Abstract
Objective
Combination antiretroviral therapy (cART) can suppress plasma HIV RNA to undetectable levels; yet reports indicate persistent HIV-associated neurocognitive disorders (HAND) among treated individuals. We sought to investigate imaging correlates of incomplete cognitive recovery among individuals with chronic HIV.
Methods
We used single voxel proton magnetic resonance spectroscopy (MRS) in four brain regions to measure changes in neuronal and glia biomarkers in cART-naïve subjects before (n=59, 27 with HAND) and after 12 months of cART.
Results
At baseline we observed elevated total choline (CHO) in the basal ganglia (BG, p=0.002) and in the posterior cingulate gyrus (PCG, p=0.022) associated with HIV-infection. Myo-inositol (MI) was elevated in the frontal white matter (FWM, p=0.040). N-acetylaspartate (NAA) was elevated in the BG (p=0.047). Using a mixed model approach among all HIV-infected individuals at 6 months, we observed decreased NAA in FWM (p =0.031), decreased creatine (CR) in PCG (p=0.026) and increased MI in FGM (p=0.023). At 12 months, we observed an increase in BG MI (p=0.038) and in FGM (p=0.021). Compared to those with normal cognition, HAND cases had higher FGM MI (p=0.014) at baseline. At 12 months, individuals that remained cognitively impaired compared to those without HAND exhibited elevated CHO in the PCG (p=0.018) and decreased GLU in both FWM (p=0.027) and BG (p=0.013).
Conclusions
cART started during chronic HIV is associated with reduced neuronal-glia and inflammatory markers. Alterations in CHO are noted among individuals who remain impaired after 12 months of cART.
Keywords: HIV, Magnetic Resonance Spectroscopy, Cognitive Disorders
INTRODUCTION
Central nervous system (CNS) dysfunction is a common characteristic of human immunodeficiency virus (HIV) infection. The underlying neuropathology is largely related to the indirect consequence of disrupted glial function since HIV does not substantially infect neurons. Instead, brain macrophages, microglia, and multinucleated giant cells become infected and instigate a reactive astrocytosis.1 Subsequent brain injury likely ensues from both toxic HIV particles and toxins from activated macrophages and astrocytes resulting in cognitive impairment as evidenced by neuropsychological testing abnormalities. Even in the era of combination antiretroviral therapy (cART) the prevalence of HIV-associated neurocognitive disorders (HAND) remains substantial.2–4 Whether these chronic deficits are due to the same mechanisms as seen in untreated HIV is incompletely understood and may be informed by non-invasive measures of glial function and CNS inflammation, as is possible with proton magnetic resonance spectroscopy (MRS).
Abnormal brain chemical concentrations in the basal ganglia (BG), frontal white matter (FWM), frontal gray matter (FGM) and the posterior cingulate gyrus (PCG) have been described in HIV.5–8 Clinical studies demonstrate abnormal axonal and neuronal markers in HIV-infected individuals with cognitive impairment and other neurological symptoms.9–11 Our earlier prospective study of the same cART-naïve chronically HIV-infected Thai subjects examined in the current work revealed associations between reservoir levels of HIV DNA in peripheral blood mononuclear cells (PBMC) enriched with those having the CD14+ cell surface marker (monocytes) and MRS abnormalities.12 In the current study, we examine MRS abnormalities associated with HIV in cART-naïve subjects and those associated with HAND. We also examined changes in MRS that were associated with cART initiation over 12 months in order to assess if MRS abnormalities could be identified in association with continued HAND despite 12 months of continuous cART.
MATERIALS AND METHODS
Participants
HIV-infected adults were enrolled at the SEARCH clinic of the Thai Red Cross AIDS Research Center in Bangkok, Thailand (protocol SEARCH 011, www.ClinicalTrials.gov NCT00782808); details are previously described.12 All met Thai Ministry Public Health criteria for initiating cART (CD4 count < 350 cells/mm3 or symptomatic disease). We enrolled participants using a stratified scheme for PBMC HIV DNA (>/< 1000 copies of HIV DNA/106 cells) and age (>/< 35 years), based on a predetermined blinded randomization scheme.13 HIV-uninfected adults were recruited in a separate normative imaging study (SEARCH 009) capturing one-time MRS data on 28 controls. HIV-infected individuals (n=59) received baseline, 6 months, and 12 months MRS, of whom 51 completed all three visits. All provided written consents approved by the Institutional Review Boards at the Chulalongkorn University in Bangkok and the University of California, San Francisco.
Cognitive assessments
HIV-infected participants completed a 60-minute battery of neuropsychological tests that included the WHO-UCLA Auditory Verbal Learning Task, Color Trails 1 and 2, Digit Symbol Modalities Test, Block Design Tasks, Grooved Pegboard for both hands, finger tapping for both hands, Timed Gait, two verbal fluency tasks (first names and animals) and the Trail Making Test A.14 We conducted consensus conferences with two of the authors (VV, RP) and a US neurologist to assign HAND diagnoses using 2007 Frascati criteria.15 We calculated a composite neuropsychological testing score (NPZ- global) as the arithmetic mean of age- and education-adjusted z-scores for performance on individual tests using normative data from over 500 Thai controls16.
Brain proton MRS
All completed axial 3D T1-weighted spoiled gradient echo MRI (TE = 7ms, TR = 11.2ms, 1mm resolution) on the same 1.5T GE MRI scanner using the same software throughout the study duration (GE Healthcare, software v12.0). An 8-channel head coil and standard body coil was used. Eight cubic centimeters voxels were placed in the normal appearing brain regions in the right BG and FWM, midline frontal gray matter (FGM) and PCG using a double spin echo data acquisition (Probe-p) (TE/TR=35/1500, number of excitations of 128 for FWM, FGM and PCG and 192 for the BG).
Voxels were carefully placed by an experienced technologist (MP) by visual inspection of the MRI screen shot of the prescription as closely monitored by our physicist (NS). The average voxel placement was consistent throughout the study with approximately 4.0 ± 0.5 mm differences between baseline, 6 and 12-month follow-up. We employed commercially available time domain fitting software (LCModel version 6.2.2) to quantify brain metabolites.17 An estimate of the variance associated (Cramer-Rao lower bounds) with time domain fitting provided by the software was used to determine reliability of the fitting and value of less than 15% was used to accept the fitting results for NAA, Cr, CHO and less than 25% is accepted for GLU.18 Brain chemical concentrations are reported in mM uncorrected for T1, T2 relaxation times and contribution of gray and white matters in each voxel. In each voxel, we measured total choline (CHO), myo-inositol (MI), glutamate (GLU), creatine (CR), and n-acetyl aspartate (NAA). The same MRS head phantom measurements were acquired at the end of each examination. The quantitative results of the MRS phantom were consistent and reproducible throughout the 12-months.
Statistical analyses
The statistical analysis was conducted by first assessing the descriptive statistics for all MRS measures at all study time points by subject’s HIV status (HIV infected vs. uninfected healthy controls). Two-sample t-test was used to assess the mean differences of MRS measures between healthy controls and HIV positive subjects. Paired t-test was used to examine the mean differences of MRS measures between baseline and each of the subsequent follow-up visits. The statistical tests were not corrected for multiple comparisons.
A mixed effect model was used to examine how the MRS measures change over time following initiation of treatment. Multivariate analyses were performed by adding age, gender, years of education and time-varying (CD4, plasma VL) as covariates in the mixed models. Pearson’s correlation coefficients were used to examine the correlation between MRS and clinical measures. The level of significance was p< 0.05. Data were analyzed using SAS version 9.2 and SPSS version 17.0 (IBM Corporation, USA).
RESULTS
Group composition
The HIV-infected and uninfected participants were similar in sex and age with mean ages of 35 and 34 years, respectively. Among HIV-infected participants, baseline mean CD4 t-lymphocyte count was 233 cells/ mm3 and the mean log10 plasma HIV RNA was 4.83 copies/ml prior to cART. After 6 and 12 months of treatment, the mean CD4 t-lymphocyte count increased to 371 and 412 cells/mm3, respectively while the log10 plasma HIV RNA levels decreased to 1.84 and 1.70, respectively (Table 1). All but three cases were undetectable (lower level of detection 50 copies/ml) at 12 months (log10 plasma HIV RNA of 2.22, 2.45, and 2.10, reduced from baseline log10 plasma HIV RNA levels of 5.64, 5.34, and 4.93 respectively). At baseline, 27 of the 59 HIV-infected participants were diagnosed with HAND (46%), including five with HIV-associated Dementia (HAD), 8 with Mild Neurocognitive Disorder (MND) and 14 with Asymptomatic Neurocognitive Impairment (ANI). All but one of the 59 HIV-infected participants was started on an NNRTI-based regimen, with the latter starting a PI-based regimen. During the first year of treatment, 12 subjects switched to an alternative regimen owing to adverse drug reactions, resistance, pregnancy, or co-enrollment in another study.
Table 1.
Participant demographics.
| HIV-infected | HIV-uninfected | p | ||
|---|---|---|---|---|
| N | 59 | 28 | ||
| Mean (SD) age, years | 35.0 (6.9) | 34.3 (6.4) | 0.47 | |
| Gender, M/F | 25/34 | 15/13 | 0.22 | |
| Education, years | 12(4.5) | 14(5.2) | 0.07 | |
| Mean/Median (SD) CD4 count cells/mm3 | ||||
| at enrollment | 233 | |||
| at 6 months | 371 | |||
| at 12 months | 412 | |||
| Mean (SD) log10 plasma HIV RNA | ||||
| at enrollment | 4.83 | |||
| at 6 months | 1.84 | |||
| at 12 months | 1.70 | |||
Cross-sectional baseline brain metabolites before treatment
We observed group difference between HIV-infected and uninfected participants in the FWM with HIV-infected participants having a higher MI (p=0.040) and CHO (p=0.002) in the BG and PCG (p=0.022) relative to HIV-uninfected participants. We also noted lower NAA in the PCG (weakly significant with p=0.051) and higher NAA in the BG (p=0.047) in the HIV-infected group (see Table, Supplemental Digital Content 1).
Effects of cART on brain metabolites
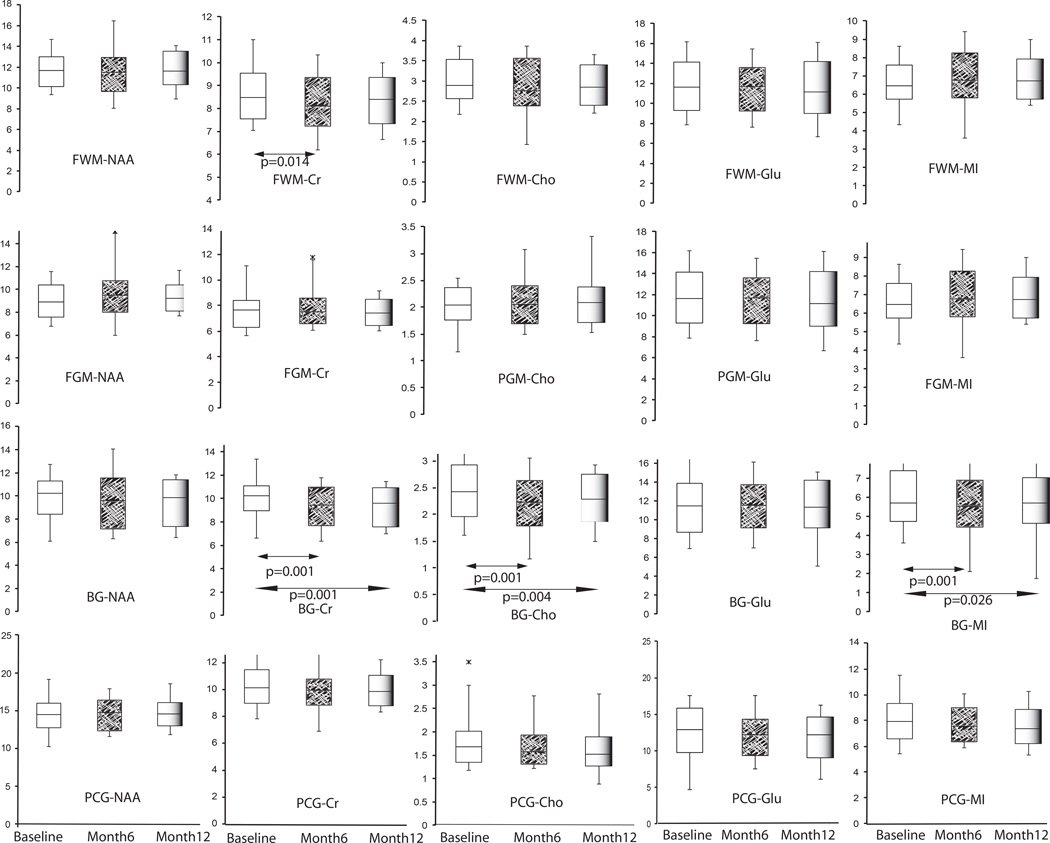
Longitudinal brain metabolite changes from pre-cART through 6 months and 12 months after the initiation of cART treatment were measured (See Figure 1). We observed a pattern of reduced inflammatory markers after 6 months of cART in the BG with statistical significance reached for decreased CR (p=0.001), CHO (p=0.001) and MI (p=0.012). After 12 months of treatment further reduction of CR (p=0.001), CHO (p=0.004) and MI (p=0.026) was observed in the BG compared to baseline. Additionally, CR was significantly decreased after 6 months of treatment in the FWM (p=0.014). There were no significant changes in NAA. Compared to uninfected controls, only CR (p=0.012) and GLU (p=0.012) were significantly lower among HIV-infected participants at 12 months. No other metabolite/voxel pairs differed at 12 months compared to controls.
Figure 1.
Longitudinal changes in MRS measures across the three time points (pre-cART, 6 months, and 12 months post cART) in HIV-infected subjects. A paired t-test is used with box plot with whiskers at 10th and 90th percentile.
Further analysis using a multivariate mixed effect model revealed changes in brain metabolites between baseline and 6 months after cART treatment (estimated coefficient) including decreased NAA (est. coef =−0.94, p=0.031) in the FWM and CR in the PCG (est. coef. =−0.73, p=0.026). We noted an increase in MI from the FGM between baseline and 6 months (est. coef. =0.39, p=0.022) and 12 months (est. coef. = 0.40, p=0.021). We also noted an increase in BG MI at 12 months compared to baseline (est. coef. =0.99, p=0.038) (see Table, Supplemental Digital Content 2).
Effects of clinical measures on brain metabolites
At baseline, there were direct correlations between plasma HIV RNA and MI in the BG (r=0.358, p=0.005) and the FGM (r=0.282, p=0.031). There were modest indirect correlations to the FWM NAA (r=−0.261, p=0.046). The pre-cART CD4 t-lymphocyte count indirectly correlated modestly with MI in the BG (r=−0.322, p=0.013) and FWM (r=−0.263, p=0.044). In separate analyses, a diagnosis of HAD at baseline (n=5) had a strong positive statistically significant correlation with BG Cr (r=0.972, p=0.006) and CHO (r=0.889, p=0.043). Further comparison among HIV-infected participants with normal cognition and those with HAND revealed increased MI in the FGM (p=0.014) at baseline. At 12 months participants who remained cognitively impaired showed increased CHO in the PCG (p=0.018) and decreased GLU in the FWM (p=0.027) and BG (p=0.013) (Table 2).
Table 2.
MRS measures from the four brain locations by impairment status at baseline and 12 months.
| BASELINE | 12 MONTHS | |||||
|---|---|---|---|---|---|---|
| HIV+/HAND− (N=32) |
HIV+/HAND+ (N=27) |
p-value | HIV+/HAND− (N=41) |
HIV+/HAND+ (N=9) |
p-value | |
| Choline | ||||||
| FWM | 2.91 (0.32) | 3.04 (0.43) | ns | 2.89 (0.38) | 2.92 (0.31) | ns |
| FGM | 2.03 (0.26) | 2.05 (0.25) | ns | 2.13 (0.31) | 2.02 (0.37) | ns |
| BG | 2.41 (0.33) | 2.43 (0.36) | ns | 2.27 (0.34) | 2.30 (0.46) | ns |
| PCG | 1.67 (0.43) | 1.72 (0.25) | ns | 1.54 (0.25) | 1.80 (0.43) | p=0.018 |
| N-acetyl aspartate | ||||||
| FWM | 11.91 (1.06) | 11.60 (1.26) | ns | 11.82 (1.31) | 11.27 (1.15) | ns |
| FGM | 9.15 (1.21) | 8.90 (1.22) | ns | 9.42 (0.83) | 9.25 (1.01) | ns |
| BG | 10.14 (1.19) | 9.87 (1.38) | ns | 9.63 (1.54) | 9.04 (1.48) | ns |
| PCG | 14.39 (1.45) | 14.30 (1.67) | ns | 14.65 (1.24) | 14.86 (1.44) | ns |
| Myo-inositol | ||||||
| FWM | 8.91 (1.62) | 8.90 (2.17) | ns | 8.24 (1.21) | 7.98 (0.89) | ns |
| FGM | 6.43 (0.67) | 6.97 (0.98) | p=0.014 | 6.85 (0.89) | 6.84 (0.64) | ns |
| BG | 5.92 (1.19) | 6.38 (1.68) | ns | 5.74 (1.17) | 5.41 (0.83) | ns |
| PCG | 7.71 (1.02) | 8.18 (1.20) | ns | 7.56 (1.09) | 7.22 (1.17) | ns |
| Glutamate | ||||||
| FWM | 10.31 (1.53) | 10.47 (2.02) | ns | 10.55 (1.92) | 8.96 (1.80) | p=0.027 |
| FGM | 11.75 (1.83) | 11.32 (2.11) | ns | 11.71 (2.00) | 10.87 (2.03) | ns |
| BG | 5.92 (1.19) | 6.38 (1.68) | ns | 11.82 (1.94) | 9.88 (2.48) | p=0.013 |
| PCG | 7.71 (1.02) | 8.18 (1.20) | ns | 13.00 (1.97) | 12.96 (1.20) | ns |
| Creatine | ||||||
| FWM | 8.43 (0.57) | 8.61 (1.00) | ns | 8.28 (0.84) | 8.22 (0.75) | ns |
| FGM | 7.43 (0.98) | 7.65 (0.92) | ns | 7.56 (0.77) | 7.19 (0.75) | ns |
| BG | 10.26 (1.04) | 10.05 (1.02) | ns | 9.59 (1.11) | 8.87 (1.34) | ns |
| PCG | 10.03 (1.07) | 10.45 (1.02) | ns | 9.80 (0.92) | 10.32 (0.54) | ns |
FWM=frontal white matter; FGM=frontal grey matter, BG=basal ganglia; PCG=posterior cingulate gyrus Cr=creatine; Glu= glutamate, MI=myo-inositol; NAA=N-acetylaspartate, Cho=total choline
Correlation between changes in NPZ-global score and changes in brain metabolites revealed that decreased BG CHO from baseline to 12 months was correlated with improvement on the NPZ-global (r=−0.32, p=0.023). A decrease in MI also correlated with improved NPZ-global in the FWM (r=−0.43, p=0.002) and BG (r=−0.42, p=0.002) (See Table, Supplemental Digital Content 3). There were no significant correlations between changes in NPZ-global score and changes in brain metabolites from baseline to 6 months.
DISCUSSION
Several studies, including our recent studies, have demonstrated persistent and continued neuronal injury in treated HIV patients using proton MRS and MRI12, 19–21. Risk factors associated with neuronal injury and cognitive impairment includes plasma HIV DNA and pre-cART CD4 t-lymphocyte cell counts. There remain large gaps in our understanding of the underlying mechanism that contributes to the persistent injury despite cART.
This study identified brain chemical differences between pre-cART HIV-infected subjects and controls and between HIV-infected subjects with and without HAND post-cART. The most robust baseline associations were with clinical parameters of plasma HIV RNA and CD4 t-lymphocyte cell counts at baseline. The elevated CHO noted at baseline between HIV-infected compared to uninfected subjects is consistent with expectations, supporting an inflammatory phenotype with untreated chronic HIV. In a preclinical study, elevated CHO/CR was observed in the frontal brain of SIV macaques at the time of peak viremia indicting early neuroinflammatory processes22. In human studies, elevated CHO/Cr has been documented in international settings21, 23–25 and in the US populations26–29. However, in similar but smaller studies, Suwanwelaa et al observed no statistical difference of CHO/CR in asymptomatic HIV-infected participants,30 while Winston et al. observed lower CHO/CR in HIV-infected participants prior to cART treatment compared to healthy volunteers31. One possible reason for this discrepancy is the assumption that CR is stable in HIV, an assumption that is not supported by our current and past data.
A pattern of improved CHO was observed across multiple voxels, and seemed to occur as early as 6 months, demonstrating more rapid recovery, which is consistent with findings from a recently reported multi-center study that identified normalization of CHO over two years.32 At 12 months, we noted no evidence of continued elevation of CHO with levels that were similar to controls across all voxels.
Creatine, a combination of creatine and phosphocreatine measured with proton MRS, is a metabolite associated with cell energy metabolism. It is widely considered to be unchanged in numerous brain pathologies and is often used as an internal reference with metabolites, which are reported as a ratio to the presumed stable CR.33–34 We observed a decrease in CR in multiple brain regions over time in these participants who initiated cART. Chang et. al. reported a similar finding in the frontal cortex of HIV-infected participants.35 The level of CR in the brain is in equilibrium with phosphocreatine via the creatine kinase enzymes activity.36 Our patients were treated with NNRTIs, which has been linked to reduced creatine kinase activity, raising concerns that they impact MRS CR; thus, it should not be considered to remain stable in this scenario.37–38 Alternatively, it has been hypothesized that changes in total CR levels are associated with intense neuronal activity and failure of energy production.39 High energy demands of infected monocytes during the early stage of HIV infection could persist in chronic HIV infection.40
Myoinositol is present mainly in glial cells and considered to be an important brain osmolyte.41 It is only observable at a short echo time MRS, as used in our study. Elevated MI has been reported at various stages of HIV infection.10, 42 In the present study, elevated MI in the FWM and slightly elevated MI in the BG at baseline reflected microglial activation during the early stage of HIV infection and tend to normalize after 12 months of treatment. This normalization suggests reduced inflammation and improved brain repair mechanisms after cART.
Unsettled issues remain around cognitive impairment in cART treated subjects, with increasing evidence of contributions from non-HIV-related comorbidity as well as HIV. In the current study, we demonstrated improved neuropsychological testing performance with cART and associations between HAND and MRS parameters. This provides further evidence of inflammatory pathogenesis to HAND. Among those who continued to have impairment at 12 months, we continued to see elevated CHO at PCG, providing evidence that, at least among a subset of subjects, inflammation is incompletely resolved with cART.
Glutamatergic neurotransmission has been associated with the pathophysiology of cognitive dysfunction in several brain conditions including HAND.43–46 Glutaminase is the enzyme that catalyzes the conversion of glutamine to glutamate and its activity has been shown to inhibit glutamate production in HIV.47 Reduced GLU may reflect mitochondria damage38, 48, enhanced synthesis of anti-oxidant49–50 or glial injury47, 51. In the longitudinal component of our study, we observed lower GLU in FWM and BG among impaired compared to unimpaired subjects at 12 months. It is important to point out that several studies exist documenting the difficulty in sorting out GLU signal without contamination of the overlapped glutamine (GLN) signal at 1.5T8, 52–53. Using short echo time proton MRS, the unresolved GLU and GLN resonances are readily reported as GLX, a combination of GLU and GLN. Previous studies reported decreased GLX in FWM in cognitively impaired HIV-infected subjects54 while increased GLX was observed in the BG in HIV participants who were on stable cART55. There are several factors that contribute to this discrepancy, which may include the scanner field strength (1.5T vs. 3T). Mohamed et al argued that changes in GLX might be due to dysfunction in the glutamate-glutamine cycle, therefore it is uncertain that increased GLX is a direct result of increase GLU or both GLU and GLN. Using a more rigorous MRS approach to measure the uncontaminated GLU signal at 3T, reduced GLU was also observed in the FWM of cognitively normal HIV-infected participants8.
In the present study, the standard deviation for the GLU metabolite quantification using the LCModel analysis was > 20% (range 9–24%) in 8% of our results at 12 months. Therefore, we adopted criteria to include results in the final analysis of < 25%, which is higher than generally accepted (< 20%)%18. This practice is not unusual and has been used in several MRS studies56–58. We also noted that by not having follow up examinations in the uninfected participants, we might have missed some inherent variability in our measurement. However, there were no significant changes observed in MRS phantom measurements acquired at the end of each examination.
In summary, we identified evidence of CNS inflammation in HIV prior to cART and among those who fail to improve cognitively at 12 months. MRS inflammatory markers are linked to important clinical measures pre-cART including plasma HIV RNA, and neuropsychological testing improvement is associated with improvement in these MRS markers.
Supplementary Material
ACKNOWLEDGEMENTS
We thank our study participants and the SEARCH 011 study group particularly the following members from Thailand: Pradit Chaisit, Yothin Chinvarun, Phayao Thongkramjaroen, Sumit Thongmuang, Sirichai Jarupittaya, Prapian Khongtia, Pornpen Methajittiphun, Pravit Mingkwanrungruang, Nittaya Phanuphak, Eugene Kroon, Nipat Terratakulpisarn, Nitiya Chomchey, Duanghathai Suttichom, Somprartthana Rattanamanee, Michittra Boonchan, Ratchapong Kanaprach and from UCSF: Elijah Mun, Nicholas Hutchings, Katherine Clifford, and Lauren Wendelken. The authors also thank Isabel Allen and Jiahong Xu for statistical assistance.
Footnotes
Disclaimer: The National Institute of Neurological Disorders and Stroke of the National Institutes of Health supported Research reported in this publication under Awards Number R01 NS061696 and R01 NS053359. The views expressed are those of the authors and should not be construed to represent the positions of the National Institutes of Health, the U.S. Army or the Department of Defense.
REFERENCES
- 1.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005 Jan;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 2.Garvey L, Winston A, Walsh J, et al. HIV-associated central nervous system diseases in the recent combination antiretroviral therapy era. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2011 Mar;18(3):527–534. doi: 10.1111/j.1468-1331.2010.03291.x. [DOI] [PubMed] [Google Scholar]
- 3.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007 Jun 1;45(2):174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 4.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010 Dec 7;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker PB, Lee RR, McArthur JC. AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology. 1995 Apr;195(1):58–64. doi: 10.1148/radiology.195.1.7892496. [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Lee PL, Yiannoutsos CT, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004 Dec;23(4):1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 7.Lentz MR, Kim WK, Kim H, et al. Alterations in brain metabolism during the first year of HIV infection. Journal of neurovirology. 2011 Jun;17(3):220–229. doi: 10.1007/s13365-011-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sailasuta N, Shriner K, Ross B. Evidence of reduced glutamate in the frontal lobe of HIV-seropositive patients. NMR Biomed. 2009 Apr;22(3):326–331. doi: 10.1002/nbm.1329. [DOI] [PubMed] [Google Scholar]
- 9.Menon DK, Baudouin CJ, Tomlinson D, Hoyle C. Proton MR spectroscopy and imaging of the brain in AIDS: evidence of neuronal loss in regions that appear normal with imaging. J Comput Assist Tomogr. 1990 Nov-Dec;14(6):882–885. doi: 10.1097/00004728-199011000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Laubenberger J, Haussinger D, Bayer S, et al. HIV-related metabolic abnormalities in the brain: depiction with proton MR spectroscopy with short echo times. Radiology. 1996 Jun;199(3):805–810. doi: 10.1148/radiology.199.3.8638009. [DOI] [PubMed] [Google Scholar]
- 11.Tracey I, Carr CA, Guimaraes AR, Worth JL, Navia BA, Gonzalez RG. Brain choline-containing compounds are elevated in HIV-positive patients before the onset of AIDS dementia complex: A proton magnetic resonance spectroscopic study. Neurology. 1996 Mar;46(3):783–788. doi: 10.1212/wnl.46.3.783. [DOI] [PubMed] [Google Scholar]
- 12.Valcour VG, Ananworanich J, Agsalda M, et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One. 2013;8(7):e70164. doi: 10.1371/journal.pone.0070164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sungkanuparph S, Techasathit W, Utaipiboon C, et al. Thai national guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2010. Asian Biomedicine. 2010 Aug;4(4):515–528. [Google Scholar]
- 14.Valcour VG, Sithinamsuwan P, Nidhinandana S, et al. Neuropsychological abnormalities in patients with dementia in CRF 01_AE HIV-1 infection. Neurology. 2007 Feb 13;68(7):525–527. doi: 10.1212/01.wnl.0000253196.78193.c7. [DOI] [PubMed] [Google Scholar]
- 15.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007 Oct 30;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaps J, Valcour V, Chalermchai T, et al. Development of normative neuropsychological performance in Thailand for the assessment of HIV-associated neurocognitive disorders. Journal of clinical and experimental neuropsychology. 2013;35(1):1–8. doi: 10.1080/13803395.2012.733682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001 Jun;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 18.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993 Dec;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 19.Sailasuta N, Ross W, Ananworanich J, et al. Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. PLoS One. 2012;7(11):e49272. doi: 10.1371/journal.pone.0049272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen RA, Harezlak J, Gongvatana A, et al. Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. J Neurovirol. 2010 Nov;16(6):435–444. doi: 10.3109/13550284.2010.520817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cysique LA, Moffat K, Moore DM, et al. HIV, vascular and aging injuries in the brain of clinically stable HIV-infected adults: a (1)H MRS study. PLoS One. 2013;8(4):e61738. doi: 10.1371/journal.pone.0061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuller RA, Westmoreland SV, Ratai E, et al. A prospective longitudinal in vivo 1H MR spectroscopy study of the SIV/macaque model of neuroAIDS. BMC Neurosci. 2004 Mar 5;5:10. doi: 10.1186/1471-2202-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvan AM, Vion-Dury J, Confort-Gouny S, Nicoli F, Lamoureux S, Cozzone PJ. Brain proton magnetic resonance spectroscopy in HIV-related encephalopathy: identification of evolving metabolic patterns in relation to dementia and therapy. AIDS Res Hum Retroviruses. 1997 Aug 10;13(12):1055–1066. doi: 10.1089/aid.1997.13.1055. [DOI] [PubMed] [Google Scholar]
- 24.Paley M, Cozzone PJ, Alonso J, et al. A multicenter proton magnetic resonance spectroscopy study of neurological complications of AIDS. AIDS Res Hum Retroviruses. 1996 Feb 10;12(3):213–222. doi: 10.1089/aid.1996.12.213. [DOI] [PubMed] [Google Scholar]
- 25.Bladowska J, Zimny A, Koltowska A, et al. Evaluation of metabolic changes within the normal appearing gray and white matters in neurologically asymptomatic HIV-1-positive and HCV-positive patients: magnetic resonance spectroscopy and immunologic correlation. Eur J Radiol. 2013 Apr;82(4):686–692. doi: 10.1016/j.ejrad.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 26.Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002 Nov;17(3):1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- 27.Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011 Mar 13;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999 Mar 23;52(5):995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- 29.Young AC, Yiannoutsos CT, Hegde M, et al. Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology. 2014 Oct 28;83(18):1592–1600. doi: 10.1212/WNL.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suwanwelaa N, Phanuphak P, Phanthumchinda K, et al. Magnetic resonance spectroscopy of the brain in neurologically asymptomatic HIV-infected patients. Magn Reson Imaging. 2000 Sep;18(7):859–865. doi: 10.1016/s0730-725x(00)00173-9. [DOI] [PubMed] [Google Scholar]
- 31.Winston A, Duncombe C, Li PC, et al. Two patterns of cerebral metabolite abnormalities are detected on proton magnetic resonance spectroscopy in HIV-infected subjects commencing antiretroviral therapy. Neuroradiology. 2012 Dec;54(12):1331–1339. doi: 10.1007/s00234-012-1061-5. [DOI] [PubMed] [Google Scholar]
- 32.Gongvatana A, Harezlak J, Buchthal S, et al. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. Journal of neurovirology. 2013 Apr 24; doi: 10.1007/s13365-013-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danielson E, Ross B. Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases. New Yord: Marcel Dekker; 1999. [Google Scholar]
- 34.Lin A, Ross BD, Harris K, Wong W. Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2005 Apr;2(2):197–214. doi: 10.1602/neurorx.2.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005 Feb;162(2):361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berg J, Tymoczko J, Stryer L. Biochemistry. 6th ed. New York, NY: WH Freeman & Company; 2007. [Google Scholar]
- 37.Streck EL, Scaini G, Rezin GT, Moreira J, Fochesato CM, Romao PR. Effects of the HIV treatment drugs nevirapine and efavirenz on brain creatine kinase activity. Metabolic brain disease. 2008 Dec;23(4):485–492. doi: 10.1007/s11011-008-9109-2. [DOI] [PubMed] [Google Scholar]
- 38.Schweinsburg BC, Taylor MJ, Alhassoon OM, et al. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol. 2005 Aug;11(4):356–364. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]
- 39.Ratai EM, Annamalai L, Burdo T, et al. Brain creatine elevation and N-Acetylaspartate reduction indicates neuronal dysfunction in the setting of enhanced glial energy metabolism in a macaque model of neuroAIDS. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2011 Sep;66(3):625–634. doi: 10.1002/mrm.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- 41.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15(3–5):289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Villegas D, Lenkinski RE, Frank I. Biochemical changes in the frontal lobe of HIV-infected individuals detected by magnetic resonance spectroscopy. Proceedings of the National Academy of Sciences of the United States of America. 1997 Sep 2;94(18):9854–9859. doi: 10.1073/pnas.94.18.9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyoo IK, Yoon SJ, Musen G, et al. Altered prefrontal glutamate-glutamine-gamma-aminobutyric acid levels and relation to low cognitive performance and depressive symptoms in type 1 diabetes mellitus. Archives of general psychiatry. 2009 Aug;66(8):878–887. doi: 10.1001/archgenpsychiatry.2009.86. [DOI] [PubMed] [Google Scholar]
- 44.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997 Apr 15;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Archives of neurology. 2006 Oct;63(10):1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 46.Potter MC, Figuera-Losada M, Rojas C, Slusher BS. Targeting the glutamatergic system for the treatment of HIV-associated neurocognitive disorders. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013 Jun;8(3):594–607. doi: 10.1007/s11481-013-9442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erdmann N, Zhao J, Lopez AL, et al. Glutamate production by HIV-1 infected human macrophage is blocked by the inhibition of glutaminase. Journal of neurochemistry. 2007 Jul;102(2):539–549. doi: 10.1111/j.1471-4159.2007.04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valcour V, Shiramizu B. HIV-associated dementia, mitochondrial dysfunction, and oxidative stress. Mitochondrion. 2004 Jul;4(2–3):119–129. doi: 10.1016/j.mito.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Mialocq P, Oiry J, Puy JY, et al. [Oxidative metabolism of HIV-infected macrophages: the role of glutathione and a pharmacologic approach] Pathol Biol (Paris) 2001 Sep;49(7):567–571. doi: 10.1016/s0369-8114(01)00214-0. [DOI] [PubMed] [Google Scholar]
- 50.Rimaniol AC, Mialocq P, Clayette P, Dormont D, Gras G. Role of glutamate transporters in the regulation of glutathione levels in human macrophages. Am J Physiol Cell Physiol. 2001 Dec;281(6):C1964–C1970. doi: 10.1152/ajpcell.2001.281.6.C1964. [DOI] [PubMed] [Google Scholar]
- 51.Ernst T, Chang L, Arnold S. Increased glial metabolites predict increased working memory network activation in HIV brain injury. Neuroimage. 2003 Aug;19(4):1686–1693. doi: 10.1016/s1053-8119(03)00232-5. [DOI] [PubMed] [Google Scholar]
- 52.Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn Reson Med. 2004 Mar;51(3):435–440. doi: 10.1002/mrm.20007. [DOI] [PubMed] [Google Scholar]
- 53.Hancu I, Zimmerman EA, Sailasuta N, Hurd RE. 1H MR spectroscopy using TE averaged PRESS: a more sensitive technique to detect neurodegeneration associated with Alzheimer's disease. Magn Reson Med. 2005 Apr;53(4):777–782. doi: 10.1002/mrm.20419. [DOI] [PubMed] [Google Scholar]
- 54.Mohamed MA, Lentz MR, Lee V, et al. Factor analysis of proton MR spectroscopic imaging data in HIV infection: metabolite-derived factors help identify infection and dementia. Radiology. 2010 Feb;254(2):577–586. doi: 10.1148/radiol.09081867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hua X, Boyle CP, Harezlak J, et al. Disrupted cerebral metabolite levels and lower nadir CD4 + counts are linked to brain volume deficits in 210 HIV-infected patients on stable treatment. Neuroimage Clin. 2013;3:132–142. doi: 10.1016/j.nicl.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim H, Catana C, Ratai EM, et al. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 2011 Jun 1;71(11):3745–3752. doi: 10.1158/0008-5472.CAN-10-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin Y, Stephenson MC, Xin L, Napolitano A, Morris PG. Investigating the metabolic changes due to visual stimulation using functional proton magnetic resonance spectroscopy at 7 T. J Cereb Blood Flow Metab. 2012 Aug;32(8):1484–1495. doi: 10.1038/jcbfm.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dydak U, Jiang YM, Long LL, et al. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ Health Perspect. 2011 Feb;119(2):219–224. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.