scraped off PDA cultures and macerated with 0.5 mm glass beads (Daintree Scientific) in a Tissue Lyser (QIAGEN). Genomic DNA was then extracted with the Gentra Puregene DNA Extraction kit (QIAGEN) according to the manufacturer’s instructions. For isolates from bitou bush, genomic DNA was extracted from mycelia scraped off ½ PDAS cultures using Mo-Bio Ultraclean Microbial DNA Isolation Kit.
The internal transcribed spacer (ITS) region of the nuclear ribosomal genes was amplified with the primers ITS4 (White et al. 1990), and V9G (de Hoog & Gerrits van den Ende 1998) or ITS1F (Gardes & Bruns 1993) for the isolates from broad acre cropping regions and bitou bush, respectively. For all isolates, the primers EF1-728 F (Carbone & Kohn 1999) and EF2 (O’Donnell et al. 1998) were used to amplify part of the translation elongation factor 1-α (TEF) gene, and the primers T1 (O’Donnell & Cigelnik 1997) and Bt2b (Glass & Donaldson 1995) were used to amplify part of the ß-tubulin (BT) gene.
The ITS region of the bitou bush isolates was amplified with Platinum Taq (Invitrogen) according to manufacturer’s instructions and the PCR conditions were 95 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min ×25 cycles. PCR products were purified with the Agencourt AMPure XP system (Beckman Coulter).
The ITS region of the broad acre cropping isolates and the BT and TEF loci of all isolates in this study were amplified with the Phusion High-Fidelity PCR Master Mix (Finnzymes) and the PCR conditions were 98 °C for 30 s, followed by 30 cycles of 98 °C for 10 s, 55 °C (ITS and TEF) or 60 °C (BT) for 30 s, 72 °C for 30 s, and a final extension at 72 °C for 5 min. The PCR products were purified with the QIAquick PCR Purification Kit (QIAGEN), and sequenced by Macrogen Incorporated (Seoul, Korea) using the amplification primers.
All unique sequences from different host-isolate combinations generated in this study were assembled using Vector NTi Advance 11.0 (Invitrogen). The ITS sequences were initially aligned with representative Diaporthe species from recent studies (Thompson et al. 2011, Udayanga et al. 2012, Gomes et al. 2013) using MAFFT alignment algorithm (Katoh et al. 2009) in the software Geneious (Biomatters Ltd). Diaporthella corylina (CBS 121124) was selected as outgroup taxon in the phylogenetic analyses based on its position as sister genus in Diaporthales (Vasilyeva et al. 2007).
A Neighbour-Joining (NJ) analysis using the Kimura-2 parameter with Gamma distribution was applied (data not shown), and the closest phylogenetic neighbours were selected for a combined analyses using BT, ITS and TEF genes. The sequences of each gene were aligned separately and manually adjusted where needed. Alignment gaps were treated as missing character states, and all characters were unordered and of equal weight. Bayesian analysis was performed with MrBayes v. 3.2.1 (Huelsenbeck & Ronquist 2001, Ronquist & Huelsenbeck 2001) in Geneious. The Markov Chain Monte Carlo (MCMC) analysis used four chains and started from a random tree topology. The sample frequency was set at 200 and the temperature of the heated chain was 0.3. Burn-in was set at 25 % after which the likelihood values were stationary. Maximum Likelihood (ML) analysis, including 1 000 bootstrap replicates, were run using RAxML v. 7.2.8 (Stamatakis & Alchiotis 2010) in Geneious. The nucleotide substitution model chosen was General Time Reversible (GTR) with a gamma-distributed rate of variation.
The concatenated alignment and resulting tree were deposited in TreeBASE (study S15707). Unique fixed nucleotides positions are used to characterise and differentiate two species from closely related phylogenetic species. For each species that was described, the closest phylogenetic neighbour was selected and this focused dataset was subjected to single nucleotide polymorphisms (SNPs) analyses. These SNPs were determined for each aligned data partition using DnaSP v. 5.10.01 (Librado & Rozas 2009).
RESULTS
Isolates
More than 500 Diaporthe isolates were recovered from live or dead plant tissues or seeds, from the crops sunflower, soybean, mungbean, lupin, maize, as well as from a range of weed species in the broad acre cropping regions of Qld and NSW (Table 2). Of these isolates, 147 could not be assigned to known taxa based on ITS sequence BLASTn search results against the GenBank database. Many of the remaining isolates recovered from a number of crop and weed hosts were identified as one of three recently described species from sunflower, namely, D. gulyae, D. kochmanii and D. kongii (Thompson et al. 2011) (data not shown). Fifteen Diaporthe isolates were recovered from the bitou bush material, including eight isolates of D. kongii (data not shown).
Table 2.
Crops and weeds from which the novel Diaporthe spp. species described in this paper were isolated.
| Plant host1 | Host family |
Diaporthe spp. |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| charlesworthii | goulteri | macintoshii | middletonii | masirevicii | miriciae | sackstonii | serafiniae | weieri | ||
| Crop | ||||||||||
| Glycine max | Fabaceae | – | – | – | – | L | L | – | – | L |
| Helianthus annuus | Asteraceae | – | S | – | – | L, S | L, S | L | L, S | L, D |
| Lupinus alba | Fabaceae | – | – | – | – | – | – | – | D | – |
| Vigna radiata | Fabaceae | – | – | – | – | L | L | – | – | – |
| Zea mays | Poaceae | – | – | – | – | L | – | – | – | L |
| Weed | ||||||||||
| Bidens pilosa | Asteraceae | – | – | – | – | – | – | – | L | – |
| Chrysanthemoides monilifera subsp. rotundata | Asteraceae | – | – | – | L | L | – | – | – | L |
| Datura ferox | Solanaceae | – | – | – | – | – | – | – | D | – |
| Gaura parviflora | Onagraceae | – | D | – | – | – | – | – | – | – |
| Malva paraflora | Malvaceae | – | – | – | – | – | – | – | L | – |
| Rapistrum rugosum | Brassicaceae | D | – | D | D | L, D | D | – | – | D |
| Sesbania cannabina | Fabaceae | – | – | – | – | L | – | – | – | – |
| Solanum nigrum | Solonaceae | – | – | – | – | – | – | – | L | – |
1 Material from which the fungi were isolated is indicated in table: L = live stem (including leaf or petiole) tissue; D = dead stem (including petiole) tissue; S = seeds.
Phylogenetic analyses
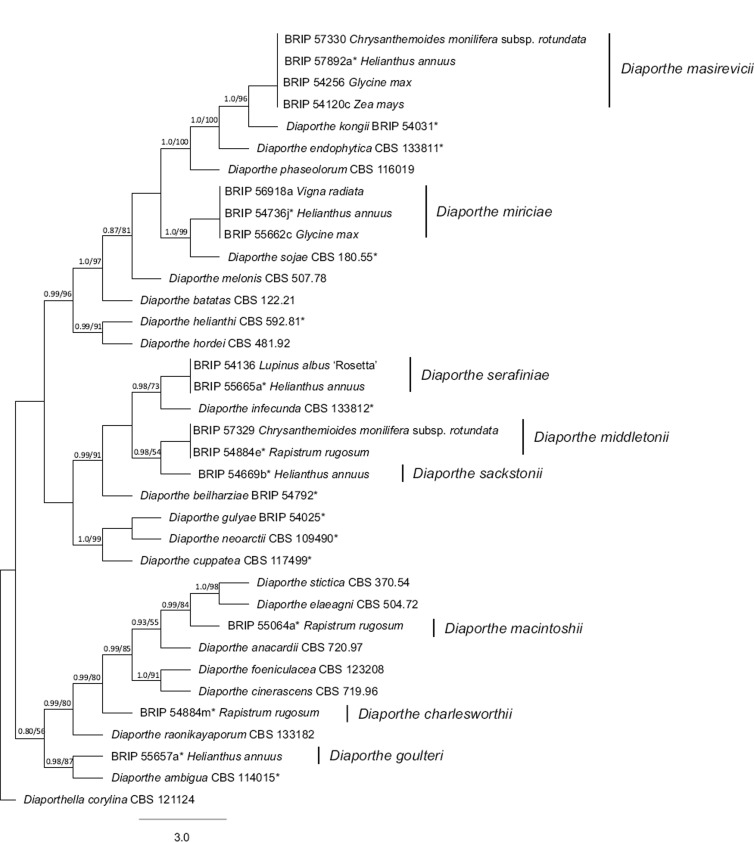
Approximately 600 bases of the ITS region were sequenced from the isolates investigated in this study and initially aligned against 116 sequences from 106 Diaporthe species, most of which were from ex-type cultures. The evolutionary relationships of these sequences were analysed using the NJ method (data not shown; TreeBASE study S15707). From this NJ phylogenetic tree, 19 Diaporthe taxa closest to the isolates in this study were selected for a combined analyses using the ITS, TEF and BT sequences. The combined sequence (ITS, TEF and BT) alignment for the Bayesian and ML analyses contained 1 642 characters from 35 isolates (including the outgroup taxon) (Table 1). The Bayesian analysis lasted 1 100 000 generations, and the consensus tree with posterior probability was calculated from 4 951 trees left after 110 000 trees were discarded at the burn-in phase. The tree topology and bootstrap values of the ML analysis supported the trees obtained from the Bayesian analysis. The multilocus phylogenetic tree (Fig. 1), along with morphological examinations (see below), support the establishment of eight novel Diaporthe species, which are described below.
Fig. 1.
Phylogenetic tree based on the combined multilocus (ITS, TEF and BT) alignment. The tree with the highest log likelihood (-8570) is shown. Bayesian posterior probabilities (pp) and RAxML bootstrap values (bs) are given at the nodes (pp/bs). Only those with bs percentage of greater than 60 are shown. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.4745)). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. Ex-type cultures are indicated by an asterisk (*).
Taxonomy
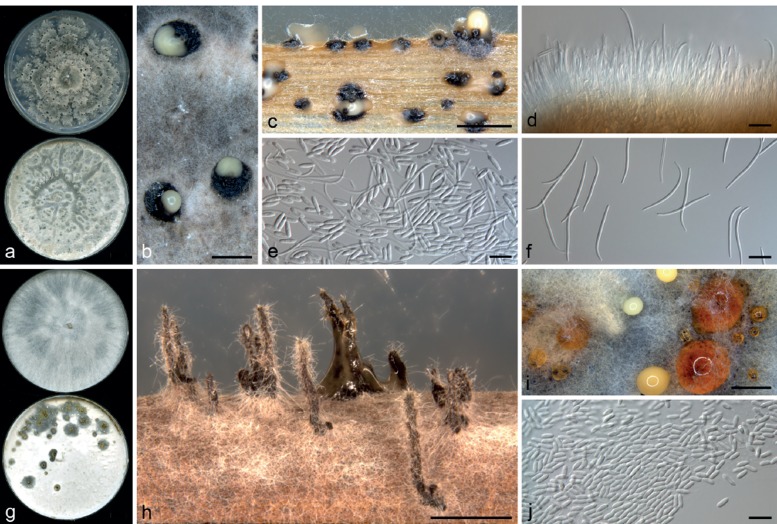
Diaporthe charlesworthii R.G. Shivas, S.M. Thomps. & Y.P. Tan, sp. nov. — MycoBank MB808668; Fig. 2a–f
Fig. 2.
Diaporthe spp. — a–f: Diaporthe charlesworthii (ex-type BRIP 54884m) after 4 wk. a. Culture on PDA (top) and OMA (bottom); b. conidiomata on OMA; c. conidiomata on PDA; d. conidiophores; e. alpha conidia and beta conidia; f. beta conidia. — g–j: Diaporthe goulteri (ex-type BRIP 55657a) after 4 wk. g. Culture on PDA (top) and OMA (bottom); h. conidiomata on sterilised wheat straw; i. conidiomata on OMA; j. alpha conidia. –– Scale bars: a, g = 1 cm; b, c, h, i = 1 mm; d–f, j = 10 μm.
Etymology. In recognition of Australian sunflower grower Kevin Charlesworth (Ryeford Qld), for his contributions to the sunflower industry and passionate advocate of research.
Conidiomata pycnidial and multilocular, scattered, abundant on PDA, OMA and WSA after 4 wk, subglobose, up to 1 mm diam, ostiolate, necks absent or up to 1 mm. Conidiophores formed from the inner layer of the locular wall, 0–2-septate, branched at septa, hyaline to subhyaline, cylindrical, 15–35 × 1.5–3 μm. Conidiogenous cells cylindrical to flexuous, tapered towards the apex, hyaline, 10–25 × 1.5–3.0 μm. Alpha conidia abundant, fusiform to cylindrical, rounded at the apex, narrowed towards the base, hyaline, (6–)7–9.5(–11) × 2–2.5 μm. Beta conidia abundant amongst the alpha conidia, flexuous to J-shaped, hyaline, 25–35 × 1.0–1.5 μm. Perithecia not seen.
Cultural characteristics — Colonies on PDA after 10 d reaching the edge of the plate, margin coralloid with feathery branches, adpressed, without aerial mycelium, with numerous irregularly zonate dark stromata up to 2 mm diam, isabelline becoming lighter towards the margin; reverse similar to the surface with zonations more apparent. On OMA covering entire plate after 10 d, with little aerial mycelium and numerous scattered pale mouse grey irregular stromata up to 1.5 cm diam, pale isabelline between the stromata; reverse irregularly mottled, cinnamon to isabelline.
Specimen examined. AUSTRALIA, Queensland, Gatton, from stem of Rapistrum rugosum, 24 Nov. 2011, S.M. Thompson (T12757Z), holotype BRIP 54884m (includes ex-type culture).
Notes — The multigene analysis of isolate BRIP 54884m was not significantly homologous to any sequences in GenBank. No morphologically similar isolates are known from Rapistrum rugosum. Therefore, this isolate is designated as representative of a new taxon. Diaporthe charlesworthii is one of three novel species isolated in this study from dead stems of R. rugosum (Brassicaceae), a widely distributed weed in eastern Australia.
Diaporthe goulteri R.G. Shivas, S.M. Thomps. & Y.P. Tan, sp. nov. — MycoBank MB808669; Fig. 2g–j
Etymology. In recognition of Australian scientist Ken Goulter, for his significant contributions to Australian sunflower pathology including the differentiation of sunflower rust races and early studies on the diversity of Diaporthe species.
Conidiomata multilocular, rare on PDA after 4 wk, abundant on OMA and WSA after 4 wk and often on a thin layer of dark textura angularis 50–100 μm thick with sharp margins on irregularly patches up to 1 cm diam, ostiolate, necks absent or less than 250 μm on PDA and OMA after 4 wk, necks up to 1.5 mm on wheat straw pieces on WA after 4 wk, abundant pale yellow conidial droplets exude from ostioles, sienna coloured droplets form on thin dark patches of textura angularis. Conidiophores formed from the inner layer of the locular wall, reduced to conidiogenous cells or 1-septate, hyaline to pale yellowish brown, filiform, 10–30 × 1.5–3 μm. Conidiogenous cells cylindrical to flexuous, tapered towards the apex, hyaline, 5–15 × 1.5–2.5 μm. Alpha conidia abundant, fusiform to cylindrical, rounded at the apex, slightly narrowed towards the base, hyaline, (6–)6.5–8(–9) × 2–2.5(–3) μm. Beta conidia not seen. Perithecia not seen.
Cultural characteristics — Colonies on PDA covering entire plate after 10 d, adpressed, white to buff; reverse buff. On OMA covering entire plate after 10 d, white tinged with pale vinaceous, with several scattered circular mouse grey patches up to 1 cm diam, these patches are sometimes confluent and at the centres have olivaceous mycelium with droplets of cinnamon coloured exudate and one or a few funiculose columns of white mycelium up to 3 mm high; reverse uniformly buff.
Specimen examined. AUSTRALIA, Queensland, Ryeford, from a seed of Helianthus annuus, 15 Feb. 2011, S.M. Thompson (T12996A); holotype BRIP 55657a (includes ex-type culture).
Notes — Cultures of D. goulteri produced a cinnamon coloured exudate under the conditions described here. It is not known if this phenotypic characteristic is taxonomically useful. A BLASTn search with the ITS sequence showed the closest match was to HQ44993 from Solidago canadensis in China, with 99 % identity (2 bp difference).
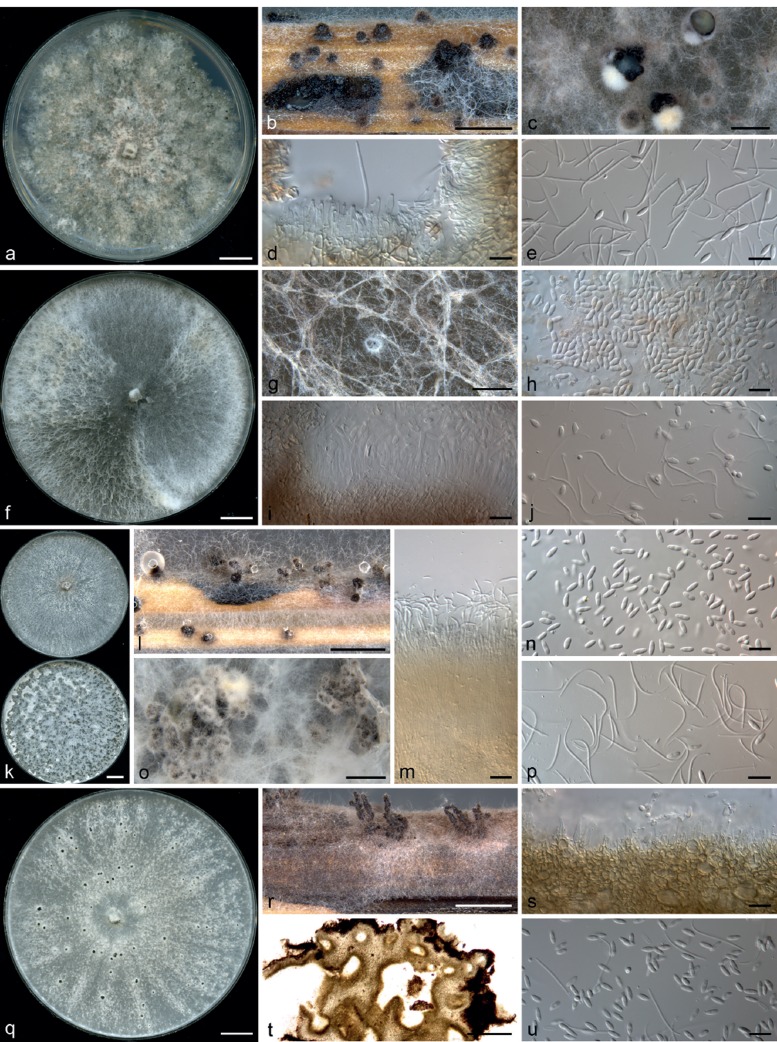
Diaporthe macintoshii R.G. Shivas, S.M. Thomps. & Y.P. Tan, sp. nov. — MycoBank MB808670; Fig. 3a–e
Fig. 3.
Diaporthe spp. — a–e: Diaporthe macintoshii (ex-type BRIP 55064a) after 4 wk. a. Culture on PDA; b. pycnidia on sterilised wheat straw; c. pycnidia on OMA; d. conidiophores; e. alpha conidia and beta conidia. — f–j: Diaporthe masirevicii (ex-type BRIP 57892a) after 4 wk. f. Culture on PDA; g. conidiomatum on OMA; h. alpha conidia; i. conidiophores; j. alpha conidia and beta conidia. — k–p: Diaporthe middletonii (ex-type BRIP 54884e) after 4 wk. k. Culture on PDA (top) and OMA (bottom); l. pycnidia on sterilised wheat straw; m. conidiophores; n. alpha conidia; o. pycnidia on OMA; p. beta conidia. — q–u: Diaporthe miriciae (ex-type BRIP 54736j) after 4 wk. q. Culture on PDA; r. conidiomata on sterilised wheat straw; s. conidiophores; t. section across conidiomatum; u. alpha and beta conidia. — Scale bars: a, f, k, q = 1 cm; b, c, g, l, o, r = 1 mm; d, e, h–j, m, n, p, s, u = 10 μm; t = 100 μm.
Etymology. In recognition of Australian agronomist Paul McIntosh, for his indefatigable and gregarious service to the Australian sunflower industry over 30 years.
Conidiomata pycnidial, solitary or aggregated in small groups, scattered, abundant on PDA, OMA and WSA after 4 wk, subglobose, up to 0.5 mm diam, ostiolate, necks absent, cream conidial droplets exuded from some ostioles. Conidiophores formed from the inner layer of the locular wall, 0–2-septate, hyaline to subhyaline, cylindrical, 10–20 × 1.5–3.5 μm. Conidiogenous cells cylindrical to flexuous, tapered towards the apex, hyaline, 10–15 × 1.5–2.5 μm. Alpha conidia abundant, fusiform to oval, narrowed towards apex and base, hyaline, (6.5–)8–11(–15) × 2–3(–3.5) μm. Beta conidia abundant amongst the alpha conidia, flexuous to hamate, hyaline, 15–30 × 1.0–1.5 μm. Perithecia not seen.
Cultural characteristics — Colonies on PDA after 10 d reaching the edge of the plate, margin coralloid, adpressed, with scattered dark stromata up to 1 mm diam, isabelline with low tufts of off white mycelium; reverse mottled buff to isabelline with darker patches corresponding to stromata. On OMA covering entire plate after 10 d, adpressed with some funiculose mycelium towards the margin, ropey, dark mouse grey, with numerous scattered dark stromata up to 2 mm diam; reverse mottled buff with irregular dark patches.
Specimen examined. AUSTRALIA, Queensland, Toowoomba, from stem of Rapistrum rugosum, 6 Dec. 2011, S.M. Thompson (T12768A); holotype BRIP 55064a (includes ex-type culture).
Notes — Diaporthe macintoshii is one of three novel species isolated in this study from dead stems of R. rugosum in south-east Qld. A BLASTn search with the ITS sequence showed the closest match was to HQ130721 from Warburgia ugandensis, with 99 % identity (6 bp difference).
Diaporthe masirevicii R.G. Shivas, L. Morin, S.M. Thomps. & Y.P. Tan, sp. nov. — MycoBank MB808671; Fig. 3f–j
Etymology. Named after the eminent Serbian plant pathologist Stevan Maširević, a distinguished member of the Yugoslavian research team who investigated the first outbreaks of D. helianthi and developed many of the techniques that are currently used to evaluate and screen sunflowers for resistance to Diaporthe.
Conidiomata pycnidial, very scarce, scattered on PDA, OMA and WSA after 4 wk, solitary, subglobose, up to 250 μm diam, ostiolate, without necks, abundant subhyaline to pale yellow conidial droplets exuded from ostioles. Conidiophores formed from the inner layer of the locular wall, 1–3-septate, hyaline to pale yellowish brown, filiform, 20–40 × 1.5–3.5 μm. Conidiogenous cells cylindrical to flexuous, tapered towards the apex, hyaline, 10–25 × 1.5–3.0 μm. Alpha conidia abundant, cylindrical, rounded at the ends, biguttulate, hyaline, (5.5–)6–7.5(–8) × 2–3 μm. Beta conidia flexuous to hamate, hyaline, 15–30 × 1.0–1.5 μm. Perithecia not seen.
Cultural characteristics — Colonies on PDA covering entire plate after 10 d, adpressed, with patches of floccose mycelium, white, sometimes with ropey hazel sectors; reverse similar to the surface. On OMA covering entire plate after 10 d, white with abundant funiculose and floccose mycelium; reverse mottled isabelline.
Specimens examined. AUSTRALIA, Queensland, Glenore Grove, from the stem of Helianthus annuus, 15 Aug. 2012, S.M. Thompson (T13228C), holotype BRIP 57892a (includes ex-type culture); Gatton, from leaf of Zea mays 31 Jan. 2011, J. McIntosh (T12539D), BRIP 54120c; unknown Queensland, from leaf of Glycine max, 20 Jan. 2011, S.M. Thompson (T12523A), BRIP 54256; New South Wales, South Bellinger Head State Park, from stem of Chrysanthemoides monilifera subsp. rotundata, 1 June 2011, L. Morin (019), BRIP 57330.
Notes — The phylogenetic inference from combined sequence data shows D. masirevicii clustered closely with D. endophytica and D. kongii (Fig. 1). Diaporthe masirevicii produced pycnidia scattered on PDA, OMA and WSA after 4 wk, compared to D. endophytica, which was sterile. Diaporthe masirevicii is distinguished from D. endophytica and D. kongii based on either ITS, TEF or BT sequences. The type of D. masirevicii was isolated from a lodged crop of sunflower, together with D. gulyae, which causes sunflower stem canker (Thompson et al. 2011). Additionally, D. masirevii is one of three novel species isolated in this study from dead stems of R. rugosum. This species was also found on Chrysanthemoides monilifera subsp. rotundata, which is an important weed of coastal dune vegetation in eastern Australia.
Diaporthe middletonii R.G. Shivas, L. Morin, S.M. Thomps. & Y.P. Tan, sp. nov. — MycoBank MB808672; Fig. 3k–p
Etymology. In recognition of Australian plant pathologist Keith Middleton, for his innovative contributions to plant pathology of summer crops, especially his early studies of sunflower rust (Puccinia helianthi) and Rhizopus sp. infection in sunflower.
Conidiomata pycnidial, up to 300 μm diam on PDA and WSA after 4 wk, aggregated in scattered groups or multilocular on a 50–100 μm thick layer of dark textura angularis with sharp margins that irregularly covers most of the agar surface on OMA after 4 wk, subglobose, ostiolate, necks absent or about 200 μm, cream conidial droplets exuded from a few ostioles. Conidiophores formed from the inner layer of the locular wall, reduced to conidiogenous cells or 1-septate, hyaline to pale yellowish brown, cylindrical, 10–25 × 1.5–3.5 μm. Conidiogenous cells cylindrical, hyaline, 5–20 × 1.5–2.5 μm. Alpha conidia abundant, fusiform to cylindrical, rounded at the apex, obconically truncate at base, mostly biguttulate, hyaline, (5–)6.0–7.5(–8) × 2–2.5(–3) μm. Beta conidia scarce abundant, flexuous, mostly J-shaped, hyaline, 20–35 × 1.0–1.5 μm. Perithecia not seen.
Cultural characteristics — Colonies on PDA covering entire plate after 10 d, with scant aerial mycelium and numerous scattered dark stromata visible as black dots, buff; reverse similar to the surface. On OMA covering entire plate after 10 d, with scattered funiculose mycelium up to 1 cm high, surface mostly leaden black with irregular faintly pale vinaceous patches towards the edge of the plate; reverse buff. Rosy vinaceous pigment produced in WA around colonised wheat straw pieces after 4 wk.
Specimens examined. AUSTRALIA, Queensland, Gatton, from stem of Rapistrum rugosum, 24 Nov. 2011, S.M. Thompson (T12757H), holotype BRIP 54884e (includes ex-type culture); New South Wales, Bongil Bongil National Park, from stem of Chrysanthemoides monilifera subsp. rotundata, 1 June 2011, L. Morin (056), BRIP 57329.
Notes — Diaporthe middletonii is one of three novel species found on R. rugosum, as well as one of three novel species found on Chrysanthemoides monilifera subsp. rotundata, which is an important weed of coastal dune vegetation in eastern Australia. A BLASTn search with the ITS sequence of the type isolate, BRIP 54884e, showed 100 % match to EF68935 from Coffea arabica in Hawaii, USA; 99 % identity (3–5 bp difference) to EU878434 from Luehea divaricata in Brazil; 99 % identity to JQ936257 from Glycine max cv. Conquista; and 99 % identity to KF467129 from Centrolobium ochroxylum in Ecuador.
Diaporthe miriciae R.G. Shivas, S.M. Thomps. & Y.P. Tan, sp. nov. — MycoBank MB808673; Fig. 3q–u
Etymology. Named after Australian scientist Elizabeth Miric, who first recognised diversity in the Australian isolates of Diaporthe (Phomopsis) on sunflower in her PhD thesis entitled: ‘Pathological, morphological and molecular studies of a worldwide collection of the sunflower pathogens Phomopsis helianthi and Phoma macdonaldii’ (University of Queensland, 2002).
Conidiomata pycnidial or multilocular, scattered or aggregated on PDA, OMA and WSA after 4 wk, solitary, ostiolate with necks up to 1 mm, pale yellow conidial droplets exuded from some ostioles. Conidiophores formed from the inner layer of the locular wall, reduced to conidiogenous cells or 1–2-septate, hyaline to subhyaline, cylindrical to obclavate, 10–20 × 1.5–3 μm. Conidiogenous cells cylindrical to obclavate, tapered towards the apex, hyaline, 5–12 × 1.5–3 μm. Alpha conidia abundant, fusiform to oval, rounded at the apex, narrowed at the base, hyaline, 6–7.5(–9) × 2–2.5(–3) μm. Beta conidia scattered or in groups amongst the alpha conidia, flexuous to hamate, hyaline, 20–35 × 1.0–1.5 μm. Perithecia not seen.
Cultural characteristics — Colonies on PDA covering entire plate after 10 d, adpressed, with a few scattered dark stromata up to 2 mm diam, buff; reverse rosy buff. On OMA covering entire plate after 10 d, ropey with a few scattered funiculose columns, white tinged with pale vinaceous with irregular pale mouse grey patches up to several cm diam associated with stromata; reverse uniformly rosy buff.
Specimens examined. AUSTRALIA, New South Wales, Premer, from stubble of Helianthus annuus, 11 Aug. 2011, S.M. Thompson (T12711M), holotype BRIP 54736j (includes ex-type culture); Queensland, Warra, from Vigna radiata, 19 Apr. 2012, S.M. Thompson (T13081F), BRIP 56918a; central Queensland, from stem of Glycine max, 28 Mar. 2012, S.M. Thompson (T13001C), BRIP 55662c.
Notes — A BLASTn search with the ITS sequence of the type isolate, BRIP 54736j, showed 100 % identity to AY148440 from Gossypium hirsutum in Australia; FJ785447 and FJ785451 from Glycine max in Mississippi, USA; KJ471541 from Melocactus ernestii in Brazil; and HF586483 from a strain identified as D. phaseolorum from a human granulomatous lesion in Brazil, although the identity of this isolate is doubtful, as the current precedent (van Rensburg et al. 2006, Mengistu et al. 2007, Santos et al. 2011, Gomes et al. 2013) is to accept strain CBS 116019 (= ATCC 64802 = FAU458) from Stokesia laevis in Mississippi, USA, as authentic for the name. Diaporthe miriciae can be easily differentiated from D. phaseolorum based on either ITS, TEF or BT loci. Diaporthe miriciae has been found on three hosts from two families and may be a widespread endophyte or saprobe in eastern Australia. Diaporthe miriciae also clusters with D. sojae, a pathogen of Glycine species, which indicates it may also be a pathogen (Fig. 1).
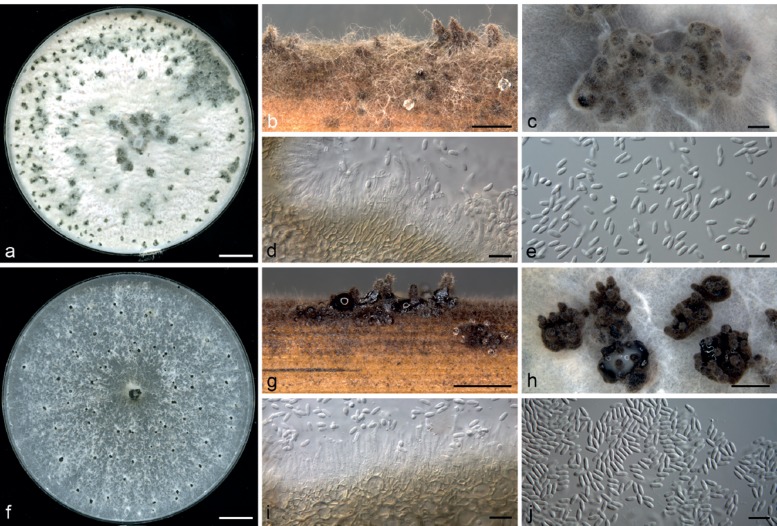
Diaporthe sackstonii R.G. Shivas, S.M. Thomps. & Y.P. Tan, sp. nov. — MycoBank MB808674; Fig. 4a–e
Fig. 4.
Diaporthe spp. — a–e: Diaporthe sackstonii (ex-type BRIP 54669b) after 4 wk. a. Culture on OMA; b. conidiomata on sterilised wheat straw; c. conidiomata on OMA; d. conidiophores; e. alpha conidia. — f–j: Diaporthe serafiniae (ex-type BRIP 55665b) after 4 wk. f. Culture on PDA; g. conidiomata on sterilised wheat straw; h. conidiomata on OMA; i. conidiophores; j. alpha conidia. –– Scale bars: a, f = 1 cm; b, g, h = 1 mm; c = 100 μm; d, e, i, j = 10 μm.
Etymology. Named after the eminent Canadian plant pathologist Waldemar E. Sackston, for his pioneering contribution to sunflower disease research on an international scale from the 1950s to the 1990s.
Conidiomata pycnidial or multilocular, solitary, scattered, scarce on PDA after 4 wk, abundant on OMA after 4 wk on a thin 50–100 μm thick layer of dark textura angularis with sharp margins that irregularly covers much of the agar surface, abundant on WSA after 4 wk, up to 1 mm diam, ostiolate, necks up to 0.5 mm, cream conidial droplets exuded from some ostioles. Conidiophores formed from the inner layer of the locular wall, reduced to conidiogenous cells or septate, filiform, 15–40 × 1.5–3 μm, hyaline to pale yellowish brown. Conidiogenous cells cylindrical to lageniform, tapered towards the apex, hyaline, 10–15 × 1.5–3.0 μm. Alpha conidia abundant, fusiform, rounded at the apex, obconically truncate at base, hyaline, 6–7(–8) × 2–2.5 μm. Beta conidia not seen. Perithecia not seen.
Cultural characteristics — Colonies on PDA covering entire plate after 10 d, adpressed, with a few scattered dark stromata up to 1 mm diam surrounded by patches of white sparse mycelium, buff; reverse isabelline with a few dark scattered stromata up to 3 mm diam. On OMA covering entire plate after 10 d, white tinged with pale vinaceous with pale mouse grey patches, with many scattered dark stromata mostly up to 4 mm diam; reverse uniformly cinnamon.
Specimen examined. AUSTRALIA, Queensland, Clermont, from a petiole of Helianthus annuus, 10 June 2011, S.M. Thompson (T12667B); holotype BRIP 54669b (includes ex-type culture).
Notes — The phylogenetic inference from the combined sequence data showed D. sackstonii clustered next to D. infecunda (Gomes et al. 2013), as well as the newly described D. serafiniae. In culture, D. sackstonii produced abundant pycnidia on PDA and OMA, compared to D. infecunda, which was sterile. Diaporthe sackstonii differs from D. serafiniae in three loci: ITS positions 40 (C), 78 (C) and 85 (G); TEF 91 % match (Identities 263/290, Gaps 8/290); BT 98 % match (Identities 635/649, Gaps 3/649).
Diaporthe serafiniae R.G. Shivas, S.M. Thomps. & Y.P. Tan, sp. nov. — MycoBank MB808675; Fig. 4f–j
Etymology. Named after the dedicated Australian agronomist Loretta Serafin, for her research on sunflower crop production and who provided the samples from which this species was isolated.
Conidiomata multilocular, scattered, abundant on PDA, OMA and WSA after 4 wk, up to 2 mm diam, ostiolate, with necks up to 1.5 mm, cream conidial droplets exuded from most ostioles. Conidiophores formed from the inner layer of the locular wall, 1-septate, hyaline to pale yellowish brown, fusiform, 15–25 × 1.5–3.5 μm. Conidiogenous cells cylindrical to flexuous, tapered towards the apex, hyaline, 5–20 × 1.5–2.5 μm. Alpha conidia abundant, fusiform, rounded at the apex, narrowed towards the base, biguttulate, hyaline, 5.5–7(–8) × 1.5–2.5(–3) μm. Beta conidia not seen. Perithecia not seen.
Cultural characteristics — Colonies on PDA covering entire plate after 10 d, adpressed, white numerous scattered dark stromata up to 2 mm diam; reverse uniformly mottled white to buff. On OMA covering entire plate after 10 d, adpressed, with numerous scattered dark stromata up to 4 mm diam; reverse uniformly isabelline.
Specimens examined. AUSTRALIA, Queensland, Glenore Grove, from seed of an ornamental variety of Helianthus annuus, 1 Apr. 2012, S.M. Thompson (T13010A), holotype BRIP 55665b (includes ex-type culture); New South Wales, from stem of Lupinus albus ‘Rosetta’, L. Serafin (T12568A), BRIP 54136.
Notes — The phylogenetic inference from the combined sequence data showed D. serafiniae clustered close to D. infecunda (Gomes et al. 2013) (Fig. 1). In culture, D. serafiniae produced abundant pycnidia on PDA and OMA, compared to D. infecunda, which was sterile.
DISCUSSION
The application of principles of genealogical concordance species concepts based on multigene phylogenetic analysis has led, in recent years, to the discovery of many new cryptic species in some important genera of plant pathogenic fungi, e.g. Colletotrichum (Damm et al. 2012a, b, Weir et al. 2012), Phyllosticta (Wikee et al. 2013) and Diaporthe (Gomes et al. 2013, Tan et al. 2013). There are about 2 000 names for Diaporthe (including Phomopsis) species in the literature (Gomes et al. 2013). Many epitypes have been recently designated for species of Diaporthe (Udayanga et al. 2012, Gomes et al. 2013), which has helped to stabilise the taxonomy of this genus. However, many Diaporthe species still lack ex-type (including epitype and neotype) cultures from which DNA is easily extracted for molecular phylogenetic analysis. Gomes et al. (2013) proposed two approaches to resolve the taxonomy of Diaporthe species – either recollect and redescribe all the existing species (which is impractical) or start again. A new start is not as daunting as it seems as the nomenclatural code that governs the naming of fungi has a tool that facilitates this approach in provision for lists of rejected as well as protected names (McNeill et al. 2012). In reality, plant pathologists and mycologists seem to have embraced a new start, as since 2010 there have been approximately 40 new species of Diaporthe described (see MycoBank, www.mycobank.org), including 12 from Australia (Thompson et al. 2011, Crous et al. 2011, 2012, Tan et al. 2013).
Colonisation of the same host plant by multiple Diaporthe species has been reported before (Farr et al. 2002, Crous & Groenewald 2005, van Niekerk et al. 2005, Thompson et al. 2011) and appears to be quite common in nature. Five of our new species were isolated from live sunflower stems. Of these five species, D. masirevicii and D. miriciae were also associated with cankers on live soybean and mungbean plants. Some new species appeared to be endophytic such as the species found on asymptomatic live maize plants and some may play a role in the dieback disease of bitou bush and tip dieback symptoms on hosts such as Sesbania cannabina and Bidens pilosa. Another group, which includes D. charlesworthi and D. macintoshii, may be primarily saprophytic, having only been isolated from decaying plant material. Detailed investigations of the pathogenicity and host range of all species are required to shed light on their ecology.
The presence of D. goulteri, D. masirevicii, D. miriciae and D. serafiniae in live crops as well as crop stubble and weed residues, highlights the potential of decaying plant material on the soil surface to act as a reservoir of inoculum for subsequent crops. It is well recognised that crop stubble aids the survival of Diaporthe species, such as D. toxica on lupins (Cowling et al. 1987), D. phaseolorum var. caulivora on soybeans (Kmetz et al. 1979), and D. helianthi on sunflower (Maširević & Gulya 1992). The role of broadleaf weed residues as an aid to survival is not well documented for many pathogenic fungal species. Our results indicate that dead weeds at the edges of cultivated fields and waterways as well as unburied weed residues, on the soil surface and amongst crop plants in low tillage systems, create a ‘brown bridge’ of dead plant material that may harbour multiple pathogenic, saprobic or endophytic species of Diaporthe. We suggest that the ‘brown bridge’ of weed residues plays as significant a role in aiding the survival of Diaporthe species. This is comparable to the ‘green bridge’ of alternative live weed hosts, such as those that facilitate survival of pathogenic Diaporthe species between cropping phases (Mihaljcevic & Muntañola-Cvetković 1985, Roy et al. 1997, Li et al. 2001, 2010, Vrandečić et al. 2010).
Of added significance for disease management is the isolation from maize of D. gulyae, a highly virulent pathogen on sunflower (Thompson et al. 2011). Diaporthe gulyae was isolated from asymptomatic maize plants, indicating endophytic colonisation. Maize is often recommended as a rotation crop to follow broadleaf crops such as sunflower, soybean and mungbean, which are susceptible to a number of damaging stem and pod cankers caused by Diaporthe species. More sampling of maize is required to confirm its possible role in the epidemiology of Diaporthe species that are pathogens of broadleaf rotational crop species. These findings support the observation by Delaye et al. (2013) and Malcolm et al. (2013) that the complex infection and survival associations between fungi and plants, including endophytic associations are poorly known.
Two species of Diaporthe isolated from sunflower, D. kongii (Thompson et al. 2011) and D. masirevicii, were also recovered from bitou bush, which is invasive in coastal dune vegetation (Vranjic et al. 2012) away from the inland broad acre cropping regions in Qld and NSW. This provides evidence that the distribution, life style and host range of many Diaporthe species may be broader than expected and more complex than currently known. Both sunflower and bitou bush belong to the Asteraceae, and whether this is significant with respect to the possible hosts and distribution of these fungi is not known.
There have been 20 species, including those from this study, of Diaporthe described from Australia since 2010 (Thompson et al. 2011, Crous et al. 2011, 2012, Tan et al. 2013). Some have been identified as significant plant pathogens although the ecological significance of most is not known. This study starts to address the case that Hyde et al. (2010) made to reassess and revise plant-associated pathogens, especially Diaporthe, in order to preserve the effective role that biosecurity agencies play in keeping unwanted plant pathogens out of Australia. Although the host range and pathogenicity of these eight newly described Diaporthe species is largely unknown, our study highlights the importance of both ‘green bridges’ and ‘brown bridges’ in the epidemiology of Diaporthe species.
Acknowledgments
Broad acre component: The authors would like to acknowledge the Queensland Department of Agriculture, Fisheries and Forestry (DAFFQ), the Grains Research and Development Corporation (GRDC), the University of Queensland (UQ), as well as the generous assistance of growers and advisors. Additionally, we acknowledge Drs Tom Gulya (USDA-ARS), Alistair McTaggart (UQ), Vu Tuan Nguyen (DAFFQ), Malcolm Ryley (DAFFQ), and Ms Ella Trembizki (DAFFQ) for their technical and philosophical support of this study. Bitou bush component: This study was supported by CSIRO, NSW National Parks and Wildlife Service, and the Australian Government National Weeds and Productivity Research Program administered by the Rural Industries Research and Development Corporation. We thank Mr Shamsul Hoque (CSIRO Plant Industry) and Ms Ruth Aveyard (CSIRO Ecosystem Sciences) for technical assistance, and also acknowledge the range of stakeholders for their support and/or permission to collect samples on their land.
REFERENCES
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Cowling WA, Hamblin J, Wood P McR, et al. 1987. Resistance to Phomopsis Stem Blight in Lupinus angustifolius L. Crop Science 27: 648–652. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Groenewald JZ. 2005. Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463–470. [Google Scholar]
- Crous PW, Groenewald JZ, Shivas RG, et al. 2011. Fungal Planet description sheets:69–91. Persoonia 26: 108–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. 2012. Fungal Planet description sheets: 107–127. Persoonia 28: 138–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. 2012a. The Colletotrichum boninense species complex. Studies in Mycology 73: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. 2012b. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaye L, Garcia-Guzman G, Heil M. 2013. Endophytes versus biotrophic and necrotrophic pathogens – are fungal lifestyles evolutionarily stable traits? Fungal Diversity 60: 125–135. [Google Scholar]
- Farr DF, Castlebury LA, Rossman AY. 2002. Morphological and molecular characterization of Phomopsis vaccinii and additional isolates of Phomopsis from blueberry and cranberry in the eastern United States. Mycologia 94: 494–504. [PubMed] [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhiza and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Glass NL, Donaldson G. 1995. Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RR, Glienke C, Videira SIR, et al. 2013. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183–189. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Hyde KD, Chommunti P, Crous PW, et al. 2010. A case for re-inventory of Australia’s pathogens. Persoonia 25: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Asimenos G, Toh H. 2009. Multiple alignment DNA sequences with MAFFT. Methods in Molecular Biology 537: 39–64. [DOI] [PubMed] [Google Scholar]
- Kmetz T, Kmetz C, Ellett W, et al. 1979. Soybean seed decay: Sources of inoculum and nature of infection. Phytopathology 69: 798–801. [Google Scholar]
- Li S, Bradley CA, Hartman GL, et al. 2001. First report of Phomopsis longicolla from velvetleaf causing stem lesions on inoculated soybean and velvetleaf plants. Plant Disease 85:1031. [DOI] [PubMed] [Google Scholar]
- Li S, Hartman GL, Boykin DL. 2010. Aggressiveness of Phomopsis longicolla and other Phomopsis spp. on soybean. Plant Disease 94: 1035–1040. [DOI] [PubMed] [Google Scholar]
- Librado P, Rozas J. 2009. DnaSP v. 5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- Malcolm GM, Kuldau GA, Gugino BK, et al. 2013. Hidden host plant associations of soilborne fungal pathogens: an ecological perspective. Phytopathology 103: 538–544. [DOI] [PubMed] [Google Scholar]
- Maširević S, Gulya TJ. 1992. Sclerotinia and Phomopsis – two devastating sunflower pathogens. Field Crops Research 30: 271–300. [Google Scholar]
- McNeill J, Barrie FR, Buck WR, et al. (eds). 2012. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). [Regnum vegetabile no. 154.] Koeltz Scientific Books, Königstein, Germany. [Google Scholar]
- Mengistu A, Castlebury LA, Smith JR, et al. 2007. Isolates of Diaporthe-Phomopsis and their effect on soybean. Canadian Journal of Plant Pathology 29: 283–289. [Google Scholar]
- Mihaljcevic M, Muntañola-Cvetković M. 1985. Responses of sunflower to different Phomopsis isolates. In: Proceedings of the XI International Sunflower Conference, Mar del Plata, Argentina: 419–424. [Google Scholar]
- Muralli TS, Suryanarayanan TS, Geeta R. 2006. Endophytic Phomopsis species: host range and implications for diversity estimates. Canadian Journal of Microbiology 52: 673–680. [DOI] [PubMed] [Google Scholar]
- Niekerk JM, van Groenewald JZ, Farr DF, et al. 2005. Reassessment of Phomopsis species on grapevines. Australasian Plant Pathology 34: 27–39. [Google Scholar]
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Gueidan C, Sink S, et al. 2009. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genetics and Biology 46: 936–948. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Humber RA, Geiser DM, et al. 2012. Phylogenetic diversity of insecticolous fusaria inferred from multilocus DNA sequence data and their molecular identification via FUSARIUM-ID and Fusarium MLST. Mycologia 104: 427–445. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Kistler HC, Cigelink E, et al. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of the United States of America 95: 2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute, Kew, UK. [Google Scholar]
- Rehner SA, Uecker FA. 1994. Nuclear ribosomal internal transcribed spacer phylogeny and host diversity in the coelomycete Phomopsis. Canadian Journal of Botany 72: 1666–1674. [Google Scholar]
- Rensburg JCJ, van Lamprecht SC, Groenewald JZ, et al. 2006. Characterisation of Phomopsis spp. associated with die-back of rooibos (Aspalathus linearis) in South Africa. Studies in Mycology 55: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Roy KW, Ratnayake S, McLean K. 1997. Colonization of weeds by Phomopsis longicolla. Canadian Journal of Plant Pathology 19: 193–196. [Google Scholar]
- Santos JM, Correia VG, Phillips AJ. 2010. Primers for mating-type diagnosis in Diaporthe and Phomopsis: their use in teleomorph induction in vitro and biological species definition. Fungal Biology 114: 255–270. [DOI] [PubMed] [Google Scholar]
- Santos JM, Vrandečić K,, Ćosić J, et al. 2011. Resolving the Diaporthe species occurring on soybean. Persoonia 27: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivas RG, Cai L. 2012. Cryptic fungal species unmasked. Microbiology Australia 33: 36–37. [Google Scholar]
- Smith D. 2002. Culturing, preservation and maintenance of fungi. In: Waller JM, Lenné JM,, Waller SJ.(eds), Plant Pathologist’s Pocketbook. 3rd ed: 384–409. CAB Publishing, UK. [Google Scholar]
- Stamatakis A, Alchiotis N. 2010. Time and memory efficient likelihood-based tree searches on phylogenomic alignments with missing data. Bioinformatics 26: i132–i139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YP, Edwards J, Grice KRE, et al. 2013. Molecular phylogenetic analysis reveals six new Diaporthe species from Australia. Fungal Diversity 61: 251–260. [Google Scholar]
- Thompson SM, Tan YP, Young AJ, et al. 2011. Stem cankers on sunflower (Helianthus annuus) in Australia reveal a complex of pathogenic Diaporthe (Phomopsis) species. Persoonia 27: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayanga D, Castlebury LA, Rossman AY, et al. 2014. Species limits in Diaporthe: molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D.rudis. Persoonia 32: 83–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayanga D, Liu XX, Crous PW, et al. 2012. A multi-locus phylogenetic evaluation of Diaporthe (Phomopsis). Fungal Diversity 56: 157–171. [Google Scholar]
- Udayanga D, Xingzhong L, McKenzie EHC, et al. 2011. The genus Phomopsis: biology, applications, species concepts and names of common pathogens. Fungal Diversity 50: 189–225. [Google Scholar]
- Vasilyeva LN, Rossman AY, Farr DF. 2007. New species of the Diaporthales from eastern Asia and eastern North America. Mycologia 99: 916–923. [DOI] [PubMed] [Google Scholar]
- Vrandečić K, Jurković D, Riccioni L, et al. 2010. Xanthium italicum, Xanthium strumarium and Arctium lappa as new hosts for Diaporthe helianthi. Mycopathologia 170: 51–60. [DOI] [PubMed] [Google Scholar]
- Vranjic J, Morin L, Reid A, et al. 2012. Integrating revegetation with management methods to rehabilitate coastal vegetation invaded by Bitou bush (Chrysanthemoides monilifera subsp. rotundata) in Australia. Australasian Ecology 37: 78–89. [Google Scholar]
- Weir BS, Johnston PR, Damm U. 2012. The Colletotrichum gloeosporioides species complex. Studies in Mycology 73: 115–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In:Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: a guide to methods and applications: 315–322. Academic Press, San Diego, USA. [Google Scholar]
- Wikee S, Lombard L, Crous PW, et al. 2013. Phyllosticta capitalensis, a widespread endophyte of plants. Fungal Diversity 60: 91–105. [Google Scholar]