Abstract
Weight loss is recommended for patients with nonalcoholic fatty liver disease (NAFLD), while metformin may lower liver enzymes in type 2 diabetics. Yet, the efficacy of the combination of weight loss and metformin in the treatment of NAFLD is unclear. We assessed the effects of metformin, caloric restriction, and their combination on NAFLD in diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Male OLETF rats (age 20 weeks; n = 6–8 per group) were fed ad libitum (AL), given metformin (300 mg·kg−1·day−1; Met), calorically restricted (70% of AL; CR), or calorically restricted and given metformin (CR+Met) for 12 weeks. Met lowered adiposity compared with AL but not to the same magnitude as CR or CR+Met (p < 0.05). Although only CR improved fasting insulin and glucose, the combination of CR+Met was needed to improve post-challenge glucose tolerance. All treatments lowered hepatic triglycerides, but further improvements were observed in the CR groups (p < 0.05, Met vs. CR or CR+Met) and a further reduction in serum alanine aminotransferases was observed in CR+Met rats. CR lowered markers of hepatic de novo lipogenesis (fatty acid synthase, acetyl-CoA carboxylase (ACC), and stearoyl-CoA desaturase-1 (SCD-1)) and increased hepatic mitochondrial activity (palmitate oxidation and β-hydroxyacyl CoA dehydrogenase (β-HAD) activity). Changes were enhanced in the CR+Met group for ACC, SCD-1, β-HAD, and the mitophagy marker BNIP3. Met decreased total hepatic mTOR content and inhibited mTOR complex 1, which may have contributed to Met-induced reductions in de novo lipogenesis. These findings in the OLETF rat suggest that the combination of caloric restriction and metformin may provide a more optimal approach than either treatment alone in the management of type 2 diabetes and NAFLD.
Keywords: caloric restriction, metformin, nonalcoholic fatty liver disease, hepatic steatosis, mitochondrial function, de novo lipogenesis
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in developed countries (Milic and Stimac 2012). The prevalence of NAFLD is 20%–30% in the general population (Bedogni et al. 2005) and 69%–87% among individuals with type 2 diabetes mellitus (Leite et al. 2009; Vernon et al. 2011). NAFLD is a progressive liver disease ranging from simple hepatic steatosis (=5% by weight for diagnosis) to nonalcoholic steato-hepatitis (NASH), advanced fibrosis, and cirrhosis (Rector and Thyfault 2011) in the absence of excess alcohol consumption (<20 g·day−1). Currently suggested therapies for NAFLD are similar to those used to combat obesity, insulin resistance, type 2 diabetes, and hyperlipidemia (Chalasani et al. 2012).
Studies have shown that lifestyle changes, including diet modification or caloric restriction and physical activity, lead to weight loss, reduced liver fat content, and improved glucose control and insulin sensitivity (Thoma et al. 2012). Caloric restriction has been shown to prevent NAFLD in rats (Rector et al. 2011) and improve NAFLD in humans (Larson-Meyer et al. 2008; Elias et al. 2010). Despite studies showing improvement in outcomes, patients have difficulty adhering to dietary modifications or caloric restriction and increasing physical activity; therefore, the identification of alternative or combinative therapies for NAFLD that may enhance the effects of dietary modification alone may be pertinent.
Recently, interest in the potential use of insulin sensitizers as treatment for NAFLD has risen because of the association between type 2 diabetes and NAFLD. One medication of interest is met-formin, a drug used as an initial treatment strategy in type 2 diabetes. Metformin is known to activate ATP-producing pathways including glycolysis, fatty acid oxidation, and mitochondrial biogenesis as a result of activation of AMP-activated protein kinase (AMPK) (Mazza et al. 2012). Recently, metformin has also been shown to affect autophagy pathways (Inokuchi-Shimizu et al. 2014; Song et al. 2015), which may contribute to improved liver health. Metformin treatment has indeed been shown to improve liver transaminase levels and (or) liver histology in humans with NAFLD and NASH (Bugianesi et al. 2005; de Oliveira et al. 2008; Loomba et al. 2009) as well as in individuals at risk for developing type 2 diabetes (Krakoff et al. 2010), but these improvements are not universal (Chalasani et al. 2012) and are often associated with metformin-induced reductions in body weight (Krakoff et al. 2010). Metformin also has been shown to lower liver triglyceride (TG) content in rodent models of diabetes and NAFLD (Lin et al. 2000; Forcheron et al. 2009; Linden et al. 2014). However, it remains unclear whether further improvements in NAFLD will be observed if metformin treatment is combined with lifestyle modifications such as moderate caloric restriction.
To assess the efficacy of the combination of moderate caloric restriction and metformin in the treatment of NAFLD, we used the Otsuka Long-Evans Tokushima Fatty (OLETF) rat model. OLETF rats are selectively bred for null expression of the cholecystokinin-1 receptor in the brain, which results in a defect in the feedback mechanism for satiety that causes hyperphagia, obesity, insulin resistance, type 2 diabetes, and NAFLD (Kawano et al. 1992; Moran and Bi 2006; Rector et al. 2010a, 2011). We have previously reported that prevention of obesity through caloric restriction prevented NAFLD in OLETF rats, while others have reported improved NAFLD in animal models (Elias et al. 2010) and humans (Larson-Meyer et al. 2008) who underwent caloric restriction. Additionally, we have recently reported that metformin treatment alone improved serum alanine aminotransferases (ALTs) and lowered liver TG content in OLETF rats. Combining metformin with exercise training did not further reduce liver TGs and actually blunted several of the exercise-associated hepatic metabolic improvements (Linden et al. 2014). However, it remains unclear whether the efficacy of metformin treatment can be further enhanced by other recommended lifestyle modifications, such as caloric restriction. Therefore, the purpose of this study was to determine the effects of metformin treatment, caloric restriction, and their combination on NAFLD-related outcomes and gain a better understanding of mechanisms that may contribute to NAFLD treatment. We hypothesized that metformin and caloric restriction would independently treat type 2 diabetes and NAFLD in OLETF rats and that their combination would lead to further improvements in these disease states.
Materials and methods
Animal protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri. Male OLETF rats were randomized at 4 weeks of age into the following groups: ad libitum feeding (AL), treatment with metformin (300 mg·kg−1·day−1; Met), caloric restriction (~70% of AL, 21 g·day−1; CR), and caloric restriction + treatment with metformin (CR+Met). Treatment began at 20 weeks of age, at which point OLETF rats present with NAFLD and type 2 diabetes with hyperglycemia, hyperinsulinemia, and insulin resistance (Rector et al. 2010a). Body mass was measured weekly throughout the study. Food for animals in the CR groups was weighed and administered daily. Food for animals allowed ad libitum access was administered and weighed weekly. Metformin (Bosche Scientific) was administered in the drinking water at a dosage of 150 mg·kg−1·day−1 in the first week and 300 mg·kg−1·day−1 thereafter. These dosages were based on previous studies with chronic metformin administration (Borst et al. 2000; Matsumoto et al. 2008), and water intake was carefully monitored. At 32 weeks of age, animals were anesthetized with sodium pentobarbital (100 mg·kg−1) and then exsanguinated by removal of the heart following a 12 h fast.
Serum assays
Fasting serum glucose, insulin, ALT, TG, and free fatty acids were assessed by Comparative Clinical Pathology Services (Columbia, Mo., USA) using commercially available assays according to the manufacturers’ instructions.
Intraperitoneal glucose tolerance tests
Animals underwent intraperitoneal glucose tolerance tests (IPGTTs) 1 week prior to sacrifice following an overnight fast. Prior to intraperitoneal glucose injection (2 g·kg−1), whole blood samples were collected from the tail vein. Samples were subsequently taken at 15, 30, 45, 60, and 120 min following glucose injection (Rector et al. 2010b). The total area under the glucose curve was calculated using the trapezoidal method (Tai 1994).
Dual-energy X-ray absorptiometry
A Hologic QDR-1000/w dual-energy X-ray absorptiometry machine was used to measure whole body composition. This machine was calibrated for rats.
Tissue collection and preparation procedure
After animals were anesthetized, retroperitoneal, epididymal, and omental fat pads were removed and weighed. Animal livers were flash frozen in liquid nitrogen, placed in 10% formalin, placed in RNAlater, or placed in ice-cold isolation buffer (100 mmol·L−1 KCl, 40 mmol·L−1 Tris-HCl, 10 mmol·L−1 Tris base, 5 mmol·L−1 MgCl2·6H2O, 1 mmol·L-1 EDTA, and 1 mmol·L-1 ATP; pH 7.4) until analysis.
Fatty acid oxidation
Fatty acid oxidation assays were performed in fresh hepatic tissue preparations using [1–14C]palmitate (American Radiochemicals), as previously described (Rector et al. 2008b). Fatty acid oxidation was measured by collecting and counting the production of 14CO2 and 14C-labeled acid-soluble metabolites, which were trapped and counted with a liquid scintillation counter, as previously described (Rector et al. 2008b). Palmitate oxidation experiments were performed in the presence (100 μmol·L−1) or absence of etomoxir (a specific inhibitor of mitochondrial carnitine palmitoyl-CoA transferase-1 and entry into the mitochondria) to examine the relative contribution of mitochondrial (-etomoxir) and extra-mitochondrial organelles (+etomoxir) in total fatty acid oxidation (Rector et al. 2011).
Assessment of mitochondrial content
Citrate synthase activity was determined using methods described by Srere (1969). The method of determining β-hydroxyacyl CoA dehydrogenase (β-HAD) activity was as described by Bass et al. (1969) and previously used by our group (Rector et al. 2008b).
Intrahepatic lipid content and liver morphology
Liver tissue was fixed in formalin and embedded in paraffin. Hematoxylin and eosin staining was done to examine liver morphology. Intrahepatic TG content was determined using a biochemical assay as previously described (Rector et al. 2008b).
Western blot analysis
Western blot analysis was performed to determine hepatic protein expression of markers related to fatty acid uptake and de novo lipogenesis. Polyclonal antibodies for the following proteins were obtained from Cell Signaling Technology (Beverly, Mass., USA): acetyl coenzyme A carboxylase (ACC), ACC phosphorylated at Ser79, fatty acid synthase (FAS), AMPK, AMPK phosphorylated at Thr172, mammalian target of rapamycin (mTOR), mTOR phosphorylated at Ser2448, regulatory-associated protein of mTOR (Raptor), Raptor phosphorylated at Ser792, autophagy marker light chain 3, serine/threonine-protein kinase ULK1, ULK1 phosphorylated at Ser317, Ser555, or Ser757, and Bcl2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3). Stearoyl-CoA desaturase-1 (SCD-1) polyclonal antibody was from Alpha Diagnostics International (San Antonio, Tex., USA). Polyclonal antibodies to CD36/fatty acid translocase and sterol regulatory element-binding protein 1c (SREBP-1c) were from Santa Cruz Biotechnology, Inc. (Dallas, Tex., USA). Orphan nuclear receptor TR4 polyclonal antibody was obtained from Abcam (Cambridge, Mass., USA). Content of phosphoproteins was calculated from the density of the phosphoprotein band divided by the density (content) of the total protein using the appropriate antibodies (Rector et al. 2008a, 2008b). Liver samples were homogenized in lysis buffer. Protein (20–40 μg) was loaded into an SDS-PAGE gel and probed with the primary antibody. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies. Protein bands were quantified using a densitometer (Bio-Rad, Hercules, Calif., USA). To control for protein loading and transfer, the membranes were then stained with 0.1% amido black (Sigma) and total protein staining was quantified and any differences were corrected (Rector et al. 2008b).
Statistical analysis
Six to eight animals were randomized to each group. One-way analysis of variance (ANOVA) was performed for each outcome measure (SPSS v. 22.0, IBM, Chicago, Ill., USA), and significant interactions were followed up using Fisher LSD post hoc comparisons. All values were reported as the mean ± standard error (SE), and statistical significance was determined as p < 0.05.
Results
Effects of metformin, caloric restriction, and their combination on body weight, adiposity, and body composition
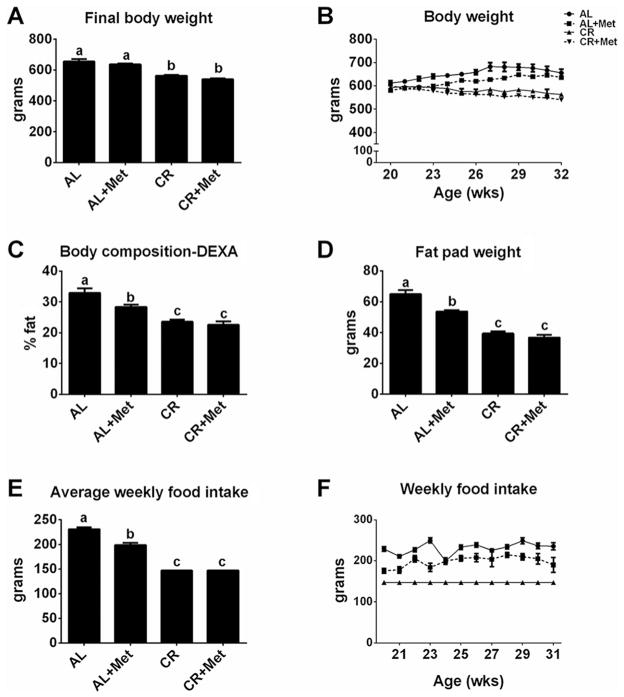
Metformin treatment did not induce weight reduction in either AL or CR OLETF rats (p > 0.05; Fig. 1A–1B). However, Met significantly improved body composition and reduced fat pad mass compared with AL (p < 0.05; Fig. 1C–1D). CR induced greater improvements in these measures than Met, but CR+Met provided no further benefits (p < 0.05). Met also induced ~15% decrease in weekly food consumption compared with AL (p < 0.001; Fig. 1E–1F); however, this reduction in food intake did not reach the level of prescribed food restriction in the CR groups.
Fig. 1.
The effects of metformin, caloric restriction, or their combination on body weight, adiposity, and food intake. Final body weight (A), weekly body weight (B), percent body fat (C), fat pad mass (omental + retroperitoneal + epididymal; D), average weekly food intake (E), and weekly food intake (F). All values are the mean ± standard error of the mean. Values with different letters are significantly different, p < 0.05. AL, ad libitum; Met, metformin; CR, calorically restricted.
Treatment effects on serum lipids and type 2 diabetes
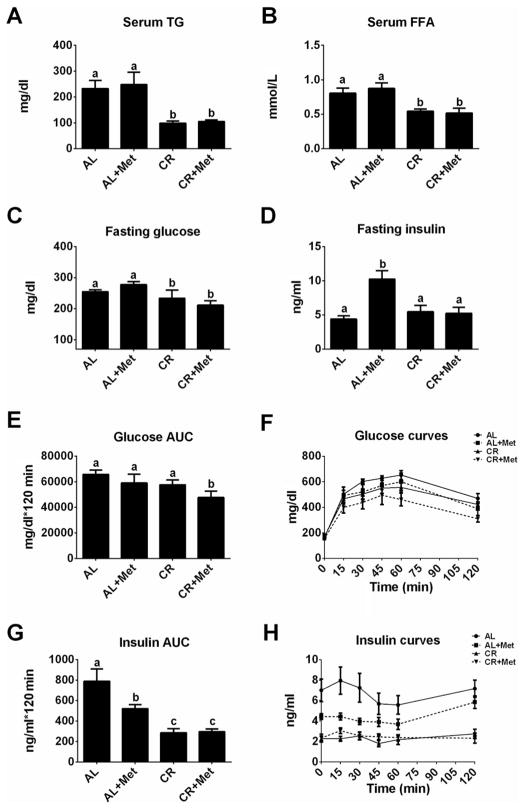
Only caloric restriction lowered serum TG (p < 0.01) and serum free fatty acids (p < 0.01) compared with AL; CR+Met provided no further improvement in these measures (Fig. 2A–2B). Additionally, CR improved fasting insulin (p < 0.01 vs. AL or Met) and glucose concentrations (p < 0.05 vs. AL); again, CR+Met provided no further benefits (p > 0.05; Fig. 2C–2D). It should be noted that insulin values in 32-week-old AL animals were significantly reduced compared with values in a subset of animals that were 20 weeks old (~60% reduction at 32 weeks vs. 20 weeks (insulin = 10.91 ± 1.41 ng·mL−1)), highlighting a transition from hyperinsulinemia to frank type 2 diabetes with pancreatic β-cell dysfunction, while CR and CR+Met rats demonstrated a normalization of insulin concentrations with reductions in blood glucose following 12 weeks of treatment. Furthermore, CR and CR+Met improved post-IPGTT insulin area under the curve (p < 0.001; Fig. 2G–2H), while only the combination of treatments effectively lowered post-IPGTT glucose area under the curve (p < 0.05; Fig. 2E–2F), suggesting that the combination of treatments may confer better protection in glycemic responses than either treatment alone.
Fig. 2.
Impact of metformin, caloric restriction, and their combination on serum lipids and glycemic control. Fasting serum triglycerides (TG; A), free fatty acids (FFA; B), glucose (C), insulin (D), glucose area under the curve after glucose challenge (AUC; E), glucose curves (F), insulin AUC (G), and insulin curves (H). All values are the mean ± standard error of the mean. Values with different letters are significantly different, p < 0.05. AL, ad libitum; Met, metformin; CR, calorically restricted.
NAFLD with metformin treatment, caloric restriction, and their combination
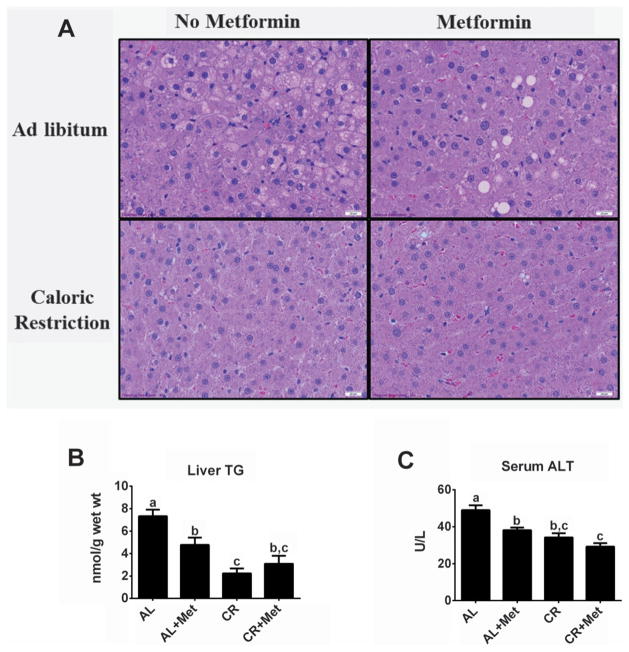
Both Met and CR reduced hepatic lipid accumulation (Fig. 3A shows representative images; note less lipid vacuolization in treatment groups compared with AL), and biochemical TG analyses revealed similar findings (Fig. 3B). Met reduced liver TG by ~40% compared with AL (p < 0.01); however, CR resulted in a larger reduction of liver TG (p < 0.01 vs. AL or Met), while no further reduction was seen with CR+Met. Both Met and CR successfully reduced serum ALT concentrations compared with AL (p < 0.01), and CR+Met provided a greater improvement than Met alone (p < 0.05, Met vs. CR+Met; Fig. 3C).
Fig. 3.
Improved liver morphology and decreased injury with metformin treatment, caloric restriction, and their combination. Liver hematoxylin and eosin staining (A), liver biochemical triglyceride analysis (TG; B), and serum alanine aminotransferase (ALT; C). All values are the mean ± standard error of the mean. Values with different letters are significantly different, p < 0.05. AL, ad libitum; Met, metformin; CR, calorically restricted.
Alterations in TR4 and mitochondrial content and function in response to metformin treatment, caloric restriction, or their combination
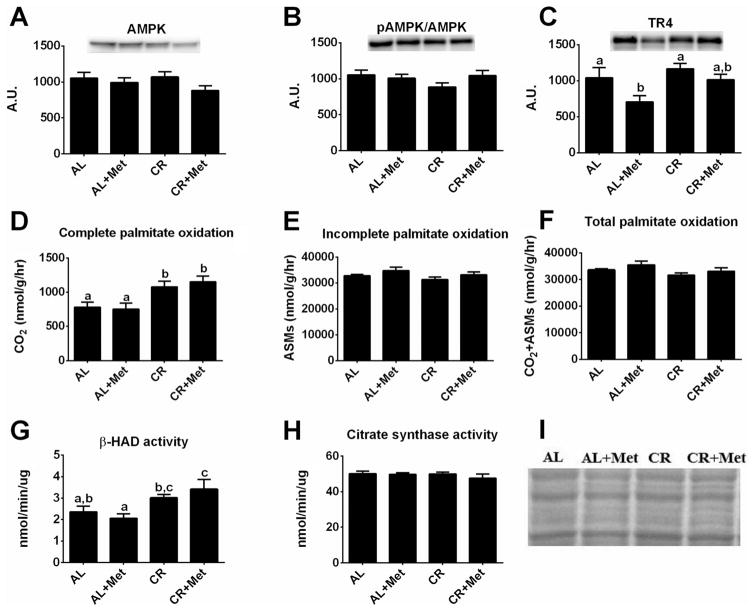
The antidiabetic effects of metformin are thought to occur through the activation of AMPK and the subsequent inhibition of the nuclear receptor TR4, which has been associated with downregulation of mitochondrial respiration and hepatic gluconeogenesis (Owen et al. 2000; Kim et al. 2011). Here, metformin treatment had no effect on AMPK protein expression or activation status (Fig. 4A–4B) but effectively reduced TR4 protein content (p < 0.05; Fig. 4C); interestingly, CR+Met increased TR4 protein expression to the level in AL rats. Metformin treatment alone also did not induce significant changes in hepatic mitochondrial content and function (complete palmitate oxidation (Fig. 4D), β-HAD activity (Fig. 4G), or citrate synthase activity (Fig. 4H)). While no differences were observed among groups for incomplete palmitate oxidation (acid-soluble metabolites, Fig. 4E), total palmitate oxidation (Fig. 4F), or measures of extra-mitochondrial oxidation (data not shown), complete palmitate oxidation was significantly increased in the CR rats (p < 0.05). Interestingly, only CR+Met significantly increased hepatic β-HAD activity (p < 0.05, CR+Met vs. AL).
Fig. 4.
Mitochondrial adaptations with metformin, caloric restriction, and their combination. AMP-activated protein kinase (AMPK; A), phospho-AMPK (B), orphan nuclear receptor TR4 (C), complete palmitate oxidation (D), incomplete palmitate oxidation (acid-soluble metabolites) (ASMs; E), total palmitate oxidation (F), β-hydroxyacyl CoA dehydrogenase (β-HAD) activity (G), citrate synthase activity (H), and representative amido black staining (I). Values (n = 6–8 per group, means ± SE) with different letters are significantly different (p < 0.05). AL, ad libitum; Met, metformin; CR, calorically restricted.
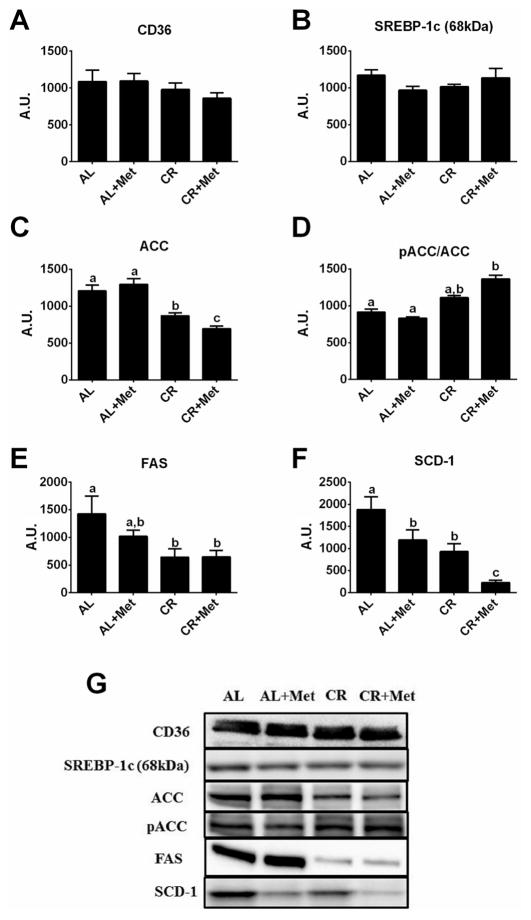
Alterations in markers of hepatic de novo lipogenesis
Several indices of hepatic fatty acid uptake and de novo lipogenesis were measured because recent evidence suggests that these processes account for >25% of hepatic TG accumulation in patients with NAFLD (Donnelly et al. 2005). No differences were observed among treatment groups in the protein content of CD36 (Fig. 5A), a protein important for fatty acid uptake, or SREBP-1c (Fig. 5B), an important transcription factor for de novo lipogenic genes. While metformin treatment alone did not alter ACC phosphorylation status (Fig. 5C–5D) or lower FAS (Fig. 5E), it lowered SCD-1 protein content by ~40% compared with AL (p < 0.05; Fig. 5F). Caloric restriction lowered FAS (p < 0.05) and SCD-1 (p < 0.01) compared with AL; however, only the combination of therapies significantly increased ACC phosphorylation (p < 0.01 vs. AL), and it reduced hepatic SCD-1 protein content by ~75% (p < 0.05) compared with either treatment alone.
Fig. 5.
Treatment effects on hepatic fatty acid uptake and de novo lipogenesis markers. Protein expression of CD36 (A), sterol regulatory element-binding protein 1c (SREBP-1c; B), acetyl-CoA carboxylase (ACC; C), phosphorylated ACC relative to total ACC (pACC/ ACC; D), fatty acid synthase (FAS; E), and stearoyl-CoA desaturase-1 (SCD-1; F), along with representative western blots (G). Values (n = 5–8 per group, means ± SE) with different letters are significantly different (p < 0.05). AL, ad libitum; Met, metformin; CR, calorically restricted.
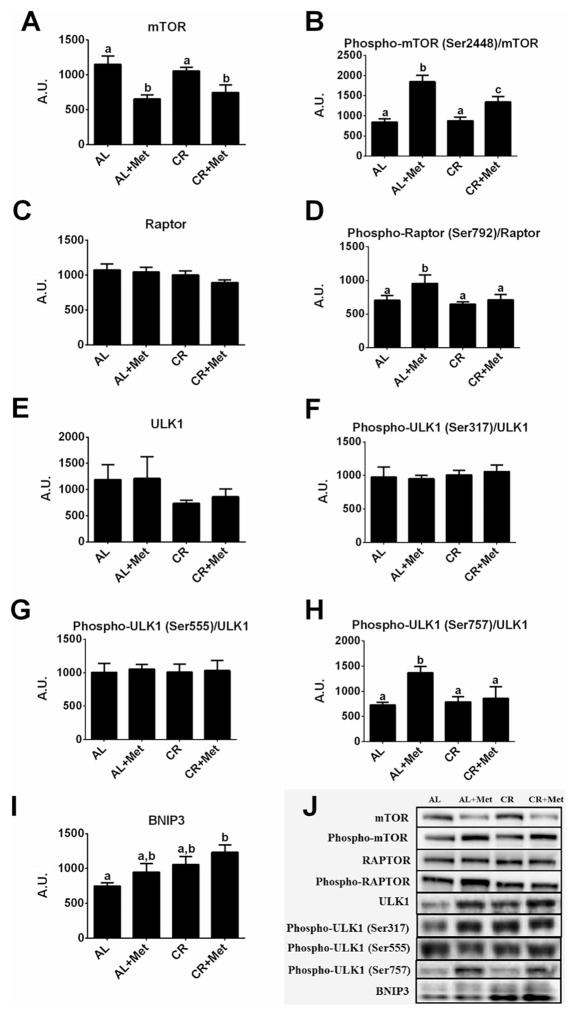
Effects of metformin, caloric restriction, and their combination on mTOR and autophagy/mitophagy-related proteins
Owing to the known role of mTOR in regulating hepatic lipogenesis, we assessed mTOR activation status. Metformin treatment significantly reduced total hepatic mTOR protein content by ~40% (p < 0.01 vs. AL; Fig. 6A) and increased the phosphorylation of mTOR (p < 0.001; Fig. 6B) compared with AL. Caloric restriction did not alter hepatic mTOR protein expression or phosphorylation of mTOR in the hyperphagic OLETF rat; however, CR+Met induced similar reductions in total mTOR protein content as Met alone but induced less of an increase in mTOR phosphorylation (p < 0.01 vs. Met). Interestingly, Met had no effect on total Raptor protein content (Fig. 6C) but did significantly increase Raptor phosphorylation compared with all other treatments (p < 0.05; Fig. 6D), which could result in mTOR complex 1 inhibition. Neither CR nor CR+Met had an effect on Raptor or phospho-Raptor compared with AL.
Fig. 6.
Alterations in mammalian target of rapamycin (mTOR) and autophagy/mitophagy-related proteins with treatment by metformin, caloric restriction, or their combination. Protein expression of mTOR (A), phospho-mTOR Ser2448 (B), regulatory-associated protein of mTOR (Raptor; C), phospho-Raptor Ser792 (D), ULK-1 (E), phospho-ULK1 Ser317 (F), phospho-ULK1 Ser555 (G), phospho-ULK1 Ser757 (H), and BNIP3 (I), and representative western blot images (J). Values (n = 6–8 per group, means ± SE) with different letters are significantly different. AL, ad libitum; Met, metformin; CR, calorically restricted.
Because AMPK and mTOR are known regulators of autophagy/ mitophagy, we assessed proteins associated with these processes. Total ULK1 protein content and phosphorylation of ULK1 at Ser317 and Ser555 (both known to be regulated by AMPK) did not differ among groups (Fig. 6E–6G). However, Met increased the phosphorylation of ULK1 at Ser 757 compared with all other treatments (p < 0.05; Fig. 6H), and interestingly, CR+Met increased BNIP3 compared with AL (p < 0.01; Fig. 6I).
Discussion
Currently, treatment recommendations for individuals with NAFLD include lifestyle modifications such as caloric restriction; however, other alternative therapies or combinations of therapies continue to be explored owing to difficulties with adherence to lifestyle modifications. One such alternative therapy is insulin sensitizers, because of the close relationship between NAFLD and type 2 diabetes. Here, we demonstrate that both CR and Met had beneficial effects for the treatment of type 2 diabetes and NAFLD, although CR resulted in greater improvements in fasting glucose, insulin, and hepatic TG accumulation as well as indices of hepatic mitochondrial function. Importantly, metformin administration in concert with reductions in overnutrition due to caloric restriction further improved post-IPGTT glucose responses, markers of hepatic de novo lipogenesis, markers of mitophagy, and markers of liver injury. These data suggest the potential for improved efficacy with the combination of lifestyle and pharmacological therapies in the management of NAFLD.
The prevalence of NAFLD is on the rise, and more than 70%–80% of type 2 diabetics have NAFLD (reviewed in Rector et al. 2008c). Lifestyle modifications, including increased physical activity and decreased caloric intake, are recommended to reduce body weight by 3.5% to improve hepatic steatosis and by 10% to improve necro-inflammation (Chalasani et al. 2012). Despite these recommendations, patients are not likely to embark on a diet and (or) exercise regimen and often turn to pharmaceutical assistance for treatment. Limited research supports the efficacy of metformin treatment as a therapy for NAFLD in adult populations at risk for the development of type 2 diabetes (Krakoff et al. 2010) and with early liver disease (Marchesini et al. 2001). These beneficial effects of metformin treatment are considered to be due in part to weight loss associated with treatment (Malin et al. 2012). While met-formin treatment in rodents improved surrogates for liver injury and histologically observed hepatic steatosis (Linden et al. 2014), studies in humans have been equivocal regarding improvement of NAFLD. Metformin has been shown to reduce transaminase levels and improve liver volume in patients with NASH (Marchesini et al. 2001). Additionally, metformin has been shown to improve hepatic necro-inflammatory activity, hepatic steatosis, hepatocyte ballooning, and acinar/portal inflammation to levels seen in patients who have undergone caloric restriction (Uygun et al. 2004). However, a randomized, placebo-controlled trial testing metformin effects in NAFLD showed limited improvement of liver histopathology (Haukeland et al. 2009). It is important to note, however, that most of these studies enrolled subjects with more advanced liver disease, including NASH, and there have been limited investigations to determine the effect of metformin on early-onset hepatic steatosis. Moreover, it remains unclear whether the combination of caloric restriction and metformin treatment can provide further benefits in type 2 diabetes and NAFLD than either treatment alone. Here, we show that met-formin treatment without weight loss improved hepatic steatosis, providing some evidence of the utility of insulin sensitizers as an alternative therapy for NAFLD. Moreover, the combination of caloric restriction and metformin treatment reduced serum ALT concentrations, a marker of liver injury, more than Met alone, highlighting the importance of a multifaceted approach in the treatment of NAFLD.
Metformin is thought to improve glycemic control through the activation of AMPK and the inhibition of nuclear receptor TR4 transactivation, which results in downregulation of mitochondrial respiration and a lowering of phosphoenolpyruvate carboxykinase and hepatic gluconeogenesis (Owen et al. 2000; Liu et al. 2007; Kim et al. 2011). Similar to our previous report (Linden et al. 2014), metformin treatment alone reduced hepatic TR4 protein content in the OLETF rat but was unable to improve glucose responses during a glucose tolerance test, which is consistent with previous studies in which metformin treatment did not improve postprandial glucose responses in both humans (Karlsson et al. 2005; Basu et al. 2008) and animals (Kosegawa et al. 1996; Faure et al. 1999). Here, we show that only CR was able to reduce dyslipidemia and lower fasting glucose and insulin, demonstrating the importance of weight maintenance and (or) weight loss in the prevention of progression to frank type 2 diabetes. Perhaps more importantly, only CR+Met led to lower glucose responses during a glucose challenge, indicating that combination therapies may be optimal when considered in the setting of a sedentary lifestyle.
Markers of mitochondrial function, hepatic fatty acid uptake, and hepatic de novo lipogenesis were assessed in the present report because of their known contributions to NAFLD (Rector and Thyfault 2011). We have previously shown that mitochondrial dysfunction precedes hepatic steatosis in sedentary OLETF rats (Rector et al. 2010a) and that chronic caloric restriction can prevent some of the mitochondrial decrements associated with obesity in these sedentary animals (Rector et al. 2011). Observations in the current investigation support this previous work and further demonstrate that treatment of NAFLD with caloric restriction improves hepatic mitochondrial function, as indicated by increased complete palmitate oxidation and β-HAD activity. As previously reported, metformin treatment did not elicit improvements in any of these indicators of hepatic mitochondrial function (Linden et al. 2015). In addition, we have previously reported that met-formin treatment actually attenuated exercise training-induced adaptations in mitochondrial content and function (Linden et al. 2014). Interestingly, in the current study, metformin did not blunt the improvements in β-HAD activity or complete palmitate oxidation induced by CR. Together, these findings suggest that mitochondrial adaptations play an important role in the treatment of NAFLD with caloric restriction but perhaps not with metformin and further support the importance of mitochondrial function in maintaining liver health (Rector et al. 2008b, 2010a, 2010b).
Fatty acid uptake and hepatic de novo lipogenesis are also associated with the development and progression of NAFLD. CD36, a transporter that promotes fatty acid uptake, has been shown to be increased in patients with NAFLD (Miquilena-Colina et al. 2011) and in obese OLETF rats (Linden et al. 2013) and may contribute to hepatic steatosis. Additionally, >25% of hepatic TG accumulation may result from de novo lipogenesis in patients with NAFLD (Donnelly et al. 2005), and this may be further exacerbated with insulin resistance (Firneisz 2014). We have previously reported that CR can prevent increases in FAS, a de novo lipogenesis marker, in the OLETF rat. Furthermore, metformin treatment lowered expression of SCD-1, a protein known to contribute to the abnormal partitioning of fatty acids through increased ACC activity and decreased fatty acid oxidation, shunting substrates to fatty acid synthesis (Dobrzyn et al. 2004; Hulver et al. 2005; Rector et al. 2011; Linden et al. 2014). Metformin-induced alterations in AMPK have been shown to regulate the phosphorylation of ACC at Ser79 (an inhibitory effect) (Foretz et al. 2010; Fullerton et al. 2013; Gowans et al. 2013), providing a potential mechanism by which metformin may produce some of its beneficial effects. Additionally, metformin-induced reductions in SCD-1 are likely the result of decreases in TR4, a known regulator of SCD-1 activity (Kim et al. 2011), and may contribute to metformin-induced improvements in NAFLD. In the present study, we observed no treatment effects on CD36; however, both caloric restriction and metformin treatment independently improved markers of de novo lipogenesis. Interestingly, the combination of caloric restriction and met-formin treatment (known activators of AMPK) resulted in further increases in phosphorylation of ACC and dramatic decreases in SCD-1 when compared with either treatment alone, perhaps contributing to less severe liver injury in these obese, hyperphagic animals.
Next, we determined the effect of caloric restriction, metformin treatment, and their combination on the mTOR pathway because of its importance in regulating lipogenesis/lipolysis. In times of nutrient excess, activation of the mTOR pathway promotes lipogenesis, in part through the regulation of SREBP (reviewed in Bakan and Laplante 2012). However, changes in energetics associated with caloric restriction and metformin treatment (Zhou et al. 2001) can increase AMPK activity and inhibit the mTOR pathway. In fact, treatment with rapamycin, an inhibitor of mTOR, and dietary restriction have been shown to decrease hepatic SREBP-1 levels (Yu et al. 2015), while inhibition of mTOR complex 1 (mTORC1) can alter expression of genes associated with lipolysis in adipocytes (Chakrabarti et al. 2010). It has previously been demonstrated that an 18-h fast and 1 h of metformin treatment can effectively inhibit mTORC1 in murine livers, as indicated by an increase in phospho-Raptor (Gwinn et al. 2008). Here, we demonstrate that 12 weeks of Met without weight loss can sustain decreases in total hepatic mTOR content and inhibition of mTORC1, as indicated by increased phosphorylation of Raptor. Although metformin-induced inhibition of mTORC1 had no effect on hepatic SREBP-1c protein content, significant reductions in the downstream de novo lipogenesis protein SCD-1 were observed. These data provide insight into a potential mechanism by which metformin treatment may decrease hepatic steatosis through inhibition of mTORC1 and decreased lipogenesis. Interestingly, CR and CR+Met had no effect on Raptor or phospho-Raptor, suggesting that regulation of mTORC1 activity may not play a prominent role in improvements in hepatic steatosis with CR.
AMPK and mTOR are known to be important regulators of autophagy, while metformin recently has been shown to increase autophagy within hepatocytes (Inokuchi-Shimizu et al. 2014; Song et al. 2015). Autophagy is an important means by which ubiquitinated proteins or damaged organelles, such as mitochondria, can be removed from tissues to maintain cellular health. Autophagy can occur when there is an interaction between functional AMPK and ULK1 (Bach et al. 2011). Interestingly, while the phosphorylation status of ULK1 Ser317 or ULK1 Ser555 (sites known to be phosphorylated by AMPK) was not different among groups, metformin treatment alone increased ULK1 phosphorylation at Ser757, a site known to inhibit autophagy. However, this enhanced phosphorylation of ULK1 at Ser757 was not apparent in the CR+Met group, and in fact, CR+Met significantly increased BNIP3 protein content. This may be an important factor in maintaining mitochondrial quality control with the combination treatment, as BNIP3 has been shown to be an important mediator of mitochondrial autophagy (mitophagy) (Rikka et al. 2011) and a loss of BNIP3 has been associated with increased lipogenesis and decreased β-oxidation within hepatocytes, which was associated with increased hepatic steatosis (Glick et al. 2012). These findings need to be followed up in the future with more thorough examination of autophagic/mitophagic flux assessments.
In summary, we have demonstrated that both caloric restriction and metformin treatment independently contribute to improved glycemic control and NAFLD outcomes in sedentary OLETF rats. Caloric restriction alone induced greater improvements in hepatic steatosis and hepatic mitochondrial adaptations compared with metformin monotherapy. Interestingly, when nutritional excess was removed with caloric restriction, administration of metformin further improved post-challenge glycemic control and further reduced markers of de novo lipogenesis, mitophagy, and liver injury compared with either therapy alone in diabetic OLETF rats. Therefore, these findings in the OLETF rat suggest the combination of caloric restriction and metformin may provide a more optimal approach than either therapy alone in the management of insulin resistance, type 2 diabetes, and NAFLD.
Acknowledgments
The authors gratefully acknowledge the excellent technical assistance of Pam Thorne and Kayla Kanosky. We would also like to thank Monica L. Kearney and Dr. Jacqueline M. Crissey for assistance with the IPGTTs. This work was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans’ Hospital in Columbia, Mo. This work was partially supported by grants NIH T32 AR 048523-07 (JAF and EMM), DK088940 (JPT), RO1HL036088 (MHL), HL73101-07 (JRS), NIH R01 HL107910-03 (JRS), VA-Merit System 0018 (JRS), and VHA-CDA2 1 IK2 BX001299 (RSR).
Footnotes
Conflict of interest statement
None of the authors have any conflicts of interest to disclose.
Contributor Information
Melissa A. Linden, Research Service, Harry S. Truman Memorial Veterans’ Hospital, Columbia, MO 65201, USA; Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO 65211, USA.
Kristi T. Lopez, Research Service, Harry S. Truman Memorial Veterans’ Hospital, Columbia, MO 65201, USA; Department of Medicine, Division of Gastroenterology and Hepatology, University of Missouri, Columbia, MO 65212, USA.
Justin A. Fletcher, Research Service, Harry S. Truman Memorial Veterans’ Hospital, Columbia, MO 65201, USA; Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO 65211, USA
E. Matthew Morris, Research Service, Harry S. Truman Memorial Veterans’ Hospital, Columbia, MO 65201, USA; Department of Medicine, Division of Gastroenterology and Hepatology, University of Missouri, Columbia, MO 65212, USA.
Grace M. Meers, Research Service, Harry S. Truman Memorial Veterans’ Hospital, Columbia, MO 65201, USA; Department of Medicine, Division of Gastroenterology and Hepatology, University of Missouri, Columbia, MO 65212, USA
Sameer Siddique, Research Service, Harry S. Truman Memorial Veterans’ Hospital, Columbia, MO 65201, USA; Department of Medicine, Division of Gastroenterology and Hepatology, University of Missouri, Columbia, MO 65212, USA.
M. Harold Laughlin, Department of Biomedical Sciences, University of Missouri, Columbia, MO 65211, USA.
James R. Sowers, Research Service, Harry S. Truman Memorial Veterans’ Hospital, Columbia, MO 65201, USA; Department of Medicine, Division of Endocrinology, University of Missouri, Columbia, MO 65212, USA
John P. Thyfault, Research Service, Harry S. Truman Memorial Veterans’ Hospital, Columbia, MO 65201, USA; Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO 65211, USA; Department of Medicine, Division of Gastroenterology and Hepatology, University of Missouri, Columbia, MO 65212, USA.
Jamal A. Ibdah, Research Service, Harry S. Truman Memorial Veterans’ Hospital, Columbia, MO 65201, USA; Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO 65211, USA; Department of Medicine, Division of Gastroenterology and Hepatology, University of Missouri, Columbia, MO 65212, USA
R. Scott Rector, Research Service, Harry S. Truman Memorial Veterans’ Hospital, Columbia, MO 65201, USA; Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO 65211, USA; Department of Medicine, Division of Gastroenterology and Hepatology, University of Missouri, Columbia, MO 65212, USA.
References
- Bach M, Larance M, James DE, Ramm G. The serine/threonine kinase ULK1 is a target of multiple phosphorylation events. Biochem J. 2011;440:283–291. doi: 10.1042/BJ20101894. [DOI] [PubMed] [Google Scholar]
- Bakan I, Laplante M. Connecting mTORC1 signaling to SREBP-1 activation. Curr Opin Lipidol. 2012;23:226–234. doi: 10.1097/MOL.0b013e328352dd03. [DOI] [PubMed] [Google Scholar]
- Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969;10:198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Basu R, Shah P, Basu A, Norby B, Dicke B, Chandramouli V, et al. Comparison of the effects of pioglitazone and metformin on hepatic and extra-hepatic insulin action in people with type 2 diabetes. Diabetes. 2008;57:24–31. doi: 10.2337/db07-0827. [DOI] [PubMed] [Google Scholar]
- Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- Borst SE, Snellen HG, Lai HL. Metformin treatment enhances insulin-stimulated glucose transport in skeletal muscle of Sprague-Dawley rats. Life Sci. 2000;67:165–174. doi: 10.1016/S0024-3205(00)00612-3. [DOI] [PubMed] [Google Scholar]
- Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes. 2010;59:775–781. doi: 10.2337/db09-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- de Oliveira CP, Stefano JT, de Siqueira ER, Silva LS, de Campos Mazo DF, Lima VM, et al. Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non-alcoholic steatohepatitis. Hepatol Res. 2008;38:159–165. doi: 10.1111/j.1872-034X.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, et al. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA. 2004;101:6409–6414. doi: 10.1073/pnas.0401627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MC, Parise ER, de Carvalho L, Szejnfeld D, Netto JP. Effect of 6-month nutritional intervention on non-alcoholic fatty liver disease. Nutrition. 2010;26:1094–1099. doi: 10.1016/j.nut.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Faure P, Rossini E, Wiernsperger N, Richard MJ, Favier A, Halimi S. An insulin sensitizer improves the free radical defense system potential and insulin sensitivity in high fructose-fed rats. Diabetes. 1999;48:353–357. doi: 10.2337/diabetes.48.2.353. [DOI] [PubMed] [Google Scholar]
- Firneisz G. Non-alcoholic fatty liver disease and type 2 diabetes mellitus: The liver disease of our age? World J Gastroenterol. 2014;20:9072–9089. doi: 10.3748/wjg.v20.i27.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcheron F, Abdallah P, Basset A, del Carmine P, Haffar G, Beylot M. Nonalcoholic hepatic steatosis in Zucker diabetic rats: spontaneous evolution and effects of metformin and fenofibrate. Obesity (Silver Spring) 2009;17:1381–1389. doi: 10.1038/oby.2008.661. [DOI] [PubMed] [Google Scholar]
- Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D, Zhang W, Beaton M, Marsboom G, Gruber M, Simon MC, et al. BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol. 2012;32:2570–2584. doi: 10.1128/MCB.00167-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukeland JW, Konopski Z, Eggesbo HB, von Volkmann HL, Raschpichler G, Bjoro K, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi-Shimizu S, Park EJ, Roh YS, Yang L, Zhang B, Song J, et al. TAK1-mediated autophagy and fatty acid oxidation prevent hepatosteatosis and tumorigenesis. J Clin Invest. 2014;124:3566–3578. doi: 10.1172/JCI74068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson HK, Hallsten K, Bjornholm M, Tsuchida H, Chibalin AV, Virtanen KA, et al. Effects of metformin and rosiglitazone treatment on insulin signaling and glucose uptake in patients with newly diagnosed type 2 diabetes: a randomized controlled study. Diabetes. 2005;54:1459–1467. doi: 10.2337/diabetes.54.5.1459. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- Kim E, Liu NC, Yu IC, Lin HY, Lee YF, Sparks JD, et al. Metformin inhibits nuclear receptor TR4-mediated hepatic stearoyl-CoA desaturase 1 gene expression with altered insulin sensitivity. Diabetes. 2011;60:1493–4503. doi: 10.2337/db10-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosegawa I, Katayama S, Kikuchi C, Kashiwabara H, Negishi K, Ishii J, et al. Metformin decreases blood pressure and obesity in OLETF rats via improvement of insulin resistance. Hypertens Res. 1996;19:37–41. doi: 10.1291/hypres.19.37. [DOI] [PubMed] [Google Scholar]
- Krakoff J, Clark JM, Crandall JP, Wilson C, Molitch ME, Brancati FL, et al. Effects of metformin and weight loss on serum alanine amino-transferase activity in the diabetes prevention program. Obesity (Silver Spring) 2010;18:1762–1767. doi: 10.1038/oby.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008;16:1355–1362. doi: 10.1038/oby.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- Linden MA, Meers GM, Ruebel ML, Jenkins NT, Booth FW, Laughlin MH, et al. Hepatic steatosis development with four weeks of physical inactivity in previously active, hyperphagic OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R763–R771. doi: 10.1152/ajpregu.00537.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden MA, Fletcher JA, Morris EM, Meers GM, Kearney ML, Crissey JM, et al. Combining metformin and aerobic exercise training in the treatment of type 2 diabetes and NAFLD in OLETF rats. Am J Physiol Endocrinol Metab. 2014;306:E300–E310. doi: 10.1152/ajpendo.00427.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden MA, Fletcher JA, Morris EM, Meers GM, Laughlin MH, Booth FW, et al. Treating NAFLD in OLETF rats with vigorous-intensity interval exercise training. Med Sci Sports Exerc. 2015;47:556–567. doi: 10.1249/MSS.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NC, Lin WJ, Kim E, Collins LL, Lin HY, Yu IC, et al. Loss of TR4 orphan nuclear receptor reduces phosphoenolpyruvate carboxykinase-mediated gluconeogenesis. Diabetes. 2007;56:2901–2909. doi: 10.2337/db07-0359. [DOI] [PubMed] [Google Scholar]
- Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172–182. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care. 2012;35:131–136. doi: 10.2337/dc11-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/S0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Noguchi E, Ishida K, Kobayashi T, Yamada N, Kamata K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol. 2008;295:H1165–H1176. doi: 10.1152/ajpheart.00486.2008. [DOI] [PubMed] [Google Scholar]
- Mazza A, Fruci B, Garinis GA, Giuliano S, Malaguarnera R, Belfiore A. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012:716404. doi: 10.1155/2012/716404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milic S, Stimac D. Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis, clinical presentation and treatment. Dig Dis. 2012;30:158–162. doi: 10.1159/000336669. [DOI] [PubMed] [Google Scholar]
- Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos S, Garcia-Mediavilla MV, Fernandez-Bermejo M, Lozano-Rodriguez T, et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steato-hepatitis and chronic hepatitis C. Gut. 2011;60:1394–1402. doi: 10.1136/gut.2010.222844. [DOI] [PubMed] [Google Scholar]
- Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol. 2006;48:360–367. doi: 10.1002/dev.20149. [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(3):607–614. doi: 10.1042/0264-6021:3480607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP. Does physical inactivity cause nonalcoholic fatty liver disease? J Appl Physiol. 2011;111:1828–1835. doi: 10.1152/japplphysiol.00384.2011. [DOI] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, et al. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol. 2008a;586:4241–4249. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008b;294:G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008c;14:185–192. doi: 10.3748/wjg.14.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010a;52:727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, et al. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab. 2010b;298:E1179–E1187. doi: 10.1152/ajpendo.00703.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, et al. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol. 2011;300:G874–G883. doi: 10.1152/ajpgi.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikka S, Quinsay MN, Thomas RL, Kubli DA, Zhang X, Murphy AN, Gustafsson AB. Bnip3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell Death Differ. 2011;18:721–731. doi: 10.1038/cdd.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YM, Lee YH, Kim JW, Ham DS, Kang ES, Cha BS, et al. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11:46–59. doi: 10.4161/15548627.2014.984271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere PA. Citrate Synthase. Methods Enzymol. 1969;13:3–5. doi: 10.1016/0076-6879(69)13005-0. [DOI] [Google Scholar]
- Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17:152–154. doi: 10.2337/diacare.17.2.152. [DOI] [PubMed] [Google Scholar]
- Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255–266. doi: 10.1016/j.jhep.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537–544. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- Yu Z, Wang R, Fok WC, Coles A, Salmon AB, Perez VI. Rapamycin and Dietary Restriction Induce Metabolically Distinctive Changes in Mouse Liver. J Gerontol A Biol Sci Med Sci. 2015;70(4):410–420. doi: 10.1093/gerona/glu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]