Abstract
Hematopoietic stem and progenitor cells (HSPC) reside in a specialized niche that regulates their proliferative capacity and their fate. There is increasing evidence for similar roles of marrow niches on controlling the behavior of leukemic cells, however whether normal HSC and leukemic cells reside in or functionally compete for the same marrow niche is unclear. We used the MLL-AF9 murine acute myeloid leukemia in a competitive repopulation model to investigate whether normal HSPC and leukemic cells functionally compete for the same marrow niches. Irradiated recipient mice were transplanted with fixed numbers of MLL-AF9 cells mixed with increasing doses of normal syngeneic whole bone marrow (WBM) or with purified HSPC (LSK). Survival was significantly increased and leukemic progression was delayed proportional to increasing doses of normal WBM or normal LSK cells in multiple independent experiments, with all doses of WBM or LSK cells studied above the threshold for rapid and complete hematopoietic reconstitution in the absence of leukemia. Confocal microscopy demonstrated nests of either leukemic cells or normal hematopoietic cells but not both in the marrow adjacent to endosteum. Early following transplantation, leukemic cells from animals receiving lower LSK doses were cycling more actively than in those receiving higher doses. These results suggest that normal HSPC and AML cells compete for the same functional niche. Manipulation of the niche could impact on response to anti-leukemic therapies, and the numbers of normal HSPC could impact on leukemia outcome, informing approaches to cell dose in the context of stem cell transplantation.
Keywords: bone marrow, niche, hematopoietic stem cells, acute myeloid leukemia, murine, competition
INTRODUCTION
Since the initial 1978 conceptualization of a bone marrow hematopoietic stem and progenitor cell (HSPC) “niche” by Schofield, and Lord’s demonstration that HSPC are not uniformly distributed throughout the marrow space, there has been intense interest and extensive recent progress in understanding the bi-directional communication pathways governing the niche-HSPC relationship.[1–4] The most primitive long-term engrafting HSPC have been localized to endosteal regions in both murine and human-murine xenografts, with specific capabilities and behavior of cells defined by their niche localization and potentially the hypoxic micro-environment.[5–7] Spatially and functionally, the number of individual niches able to support and protect HSPCs is finite, as demonstrated via murine competitive repopulation assays and the requirement for niche-emptying conditioning in order to facilitate engraftment of transplanted HSPCs.[8, 9] An understanding of HSPC-niche interactions and the mirror-image processes of HSPC niche mobilization has significant impact for improving outcomes in HSPC transplantation.
The interactions between leukemic cells and marrow microenvironmental niches has also begun to be explored, but are less well-defined.[10] An understanding of any such interactions has therapeutic importance, and may also help explain the occurrence of cytopenias that can predate overt leukemia in patients with both myeloid and lymphoid leukemias. Leukemia may represent in part a loss of niche-dependence and homeostatic controls, but conversely leukemia cells, particularly leukemia stem cells (LSC) may be able to evade cytotoxic therapies by sheltering in quiescence-inducing niches. Targeting LSCs in the marrow niche has been proposed as a possible treatment approach for some types of leukemia.[11] [12] Mapping of human myeloid leukemia cell homing in murine xenografts has found a similar pattern of distribution to normal HSPCs, specifically endosteal areas in the epiphyseal regions.[13, 14] A number of recent studies have found that human acute lymphoid leukemia cells disrupt xenogenic niches for normal HSPC, via cytokine secretion, or physical changes in niche characteristics.[15, 16]
However, previous studies have not directly asked whether normal HSPC and leukemic cells compete for and reside in the same functional niches. This question has many implications for design of rational leukemic therapies, particularly regarding both autologous and allogeneic stem cell transplantation. We utilized the MLL-AF9 murine myeloid leukemia model to investigate the impact of normal murine HSPC cell dose on leukemia engraftment and progression in a competitive transplantation model.
MATERIALS AND METHODS
Derivation and passage of the Mixed Lineage Leukemia-AF9 (MLL-AF9) cell line
The MLL-AF9 leukemia cells utilized in these studies were obtained from the laboratory of Dr. James Mulloy at Cincinnati Children’s Hospital Medical Center and were derived as described in prior publications.[17, 18] In brief, C57BL/6 murine bone marrow progenitors were transduced with a replication-incompetent retroviral vector expressing GFP and the MLL-AF9 oncogenic fusion gene. These cells can be passaged in vivo in recipient mice, and rapidly induce an acute myelogenous leukemia following transplantation. MLL-AF9 leukemic blasts can be harvested from recipient spleens and bone marrow and utilized for transplantation or in vitro studies.
For these studies, cryopreserved MLL-AF9 cells isolated from spleens of leukemic mice were thawed and passaged in vivo by tail-vein injection of one million viable thawed cells into a lethally irradiated (1000 cGy, cesium source) C57BL/6 recipient mice. Approximately two weeks later, leukemic cells were obtained by flushing the bone marrow from femurs and tibias with saline. Red cells were lysed with ACK buffer (Quality Biological Inc., Gaithersburg, MD), resulting in a pure MLL-AF9 cell source for the competitive transplantation studies described below (Quality Biological Inc., Gaithersburg, MD).
Animal care and transplantation
Inbred C57BL/6J (B6) and transgenic B6.Cg-Tg(CAG-DsRed*MST)1Nagy/J (B6-DsRed) mice were obtained from the Jackson Laboratory (Bar Harbor, ME), and were bred and raised in National Institutes of Health animal facilities under standard care and nutrition. Mice were used at 8–18 weeks of age as donors and recipients for imaging and transplantation experiments. All animal studies were approved by the Institutional Animal Care and User Committee at National Heart, Lung, and Blood Institute.
For transplantation studies, B6 mice were pre-irradiated with 10 Gy total body irradiation (TBI) using a 137cesium gamma source (J. L. Shepherd & Associates, Glandale, CA, USA). Four to six hours later, each irradiated recipient was injected with 1) 0.9% saline, or 2) increasing doses of B6 whole bone marrow cells (105, 106, 107 and 5×107) mixed with 4×105 AF9-MLL-GFP cells, or 3) increasing doses of B6 LSK cells (102, 103, 104, and 5×104) alone or mixed with 4×105 AF9-MLL-GFP cells.
Post-transplantation, animals were euthanized for analysis at days 7 and 12 after cell transplantation, or at the stage when animals become moribund with decreased activity, hunched posture, ruffled hair, and inability to eat or drink. Bone marrow cells were extracted from bilateral femurs using a small gauge needle and PBS supplemented with 1% BSA (Gibco® Life Technologies, Carlsbad, CA). Leukemic engraftment was assessed via flow cytometry for the percentage of GFP positive cells.
Enrichment of primary hematopoietic stem cells from adult mouse bone marrow
An enriched hematopoietic stem cell population was obtained as described.[19] In brief, bone marrow cells from the femurs and tibias of adult C57BL/6 mice underwent red cell lysis (ACK buffer, Quality Biological) and ~109 cells were lineage depleted (lin−) with a panel of antibody-conjugated goat anti-Rat IgG BioMag beads (Qiagen), and subsequently stained with anti-murine cKit-APC and Sca1-PE antibodies (eBioscience, San Diego, CA). Cells were sorted on a BD FACS Aria instrument. The HSPC population (Sca+cKit+) constituted ~1.2% of lin− cells. Population enrichment was confirmed by soft agar clonal expansion. Lineage depletion rate anti-mouse antibodies utilized included anti- CD4, CD8a, IL7Ra, CD11b, Ly-6G, Ter119, and CD45R (eBioscience).
Fixation, permeabilization, and flow cytometric analysis
Harvested bone marrow or spleen cells were fixed and permeabilized using a Fixation/Permeabilization Concentrate (eBiosiences) according to the manufacture’s protocol. Intracellular staining was performed with an anti-mouse/rat Ki67 PE-Cy7 (clone SolA15) antibody (eBiosience). Following incubation of permabilized cells with the antibody for 20 min at 37 °C, cells were washed once in PBS containing 2% BSA, and then analyzed and/or sorted for GFP positive, Ki67 positive and Ki67 negative populations on an AriaII instrument (BD Biosiences).
Confocal microscopy
Confocal imaging was performed on bisected sternal whole mounts as previously described.[20, 21] Sternums were bisected sagittally and placed cut-face down onto a 35-mm coverglass culture dish (MatTek, Ashland, MA). Images were acquired directly using confocal light scanning microscopy on Zeiss LSM 510 and LSM 710 confocal systems (Carl Zeiss MicroImaging, Peabody, MA). At the time of image acquisition, the marrow tissue beneath the bone edge was located and positioned using differential interference contrast (DIC) illumination. Fluorescence images were captured sequentially, using a 488-nm laser line and emission between 505 and 550 nm for GFP and DIC, a 561-nm laser line and emission between 575 and 615 nm for DsRed. Confocal reflection microscopy was utilized to visualize the bone. 3D tiled-images comprising the entire sternum fossae volume were collected for assessing numbers and distributions of normal hematopoietic stem cells and leukemia cells, as well as comparisons between different experimental conditions.
Histopathology
Tissue and organ samples were immediately removed and fixed in 10% formalin before being embedded in paraffin, sectioned, and stained with hematoxylin and eosin (Histoserve, Inc. MD).
Statistical Analyses
Kaplan–Meier overall survival curves were generated using GraphPad Prism 6 (GraphPad, La Jolla, CA) and logrank testing was applied to analyze for survival differences. For all analyses, a p value of less than 0.05 was considered significant. Prism 6 software was also used to perform student t tests for direct comparisons of biologic outcomes between groups.
RESULTS
Dose escalation of normal bone marrow cells or purified HSPCs increases survival of leukemic mice
We asked whether normal murine bone marrow cells and murine MLL-AF9 myeloid leukemia cells functionally compete for the same bone marrow niche by performing competitive transplantation experiments, co-infusing a fixed dose of MLL-AF9 cells along with escalating doses of normal bone marrow cells (WBM) into recipient mice conditioned with 1000 rads total body irradiation. We first identified the minimum dose of WBM ensuring rescue from irradiation-induced lethality, finding that cell doses of 1 × 105 WBM/mouse or greater ensured marrow recovery and 100% survival to day 90 in the absence of administration of leukemia cells, with death from lack of hematopoietic recovery at day 10–11 with insufficient doses (Figure S1A).
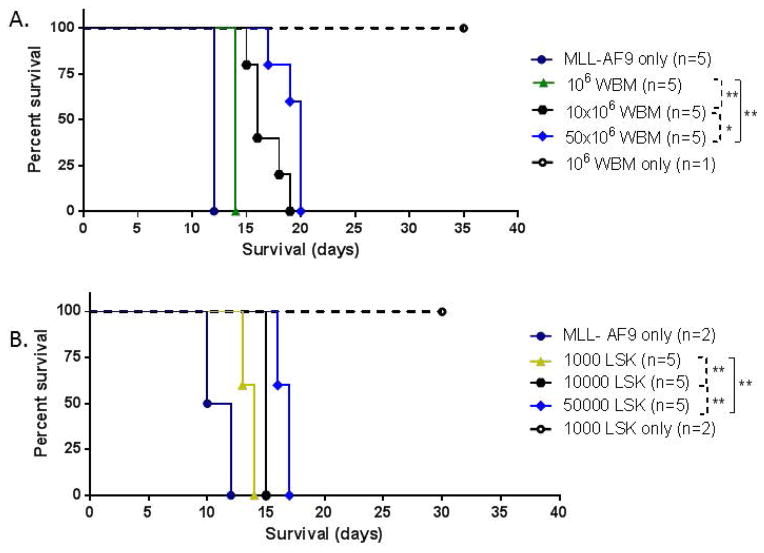
We then co-transplanted 4 × 105 MLL-AF9 myeloid leukemia cells mixed with graded doses of WBM escalating from 1 × 106/mouse up to 5 × 107/mouse and monitored survival, starting at a dose an order of magnitude greater than required to ensure engraftment. As shown in three independent experiments (Figure 1A and Figure S2 A and B), co-transplantation of higher doses of WBM cells/mouse increased survival significantly as compared to lower WBM doses. Animals receiving insufficient WBM, or MLL-AF9 cells alone without WBM, die due to lack of hematopoietic recovery on day 10–11 following 1000 rads irradiation and transplantation. Animals receiving MLL-AF9 cells plus 106 WBM cells had a median survival of 14 days in the experiment shown in Figure 1A, compared to 16 days for MLL-AF9 plus 107 WBM cells and 20 days for MLL-AF9 plus 5 × 107 WBM cells, with significant p values for comparisons between doses as shown (Figure 1A and figure S2). The number of circulating GFP+ blasts were measured in one experiment at day 12, and found to be significantly lower in higher versus lower dose WBM groups (9,490/ul+/−3,965 for 106 WBM cells versus 1,058/ul +/− 776 for 107 WBM cells versus 384/ul +/−68 for 5 × 107 WBM cells, all comparison p<0.05).
Figure 1. Impact of normal bone marrow cell dose on survival following MLL-AF9 leukemia initiation.
Kaplan-Meier survival curves for recipient C57L/6J mice co-transplanted with fixed-numbers of MLL-AF9 cells (4 × 105) mixed with increasing numbers of normal marrow hematopoietic cells, either whole bone marrow (WBM, panel A), or purified hematopietic stem and progenitor cells (LSK, panel B). Controls in each experiment received MLL-AF9 alone or normal hematopoietic cells alone. Animals were euthanized when premorbid based on predefined objective criteria. (A) Whole bone marrow. (B) Purified LSK cells. P values are shown comparing survivals between groups via logrank testing. Comparisons with p <0.05 are considered significant and are designated “*” or “**” for p <0.025.
We next confirmed that this effect on survival was specifically due to functional competition of hematopoietic stem and early progenitor cells with leukemic cells, as compared to more mature hematopoietic elements, by repeating these experiments utilizing purified lineage negative, Sca1+, c-kit+ cells (LSK cells). Transplantation of even 100 of these LSK cells resulted in hematopoietic recovery and long-term survival in the majority of mice (Figure S1B). When competitive transplantation was performed with 4 × 105 MLL-AF9 cells mixed with graded doses of LSK cells escalating from 1000 cells/mouse up to 50,000 cells/mouse, survival was enhanced as compared to lower LSK cell doses (Figure 1B).
Dose escalation of normal bone marrow cells or purified HSPCs retards leukemia progression
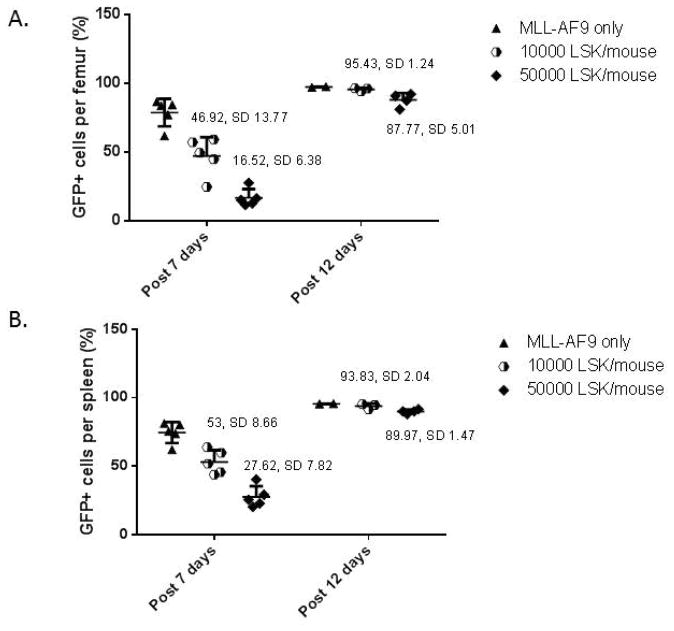
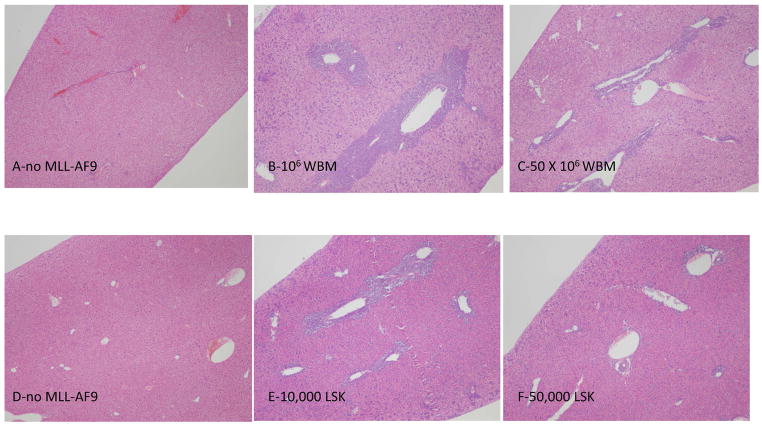
We repeated the competitive transplantation experiments with LSK cells co-transplanted with MLL-AF9 cells. Transplantations were designed to compare doses of 10,000 versus 50,000 LSK (associated with a significant difference in survival as shown above), co-transplanted with 4 × 105 MLL-AF9 cells, and sacrificed animals at set times post-transplantation to assess leukemic cell localization, leukemia burden and cycling properties. As shown in Figure 2, the level of leukemic cells in femurs and spleens at day 7 were significantly lower in the animals co-transplanted with 50,000 compared to 10,000 LSK cells. By day 12, the leukemia cells had almost completely replaced normal hematopoiesis in the marrow and spleen in all groups. Survival was significantly different, likely based on more prolonged active leukemia with tissue disruption and cytopenias in the lower dose LSK group. Liver sections clearly demonstrated much less leukemic infiltration around vessels in animals receiving the higher versus lower doses of either WBM or LSK cells (Figure 3).
Figure 2. Impact of normal hematopoietic stem and progenitor cell dose on MLL-AF9 leukemic burden.
Groups of recipients were co-transplanted with 4 × 105 MLL-AF9 cells alone or 4 × 105 MLL-AF9 cells and increasing numbers of purified LSK cells, and then assessed for leukemia burden (via % GFP + MLL-AF9 cells) in the femoral bone marrow (A) and spleen (B) at 7 and at 12 days following transplantation. At 7 days there was a significant reduction in leukemic infiltration comparing 50,000 versus 10,000 LSK cells for both femurs (A) p=2.71×10−6 and spleens (B) p= 1.152×10−5. By day 12, all groups were grossly leukemic. As expected, MLL-AF9 cells administered alone rapidly constituted the entirety of the marrow, due to the ablation of endogenous hematopoiesis via the total body irradiation.
Figure 3. Impact of normal hematopoietic cell dose on organ leukemic infiltration.
Representative hematoxylin and eosin stained sections of liver from mice transplanted with normal WBM alone (A), fixed numbers of MLL-AF9 cells (4 ×105) mixed with 1 or 50 million WBM cells (panels B and C), purified LSK cells alone (D), or fixed numbers of MLL-AF9 mixed with 10,000 (E) or 50,000 (F) LSK cells. Magnification 100X. Animals were euthanized on day 8 for WBM and day 7 for LSK.
Impact of HSPC cell dose on cycling of leukemic cells
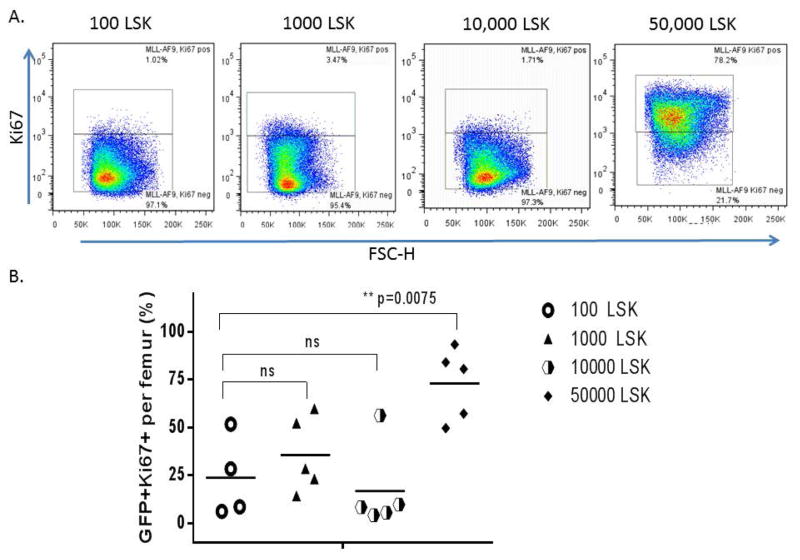
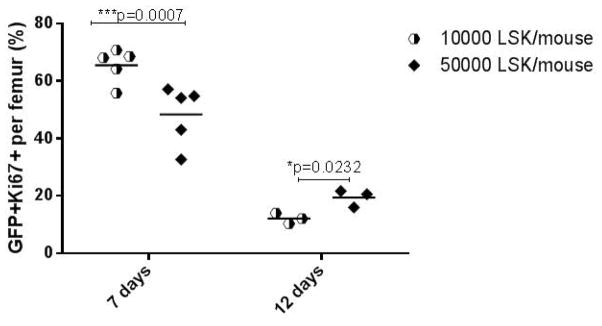
The cycling status of MLL-AF9 leukemic cells was first assessed via measuring the fraction of Ki67-positive cells within the GFP+ leukemic cell gate in the bone marrow of animals in the competitive repopulation LSK cell survival study, at the time of sacrifice due to a a pre-morbid state. The fraction of Ki67+ and thus cycling leukemic cells was approximately 3-fold increased in the animals receiving 50,000 as compared to lower doses of LSK cells plus MLL-AF9 cells (Figure 4). Cycling status was then assessed in bone marrow leukemic cells at defined earlier time points in animals receiving MLL-AF9 cells plus 10,000 or 50,000 LSK cells. As shown in Figure 5, cycling of leukemic cells was decreased at day 7 with the higher LSK cell dose. However, by day 12 the leukemic cells in the animal receiving the higher cell dose had increased again, similar to what was found at the time of death and described above (Figure 5).
Figure 4. Impact of normal hematopoietic cell dose on cycling of leukemia cells at time of disease progression.
At the time of euthanasia due to a pre-morbid state, the cycling status of marrow leukemic cells was measured. The % of GFP+ leukemic cells that expressed Ki67 is shown. (A) Representative Ki67 staining of the GFP+ leukemic cells. (B) Comparison of % Ki67+ tumor cells at different LSK doses at the time of euthanasia. A dose of 50,000 purified LSKs resulted in a significantly increased fraction of Ki67+ leukemic cells, compared to groups transplanted with lower doses of purified LSKs (p=0.0075).
Figure 5. Impact of normal hematopoietic cell dose on cycling of leukemia cells early post-transplantation.
When animals were sacrificed at set times post-transplantation, the proportion of Ki67+ leukemic cells was reduced at higher (50,000 LSK) versus lower (10,000 LSK) normal cell doses at day 7 (p=0.0007, marked “***”), but by day 12 had increased compared to the lower cell dose (p=0.0232, marked “**”).
Imaging of HSPC and leukemic cells in the bone marrow niche
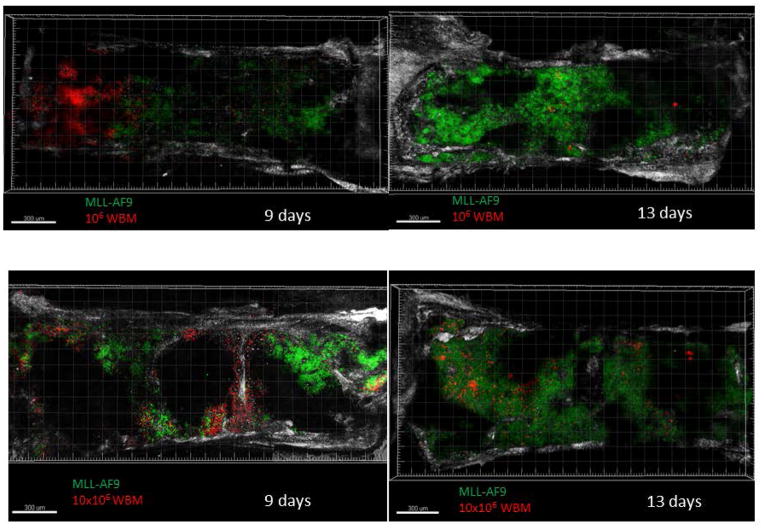
In order to explore localization of MLL-AF9 leukemic cells versus hematopoiesis derived from normal HSPC in marrow niches, we performed competitive transplantation followed by confocal combined with two photon imaging of the sternal bone marrow. Recipient mice were co-transplanted with 4×105 GFP+ MLL-AF9 cells, along with 1×106 or 10×106 WBM cells ex pressing dsRED, respectively. Imaging at day 9 post-transplantation demonstrated distinct foci of either GFP+ or dsRed+ cells (Figure 6), suggesting that each niche was occupied by one cell type or the other, but not both. As previously reported for normal hematopoiesis early after transplant [21], each focus of hematopoiesis was large, likely clonal, and seemed to spread contiguously. When the marrow was imaged 13 days following transplantation, the MLL-AF9 cells (marked by GFP) now occupied most of the bone marrow volume, with only small residual foci of dsRed normal hematopoietic cells (Figure 6).
Figure 6. Sternal bone marrow confocal combined with two-photon imaging demonstration of leukemic and normal HSCs foci.
Representative micrographs of sternal marrow sections from mice transplanted with normal WBM (marked by dsRED) only compared with fixed amount of MLL-AF9 cells (marked by GFP) along with different LSKs doses. Scale bars indicate 300 μm. Two mice were imaged for each time point in each group, in two independent experiments.
DISCUSSION
We have demonstrated that normal mouse bone marrow cells functionally compete with MLL-AF9 myeloid leukemia-initiating cells. Co-transplantation of increasing doses of either whole bone marrow or highly HSC-enriched LSK cells along with leukemic cells lengthened survival via inhibiting progression of the leukemia. We hypothesize this effect resulted from direct competition for physical niche occupancy, given the similar effect of purified LSK cells and whole bone marrow; however, the findings could also result from competition for supportive cytokines, or the production of a factor by normal HSPC able to inhibit leukemia cell growth or survival. Whole sternal imaging early after transplanting the cells demonstrated that discrete foci consisted of either leukemic cells or normal hematopoiesis, suggesting niche occupancy by one stem/progenitor type or the other, but not both simultaneously.
Cycling of leukemic cells was lower early after transplantation in the presence of higher HSPC doses, suggesting a limitation of leukemic expansion in the presence of more competitor HSPC, we hypothesize due to lack of available niches to support leukemic cells expansion/self renewal in the presence of high HSPC doses. At later time points, once the leukemia was established in all dose groups, there was actually somewhat higher cycling of the leukemic cells in animals receiving higher HSPC doses. We hypothesize this finding may reflect localization of leukemic cells outside niches, with cycling cells perhaps less effective at spread to other tissues. Most importantly, animals receiving higher HSPC doses, whether whole bone marrow or purified HSPC, survived longer, with all HSPC doses utilized beyond those required for initial survival and hematopoietic reconstitution in the absence of leukemic cells. Longer survival was likely linked to less leukemic organ infiltration and more sustained production of normal hematopoietic cells to prevent infection and bleeding in the groups receiving higher versus lower doses of normal HSPC.
A recent publication demonstrated similar niche competition between normal and myeloid leukemia cells in a xenograft model, showing dose-dependent cord blood HSC competitive inhibition of engraftment and potency of co-transplanted human acute myeloid leukemia cells.[22] Most interestingly is that cytokine-induced mobilization of leukemia initiating cells from the marrow in this model facilitated replacement of leukemic cells with normal HSPC, an observation with potential therapeutic importance. However, no prior studies have reported similar findings outside a xenograft model, where niche interactions with either normal HSPC or leukemic cells across species barriers may be abnormal. Our findings, along with those of Boyd et al, suggest that manipulations designed to favor normal HSPC niche occupancy over that of leukemic cells may be therapeutically beneficial.
Since the concept of a marrow or stem cell niche was introduced over thirty years ago [1], investigators have focused on the basic mechanisms of interaction between HSPCs and the marrow microenvironment and the clinical relevance of these relationships, particularly as applied to mobilization or engraftment of HSPCs in the transplantation setting.[3] Niche functional capacity has been demonstrated to be finite, and niche occupancy a limiting factor in transplantation of normal HSCs.[23, 24] More recently, sophisticated imaging studies have localized normal murine HSCs to marrow endosteal regions, with the most functionally-robust cells present in the trabecular metaphysis as compared to non-trabecular long bone areas.[5–7] Much less has been known about the relationship between leukemic cells and marrow niches.[10] An elegant study focused on the intricate interaction between the marrow niche and the self-renewal capability of HSPC, specifically in the setting of leukemia.[25] They found that self-renewal potency of the most primitive HSC can be reversibly suppressed by the leukemic microenvironment in a Notch1-induced T-ALL model, despite proliferation and exhaustion of more mature progenitors. These HSC can re-enter cell cycle and self-renew when transplanted into a non-leukemic host.
Do leukemias replace normal marrow function via indirect impact on the niches occupied by normal HSPC, occupying separate niches, or do they share and thus compete for the same niches? These questions have clinical relevance, particularly in the context of the concept of leukemic stem cells, and resistance to cytotoxic therapies, potentially via niche protection.[26] Several reports found similar distribution patterns for normal human CD34+ cord blood cell and human primary acute myeloid leukemia blasts in murine xenografts, with both cell types accumulating in cancellous trabecular bone, closely adjacent to endosteum.[22] [13] It is interesting to note that the type of leukemia may determine the specific niche occupied: for instance in one report, murine chronic BCR-ABL-induced chronic myeloid leukemic cells occupied different niches than MLL-AF9-induced acute myeloid leukemia cells, with osteoblastic stimulation enhancing the acute myeloid leukemia but inhibiting the chronic myeloid leukemia.[11] The concept that leukemic cells can alter marrow niches has also been supported via studies demonstrating that human acute lymphoid leukemia (ALL) cells actually alter the localization of normal human CD34+ cells via secretion of cytokines, and that ALL cells can stimulate formation of walled-off abnormal niches.[15, 16]
These findings have a number of important potential clinical implications. Outcomes following allogeneic hematopoietic stem cell transplants from HLA-matched donors were studied in relationship to CD34+ cell dose in patients with primarily myeloid leukemias, and leukemia relapse was significantly lower in patients transplanted with high versus low CD34+ dosages in two very large registry studies.[27, 28] This effect did not appear to be due to more robust graft-versus-leukemia effects with higher cell doses. This dose effect was not seen in human autologous transplantation for AML, presumably due to proportional increases in both normal and leukemic cells in the setting of transplanting autologous marrow, even harvested from a patient in a morphologic complete remission.[29] Human allogeneic transplants do not run the risk of co-transplantation with leukemic cells, in contrast to our murine studies. However, normal HSPC and presumably leukemic cells cycle in and out of niches, leaving a proportion of niches empty at any given moment.[30] Therefore it is reasonable to hypothesize that at least one explanation for the impact of CD34+ cell dose on leukemic relapse could be niche competition, and high cell dose allografts should be utilized if possible, at least for myeloid leukemias.
Pharmacologic or antibody-mediated emptying of niches just prior to transplantation may increase the likelihood that infused normal HSCs will have a chance to outcompete residual endogenous leukemic cells.[8, 9] Boyd and coworkers demonstrated the potential of this approach experimentally in their xenograft model, and their result and our own support further translational development of this concept.[22] It is unknown whether our findings would be relevant in a non-myeloablative setting: it is possible that irradiation damages niches and makes niche availability a limiting factor only in this setting. Performing additional studies in the setting of a stem cell deficient murine strain such as W (c-kit mutant) mice would be of interest. Finally, our results may help explain the occurrence of cytopenias in some patients long before overt leukemia manifests in the peripheral blood. Normal HSCs may already be displaced or inhibited by leukemic cells in marrow niches.
Supplementary Material
Significance Statement.
Our study demonstrated that normal mouse bone marrow cells functionally compete with MLL-AF9 myeloid leukemia-initiating cells for physical niche occupancy. No prior studies have reported similar findings outside a xenograft model, where niche interactions with either normal HSPC or leukemic cells across species barriers may be abnormal. Much less has been known about the relationship between leukemic cells and marrow niches. Do leukemias replace normal marrow function via an ability function outside of a specific and limited supportive niche, or do they occupy specific niches that are shared or completely different from those harboring normal stem cells? By following these questions, our study has clinical relevance, particularly in the context of the concept of leukemic stem cells, and resistance to cytotoxic therapies, potentially via niche protection.
Acknowledgments
Research Support: This project was funded by the intramural programs of the National Heart, Lung, and Blood Institute and the National Human Genome Research Institute.
This work was funded by the intramural programs of the National Heart, Lung, and Blood Institute and the National Human Genome Research Institute. The authors thank the NHLBI Light Microscopy Core, and the Hematology Branch flow cytometry facility and Keyvan Keyvanfar for his valuable assistance.
Footnotes
Authorship contributions:
Chen Glait-Santar: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
Ronan Desmond: conception and design, collection and assembly of data, data analysis and interpretation
Xingmin Feng: collection and assembly of data, data analysis and interpretation
Taha Bat: collection and assembly of data
Jichun Chen: collection and assembly of data
Elisabeth Heuston: provision of study material
Benjamin Mizukawa: provision of study material
James Mulloy: provision of study material, data interpretation
Andre Larochelle: conception and design, data analysis and interpretation
David Bodine: provision of study material
Cynthia Dunbar: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Frassoni F, Testa NG, Lord BI. The relative spatial distribution of erythroid progenitor cells (BFUe and CFUe) in the normal mouse femur. Cell Tissue Kinet. 1982;15:447–455. doi: 10.1111/j.1365-2184.1982.tb01062.x. [DOI] [PubMed] [Google Scholar]
- 3.Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 6.Nombela-Arrieta C, Pivarnik G, Winkel B, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nature cell biology. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guezguez B, Campbell CJ, Boyd AL, et al. Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell Stem Cell. 2013;13:175–189. doi: 10.1016/j.stem.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Czechowicz A, Kraft D, Weissman IL, et al. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Larochelle A, Fricker S, et al. Mobilization as a preparative regimen for hematopoietic stem cell transplantation. Blood. 2006;107:3764–3771. doi: 10.1182/blood-2005-09-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114:1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krause DS, Fulzele K, Catic A, et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. 2013;19:1513–1517. doi: 10.1038/nm.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sipkins DA. Rendering the leukemia cell susceptible to attack. N Engl J Med. 2009;361:1307–1309. doi: 10.1056/NEJMcibr0904291. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa F, Yoshida S, Saito Y, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 14.Ninomiya M, Abe A, Katsumi A, et al. Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia. 2007;21:136–142. doi: 10.1038/sj.leu.2404432. [DOI] [PubMed] [Google Scholar]
- 15.Colmone A, Amorim M, Pontier AL, et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 16.Duan CW, Shi J, Chen J, et al. Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell. 2014;25:778–793. doi: 10.1016/j.ccr.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Mizukawa B, Wei J, Shrestha M, et al. Inhibition of Rac GTPase signaling and downstream prosurvival Bcl-2 proteins as combination targeted therapy in MLL-AF9 leukemia. Blood. 2011;118:5235–5245. doi: 10.1182/blood-2011-04-351817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyama S, Schibler J, Cunningham L, et al. Transcription factor RUNX1 promotes survival of acute myeloid leukemia cells. J Clin Invest. 2013;123:3876–3888. doi: 10.1172/JCI68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogart A, Lichtenberg J, Ajay SS, et al. Genome-wide DNA methylation profiles in hematopoietic stem and progenitor cells reveal overrepresentation of ETS transcription factor binding sites. Genome research. 2012;22:1407–1418. doi: 10.1101/gr.132878.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takaku T, Malide D, Chen J, et al. Hematopoiesis in 3 dimensions: human and murine bone marrow architecture visualized by confocal microscopy. Blood. 2010;116:e41–55. doi: 10.1182/blood-2010-02-268466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malide D, Metais JY, Dunbar CE. Dynamic clonal analysis of murine hematopoietic stem and progenitor cells marked by 5 fluorescent proteins using confocal and multiphoton microscopy. Blood. 2012;120:e105–116. doi: 10.1182/blood-2012-06-440636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd AL, Campbell CJ, Hopkins CI, et al. Niche displacement of human leukemic stem cells uniquely allows their competitive replacement with healthy HSPCs. J Exp Med. 2014;211:1925–1935. doi: 10.1084/jem.20140131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart FM, Crittenden RB, Lowry PA, et al. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood. 1993;81:2566–2571. [PubMed] [Google Scholar]
- 24.Colvin GA, Lambert JF, Abedi M, et al. Murine marrow cellularity and the concept of stem cell competition: geographic and quantitative determinants in stem cell biology. Leukemia. 2004;18:575–583. doi: 10.1038/sj.leu.2403268. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Shen H, Tian C, et al. Kinetics of normal hematopoietic stem and progenitor cells in a Notch1-induced leukemia model. Blood. 2009;114:3783–3792. doi: 10.1182/blood-2009-06-227843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 27.Rocha V, Labopin M, Gluckman E, et al. Relevance of bone marrow cell dose on allogeneic transplantation outcomes for patients with acute myeloid leukemia in first complete remission: results of a European survey. J Clin Oncol. 2002;20:4324–4330. doi: 10.1200/JCO.2002.11.058. [DOI] [PubMed] [Google Scholar]
- 28.Ringden O, Barrett AJ, Zhang MJ, et al. Decreased treatment failure in recipients of HLA-identical bone marrow or peripheral blood stem cell transplants with high CD34 cell doses. Br J Haematol. 2003;121:874–885. doi: 10.1046/j.1365-2141.2003.04364.x. [DOI] [PubMed] [Google Scholar]
- 29.Gorin NC, Labopin M, Reiffers J, et al. Higher incidence of relapse in patients with acute myelocytic leukemia infused with higher doses of CD34+ cells from leukapheresis products autografted during the first remission. Blood. 2010;116:3157–3162. doi: 10.1182/blood-2009-11-252197. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharya D, Czechowicz A, Ooi AG, et al. Niche recycling through division-independent egress of hematopoietic stem cells. J Exp Med. 2009;206:2837–2850. doi: 10.1084/jem.20090778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.