Abstract
Background and study aims: Endoscopic submucosal dissection (ESD) is a standard treatment for early gastric cancer (EGC) without lymph node metastasis. However, some patients undergo noncurative ESD. The aim of the present study was to assess the long-term clinical outcomes of noncurative ESD with or without additional surgery.
Patients and methods: We investigated the chart data from all patients who had undergone ESD for EGC at Saga Medical School Hospital and Saga Prefectural Medical Centre Koseikan between 2001 and 2012. A total of 957 cases (1047 lesions) of EGC underwent ESD, and 99 had noncurative ESD. In total, 20 cases were excluded because their follow-up period was < 3 years. We divided the patients into observation and additional surgery groups, and we compared the survival rate and related factors between the groups.
Results: After noncurative ESD, 28 /79 patients (35.4 %) underwent additional surgery and 51/79 (64.6 %) were followed up without surgery. The average age of patients in the observation group was higher than that of the additional surgery group (75.9 vs. 71.6 years; P = 0.03). The incidence of hypertension was significantly higher in the observation group compared with the additional surgery group (51.0 vs. 25.9 %; P = 0.03). The overall survival rate of the additional surgery group was longer than that of the observation group. However, only one patient died from gastric cancer in the observation group. The disease-specific survival rate did not differ significantly between the groups.
Conclusions: It might be acceptable to follow up without additional surgery for some patients with comorbidity and who were elderly after noncurative ESD for EGC.
Introduction
In Japan, endoscopic submucosal dissection (ESD) is a standard treatment for early gastric cancer (EGC) with a negligible risk of lymph node metastasis. According to the Japanese Gastric Cancer Treatment Guidelines 2010 (version 3), ESD is acceptable according to the guideline and expanded criteria [1]. The guideline criteria include cases with differentiated type adenocarcinoma in which the depth of invasion is T1a and the diameter is ≤ 2 cm, without ulcerative findings. The expanded criteria include the following: (1) differentiated mucosal cancer without ulcerative findings, > 2 cm in diameter; (2) differentiated mucosal cancer with ulcerative findings, ≤ 3 cm in diameter; (3) differentiated submucosal invasive cancer < 500 µm, ≤ 3 cm in diameter; and (4) poorly differentiated mucosal cancer without ulcerative findings, ≤ 2 cm in diameter. EGC patients who meet the guideline or expanded criteria are usually treated by ESD, and further treatment is not required in the case of pathologically proven cancer restricted to the resected specimen. It is reported that the long-term outcomes of ESD for EGC are favorable in patients within the guideline and expanded criteria [2 – 11].
Patients who have undergone noncurative ESD are considered for additional surgery. Some reports recommend additional surgery to prevent lymph node or distant metastasis in patients who have undergone noncurative ESD [8, 12 – 15]. However, in the aged population in Japan, some patients with noncurative ESD are followed up without additional surgery, because of the risk associated with comorbid disease, low performance status, or patients’ refusal of additional treatment. Aged patients who undergo noncurative ESD sometimes die of diseases other than cancer recurrence. Thus, there is reason to examine the efficacy of additional surgery. The aim of the present study was to assess the long-term clinical outcomes of noncurative ESD with or without additional surgery.
Methods
Patients
We reviewed the chart data of all patients who underwent ESD for EGC at Saga Medical School Hospital and Saga Prefectural Hospital between January 2001 and December 2012. A total of 957 patients (1047 lesions) with EGC underwent ESD: 858 had curative ESD and 99 had noncurative ESD. Among the latter 99 patients, 20 were excluded because their follow-up period was < 3 years. The remaining 79 patients with noncurative ESD were divided into two groups. Patients in the first group received additional surgery after noncurative ESD. Patients in the second group did not receive additional surgery after noncurative ESD and were followed up periodically with routine examination alone. Patients’ characteristics, clinicopathological characteristics of cancerous lesions, adverse events, and long-term outcomes were retrospectively assessed. Patient characteristics included age, sex, and concomitant disease. Chronic hepatitis B and C were included in chronic liver disease. Chronic kidney disease was defined as an estimated glomerular filtration rate < 30 mL/min/1.73 m2.
The clinicopathological characteristics and adverse events were evaluated: ESD adaptation; en bloc resection; bleeding that required hemostasis and/or blood transfusion; and perforation during ESD. Pathological findings from the resected specimens included tumor size, histological type, ulcerative findings (Ul), depth of invasion, lymphatic (ly) and/or venous (v) invasion, and resection margins [horizontal margin (HM) and vertical margin (VM)]. In the additional surgery group, the types of operative procedure performed after noncurative ESD were studied. In the observation group, avoidance of additional surgery was based on the condition of the patients or their refusal. The long-term outcomes were analyzed: overall survival and disease-specific survival, including local recurrence; metachronous gastric cancer; and lymph node/distant metastasis. According to the Japanese Gastric Cancer Treatment Guidelines 2010 (version 3), a curative resection was judged when the specimen was evaluated histologically: en block resection, tumor size ≤ 2 cm, differentiated type, pT1a, ly(–), v(–), HM0 (horizontal margin negative), VM0 (vertical margin negative). In addition, lesions that met the expanded criteria were applicable to curative resection: en block resection, ly(–), v(–), HM0, VM0, and (1) tumor size > 2 cm, differentiated type, intramucosal cancer, Ul(–) histologically; (2) tumor size ≤ 3 cm, differentiated type, intramucosal cancer, Ul(+) histologically; (3) tumor size ≤ 2 cm, undifferentiated type, intramucosal cancer, Ul(–) histologically; (4) tumor size ≤ 3 cm, differentiated type, sm1 (submucosal invasion of less than 500 µm) histologically. Cases that deviated from these criteria were considered noncurative resection.
This retrospective study was approved by the Saga Medical School Hospital Ethics Committee (2014 – 04 – 05) and conducted in accordance with the Declaration of Helsinki.
Indications for ESD
Indications for ESD for EGC were as follows: (1) differentiated carcinoma; (2) no findings of submucosal invasion; and (3) tumor size ≤ 3 cm with ulcerative findings. We usually performed endoscopy and abdominal computed tomography (CT) to assess depth of invasion and tumor staging. Radiologic examination of the upper gastrointestinal tract or endoscopic ultrasonography was additionally performed as required. In total, 17 patients who did not meet these indications were treated with ESD because of comorbidity, old age, or at their own request.
ESD procedure
We used white light endoscopy, chromoendoscopy with indigo carmine solution, and narrow-band imaging to determine the demarcation of EGC. Marking was performed by a needle-type knife, and was maintained at 5 mm lateral from the lesion. Hypertonic saline mixed with epinephrine (1 : 10 000) and sodium hyaluronate were injected into the submucosal layer to lift the lesion. Mucosal incision and submucosal dissection were performed with an IT Knife (Olympus Medical Systems, Tokyo, Japan), IT Knife 2 (Olympus Medical Systems), Hook Knife (Olympus Medical Systems), Dual Knife (Olympus Medical Systems), and Flush Knife (Fujifilm, Tokyo, Japan) to achieve complete resection. High-frequency generators (ICC 200 or VIO 300D; ERBE Electromedizin GmbH, Tübingen, Germany) were used during ESD.
Post-ESD management
Patients who underwent noncurative ESD were evaluated as to whether additional surgery with lymph node dissection was possible. Usually, the surveillance endoscopy was performed annually. In addition, CT was performed twice yearly for at least 5 years after surgery. Patients with noncurative ESD without additional surgery due to concomitant disease, old age, or refusal were followed up closely. Surveillance esophagogastroduodenoscopy was performed at 6, 12, 18, and 24 months after noncurative ESD. After that, surveillance endoscopy was performed annually. In addition, abdominal CT was performed once or twice yearly to detect lymph node or distant metastasis.
Statistical analysis
Categorical variables were expressed as the actual number (percentage), and continuous variables were expressed as means (standard deviation). To evaluate the differences in patients’ characteristics, clinicopathological characteristics, and adverse events between the two groups, the χ 2 test or Fisher’s exact test was performed for categorical variables, and Student’s t test or Mann-Whitney U test for continuous variables. Overall and disease-specific survival curves were calculated using the Kaplan – Meier method, and analyzed by log-rank test. In addition, Cox proportional hazard model was proposed for adjusting possible confounding variables such as age, sex, concomitant disease, sm2 (submucosal invasion of more than 500 µm), and lymphovascular invasion. A P value < 0.05 was considered a statistically significant difference. All statistical analyses were performed with SPSS version 22 (SPSS, Tokyo, Japan).
Results
Patient characteristics
Among 957 cases of EGC, 858 (89.7 %) underwent successful curative ESD and 99 (10.3 %) had noncurative ESD. Among the latter 99 patients, 20 were excluded. There were eight cases with follow-up periods < 3 years because the ESD was performed in 2012, and 12 cases were lost during the follow-up period. Of the remaining 79 patients with noncurative ESD, 28 (35.4 %) underwent additional surgery and 51 (64.6 %) were followed up without surgery (Fig. 1). Patients’ characteristics are summarized in Table 1. The mean age of patients with additional surgery was 71.6 ± 1.6 years compared with 75.9 ± 1.1 years in those without additional surgery. The sex ratio (men/women) was 20 : 8 and 40 : 11 in those with and without additional surgery, respectively, and did not differ significantly between the groups. The incidence of hypertension was significantly higher in the observation alone group compared with the additional surgery group (51.0 vs. 25.9 %; P = 0.03). The incidence of other comorbidities, such as cardiac disease, cerebrovascular disease, chronic liver disease, chronic kidney disease, and diabetes did not differ between the two groups. There was no case with concomitant cancer in other organs at the time of deciding on ESD.
Fig. 1.
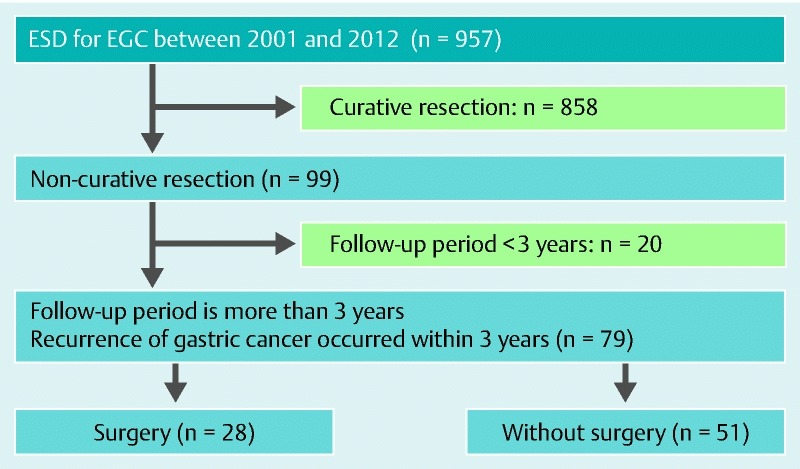
Flowchart for the patients included in this study. ESD, endoscopic submucosal dissection; ECG, early gastric cancer.
Table 1. Patient characteristics.
| Noncurative ESD with surgery (n = 28) |
Noncurative ESD without surgery (n = 51) |
P value | |
| Age, mean (SD) | 71.6 (1.6) | 75.9 (1.1) | 0.031 |
| Gender ratio, men/women | 20 : 8 | 40 : 11 | 0.49 |
| Concomitant disease (%) | |||
| Hypertension | 7 (25.9) | 25 (51.0) | 0.031 |
| Cardiac disease | 5 (18.5) | 12 (24.5) | 0.55 |
| Cerebrovascular disease | 2 (7.4) | 6 (12.2) | 0.51 |
| Chronic liver disease | 3 (11.1) | 4 (8.2) | 0.67 |
| Chronic kidney disease | 2 (7.4) | 3 (6.1) | 0.83 |
| Diabetes | 6 (22.2) | 10 (20.4) | 0.85 |
| Other diseases | 1 (3.7) | 5 (10.2) | 0.32 |
ESD, endoscopic submucosal dissection.
P < 0.05.
Clinicopathological characteristics and adverse event of ESD
Clinicopathological findings of the two groups are included in Table 2. The evaluation of ESD adaptation did not differ between the two groups. Tumor size, type of differentiation, en bloc resection, ulcerative findings, depth of invasion, resection margin, and lymph and/or venous invasion did not differ significantly between the groups. Although the rate of positive VM did not differ significantly, patients who had positive VMs tended to undergo additional surgery (21.4 vs. 7.8 %; P = 0.08). There was no patient whose noncurative factor was a positive horizontal margin or piecemeal resection only.
Table 2. Clinicopathological characteristics and adverse event of ESD.
| Noncurative ESD with surgery (n = 28) |
Noncurative without surgery (n = 51) |
P value | |
| ESD adaptation | |||
| Guideline criteria/expanded criteria/outside the guideline | 15 : 8 : 5 | 23 : 16 : 12 | 0.75 |
| Tumor size (SD) | 25.0 (2.3) | 25.6 (1.7) | 0.83 |
| Histology | |||
| tub1, tub2, pap/por, sig | 27 : 1 | 44 : 7 | 0.12 |
| En bloc resection (%) | 28 (100) | 50 (98.0) | 0.35 |
| Ulcerative findings (%) | 1 (3.6) | 9 (17.7) | 0.07 |
| Depth | |||
| m/sm1/sm2 | 3 : 3 : 22 | 6 : 14 : 31 | 0.20 |
| HM(+) (%) | 0 (0) | 2 (3.9) | 0.29 |
| VM(+) (%) | 6 (21.4) | 4 (7.8) | 0.08 |
| ly(+) or/and v(+) (%) | 13 (46.4) | 19 (37.2) | 0.43 |
| Perforation (%) | 2 (7.1) | 6 (11.8) | 0.51 |
| Delayed bleeding (%) | 2 (7.1) | 3 (5.9) | 0.83 |
ESD, endoscopic submucosal dissection; tub1, well differentiated adenocarcinoma; tub2, moderately differentiated adenocarcinoma; pap, papillary adenocarcinoma; por, poorly differentiated adenocarcinoma; sig, signet ring cell carcinoma; HM, horizontal margin; VM, vertical margin; ly, lymphatic invasion; v, venous invasion.
The rate of adverse events after ESD, such as perforation and delayed bleeding, did not differ significantly between the additional surgery and observation groups (7.1 vs. 11.8 %, and 7.1 vs. 5.9 %, respectively).
Additional surgery was performed in 28 patients (35.4 %) who had noncurative ESD. Among the patients who underwent additional surgery, 10 (35.7 %) received laparoscopy-assisted distal gastrectomy, four (14.3 %) underwent laparoscopy-assisted proximal gastrectomy, two (7.1 %) underwent laparoscopy-assisted total gastrectomy, 10 (35.7 %) underwent distal gastrectomy, and one each (3.6 %) underwent proximal gastrectomy and total gastrectomy. One patient had lymph node metastasis and two had residual cancer according to postoperative histological examination. The main reasons for no additional surgery after noncurative ESD were patients’ decision (46/51; 90.2 %) and surgeons’ rejection (5/51; 9.8 %) because of comorbidity, or old age.
Clinical course and survival
Table 3 summarizes the clinical course and long-term outcome after noncurative ESD. The median follow-up period of patients with additional surgery and those with follow-up alone was 59.0 and 51.0 months, respectively. During follow-up, local recurrence was not detected among patients with additional surgery and none died from recurrent gastric cancer. In contrast, four patients (7.8 %) who received follow-up alone had local recurrence. Among the recurrence group, two patients underwent argon plasma coagulation therapy, one underwent yttrium aluminum garnet laser ablation, and one did not receive additional treatment. Metachronous cancerous lesions were observed in one patient (3.6 %) in the additional surgery group and in two (3.9 %) in the observation alone group. These three patients underwent repeat ESD for metachronous gastric cancer. Lymph node metastasis was observed in one case in the observation alone group at 17 months after ESD. Although total gastrectomy was performed later, the patient died from recurrent gastric cancer with lymph node metastasis and peritoneal dissemination. The patient was an 80-year-old man at the time of first ESD. Lesion characteristics were as follows: tumor size, 25 mm; histology, differentiated type; depth of invasion, sm2; lymphovascular involvement, ly+/v–; tumor margin, HM0 /VM0. He died 2 years after the first ESD.
Table 3. Cancer recurrence, and long-term outcomes post-ESD.
| Noncurative ESD with surgery (n = 28) |
Noncurative ESD without surgery (n = 51) |
P value | |
| Median follow-up period, months | 59.0 | 51.0 | 0.37 |
| Recurrence type (%) | |||
| Local tumor recurrence | 0 (0) | 4 (7.8) | 0.13 |
| Metachronous gastric cancer | 1 (3.6) | 2 (3.9) | 0.94 |
| Lymph node metastasis | 0 (0) | 1 (2.0) | 0.46 |
| Cause of death (%) | |||
| Gastric cancer | 0 (0) | 1 (2.0) | 0.46 |
| Other diseases | 2 (7.1) | 13 (25.5) | < 0.051 |
ESD, endoscopic submucosal dissection.
P < 0.05.
Fig. 2 shows the long-term survival rate by Kaplan – Meier method. The overall 5-year survival rate of patients with additional surgery was 91.7 %, and that of patients without additional surgery was 75.3 %. There was a significant difference in overall survival rate between the two groups (P = 0.04) (Fig. 2 a). However, when we focus on disease-specific survival, the 5-year survival rate of patients with additional surgery was 100 % and that of patients without additional surgery was 97.8 %. There was no significant difference between the two groups (P = 0.44) (Fig.2 b). Eventually, 16 patients (20.3 %) in the two groups died during the study. Fifteen of these patients died from diseases other than gastric cancer. Causes of death were lung cancer (n = 3), colon cancer (n = 2), peritoneal cancer (n = 1), pneumonia (n = 4), liver cirrhosis (n = 1), senility (n = 1), respiratory failure (n = 1), sudden death of unknown cause (n = 1), and chronic heart failure (n = 1). Only one patient with noncurative ESD who received observation alone died from gastric cancer. Table 4 shows multivariate analysis using a Cox proportional hazards model. There was no significant difference in overall survival between the two groups (hazard ratio [95 % confidence interval]: 0.29 [0.06 – 1.45]).
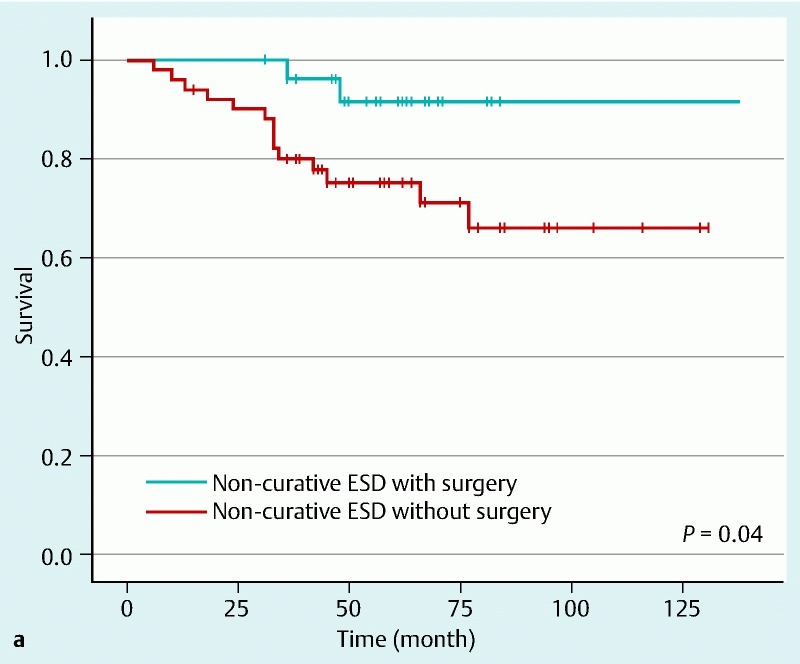
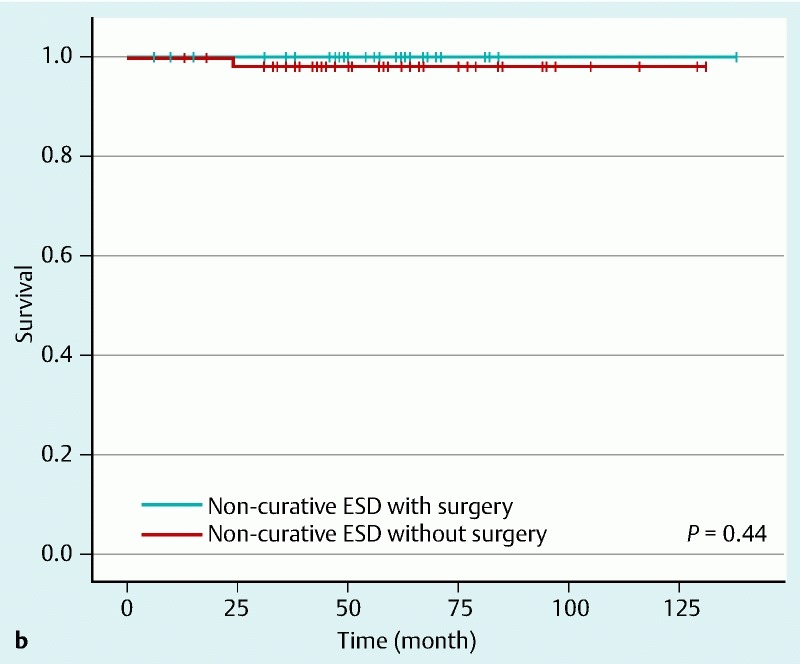
Table 4. Hazard ratio and 95 % confidence intervals of overall survival using Cox proportional hazards model.
| Number of deaths (death from gastric cancer) | Person-years | Univariate analysis | Multivariate analysis1 | |||
| HR | 95 %CI | HR | 95 %CI | |||
| Noncurative ESD without surgery (n = 51) | 14 (1) | 242.2 | 1.0 | 1.0 | ||
| Noncurative ESD with surgery (n = 28) | 2 (0) | 141.4 | 0.24 | 0.05 – 1.05 | 0.29 | 0.06 – 1.45 |
ESD, endoscopic submucosa dissection; HR, hazard ratio; CI, confidence interval.
Adjusted for age, sex, concomitant disease, sm2 invasion, and lymphovascular invasion.
Fig. 2 Kaplan-Meier survival curves. Solid lines show survival of patients who underwent additional surgery after noncurative ESD. Broken lines show survival of patients who did not undergo additional surgery. a Overall 5-year survival rate of patients with additional surgery was 91.7 %, and that of patients without additional surgery was 75.3 %. There was a significant difference in overall survival rate between the groups (P = 0.04). b When the focus was on disease-specific survival, the 5-year survival rate of patients with additional surgery was 100 % and that of patients without additional surgery was 97.8 %. There was no significant difference between the groups (P = 0.44).
Discussion
This retrospective study showed that the overall survival rate of EGC patients who underwent noncurative ESD with additional surgery was higher than that of patients without additional surgery who received follow-up alone. However, the disease-specific survival rate did not differ significantly between the groups. In addition, there were no significant differences in overall survival between the groups using multivariate analysis.
According to a previous retrospective analysis of a large number of surgical cases of EGC, patients who met the guideline or expanded criteria had a negligible risk of lymph node metastasis [16, 17]. The long-term outcomes of gastric ESD have been investigated previously [2 – 11]. Long-term survival of EGC patients undergoing ESD with expanded criteria was similar to that in patients undergoing ESD with guideline criteria. In addition, ESD can achieve similar oncological and better perioperative outcomes when compared with radical gastrectomy for treatment of EGC [7, 18]. Therefore, ESD is accepted as an initial treatment for EGC with a negligible risk of lymph node metastasis. Patients with noncurative ESD should undergo additional surgery, considering the risk of lymph node metastasis. However, some patients with noncurative ESD are followed up without additional surgery because of the risk of comorbidity, low performance status, or patients’ refusal of additional treatment. Some studies have reported that additional surgery should be considered after noncurative ESD because of the possibility of lymph node metastasis [12, 19]. However, disease-specific survival was not significantly different between the additional surgery and observation groups in our study. This may have been because the mean patient age and rate of concomitant disease were higher than those in previous studies. It is generally considered that prognosis is worse in elderly cancer patients because of the causes of high mortality other than cancer. In our series, the mortality rate from other diseases in the observation alone group was significantly higher than in the patients with additional surgery (25.5 vs. 7.1 %; P < 0.05). Therefore, one of the reasons for the lack of a significant difference in disease-specific survival between the two groups was death from other diseases before gastric cancer recurrence.
Another study that was limited to patients aged > 75 years reported that additional surgery improved overall and disease-free survival compared with observation alone in patients with noncurative ESD [14]. In that study, death from gastric cancer recurrence was observed in 4.9 % of the group of noncurative ESD patients without additional surgery. A large number of patients without additional surgery died from other diseases (24.4 %). In the present study, mortality due to gastric cancer was observed in only one (2.0 %) patient in the observation alone group. This difference in gastric cancer mortality might have been caused by the difference in comorbidity. Some comorbidity was observed in 65.8 % of patients in our study. According to a recent study from Korea on the outcome of noncurative ESD for EGC, the overall and disease-free survival did not differ significantly between patients who were simply followed up and those who received additional surgery [20]. Although that study focused on submucosal invasive gastric cancer that resulted in noncurative ESD, and the mean age was younger than in our study, the results were similar to the present study. There are problems in that the mean life expectancy differs between Japan and Korea, and comorbidity was not described in the previous study. Considering recurrence, additional surgery should be performed in patients who have undergone noncurative ESD. However, most deaths were due to other diseases in both groups (19 %; 15/79). Our postoperative histological findings showed that two patients (7.1 %) had residual cancer and one (3.6 %) had lymph node metastasis among those treated with additional surgery for noncurative ESD. Residual cancer or lymph node metastasis was only observed in these patients. Based on these results, additional surgery for noncurative ESD may not always be necessary.
The number of elderly people is increasing rapidly worldwide [21, 22]. Although the incidence of gastric cancer has decreased in the general population, it is the third largest cause of cancer mortality worldwide [23]. Also, gastric cancer mortality among aged people is increasing as a result of prolonged life expectancy. Considering these facts, there is likely to be an increase in noncurative ESD for EGC, and in the number of patients who are followed up without additional surgery because of their old age or comorbidity. Previous studies have reported that diseases other than cancer accounted for 34 – 37 % of total deaths in elderly patients with gastric cancer [24, 25]. In our present study, most of the patients achieved cancer-free survival even in cases of noncurative ESD without additional surgery. Therefore, indications for additional surgery after noncurative ESD should be considered carefully to improve long-term prognosis. The natural history of EGC is still unclear. It has not been fully investigated whether ESD for EGC improves longevity or quality of life for elderly patients who have comorbidity. It is necessary to investigate whether ESD for EGC is really adequate for these patients.
The present study had several limitations. First, this was a retrospective study and had a potential for selection bias. Patients in the observation group may have included patients in poorer condition than in the additional surgery group. The average age of the observation alone group was higher than that of the additional surgery group. However, selection bias can be minimized by multivariate analysis. Second, because this study was conducted in two centers, endoscopic diagnosis before ESD and ESD procedures was performed by independent endoscopists. The indications for ESD and ESD procedures may have differed between the facilities. However, all doctors involved in this study belonged to the Japan Gastroenterological Endoscopy Society and ESD procedures were performed by specialists authorized by the Society. Third, it was difficult to investigate the prognostic factors for gastric cancer recurrence because there was a low frequency of lymph node or distant metastasis after noncurative ESD. In future, a large prospective cohort study is required to investigate these questions.
In conclusion, although the risk of gastric cancer recurrence remains, survival of patients with noncurative ESD for EGC and follow-up without additional surgery is comparable to that in patients with additional surgery.
It might be acceptable to follow up without additional surgery for some EGC patients with comorbidity and old age who have undergone noncurative ESD. However, further studies are required to establish the appropriate treatment strategy.
Footnotes
Competing interests: None
References
- 1.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 2.Jang J S, Choi S R, Qureshi W. et al. Long-term outcomes of endoscopic submucosal dissection in gastric neoplastic lesions at a single institution in South Korea. Scand J Gastroenterol. 2009;44:1315–1322. doi: 10.3109/00365520903254304. [DOI] [PubMed] [Google Scholar]
- 3.Isomoto H, Shikuwa S, Yamaguchi N. et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 4.Choi M K, Kim G H, Park do Y. et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc. 2013;27:4250–4258. doi: 10.1007/s00464-013-3030-4. [DOI] [PubMed] [Google Scholar]
- 5.Ohnita K, Isomoto H, Shikuwa S. et al. Early and long-term outcomes of endoscopic submucosal dissection for early gastric cancer in a large patient series. Exp Ther Med. 2014;7:594–598. doi: 10.3892/etm.2014.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oda I, Oyama T, Abe S. et al. Preliminary results of multicenter questionnaire study on long-term outcomes of curative endoscopic submucosal dissection for early gastric cancer. Dig Endosc. 2014;26:214–219. doi: 10.1111/den.12141. [DOI] [PubMed] [Google Scholar]
- 7.Kim D Y, Hong S J, Cho G S. et al. Long-term efficacy of endoscopic submucosal dissection compared with surgery for early gastric cancer: a retrospective cohort study. Gut Liver. 2014;8:519–525. doi: 10.5009/gnl13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotoda T, Iwasaki M, Kusano C. et al. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010;97:868–871. doi: 10.1002/bjs.7033. [DOI] [PubMed] [Google Scholar]
- 9.Kosaka T, Endo M, Toya Y. et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc. 2014;26:183–191. doi: 10.1111/den.12099. [DOI] [PubMed] [Google Scholar]
- 10.Tanabe S, Ishido K, Higuchi K. et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer. 2014;17:130–136. doi: 10.1007/s10120-013-0241-2. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H Oda I Abe S et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection Gastric Cancer24. 1. 2015 [Epub ahead of print] 10.1007/s10120-015-0469-0 [DOI] [PubMed] [Google Scholar]
- 12.Oda I, Gotoda T, Sasako M. et al. Treatment strategy after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2008;95:1495–1500. doi: 10.1002/bjs.6305. [DOI] [PubMed] [Google Scholar]
- 13.Gotoda T. Should the elderly patients undergo additional surgery after non-curative endoscopic resection? Nihon Ronen Igakkai Zasshi. 2010;47:281–284. doi: 10.3143/geriatrics.47.281. [DOI] [PubMed] [Google Scholar]
- 14.Kusano C, Iwasaki M, Kaltenbach T. et al. Should elderly patients undergo additional surgery after non-curative endoscopic resection for early gastric cancer? Long-term comparative outcomes. Am J Gastroenterol. 2011;106:1064–1069. doi: 10.1038/ajg.2011.49. [DOI] [PubMed] [Google Scholar]
- 15.Lee J H, Kim J H, Kim D H. et al. Surgical treatment necessary after non-curative endoscopic resection for early gastric cancer? J Gastric Cancer. 2010;10:182–187. doi: 10.5230/jgc.2010.10.4.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gotoda T, Yanagisawa A, Sasako M. et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 17.Hirasawa T, Gotoda T, Miyata S. et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–152. doi: 10.1007/s10120-009-0515-x. [DOI] [PubMed] [Google Scholar]
- 18.Chiu P W, Teoh A Y, To K F. et al. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc. 2012;26:3584–3591. doi: 10.1007/s00464-012-2371-8. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Oda I, Nonaka S. et al. Is endoscopic submucosal dissection an effective treatment for operable patients with clinical submucosal invasive early gastric cancer? Endoscopy. 2013;45:93–97. doi: 10.1055/s-0032-1325929. [DOI] [PubMed] [Google Scholar]
- 20.Choi J Y, Jeon S W, Cho K B. et al. Non-curative endoscopic resection does not always lead to grave outcomes in submucosal invasive early gastric cancer. Surg Endosc. 2015;29:1842–1849. doi: 10.1007/s00464-014-3874-2. [DOI] [PubMed] [Google Scholar]
- 21.DePinho R A. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 22.Monson K, Litvak D A, Bold R J. Surgery in the aged population: surgical oncology. Arch Surg. 2003;138:1061–1067. doi: 10.1001/archsurg.138.10.1061. [DOI] [PubMed] [Google Scholar]
- 23.Stewart B W Wild C World Cancer Report 2014 WHO IARC Press 2014. Available from:http://www.who.int/mediacentre/factsheets/fs297/en/[Accessed 10 April 2015] [Google Scholar]
- 24.Kitamura K, Yamaguchi T, Taniguchi H. et al. Clinicopathological characteristics of gastric cancer in the elderly. Br J Cancer. 1996;73:798–802. doi: 10.1038/bjc.1996.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriguchi S, Maehara Y, Korenaga D. et al. Relationship between age and the time of surgery and prognosis after gastrectomy for gastric cancer. J Surg Oncol. 1993;52:119–123. doi: 10.1002/jso.2930520213. [DOI] [PubMed] [Google Scholar]


