Abstract
Importance
Evolving data on the effectiveness of post-mastectomy radiation therapy (PMRT) have led to changes in NCCN recommendations, counseling providers to “strongly consider” PMRT for breast cancer patients with tumors ≤5cm and 1-3 positive nodes; however, anticipated PMRT may lead to delay or omission of reconstruction which can have cosmetic, quality of life, and complication implications for patients.
Objective
To determine whether revised guidelines have increased PMRT and impacted receipt of breast reconstruction. We hypothesized that: 1) PMRT would increase for women affected by the revised guidelines while remaining stable in other cohorts, and 2) that these women would have decreased receipt of breast reconstruction while reconstruction increased in other groups.
Design
A retrospective, population-based cohort study
Setting
Surveillance, Epidemiology, and End Results (SEER) data from 2000 – 2011.
Participants
Women with stage I-III breast cancer undergoing mastectomy were identified. Our analytic sample (n=62,442) was divided into cohorts based on current NCCN radiation recommendations: “Radiation Recommended” (tumors >5 cm or ≥4 positive lymph nodes), “Strongly Consider Radiation” (tumor ≤5cm, 1-3 positive nodes), and “Radiation Not Recommended” (tumors ≤5cm, no positive nodes).
Main Outcome Measure(s)
We used joinpoint regression analysis to evaluate temporal trends in our outcomes of interest: receipt of PMRT and receipt of breast reconstruction.
Results
Rates of PMRT were unchanged in the “Radiation Recommended” and “Radiation Not Recommended” cohorts over the study period. In contrast, receipt of PMRT for the “Strongly Consider Radiation” cohort was unchanged until 2007, then significantly increased (APC 9.0%, p=0.013).
Breast reconstruction increased across all cohorts. Despite increasing receipt of PMRT, the “Strongly Consider Radiation” cohort maintained a consistent increase in reconstruction (APC 7.5%) throughout the study period. This is similar to the increase in reconstruction observed for the “Radiation Recommended” (10.7%) and “Radiation Not Recommended (8.4%) cohorts.
Conclusions and Relevance
NCCN guideline changes have increased PMRT receipt for patients with tumors ≤5cm and 1-3 positive nodes without an associated decrease in receipt of reconstruction. This may represent increasing provider comfort with the prospect of irradiating a new breast reconstruction, and may have significant cosmetic and quality of life implications for patients.
Introduction
In the past decade, indications for the use of post-mastectomy radiation therapy (PMRT) have expanded. Prior to the year 2000, several trials demonstrated decreased loco-regional recurrence as well as improved survival in breast cancer patients with tumors >5 cm, positive lymph nodes, and/or invasion of skin or pectoral fascia who received PMRT plus mastectomy and axillary clearance versus mastectomy and axillary clearance alone,1-3 establishing a standard of care for who should be considered for PMRT. In subgroup analyses of these initial studies, the observed benefits of PMRT persisted in patients with 1-3 positive lymph nodes, with a decrease in loco-regional recurrence from 27% to 4% (p<0.001) and a corresponding increase in overall survival from 48% to 57% (p=0.03)4. Further data supporting the benefit of PMRT for patients with 1-3 positive lymph nodes was presented by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) in 2005. Although the magnitude of the absolute reduction in loco-regional recurrence was lower in this meta-analysis (11.6%) than in the RCTs, similar trends were observed with a 4.4% improvement in 15-year breast cancer survival for patients who underwent mastectomy, axillary clearance, and PMRT compared to surgery alone.5 Based on these findings, the National Comprehensive Cancer Network (NCCN) expanded its treatment guidelines to “strongly consider” PMRT for patients with tumors ≤5 cm and 1-3 positive lymph nodes.6 However, the role of PMRT for patients with 1-3 positive lymph nodes remains controversial due to the relatively high rate of local recurrence observed in these trials combined with advances in systemic and targeted therapies since completion of the trial.
Concurrently, there has been a rapid expansion in the use of immediate breast reconstruction over the past two decades.7,8 Breast reconstruction appears to significantly improve quality of life,9,10 and immediate reconstruction reduces the adverse psychosocial effects associated with mastectomy,11 can streamline treatment by reducing the number of necessary surgeries, and is favored by women compared to delayed reconstruction.12 However, in the setting of anticipated PMRT, reconstruction decision-making becomes more complicated: prior studies suggest that both radiation oncologists and plastic surgeons have reservations about the use of immediate reconstruction in the setting of PMRT. The majority of radiation oncologists believe that immediate breast reconstruction challenges their ability to effectively deliver radiation to the chest wall,13 and the majority of reconstructive surgeons would prefer to delay reconstruction in the setting of anticipated PMRT.14 PMRT appears to be associated with increased risk of reconstruction-related complications such as implant removal 15,16 and fat necrosis of autologous tissue reconstructions;16 however, there is no clear association between PMRT and reduced patient satisfaction.15,17 Currently, there is no consensus on optimal management and timing of breast reconstruction in the setting of possible PMRT. Consequently, while the new NCCN guidelines urging strong consideration of PMRT in patients with tumors ≤5 cm with 1-3 positive lymph nodes have the potential to significantly impact oncologic outcomes, they may also lead healthcare providers to discourage immediate breast reconstruction, resulting in poorer patient satisfaction and quality of life.
We therefore sought to determine whether changing guidelines have increased receipt of PMRT in patients with tumors ≤5 cm and 1-3 positive lymph nodes, and whether any changes in receipt of PMRT have impacted rates of breast reconstruction. We hypothesized that: 1) PMRT would increase in the cohort of patients for whom NCCN guidelines have changed (i.e. patients with tumors ≤5 cm and 1-3 positive lymph nodes) while utilization of PMRT in those with clear indications for (tumors > 5cm or 4 or more positive lymph nodes) or against (tumors ≤5cm with negative lymph nodes) would remain stable, and that 2) new guidelines would result in a decrease in the receipt of breast reconstruction in patients for whom NCCN guidelines for PMRT have changed relative to those for whom the NCCN PMRT guidelines have remained the same.
Methods
Patients who underwent mastectomy for stage I-III breast cancer from 2000-2011 were identified in the Surveillance, Epidemiology, and End Results (SEER) database (N=104,433). Patients were excluded if they were male (N=2,003), had prior cancers (including prior breast cancer) (N=37,214), or had previously received radiation (N=2,774). The final sample size was 62,442 patients.
Patients were grouped into three cohorts based on current NCCN recommendations for receipt of PMRT (Table 1). The “Radiation Recommended” cohort (n=15,599) represents patients with four or more positive lymph nodes, regardless of tumor size, and patients with tumors >5 cm, regardless of nodal status. The “Strongly Consider Radiation” cohort (n=15,006) represents patients for whom NCCN guidelines have changed over the study period: those with tumors ≤5 cm and 1-3 positive lymph nodes. The last cohort, “Radiation Not Recommended” (n=31,837), represents patients with tumors ≤5 cm and negative lymph nodes. Socio-demographic data (age, race/ethnicity, marital status), tumor characteristics (tumor size, number of positive lymph nodes, estrogen and progesterone receptor status) and receipt of PMRT and immediate reconstruction as reported by SEER were evaluated for the overall sample and by cohort using Stata 12.1 (StataCorp, College Station, TX). As defined by SEER guidelines, nodal status and tumor size are coded according to the most advanced stage (pathologic or clinical) identified for a given patient. Breast reconstruction included any reconstruction within 4 months of mastectomy, as defined by SEER.
Table 1.
Cohort Creation Based on National Comprehensive Cancer Network Guidelines for Receipt of Post-Mastectomy Radiation Therapy.
| Study Cohort | Tumor Characteristics | N |
|---|---|---|
| Radiation Recommended | Tumor >5 cm in size (regardless of nodal status) Four or more positive lymph nodes (regardless of primary tumor size) |
15,599 |
| Strongly Consider Radiation | Tumor ≤5 cm with 1-3 positive lymph nodes | 15,006 |
| Radiation Not Recommended | Tumor ≤5 cm with 0 positive lymph nodes | 31,837 |
Outcomes
The aim of our study was to investigate rates of receipt of PMRT and breast reconstruction in women for whom NCCN guidelines regarding PMRT changed over the study period, relative to that of women for whom NCCN guidelines for PMRT have remained unchanged.
Statistical Analysis
Differences between the three cohorts in socio-demographic and tumor characteristics were assessed with Pearson's chi-squared test. A two-tailed p-value < 0.05 was considered statistically significant. Exploratory analyses using logistic regression were performed to evaluate associations between demographic and tumor characteristics with receipt of reconstruction and PMRT.
Temporal trends in receipt of PMRT and breast reconstruction were evaluated for our cohorts using Joinpoint regression software (National Cancer Institute, Bethesda, MD). Joinpoint regression analysis is increasingly used 18,19 to evaluate temporal trends in an outcome of interest by evaluating changes in the rates of that outcome over time. Joinpoint regression analysis determines whether multiple regression lines provide a better fit for the data than a single straight line, suggestive of changing trends in the data. If a multi-segmented line represents a better fit, this means that the rate of change (the slope of the line) is different before and after one or more points in time, and the program provides statistical estimation of when the change(s) in slope occurred, with a p-value < 0.05 considered statistically significant. It also calculates the slope of all line segments, called the annual percentage change (APC), and the likelihood that this APC is significantly different from zero, or represents a true trend (p < 0.05). The APC represents the change in rate on an annual basis – for example, an APC of 0 would reflect no change over time and would be represented by a horizontal line on the graph. An APC of any value would not be considered significant if the software is unable to definitively identify a trend in the data. For these reasons, a small APC associated with a definitive trend (for example, 0.4%) may be considered statistically significant while a larger APC (for example, 10%) associated with more variable data may not.
Sensitivity Analyses
Older women are less likely to undergo breast reconstruction 7,20 and tend to have less aggressive and lower stage tumors, making them over-represented in our Radiation Not Recommended cohort. Because we were interested in the relationship between radiation and reconstruction, we wanted to ensure that age was not confounding our results. We therefore performed a sensitivity analysis evaluating trends in both PMRT and breast reconstruction, considering only those patients younger than 65.
Results
Table 2 provides a summary of differences in sociodemographic and tumor characteristics for our overall cohort and by NCCN PMRT recommendations. Approximately half of patients were in the “Radiation Not Recommended” cohort, with the remaining half split nearly equally between the “Radiation Recommended” and “Strongly Consider Radiation” cohorts. Patients in the “Radiation Not Recommended” cohort were more likely to be older (p<0.001). In addition to the expected differences in tumor size and lymph node status between the radiation recommendation cohorts, patients in the “Radiation Recommended” cohort were more likely to be estrogen- and progesterone-receptor negative.
Table 2.
Demographic and Tumor Characteristic Information for Female Patients Undergoing Mastectomy for Invasive Breast Cancer, SEER Database, 2000 – 2011 (n=62,442). Data shown for all patients and for cohorts based on NCCN guidelines for receipt of PMRT.
| NCCN Radiation Recommendation Cohorts* | |||||
|---|---|---|---|---|---|
| All patients | Recommended | Strongly Consider | Not Recommended | ||
| N | 62,442 | 15,599 | 15,006 | 31,837 | |
| Age | <45 | 17.2% | 19.8% | 19.5% | 14.5% |
| 45-54 | 25.8% | 27.5% | 28.1% | 24.0% | |
| 55-64 | 22.5% | 22.8% | 22.3% | 22.5% | |
| ≥ 65 | 34.5% | 29.9% | 30.2% | 38.8% | |
| Race | White | 80.4% | 77.9% | 81.0% | 81.4% |
| Black | 9.1% | 12.2% | 9.4% | 7.5% | |
| Other | 9.9% | 9.6% | 9.1% | 10.5% | |
| Marital Status | Married | 57.4% | 55.6% | 59.3% | 57.5% |
| Single | 12.9% | 14.7% | 12.4% | 12.1% | |
| Divorced, separated, or widowed | 25.7% | 26.0% | 24.6% | 26.0% | |
| Tumor size | 0 - 2 cm | 43.0% | 9.3% | 37.5% | 62.2% |
| 2 - 5 cm | 43.4% | 36.4% | 62.5% | 37.8% | |
| >5 cm or diffuse/inflammatory | 13.6% | 54.3% | 0% | 0% | |
| Positive Lymph nodes | 0 | 54.8% | 15.4% | 0% | 100% |
| 1-3 | 28.1% | 16.3% | 100% | 0% | |
| ≥ 4 | 17.1% | 68.3% | 0% | 0 % | |
| ER Status | Positive | 73.3% | 68.8% | 76.1% | 74.2% |
| Negative | 21.5% | 26.5% | 19.6% | 19.6% | |
| PR Status | Positive | 62.4% | 57.0% | 65.7% | 63.4% |
| Negative | 32.0% | 37.9% | 29.7% | 30.3% | |
| Receipt of PMRT | 27.1% | 67.6% | 29.9% | 7.6% | |
| Receipt of Breast Reconstruction | 21.7% | 15.6% | 22.1% | 24.1% | |
Note: Percentages sum within columns for each category (age, race, marital status). Missing data not shown; percentages may not sum to 100%.
NCCN, National Comprehensive Cancer Center. ER, estrogen receptor. PR, progesterone receptor. PMRT, post-mastectomy radiation therapy.
all demographic and tumor characteristics were significantly different amongst the three cohorts (p < 0.001)
Radiation was received by 67.6% of patients in the “Radiation Recommended” cohort, 29.9% in the “Strongly Consider Radiation” cohort, and 7.4% in the “Radiation Not Recommended” cohort. Age was strongly associated with receipt of radiation for both the “Radiation Recommended” and “Strongly Consider Radiation” cohorts. For example, in the “Radiation Recommended” cohort, patients aged 55-64 (OR 0.77 [0.69-0.87]) and >=65 years of age (0.44 [0.40-0.50]) were significantly less likely to receive PMRT than patients younger than 45 years of age (p<0.0001). Tumor characteristics also influenced receipt of radiation. In the “Radiation Recommended” cohort, patients who were recommended PMRT based on tumor size alone (i.e T3N0) were less likely to receive PMRT than those with 4 or more positive nodes (OR 0.43 [0.39-0.47]). In the “Strongly Consider Radiation” cohort, patients with smaller tumor sizes (<2 cm versus 2-5 cm) and lower tumor grade (grade1 versus grade 2/2) were less likely to receive PMRT (p<0.0001).
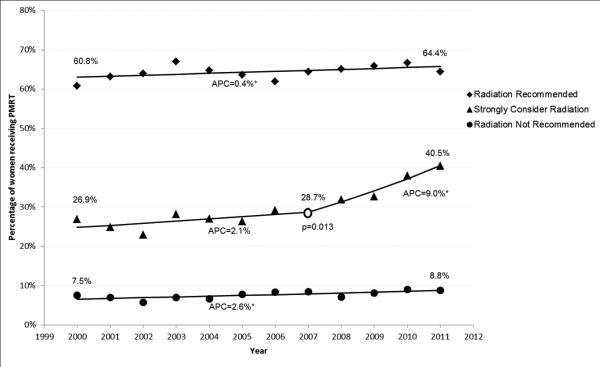
Post-Mastectomy Radiation Therapy
Use of PMRT increased over the study period from 24.7% in 2000 to 30.0% in 2011. Results of the joinpoint regression analysis of receipt of PMRT indicted that the “Radiation Recommended” and “Radiation Not Recommended” cohorts demonstrated small but steady increases in receipt of PMRT over the study period, with APCs of 0.4% and 2.6%, respectively (Figure 1(a)). Receipt of PMRT in the “Strongly Consider Radiation” cohort was statistically unchanged until 2007. At that time, a significant change in the APC was observed (p=0.019) with an increase in APC to 9.0% through the end of the study period.
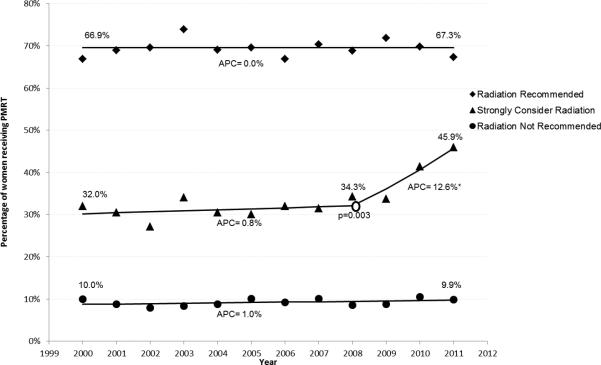
Figure 1.
Joinpoint regression analysis of Post-Mastectomy Radiation Therapy. (a) All patients (N=62,442) (b) Patients < 65 years of age (N=30,605).
APC, Annual Percentage Change, represents the change in rate on a yearly basis. An APC of 0 would mean no change in the rate, represented by a horizontal line. Asterisk indicates APC is significantly different from zero (p<0.05).
Open circle indicates that a change in slope has occurred; associated p-value indicates the statistical significance associated with that change in slope
Given the observed difference in age between the radiation cohorts, we assessed trends in a subgroup of women < age 65 (n=30,605), with similar findings observed (Figure 1(b)). PMRT for the “Radiation Recommended” and “Radiation Not Recommended” cohorts was stable throughout the study period. The “Strongly Consider Radiation” cohort demonstrated statistically unchanged receipt of PMRT until 2008, followed by a change in slope (p=0.003) and a subsequent APC of 12.6% until the end of the study period.
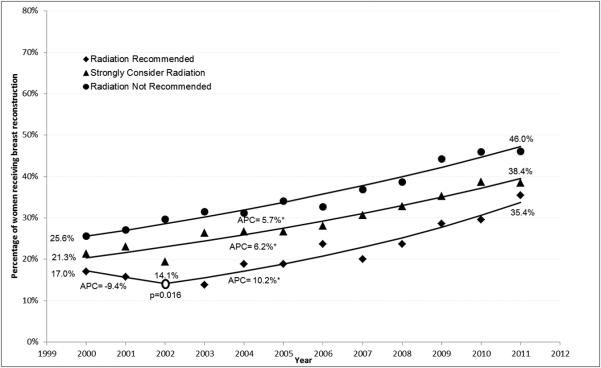
Breast Reconstruction
Receipt of breast reconstruction increased during the study period from 14.8% to 31.9% overall. Younger age, white race, smaller tumor size, negative lymph node status, and later year of diagnosis were all associated with receipt of reconstruction (p<0.0001). Results from the joinpoint regression analysis (Figure 2(a)) indicate that the “Radiation Not Recommended” and “Strongly Consider Radiation” cohorts experienced steadily increasing rates of breast reconstruction with APCs of 8.4% and 7.5%, respectively, throughout the study period. The “Radiation Recommended” cohort experienced unchanged rate of receipt of reconstruction between 2000 and 2002, at which point there was a change in slope (p=0.002) and the APC increased to 10.7% for the remainder of the study period. Results were similar when the sample was restricted to patients <65 years old (n=30,605) (Fig 2(b)).
Figure 2.
Joinpoint regression analysis of Immediate Breast Reconstruction. (a) All patients (N=62,442) (b) Patients < 65 years of age (N=30,605).
APC, Annual Percentage Change, represents the change in rate on a yearly basis. An APC of 0 would mean no change in the rate, represented by a horizontal line. Asterisk indicates APC is significantly different from zero (p<0.05).
Open circle indicates that a change in slope has occurred; associated p-value indicates the statistical significance associated with that change in slope
Discussion
This study used national patient data to examine temporal trends in the receipt of PMRT and breast reconstruction based on current NCCN guidelines for PMRT. As expected, receipt of PMRT by women for whom guideline recommendations did not change (i.e. the “Radiation Not Recommended” and “Radiation Recommended” cohorts) demonstrated minimal changes in receipt of PMRT over time, while women for whom the guidelines changed (i.e. “Strongly Consider Radiation” cohort) initially demonstrated statistically unchanged rates of PMRT, followed by a significant increase in PMRT after 2007. This would suggest that as expected, guidelines are impacting clinical practice patterns with increased utilization of PMRT in this group.
Breast reconstruction increased significantly over the study time period overall. The rates observed were consistent with findings of other population based studies, especially when taking into consideration our inclusion of older patients (who are less likely to receive reconstruction and exclusion of patients with DCIS (who are more likely to receive reconstruction).20-23 Although rates of breast reconstruction increased for all three cohorts, the baseline and final rates of reconstruction differed based on likelihood of receiving PMRT. This suggests that surgeons may be using anticipated receipt of PMRT to guide decision-making regarding recommendations for immediate reconstruction. This is supported by previous literature showing receipt of PMRT to be a negative predictor for both immediate 24 and overall 25 breast reconstruction. However, in contrast to our expectations, rates of breast reconstruction for women in the “Strongly Consider Radiation” cohort (those with tumors ≤5cm and 1-3 positive lymph nodes) did not have a change in rate of breast reconstruction to correspond to the observed increase in PMRT. . Instead, breast reconstruction continued to increase over time, at a rate similar to that of women in both the “Radiation Not Recommended” and “Radiation Recommended” cohorts (for whom receipt of PMRT was stable).
Patients with locally advanced tumors have an overall poorer prognosis from their cancer, and are likely to be recommended PMRT by their providers. Given concerns expressed by plastic surgeons and radiation oncologists about PMRT in the setting of reconstruction, deferring or recommending against reconstruction in this clinical scenario may be reasonable. Similarly, patients with small, node negative tumors are not likely to be offered the option of radiation and providers may be more comfortable recommended immediate reconstruction. However, how these clinical factors influence decision-making for patients who fall into the “Strongly Consider Radiation” cohort is difficult to determine. The majority of these women are likely clinically node-negative at the time of surgery and are identified as eligible for PMRT post-operatively, after reconstruction decision-making has already occurred. This may explain why the dramatic changes in rates of PMRT observed in this cohort did not translate into changes in the rate of breast reconstruction.
In the “Radiation Recommended” cohort, the first few years of the study demonstrated statistically flat rates of breast reconstruction prior to a trend upwards. Although graphically the rate of reconstruction appears to decrease over these years, the trend was not strong enough to be considered significant in our analysis. This finding may represent the tail end of a trend in which women with more advanced tumors were less likely to be offered or undergo breast reconstruction. The reversal of this trend could be related to the introduction of new therapies, such as trastuzumab, which significantly improved prognosis and made consideration of reconstruction more relevant for these women. Additionally, introduction of new techniques for reconstruction may have provided surgeons with alternative options in the setting of anticipated PMRT. This latter explanation would also help to explain the observed stable increase in rates of reconstruction despite a significant increase in the utilization of PMRT for the “Strongly Consider Radiation” cohort.
Although there are a number of strengths to the current study, including a nationally representative sample of breast cancer cases with validated assessments of treatment received, a few limitations should be noted. Under-ascertainment of radiation therapy is an acknowledged weakness of SEER registry data. However, this should not affect evaluation of temporal trends within each cohort, which is the focus of this paper. SEER registry data do not allow us to determine the proportion of patients undergoing immediate versus early-delayed reconstruction, as all reconstructive surgeries within four months of initiation of treatment are captured together. Some patients may be receiving early-delayed reconstruction, where surgeons defer reconstruction at the time of mastectomy to await pathology results, and then return to the operating room after a short interval for definitive reconstruction if PMRT is not indicated. This clinical scenario may be especially true for the “Strongly Consider Radiation” cohort, leading to an underestimation of the impact of current NCCN guidelines for PMRT on decision-making surrounding reconstruction. However, recent literature indicates that the majority of reconstructions performed in the U.S. are immediate (>75%).20-23,27,28 Therefore, despite our inability to separate immediate from early-delayed reconstruction captured in SEER data, we are confident that the majority of the reconstructions identified in SEER represent immediate reconstruction. Additionally, the current analysis did not include an examination of temporal trends in the types of reconstruction (autologous tissue flap, implant, or combination procedures) or whether type of reconstruction varied by a patient's likelihood of receiving PMRT. It is possible that surgeons are offering different reconstruction options to patients who may be candidates for PMRT (i.e. less likely to offer tissue expander and implant reconstruction); this may have implications on patients’ out-of-pocket costs of reconstruction, cosmesis, and overall satisfaction with their reconstruction, and our study may therefore underestimate the influence of changes to PMRT recommendations on the breast reconstruction patients experience. However, in order to fully assess trends in the type of reconstruction received, it would be important to have complete information on all reconstruction, including those that occur in a delayed fashion. Given that SEER does not capture delayed reconstruction, we were unable to assess this in this study. Finally, these data cannot assess the contributions of surgeon practice patterns and patients’ values and preferences on decision-making for breast reconstruction.
The multi-disciplinary treatment of women with breast cancer is complex and continues to evolve. Numerous factors influence the receipt of breast reconstruction, including non-clinical factors such as the availability of reconstructive surgeons, institutional and physician practice patterns, and patients’ values and preferences. We examined one important clinical component, the expanded use of PMRT, as the decision for PMRT requires breast cancer providers and patients to weigh improved cancer outcomes (local-regional recurrence and survival) associated with PMRT against the potential for negative implications on breast reconstruction. It is encouraging that the national increases in PMRT we observed were not accompanied by declining use of breast reconstruction, despite prior evidence that reconstructive surgeons would prefer to delay reconstruction in the setting of anticipated PMRT. Further research is needed to understand how patients and providers reach consensus on this topic, and how receipt of PMRT may be impacting type of reconstruction received and patient centered outcomes including cosmesis and quality of life.
Acknowledgements
Lane Frasier is currently supported by AHRQ F32 HS022403 and the AAS Research Fellowship Award. She previously received support via NIH/ National Cancer Institute T32 CA90217. Heather Neuman is supported through the Building Interdisciplinary Research Careers in Women's Health Scholar Program (NIH K12 HD055894). All funding sources provide salary or stipend support and had no role in, or influence on, the design and conduct of the study, data collection, management, analysis, or interpretation, and did not review or approve the manuscript.
Footnotes
These data have been presented as oral presentations at the Academic Surgical Congress in Las Vegas, Nevada in February 2015. They have not been otherwise published, nor are they under consideration for publication through any other journal.
The authors have no relevant financial interests, activities, relationships, and affiliations that could represent a potential or perceived conflict of interest.
References
- 1.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337(14):949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 2.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:957–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 3.Overgaard M, Jensen M-J, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 4.Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82:247–253. doi: 10.1016/j.radonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Group EBCTC Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 6.Network NCC. Breast Cancer: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 7.Yang RL, Newman AS, Line IC, Reinke CE, Karakousis GC. Trends in immediate breast reconstruction across insurance groups after enactment of breast cancer legislation. Cancer. 2013;119:2462–2468. doi: 10.1002/cncr.28050. [DOI] [PubMed] [Google Scholar]
- 8.Reuben BC, Manwaring J, Neumayer LA. Recent trends and predictors in immediate breast reconstruction after mastectomy in the United States. Am J Surg. 2009;198:237–243. doi: 10.1016/j.amjsurg.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Stevens LA, McGrath MH, Druss RG, Kister SJ, Gump FE, Forde KA. The psychological impact of immediate breast reconstruction for women with early breast cancer. Pla Reconstr Surg. 1984;73:619–628. doi: 10.1097/00006534-198404000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Schain WS. Breast reconstruction. Update of psychosocial and pragmatic concerns. Cancer. 1991;68:1170–1175. doi: 10.1002/1097-0142(19910901)68:5+<1170::aid-cncr2820681309>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Elder EE, Brandberg Y, Bjorkund T, et al. Quality of life and patient satisfaction in breast cancer patients after immediate brast reconstruction: a prospective study. Breast. 2005;14:201–208. doi: 10.1016/j.breast.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ghazal SK, Sully L, Fallowfield L, Blamey RW. The psychological impact of immediate rather than delayed breast reconstruction. Eur J Surg Oncol. 2000;26:17–19. doi: 10.1053/ejso.1999.0733. [DOI] [PubMed] [Google Scholar]
- 13.Chen SA, Hiley C, Nickleach D, et al. Breast reconstruction and post-mastectomy radiation practice. [19 January 2015];Radiat Oncol. 2013 8:9. doi: 10.1186/1748-717X-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurunluoglu R, Gurunluoglu A, Williams SA, Tebockhorst S. Current trends in breast reconstruction. Survey of American Society of Plastic Surgeons 2010. Ann Plas Surg. 2013;70(1):103–110. doi: 10.1097/SAP.0b013e31822ed5ce. [DOI] [PubMed] [Google Scholar]
- 15.Anker CJ, Hymas RVA R, Kokeny KE, et al. The effect of radiation on complication rates and patient satisfaction in breast reconstruction using temporary tissue expanders and permanent implants. Breast J. 2015;21(3):233–240. doi: 10.1111/tbj.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer. A claims-based analysis. Ann Surg. 2015;00:1–9. doi: 10.1097/SLA.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagsi R, Li Y, Morrow M, et al. Patient-reported quality of life and satisfaction with cosmetic outcomes after breast conservation and mastectomy with and without reconstruction. Results of a survey of breast cancer survivors. Ann Surg. 2015;261:1198–1206. doi: 10.1097/SLA.0000000000000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu D, Katanoda K, Marugame T, Sobue T. A Joinpoint regression analysis of long-term trends in cancer mortality in Japan (1958-2004). Int J Cancer. 2009;124:443–448. doi: 10.1002/ijc.23911. [DOI] [PubMed] [Google Scholar]
- 19.Bartlett EK, Simmons KD, Wachtel H, et al. The rise in metastectomy across cancer types over the past decade. Cancer. 2014 doi: 10.1002/cncr.29134. [DOI] [PubMed] [Google Scholar]
- 20.Alderman AK, Hawley ST, Morrow M, et al. Receipt of delayed breast reconstruction after mastectomy: do women revisit the decision? Ann Surg Oncol. 2011;18:1748–1756. doi: 10.1245/s10434-010-1509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alderman AK, Hawley ST, Janz NK, et al. Racial and ethnic disparities in the use of postmastectomy breast reconstruction: results from a population-based study. J Clin Oncol. 2009;27(32):5325–5330. doi: 10.1200/JCO.2009.22.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruciton in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32(9):919–926. doi: 10.1200/JCO.2013.52.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationalwide trends in mastectomy for early-sage breast cancer. JAMA Surgery. 2015;150(1):9–16. doi: 10.1001/jamasurg.2014.2895. [DOI] [PubMed] [Google Scholar]
- 24.Roder D, Zorbas H, Kollias J, et al. Factors predictive of immediate breast reconstruction following mastectomy for invasive breast cancer in Australia. Breast. 2013;22:1220–1225. doi: 10.1016/j.breast.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Brennan ME, Spillane AJ. Uptake and predictors of post-mastectomy reconstruction in women with breast malignancy - systematic review. Eur J Surg Oncol. 2013;39(6):527–541. doi: 10.1016/j.ejso.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Jagsi R, Abrahamse P, Hawley ST, Graff JJ, Hamilton AS, Katz SJ. Underascertainment of radiatiotherapy receipt in SEER registry data. Cancer. 2012;118(2):333–341. doi: 10.1002/cncr.26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christian CK, Niland J, Edge SB, et al. A multi-institutional analysis of the socioeconomic determinants of breast reconstruction A study of the National Comprehensive Cancer Network. Ann Surg. 2006;243(2):241–249. doi: 10.1097/01.sla.0000197738.63512.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez-Boussard T, Zeidler K, Barzin A, Lee G, Curtin C. Breast reconstruction national trends and healthcare implications. Breast J. 2013;19(5):463–469. doi: 10.1111/tbj.12148. [DOI] [PubMed] [Google Scholar]