Abstract
Background and aims: Submucosal injection is standard practice in endoscopic mucosal resection of gastrointestinal lesions. Several solutions are used. Our aim was to systematically review their efficacy and safety.
Patients and methods: We performed a systematic review and meta-analysis using a random effects model of randomized controlled trials (RCTs) from MEDLINE. Studies in animal models were qualitatively assessed for efficacy and safety.
Results: In total, 54 studies were qualitatively assessed. Eleven RCTs were analyzed, two of which were on endoscopic submucosal dissection (ESD). The quantitative synthesis included nine RCTs on endoscopic mucosal resection (EMR), comprising 792 subjects and 793 lesions. Mean lesion size was 20.9 mm (range 8.5 – 46 mm). A total of 209 lesions were randomized to sodium hyaluronate (SH) vs normal saline (NS), 72 to 50 % dextrose (D50) vs NS, 82 to D50 vs SH, 43 to succinylated gelatin, 25 to hydroxyethyl starch and 36 to fibrinogen. In total, 385 were randomized to NS as controls. NS and SH are the best studied solutions and seem to be equally effective in achieving complete resection (OR 1.09; 95 %CI 0.82, 1.45). No solution was proven to be superior in complete resection rate, post-polypectomy bleeding or coagulation syndrome/perforation incidence. Many solutions have been tested in animal studies and most seem more effective for mucosal elevation than NS.
Conclusions: There are several solutions in clinical use and many more under research, but most are poorly studied. SH seems to be clinically equivalent to NS. There are no significant differences in post-polypectomy complications. Larger RCTs are needed to determine any small differences that may exist between solutions.
Introduction
Gastrointestinal tract cancer represents the leading cause of cancer death worldwide, with an estimated mortality over 1.75 million 1. Early endoscopic detection and treatment of potentially curable cancers or precancerous lesions could potentially lead to a reduction of gastrointestinal cancer incidence and cancer related mortality 2 3 4 5. In the past decades, endoscopic resection therapies have gradually improved and gained more importance for premalignant lesions and noninvasive early cancers with a low risk of lymph node metastasis. The survival after endoscopic removal of an early cancer may be similar to that after surgical resection, providing the rationale for this approach 6 7.
Resection-based modalities consist of endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). Injection-assisted EMR was first introduced in 1955 for rigid sigmoidoscopy 8 and then in 1973 for flexible colonoscopy 9. In the following years, improvements in the EMR techniques, such as cap-assisted EMR and ligation method, have been introduced 10 11, and nowadays, EMR is a widely used and useful method to resect minimally invasive benign and early malignant lesions of the gastrointestinal tract 12. However, despite its efficacy, this method is sometimes associated with local recurrence, especially when lesions larger than 40 mm are resected in a piecemeal fashion 13. To overcome this limitation, ESD has been developed, allowing en bloc resection of superficial neoplasms and providing better histopathological diagnosis and decreased local recurrence rates 14 15 16.
Endoscopic resection techniques are aided by mucosal elevation through the injection of a solution into the submucosal space. This technique may reduce complications, such as perforation or bleeding and improve the technical feasibility of the procedure. The volume of injected fluid is highly variable and depends on the size and location of the lesion, and repeated injections may be needed for complete removal.
Several solutions have been used to lift the mucosal lesion, but the optimal solution is still a matter of debate. It is accepted that the “ideal” solution for submucosal injection should provide a thick submucosal fluid cushion, remain in the submucosal space long enough to safely allow EMR or ESD, and preserve tissue specimens and allow for precise pathologic staging.
In this setting, normal saline (NS) has been the most widely used solution as it is simple to use and available at a low cost. However, the mucosal protrusion created by the submucosal injection of normal saline solution is only maintained for a short period of time. This may not have a significant impact on the removal of small lesions but, when performing longer procedures or resecting larger lesions, the need for repeated injections in order to maintain the cushion may become problematic and the risk of perforation may be higher. In order to overcome these limitations and to improve the technical feasibility of EMR and ESD, several solutions have been studied. Submucosal injection of glucose solution, glycerol, sodium hyaluronate (SH), colloids, hydroxypropyl methylcellulose, fibrinogen solution, autologous blood, and other alternatives have been investigated in different contexts. Nevertheless, these solutions are also associated with some caveats: they can be difficult to prepare or administer, available at a high cost or not readily available, or may be associated with toxicity.
In the past few years, several substances with different properties have been studied in ex vivo and in vivo studies. Among these, only a few have been evaluated in clinical trials. At the present time, no definitive proof of the superiority of any solution has been provided and there is no systematic review or meta-analysis on this topic.
Objectives
The primary objective of this review was to identify and evaluate the safety and effectiveness of the available solutions for submucosal injection in endoscopic mucosal resection techniques (polypectomy, mucosal resection, and submucosal dissection) in human patients. As secondary objectives, we aimed to evaluate the duration of the effect, and the local deleterious effects of the solutions on the submucosal tissue, including those studies performed on animals.
Material and methods
We performed a systematic review and a meta-analysis to evaluate the effectiveness and safety of existing solutions for submucosal injection in endoscopic mucosal resection or dissection. This review was registered on the International prospective register of systematic reviews, PROSPERO: CRD42014009577.
We considered all published randomized controlled trials for the quantitative synthesis. We performed a separate analysis for ESD and EMR. For the overall qualitative synthesis, we included non-randomized trials, and observational studies (cohort, case-control, case series and case reports) evaluating the safety and effectiveness of submucosal injection solutions, regardless of blinding and language.
For the primary outcome, we included studies with humans submitted to upper or lower gastrointestinal endoscopy. For the secondary outcomes, we also included animal studies (including ex vivo).
We included procedures where polypectomy, EMR or ESD were performed after the injection of submucosal solutions had taken place, either in the esophagus, stomach, colon or rectum.
Primary outcome
Complete resection of the lesion – histological determination of en bloc lesion free margins or endoscopic determination of no residual lesion. Endoscopic determination included the lack of residual lesion as reported by the endoscopist (with or without chromoendoscopy) or the inclusion of resection marks in the resected specimen or negative follow-up with tissue forceps biopsies from the resection site.
Secondary outcomes
Number of injections given; volume injected; duration of submucosal cushion; procedure time; endoscopic complications; residual lesion at follow-up; tissue injury.
Search strategy
We individually searched MEDLINE and included all studies published until March 2014. The electronic search was performed using the following key words: submucosal injection AND (endoscopic AND resection OR EMR OR ER OR mucosectomy OR endoscopic submucosal dissection OR ESD OR polypectom*) AND (solution* OR saline OR hyaluron* OR glycerol OR hypertonic OR fibrinogen OR epinephrine OR adrenaline OR dextrose OR blood OR gelatin OR jelly OR mannitol OR sodium alginate OR carboxymethylcellulose OR albumin OR succiny* OR indigo OR methylene) AND (complete resection OR R0 OR adverse event* OR complication* OR injection* OR volume OR duration).
Study selection
Two authors (AF, JM) independently scanned all titles and abstracts for relevance by electronic search. A third author (JT) intervened in case of disagreement.
Data extraction
Data extraction was performed independently by two authors (AF, JM) using a data extraction form to evaluate risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions. Studies were classified as high risk, low risk or unclear risk of bias.
The end points were rate of complete resection (primary end point), number of submucosal injections, total volume (mL) used, duration of submucosal cushion (min), procedural time (min), rate of en bloc resection, incidence of endoscopic complications (perforation and bleeding), recurrence rate at follow-up and incidence of tissue injury or fibrosis.
Data synthesis
We provide a description of the findings including a summary of the study’s results by intervention.
We performed the analysis in STATA 13 (Stata Corp., Texas, United States) and the flow diagram using Review Manager 5. We meta-analyzed the complete resection rate and the incidence of adverse events (bleeding and perforation), using both random-effects and fixed-effect meta-analyses but we only report the random-effects meta-analyses, since the two methods concurred. We present odds ratios with a 95 % confidence interval. Heterogeneity was assessed using the I 2 statistic. We produced a summary of findings table, rating the quality of evidence of the primary outcome.
Results
The electronic search resulted in a total of 159 published manuscripts that were scanned based on the title and abstract; 105 did not meet the inclusion criteria. The remaining 54 were assessed for eligibility using the full text articles and 11 were initially included for quantitative analysis. The flow diagram is shown in Fig. 1, and the details of the studies are shown in Table 1.
Fig. 1 .
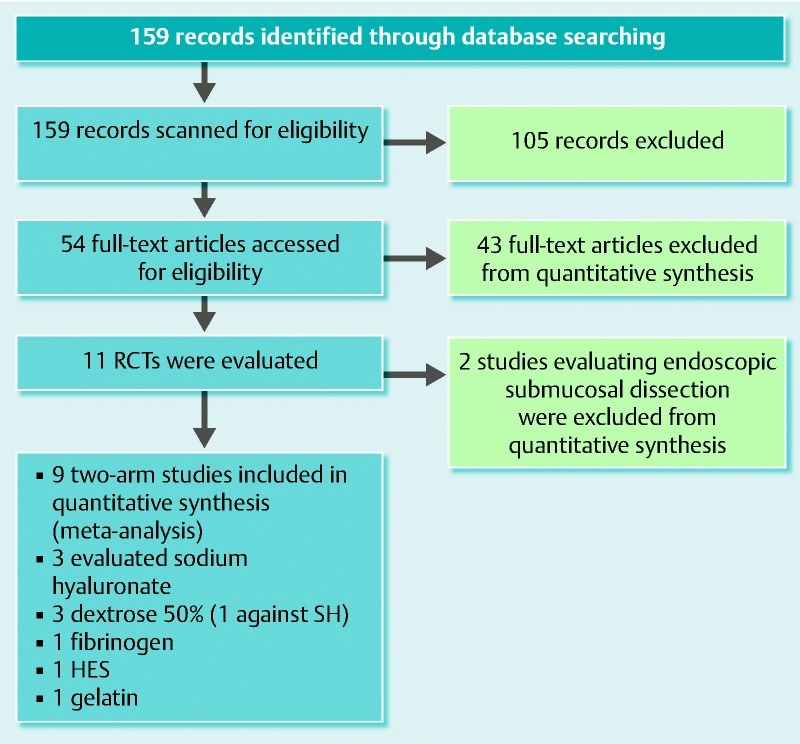
Flow diagram with the selected studies for the meta-analysis.
Table 1. Characteristics of the randomized controlled trials included in the meta-analysis.
| Study (reference) | Country | n | Lesion size, mm | Intervention | Control | R0 A, % | R0 B, % | P value |
| Stomach (ESD) | ||||||||
| Sumiyama et al. (2014) 17 | Japan | 100 | 18.29 | Mesna | NS | 100 | 98.8 | NS |
| Kim et al. (2013) 18 | South Korea | 63 | 13.84 | SH | NS | 90.9* | 61.1* | 0.004 |
| Stomach (EMR) | ||||||||
| Yamamoto et al. (2008) 22 | Japan | 140 | 5 – 20 | SH | NS | 92.8 | 94.3 | 0.745 |
| Stomach and colon (EMR) | ||||||||
| Varadarajulu et al. (2006) 24 | USA | 60 | 22.5 | D50 | NS | 96.3 | 80.0 | 0.09 |
| Lee et al. (2006) 25 | South Korea | 72 | 17.98 | Fibrinogen | NS | 86.1 | 80.6 | 0.53 |
| Colorectal (EMR) | ||||||||
| Kishihara et al. (2012) 21 | Japan | 94 | – | SH | NS | 97.8 | 93.8 | 0.06 |
| Yoshida et al. (2012) 20 | Japan | 189 | 8.54 | SH | NS | 79.5 | 65.6 | 0.03 |
| Fasoulas et al. (2012) 26 | Greece | 49 | 46 | HES | NS | 96.0 | 95.8 | 0.94 |
| Moss et al. (2010) 27 | Australia | 80 | 37.5 | SG | NS | 90.0 | 90.0 | 1.0 |
| Katsinelos et al. (2008) 23 | Greece | 92 | 23 | D50 | NS | 93.3 | 87.2 | 0.13 |
| Hurlstone et al. (2008) 19 | UK | 163 | 19.1 | D50 | SH | 72.0 | 69.1 | > 0.01 |
R0 A, complete resection rate in active group; R0 B, complete resection rate in control group; ESD, endoscopic submucosal dissection; EMR, endoscopic mucosal resection; SH, sodium hyaluronate; NS, normal saline; D50, 50 % dextrose; SG, succinylated gelatin.
These proportions refer to clinical usefulness rate (complete resection within one additional submucosal injection).
Since there were only two studies on ESD and with different solutions (Mesna and SH) 17 18, a meta-analysis was not performed. In these studies, 53 lesions were randomized to Mesna (vs NS) and 33 to SH (vs NS). There were 88 lesions randomized as controls. In the Mesna RCT 17, Sumiyama and colleagues aimed to evaluate the procedural time with Mesna compared to NS for gastric epithelial lesions. There was no statistically significant difference in this outcome. There were no differences in other outcomes such as R0 resection rate and adverse events (bleeding and perforation). Kim et al. 18 designed an RCT to compare SH to NS with “clinical usefulness” (a combination of en bloc resection and the need for additional injection) as the primary outcome. They randomized 76 gastric lesions and demonstrated a significant effect of SH in increasing the usefulness rate (90.9 % vs 61.1 %; P = 0.004).
The nine EMR studies were all two-arm RCTs; eight of them used NS as the control group and only one used SH as the control 19. Three trials evaluated SH solutions 20 21 22, three trials evaluated D50 19 23 24, and the others evaluated fibrinogen 25, hydroxyethyl starch (HES) 26, and succinylated gelatin (SG) 27.
The three studies that were excluded from the meta-analysis did not report the outcome of interest 28 29 30.
Quality assessment of the nine RCT determined that six had a low risk of bias on the generation of the randomization sequence and allocation concealment; six had kept double blinding, while two studies failed to report adequate blinding of the subjects and personnel, and one reported no blinding.
In the EMR studies, a total of 792 subjects and 793 lesions were included for analysis. The majority were male patients (56.7 %) and their mean age was 63.6 ± 3.9 years. Mean lesion size was 20.9 mm (range 8.5 – 46 mm).
After pooling, 209 lesions were randomized to SH (vs NS), 72 to D50 (vs NS), 82 to D50 (vs SH), 43 to SG, 25 to HES and 36 to fibrinogen. In total, 385 were randomized to NS as controls.
Six studies were performed on colorectal lesions, one on gastric, and two using both gastric and colorectal lesions.
Meta-analysis results
Complete resection rate
All the nine studies included in the meta-analysis reported the resection efficacy and explicitly provided the complete resection rate (either by endoscopic evaluation or histological confirmation). The analysis results are shown as a forest plot in Fig. 2, with the studies having a low heterogeneity. The results indicate that the solution used does not have a significant impact on the resection efficacy. However, most solutions were only tried in one RCT which may limit the sensitivity to detect small effects. SH is the best studied solution and was compared to NS in three RCTs (423 patients) and the pooled results fail to suggest a difference between SH and NS with OR (95 %CI) 1.09 (0.82, 1.45). The overall plot indicates that the pooled results of the interventions (SH, HES, SG, D50, and fibrinogen) were not superior to the comparator, which was always NS with the exception of Hurlstone’s trial which compared D50 to SH with OR (95 %CI) 1.07 (0.88, 1.29).
Fig. 2.
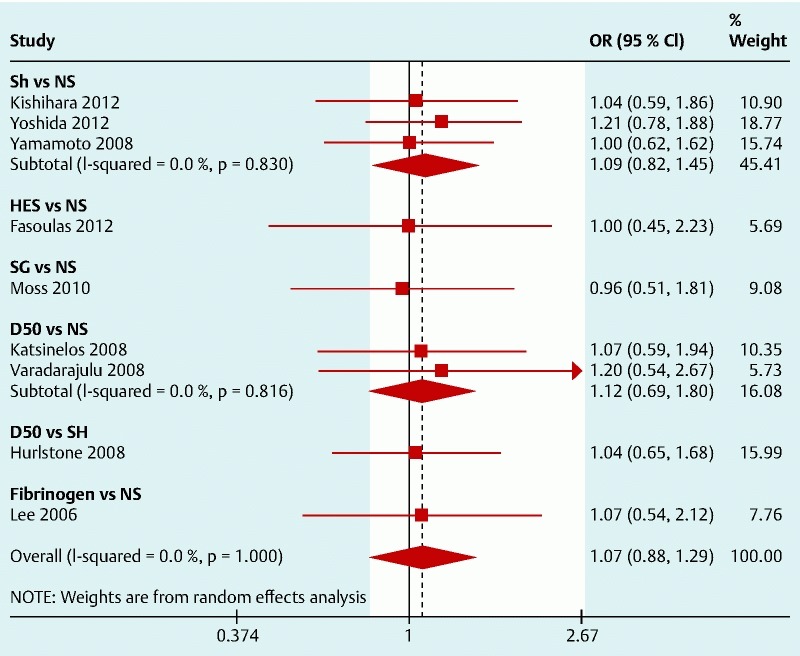
Forest plot for complete resection (right side favors intervention).
Bleeding rate
All the studies reported the post-polypectomy bleeding rate. Even though the bleeding definition was different across studies, the heterogeneity of the results was low. The pooled results are shown in Fig. 3. No single solution was shown to be more effective in decreasing the post-polypectomy bleeding rate but HES, SG, and fibrinogen have shown a non-significant favorable trend against NS with a pooled OR (95 %CI) 0.59 (0.34, 1.01). Pooled results for SH suggest that there is no beneficial effect on the bleeding risk when using this agent.
Fig. 3.
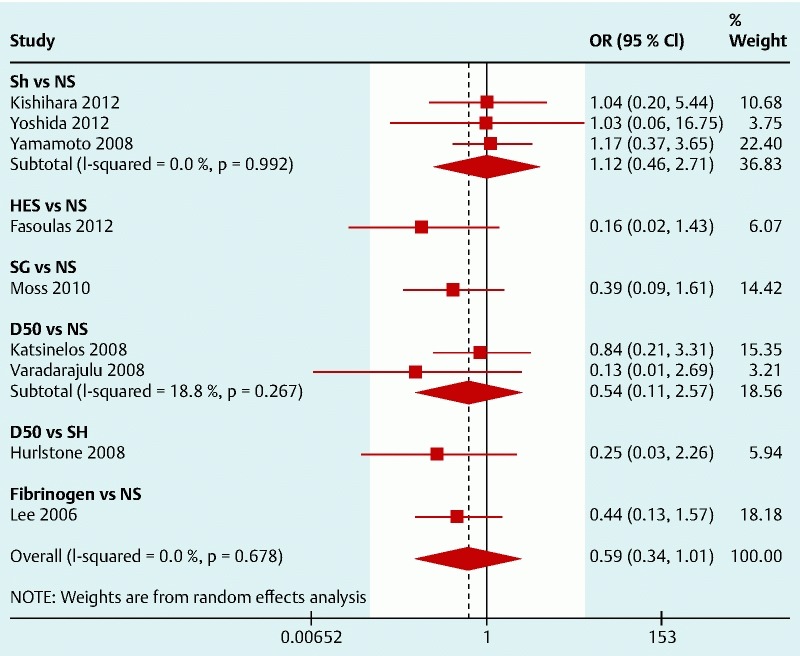
Forest plot for post-polypectomy bleeding (left side favors intervention).
Post-polypectomy coagulation syndrome/perforation rate
Only four studies reported the occurrence of perforations or coagulation syndrome. The results are shown in Fig. 4. There is only one RCT for each solution and none for SH. These studies were underpowered to detect significant differences in this specific outcome but the pooled analyses seem to suggest that NS may be effective in preventing perforations and coagulation syndrome (Fig. 5) with an OR (95 %CI) 0.27 (0.06, 1.19), especially when compared to HES (OR 0.15; 95 %CI 0.007, 3.03) and D50 (OR 0.16; 95 %CI 0.02, 1.38).
Fig. 4.
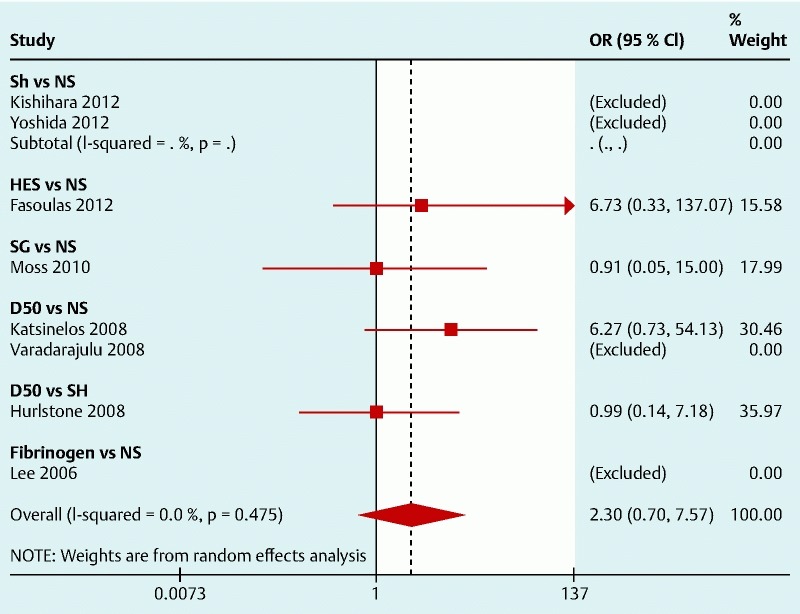
Forest plot for post-polypectomy coagulation syndrome/perforation (left side favors intervention).
Fig. 5.
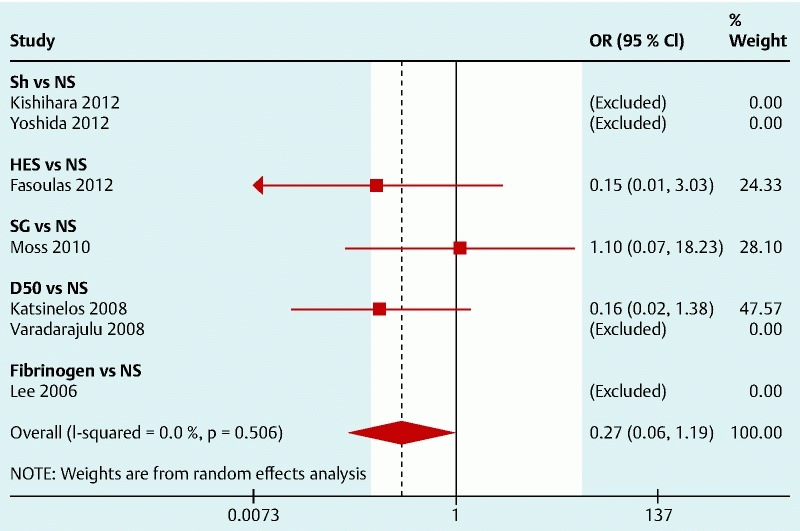
Forest plot for post-polypectomy coagulation syndrome/perforation with specific solutions compared with normal saline (left side favors intervention).
Other secondary end points
Due to the lack of data and heterogeneity of definitions, it was not possible to analyze the other proposed end points, such as number of submucosal injections, total volume (mL) used, duration of submucosal cushion (min), procedural time (min), rate of en bloc resection, recurrence rate at follow-up, and incidence of tissue injury or fibrosis.
Descriptive analysis
This section will evaluate the 54 studies included in the systematic review in order to assess the proposed outcomes. A summary of these studies is available in the Appendix.
Sodium hyaluronate (SH) solution is widely used as an endoscopic submucosal injection material. It was first reported in animal models that the submucosal fluid cushion created by SH persists for longer periods of time than other available submucosal solutions 31 32 33 34. Its efficacy in EMR and ESD was also reported in clinical practice. Using 0.4 % SH as a submucosal injection solution in endoscopic resection enabled an effective lifting of a colorectal intramucosal lesion, reducing the need for additional injections 22. Fujishiro et al. 35 reported that a mixture of a high concentration of SH and glycerine had good results in ESD. SH was compared with NS in two randomized controlled trials that included patients with colorectal lesion < 20 mm managed with EMR. Yoshida et al. 20 concluded that EMR using 0.13 % SH applied to colon lesions of less than 20 mm diameter is more effective than NS for complete resection and maintenance of mucosal elevation, since complete resection was achieved in 74 of 93 lesions (79.5 %) in the SH group and 63 of 96 lesions (65.6 %) in the NS group (P < 0.05) and high mucosal elevation was maintained in 83.9 % of procedures in the SH group and 54.1 % in the NS group (P < 0.01). Kishihara et al. 21 also reported the superiority of NS solution for the ease of submucosal injection and snaring with less variability (P < 0.05). Finally, SH was compared to NS in a randomized controlled trial with gastric lesions proposed for ESD and it was shown that the usefulness rate and the volume of solution injected were significantly better in the 0.4 % SH group 18. However, SH still faces some problems, namely its higher cost, requirement of an air-sealed container for storage, and the conflicting data concerning stimulation of tumor growth 36 37.
Sodium alginate is an inexpensive high viscosity solution. Eun et al. demonstrated that mucosa-elevating capacity was comparable between 1 % sodium alginate solution and 0.5 % SH solution 38. It also showed greater elevation when compared to that created by NS solution 39. In a clinical study, 0.4 % SH solution exhibited no significant difference in catheter injectability but significant superiority in mucosa-elevating capacity over 0.6 % sodium alginate solution, with no findings indicative of tissue injury. En bloc resection was achieved in all cases, no adverse events were observed, and no case showed recurrence 40. Further investigation is needed on the usefulness of this material as a submucosal injection solution for endoscopic procedures.
With regard to dextrose solution, in a prospective, uncontrolled clinical study, Katsinelos et al. 41 first investigated the effectiveness of EMR using a hypertonic dextrose plus epinephrine solution as a submucosal cushion agent for the resection of 59 large sessile colorectal polyps, showing that 23/59 (39 %) were resected en bloc and 36/59 (61 %) in a piecemeal fashion. Also, Varadarajulu et al. 24 compared D50 and NS for injection assisted resection of 52 sessile gastrointestinal lesions. Compared with NS, lower volumes (median 2 vs 1 mL; P = 0.03) were required. Even after completion of resection, submucosal elevation persisted in 36 % of the patients randomly assigned to D50 compared with 20 % of those randomized to NS (P < 0.001). There were no significant differences in the rates of complete resection. Later, Katsinelos et al. 23 performed a prospective, double-blind, randomized study that compared EMR of 92 sessile rectosigmoid lesions ( > 10 mm) using D50 plus epinephrine or NS plus epinephrine. Injected solution volumes and number of injections were lower in the D50 group (P = 0.033 and P = 0.028, respectively). Submucosal elevation had a longer duration in the D50 group (P = 0.043). This difference mainly included large (≥ 20 mm) and giant (> 40 mm) lesions. There were 6 cases versus 1 case of post-polypectomy syndrome in the D50 and NS groups (P = 0.01). Dextrose solution was also compared with SH 19 in a RCT including 174 patients. R0 resection was achieved in 59 of the 82 lesions (72 %) in the dextrose group and in 56 of the 81 lesions (69 %) in the SH group (P > 0.1). Nevertheless, Fujishiro et al. 33 showed that injection of 20 % submucosal dextrose in an animal model was associated with mucosal and muscle damage on the day of injection, with ulceration extending to the submucosal layer within a week after injection.
Glycerol was first evaluated for mucosal elevation in porcine esophagus, showing a longer disappearance time when compared with NS 34, and later in EMR of colorectal laterally spreading tumors (LSTs) 42. In this clinical study, particularly for non-granular, laterally spreading tumors (LST-NGs) < 20 mm, the glycerol group had a higher en bloc resection rate than the NS group (P < 0.01), however a similar recurrence rate and complications were achieved and there was no difference between en bloc resection for LST ≥ 20 mm.
Sodium carboxymethylcellulose is a water-soluble polymer derived from cellulose. In vitro, the submucosal injection of sodium carboxymethylcellulose solution was able to dissect by itself most of the mucosal layer from the muscular layer at a concentration above 2.0 %. In vivo, three specimens were resected with 2.5 % sodium carboxymethylcellulose without difficulty. There were no procedure-related complications and histologic examination revealed no tissue damage 43.
Hydroxypropyl methylcellulose is a high viscosity agent that has been considered to be a good and low cost option readily available in the United States. Its superiority over NS solution in height and duration of mucosal elevation has been shown in animal studies 31 32. Further studies are needed to clarify the real benefits of this synthetic agent.
Photocrosslinkable chitosan in DMEM/F12 medium is a viscous solution that crosslinks UV irradiation, resulting in an insoluble hydrogel. Photocrosslinkable chitosan hydrogel injection led to a longer lasting elevation with clearer margins compared with NS or SH solutions 44, and was useful when used in ESD 44. Furthermore, photocrosslinkable chitosan hydrogel may contribute to the healing of artificial ulcers after EMR and ESD 45, which makes it a promising agent for endoscopic procedures and it should be evaluated in clinical trials after biocompatibility has been established.
Succinylated gelatin (SG) is a widely available, inexpensive, safe, colloidal solution that exerts an oncotic pressure comparable with that of human albumin, with a favorable safety profile. In an animal study 46, the mean EMR specimen dimension and surface area were significantly larger and the duration of mucosal elevation was significantly longer for SG (P = 0.005). Three perforations were recorded, two with SG and one with NS (P = 1.0). However, these perforations occurred in the proximal porcine colon which is thinner than distal porcine colon and human colon. The clinical efficacy of SG was evaluated by Moss et al. in a randomized double-blind trial, conducted to compare the performance of EMR with SG or NS for sessile lesions of the colon sized ≥ 20 mm 27.
The “Sydney Resection Quotient” (defined as lesion size in millimeters divided by the number of pieces to resect) was significantly different between groups, favoring SG; fewer injections per lesion (P = 0.002), lower injection volume (P = 0.009), and shorter procedure duration (P = 0.006) were reported with the SG group. There was also a non-significant trend towards higher en bloc resection rate with SG (30 % vs 15 %, P = 0.137). There were no perforations.
Mesna (sodium-2-mercaptoethanesulfonate [C2H5NaO3S2]) is a mucolytic agent that acts by cleaving disulfide bonds in proteins, thereby breaking down the connective tissue between anatomical planes. A preliminary clinical study that used submucosal mesna injection for ESD demonstrated the feasibility and safety of the procedure 47. In an animal study comparing it with NS, there were no differences between groups related to ESD procedure time and en bloc resection, but mesna injection was associated with a non-significant lower incidence of intraprocedural bleeding (P = 0.09) 48. Recently, mesna solution was compared to NS in a randomized controlled trial and it showed that ESD time was not significantly different between groups, but multivariate analysis indicated that mesna reduced procedural challenges associated with submucosal dissection 17.
Autologous blood is readily available at low cost. Previous human and animal studies have demonstrated that autologous whole blood produced the longest durable cushion compared with standard agents 49. The feasibility of EMR with blood submucosal injection was also reported with no complications 29 50. Regarding tissue injury, a study has shown that blood produces less tissue injury (measured as hydrops and tears) than NS 29. However, some potential problems need to be clarified, namely the fact that autologous blood could hamper the specialist’s view during the procedure and the possibility for blood coagulation 51.
Other agents such as fibrinogen mixtures, poloxamers, and photocrosslinkable chitosan have been reported for EMR with great enthusiasm. Compared with SH, fibrinogen mixtures and poloxamer solutions are significantly less expensive but remain substantially more expensive than NS 25. A study that included EMR of 35 early gastric neoplasms showed that, after an initial injection of fibrinogen mixture, additional submucosal injection was not required for any lesion. The rates of en bloc resection and complete resection were, respectively, 82.9 % and 88.6 %. The en bloc resection rate was significantly lower for lesions over 20 mm in diameter (60 % vs. 92 %; P < 0.05) and for lesions on the lesser curvature or posterior wall of the stomach compared with those on the greater curvature or anterior wall (55.6 % vs. 92.3 %; P < 0.05). During follow-up, recurrence was noted in only one patient in whom the lesion had been resected piecemeal 52.
Later, the clinical efficacy of the fibrinogen mixture was evaluated in a RCT, comparing it with NS in EMR of early gastric neoplasms 25. This study did not show differences between the two groups in the rates of en bloc resection and recurrence rate, but mean procedure time was significantly shorter in the fibrinogen group and additional submucosal injection to maintain elevation of the lesion was less frequently required in the fibrinogen group (P < 0.05). In addition, the use of fibrinogen mixtures for endoscopic resections still needs to be critically considered with regard to their potential to transfer infections. The poloxamer solution PS137 – 25 was studied in porcine models, comparing it with NS and hydroxypropyl methylcellulose 53, showing greater height of the initial mucosal elevation and longer mucosal elevation. Five EMRs were successfully performed after one injection of PS137 – 25, with no thermal injury or perforations.
Recently, other alternatives have been presented. A novel injectable drug eluting elastomeric biodegradable polymer (iDEEP) was developed to overcome the limitations of previous solutions, using both viscosity and gel formation through redox initiated crosslinking 54, and showing more durable cushions than those formed with NS and SH. Carbon dioxide (CO2) was also tested as an injection agent. Uraoka et al. 55 performed an animal study that showed the safety and efficacy of CO2 as a satisfactory submucosal injection agent during ESD, the submucosal elevation created by CO2 being longer than with either NS or sodium hyaluronic acid (P < 0.001). Creating and maintaining a CO2 submucosal cushion of sufficient elevation was achieved combined with partial physical dissection of the submucosal layer, followed by complete endoscopic dissection of the CO2 submucosal layer with ESD, resulting in successful en bloc resection with no complications.
Cook Medical’s (Bloomington, IN, United States) submucosal lifting gel consists of a proprietary combination of known biocompatible components that appears to be a promising safe and effective substance for submucosal injection. In an animal study, every injection resulted in adequate mucosal lifting, with no evidence of perforation, bleeding, gel extravasation through the serosal surface, or damage to surrounding tissue or organs 56.
Discussion
EMR and ESD are minimally invasive endoscopic procedures now accepted worldwide as a treatment modality in the removal of dysplastic and early malignant lesions limited to the superficial layers of the gastrointestinal tract 6 7. Endoscopic resection techniques are aided by mucosal elevation through the injection of a solution into the submucosal space in order to reduce complications. In this study, we tried to identify the best solution to use to lift the mucosal lesion. Our primary outcome was to evaluate complete resection of the lesion. All studies included in the meta-analysis 19 20 21 22 23 24 25 26 27 provided the complete resection rate. SH is the best studied solution, being compared with NS in three RCTs 20 21 22. The remaining solutions, namely fibrinogen mixture 25, hydroxyethyl starch 26, and succinylated gelatin 27, were only studied in one RCT each. Our study shows that the available evidence does not allow a robust conclusion to be drawn on the solution’s effect on resection rate (OR 1.07; 95 %CI 0.88, 1.29) and, particularly, there is no difference between SH and NS (OR 1.09; 95 %CI 0.82, 1.45) ( Fig.3).
Regarding the complications, bleeding rate was reported in all studies, but the definition of bleeding was different across studies. We found that no single solution was shown to be more effective in decreasing the post-polypectomy bleeding rate, but HES, SG, and fibrinogen have shown a non-significant favorable trend against NS. The post-polypectomy coagulation syndrome/perforation rate was evaluated in four studies 19 23 26 27. From the analysis, we infer that NS may have a beneficial effect in preventing perforations and coagulation syndrome ( Fig.5) with an OR (95 %CI) 0.27 (0.06, 1.19), especially when compared to HES (OR 0.15; 95 %CI 0.007, 3.03) and D50 (OR 0.16; 95 %CI 0.02, 1.38). However, these are rare events and a much larger sample size would be needed to determine a more precise effect estimate.
In the descriptive analysis section, we analyzed several solutions with different properties. Many solutions have been tested in animal studies and most seem more effective for mucosal elevation than NS, without significant differences in complication rates. We highlight that the superiority of these solutions must be evaluated in RCTs.
According to our results, no solution was proven to be superior in complete resection rate, post-polypectomy bleeding, or coagulation syndrome/perforation incidence. We emphasize the need for continuing research in this topic.
Potential biases and limitations
Our conclusions are limited by the small number of published RCTs and because there are several solutions being evaluated and different control groups.
There is a potential bias in the analysis as many studies were not clear as to whether they report the intention-to-treat (ITT) or the per protocol analysis. Also two of the RCTs were not adequately blinded. The studies include lesions in the stomach, in the colon or rectum, and the effect of the submucosal injection may be different according to the anatomical site. In addition, the size of the lesions was quite different between studies, ranging from 8.5 mm to 46 mm lesions (EMR studies) and this represents a heterogeneous sample to pool.
We chose to consider complete resection as either endoscopic or histologically assessed in the original studies even though they may not be perfectly correlated. In the adverse event reporting, there were also a wide range of definitions for post-polypectomy bleeding and some of the studies reported immediate and/or delayed bleeding rates, while we counted the totals.
Conclusions
In summary, there are many solutions being commonly used for submucosal injection and many more under research. There is a lack of high quality evidence. According to the present meta-analysis, it is not possible to select one solution over the others by considering complete resection rates and procedural safety. There was a trend towards a higher risk of bleeding and a lower risk of perforation/post-polypectomy syndrome with NS.
More trials may be needed to select the best solution. At the moment, RCTs should use NS as the control group.
Appendix. Summary of the included studies.
| Study (reference) | Country | Type of study | Type of lesion | End points | Specimen | Intervention 1 | Intervention 2 | Intervention 3 | Intervention 4 | Intervention 5 | Intervention 6 | Intervention 7 | Conclusions |
| Polymeros 2010 31 | Greece | Animal study (ex-vivo) | No lesions | Median time (min) of mucosal elevation of some solutions in comparison with NS and SH | Stomach | NS | 0.4 % SH | 25 % HA | 0.3 % HPMC / 70 % dextran | 6 % HES | The median duration of mucosal elevation was longer with HPMC/dextran, HES and SH compared with NS (P < 0.05). There were no significant differences between SH and HPMC/dextran and HES (P > 0.05). | ||
| 12 | 41.5 | 23 | 29 | 38 | |||||||||
| Hyun 2006 32 | Korea | Animal study (ex-vivo) | No lesions | Colon | NS | 0.1 % SH | 0.3 % HPMC | 2 % Fibrinogen | Mannitol | The mucosal elevation lasted longer with SH, HPMC and fibrinogen than with mannitol or NS. | |||
| Height of initial mucosal elevation (mm) | 6.52 ± 0.26 | 6.92 ± 0.09 | 6.90 ± 0.08 | 6.90 ± 0.08 | 6.87 ± 0.05 | ||||||||
| Time to be reduced more than half the initial height (min) | 20 | > 60 min | > 60 min | > 60 min | 30 min | ||||||||
| Fujishiro 2005 33 | Japan | Animal study (in-vivo) | No lesions | Tissue damage seen by histology | Stomach | NS | SH | Glyceol | 5 – 50 % DW | Use of hypertonic solutions, except Glyceol, is not recommended with respect to tissue damage. | |||
| No tissue damage | No tissue damage | No tissue damage | ≥ 20 % DW showed tissue damage, including muscle damage | ||||||||||
| Conio 2002 34 | Italy | Animal study (in-vivo) | No lesions | Mean (±SD) disappearance time (min) | Esophagus | NS | NS + epinephrine | 50 DW | Glycerol | SH | The disappearance time for SH was longer when compared with all other solutions (P = 0.0001). | ||
| 2.6 ± 0.6 | 2.9 ± 1.2 | 5.3 ± 2.5 | 5.2 ± 2.6 | 23 ± 10.5 | |||||||||
| Yoshida 2011 57 | Japan | Animal study (ex-vivo) | No lesions | Mean mucosal elevation (mm) measured at 0, 2, 4 and 6 min | Colon | NS | 0.1 % SH | 0.13 % SH | 0.2 % SH | 0.4 % SH | |||
| 9.5, 7, 5.3 and 4 | 11, 9, 7 and 6 | 11, 10, 8 and 7 | 11, 10, 9 and 9 | 13, 12, 11.6 and 11.3 | The initial mucosal elevations of all SH concentrations were higher than NS (P < 0.05) and all concentrations of SH maintained a greater degree of mucosal elevation than NS (P < 0.05). | ||||||||
| Esophagus | 8.2, 6.8, 5.4 and 3.8 | 9, 7, 6 and 5 | 10.5, 8.5, 8.5 and 7 | 11.5, 10, 9.5 and 9 | 12, 11, 9 and 9 | The initial mucosal elevation of 0.13 %, 0.2 %, and 0.4 % SH were higher than NS (P < 0.05) and 0.13 %, 0.2 %, and 0.4 % SH maintained a greater degree of mucosal elevation than NS (P < 0.05). | |||||||
| Animal study (in-vivo) | Evaluation of mucosal elevation (classified into 3 macroscopic types: kept (K) type; slightly-kept (SK) type; diminished (D) type) at 1, 2, 3 and 4 min | Colon | SK; D; D and D | SK; SK; D and D | K; K; SK and D | K; K; SK and SK | K; K; K and K | Initial mucosal elevation of 0.13 % SH was higher than that of NS and remained greater 2 min after injection. | |||||
| Fujishiro 2006 35 | Japan | Clinical study (case series) | 67 gastrointestinal tumors | Esophagus Stomach Duodenum Colorectum | Mixture of a 1 % 1900 KDa SH preparation and Glycerol | ESD when using a mixture of Suvenyl and Glyceol results in excellent outcomes. | |||||||
| Endoscopic en bloc resection | 94 % (63 /67) (esophagus: 10 /10 (100 %); stomach: 26 /26 (100 %); duodenum: 1 /1 (100 %); colorectum: 26 /30 (87 %)) | ||||||||||||
| Histologic en bloc resection | 78 % (52 /67) (esophagus: 8 /10 (80 %); stomach: 24 /26 (92 %); duodenum: 1 /1 (100 %); colorectum: 19 /30 (63 %)) | ||||||||||||
| Complications | No patient had massive hemorrhage that needed blood transfusion and perforation was experienced in a patient. | ||||||||||||
| Recurrence after ESD | Short-term follow-up of 12 months revealed no local or distant recurrence | ||||||||||||
| Eun 2007 38 | Korea | Animal study (ex-vivo) | No lesions | Mean height of mucosal elevation (mm) at 5, 10, 15, 20, 25and 30 minutes after injection | Stomach | NS | 10 DW | 1 % SA | 0.5 % SH | There was no significant difference in the height of mucosal elevation between SA and SH and both maintain a higher mucosal elevation between 5 and 30 minutes when compared to other solutions (P < 0.05). | |||
| Animal study (in-vivo) | Histologic findings of the submucosal cushion, effect of injection on the tissue and effect on healing process of EMR-induced ulcers | 1 % SA | A clear separation of the mucosal layer from the proper muscle layer was achieved by injecting SA solution. Histological examination of EMR-induced artificial ulcers revealed no apparent tissue damage and showed normal healing process. | ||||||||||
| Akagi 2011 39 | Japan | Animal study (ex-vivo) | No lesions | Mean mucosal elevation (mm) observed immediately and at 5, 10, 15, 30, 45 and 60 minutes after injection (average of initial mucosal thickness (mm)) | Stomach | 2.0 % SA | 3.0 % SA | 4.0 % SA | 0.4 % SH | NS | The elevation created by the 2 %, 3 % and 4 % SA solutions and the SH solution was significantly greater and maintained a greater degree of elevation than NS (P < 0.05). | ||
| 6.0 | 6.1 | 6.2 | 6.1 | 4.0 | |||||||||
| Clinical study (case series) | 11 EGC | 3.0 % SA | En bloc resection and a negative resection margin were obtained in all patients without major complications (3 patients had minor bleeding) and there were no tissue damage. None showed recurrence during the follow-up period (28 months). | ||||||||||
| Endoscopic and histologic en bloc resection | 11 /11 (100 %) | ||||||||||||
| Complications | There were no major complications; minor bleeding was reported in 3 patients | ||||||||||||
| Recurrence after ESD | No patients showed recurrence during a median follow-up period of 28 months | ||||||||||||
| Kusano 2014 40 | Japan | Animal study (ex-vivo) | No lesions | Subjective assessment of injectability of SA solution into catheter and its mucosa-elevating capacity | Stomach | 0.3 – 0.8 % SA and 0.4 % SH | Compared with 0.4 % SH, 0.6 % SA solution exhibited no significant difference in catheter injectability and created an excellent mucosal elevation in height immediately after injection (P < 0.01) and a significantly higher mucosal elevation was maintained at all time points until 30 min later (P < 0.05). | ||||||
| Animal study (in-vivo) | Tissue injury | 0.6 % SA | NS | 50 DW | 0.6 % SA solution or NS, which served as a negative control, showed no findings suggestive of tissue injury. | ||||||||
| Clinical study (case series) | 10 EGC | Complications, operation time, rate of en-bloc resection, recurrence rate and ulcer healing process | 0.6 % SA | None of patients had bleeding or gastric perforation. Operation time was 130.5 ± 92.1 min and endoscopic and histologic en-bloc resection was done in all cases. There were no cases of recurrence during the follow-up period (14.5 months). All cases showed excellent healing of the artificial ulcer and scarring. | |||||||||
| Uraoka 2005 42 | Japan | Clinical study (case-control) | 110 colorectal LSTs | Colon | Glycerol | NS | |||||||
| En bloc resection rate | 70 /110 (63.6 %) | 55 /113 (48.7 %) | P = 0.03 | ||||||||||
| Complete resection rate | 50 /110 (45.5 %) | 28 /113 (24.8 %) | P < 0.01 | ||||||||||
| Complications (bleeding and perforation rates) | 8 /110 (7.3 %) and 0 /110 (0 %) | 12 /113 (10.6 %) and 1 /113 (0.9 %) | P = 0.48 | ||||||||||
| Yamasaki 2006 43 | Japan | Animal study (ex-vivo) | No lesions | Dissection the mucosal layer from the muscular layer evaluated by EUS | Stomach | 0.5 – 3.5 % SCMC | Submucosal injection of the SCMC solution dissected most of the mucosal layer from the muscular layer at the concentration above 2.0 %. | ||||||
| Animal study (in-vivo) | Efficacy and safety of SCMC for ESD | 2.5 % SCMC | 2.5 % SCMC dissected most of the mucosal layer from the muscular layer and there were no complications. Histologic examination revealed no significant alterations in the muscular layer and surrounding tissue. | ||||||||||
| Lee 2004 52 | Korea | Clinical study (case series) | 35 EGC | Stomach | Fibrinogen mixture | The en bloc resection rate was significantly lower for lesions over 20 mm in diameter (60 % vs 92 %; P < 0.05) and for lesions on the lesser curvature or posterior wall of the stomach compared with those on the greater curvature or anterior wall (55.6 % vs 92.3 %; P < 0.05). There were no major complications. | |||||||
| En bloc resection rate | 29 /35 (82.9 %) | ||||||||||||
| Complete resection rate | 31 /35 (88.6 %) | ||||||||||||
| Complications | 2 /35 (5.7 %) | ||||||||||||
| Recurrence after EMR | 1 /31 (3.2 %) | ||||||||||||
| Fernandez-Esparrach 2009 53 | USA | Animal study (ex-vivo) | No lesions | Stomach | NS | HPMC | PS137 – 25 | ||||||
| Mean height (mm) and duration (min) of submucosal cushions | 8.3 mm/20.9 min | 9.05 mm/89 min | 10.3 mm/> 120 min | The height of mucosal elevation was greater with PS137 – 25 than with NS solution (P < 0.01) or HPMC (not significant). All the mucosal elevations with PS137 – 25 lasted longer than with NS and HPMC (P < 0.01). | |||||||||
| Animal study (in-vivo) | Usefulness of PS137 – 25 evaluated in vivo en bloc resection | PS137 – 25 | All 5 EMRs were successfully performed after 1 injection of PS137 – 25. No repeat injections were needed. No thermal injury was observed in the serosa, muscularis propia or mucosal surface. | ||||||||||
| Kumano 2012 44 | Japan | Animal study (in-vivo) | No lesions | Esophagus | PCH | SH | Hypertonic saline | PCH injection led to a longer-lasting elevation with clearer margins compared with controls, thus enabling precise ESD along the margins of the elevated mucosa without complications. The aspects of wound repair were similar between solutions. Biodegradation of PCH was confirmed to be almost completed within 8 weeks. Statistically significant differences were not observed in esophageal constriction. | |||||
| Changes in elevation at the injection sites observed endoscopically 0, 5, 60, and 120 minutes after the injections | |||||||||||||
| Biodegradation of PCH injected into the submucosa | |||||||||||||
| Constriction of the esophagus (on the basis of endoscopic and histologic observations) | |||||||||||||
| Ishizuka 2009 58 | Japan | Animal study (in-vivo) | No lesions | Stomach | PCH | Hypertonic saline | PCH injection led to a longer−lasting elevation with clearer margins compared with hypertonic saline, enabling precise ESD along the margins of the elevated mucosa. The endoscopic appearance after ESD was similar in both groups. PCH biodegradationwas completed within 8 weeks. | ||||||
| Elevation of the submucosal layer at 0, 5, 10, 30 and 60 min | |||||||||||||
| Wound healing after ESD | |||||||||||||
| Biodegradation of PCH injected into the submucosa (on the basis of endoscopic and histologic observations) | |||||||||||||
| Hayashi 2004 45 | Japan | Animal study (in-vivo) | No lesions | Stomach | PCH | NS | |||||||
| Mean thickness of the submucosal layer (mm) 30 min after injection | 3.8 | 2.0 | P < 0.005 | ||||||||||
| Mean cumulative blood loss (mg) 30 min after injection | 113 | 1682 | P < 0.001 | ||||||||||
| Moss 2010 46 | Australia | Animal study (in-vivo) | No lesions | Colon | Succinylated gelatin | NS | |||||||
| Mean EMR specimen size (surface area (cm2)) | 9.5 | 6.7 | P = 0.044 | ||||||||||
| Mean duration (min) of submucosal cushion and of procedure | 60 and 2.5 | 15 and 2.5 | P = 0.005 and P = 0.515 | ||||||||||
| Perforations | 2 | 1 | P = 1.0 | ||||||||||
| Sumiyama 2010 47 | Japan | Clinical study (case series) | 16 EGC and 4 adenomas | Stomach | Mesna | The use of mesna solution facilitates and expedites mechanical submucosal dissection. | |||||||
| En bloc resection rate | 20 /20 (100 %) | ||||||||||||
| Mean sampled specimen size (mm) | 38.25 | ||||||||||||
| Mean ESD procedure time (min) | 21.17 | ||||||||||||
| Mean volume of mesna solution used (ml) | 7.4 | ||||||||||||
| von Renteln 2011 48 | USA | Animal study (in-vivo) | No lesions | Stomach | Mesna | NS | |||||||
| Mean time to dissect the submucosal plane (min) | 15 | 16 | P = 1.0 | ||||||||||
| Mean overall ESD time (min) | 24 | 28 | P = 0.48 | ||||||||||
| En bloc resection | 6 /6 (100 %) | 6 /6 (100 %) | |||||||||||
| Bleeding | 0 | 4 | P = 0.09 | ||||||||||
| Giday 2006 49 | USA | Animal study (in-vivo) | No lesions | Esophagus | Blood | HPMC | Albumin 25 % | Albumin 12.5 % | NS + E | Injection of blood resulted in significantly longer mucosal elevation than any other solution (P < 0.0007). | |||
| Mean time to dissipation of the submucosal cushion (min) | 38.6 | 9.77 | 7.83 | 5.68 | 2.87 | ||||||||
| Al-Taie 2012 51 | Germany | Animal study (ex-vivo) | No lesions | Stomach | NS | SH | Glyceol | Hydroxyethyl | Blood | Serum | Plasma | Whole blood is more effective in generating long-lasting mucosa elevation than any other commonly used solution (P < 0.05). | |
| Mean mucosa elevation before and up to 60 minutes after injection | A significantly longer duration was obtained after injection of hydroxyethyl starch, 0.25 % and 0.5 % hyaluronic acid, serum, and plasma. However, whole blood generated a longer-lasting mucosa elevation than all other agents. | ||||||||||||
| Sato 2006 50 | Japan | Clinical study (case series) | 35 colorectal polyps | Colon | Blood | EMR assisted by submucosal injection of autologous blood can be performed safely, easily and economically. | |||||||
| Lesion lifting sufficient to perform the EMR | 34 /35 (97.1 %) | ||||||||||||
| En bloc resection | 31 /34 (91.2 %) | ||||||||||||
| Overall complete resection | 33 /34 (97.1 %) | ||||||||||||
| Bleeding / perfuration | 0 /34 (0 %) | ||||||||||||
| Tran 2012 54 | USA | No lesions | Stomach | NS | SH | IDEEP | |||||||
| Animal study (ex-vivo) | Mean maximum injection pressure (PSI) | 6.0 | 29.5 | 28.8 | P > 0.05 | ||||||||
| Mean submucosal elevation duration 30 min after injection (mm) | 2.8 | 4.2 | 5.7 | P < 0.05 | |||||||||
| Animal study (in-vivo) | Assessment of in vivo efficacy by en bloc resection | All EMR were successfully performed after injection of iDEEP and a large gel cushion was noted after the resection procedure. | |||||||||||
| Uraoka 2011 55 | Japan | No lesions | NS | CO2 | SH | The efficacy of using CO2 as a satisfactory submucosal injection agent during ESD procedures was successfully demonstrated. | |||||||
| Animal study (ex-vivo) | Mean volume of injection agent needed to create the initial 2 cm submucosal elevation height (ml) | Stomach | 12.4 | 41.0 | 8.1 | ||||||||
| Mean submucosal elevation (mm) | Submucosal elevation was significantly longer lasting than either NS or SH (P < 0.001) | ||||||||||||
| Animal study (in-vivo) | Assessment of in vivo the efficacy of EMR using CO2 | Stomach | Resected CO2 specimens measured 25 and 33 mm from the gastric body and 15 mm from the rectum with a mean size of 24.3 mm vs 20 mm for those in the gastric body using NS. | ||||||||||
| Rectum | |||||||||||||
| Chandrasekhara 2013 56 | USA | Animal studu (in-vivo) | No lesions | Stomach | Cook Medical Gel | The gel appears to be a safe injectate that provides a submucosal cushion with a duration that is longer than other available injectates for EMR and ESD. | |||||||
| Evaluation of submucosal cushion | Every injection resulted in adequate mucosal lifting with a shoulder and defined margin and no cases of gel extravasation. The submucosal cushion was still present at the time of organ extraction without evidence of perforation, bleeding or tissue damage. | ||||||||||||
| Complications | Necropsy demonstrated no evidence of perforation, bleeding or gel extravasation. | ||||||||||||
| Katsinelos 2006 41 | Greece | Clinical study (case series) | 59 colorectal polyps | Colon | 50 D + E | EMR after submucosal hypertonic + epinephrine solution injection, with an intensive follow-up program, seems to be a safe and effective treatment for large colorectal polyps. | |||||||
| Mean amount of solution injected (ml) | 24.42 ± 17.52 | ||||||||||||
| Mean disapperance time (min) | 13.61 ± 5.21 | ||||||||||||
| En bloc and piecemeal resection rate | 23 /59 (39 %) and 36 /59 (61 %) | ||||||||||||
| Complications rate (bleeding) | 4 / 59 (6.8 %) | ||||||||||||
| Recurrence rate after 1 year or longer (en bloc and piecemeal resection) | 23 /23 (100 %) and 30 /31 (96.78 %) | ||||||||||||
| Wen 2012 29 | China | Blood | NS | ||||||||||
| Animal study | No lesions | Mean lifting time of the injection (min) | Colon | 18.25 ± 5.44 | 6.5 ± 2.38 | P = 0.007 | |||||||
| Degree of hydrops | The hydrops in the NS group were more extensive than those in the plasma solution injected group (P = 0.011) | The in vivo animal and human study demonstrated that whole blood or plasma solution may outperform normal saline due to its unique lifting ability, less tissue damage and marked effective submucosal blunt dissection. | |||||||||||
| Degree of tears | Tearing in the NS group was less than that in the plasma injected group (P = 0.008) | ||||||||||||
| Mean time of ESD (min) | Stomach | 7.0 ± 2.12 | 5.25 ± 0.96 | ||||||||||
| Clinical study (case-control) | 38 gastrointestinal lesions (35 polyps, 2 EGC, 1 cystic gastritis) | Colon Stomach | 7 | 31 | |||||||||
| Degree of hydrops | The degree of hydrops in the NS group was more extensive than that in the group with whole blood (P < 0.001). | ||||||||||||
| Degree of tears | The effective submucosal tearing in the group with NS was less than that in the group with blood (P < 0.001). | ||||||||||||
Abbreviations: min – minutes; NS – normal saline; SH – sodium hyaluronate; HA – human albumin; HPMC – hydroxypropyl methylcellulose; HES – hydroxyethyl starch; DW – dextrose water; SD – standard deviation; kDa – kiloDaltons; ESD – endoscopic submucosal dissection; SA – sodium alginate; EMR – endoscopic mucosal resection; EGC – early gastric cancer; LSTs – laterally spreading tumors; SCMC – sodium carboxymethylcellulose; EUS – endoscopic ultrasonography; PS137 – 25 – poloxamer solution PS137 – 25; PCH – Photocrosslinkable chitosan hydrogel; mesna – sodium-2-mercaptoethanesulfonate; E – epinephrine; iDEEP – injectable drug-eluting elastomeric polymer; 50 D + E – 50 % dextrose plus epinephrine
Competing interests: None of the authors has any relevant conflict of interest to disclose.
These authors contributed equally to the study.
References
- 1.Ferlay J, Shin H R, Bray F. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Zauber A G, Winawer S J, O'Brien M J. et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. NEJM. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winawer S J, Zauber A G, Ho M N. et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. NEJM. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 4.Hartgrink H H, Jansen E P, van Grieken N C. et al. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajani J A, Bentrem D J, Besh S. et al. Gastric cancer, version 2. 2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 6.Isomoto H, Shikuwa S, Yamaguchi N. et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 7.Choi K S, Jung H Y, Choi K D. et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942–948. doi: 10.1016/j.gie.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg N. Submucosal saline wheal as safety factor in fulguration or rectal and sigmoidal polypi. AMA Arch Surg. 1955;70:120–122. doi: 10.1001/archsurg.1955.01270070122021. [DOI] [PubMed] [Google Scholar]
- 9.Deyhle P, Jenny S, Fumagalli I. [Endoscopic polypectomy in the proximal colon. A diagnostic, therapeutic (and preventive?) intervention] Dtsch Med Wochenschr. 1973;98:219–220. doi: 10.1055/s-0028-1106782. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama M, Ota M, Nakajima H. et al. Endoscopic mucosal resection of gastric neoplasms using a ligating device. Gastrointest Endosc. 1997;45:182–186. doi: 10.1016/s0016-5107(97)70245-1. [DOI] [PubMed] [Google Scholar]
- 11.Inoue H, Takeshita K, Hori H. et al. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58–62. doi: 10.1016/s0016-5107(93)70012-7. [DOI] [PubMed] [Google Scholar]
- 12.Kantsevoy S V, Adler D G, Conway J D. et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11–18. doi: 10.1016/j.gie.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 13.Moss A, Bourke M J, Williams S J. et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909–1918. doi: 10.1053/j.gastro.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 14.Park Y M, Cho E, Kang H Y. et al. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666–2677. doi: 10.1007/s00464-011-1627-z. [DOI] [PubMed] [Google Scholar]
- 15.Lian J, Chen S, Zhang Y. et al. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763–770. doi: 10.1016/j.gie.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Liao C, Tan A. et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751–757. doi: 10.1055/s-0029-1215053. [DOI] [PubMed] [Google Scholar]
- 17.Sumiyama K, Toyoizumi H, Ohya T R. et al. A double-blind, block-randomized, placebo-controlled trial to identify the chemical assistance effect of mesna submucosal injection for gastric endoscopic submucosal dissection. Gastrointest Endosc. 2014;79:756–764. doi: 10.1016/j.gie.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y D, Lee J, Cho J Y. et al. Efficacy and safety of 0.4 percent sodium hyaluronate for endoscopic submucosal dissection of gastric neoplasms. World J Gastroenterol. 2013;19:3069–3076. doi: 10.3748/wjg.v19.i20.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurlstone D P, Fu K I, Brown S R. et al. EMR using dextrose solution versus sodium hyaluronate for colorectal Paris type I and 0-II lesions: a randomized endoscopist-blinded study. Endoscopy. 2008;40:110–114. doi: 10.1055/s-2007-966987. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida N, Naito Y, Inada Y. et al. Endoscopic mucosal resection with 0.13% hyaluronic acid solution for colorectal polyps less than 20 mm: a randomized controlled trial. J Gastroenterol Hepatol. 2012;27:1377–1383. doi: 10.1111/j.1440-1746.2012.07166.x. [DOI] [PubMed] [Google Scholar]
- 21.Kishihara T, Chino A, Uragami N. et al. Usefulness of sodium hyaluronate solution in colorectal endoscopic mucosal resection. Dig Endosc. 2012;24:348–352. doi: 10.1111/j.1443-1661.2012.01244.x. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto H, Yahagi N, Oyama T. et al. Usefulness and safety of 0.4% sodium hyaluronate solution as a submucosal fluid "cushion" in endoscopic resection for gastric neoplasms: a prospective multicenter trial. Gastrointest Endosc. 2008;67:830–839. doi: 10.1016/j.gie.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 23.Katsinelos P, Kountouras J, Paroutoglou G. et al. A comparative study of 50% dextrose and normal saline solution on their ability to create submucosal fluid cushions for endoscopic resection of sessile rectosigmoid polyps. Gastrointest Endosc. 2008;68:692–698. doi: 10.1016/j.gie.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 24.Varadarajulu S, Tamhane A, Slaughter R L. Evaluation of dextrose 50% as a medium for injection-assisted polypectomy. Endoscopy. 2006;38:907–912. doi: 10.1055/s-2006-944664. [DOI] [PubMed] [Google Scholar]
- 25.Lee S H, Park J H, do Park H. et al. Clinical efficacy of EMR with submucosal injection of a fibrinogen mixture: a prospective randomized trial. Gastrointest Endosc. 2006;64:691–696. doi: 10.1016/j.gie.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 26.Fasoulas K, Lazaraki G, Chatzimavroudis G. et al. Endoscopic mucosal resection of giant laterally spreading tumors with submucosal injection of hydroxyethyl starch: comparative study with normal saline solution. Surg Laparosc Endosc Percutan Tech. 2012;22:272–278. doi: 10.1097/SLE.0b013e318251553c. [DOI] [PubMed] [Google Scholar]
- 27.Moss A, Bourke M J, Metz A J. A randomized, double-blind trial of succinylated gelatin submucosal injection for endoscopic resection of large sessile polyps of the colon. Am J Gastroenterol. 2010;105:2375–2382. doi: 10.1038/ajg.2010.319. [DOI] [PubMed] [Google Scholar]
- 28.Lee S H, Chung I K, Kim S J. et al. Comparison of postpolypectomy bleeding between epinephrine and saline submucosal injection for large colon polyps by conventional polypectomy: a prospective randomized, multicenter study. World J Gastroenterol. 2007;13:2973–2977. doi: 10.3748/wjg.v13.i21.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen W, Shi C, Shi Y. et al. A pilot animal and clinical study of autologous blood solution compared with normal saline for use as an endoscopic submucosal cushion. Exp Ther Med. 2012;4:419–424. doi: 10.3892/etm.2012.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobrowolski S, Dobosz M, Babicki A. et al. Prophylactic submucosal saline-adrenaline injection in colonoscopic polypectomy: prospective randomized study. Surg Endosc. 2004;18:990–993. doi: 10.1007/s00464-003-9214-6. [DOI] [PubMed] [Google Scholar]
- 31.Polymeros D, Kotsalidis G, Triantafyllou K. et al. Comparative performance of novel solutions for submucosal injection in porcine stomachs: An ex vivo study. Dig Liver Dis. 2010;42:226–229. doi: 10.1016/j.dld.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Hyun J J, Chun H R, Chun H J. et al. Comparison of the characteristics of submucosal injection solutions used in endoscopic mucosal resection. Scand J Gastroenterol. 2006;41:488–492. doi: 10.1080/00365520500325994. [DOI] [PubMed] [Google Scholar]
- 33.Fujishiro M, Yahagi N, Kashimura K. et al. Tissue damage of different submucosal injection solutions for EMR. Gastrointest Endosc. 2005;62:933–942. doi: 10.1016/j.gie.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 34.Conio M, Rajan E, Sorbi D. et al. Comparative performance in the porcine esophagus of different solutions used for submucosal injection. Gastrointest Endosc. 2002;56:513–516. doi: 10.1067/mge.2002.128107. [DOI] [PubMed] [Google Scholar]
- 35.Fujishiro M, Yahagi N, Nakamura M. et al. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63:243–249. doi: 10.1016/j.gie.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Sohn D K, Chang H J, Choi H S. et al. Does hyaluronic acid stimulate tumor growth after endoscopic mucosal resection? J Gastroenterol Hepatol. 2008;23:1204–1207. doi: 10.1111/j.1440-1746.2008.05470.x. [DOI] [PubMed] [Google Scholar]
- 37.Matsui Y, Inomata M, Izumi K. et al. Hyaluronic acid stimulates tumor-cell proliferation at wound sites. Gastrointest Endosc. 2004;60:539–543. doi: 10.1016/s0016-5107(04)01890-5. [DOI] [PubMed] [Google Scholar]
- 38.Eun S H, Cho J Y, Jung I S. et al. Effectiveness of sodium alginate as a submucosal injection material for endoscopic mucosal resection in animal. Gut Liver. 2007;1:27–32. doi: 10.5009/gnl.2007.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akagi T, Yasuda K, Tajima M. et al. Sodium alginate as an ideal submucosal injection material for endoscopic submucosal resection: preliminary experimental and clinical study. Gastrointest Endosc. 2011;74:1026–1032. doi: 10.1016/j.gie.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 40.Kusano T, Etoh T, Akagi T. et al. Evaluation of 0.6% sodium alginate as a submucosal injection material in endoscopic submucosal dissection for early gastric cancer. Dig Endosc. 2014;26:638–645. doi: 10.1111/den.12268. [DOI] [PubMed] [Google Scholar]
- 41.Katsinelos P, Paroutoglou G, Beltsis A. et al. Endoscopic mucosal resection of lateral spreading tumors of the colon using a novel solution. Surg Laparosc Endosc Percutan Tech. 2006;16:73–77. doi: 10.1097/00129689-200604000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Uraoka T, Fujii T, Saito Y. et al. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736–740. doi: 10.1016/s0016-5107(05)00321-4. [DOI] [PubMed] [Google Scholar]
- 43.Yamasaki M, Kume K, Yoshikawa I. et al. A novel method of endoscopic submucosal dissection with blunt abrasion by submucosal injection of sodium carboxymethylcellulose: an animal preliminary study. Gastrointest Endosc. 2006;64:958–965. doi: 10.1016/j.gie.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 44.Kumano I, Ishihara M, Nakamura S. et al. Endoscopic submucosal dissection for pig esophagus by using photocrosslinkable chitosan hydrogel as submucosal fluid cushion. Gastrointest Endosc. 2012;75:841–848. doi: 10.1016/j.gie.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi T, Matsuyama T, Hanada K. et al. Usefulness of photocrosslinkable chitosan for endoscopic cancer treatment in alimentary tract. J Biomed Mater Res B Appl Biomater. 2004;71:367–372. doi: 10.1002/jbm.b.30099. [DOI] [PubMed] [Google Scholar]
- 46.Moss A, Bourke M J, Kwan V. et al. Succinylated gelatin substantially increases en bloc resection size in colonic EMR: a randomized, blinded trial in a porcine model. Gastrointest Endosc. 2010;71:589–595. doi: 10.1016/j.gie.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 47.Sumiyama K, Tajiri H, Gostout C J. et al. Chemically assisted submucosal injection facilitates endoscopic submucosal dissection of gastric neoplasms. Endoscopy. 2010;42:627–632. doi: 10.1055/s-0029-1244223. [DOI] [PubMed] [Google Scholar]
- 48.von Renteln D, Dulai P S, Pohl H. et al. Endoscopic submucosal dissection with a flexible Maryland dissector: randomized comparison of mesna and saline solution for submucosal injection (with videos) Gastrointest Endosc. 2011;74:906–911. doi: 10.1016/j.gie.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 49.Giday S A, Magno P, Buscaglia J M. et al. Is blood the ideal submucosal cushioning agent? A comparative study in a porcine model. Endoscopy. 2006;38:1230–1234. doi: 10.1055/s-2006-944971. [DOI] [PubMed] [Google Scholar]
- 50.Sato T. A novel method of endoscopic mucosal resection assisted by submucosal injection of autologous blood (blood patch EMR) Dis Colon Rectum. 2006;49:1636–1641. doi: 10.1007/s10350-006-0680-5. [DOI] [PubMed] [Google Scholar]
- 51.Al-Taie O H, Bauer Y, Dietrich C G. et al. Efficacy of submucosal injection of different solutions inclusive blood components on mucosa elevation for endoscopic resection. Clin Exp Gastroenterol. 2012;5:43–48. doi: 10.2147/CEG.S29704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S H, Cho W Y, Kim H J. et al. A new method of EMR: submucosal injection of a fibrinogen mixture. Gastrointest Endosc. 2004;59:220–224. doi: 10.1016/s0016-5107(03)02689-0. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez-Esparrach G, Shaikh S N, Cohen A. et al. Efficacy of a reverse-phase polymer as a submucosal injection solution for EMR: a comparative study (with video) Gastrointest Endosc. 2009;69:1135–1139. doi: 10.1016/j.gie.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 54.Tran R T, Palmer M, Tang S J. et al. Injectable drug-eluting elastomeric polymer: a novel submucosal injection material. Gastrointest Endosc. 2012;75:1092–1097. doi: 10.1016/j.gie.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uraoka T, Kawahara Y, Ohara N. et al. Carbon dioxide submucosal injection cushion: an innovative technique in endoscopic submucosal dissection. Dig Endosc. 2011;23:5–9. doi: 10.1111/j.1443-1661.2010.01038.x. [DOI] [PubMed] [Google Scholar]
- 56.Chandrasekhara V, Sigmon J C Jr, Surti V C. et al. A novel gel provides durable submucosal cushion for endoscopic mucosal resection and endoscopic submucosal dissection. Surg Endosc. 2013;27:3039–3042. doi: 10.1007/s00464-013-2813-y. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida N, Naito Y, Kugai M. et al. Efficacy of hyaluronic acid in endoscopic mucosal resection of colorectal tumors. J Gastroenterol Hepatol. 2011;26:286–291. doi: 10.1111/j.1440-1746.2010.06505.x. [DOI] [PubMed] [Google Scholar]
- 58.Ishizuka T, Ishihara M, Aiko S. et al. Experimental evaluation of photocrosslinkable chitosan hydrogel as injection solution for endoscopic resection. Endoscopy. 2009;41:25–28. doi: 10.1055/s-0028-1103483. [DOI] [PubMed] [Google Scholar]


