Abstract
Horizontal gene transfer (HGT) allows organisms to rapidly acquire adaptive traits1. Though documented instances of HGT from bacteria to eukaryotes remain rare, bacteria represent a rich source of new functions potentially available for co-option2. One benefit that genes of bacterial origin could provide to eukaryotes is the capacity to produce anti-bacterials, which have evolved in prokaryotes as the result of eons of interbacterial competition. The type VI secretion amidase effector (Tae) proteins are potent bacteriocidal enzymes that degrade the cell wall when delivered into competing bacterial cells by the type VI secretion system (T6SS)3. Here we show that tae genes have been transferred to eukaryotes on at least six occasions, and that the resulting domesticated amidase effector (dae) genes have been preserved for hundreds of millions of years via purifying selection. We show that the dae genes acquired eukaryotic secretion signals, are expressed within recipient organisms, and encode active antibacterial toxins that possess substrate specificity matching extant Tae proteins of the same lineage. Finally, we show that a dae gene in the deer tick Ixodes scapularis limits proliferation of Borrelia burgdorferi, the etiologic agent of Lyme disease. Our work demonstrates that a family of horizontally acquired toxins honed to mediate interbacterial antagonism confers previously undescribed antibacterial capacity to eukaryotes. We speculate that the selective pressure imposed by competition between bacteria has produced a reservoir of genes encoding diverse antimicrobial functions that are tailored for facile co-option by eukaryotic innate immune systems.
Eukaryotes can acquire new functions through the exchange of genetic material with other domains of life1. Indeed, Bacteria-to-Eukarya HGT underlies the adaptation and diversification of many microbial eukaryotes, such as algae, choanoflagellates, and protozoa4,5. The acquisition of bacterial genes by metazoans is rare. Among the transferred genes, many are not expressed and have no known function6, while others have roles in endosymbiont maintenance7,8. Relatively few reports provide evidence of transferred elements conferring traits directly beneficial strictly to their metazoan recipients2. One recent example is the discovery that phytophagous mites and Lepidoptera species exploit a horizontally acquired bacterial cysteine synthase in order to feed on plants producing cyanogenic defense compounds9.
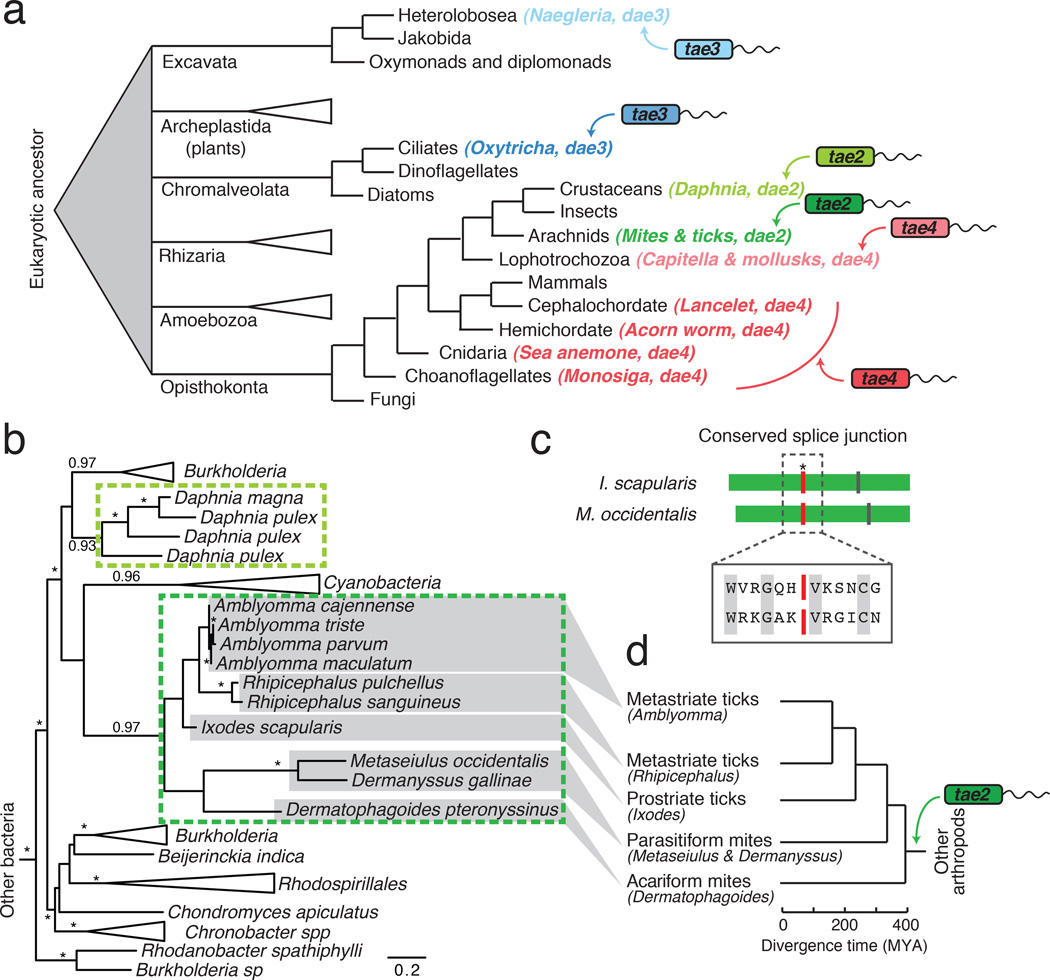
Genes that can independently provide new functionality to a recipient organism are strong candidates for domestication following HGT6,10. The Tae proteins are small, single domain enzymes that can rapidly digest the bacterial cell wall11. These proteins comprise four phylogenetically distinct families (Tae1–4) that share no overall sequence homology and display unique specificities against peptidoglycan (PG)3,12. In the course of probing tae distribution, we made the serendipitous observation that homologs are found in distantly related eukaryotic genomic and expression datasets ranging from unicellular protozoa to multicellular metazoans (Fig. 1a). The genes did not appear to derive from contaminating bacterial DNA; most contain introns and are located in genomic regions flanked by eukaryotic genes (Extended Data Fig. 1)13. We therefore refer to these eukaryotic loci as domesticated amidase effector (dae) genes, and hypothesized they encode antibacterial toxins horizontally acquired from bacteria. Maximum likelihood and Bayesian phylogenetic analyses revealed that trees of bacterial tae2–4 families each contained two distinct monophyletic clades of eukaryotic dae genes (Fig. 1b; Extended Data Figs. 2–4). Thus, we conclude that three of the four known tae gene families have been acquired by eukaryotes from diverse bacteria in at least six HGT events (Fig. 1a). Our survey is biased by the status of genome sequencing efforts; therefore, these six instances are likely an underestimate of eukaryotic tae acquisitions.
Figure 1. Recurrent horizontal gene transfer of type VI amidase effector (tae) genes into diverse eukaryotic lineages.
a, Schematized phylogenetic tree of basal eukaryotic lineages28 showing instances of tae transfer (arrows) from bacteria to eukaryotes, coded by color (tae family) and shading (acquisition events). b, Maximum likelihood phylogenetic tree of tae2 and dae2 genes. Representatives are boxed and color-coded according to Fig. 1a. Branch support > 0.7 indicated by asterisks or numbers. Scale bar shows estimated divergence in amino acid changes per residue. Dashed lines highlight separate HGT events. c, Schematic alignment of tick (I. scapularis) and mite (M. occidentalis) dae2 genes with shared (red line, asterisk) and unique (vertical lines) intron positions denoted. Aligned residues surrounding the splice site are shown (boxed) with conserved amino acids indicated (grey). d, Tick and mite phylogeny with approximate dates of divergence based on concordance with the dae2 gene tree (c)14.
Three of the dae genes we found are limited to individual or closely related eukaryotes (light green, light blue and dark blue; Fig. 1a). These could represent recent HGT events, or reflect limited genomic and transcriptomic sampling of related species. The remaining three dae genes appear to be the result of ancient HGT events. For instance, we found dae2 in 10 species of ticks and mites (Fig. 1b). This dense sampling, a shared intron between the dae2 genome sequence of I. scapularis and Metaseiulus occidentalis, and the fact that the tick and mite dae2 gene phylogeny closely resembles the established phylogeny of these organisms, lead us to conclude that vertical transmission followed a single HGT event of a bacterial tae2 gene to the common ancestor of ticks and mites approximately 400 million years ago (MYA) (Figs. 1b–d; Extended Data Fig. 1, Fig. 5a and 5b)14. The complete genome sequence of the Acariform mite Tetranychus urticae does not possess dae2, indicating loss of the gene has also occurred. Partial dae2 sequences in the genomes of two scorpions species and the horseshoe crab share an intron position with dae2 from ticks and mites, suggesting that dae2 introduction into arthropods may have occurred as early as 550 MYA (Extended Data Fig. 5c). Similarly, dense sampling of dae4 genes in gastropod and bivalve mollusks, as well as a shared dae4 intron position across all sampled mollusks and an annelid, dates the origin of dae4 in these animals to at least 400 MYA (light red; Fig. 1a and Extended Data Fig. 1 and Fig. 4)15. Finally, a second dae4 present in a species of choanoflagellates, sea anemones, acorn worms and lancelets is most parsimoniously explained by a single HGT event followed by vertical inheritance and loss in multiple lineages, dating this dae4 acquisition prior to the base of the metazoan lineage (>800 MYA) (dark red; Fig. 1a and Extended Data Fig. 4). However, owing to sparse sampling and lack of evidence of shared synteny, we cannot rule out more recent HGT to and between these eukaryotic lineages4. In summary, we find compelling evidence that at least two animal lineages have retained a bacterially derived antibacterial gene for hundreds of millions of years.
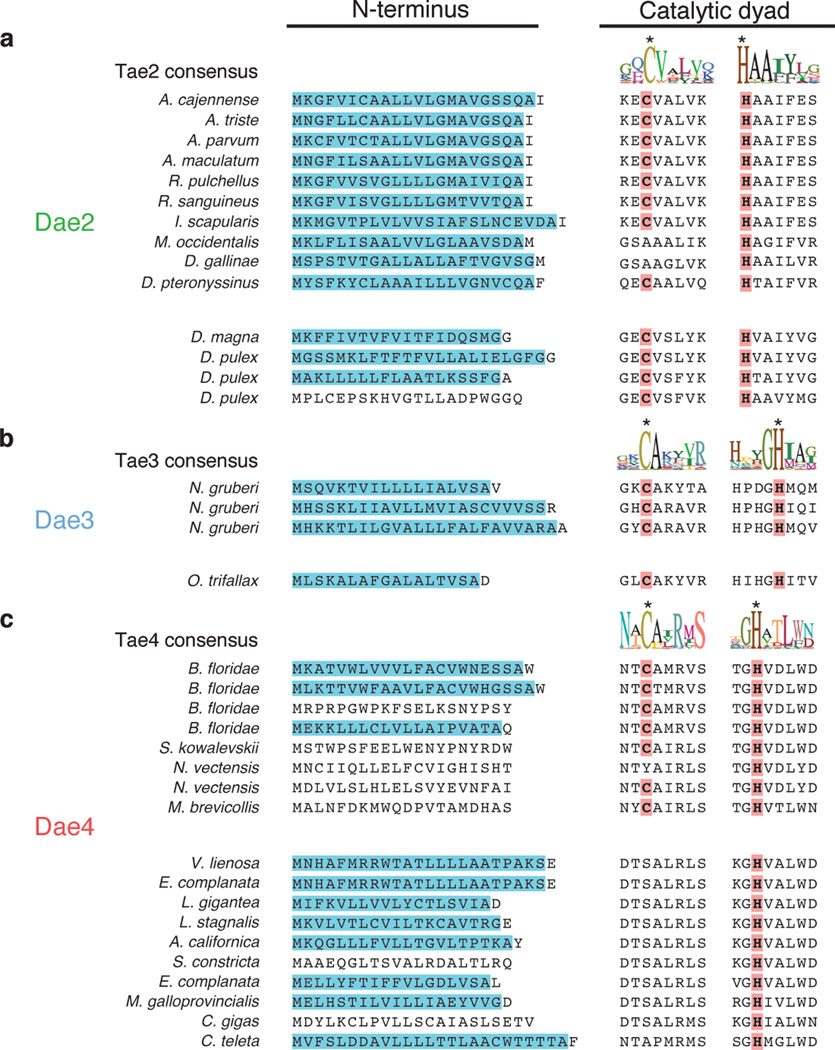
Several lines of evidence led us to hypothesize that dae genes provide an adaptive function to their eukaryotic hosts. We found strong signatures of purifying selection acting on dae2 and dae4 genes (Extended data Table 1). Additionally, eukaryotic Sec signals were identified in the majority of Dae proteins, including representatives from each of the predicted HGT events (Extended data Fig. 6). Secretion of bacterial Tae proteins occurs through the Sec-independent T6SS; thus, acquisition of a Sec signal is indicative of functional specialization involving export from eukaryotic cells. Lastly, the majority of Dae proteins possess the cysteine–histidine catalytic dyad and flanking motifs of their corresponding Tae families, consistent with retention of enzymatic activity (Extended data Fig. 6).
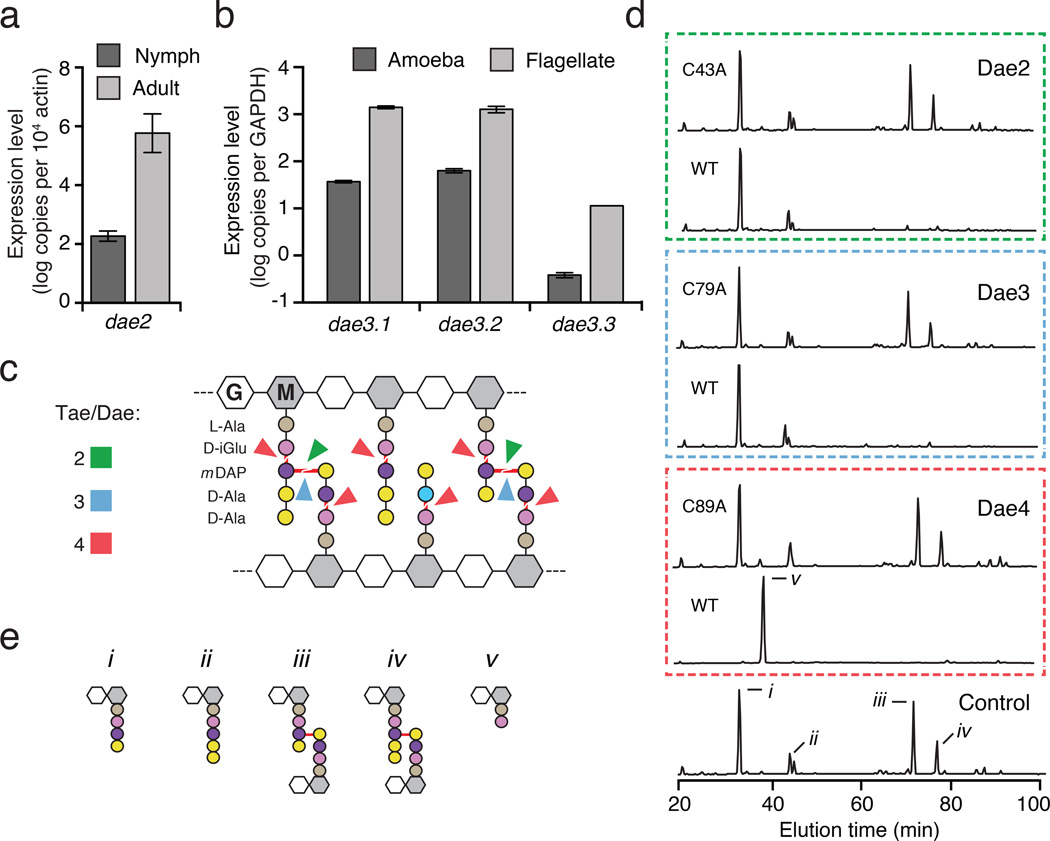
We next sought evidence of expression for eukaryotic dae homologs belonging to each of the transferred bacterial tae families. We found dae2 expression during both the unfed nymphal and unfed adult life stages of the hard tick I. scapularis, with levels significantly elevated in adults (Fig. 2a). In the amoeba Naegleria gruberi, we observed a basal level of expression of each of the three dae3 homologs in trophozoite (amoeba) cells, which increased during differentiation into flagellates (Fig. 2b). A published expression profile of the lancelet Branchiostoma floridae indicates that expression of dae4 is enriched at the neurula stage of development16. Together, these data strongly support the hypothesis that dae genes have been functionally integrat2 ed into recipient physiology.
Figure 2. Eukaryotic dae genes encode differentially-expressed PG amidases with conserved specificity.
a, b, Expression profile of I. scapularis and N. gruberi dae genes at the indicated life stages as measured by qRT-PCR. Levels of each N. gruberi dae3 genes (Dae3.1–3.3) were determined. Error bars +/− s.d., n=3. c, Schematic representation of typical Gram-negative PG showing cleavage sites (red lines) for Tae and Dae families 2–4 (colors correspond to Fig. 1a). d, Partial HPLC chromatograms of E. coli PG sacculi products resulting from incubation with buffer (control), native and catalytically inactive (C43A, C79A, C89A) Dae enzymes and cellosyl. e, Major HPLC peaks assigned previously by mass spectrometry correspond to disaccharide-linked tetrapeptide (i), pentapeptide (ii), tetrapeptide– tetrapeptide (iii), pentapeptide–tetrapeptide (iv), and dipeptide (v)3.
The Tae families display unique specificities against PG. Within PG typified by Gram-negative Proteobacteria, enzymes from families 1 and 4 cleave at the γ-D-glutamyl-meso-diaminopimelic acid (mDAP) bond, whereas those from 2 and 3 cleave the mDAP-D-alanine bond crosslinking the peptide stems (Fig. 2c)3,12,17. To test whether Dae proteins can hydrolyze PG, we incubated purified Dae2–4 representatives from I. scapularis, N. gruberi, and B. floridae, respectively, with isolated E. coli PG sacculi. HPLC analysis of reaction products demonstrated that each of the enzymes hydrolyzes PG (Fig. 2d and Fig. 2e). Remarkably, Dae2, Dae3, and Dae4 display substrate specificity matching that of the characterized extant Tae homologs within corresponding families (Fig. 2c). These data support the hypothesis that dae homologs, derived from three tae families, have been retained in eukaryotic genomes due to their PG amidase activity. We did not find evidence supporting the transfer of housekeeping bacterial amidases, leading us to speculate that genes encoding T6S effectors – enzymes that intoxicate recipient cells at exceedingly low concentrations – might be especially amenable to preservation following HGT18.
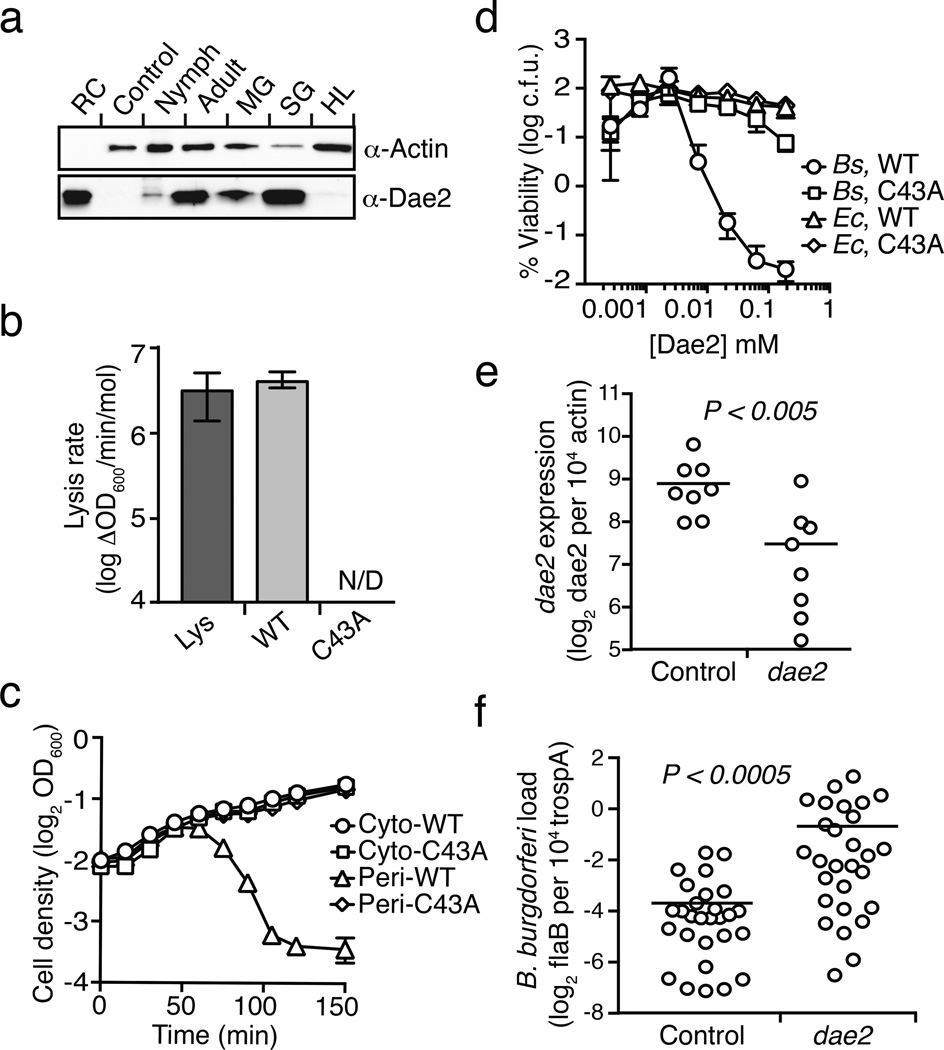
Within eukaryotes, enzymes with PG-degrading activity might play immuno-regulatory roles, or act directly as antibacterial factors like the Tae toxins19. To explore the functional significance of a domesticated tae, we focused on dae2 from the deer tick I. scapularis, an important vector for numerous diseases, including Lyme borreliosis and anaplasmosis20. Western blot analysis of adult I. scapularis demonstrated that Dae2 is present in the salivary glands and midgut (Fig. 3a). I. scapularis is an ectoparasite that requires a blood meal for life stage transitions; pathogens are typically acquired during feeding and transmitted to a new host at the next blood meal. Accordingly, the midgut and salivary glands interface with bacterial pathogens and influence their transmission21.
Figure 3. Dae2 is a bacteriolytic toxin that restricts the proliferation of B. burgdorferi in the tick I. scapularis.
a, Western blot analysis of Dae2 in unfed adult and nymphal total tissue (total), midgut (MG), salivary gland (SG), and hemolymph (HL) extracts from I. scapularis. Recombinant Dae2 protein (RC) and tissue from a closely related species, Dermacentor variablis (Control), were included. Actin levels were examined as a loading control. b, Lytic activity of lysozyme (Lys) and Dae3 (WT, C43A) proteins against permeabilized E. coli. Error bars +/− s.d., n=3. c, Growth of E. coli expressing native (cyto-) or periplasm-targeted (peri-) Dae2 proteins. Error bars +/− s.d., n=3. d, Bacterial killing activity of indicated proteins against B. subtilis (Bs) and E. coli (Ec) cells. Error bars +/− s.d., n=3. e, Dae2 transcript levels quantified by qRT-PCR in RNAi-treated engorged ticks. f, At 2-weeks post-engorgment, spirochaete levels were quantified in ticks that had received the indicated RNAi treatments, using qPCR analysis of flaB, a B. burgdorferi-specific gene, and normalized to trospa, a tick-specific gene. n=20. Each data point in Figs 3e and 3f represents 3 nymphs. Horizontal bars represent mean values, which were significantly different in a two-tailed nonparametric Mann-Whitney test (p<0.05).
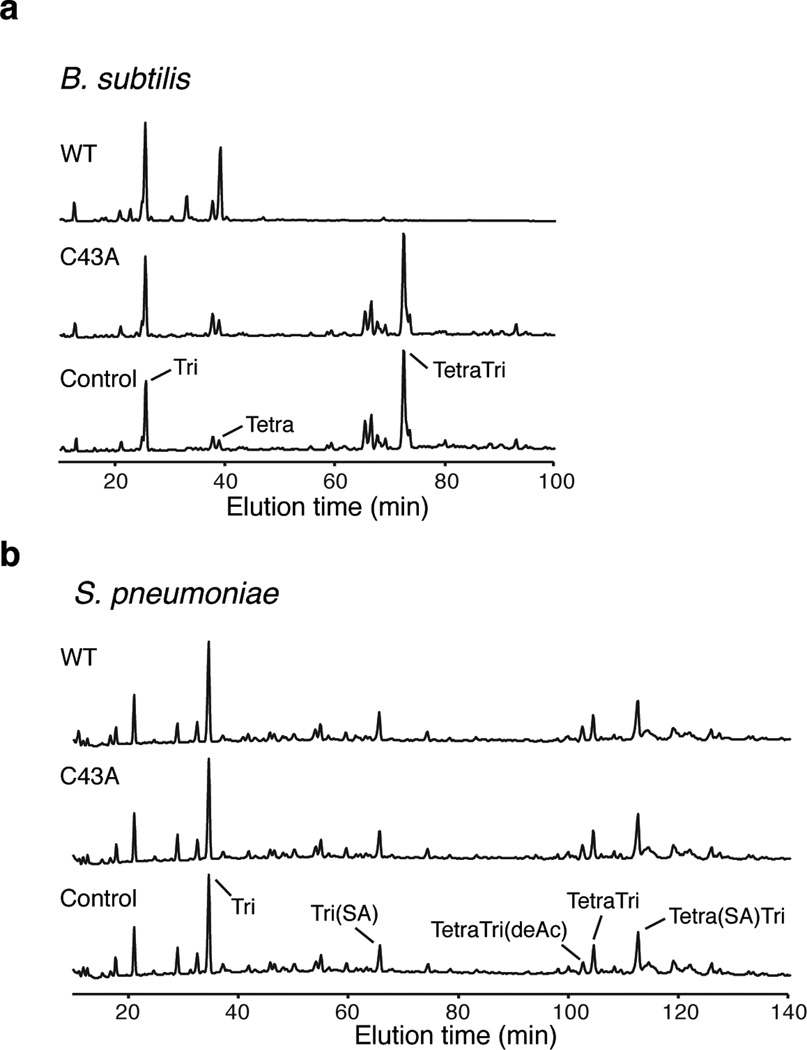
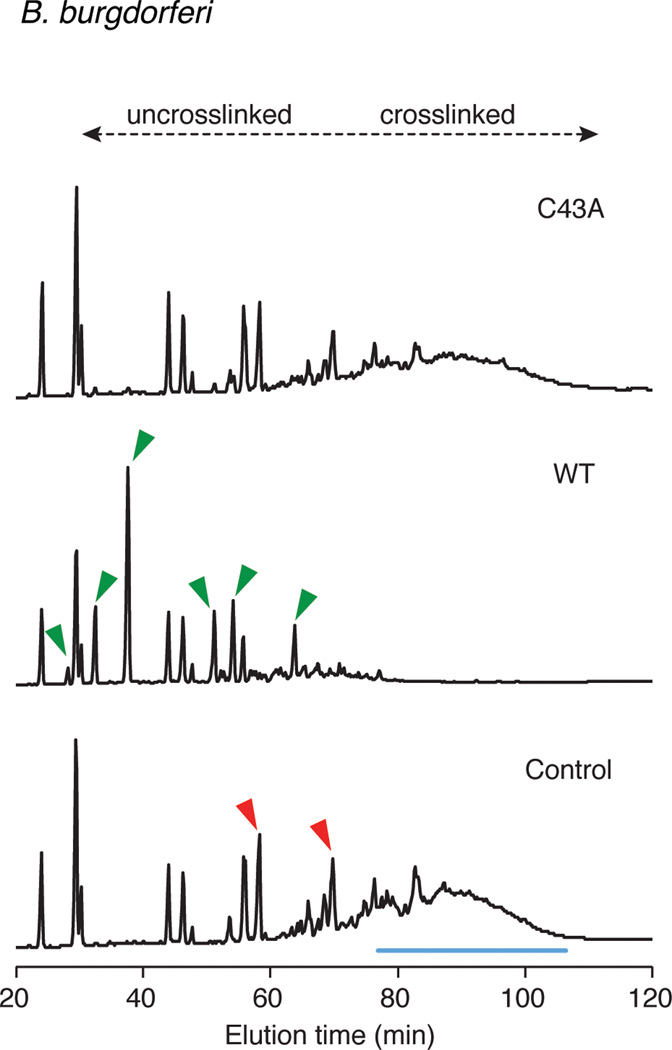
To understand how Dae2 could contribute to innate bacterial defense within I. scapularis, we tested its capacity to cleave diverse PG structures representative of bacteria the organism encounters in the environment22. Consistent with its ability to degrade E. coli PG, we found that Dae2 degrades a related form of the cell wall present in Firmicutes belonging to the class Bacilli (Extended data Fig. 7a)17. We did not detect cleavage of the lysine-type PG found in Streptococcus pneumoniae, which represents the second major PG-type found in Firmicutes (Extended data Fig. 7b). Though the ultrastructure of the B. burgdoferi sacculus is not well defined, its amino acid composition appears to differ from that of well-characterized bacterial cell walls23. Incubation of B. burgdorferi sacculi with Dae2 led to the accumulation of specific enzymatic degradation products, indicating that the cell wall of this organism is also a substrate of the amidase (Extended data Fig. 8).
The Dae proteins are reminiscent of an evolutionarily conserved group of bacteriophage-related eukaryotic innate immune amidases, the PG recognition proteins (PGRPs)19. Some PGRPs are directly bacteriocidal and act by hydrolyzing PG, whereas others exert antibacterial activity through alternative mechanisms24. We found that exogenous Dae2 is not toxic to intact E. coli cells. In contrast, Dae2, but not a catalytically-inactive variant of the enzyme (C43A), administered to outer membrane (OM)-permeabilized E. coli or targeted to the periplasm via the Sec pathway is highly lytic (Figs 3b–d). Moreover, exogenous Dae2 is bacteriocidal against B. subtilis, which has cell surface exposed PG (Fig. 3d). Together, these results strongly suggest Dae2-dependent antibiosis is solely the result of its amidase activity and that the enzyme would require OM permeabilizing agents such as antimicrobial peptides to act in vivo.
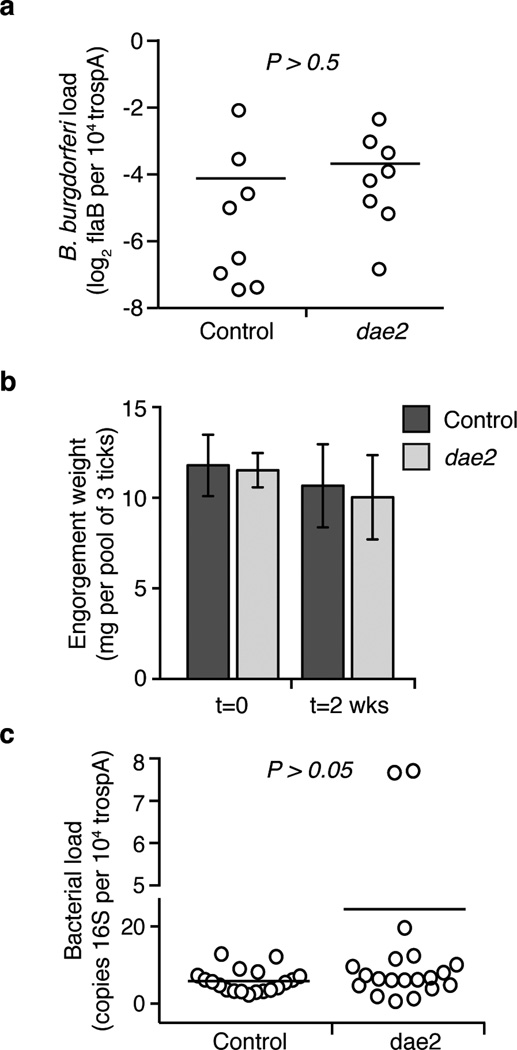
B. burgdorferi is the causative agent of Lyme disease, the most prevalent vector-borne illness in the United States25. Given the antibacterial activity of Dae2 (Figs 3b–d), its ability to cleave B. burgdorferi PG in vitro (Extended Data Fig. 8), and its localization to sites that interface with bacteria (Fig. 3a), we hypothesized that Dae2 could play a role in regulating B. burgdorferi populations in I. scapularis. We tested this possibility using RNAi-mediated knockdown of dae2 (Fig. 3e). RNAi-treated nymphal ticks were fed to repletion on B. burgdorferi-infected mice, and spirochaete load was assessed at engorgement and again after 2 weeks. At repletion, we observed no detectable difference in B. burgdorferi levels in control and experimental RNAi-treated ticks, indicating Dae2 activity does not limit initial acquisition of the bacterium (Extended Data Fig. 9a). In contrast, at 2 weeks post-engorgement, B. burgdorferi levels were significantly elevated in the dae2 knockdown group (Fig. 3f). The effect of Dae2 disruption on B. burgdorferi levels is unlikely to be due to variations in tick feeding or general fitness, as we observed no difference between the groups in engorgement weights at either time point (Extended Data Fig. 9b). Furthermore, overall bacterial load was similar between groups, suggesting that the increase in B. burgdorferi did not result from gross changes in populations of tick-associated microbes (Extended Data Fig. 9c). The ability of Dae2 to act on a wide range of bacterial cell walls leaves open the possibility that compositional changes to the tick microbiome may contribute to the effect of the knockdown on B. burgdorferi26. We conclude based on these findings that Dae2 contributes to the innate ability of I. scapularis to control B. burgdorferi levels following acquisition. This has potential ramifications for Lyme disease transmission, as spirochaete load in the tick can influence transmission efficiency27.
Here, we demonstrate that bacterial genes encoding antibacterial effectors of the T6SS have been horizontally transferred to diverse eukaryotes. The recurrent and independent transfer of tae genes to distinct eukaryotic lineages suggests that these toxins can confer immediate fitness benefits by supplying new function to the innate immune system10. Recent studies have revealed that the number and diversity of factors mediating interbacterial antagonism is greater than once appreciated. Thus, we speculate that competition between bacteria generates a reservoir of genes – beyond the tae superfamily – with the potential to confer antimicrobial capacity to eukaryotes upon acquisition.
Extended Data
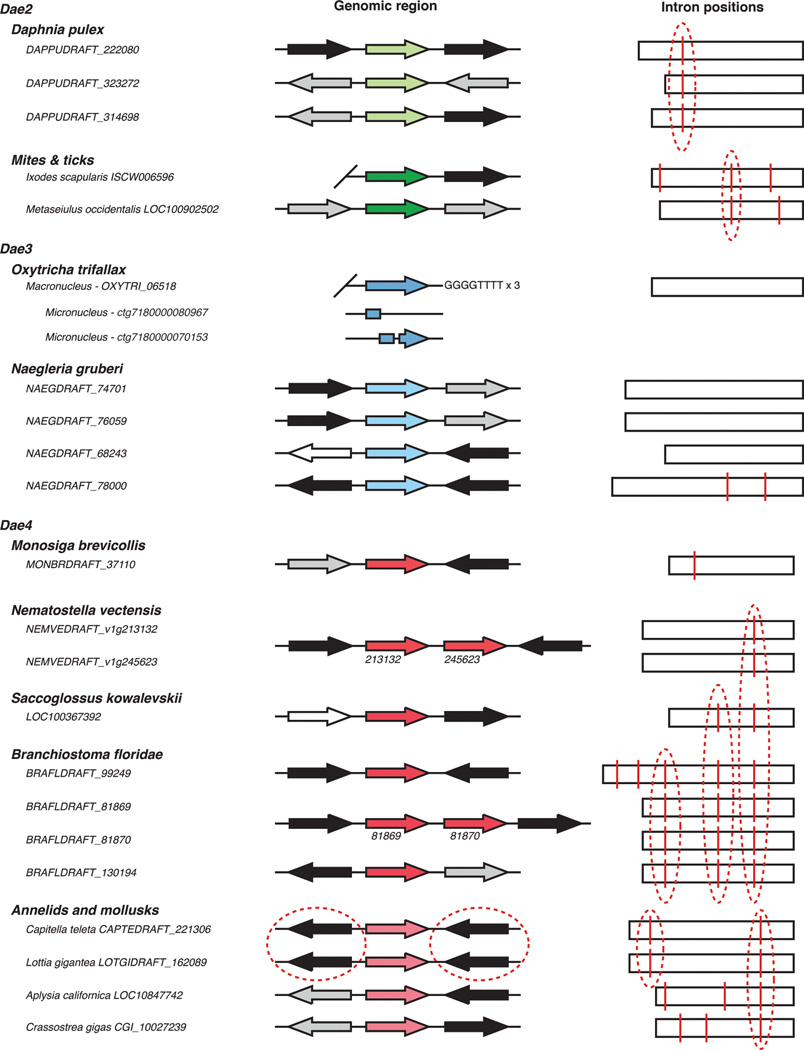
Extended Data Figure 1. Genomic evidence for validated eukaryotic dae genes.
Eukaryotic dae genes from the indicated organisms are listed adjacent to schematic representations of available predicted open reading frames (color-coded according to family as in Fig. 1a) and corresponding genomic context of dae genes. Flanking genes are colored-coded according to organisms that homologs of these genes are found in (broadly in eukaryotes, black; only closely related eukaryotic species, grey; both bacteria and eukaryotes, white). Diagonal lines denote ends of genomic contigs. In the right column, splice sites (red vertical lines) and conserved intron positions (red dashed circles) are shown. In Oxytricha trifallax, the somatic nucleus (macronucleus) contains ~16,000 chromosomes and is a rearranged form of the germline nucleus (micronucleus)13. The complete dae3 gene in Oxytricha is found in the macronucleus on a chromosome with three characteristic GGGGTTTT telomere sequences. Three fragments comprising the dae3 gene are found in the micronuclear genome (http://oxy.ciliate.org/). In Nematostella vectensis and Branchiostoma floridae, lineage-specific duplication events have resulted in two adjacent dae4 paralogs with gene names labeled (numbers). In Capitella teleta and Lottia gigantea, shared synteny on both sides of the dae4 gene is indicated (red dashed circles).
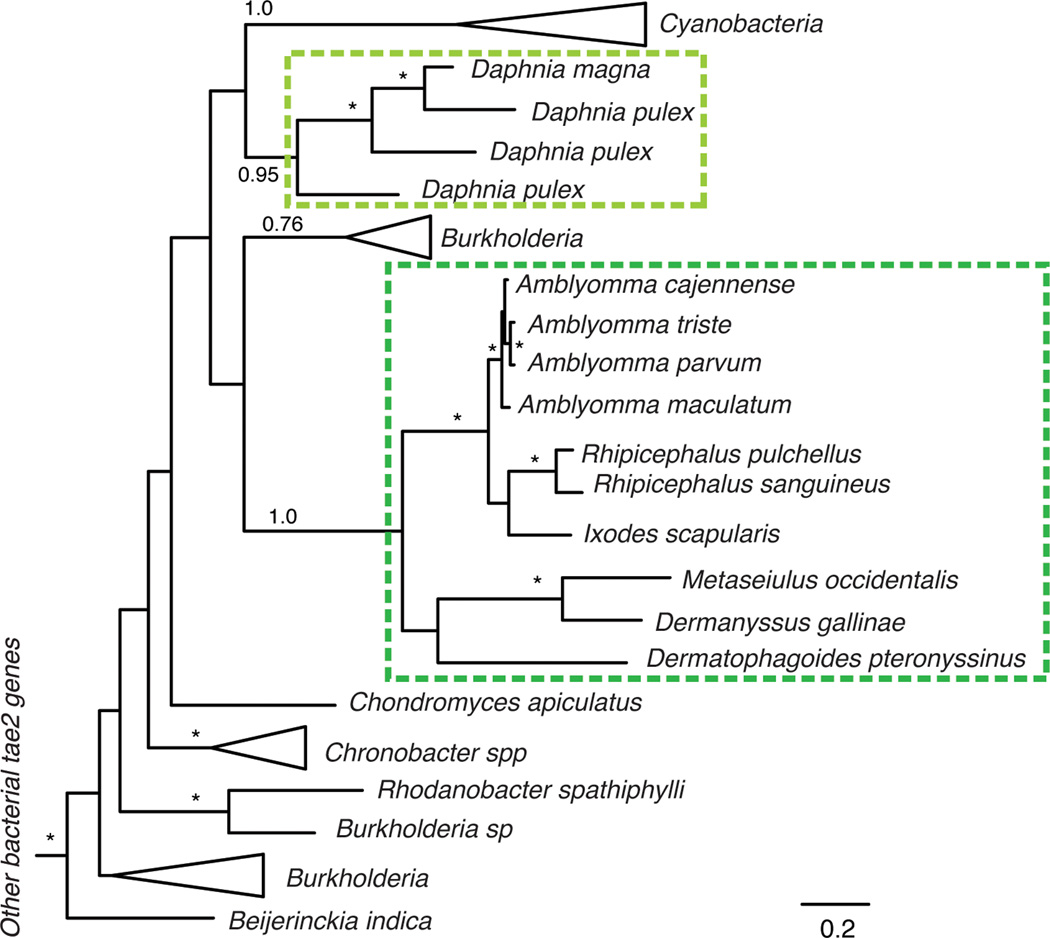
Extended Data Figure 2. Phylogenetic tree of bacterial tae2 and eukaroytic dae2 genes.
A phylogenetic tree was constructed using Bayesian methods in MrBayes34 to compare to the maximum likelihood tree shown in Fig. 1b. Branch support > 0.7 is indicated by asterisks or by numbers. The scale bar shows estimated divergence in amino acid changes per residue. Eukaryotic dae2 genes are indicated by dashed boxes, which highlight two separate HGT events. In both phylogenetic trees, the two eukaryotic dae2 clades are well supported as monophyletic clades, supporting our conclusion of two HGT events. Likewise, many major bacterial groups are well supported in both trees. Differences in the overall topology of the trees, mostly owing to changes in deep branches that are not well supported in either phylogenetic tree, reflect uncertainty in the ancient history of these genes and should therefore be treated with caution.
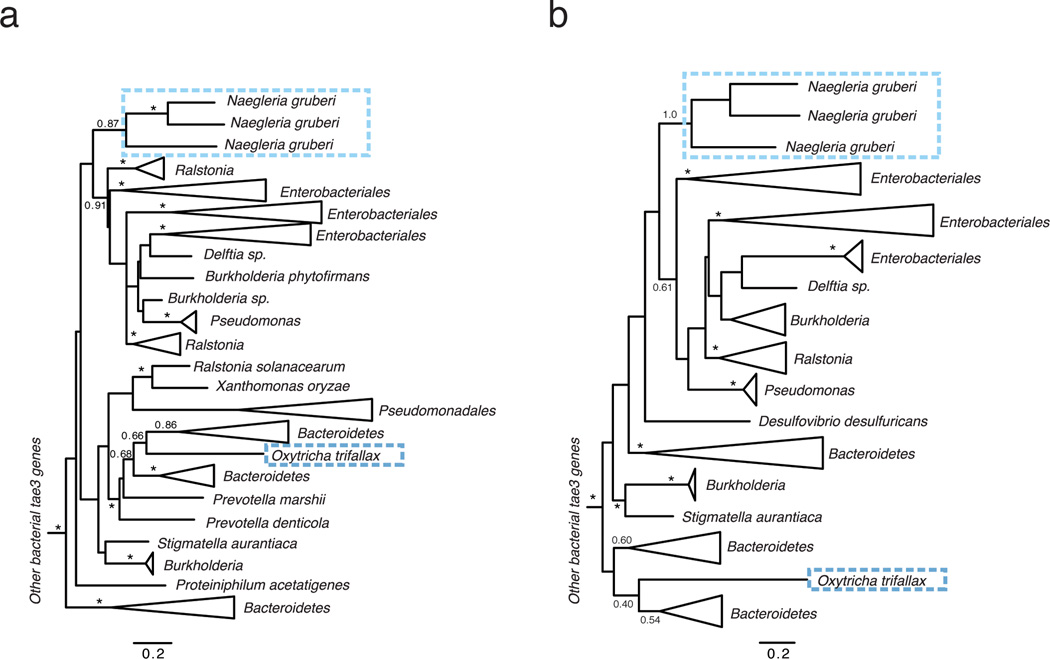
Extended Data Figure 3. Phylogenetic tree of bacterial tae3 and eukaryotic dae3 genes.
Phylogenetic trees were constructed using either maximum likelihood methods (a) or Bayesian methods (b). Branch support > 0.7 is indicated by asterisks or by numbers. The scale bar shows estimated divergence in amino acid changes per residue. Eukaryotic dae3 genes are indicated by dashed boxes, which highlight two separate HGT events. In both trees, the two eukaryotic dae3 clades are well supported as monophyletic clades, supporting our conclusion of two separate HGT events. Likewise, many major bacterial groups are well supported in both trees. Differences in the overall topology of the trees, mostly owing to changes in deep branches that are not well supported in either phylogenetic tree, reflect uncertainty in the ancient history of these genes and should therefore be treated with caution.
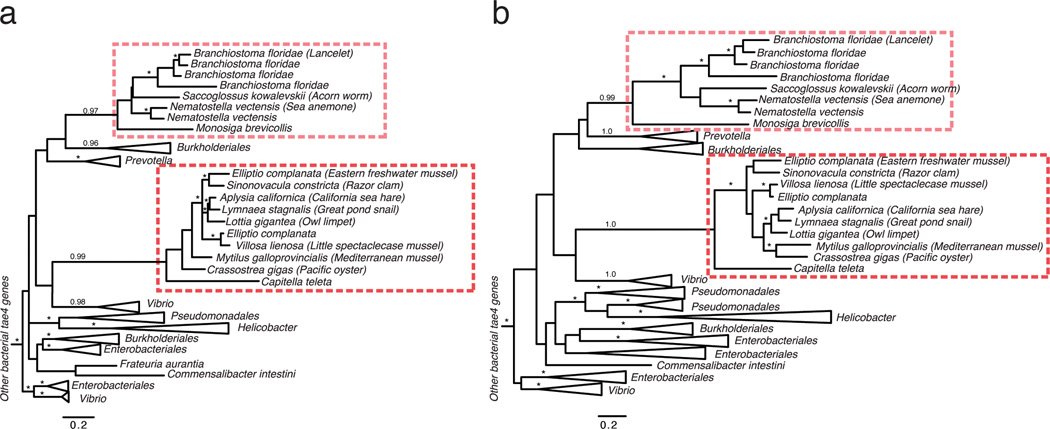
Extended Data Figure 4. Phylogenetic tree of bacterial tae4 and eukaryotic dae4 genes.
Phylogenetic trees were constructed using either maximum likelihood methods (a) or Bayesian methods (b). Branch support > 0.7 is indicated by asterisks or by numbers. The scale bar shows estimated divergence in amino acid changes per residue. Eukaryotic dae4 genes are indicated by dashed boxes, which highlight two separate HGT events. In both trees, the two eukaryotic dae4 clades are well supported as monophyletic clades, supporting our conclusion of two separate HGT events. Likewise, many major bacterial groups are well supported in both trees. Differences in the overall topology of the trees, mostly owing to changes in deep branches that are not well supported in either phylogenetic tree, reflect uncertainty in the ancient history of these genes and should therefore be treated with caution.
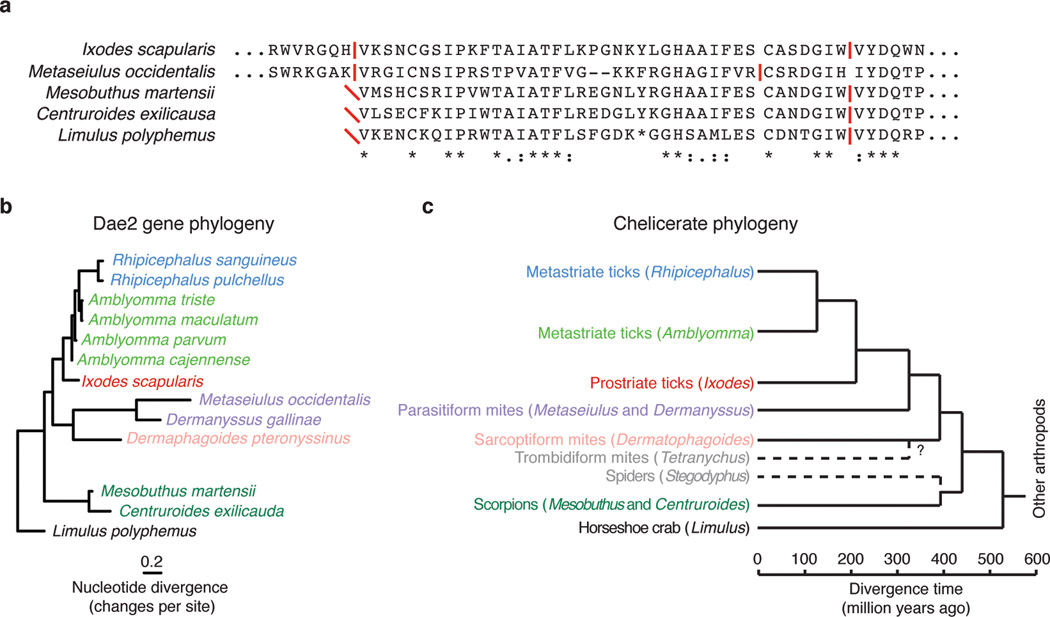
Extended Data Figure 5. Evidence for dae2 in other chelicerates.
a, Phylogenetic tree based on partial nucleotide sequences of dae2 from the indicated chelicerate species. Scale bar shows estimated divergence, in substitutions per nucleotide. b, Chelicerate phylogeny with approximate dates of divergence14. The unknown divergence time of sarcoptiform and trombidiform mites is indicated by a question mark. We find no evidence for dae2 in the complete genome of the trombidiform mite Tetranychus urticae nor in the partial (several species) or complete genome (Stegodyphus mimosarum) of any spider. Putative dae2 gene loss events in trombidiform mites and spiders are denoted (dashed lines). c, Alignment of Dae2 from ticks and mites (I. scapularis and M. occidentalis) with Dae2 sequences from partially assembled genomes of two scorpions (Mesobuthus martensii and Centruroides exilicauda) and the horsehoe crab (Limulus polyphemus). Splice junctions are denoted (horizontal red lines). All three alignable partial sequences start (red diagonal slashes) in the same position as the shared splice site in tick and mite dae2 genes, suggesting this is likely the beginning of the exons in all dae genes shown. A second intron position is shared between the tick, scorpion and horseshoe crab dae genes and is nearby the mite intron position.
Extended Data Figure 6. Evidence for retention of important catalytic motifs and recurrent eukaroytic-specific addition of secretion signals.
a-c, Alignments for the predicted Dae N-terminal signal sequences (shaded blue) and catalytic motifs (right) are shown for each of the families. The consensus sequence logo of residues surrounding the cysteine and histidine positions of catalytic dyads from extant Tae enzymes are shown above alignments from each family. Below are aligned eukaroytic Dae proteins in these same regions. Representatives derived from distinct HGT events are separated by a space. Predicted N-terminal secretion signals (blue) and predicted catalytic residues (red) are colored. Lowering the cutoff value in SignalP36 from the default value of 0.45 to the 'sensitive' value of 0.34 predicted a signal peptide in residues 1-21 of C. gigas Dae4.
Extended Data Figure 7. Dae2 degrades mDAP- but not Lys-type PG.
a,b, Partial HPLC chromatograms of sodium borohydride-reduced soluble PG fragments (muropeptides) from Bacillus subtilis (a) or Streptococcus pneumoniae (b). PG sacculi products resulting from incubation with buffer (Control) or the indicated Dae2 proteins (WT or C43A), followed by cellosyl digestion are shown. Major peaks are labeled. a, Muropeptides from B. subtilis include Tri (Glc-NAc–MurNAc(reduced (r))–L-Ala–D-γ-Glu–mDAP(amidated (NH2))), Tetra (GlcNAc–MurNAc(r)–L-Ala–D-γ-Glu–mDAP(NH2)–D-Ala), and TetraTri (GlcNAc–MurNAc–L-Ala–D-γ-Glu–mDAP(NH2)–D-Ala–mDAP(NH2)–D-γ-Glu–L-Ala–MurNAc(r)–GlcNAc). b, Muropeptides from S. pneumoniae include Tri (GlcNAc–MurNAc(r) –L-Ala–D-γ-Gln–L-Lys) and TetraTri (GlcNAc–MurNAc–L-Ala–D-γ-Gln–L-Lys–D-Ala–L-Lys–D-γ-Gln–L-Ala–MurNAc(r) – GlcNAc). L-Ser–L-Ala branch is indicated by (SA) and deacetylation by (deAc).
Extended Data Figure 8. Dae2 is active against B. burgdorferi PG.
HPLC elution profiles of B. burgdorferi sacculi incubated with buffer (Control) or the indicated Dae2 proteins (WT or C43A), followed by cellosyl digestion are shown. Discrete peaks lost (red) or produced (green) upon digestion by Dae2 are denoted with arrowheads in Control and WT chromatograms, respectively. Unresolved peaks, likely corresponding to a complex mixture of multi-crosslinked species cleaved by Dae2, are also highlighted (blue line). B. burgdorferi PG composition is complex and not yet resolved, thus approximate elution times of uncrosslinked versus crosslinked species are based on E. coli muropeptides in the same solvent system.
Extended Data Figure 9. Disruption of dae2 expression does not significantly alter tick physiology at repletion.
a, Knockdown of dae2 does not increase the B. burgdorferi burden in infected nymphs at engorgement. Loads were quantified by qPCR analysis of flaB, a B. burgdorferi-specific gene, and normalized to trospa, a tick-specific gene. n=20. For this and subsequent panels, each data point represents a pool of 3 nymphs, and horizontal bars represent mean values, which were not significantly different in a two-tailed nonparametric Mann-Whitney test (p>0.5). b, Disruption of dae2 expression did not affect engorgement weights of nymphal ticks fed on B. burgdorferi-infected mice. Tick weights were measured at repletion and 2 weeks post-repletion. Error bars +/− s.d., n=8. c, Overall bacterial load was not affected by knockdown of dae2. Bacterial load was assessed by qPCR analysis of the 16S rRNA normalized against tick-specific gene, trospA. Load is represented on both a linear (below) and log2 (above) scale, which is denoted by a gap on y-axis.
Evolutionary analyses of dae and tae gene families.
Summary of results from maximum likelihood tests of aligned dae or tae sequences from the indicated species, using SLAC in the HyPhy software package35. The overall gene dN/dS ratio (ratio of non-synonymous changes to synonymous changes) is shown, indicating an overall signature of purifying selection. Individual codons with a statistically significant signature of purifying selection (p<0.05) were also calculated and are expressed as a percentage of the total number of codons used in the analysis. In the same analyses, no codons were found with a statistically significant signature of positive selection.
| Gene family |
Species group |
Number of species |
dN/dS | Codons evolving under purifying selection |
|
|---|---|---|---|---|---|
| Eukaryotic amidases | dae2 | Ticks & mites | 10 | 0.20 | 21% (25 of 120) |
| dae4 | Mollusks | 9 | 0.18 | 40% (59 of 149) | |
| Prokaryotic amidases | tae2 | Cronobacter | 10 | 0.12 | 23% (30 of 129) |
| tae3 | Acinetobacter | 10 | 0.08 | 30% (45 of 150) | |
| tae4 | Pseudomonas | 12 | 0.15 | 46% (75 of 163) |
Supplementary Material
Acknowledgements
We thank L. Holland for assistance with transcriptome analysis, D. Vollmer and C. Aldridge for PG preparation, J. Parrish for microinjection assistance, J. Young for assistance with phylogenetic analyses, H. Merrikh for sharing equipment, and T. Alber, C. Fuqua, K. Clay, E. Rynkiewicz, U. Pal, C. Grundner, G. Nester, P. Singh and members of the Malik and Mougous laboratories for helpful discussions. This work was funded by the NIH (AI080609 to J.D.M), the Defense Threat Reduction Agency (HDTRA-1-13-014 to J.D.M.), and the BBSRC (BB/I020012/1 to W.V.). S.C. was supported by an HHMI-sponsored Life Sciences Research Foundation fellowship, M.A.F. by the ASM Undergraduate Research Fellowship, and M.D.D. by an Irvington Institute Fellowship from the Cancer Research Institute. C.J.-W. and H.S.M. are investigators of the Howard Hughes Medical Institute. J.D.M. holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
Author Contributions
S.C., M.D.D., H.S.M. and J.D.M. designed the study. S.C., M.D.D., S.B.P., J.B., Y.Y., B.L.J., L.K.F.-L., M.A.F., B.N.H., C.J.-W., X.F.Y., W.V., H.S.M., and J.D.M. performed experiments, analyzed data, and provided intellectual input into aspects of this study. S.C., M.D.D., S.B.P., H.S.M., and J.D.M. wrote the manuscript; all authors contributed to its editing.
The authors declare no competing financial interests.
References
- 1.Boto L. Horizontal gene transfer in the acquisition of novel traits by metazoans. Proc Biol Sci. 2014;281:2013–2450. doi: 10.1098/rspb.2013.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunning Hotopp JC. Horizontal gene transfer between bacteria and animals. Trends Genet. 2011;27:157–163. doi: 10.1016/j.tig.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell AB, et al. A widespread bacterial type VI secretion effector superfamily identified using a heuristic approach. Cell host & microbe. 2012;11:538–549. doi: 10.1016/j.chom.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson JO. Gene transfer and diversification of microbial eukaryotes. Annual review of microbiology. 2009;63:177–193. doi: 10.1146/annurev.micro.091208.073203. [DOI] [PubMed] [Google Scholar]
- 5.Schonknecht G, et al. Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote. Science. 2013;339:1207–1210. doi: 10.1126/science.1231707. [DOI] [PubMed] [Google Scholar]
- 6.Keeling PJ, Palmer JD. Horizontal gene transfer in eukaryotic evolution. Nature reviews. Genetics. 2008;9:605–618. doi: 10.1038/nrg2386. [DOI] [PubMed] [Google Scholar]
- 7.Husnik F, et al. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell. 2013;153:1567–1578. doi: 10.1016/j.cell.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 8.Nikoh N, Nakabachi A. Aphids acquired symbiotic genes via lateral gene transfer. BMC Biol. 2009;7:12. doi: 10.1186/1741-7007-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wybouw N, et al. A gene horizontally transferred from bacteria protects arthropods from host plant cyanide poisoning. Elife. 2014;3:e02365. doi: 10.7554/eLife.02365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran Y, Fredman D, Szczesny P, Grynberg M, Technau U. Recurrent horizontal transfer of bacterial toxin genes to eukaryotes. Molecular biology and evolution. 2012;29:2223–2230. doi: 10.1093/molbev/mss089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell AB, et al. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475:343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou S, et al. Structure of a Peptidoglycan Amidase Effector Targeted to Gram-Negative Bacteria by the Type VI Secretion System. Cell Rep. 2012;1:656–664. doi: 10.1016/j.celrep.2012.05.016. doi:S2211-1247(12)00141-6 [pii] 10.1016/j.celrep.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swart EC, et al. The Oxytricha trifallax macronuclear genome: a complex eukaryotic genome with 16,000 tiny chromosomes. PLoS Biol. 2013;11:e1001473. doi: 10.1371/journal.pbio.1001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeyaprakash A, Hoy MA. First divergence time estimate of spiders, scorpions, mites and ticks (subphylum: Chelicerata) inferred from mitochondrial phylogeny. Exp Appl Acarol. 2009;47:1–18. doi: 10.1007/s10493-008-9203-5. [DOI] [PubMed] [Google Scholar]
- 15.Warnke KM, Meyer A, Ebner B, Lieb B. Assessing divergence time of Spirulida and Sepiida (Cephalopoda) based on hemocyanin sequences. Mol Phylogenet Evol. 2011;58:390–394. doi: 10.1016/j.ympev.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Yu JK, et al. A cDNA resource for the cephalochordate amphioxus Branchiostoma floridae. Dev Genes Evol. 2008;218:723–727. doi: 10.1007/s00427-008-0228-x. [DOI] [PubMed] [Google Scholar]
- 17.Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. doi:FMR094 [pii] 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 18.Russell AB, Peterson SB, Mougous JD. Type VI secretion system effectors: poisons with a purpose. Nature reviews. Microbiology. 2014;12:137–148. doi: 10.1038/nrmicro3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dziarski R, Gupta D. The peptidoglycan recognition proteins (PGRPs) Genome biology. 2006;7:232. doi: 10.1186/gb-2006-7-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonenshine DE, Roe RM. Biology of ticks. 2nd edn. Oxford University Press; 2013. [Google Scholar]
- 21.Hajdusek O, et al. Interaction of the tick immune system with transmitted pathogens. Front Cell Infect Microbiol. 2013;3:26. doi: 10.3389/fcimb.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawlena H, et al. The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. The ISME journal. 2013;7:221–223. doi: 10.1038/ismej.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck G, Benach JL, Habicht GS. Isolation, preliminary chemical characterization, and biological activity of Borrelia burgdorferi peptidoglycan. Biochem Bioph Res Co. 1990;167:89–95. doi: 10.1016/0006-291x(90)91734-a. [DOI] [PubMed] [Google Scholar]
- 24.Kashyap DR, et al. Peptidoglycan recognition proteins kill bacteria by activating protein-sensing two-component systems. Nat Med. 2011;17:676–683. doi: 10.1038/nm.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nature reviews. Microbiology. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narasimhan S, et al. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the lyme disease spirochete. Cell host & microbe. 2014;15:58–71. doi: 10.1016/j.chom.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, et al. Molecular interactions that enable movement of the Lyme disease agent from the tick gut into the hemolymph. PLoS pathogens. 2011;7:e1002079. doi: 10.1371/journal.ppat.1002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.