Abstract
A growing line of research has shown positive treatment outcomes from technology-based therapy for substance use disorders (SUDs). However, little is known about the effectiveness of technology-based SUD interventions for persons who already had numerous prior SUD treatments. We conducted a secondary analysis on a 12-month trial with patients (N = 160) entering methadone maintenance treatment (MMT). Patients were randomly assigned to either standard MMT treatment or a model in which half of standard counseling sessions were replaced with a computer-based intervention, called Therapeutic Education System (standard + TES). Four treatment history factors at baseline, the number of lifetime SUD treatment episodes, detoxification episodes, and inpatient/outpatient treatment episodes were categorized into three levels based on their tertile points, and analyzed as moderators. Dependent variables were urine toxicology results for opioid and cocaine abstinence for 52-weeks. The standard + TES condition produced significantly better opioid abstinence than standard treatment for participants with 1) a moderate or high frequency of lifetime SUD treatment episodes, and 2) those with all three levels (low, moderate and high) of detoxification and inpatient/outpatient treatment episodes, ps < .01. The standard + TES condition enhanced cocaine abstinence compared to standard treatment among people with 1) a moderate or high frequency of lifetime SUD treatment episodes, 2) a high level of detoxification episodes, and 3) a moderate or high level of inpatient treatment history, ps < .01. We found that including technology-based behavioral therapy as part of treatment can be more effective than MMT alone, even among patients with a history of multiple addiction treatment episodes.
Keywords: Computer-based health intervention, Behavioral therapy, Treatment history, Opioid dependence, Cocaine dependence
1. Introduction
Many persons with substance use disorders (SUDs) suffer from continuing abuse of drugs and relapse during their lifetimes, even after successfully completing addiction treatment (McLellan, 2002; Witkiewitz & Marlatt, 2004). About 40%to 60% of the people discharged from substance abuse treatment programs return back to active substance use within a year (Brecht & Herbeck, 2014; McLellan, Lewis, O'Brien, & Kleber, 2000). McLellan et al. (2000) argue that substance dependence and recovery processes should be treated and monitored like chronic illness through ongoing care and personalized support to optimize recovery outcomes. Treatment-resistant patients with continuous relapses despite multiple episodes of treatment may require reinstatement or adjustment in their routine treatments (White et al., 2014). On one hand, it is plausible to argue that patients with extensive treatment histories need intensive, in-person treatment with professional monitoring and customized care from highly skilled counselors (McGovern, Wrisley, & Drake, 2005). On the other hand, it is also reasonable to predict that patients with treatment-resistant SUDs may need a novel approach, such as a technology-based treatment as part of their care in order to offer highly personalized, evidence-based content as well as interactive, constantly available care. There is, however, no empirical evidence to gauge these alternative predictions. Understanding optimal models of care (clinician-delivered treatment vs. technology-assisted treatment) is an important area of research to better understand how to help these difficult cases of patients with chronic, treatment-resistant SUDs.
Technology-based interventions for SUDs are becoming increasingly popular (Dallery, Jarvis, Marsch, & Xie, 2015; Litvin, Abrantes, & Brown, 2013). Technology-based therapeutic tools can promote just-in-time monitoring and support (Cucciare, Weingardt, Greene, & Hoffman, 2012; Ondersma, Grekin, & Svikis, 2011), highly tailored, personalized content with easy access (Litvin et al., 2013), as well as patients' coping competence and fluency in recovery (Marsch, Carroll, & Kiluk, 2014a). These therapeutic benefits may be particularly helpful for those with a long history of SUD treatments and chronic relapses. Despite the considerable promise of technology-assisted SUD therapeutic tools, surprisingly very little is known as to whether and to what extent these tools can work for persons with chronic relapse who have already received multiple addiction treatments.
1.1. Secondary analysis using treatment history variables as moderators
Our investigation is designed to fill this gap by exploring whether a technology-based therapy will generate better or worse treatment outcomes for patients with a long history of relapses and multiple episodes of addiction treatment. This aim centers on a critical examination of not just whether technology-based interventions for SUDs work, but for whom they work (Kim, Marsch, Guarino, Acosta, & Aponte-Melendez, 2015). We conducted an exploratory secondary analysis to systematically examine the role of treatment history as a moderating factor of treatment outcomes for technology-assisted therapies. This analysis offers an unprecedented insight into the subgroups of patients with shorter vs. longer treatment histories that benefit or do not benefit from technology-based SUD therapy (Kazdin, 2007). In this investigation, we explored various treatment histories, such as lifetime SUD treatment, inpatient vs. outpatient treatment episodes, and detoxification-only treatment episodes. This level of specification was intended to allow us to distinguish whether a technology-assisted treatment, compared to standard alone treatment, works differently across subgroups of patients with various types and levels of treatment histories.
1.2. Technology-based intervention for SUD: therapeutic education system
Our data are from a clinical trial (Marsch et al., 2014b) testing the Therapeutic Education System (TES), a widely studied and empirically supported web-based intervention for SUD treatment. The primary outcomes from this trial have been published elsewhere (Marsch et al., 2014b). TES is grounded in the Community Reinforcement Approach (CRA) and the Cognitive Behavior Therapy (CBT) approaches to SUD treatment (Bickel, Marsch, Buchhalter, & Badger, 2008), and based on fluency-building information technology and an interactive learning process with 67 modules. TES incorporates these behavior change principles in a systematic, individualized manner, to deliver evidence-based care with high fidelity (Marsch et al., 2014a). The content and behavioral therapy principles on which TES is based are reported in the primary outcomes paper in greater detail (Marsch et al., 2014b).
The TES intervention has demonstrated efficacy and effectiveness in the treatment for SUDs in several randomized clinical trials. Bickel et al. (2008) found that TES produced weeks of opioid and cocaine abstinence that were comparable to the treatment outcomes achieved by highly trained therapists. Campbell et al. (2012, 2014b) conducted a multisite randomized clinical trial within the National Drug Abuse Treatment Clinical Trials Network (CTN) evaluating the effectiveness of TES among more than 500 participants in outpatient treatment for SUD across 10 US states over 12 weeks. Campbell et al. (2014b) found that the integration of TES had a significant effect on reducing treatment dropout while enhancing abstinence compared to the treatment-as-usual group. In a recent randomized controlled clinical trial, Marsch et al. (2014b) found that computer-based TES, when it replaced half of the face-to-face therapeutic sessions in methadone maintenance treatment (MMT), resulted in significantly greater opioid abstinence (as measured via urine testing) compared to the treatment-as-usual condition over the 12 month evaluation period.
Despite promising findings from the web-based TES and its documented advantages over standard alone treatment, no research has directly examined whether this technology-based therapeutic tool can enhance treatment outcomes for patients with a long history of repetitive participation in SUD treatment. In secondary analyses, we directly examine the interaction effects of treatment condition and different SUD treatment histories on drug abstinence. Given the lack of published data on this topic, we conduct exploratory secondary analyses to address this research question rather than proposing a directional hypothesis.
2. Methods
2.1. Study setting and random assignment
The study was conducted in a methadone maintenance treatment (MMT) program in a large urban area in the northeastern United States. To be eligible, participants at the study site had to be ≥18 years of age, had to meet DSM criteria for opioid dependence, and be within their first 30 days of MMT program entry. Sufficient English language skills were required for study participation in order to comprehend and respond to the intervention content and study assessments. Individuals who entered the MMT program for detoxification only were not eligible to participate. The study protocol was approved by the Institutional Review Board at the National Development and Research Institutes. All the participants (N = 160) provided written informed consent before study participation.
Participants were randomly assigned to either the standard treatment condition (control group, hereafter) or the reduced standard treatment + computer-based TES condition (experimental group,hereafter) in an intent-to-treat design. Randomization was not blinded. The CONSORT diagram and study procedure are reported elsewhere (Marsch et al., 2014b).
2.1.1. Control group: standard treatment
Participants in standard treatment received daily maintenance doses of methadone (ranging from approximately 80–100 mg/day) and individual counseling at the study MMT site. Each counseling session lasted up to approximately 60 min and occurred once per week for the first four weeks, and every other week thereafter (although participants with persistent illicit drug use continued to meet with their counselor on a weekly basis). Counseling therapy sessions were delivered by experienced Certified Alcohol and Substance Abuse Counselors (CASACs), and largely focused on helping patients understand and comply with program guidelines, resolving personal problems (e.g., employment), teaching cognitive coping skills, monitoring treatment progress (e.g., abstinence), and providing weekly group supervision. The content of standard treatment was consistent with that provided by the majority of MMT programs (McLellan, Arndt, Metzger, Woody, & O'Brien, 1993).
2.1.2. Experimental group: reduced standard treatment + computer-based TES
Participants in this group received the same daily maintenance doses of methadone and the same therapeutic content and substance abuse in-person counseling as those in the control group, except that approximately 30 min of each counseling session was replaced with computer-based TES onsite over the entire 52-week intervention period. Upon first accessing the system, each participant completed a computerized training module and a risk assessment survey. The selection of TES modules was customized for each participant based on responses to the risk assessment survey, but participants could electively complete any optional modules. Each module took approximately 15 min to complete. Participants were asked to complete about two modules per session to ensure the amount of time spent in the reduced standard counseling and TES modules (about one hour in total) was equivalent to that in the standard treatment. TES was provided to participants in this trial without any contingency management interventions; that is, no incentives were provided to participants for treatment progress (such as negative urines; completion of treatment sessions).
2.2. Measures
2.2.1. Treatment outcome: urine toxicology results
The present study reports objectively measured abstinence results for two substances: urine toxicology results for opioids and cocaine (the most common illicit drugs used by participants of this sample) were measured over 12-months using Drug Check Drug Test cups (Drug Test Systems, Dover, NH). Opioid abstinence was determined by negative urine results for opiates, propoxyphene, and oxycodone. In order to be considered opioid-negative, all three tests had to be negative. We calculated abstinence based on the number of weeks with opioid or cocaine abstinence out of the number of total study weeks. This method conservatively treated a missed test prior to study dropout as drug-positive when aggregating urine test results across study weeks. Note that the experimental group had more missing data prior to study dropout (untested weeks, M = 5.99 weeks, SD = 5.42) than the control group (M = 4.40 weeks, SD = 4.58), t(158) = 2.00, p = .05, d = 0.32. Thus, missing data from this experimental group were replaced with more drug-positive results compared to the standard condition.
2.2.2. Treatment history at baseline (moderator)
We used the 5th Edition of the Addiction Severity Index (ASI) (Bovasso, Alterman, Cacciola, & Cook, 2001; McLellan, Cacciola, Alterman, Rikoon, & Carise, 2006; McLellan, Luborsky, Woody, & O'Brien, 1980) with some additions inquiring about the prior treatments for opiate abuse and cocaine abuse. We measured 1) the number of lifetime SUD treatment episodes for opioids and cocaine at baseline first, and asked participants to specify 2) the number of detoxification only treatment episodes for opioids and cocaine among those lifetime SUD treatment episodes;3) the number of inpatient treatment episodes for opioids and cocaine among those lifetime SUD treatment episodes; and 4) the number of outpatient treatment episodes for opioids and cocaine among those lifetime SUD treatment episodes. Aligned with the standard ASI protocol, this approach was intended to account for any potentially unique moderating role of various kinds of treatment facilities. The lifetime SUD treatment episode variable overlaps with other three treatment variables, detoxification only, inpatient, and outpatient treatments.
2.3. Data analysis
Note that the present secondary analyses were not to emphasize between-groups main effects (reported in Marsch et al., 2014b), but a summary of the reported treatment outcomes were presented here only to provide an important context for interpreting the central moderator analysis. Descriptive statistics, central tendency tests, and randomization checks were conducted to characterize the sample. A generalized linear model applying a binomial logit link function with maximum likelihood was used to examine the moderating role of participants' treatment histories on treatment outcome (total study weeks with opioid abstinence and total study weeks with cocaine abstinence over a possible 52-week period) across the two study arms. In this modeling computation, abstinence rates were calculated as proportions (events/trials with logit function), using only the data prior to dropping out in an aggregated manner. The proportion of abstinence was based on all participants' actual length in the study and thus dropout did not influence the computation of the model algorithms.
All treatment history variables were mean-centered prior to computing interaction terms (Aiken & West, 1991) in order to make the regression coefficients more interpretable than raw scores without changing the strength of the testing association (Cohen, Cohen, West, & Aiken, 2003; Whisman & McClelland, 2005). After examining coefficients in the context of the generalized linear models, mean-centered values for each treatment history variable were rank-ordered from low to high and classified into three categories: 1) values that fell within the lowest third of the distribution (from zero to 33.30 percentiles); 2) values that fell within the middle third (from 33.40 to 66.60 percentiles); and 3) values that fell within the highest third (from 66.70 to 100 percentiles), representing low-, moderate-, and high-severity categories (Aiken & West, 1991). The inference by eye technique, comparing 95% confidence intervals (CIs) of three-subgroup means across two study arms (Cumming, 2009), was then used to detect statistically significant differences across the levels of treatment history by study condition. The inference by eye approach is a straightforward way to convey an overall pattern of results (Cumming, 2009; Cumming & Finch, 2005). When two 95% CIs overlap by about half the length of one side CI arm, there is a statistically significant difference between the means at a p-value < .05. When the two CIs just touch, the two-tailed p-value becomes <.01 (Cumming, 2009; Cumming & Finch,2005).
3. Results
3.1. Participants and ASI treatment history
Participants (N = 160) were between 20 and 64 years old (M = 40.66, SD = 9.83). The majority of participants were male (75%) and white (44.3%) with an average of 12.2 years of education (SD = 2.4). Opioids were self-reported as the primary substance causing major life problems (n = 123; 76.9%) followed by poly-drugs, cocaine, and alcohol (n = 26). Participants in both conditions reported that they regularly used opioids for an average of approximately 15 years (SD = 11.72), cocaine and crack more than 7 years (SD = 8.99), and poly-drugs longer than 9 years (SD = 9.58; Table 1). Descriptive statistics on tertile-based categorizations for treatment histories are reported in Table 2.
Table 1.
Lifetime regular use of drugs and alcohol.
| TES (n = 80) | Standard (n = 80) | Total (N = 160) | |
|---|---|---|---|
| Alcohol | 10.44 (16.29) | 9.05 (14.83) | 9.74 (15.54) |
| Opioid | 15.21 (12.53) | 14.70 (10.93) | 14.95 (11.72) |
| Barbiturates | 0.06 (0.46) | 0.00 | 0.03 (0.33) |
| Other sedatives, hypnotics, tranquilizers | 1.95 (4.43) | .74 (2.12) | 1.34 (3.52) |
| Cocaine or crack | 8.05 (9.76) | 6.49 (8.15) | 7.27 (8.99) |
| Amphetamines | 0.15 (0.81) | 0.25 (1.32) | 0.20 (1.09) |
| Marijuana | 6.71 (8.93) | 4.40 (6.41) | 5.56 (7.83) |
| Hallucinogens | 0.31 (1.05) | 0.40 (1.24) | 0.36 (1.15) |
| Inhalants | 0.03 (0.23) | 0.00 | 0.01 (0.16) |
| More than 1 substance/day | 10.18 (9.75) | 8.46 (9.39) | 9.31 (9.58) |
Note: The unit of analysis is year. Standard deviations are presented in parentheses. The severity of SUD at baseline was measured by the total number of years in their lifetime they have regularly used each of the above substances. Substance use history at baseline did not differ between study conditions, verifying successful randomization.
Table 2.
Lifetime treatment history at baseline by study conditions.
| TES (n = 80) | Standard (n = 80) | Total (N = 160) | Low | Moderate | High | |
|---|---|---|---|---|---|---|
| Num. of SUD treatment episodes | 9.86 (10.41) | 10.44 (10.28) | 10.15 (10.32) | 2.55 (1.05) [0–4] | 7.13 (1.87) [5–11] | 21.55 (11.30) [12–60] |
| Num. of detox treatment episodes | 4.49 (7.60) | 5.16 (6.77) | 4.83 (7.18) | .06 (.49) [0–1] | 2.67 (.73) [2–4] | 11.96 (9.41) [5–58] |
| Num. of inpatient treatment episodes for SUD | 6.50 (9.32) | 7.28 (8.64) | 6.89 (8.96) | 1.10 (.82) [0–2] | 4.19 (1.12) [3–6] | 16.72 (10.49) [7–57] |
| Num. of outpatient treatment episodes for SUD | 3.49 (2.46) | 3.46 (2.80) | 3.48 (2.63) | 1.55 (.55) [0–2] | 3.42 (.50) [3–4] | 6.82 (2.51) [5–14] |
Note: Numbers represent the mean values of treatment episodes. Numbers in parentheses indicate values of standard deviations. Treatment history at baseline did not differ between study conditions, verifying successful randomization. Numbers in the square brackets indicate the range of each subgroup.
3.2. Generalized linear models
3.2.1. Total weeks of opioid abstinence
For lifetime SUD treatment episodes at baseline, the experimental group showed .51 times better opioid abstinence than the standard treatment group when mean-centered scores of lifetime SUD treatment episodes (M = 10.15, SD= 10.32) were held constant, p< .01 (Table 3). Small but significant interaction effects were observed between treatment intervention and total weeks of opioid abstinence for detoxification episodes. During the time patients stayed in the program, there was a 2% increase in opioid abstinence in the experimental group when the number of lifetime detoxification episodes increased by one unit (OR = 1.02). That is, if someone has 40 times of lifetime detoxification episodes at baseline, s/he is likely to have 70% better opioid abstinence rates when assigned to the experimental group compared to someone who has 5 times of lifetime detoxification episodes at baseline.
Table 3.
Effects of intervention conditions × treatment history variables on treatment outcomes.
| Total weeks with opioid abstinence | Total weeks with cocaine abstinence | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| β | (SE) | OR | 95% C.I. | β | (SE) | OR | 95% C.I. | |
| TES condition (vs. standard) | 0.51 | 0.06 | 1.66 | 1.48–1.85 | 0.18 | 0.06 | 1.20 | 1.08–1.34 |
| Lifetime treatment episodes | 0.02 | 0.00 | 1.02 | 1.01–1.03 | −0.01 | 0.00 | 0.99 | 0.98–1.00 |
| TES * lifetime treatment episodes | 0.01 | 0.06 | 1.01 | 1.00–1.02 | 0.03 | 0.01 | 1.03 | 1.02–1.04 |
| TES condition (vs. standard) | 0.52 | 0.06 | 1.69 | 1.51–1.88 | 0.18 | 0.06 | 1.20 | 1.07–1.34 |
| Detox treatment episodes | 0.02 | 0.01 | 1.02 | 1.01–1.04 | −0.03 | 0.01 | 0.97 | 0.96–0.99 |
| TES * detox treatment episodes | 0.02 | 0.01 | 1.02 | 1.00–1.03 | 0.06 | 0.01 | 1.06 | 1.04–1.08 |
| TES condition (vs. standard) | 0.53 | 0.06 | 1.70 | 1.52–1.90 | 0.18 | 0.06 | 1.20 | 1.07–1.34 |
| Lifetime inpatient treatment episodes | 0.02 | 0.00 | 1.03 | 1.02–1.03 | −0.02 | 0.00 | 0.98 | 0.97–0.99 |
| TES * lifetime inpatient treatment episodes | 0.01 | 0.00 | 1.01 | 1.00–1.02 | 0.04 | 0.01 | 1.04 | 1.03–1.05 |
| TES condition (vs. standard) | 0.48 | 0.06 | 1.61 | 1.45–1.80 | 0.17 | 0.06 | 1.18 | 1.06–1.32 |
| Lifetime outpatient treatment episodes | −0.03 | 0.01 | 0.97 | 0.94–1.00 | −0.04 | 0.01 | 0.96 | 0.94–0.99 |
| TES * lifetime outpatient treatment episodes | 0.00 | 0.02 | 1.00 | 0.96–1.04 | 0.09 | 0.02 | 1.10 | 1.05–1.14 |
Note: Interaction effects can be interpreted as the difference in the slopes of the moderating variables across the two intervention groups. Values in bold indicate p < .05, and values in bold and underlined indicate p < .01.
3.2.2. Total weeks of cocaine abstinence
The odds ratio of cocaine abstinence changed as a function of study interventions and lifetime SUD treatment episodes: during the time patients stayed in their treatment program, there was a 3% increase in cocaine abstinence in the experimental group when the number of lifetime SUD episodes increased by one unit (OR = 1.03, 95% CI = 1.02–1.04).
The urinalysis results for cocaine abstinence indicate that during the time patients stayed in the study there was a 6% increase in cocaine abstinence in the experimental group when the number of detoxification episodes at baseline increased by one unit (OR = 1.06, 95% CI = 1.04–1.08). Also, over the time patients stayed in their treatment program, there was a 4% increase in cocaine abstinence in the experimental group when the number of lifetime inpatient treatment episodes at baseline increased by one unit (OR = 1.04, 95% CI = 1.03–1.05). The strength and direction of the relationship between the experimental group and cocaine abstinence changed as a function of lifetime outpatient treatment episodes at baseline: during the time patients stayed in the study, there was a 9% increase in cocaine abstinence in the experimental group when the number of lifetime outpatient treatment episodes at baseline increased by one unit (OR = 1.10, 95% CI = 1.05–1.14, Table 3).
3.3. 95% CI's between three-level categorized moderators across intervention conditions
3.3.1. Total weeks of opioid abstinence
The experimental group generated significantly better opioid abstinence compared to the control group among people with a moderate or high level of lifetime SUD treatment episodes prior to the treatment admission (Fig. 1). The intervention effect was not different for those with a low level of lifetime SUD treatment episodes at baseline. For all three levels of detoxification treatment history as well as inpatient and outpatient treatment episodes at baseline, the experimental group led to significantly better opioid abstinence compared to standard treatment at a p-values < .01.
Fig. 1.
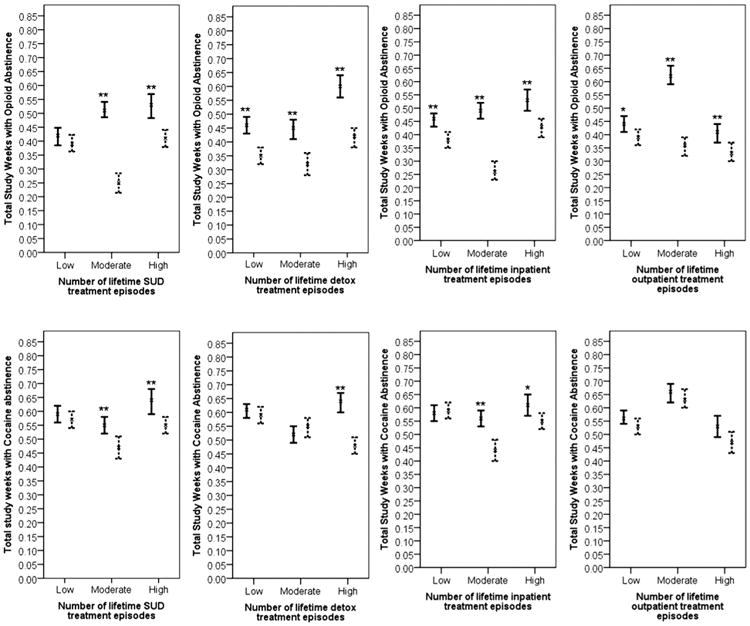
95% Confidence intervals for opioid abstinence (the first row) and cocaine abstinence (the second row) by moderator variables across study conditions. Lines = reduced standard + TES treatment, dotted lines = standard treatment. **p < .01. *p < .05.
3.3.2. Total weeks of cocaine abstinence
The experimental group generated significantly better cocaine abstinence compared to standard treatment among people with moderate or high levels of lifetime SUD treatment episodes at baseline (Fig. 1). The experimental group was significantly more effective than the control group for those with a high level of detoxification treatment episodes at baseline (p< .01).
For those with moderate or high levels of inpatient treatment history at baseline, participants in the experimental group showed significantly better cocaine abstinence compared to those in the standard treatment (Fig. 1).
4. Discussion
The present study examined whether different treatment histories at baseline moderated the relative effectiveness of clinician-delivered SUD behavior therapy versus a model where half of each counseling session was substituted with a computer-based behavioral intervention. Evaluating the moderating effect of treatment history allowed us to examine if a technology-based therapeutic tool can be effective for those with intensive levels of prior treatment involvement who may be considered treatment resistant (Fairchild & MacKinnon, 2009).
We found that some of the significant interaction coefficients from generalized linear models are as small as .02. However, given that treatment history variables have a wide range in their scores (e.g., 0–60), it should be noted that those small coefficients are not clinically non-trivial. For example, the lifetime SUD treatment episodes ranged from zero to 60, and if someone has 30 times of lifetime SUD treatment episodes at baseline, s/he is likely to have 75% better cocaine abstinence rates when assigned to the experimental group compared to someone who has 5 times of lifetime SUD treatment episodes at baseline.
Furthermore, when the treatment history characteristics were categorized into three levels, better opioid and cocaine abstinence outcomes over 12 months were pronounced among patients with moderate or high levels of prior SUD treatment episodes at baseline when they received computer-based TES as part of their care. These consistent findings, in favor of the model of treatment that included TES, are promising in light of the scientific literature demonstrating the need for more effective treatment approaches for treatment resistant patients (McLellan et al., 2000). On that note, evidence-based behavioral interventions mediated via technology may provide a platform for changing the way that care for SUDs is offered. For example, remotely accessed computer-based treatment programs can provide greater anonymity, which can be advantageous for patients with extensive treatment histories and relapses who are concerned with social stigmatization (Campbell et al., 2014a; Rooke, Gates, Norberg, & Copeland, 2014; Sinadinovic, Wennberg, & Berman, 2012).
Adaptive educational modules, automated and personalized features, and human–computer interactivity have been shown to enhance learning outcomes (Cook, Levinson, & Garside, 2010; Walther, Pingree, Hawkins, & Buller, 2005), and these features were pivotal elements implemented in TES. Our findings indicate that patients with a substantial history of lifetime treatment episodes at baseline might have needed a novel approach to treatment, such as that afforded by computer-based TES. Additionally, TES may have offered a more engaging, interactive experience relative to traditional therapy within the context of MMT. Although there have been concerns about possible resistance toward treatment among patients with repeated exposure to similar care and treatment content, the present findings suggest that even patients with extensive histories of substance abuse treatment can benefit from additional computer-based behavioral therapy.
Our findings should be understood with limitations imposed by study attrition. The sample became smaller over the intervention period. However, the sample sizes for each condition over the follow-up period were similar across study arms, and at the 12-month follow-up, the sample size across study arms is identical (Marsch et al., 2014b). Also, we used generalized linear models applying a logit link function with maximum likelihood. The maximum likelihood algorithm is straightforward and applies the log-likelihood function, using a reduced form of the distribution (Allison, 2002). This approach removes potential confounding effects of study attribution on dependent variables, and regression coefficients should be interpreted as effects before dropout.
5. Conclusions
We examined the potential utility and effectiveness of technology-based SUD therapy among persons with various levels and types of SUD treatment histories who may struggle with continuing relapse and treatment resistance. We found that, at least for the time they stayed in their MMT program, patients with an extensive history of prior SUD treatments had better opioid and cocaine abstinence when assigned to the reduced standard treatment + computer-based TES condition rather than standard alone treatment. In particular, patients with a moderate and high level of lifetime SUD treatment episodes, a high level of detox treatment episodes, and a moderate and high levels of inpatient treatment episodes reported both better opioid and cocaine abstinence when they stayed in the experimental group compared to standard alone treatment. Our empirical evidence underscores the finding that the personalized computer-based SUD therapy offers promise for treatment resistant patients who may require prolonged treatment and care.
Highlights.
It is largely unknown for whom technology-based interventions for SUDs work.
We found interaction effects of TES vs. control in relation to treatment histories.
Drug abstinence was moderated by study arms and SUD treatment history.
Computer-based TES worked well even among persons with long treatment histories.
Acknowledgments
Role of funding sources: The study was supported by NIH/NIDA R01 DA021818. The preparation of this manuscript was partially supported by NIH/NIDA P30DA029926. NIDA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Contributors: LAS, MCA, and HG designed the study and wrote the protocol. LAM served as Principal Investigator on the clinical trial. HG and MCA helped manage the clinical trial. YA-M collected data. SJK and LAM conceptualized the study. SJK conducted literature searches. SJK analyzed the data, and wrote the first and final drafts. SHG, MCA, and YA-M provided feedback on the first draft. SJK and LAM contributed to the writing and review of the paper.
Conflict of interest: None. In addition to her academic affiliation, Dr. Marsch is affiliated with HealthSim, LLC, the health-promotion software development organization that developed the web-based Therapeutic Education System referenced in this manuscript. Dr. Marsch has worked extensively with her institutions to manage any potential conflict of interest. All research data collection, data management, and statistical analyses were conducted by individuals with no affiliation to HealthSim, LLC. All other authors declare that they have no conflicts of interest.
Contributor Information
Sunny Jung Kim, Email: Sunny.J.Kim@dartmouth.edu.
Lisa A. Marsch, Email: Lisa.A.Marsch@dartmouth.edu.
Michelle C. Acosta, Email: acosta@ndri.org.
Honoria Guarino, Email: guarino@ndri.org.
Yesenia Aponte-Melendez, Email: aponte-melendez@ndri.org.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, Calif.: Sage Publications; 1991. [Google Scholar]
- Allison PD. Missing data. Thousand Oaks, CA: Sage Publications, Inc; 2002. [Google Scholar]
- Bickel WK, Marsch LA, Buchhalter AR, Badger GJ. Computerized behavior therapy for opioid-dependent outpatients: A randomized controlled trial. Experimental and Clinical Psychopharmacology. 2008;16(2):132–143. doi: 10.1037/1064-1297.16.2.132. http://dx.doi.org/10.1037/1064-1297.16.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovasso GB, Alterman AI, Cacciola JS, Cook TG. Predictive validity of the addiction severity index's composite scores in the assessment of 2-year outcomes in a methadone maintenance population. Psychology of Addictive Behaviors. 2001;15(3):171–176. [PubMed] [Google Scholar]
- Brecht ML, Herbeck D. Time to relapse following treatment for methamphetamine use: A long-term perspective on patterns and predictors. Drug and Alcohol Dependence. 2014;139:18–25. doi: 10.1016/j.drugalcdep.2014.02.702. http://dx.doi.org/10.1016/j.drugalcdep.2014.02.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ANC, Nunes EV, Matthews AG, Stitzer M, Miele GM, Polsky D, Ghitza UE, et al. Internet-delivered treatment for substance abuse: A multisite randomized controlled trial. American Journal of Psychiatry. 2014b;171(6):683–690. doi: 10.1176/appi.ajp.2014.13081055. http://dx.doi.org/10.1176/appi.ajp.2014.13081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AN, Nunes EV, Miele GM, Matthews A, Polsky D, Ghitza UE, Crowell AR, et al. Design and methodological considerations of an effectiveness trial of a computer-assisted intervention: An example from the NIDA clinical trials network. Contemporary Clinical Trials. 2012;33(2):386–395. doi: 10.1016/j.cct.2011.11.001. http://dx.doi.org/10.1016/j.cct.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AN, Turrigiano E, Moore M, Miele GM, Rieckmann T, Hu MC, Nunes EV, et al. Acceptability of a web-based community reinforcement approach for substance use disorders with treatment-seeking American Indians/Alaska natives. Community Mental Health Journal. 2014a doi: 10.1007/s10597-014-9764-1. http://dx.doi.org/10.1007/s10597-014-9764-1. [DOI] [PMC free article] [PubMed]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd. NJ: Erlbaum (Mahwah); 2003. [Google Scholar]
- Cook DA, Levinson AJ, Garside S. Time and learning efficiency in internet-based learning: A systematic review and meta-analysis. Advances in Health Sciences Education: Theory and Practice. 2010;15(5):755–770. doi: 10.1007/s10459-010-9231-x. http://dx.doi.org/10.1007/s10459-010-9231-x. [DOI] [PubMed] [Google Scholar]
- Cucciare MA, Weingardt KR, Greene CJ, Hoffman J. Current trends in using internet and mobile technology to support the treatment of substance use disorders. Current Drug Abuse Reviews. 2012;5(3):172–177. doi: 10.2174/1874473711205030172. [DOI] [PubMed] [Google Scholar]
- Cumming G. Inference by eye: Reading the overlap of independent confidence intervals. Statistics in Medicine. 2009;28(2):205–220. doi: 10.1002/sim.3471. http://dx.doi.org/10.1002/sim.3471. [DOI] [PubMed] [Google Scholar]
- Cumming G, Finch S. Inference by eye: Confidence intervals and how to read pictures of data. The American Psychologist. 2005;60:170–180. doi: 10.1037/0003-066X.60.2.170. http://dx.doi.org/10.1037/0003-066X.60.2.170. [DOI] [PubMed] [Google Scholar]
- Dallery J, Jarvis B, Marsch L, Xie H. Mechanisms of change associated with technology-based interventions for substance use. Drug and Alcohol Dependence. 2015;150:14–23. doi: 10.1016/j.drugalcdep.2015.02.036. http://dx.doi.org/10.1016/j.drugalcdep.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prevention Science. 2009;10:87–99. doi: 10.1007/s11121-008-0109-6. http://dx.doi.org/10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. http://dx.doi.org/10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Marsch LA, Guarino H, Acosta MC, Aponte-Melendez Y. Predictors of outcome from computer-based treatment for substance use disorders: Results from a randomized clinical trial. Drug and Alcohol Dependence. 2015 doi: 10.1016/j.drugalcdep.2015.09.019. http://dx.doi.org/10.1016/j.drugalcdep.2015.09.019. [DOI] [PMC free article] [PubMed]
- Litvin EB, Abrantes AM, Brown RA. Computer and mobile technology-based interventions for substance use disorders: An organizing framework. Addictive Behaviors. 2013;38(3):1747–1756. doi: 10.1016/j.addbeh.2012.09.003. http://dx.doi.org/10.1016/j.addbeh.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Carroll KM, Kiluk BD. Technology-based interventions for the treatment and recovery management of substance use disorders: A JSAT special issue. Journal of Substance Abuse Treatment. 2014a;46(1):1–4. doi: 10.1016/j.jsat.2013.08.010. http://dx.doi.org/10.1016/j.jsat.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Guarino H, Acosta M, Aponte-Melendez Y, Cleland C, Grabinski M, Edwards J, et al. Web-based behavioral treatment for substance use disorders as a partial replacement of standard methadone maintenance treatment. Journal of Substance Abuse Treatment. 2014b;46(1):43–51. doi: 10.1016/j.jsat.2013.08.012. http://dx.doi.org/10.1016/j.jsat.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern MP, Wrisley BR, Drake RE. Relapse of substance use disorder and its prevention among persons with co-occurring disorders. Psychiatric Services. 2005;56(10):1270–1273. doi: 10.1176/appi.ps.56.10.1270. http://dx.doi.org/10.1176/appi.ps.56.10.1270. [DOI] [PubMed] [Google Scholar]
- McLellan AT. Have we evaluated addiction treatment correctly?: Implications from a chronic care perspective. Addiction. 2002;97(3):249–252. doi: 10.1046/j.1360-0443.2002.00127.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Arndt IO, Metzger DS, Woody GE, O'Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269(15):1953–1959. [PubMed] [Google Scholar]
- McLellan AT, Cacciola JC, Alterman AI, Rikoon SH, Carise D. The addiction severity index at 25: Origins, contributions and transitions. American Journal on Addictions. 2006;15(2):113–124. doi: 10.1080/10550490500528316. http://dx.doi.org/10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Ondersma SJ, Grekin ER, Svikis D. The potential for technology in brief interventions for substance use, and during-session prediction of computer-delivered brief intervention response. Substance Use and Misuse. 2011;46(1):77–86. doi: 10.3109/10826084.2011.521372. http://dx.doi.org/10.3109/10826084.2011.521372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooke SE, Gates PJ, Norberg MM, Copeland J. Applying technology to the treatment of cannabis use disorder: Comparing telephone versus internet delivery using data from two completed trials. Journal of Substance Abuse Treatment. 2014;46(1):78–84. doi: 10.1016/j.jsat.2013.08.007. http://dx.doi.org/10.1016/j.jsat.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Sinadinovic K, Wennberg P, Berman AH. Targeting problematic users of illicit drugs with Internet-based screening and brief intervention: A randomized controlled trial. Drug and Alcohol Dependence. 2012;126:42–50. doi: 10.1016/j.drugalcdep.2012.04.016. http://dx.doi.org/10.1016/j.drugalcdep.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Walther JB, Pingree S, Hawkins RP, Buller DB. Attributes of interactive online health information systems. Journal of Medical Internet Research. 2005;7(3):e33. doi: 10.2196/jmir.7.3.e33. http://dx.doi.org/10.2196/jmir.7.3.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA, McClelland GH. Designing, testing, and interpreting interactions and moderator effects in family research. Journal of Family Psychology. 2005;19(1):111–120. doi: 10.1037/0893-3200.19.1.111. http://dx.doi.org/10.1037/0893-3200.19.1.111. [DOI] [PubMed] [Google Scholar]
- White WL, Campbell MD, Spencer RD, Hoffman HA, Crissman B, DuPont RL. Patterns of abstinence or continued drug use among methadone maintenance patients and their relation to treatment retention. Journal of Psychoactive Drugs. 2014;46(2):114–122. doi: 10.1080/02791072.2014.901587. http://dx.doi.org/10.1080/02791072.2014.901587. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: That was Zen, this is Tao. American Psychologist. 2004;59(4):224–235. doi: 10.1037/0003-066X.59.4.224. http://dx.doi.org/10.1037/0003-066x.59.4.224. [DOI] [PubMed] [Google Scholar]


