Abstract
Objective(s)
To investigate differences in subclinical coronary atherosclerotic plaque and markers of immune activation among HIV-infected and non-HIV-infected women categorized by degree of ovarian reserve and menopause status.
Design
Cross-sectional evaluation.
Methods
Seventy-four women (49 HIV-infected, 25 non-HIV-infected) without known CVD were classified as premenopausal, premenopausal with reduced ovarian reserve, or postmenopausal based on menstrual history and antimullerian hormone (AMH) levels. Participants underwent contrast enhanced coronary computed tomography angiography (CCTA) and immune phenotyping. Comparisons in coronary atherosclerotic plaque burden and immune markers were made between the HIV-infected and non-HIV-infected women overall and within the HIV-infected and non-HIV-infected women by reproductive classification group.
Results
Among the overall group of HIV-infected women, the women with reduced ovarian reserve (undetectable AMH) had a higher prevalence of coronary atherosclerotic plaque (52% versus 6%, p=0.0007) and noncalcified plaque (48% versus 6%, p=0.002), as well as higher levels of log sCD163 (p=0.0004) and log MCP-1 (p=0.006), compared with the premenopausal women with measurable AMH. Furthermore, reduced ovarian reserve in the HIV-infected group related to noncalcified plaque, controlling for traditional CVD risk factors (p=0.04) and sCD163 (p=0.03).
Conclusions
HIV-infected women with reduced ovarian reserve have increased subclinical coronary atherosclerotic plaque compared with premenopausal women in whom AMH is measurable. This relationship holds when controlling for CVD risk factors (including age) and immune activation. Our findings demonstrate that reduced ovarian reserve may contribute to CVD burden in HIV-infected women and support a comprehensive assessment of CVD risk prior to completion of menopause in this population.
Keywords: Women, HIV, Ovarian Reserve, Menopause, Cardiovascular Diseases
Introduction
In the United States, HIV-infected women face a three-fold increased rate of myocardial infarction and two-fold increased rate of stroke compared to non-HIV-infected women [1] [2] [3]. With respect to mechanisms underlying this heightened risk, HIV-infected women have higher rates of select traditional risk cardiovascular disease (CVD) risk factors than are seen in the general population [4]. Compared with non-HIV-infected women, HIV-infected women on antiretroviral therapy (ART) also have higher-level immune activation [5], a potential non-traditional risk factor for HIV-associated CVD. Further, among HIV-infected women without known CVD, immune activation is increased in relation to presence of subclinical noncalcified coronary atherosclerotic plaque [5]. In regions where ART is readily accessible, the percentage of HIV-infected adults greater than 50 years old is steadily increasing [6]. Thus, it will be critical to understand the ways in which reproductive aging contributes to CVD risk in this population.
Reproductive aging leading to menopause (cessation of menses ≥12 months) is characterized by loss of ovarian reserve. Ovarian reserve may be quantified through measurement of antimullerian hormone (AMH), a protein encoded by the AMH gene and secreted by ovarian granulosa cells [7]. AMH levels decline as women transition into menopause and levels may drop nearly five years before the final menstrual period [8]. Indeed, AMH levels may be useful in predicting age at menopause [8] [9]. Among HIV-infected women (versus non-HIV-infected women), the menopausal transition appears to occur earlier [10] [11], and age-adjusted AMH levels are lower in HIV-infected women versus uninfected women [12]. Prior studies suggest a potential relationship between reduced ovarian reserve and increased CVD risk [13] [14]. However, the relationship between reduced ovarian reserve, postmenopausal status, and CVD risk among HIV-infected women remains unknown. Moreover, there is incomplete understanding as to how the hormonal milieu in aging HIV-infected women influences immune activation/immunosenescence and whether changes in immune function brought about by reproductive aging contribute to HIV-associated CVD.
In this study, we investigate differences in CVD risk parameters, including immune markers and subclinical atherosclerotic plaque, among HIV-infected and non-HIV-infected women carefully characterized by menopause status and degree of ovarian reserve. CVD risk parameters among HIV-infected women in sequential stages of reproductive aging are assessed.
Methods
Study Participants
A prior investigation of coronary plaque burden and immune activation among a cohort of sixty HIV-infected and thirty non-HIV-infected women was previously published [5]. Seventy-four women from the original study cohort (49 HIV-infected and 25 non-HIV-infected) with data regarding date of last menstrual period and specimens for processing hormones were included in this analysis. As previously described [5], participants were recruited from HIV clinics, community health centers and newspaper advertisements. Women aged 18–60, without known CVD were eligible. Women with renal disease were excluded to reduce risk related to contrast-enhanced computed tomography angiography (CCTA). Participants receiving ART were on stable therapy for >3 months [5]. Data from women who reported current use of medroxyprogesterone (pharmacologic amenorrhea) were excluded. This study was approved by the Partners Institutional Review Board, and informed consent was provided by all participants.
Calculation of Cardiovascular Risk Scores
Framingham Point Score and 10-year ASCVD risk score (Pooled Cohort Equation) were calculated. 10-year ASCVD risk score was calculated for individuals age ≥40. For individuals whose total cholesterol, high density lipoprotein (HDL) cholesterol, and/or systolic blood pressure fell outside the bounds defined by the risk score calculator, the value closest to the acceptable bound was used.
Lipids and Chemistries
Total cholesterol, low density lipoprotein (LDL), HDL, triglyceride, and creatinine levels were measured via standard techniques after a twelve hour fast.
Hormone Parameters
Antimullerian hormone (AMH) was measured via enzyme linked immunuosorbent assay (ELISA, Ansh Labs; <0.023 ng/mL is below the limit of detection). Serum estradiol and follicle stimulating hormone (FSH) levels were measured via Access Chemiluminescent Immunoassay (Beckman Coulter, Fullerton, California) and were not timed to menstrual cycle phase.
Paradigm for Characterizing Reproductive Aging in Women
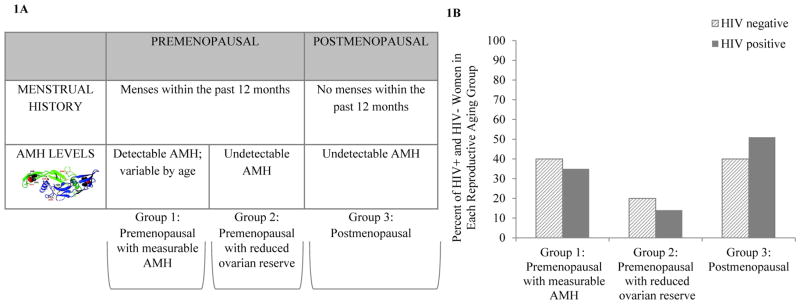
A prospectively delineated 3-group paradigm for characterizing reproductive aging was applied to our study population. This paradigm is based on traditional historical data (menstrual history) and levels of AMH – a marker of ovarian reserve (Figure 1a).
Figure 1.
Figure 1AReproductive Aging Classification Scheme. Women with menses within the past 12 months and with detectable AMH levels (≥0.023 ng/mL) were classified as premenopausal with measurable AMH (Group 1). Women with menses within the past 12 months and with undetectable AMH levels (< 0.023 ng/mL) were classified as premenopausal with reduced ovarian reserve (Group 2). Women with greater than or equal to 1 year of amenorrhea and undetectable AMH levels (< 0.023 ng/mL) were classified as postmenopausal (Group 3).
Figure 1B: Reproductive Aging Classification of Women with and without HIV Infection. Group 1 represents premenopausal women with measurable AMH (35% HIV-infected, 40% non-HIV-infected); Group 2 represents premenopausal women with reduced ovarian reserve/undetectable AMH (14% HIV-infected, 20% non-HIV-infected); Group 3 represents postmenopausal women (51% HIV-infected, 40% non-HIV-infected).
Markers of Immune Activation and HIV-associated Parameters
Soluble CD163 (sCD163) (Trillium), soluble CD14 (sCD14) (R&D), monocyte chemoattractant protein-1 (MCP-1) (R&D), and CXCL10 (R&D) were determined by enzyme-linked immunosorbent assay (ELISA). HIV viral load was measured by ultrasensitive reverse-transciption polymerase chain reaction (RT PCR) (Roche COBAS Amplicor; lower limit of detection, 50 copies/mL). CD4 count was assessed by flow cytometry.
Contrast-Enhanced Computed Tomography Coronary Angiography (CCTA)
CCTA imaging was performed using a 64-slice CT scanner (Siemens Medical Solution). A standardized protocol was implemented for assessment of calcified and noncalcified plaque [5] [15] [16].
Statistical Analysis
Distribution of variables was assessed via histograms and application of the Shapiro-Wilk test. Normally distributed data were presented as mean ± standard deviation while non-normally distributed data were presented as median (interquartile range, IQR).
First, comparisons were made between HIV-infected and non-HIV-infected groups. For these 2-group comparisons: Normally distributed data were analyzed via Student’s t-test. Non-normally distributed data were evaluated by the Wilcoxon rank-sum test. Categorical variables were analyzed using the Chi-square (Chi2) test.
Within the HIV-infected and non-HIV-infected groups, comparisons were made by reproductive aging group. First, comparisons were made across all three reproductive aging groups. ANOVA was used for comparison of normally distributed data while Kruskal-Wallis test was used for comparison of non-normally distributed data. Categorical variables were analyzed using the Chi2 test. Next, comparisons were made between premenopausal women with measurable AMH (Group 1) versus women with reduced ovarian reserve (undetectable AMH: Groups 2 and 3) by Student’s t-test, Wilcoxon rank-sum test, or Chi2 test, as appropriate. Finally, comparisons were made between premenopausal (Groups 1 and 2) versus postmenopausal (Group 3) women.
Multivariate modeling was performed for number of noncalcified plaque segments. Among the HIV-infected women, three models were constructed – the first with reduced ovarian reserve and Framingham Point Score (traditional CVD risk), second with reduced ovarian reserve and log sCD163 (as a measure of non-traditional immune-based CVD risk), and the third with reduced ovarian reserve and Hepatitis C virus (HCV) co-infection. In the second model among HIV-infected individuals, the interaction between reduced ovarian reserve and log sCD163 was tested. Among the non-HIV-infected group, reduced ovarian reserve and Framingham Point Score were entered into the model. Framingham Point Score was selected over 10-year ASCVD risk score because 10-year ASCVD risk score could not be characterized among female participants <40 years old. SAS JMP software (version 11.0, SAS Institute) was used to perform all statistical analyses.
Results
Demographic, Clinical, and Reproductive Aging Characteristics among HIV-infected and Non-HIV-infected Women
The demographic, clinical, and reproductive aging characteristics of HIV-infected and non-HIV-infected women in our cohort are described in Table 1. The median age in both groups was 47. The ethnic make-up of both groups was similar. There were no significant differences between groups in traditional CVD factors. Summative traditional CVD scores (Framingham Point Score and 10-year ASCVD risk score) were similar between groups and relatively low in both groups. The HIV-infected group demonstrated higher levels of select immune activation markers including sCD163, sCD14, and CXCL10. Consistent with prior data [5], the overall prevalence of any subclinical atherosclerotic plaque on CCTA was similar between groups (33% non-HIV versus 34% HIV, p=0.95); however, among the HIV-infected women, the percent of plaque segments which were noncalcified was higher (0% non-HIV versus 75% HIV, p=0.03) (Table 1).
TABLE 1.
Demographic, Clinical, and Reproductive Aging Characteristics of HIV-infected and Non-HIV-infected Women
| Non-HIV (N=25) | HIV (N=49) | P values | |
|---|---|---|---|
| DEMOGRAPHICS AND TRADITIONAL CV RISK PARAMETERS | |||
|
| |||
| Race/Ethnicity | 0.85 | ||
| White | 28% (7/25) | 27% (13/49) | |
| Black/African-American | 64% (16/25) | 59% (29/49) | |
| Hispanic | 4% (1/25) | 8% (4/49) | |
| Other | 4% (1/25) | 6% (3/49) | |
| Age (years) | 47 (46, 51) | 47 (44, 53) | 1.0 |
| Current Statin Use | 4% (1/25) | 8% (4/49) | 0.48 |
| Current HTN | 24% (6/25) | 12% (6/49) | 0.20 |
| Current DM | 16% (4/25) | 12% (6/49) | 0.66 |
| Current Smoking | 56% (14/25) | 47% (23/49) | 0.46 |
| Total Cholesterol (mmol/L) | 4.7 ± 0.7 | 4.9 ± 1.1 | 0.53 |
| LDL Cholesterol (mmol/L) | 2.6 ± 0.7 | 2.8 ± 1.0 | 0.44 |
| HDL Cholesterol (mmol/L) | 1.6 ± 0.5 | 1.6 ± 0.5 | 0.87 |
| Triglycerides (mmol/L) | 0.9 (0.7, 1.4) | 1.0 (0.8, 1.3) | 0.62 |
| SBP (mm Hg) | 122 ± 17 | 114 ± 13 | 0.04 |
| Current IVDU | 4% (1/25) | 2% (1/49) | 0.63 |
| Active Cocaine Use | 0% (0/25) | 10% (5/49) | 0.04 |
| Creatinine (μmol/L) | 71 (62, 71) | 71 (62, 80) | 0.12 |
| BMI (kg/m2) | 29 ± 5 | 28 ± 5 | 0.42 |
| WHR (iliac waist) | 0.91 ± 0.07 | 0.91 ± 0.06 | 0.74 |
| Framingham Point Score | 11 ± 5 | 10 ± 5 | 0.86 |
| ASCVD Risk Score (%)* | 2.0 (0.8, 4.4) | 1.4 (0.8, 3.0) | 0.40 |
| HCV Co-Infection | 20% (5/25) | 31% (15/49) | 0.32 |
|
| |||
| HIV-SPECIFIC PARAMETERS | |||
|
| |||
| Years Since HIV | 15 ± 6 | ||
| Currently on ART | 98% (48/49) | ||
| Duration ART (years) | 8 ± 5 | ||
| Current PI | 59% (29/49) | ||
| Duration PI (years) | 2 (0, 8) | ||
| Current NRTI | 90% (44/49) | ||
| Duration NRTI (years) | 7 ± 5 | ||
| Current NNRTI | 18% (9/49) | ||
| Duration NNRTI (years) | 0 (0, 2) | ||
| CD4 Count (cells/mm3) | 518 (405, 712) | ||
| Nadir CD4 (cells/mm3) | 198 (57, 250) | ||
| Log VL (copies/mL) | 4.1 ± 0.9 | ||
| VL Undetectable | 83% (38/46) | ||
|
| |||
| HORMONAL PARAMETERS | |||
|
| |||
| Estradiol (pmol/L) | 132 (29, 367) | 77 (22, 246) | 0.37 |
| FSH (mIU/mL) | 21 (7, 47) | 45 (8, 68) | 0.22 |
| AMH (ng/mL) | <0.023 (<0.023, 0.53) | <0.023 (<0.023, 0.45) | 0.61 |
|
| |||
| IMMUNE PARAMETERS | |||
|
| |||
| Log sCD163 (ng/mL) | 3.0 ± 0.3 | 3.2 ± 0.2 | 0.006 |
| Log sCD14 (ng/mL) | 2.9 ± 0.5 | 3.2 ± 0.4 | 0.02 |
| Log MCP-1 (pg/mL) | 2.3 ± 0.2 | 2.4 ± 0.2 | 0.14 |
| Log CXCL10 (pg/mL) | 2.1 ± 0.3 | 2.4 ± 0.3 | 0.0008 |
|
| |||
|
CARDIAC CT PARAMETERS
| |||
| Any Plaque | 33% (8/24) | 34% (15/44) | 0.95 |
| # Plaque Segments | 0 (0, 2.8) 1.2 ± 2.2 |
0 (0, 2.0) 1.3 ± 2.4 |
0.94 |
| Any Noncalcified Plaque Segments | 13% (3/24) | 32% (14/44) | 0.07 |
| # Noncalcified Plaque Segments | 0 (0, 0) 0.4 ± 1.5 |
0 (0, 1.8) 0.8 ± 1.4 |
0.09 |
| % of Plaque Segments which are Noncalcified | 0 (0, 67) | 75 (50, 100) | 0.03 |
Normally distributed data are presented as means ± SDs or percentages; non-normally distributed data are presented as median (interquartile range). Abbreviations: HTN, hypertension; DM, diabetes mellitus; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SBP, systolic blood pressure; BMI, body mass index; WHR, waist hip ratio; ASCVD, atherosclerotic cardiovascular disease risk score; HCV, hepatitis C virus; ART, antiretroviral therapy; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor; VL, viral load; FSH, follicle stimulating hormone; AMH antimullerian hormone; MCP-1, monocyte chemoattractant protein-1. P ≤ 0.05 indicates statistical significance.
Missing data on 8 subjects.
Levels of AMH, estradiol, and FSH were not significantly different between HIV and non-HIV-infected women (Table 1). A prospectively delineated paradigm for characterizing reproductive aging was applied to both groups, as per the methods section. Of note, one participant who had undergone hysterectomy and who had a measurable AMH level was classified as Group 1. Four participants who had undergone hysterectomy and who had undetectable AMH levels were classified as Group 3. Overall, the distribution of women into reproductive aging categories was similar between HIV-infected and non-HIV-infected women (Figure 1b).
Comparison of Traditional and Non-traditional CVD Risk Parameters and Subclinical Atherosclerotic Plaque Among Women in Sequential Stages of Reproductive Aging
HIV-Infected Women
A comparison of CVD risk parameters and plaque parameters among women in sequential stages of reproductive aging is shown in Table 2. AMH levels were undetectable in the premenopausal women with reduced ovarian reserve and in the postmenopausal women (Groups 2 and 3). As would be expected, estradiol levels were higher and FSH levels were lower in the premenopausal women with measurable AMH (Group 1 versus Group 2 and Group 3). Also as expected, chronological age increased along the reproductive aging spectrum: Group 1 (40 years (36, 45)), Group 2 (47 years (47, 49)), and Group 3 (52 years (48, 57)); p<0.0001. There were no statistically significant differences in hypertension, diabetes, or smoking among HIV-infected women in our cohort across reproductive aging groups. The Framingham Point Score increased along the reproductive aging spectrum, and there was a trend toward an increase in 10-year ASCVD risk score. With respect to HIV-specific risk factors, the percent of women with undetectable viral load was similar between groups, as was the CD4 count. A higher proportion of women in Groups 2 and 3 were co-infected with HCV (Table 2).
TABLE 2.
Comparison of Traditional and Non-traditional CV Risk Parameters and Subclinical Coronary Atherosclerotic Plaque Among HIV-infected Women in Sequential Stages of Reproductive Aging
| Premenopausal with Measurable AMH (Group 1) (N=17) | Premenopausal with Reduced Ovarian Reserve (Group 2) (N=7) | Post-menopausal (Group 3) (N= 25) | P values | |
|---|---|---|---|---|
| DEMOGRAPHICS AND TRADITIONAL CV RISK PARAMETERS | ||||
|
| ||||
| Race/Ethnicity | 0.62 | |||
| White | 23% (4/17) | 43% (3/7) | 24% (6/25) | |
| Black/African-American | 65% (11/17) | 57% (4/7) | 56% (14/25) | |
| Hispanic | 12% (2/17) | 0% (0/7) | 8% (2/25) | |
| Other | 0% (0/17) | 0% (0/7) | 12% (3/25) | |
| Age (years) | 40 (36, 45) | 47 (47, 49) | 52 (48, 57) | <0.0001 |
| Current Statin Use | 12% (2/17) | 0% (0/7) | 8% (2/25) | 0.48 |
| Current HTN | 12% (2/17) | 0% (0/7) | 16% (4/25) | 0.34 |
| Current DM | 6% (1/17) | 0% (0/7) | 20% (5/25) | 0.15 |
| Current Smoking | 47% (8/17) | 71% (5/7) | 40% (10/25) | 0.33 |
| TotalCholesterol (mmol/L) | 4.9 ± 1.1 | 4.2 ± 0.8 | 5.1 ± 1.2 | 0.21 |
| LDL Cholesterol (mmol/L) | 2.9 ± 1.0 | 2.2 ± 0.5 | 2.8 ± 1.0 | 0.27 |
| HDL Cholesterol (mmol/L) | 1.4 ± 0.4 | 1.6 ± 0.7 | 1.6 ± 0.5 | 0.51 |
| Triglycerides (mmol/L) | 0.9 (0.8, 1.6) | 1.1 (0.7, 1.1) | 1.0 (0.8, 1.4) | 0.83 |
| BMI (kg/m2) | 29 ± 5 | 27 ± 5 | 27 ± 5 | 0.41 |
| Framingham Point Score | 8 ± 5 | 11 ± 4 | 12 ± 4 | 0.009 |
| ASCVD Risk Score (%)* | 0.8 (0.5, 1.8) | 1.2 (0.9, 3.2) | 1.8 (0.9, 4.4) | 0.11 |
| HCV Co-Infection | 6% (1/17) | 43% (3/7) | 44% (11/25) | 0.01 |
|
| ||||
| HIV SPECIFIC PARAMETERS | ||||
|
| ||||
| Years Since HIV | 13 ± 4 | 14 ± 3 | 16 ± 7 | 0.39 |
| Currently on ART | 100% (17/17) | 86% (6/7) | 100% (25/25) | 0.13 |
| Duration ART (years) | 8 ± 4 | 6 ± 5 | 8 ± 5 | 0.61 |
| Current PI | 47% (8/17) | 71% (5/7) | 64% (16/25) | 0.43 |
| Duration PI (years) | 2 (0, 9) | 2 (0, 11) | 3 (1, 8) | 0.84 |
| Current NRTI | 94% (16/17) | 71% (5/7) | 92% (23/25) | 0.31 |
| Duration NRTI (years) | 7 ± 4 | 4 ± 5 | 7 ± 5 | 0.33 |
| Current NNRTI | 35% (6/17) | 14% (1/7) | 8% (2/25) | 0.08 |
| Duration NNRTI (years) | 2 (0, 4) | 0 (0, 2) | 0 (0, 0) | 0.01 |
| CD4 Count (cell/mm3) | 535 (415, 723) | 602 (416, 706) | 513 (389, 737) | 0.99 |
| Nadir CD4 (cells/mm3) | 140 (63, 250) | 249 (29, 350) | 180 (50, 200) | 0.47 |
| Log VL (copies/mL) | 4.0 ± 0.8 | 5.0 ± 1.9 | 3.9 ± 0.3 | 0.03 |
| VL Undetectable | 81% (13/16) | 71% (5/7) | 87% (20/23) | 0.64 |
|
| ||||
| HORMONAL PARAMETERS | ||||
|
| ||||
| Estradiol (pmol/L) | 345 (128, 606) | 40 (22, 70) | 33 (22, 88) | <0.0001 |
| FSH (mIU/mL) | 7 (6, 11) | 57 (50, 71) | 64 (43, 78) | <0.0001 |
| AMH <0.023 ng/mL | 0% (0/17) | 100% (7/7) | 100% (25/25) | <0.0001 |
|
| ||||
| IMMUNE PARAMETERS | ||||
|
| ||||
| Log sCD163 (ng/mL) | 3.1 ± 0.2 | 3.3 ± 0.2 | 3.3± 0.2 | 0.002 |
| Log sCD14 (ng/mL) | 3.4 ± 0.3 | 3.0 ± 0.6 | 3.2± 0.4 | 0.13 |
| Log MCP-1 (pg/mL) | 2.2 ± 0.2 | 2.4 ± 0.2 | 2.4± 0.2 | 0.02 |
| Log CXCL10 (pg/mL) | 2.2 ± 0.3 | 2.3 ± 0.3 | 2.5± 0.4 | 0.13 |
|
| ||||
| CARDIAC CT PARAMETERS | ||||
|
| ||||
| Any Plaque | 6% (1/17) | 67% (4/6) | 48% (10/21) | 0.002 |
| # Plaque Segments | 0 (0, 0) 0.1 ± 0.2 |
2.0 (0, 4.5) 2.3 ± 2.4 |
0 (0, 3.5) 2.0 ± 3.0 |
0.005 |
| Any Noncalcified Plaque | 6% (1/17) | 67% (4/6) | 43% (9/21) | 0.004 |
| # Noncalcified Plaque Segments | 0 (0, 0) 0.1 ± 0.2 |
1.5 (0, 2.3) 1.3 ± 1.2 |
0 (0, 2.5) | 0.009 |
| % of Plaque Segments which are Noncalcified | 100 (100, 100) | 58 (50, 92) | 75 (58, 100) | 0.41 |
Normally distributed data are presented as means ± SDs or percentages; non-normally distributed data are presented as median (interquartile range). Abbreviations: HTN, hypertension; DM, diabetes mellitus; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BMI, body mass index; ASCVD, atherosclerotic cardiovascular disease risk score; HCV, hepatitis C virus; ART, antiretroviral therapy; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor; VL, viral load; FSH, follicle stimulating hormone; AMH antimullerian hormone; MCP-1, monocyte chemoattractant protein-1. P ≤ 0.05 indicates statistical significance.
Missing data on 7 subjects.
With respect to subclinical coronary atherosclerotic plaque, there was an increase across the reproductive aging spectrum in parameters including any plaque, any noncalcified plaque, and number of noncalcified plaque segments. Specifically, the prevalence of any subclinical coronary atherosclerotic plaque increased significantly across the reproductive aging spectrum (6% in Group 1, 67% in Group 2, and 48% in Group 3, p=0.002). Importantly, the “step-up” for these parameters was between Group 1 (premenopausal women with measurable AMH) and Group 2 (premenopausal women with reduced ovarian reserve/undetectable AMH). A second step-up in these parameters was not observed between Group 2 and Group 3 (postmenopausal women). Significant trends with respect to levels of immune activation markers among women along the reproductive aging spectrum also emerged. Specifically, levels of sCD163 and MCP-1 increased across stages of reproductive aging; levels of CXCL10 also tended to increase (Table 2).
Non-HIV-infected Women
Among non-HIV-infected women, the prevalence of subclinical coronary atherosclerotic plaque increased significantly across the reproductive aging spectrum (10% in Group 1, 25% in Group 2, and 60% in Group 3, p=0.05); however, the largest step-up was between premenopausal women with reduced ovarian reserve/undetectable AMH and postmenopausal women. Immune activation markers assessed did not increase significantly across the reproductive aging spectrum (data not shown).
Comparison of Premenopausal Women with Measurable AMH (Group 1) and Women with Reduced Ovarian Reserve (Groups 2 and 3)
HIV-Infected Women
Select, but not all, traditional CVD risk factors differed in the comparison of Groups 1 versus Group 2 and 3 (Table 3). As would be expected, the group with reduced ovarian reserve (versus the group with measurable AMH) was older (49 years (47, 55) versus 40 years (36, 45), p<0.0001) and had a higher Framingham Point Score (12±4 versus 8±5, p=0.006) and 10-year ASCVD Risk Score (1.7% versus 0.8%, p=0.04). Prevalence of hypertension and cigarette smoking was similar between groups. BMI was also similar between groups. HIV-specific parameters were similar, except for HCV Co-Infection, which was more prevalent among women with reduced ovarian reserve in this group (44% versus 6%, p=0.003).
TABLE 3.
Comparison of Traditional and Non-traditional CVD Risk Parameters and Subclinical Coronary Atherosclerotic Plaque Among HIV-infected Premenopausal Women with Measurable AMH (Group 1) and HIV-infected Women with Reduced Ovarian Reserve (Groups 2 and 3)
| Premenopausal with Measurable AMH (Group 1) (N=17) | Reduced Ovarian Reserve (undetectable AMH) (Groups 2 and 3) (N=32) | P Values | |
|---|---|---|---|
| DEMOGRAPHICS AND TRADITIONAL CV RISK PARAMETERS | |||
|
| |||
| Race/Ethnicity | 0.53 | ||
| White | 23% (4/17) | 28% (9/32) | |
| Black/African-American | 65% (11/17) | 56% (18/32) | |
| Hispanic | 12% (2/17) | 6% (2/32) | |
| Other | 0% (0/17) | 10% (3/32) | |
| Age (years) | 40 (36, 45) | 49 (47, 55) | <0.0001 |
| Current Statin Use | 12% (2/17) | 6% (2/32) | 0.51 |
| Current HTN | 12% (2/17) | 13% (4/32) | 0.94 |
| Current DM | 6% (1/17) | 16% (5/32) | 0.30 |
| Current Smoking | 47% (8/17) | 47% (15/32) | 0.99 |
| Total Cholesterol (mmol/L) | 4.9 ± 1.1 | 4.9 ± 1.1 | 0.96 |
| LDL Cholesterol (mmol/L) | 2.9 ± 1.0 | 2.7 ± 1.0 | 0.61 |
| HDL Cholesterol (mmol/L) | 1.4 ± 0.4 | 1.6 ± 0.5 | 0.23 |
| Triglycerides (mmol/L) | 0.9 (0.8, 1.6) | 1.0 (0.8, 1.3) | 0.73 |
| BMI (kg/m2) | 29 ± 5 | 27 ± 5 | 0.18 |
| Framingham Point Score | 8 ± 5 | 12 ± 4 | 0.006 |
| ASCVD Risk Score (%)* | 0.8 (0.5, 1.8) | 1.7 (0.9, 3.5) | 0.04 |
| HCV Co-Infection | 6% (1/17) | 44% (14/32) | 0.003 |
|
| |||
| HIV SPECIFIC PARAMETERS | |||
|
| |||
| Years Since HIV | 13 ± 4 | 15 ± 6 | 0.20 |
| Currently on ART | 100% (17/17) | 97% (31/32) | 0.35 |
| Duration ART (years) | 8 ± 4 | 7 ± 5 | 0.75 |
| CD4 Count (cells/mm3) | 535 (415, 723) | 516 (403, 714) | 0.98 |
| Nadir CD4 (cells/mm3) | 140 (63, 250) | 199 (45, 249) | 0.71 |
| Log VL (copies/mL) | 4.0 ± 0.8 | 4.2 ± 1.0 | 0.63 |
| VL Undetectable | 81% (13/16) | 83% (25/30) | 0.86 |
|
| |||
| IMMUNE PARAMETERS | |||
|
| |||
| Log sCD163 (ng/mL) | 3.1 ± 0.2 | 3.3 ± 0.2 | 0.0004 |
| Log sCD14 (ng/mL) | 3.4 ± 0.3 | 3.1 ± 0.4 | 0.04 |
| Log MCP-1 (pg/mL) | 2.2 ± 0.2 | 2.4 ± 0.2 | 0.006 |
| Log CXCL10 (pg/mL) | 2.2 ± 0.3 | 2.4 ± 0.4 | 0.07 |
|
| |||
| CARDIAC CT PARAMETERS | |||
|
| |||
| Any Plaque | 6% (1/17) | 52% (14/27) | 0.0007 |
| # Plaque Segments | 0 (0, 0) 0.1 ± 0.2 |
1.0 (0, 4.0) 2.1 ± 2.8 |
0.002 |
| Any Noncalcified Plaque Segments | 6% (1/17) | 48% (13/27) | 0.002 |
| # Noncalcified Plaque Segments | 0 (0, 0) 0.1 ± 0.2 |
0 (0, 2.0) 1.3 ± 1.7 |
0.003 |
| % of Plaque Segments which are Noncalcified | 100 (100, 100) | 71 (50, 100) | 0.29 |
Normally distributed data are presented as means ± SDs or percentages; non-normally distributed data are presented as median (interquartile range). Abbreviations: HTN, hypertension; DM, diabetes mellitus; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BMI, body mass index; ASCVD, atherosclerotic cardiovascular disease risk score; HCV, hepatitis C virus; ART, antiretroviral therapy; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor; VL, viral load; MCP-1, monocyte chemoattractant protein-1. P ≤ 0.05 indicates statistical significance.
Missing data on 7 subjects.
Among women with reduced ovarian reserve (versus the group with measurable AMH), there was an increased prevalence of any subclinical coronary atherosclerotic plaque (52% versus 6%, p=0.0007) and an increased number of coronary atherosclerotic plaque segments per participant (2.1±2.8 versus 0.1±0.2, p=0.002) (Table 3). There was also an increased prevalence of noncalcified coronary atherosclerotic plaque (48% versus 6%, p=0.002) (Supplemental Figure 2) and an increased number of noncalcified coronary atherosclerotic plaque segments (1.3±1.7 versus 0.1±0.2, p=0.003). Immune parameters also differed between groups: Among women with reduced ovarian reserve (versus women with measurable AMH), there were higher levels of log sCD163 (p=0.0004) and log MCP-1 (p=0.006), and there was a trend toward higher levels of log CXCL10 which did not reach statistical significance. In contrast, log sCD14 was higher among women with measurable AMH as compared with women with reduced ovarian reserve (p=0.04).
Non-HIV-infected Women
Among non-HIV-infected women, women with reduced ovarian reserve had a higher prevalence of plaque compared with women with measurable AMH (50% versus 10%, p=0.03), but no other between-group differences in plaque parameters reached statistical significance (Supplemental Table 1). There were no statistically significant differences between groups in markers of immune activation (Supplemental Table 1).
Comparison of Premenopausal Women (Groups 1 and 2) and Postmenopausal Women (Group 3)
HIV-Infected Women
Comparing CVD risk factors between Groups 1 and 2 versus Group 3, the postmenopausal group was older (52 years (48, 57) versus 44 years (38, 47) p<0.0001) and had a higher total Framingham Point Score (12±4 versus 9±5, p=0.01) (Supplemental Table 2). Prevalence of cigarette smoking was similar between groups. There was a trend toward an increased prevalence of hypertension and diabetes in the postmenopausal group. HIV-specific parameters were similar, save for HCV co-infection, which was more prevalent among postmenopausal women in this cohort.
In contrast with the significant trends in subclinical coronary atherosclerotic plaque parameters between women with reduced ovarian reserve versus women with measurable AMH (Table 3), no statistically significant differences in plaque parameters were observed in premenopausal versus postmenopausal women (Supplemental Table 2). There was, however, a trend toward higher prevalence of any plaque, number of plaque segments, prevalence of any noncalcified plaque, and number of noncalcified plaque segments among the postmenopausal (versus premenopausal) women. With respect to immune parameters, among postmenopausal (versus premenopausal) women, increased levels of several immune activation markers were observed including log sCD163 (p=0.05), log MCP-1 (p=0.02), and log CXCL10 (p=0.05).
Non-HIV-infected Women
Among non-HIV-infected women, those who were postmenopausal (versus premenopausal) had a significantly higher prevalence of plaque (60% versus 14%, p=0.02) and number of plaque segments, as well as a higher prevalence of noncalcified plaque and number of noncalcified plaque segments (Supplemental Table 3).There were no significant between-group differences in levels of immune activation parameters (Supplemental Table 3).
Multivariate modeling for number of noncalcified plaque segments
Among HIV-infected women, reduced ovarian reserve remained significantly related to number of noncalcified plaque segments controlling for Framingham Point Score (Supplemental Table 4, Model 1a). Among HIV-infected women, reduced ovarian reserve also remained significantly related to noncalcified coronary atherosclerotic plaque controlling for immune activation (sCD163), and there was not a significant interaction between reduced ovarian reserve and immune activation (Supplemental Table 4, Model 1b). Finally, among HIV-infected women, reduced ovarian reserve remained significantly related to noncalcified coronary atherosclerotic plaque controlling for HCV co-infection (overall R2 0.18, p value for overall model 0.02, p value for reduced ovarian reserve 0.006). HCV did not remain significantly related to noncalcified plaque in this model (p=0.72). In contrast, among non-HIV-infected women, Framingham Point Score trumped reduced ovarian reserve in contribution to number of noncalcified plaque segments (Supplemental Table 4, Model 2).
Discussion
In this study, we show for the first time that HIV-infected women with reduced ovarian reserve defined by undetectable levels of AMH have a higher prevalence and burden of subclinical coronary atherosclerotic plaque and noncalcified plaque versus premenopausal HIV-infected with measurable AMH. We further show that among HIV-infected women, reduced ovarian reserve relates to noncalcified coronary atherosclerotic plaque even after controlling for traditional CVD risk factors – including chronological age – encompassed in the Framingham Point Score. We also demonstrate that among HIV-infected women, levels of select markers of immune activation including sCD163 and MCP-1 increase across the reproductive aging spectrum. Ongoing work is needed to characterize mechanisms through which reproductive aging influences immune activation and coronary atherosclerotic plaque among HIV-infected women.
In the general population, postmenopausal women are at increased risk of CVD (versus premenopausal women) but the mechanisms underlying this heightened risk remain unclear [17]. Compared with premenopausal women, postmenopausal women have alterations in lipid metabolism; a higher prevalence of hypertension and diabetes; increased BMI and visceral adiposity; increased endothelial dysfunction and decreased arterial dispensability; and alterations in immune function and oxidative stress potentially relevant to atherogenesis [18] [19] [20] [21]. Among aging women, the relative contributions of chronological/somatic aging versus reproductive aging (altered hormonal milieu [22]) to increased atherogenicity remain unclear. Importantly, numerous atherogenic changes contributing to heightened CVD risk occur along a continuum among aging women and begin well before the menopause transition is complete [23]. Thus, imperatives exist to refine strategies for identifying women at heightened risk for CVD and to elucidate mechanisms underlying this risk.
Through our work, we compare CVD risk across reproductive aging categories employing a carefully designed classification scheme based on menstrual history and levels of AMH, which are not dependent on menstrual cycle phase [7]. The most widely accepted traditional reproductive aging classification scheme is based on the STRAW criteria [24]. These criteria are reliant on menstrual history as well as levels of FSH and E2 timed to menstrual cycle day 3. There are challenges, however, to applying the STRAW criteria to HIV-infected women. Among HIV-infected women, menstrual irregularity/anovulation is frequently observed [25]. Thus, timing hormonal assessments to day 3 of the menstrual cycle represents a serious logistic challenge for clinic patients and clinical trial participants alike. Indeed, the original STRAW system was initially developed and validated in a cohort of healthy women, and the North American Menopause Society has advised against application among women with chronic illness and/or menstrual irregularity [24]. The above caveats also apply to the updated STRAW-10 criteria [26].
Synthesizing data on AMH with data on menstrual cycles, as we do, permits us to distinguish between women with reduced ovarian reserve who have not yet completed the menopausal transition (cycles within the year and undetectable AMH) and women who have indeed completed menopause (no cycles ≥1 year and undetectable AMH). Our paradigm may be usefully applied to future studies characterizing effects of reproductive aging on CVD risk in HIV. Such studies are much needed, as our work suggests that CVD risk significantly increases prior to completion of the menopause transition among HIV-infected women.
Whether reduced ovarian reserve relates causally to increased subclinical atherosclerosis and CVD risk among HIV-infected individuals remains to be determined. Intriguingly, a study among female cynomolgus macaques fed an atherogenic diet revealed that those monkeys with the lowest tertile of baseline AMH went on to develop the largest atherosclerotic plaques [14]. In humans, no such studies relating baseline AMH levels to development of atherosclerotic plaque over time have been conducted. However, a recent study suggested that reduced duration of ovarian hormone exposure (menarche to menopause) increased a woman’s risk of developing acute myocardial infarction, ischemic heart disease, or stroke [27]. Among women with HIV, the menopausal transition may occur earlier than in women without HIV [10] [11]. Whether AMH falls to undetectable levels earlier among premenopausal HIV-infected women (versus premenopausal non-HIV-infected women) and whether AMH levels predict time-to-menopause among HIV-infected women remains unknown.
Heightened immune activation represents a theoretical intermediary between reduced ovarian reserve and heightened subclinical atherosclerosis among women with HIV. We found that among women with HIV, select immune activation markers (sCD163, MCP-1) increase along the reproductive aging spectrum [5]. Situating this finding, Fitch et al. demonstrated synergistic effects of age, sex, and HIV serostatus on levels of sCD163[5]. Martin et. al further showed that age-associated changes in immune activation are accelerated among HIV-infected women (versus uninfected women) [28]. Non-HIV studies have suggested that menopause is associated with immune activation/immunosenescence [29], and work from our group and others has suggested that these processes may promote atherogenesis in HIV [30] [31] [32]. Indeed, the recently launched REPRIEVE trial, a randomized clinical trial of pitavastatin versus placebo among 6500 HIV-infected individuals with low-to-moderate traditional CVD risk, tests the hypothesis that statin therapy will reduce ASCVD events in HIV in part through effects to dampen immune activation. It is worth noting, however, that while reduced ovarian reserve may plausibly exacerbate immune activation and subclinical atherosclerosis in HIV, the reverse causality may also be entertained [33]. That is, among HIV-infected individuals, vasculopathy and/or associated immune activation may contribute to accelerated loss of ovarian reserve. Future, prospective studies among HIV-infected women are needed to tease out the complex interplay between reduced ovarian reserve, immune activation, and accelerated atherosclerosis.
Loss of endogenous estrogen may be another theoretical intermediary between reduced ovarian reserve and heightened subclinical atherosclerosis among women with HIV. Numerous studies suggest endogenous estrogen may protect against atherogenesis, favorably influencing lipid homeostasis as well as endothelial function[34]. In our study, estradiol levels were assessed but blood sampling was not timed to the menstrual cycle, precluding inferences about the relationship between estradiol levels and subclinical coronary atherosclerosis. Future research analyzing the relationship between reduced ovarian reserve, decreased endogenous estrogen production, and atherogenesis among HIV-infected women is needed. Future research is also needed to ascertain how different non-traditional CVD risk factors (such as cocaine use[35] [36]) influence atherogenesis among HIV-infected women across the reproductive aging spectrum.
Results from our multivariate modeling suggest that among women with HIV, reduced ovarian reserve contributes to the burden of noncalcified coronary atherosclerotic plaque even when controlling for Framingham Point Score (which encompasses chronologic age). Moreover, in the HIV-infected group, the relationship between reduced ovarian reserve and noncalcified coronary atherosclerotic plaque does not appear to be mediated entirely through heightened immune activation. Additionally, in the HIV-infected group, reduced ovarian reserve relates to noncalcified coronary atherosclerotic plaque controlling for HCV co-infection. These findings are important, as noncalcified atherosclerotic plaque has been shown in the general population to be more vulnerable to rupture and result in myocardial infarction, as compared with calcified coronary atherosclerotic plaque [37]. Of note, in our cohort, parallel modeling among non-HIV-infected women suggests that Framingham Point Score relates strongly to the burden of noncalcified coronary atherosclerotic plaque in this group, overshadowing the potential contribution of reduced ovarian reserve. The overall predictive value of the model is stronger in the non-HIV-infected group. These findings reinforce that increased subclinical plaque may be seen among HIV-infected men and women with low traditional CVD risk indices [15] [32] [5].
Strengths of our study include the application of a reproductive aging categorization paradigm (incorporating both menstrual history data and cycle-independent AMH data) to relate loss of ovarian reserve and/or menopause to CVD risk in HIV. This paradigm may be employed in future studies of cardiometabolic health among HIV-infected women. Limitations of our study include the relatively small sample size, the recruitment of subjects from a single demographic region, and the cross-sectional design, which precludes determinations of causality.
Our finding that reduced ovarian reserve among women with HIV relates to subclinical atherosclerotic plaque even after controlling for traditional CVD risk (including age) is novel and clinically relevant. This finding suggests that careful CVD risk assessment may be usefully directed to HIV-infected women well before they complete the menopause transition, along with strategies to manage modifiable CVD risk factors and counseling to report cardiovascular symptoms. Moreover, our observations that markers of immune activation increase along the reproductive aging spectrum among HIV-infected women provides direction for future work analyzing the mechanistic relationship between reduced ovarian reserve and atherosclerosis in HIV.
Supplementary Material
HIV-infected women with reduced ovarian reserve have a greater prevalence of noncalcified coronary atherosclerotic plaque compared to premenopausal HIV-infected women with measurable AMH (48% versus 6%, p=0.0002).
Acknowledgments
Funding: This work was supported by Bristol Myers Squibb, Inc and the National Institutes of Health and R01 HL095123-04 to S.G., M01-RR-01066 and 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resources and P30 DK040561, Nutrition Obesity Research Center at Harvard. Funding sources had no role in the design of the study, data analysis or the writing of the manuscript.
We would like to thank the research participants and the staff from the MGH Clinical Research Center for their dedicated patient care.
Footnotes
Author Contributions
Study design (S.E.L., S.G, M.V.Z.), data collection (S.E.L., K.V.F., J.L.), data interpretation (S.E.L., K.V.F., S.S., J.L., J.C.C., S.G., M.V.Z.), drafting of manuscript (S.E.L., D.R., A.M., M.V.Z.), critical revision of manuscript (S.E.L., K.V.F., S.S., J.L., D.R., A.M., J.C.C., S.G, M.V.Z.). All authors have read and approved the submitted manuscript.
Clinical Trial Registration Number: NCT00455793
Disclosures: SG received research funding for this investigator-initiated research project through Bristol Myers Squibb, Inc. SG has served as a consultant to Navidea, Theratechnologies, Bristol Myers Squibb, Merck and Gilead, all unrelated to this project
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Womack JA, Chang CC, So-Armah KA, Alcorn C, Baker JV, Brown ST, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3:e001035. doi: 10.1161/JAHA.114.001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60:351–358. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triant VA, Regan S, Grinspoon SK. MACE Incidence Among HIV and Non-HIV-Infected Patients in a Clinical Care Cohort. CROI 2014. 2014 [Google Scholar]
- 5.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208:1737–1746. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. HIV and Aging. 2013 http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/20131101_JC2563_hiv-and-aging_en.pdf.
- 7.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 8.Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of antimullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause. 2009;16:797–802. doi: 10.1097/gme.0b013e318193e95d. [DOI] [PubMed] [Google Scholar]
- 9.Nair S, Slaughter JC, Terry JG, Appiah D, Ebong I, Wang E, et al. Anti-mullerian hormone (AMH) is associated with natural menopause in a population-based sample: The CARDIA Women’s Study. Maturitas. 2015 doi: 10.1016/j.maturitas.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoenbaum EE, Hartel D, Lo Y, Howard AA, Floris-Moore M, Arnsten JH, et al. HIV infection, drug use, and onset of natural menopause. Clin Infect Dis. 2005;41:1517–1524. doi: 10.1086/497270. [DOI] [PubMed] [Google Scholar]
- 11.Boonyanurak P, Bunupuradah T, Wilawan K, Lueanyod A, Thongpaeng P, Chatvong D, et al. Age at menopause and menopause-related symptoms in human immunodeficiency virus-infected Thai women. Menopause. 2012;19:820–824. doi: 10.1097/gme.0b013e31824cfc0f. [DOI] [PubMed] [Google Scholar]
- 12.Scherzer R, Bacchetti P, Messerlian G, Goderre J, Maki PM, Seifer DB, et al. Impact of CD4+ lymphocytes and HIV infection on Anti-Mullerian Hormone levels in a large cohort of HIV-infected and HIV-uninfected women. Am J Reprod Immunol. 2015;73:273–284. doi: 10.1111/aji.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Kat AC, Broekmans FJ, Laven JS, van der Schouw YT. Anti-Mullerian Hormone as a marker of ovarian reserve in relation to cardio-metabolic health: a narrative review. Maturitas. 2015;80:251–257. doi: 10.1016/j.maturitas.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Appt SE, Chen H, Clarkson TB, Kaplan JR. Premenopausal antimullerian hormone concentration is associated with subsequent atherosclerosis. Menopause. 2012;19:1353–1359. doi: 10.1097/gme.0b013e31825b4fe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 18.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meadows JL, Vaughan DE. Endothelial biology in the post-menopausal obese woman. Maturitas. 2011;69:120–125. doi: 10.1016/j.maturitas.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol. 2009;5:553–558. doi: 10.1038/nrendo.2009.166. [DOI] [PubMed] [Google Scholar]
- 21.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens. 2011;24:740–749. doi: 10.1038/ajh.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Azzawi F, Palacios S. Hormonal changes during menopause. Maturitas. 2009;63:135–137. doi: 10.1016/j.maturitas.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 25.Clark RA, Mulligan K, Stamenovic E, Chang B, Watts H, Andersen J, et al. Frequency of anovulation and early menopause among women enrolled in selected adult AIDS clinical trials group studies. J Infect Dis. 2001;184:1325–1327. doi: 10.1086/323999. [DOI] [PubMed] [Google Scholar]
- 26.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung KJ, Kim MR, Yun YD, Kim HC, Jee SH. Duration of ovarian hormone exposure and atherosclerotic cardiovascular disease in Korean women: the Korean Heart Study. Menopause. 2015 doi: 10.1097/GME.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 28.Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, et al. Age-Associated Changes in Monocyte and Innate Immune Activation Markers Occur More Rapidly in HIV Infected Women. PLoS One. 2013;8:e55279. doi: 10.1371/journal.pone.0055279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gameiro CM, Romao F, Castelo-Branco C. Menopause and aging: changes in the immune system--a review. Maturitas. 2010;67:316–320. doi: 10.1016/j.maturitas.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11:728–741. doi: 10.1038/nrcardio.2014.167. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, et al. Increased Coronary Atherosclerotic Plaque Vulnerability by Coronary Computed Tomography Angiography in HIV-Infected Men. AIDS. 2013 doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kok HS, van Asselt KM, van der Schouw YT, van der Tweel I, Peeters PH, Wilson PW, et al. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47:1976–1983. doi: 10.1016/j.jacc.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 34.Resanovic I, Rizzo M, Zafirovic S, Bjelogrlic P, Perovic M, Savic K, et al. Anti-atherogenic effects of 17beta-estradiol. Horm Metab Res. 2013;45:701–708. doi: 10.1055/s-0033-1343478. [DOI] [PubMed] [Google Scholar]
- 35.Lai S, Lima JA, Lai H, Vlahov D, Celentano D, Tong W, et al. Human immunodeficiency virus 1 infection, cocaine, and coronary calcification. Arch Intern Med. 2005;165:690–695. doi: 10.1001/archinte.165.6.690. [DOI] [PubMed] [Google Scholar]
- 36.Lai S, Fishman EK, Lai H, Moore R, Cofrancesco J, Jr, Pannu H, et al. Long-term cocaine use and antiretroviral therapy are associated with silent coronary artery disease in African Americans with HIV infection who have no cardiovascular symptoms. Clin Infect Dis. 2008;46:600–610. doi: 10.1086/526782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou ZH, Lu B, Gao Y, Jiang SL, Wang Y, Li W, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990–999. doi: 10.1016/j.jcmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HIV-infected women with reduced ovarian reserve have a greater prevalence of noncalcified coronary atherosclerotic plaque compared to premenopausal HIV-infected women with measurable AMH (48% versus 6%, p=0.0002).