Abstract
Pre-exposure prophylaxis (PrEP), taken as a single daily co-formulated pill containing tenofovir-emtricitabine, is a promising intervention to reduce the likelihood of HIV acquisition in at-risk individuals, including men who have sex with men (MSM). Little is known about the acceptability of less than daily, intermittent PrEP (iPrEP) regimens. We conducted an online survey of North American MSM to characterize their sexual frequency and planning behaviors and correlate these with PrEP dosing preferences. Of the 3,217 respondents who completed the survey, 46% reported engaging in unplanned condomless anal intercourse (CAI) at least once in the prior 3 months and 8% reported engaging in CAI more than once per week. In multivariable analysis, reporting unplanned CAI was associated with lower educational level, identifying as homosexual/gay as compared to bisexual, being in a monogamous relationship, having a higher self-perceived risk of HIV acquisition, higher income, engaging in CAI more than five times in the last 3 months, and not having visited a healthcare provider in the previous year. Frequent CAI (>1×/week) was associated with being younger, identifying as homosexual/gay as compared to bisexual, being in a monogamous relationship, and having a higher self-perceived risk of HIV. Having had only planned sex over the last 3 months was associated with a preference for event-based PrEP, while having frequent or unplanned CAI was associated with a preference for daily or time-driven PrEP regimens, respectively. Our findings suggest that preferences for different PrEP regimens are associated with the sexual frequency and planning behaviors of potential users.
Keywords: PrEP, intermittent PrEP, HIV prevention
INTRODUCTION
Men who have sex with men (MSM) continue to have high rates of incident HIV infection in the United States and worldwide (1,2). For this reason, robust preventative strategies are needed to limit the number of new infections in this population. Oral antiretroviral pre-exposure prophylaxis (PrEP) offers promise as a new prevention modality. In 2010, the iPrEx study demonstrated that a once daily fixed dose combination tablet containing tenofovir disoproxil fumarate and emtricitabine (TDF/FTC) reduced the risk of HIV acquisition among at-risk men and transgendered women who have sex with men compared to placebo by 44% (3). Other studies have demonstrated the efficacy of daily PrEP in reducing the risk of HIV acquisition in several populations, including HIV sero-discordant heterosexual couples (4,5) and persons who inject drugs (6). Two oral PrEP studies in African women did not demonstrate protection with PrEP (7,8); however these trials suffered from low rates of adherence. Retrospective analysis of these recent trial results found that PrEP efficacy is primarily correlated with medication adherence (9). Indeed, in the iPrEx study, the protective effect of TDF/FTC was estimated at 92% in those who had detectable levels of drug in their serum and over 99% for those with drug levels consistent with daily use (10).
In light of trial results that were positive, the U.S. Center for Disease Control and Prevention (CDC) issued guidance for healthcare practitioners to consider providing PrEP for HIV prevention in high-risk MSM in 2011 and in at-risk heterosexual individuals in 2012 (11,12). Despite these developments, PrEP has had low uptake amongst consumers and clinicians (13). A few barriers to widespread uptake of PrEP include concerns about cost, given medication expenses of over $10,000 annually, potential toxicities associated with long-term use of daily medications, and how best to optimize adherence, given the clear correlation between adherence and efficacy seen in trials (14,15). One possible solution is the development of less frequent dosing regimens that would reduce pill burden and limit potential side effects and toxicities associated with daily use. Intermittent dosing would theoretically cost less and could be appealing to those who find taking a daily pill difficult or whose risk for HIV acquisition varies over time (16).
The efficacy of intermittent PrEP was demonstrated in the CAPRISA 004 trial, which showed a 39% reduction in HIV acquisition in women who used 1% tenofovir vaginal gel before and after sexual encounters (17). The positive results of this trial were not replicated in the larger FACTS 001 trial, which suffered from low rates of adherence (18). Two studies have been undertaken to determine if oral intermittent PrEP (iPrEP) regimens are safe, efficacious, and acceptable. The IPERGAY study examined the safety and efficacy of “event driven” PrEP, defined as PrEP taken around episodes of sexual contact (19). In October, 2014, study investigators began offering PrEP to all study participants as interim analyses demonstrated that the incidence of HIV infection was significantly lower among MSM randomized to use TDF/FTC episodically as compared to placebo (20). It was recently reported that those in the “event driven” TDF/FTC arm had an 86% relative reduction in HIV incidence over the approximately 9 month follow-up period of randomization as compared to those in the placebo arm (21). The ADAPT (HPTN067) study is comparing acceptability and tolerability of several PrEP dosing regimens including daily dosing, “time driven” dosing (i.e. fixed interval) and “event driven” dosing (22). Preliminary analysis indicated greater adherence with daily dosing regimens amongst the cohort of heterosexual women in Cape Town in this study (23). The final study results, which included two cohorts of MSM, one recruited in New York City and one in Bangkok, are expected to be presented in the second half of 2015 (24). Although the complete results of these studies of less than daily PrEP are not available, the implementation of new dosing strategies will require a deeper understanding of the patterns of sexual behaviors among at-risk persons and their attitudes towards the use of iPrEP. As 2/3 of all new HIV infections in the US occur among MSM (25), and MSM who engage in online social networking may be at increased risk for HIV acquisition (26–28) and report interest in utilizing PrEP (29), additional studies to understand PrEP dosing preferences and likely adherence among subgroups of MSM who meet their partners online are needed.
The current study characterizes sexual frequency and planning behaviors in a North American population of MSM using a survey conducted through a popular online sexual networking site for MSM. We also present information regarding preferences for different PrEP dosing regimens and identify sociodemographic and behavioral factors associated with these preferences.
METHODS
Study Population
North American members of a large, multinational social networking site for MSM were invited to complete an anonymous online survey shortly after the release of the iPrEx results (December–January, 2011). Participants were eligible if they were biologically male at birth, at least 18 years of age, HIV-uninfected by self-report, and able to read/understand English and use a computer. The methods for study recruitment have been described in detail previously (29). Email broadcasts were sent out to members of the networking site in U.S. and Canada with an invitation to complete surveys assessing their knowledge of and interest in using PrEP. Previously, we reported differences in PrEP awareness and interest before and after the publication of the iPrEx study findings. For the current analysis, data was used from recruitment after the publication of iPrEx. Of the 107,559 members who received and opened emails, 18,710 (17.3%) clicked through to the survey, 6,993 (37.3%) consented to complete a pre-screening questionnaire, 6,009 (85.9%) met eligibility criteria, and 5,079 (84.5%) consented to enroll in the study and have their data analyzed; 1,862 (36.6%) participants abandoned the study before completion. Data was analyzed for the remaining 3,217 who completed the survey (supplemental figure 1).
Measures
Sociodemographics
Analyses included age, race, education level, annual income, sexual orientation, relationship status, and country of residence.
Psychosocial factors and engagement in healthcare
Problematic alcohol use was assessed by administering the 4-item CAGE questionnaire (30,31). We screened for depression by using the Centers for Epidemiologic Studies Depression (CES-D) scale, with a score of 10 or greater considered a positive screen (32). To assess engagement in healthcare, participants were asked if they had visited a provider in the last 12 months.
Self-Perceived Risk of HIV Acquisition
Self-perceived risk of HIV was assessed by asking: “Based on your sexual experiences in the past 3 months with male sex partners, if you were to rate your risk of getting HIV on a scale of 1–10, with 1 being not risky at all to 10 being extremely risky, how would you rate yourself? (1–10)” (33).
Sexual Planning
To characterize sex planning, participants were asked, “Over the past 3 months, I had sex defined as insertive or receptive anal sex with a man that was not ‘planned’ and did not use a condom at least once (true, false, unsure)”, and “Over the past 3 months, all the sex I had was planned (true, false, unsure).” “Planned” sex was defined as having an intentional arrangement to have sex with a man, such as making an appointment to have sex, or going out to a sex party, bar or club to have sex (34).
Sexual Frequency
To characterize sexual frequency, participants were asked: “On average, how often do you have anal sex with a man without a condom (i.e. his penis in your anus without a condom OR your penis in his anus without a condom)? (more than once per day, once per day, several times per week, once per week, and less than once per week).”
Sexual frequency/planning throughout the week
Respondents were asked: “Over the past week, on which days did you have anal sex with a man without a condom? Monday–Sunday (check all that apply),” followed by “Please indicate if the first act of anal sex with a man without a condom on each of those days was planned.” Respondents were also asked whether, over the last 3 months, all the sex they had was only on weekends (true/false/unsure).
Regimen Preferences
Interest in using different PrEP regimens was assessed with the following question: “Would you take PrEP (ie pre-exposure prophylaxis, medication taken by mouth before sex as protection against HIV infection) if: (a) you had to take a pill once a day for it to work (daily PrEP);? (b) you only had to take a pill every Monday and Friday plus another pill soon after sex for it to work (time driven PrEP);? and (c) you had to take a pill right before sex plus another pill soon after sex for it to work (event driven PrEP)? (yes, no, unsure).”
Statistical Analysis
Descriptive statistics for sociodemographics, self-perceived risk of HIV infection, psychosocial factors (depression, alcohol use), and healthcare access were calculated with proportions for categorical variables and medians and interquartile ranges (IQR) for continuous variables. These factors were calculated for the overall sample, and for those reporting CAI and frequent CAI (>1 one time per week). To assess factors associated with 1) engagement in CAI and 2) frequent CAI, logistic regression models were fit using sociodemographic, psychosocial, and healthcare-related covariates as independent variables. First, bivariate logistic regression models were fit for each outcome of interest. Second, multivariable logistic regression models containing all of the original covariates were fit to assess independent associations between covariates and outcomes of interest. We then built a series of logistic regression models to determine associations between sexual behavior patterns and interest in daily, event-driven, and time-driven PrEP. Independent variables included engagement in any unplanned CAI in the previous 3 months, frequent CAI, and only having planned sex over the previous 3 months. A bivariate logistic regression model was first built for each dependent-independent association of interest, followed by a multivariable logistic regression model adjusting for factors conceptualized to potentially confound these relationships, including age, education, income, sexual orientation, relationship status, depression and alcohol use, healthcare access, and nation of residence. An alpha level of 0.05 or less was considered statistically significant. All analyses were run in Stata 13.1 (StataCorp, College Station, TX).
Ethics Statement
The Institutional Review Boards of the Fenway Institute and Beth Israel Deaconess Medical Center approved the study procedures. All participants provided internet-based informed consent. As all data were collected and analyzed anonymously, documentation of written informed consent was waived by these Institutional Review Boards.
RESULTS
Demographics
The baseline demographics of the sample are reported in Table 1. The median age was 40 (IQR 28–49) and 84% identified as White; 68% had a college degree or higher, 83% identified as homosexual/gay, and 11.9% identified as being in a monogamous relationship.
Table 1.
Factors associated with engaging in unplanned condomless anal intercourse (CAI) at last once in the last 3 months (N=3,217)
| Total (N=3,217) | Engaged in Unplanned CAI (N=1,471) | Bivariate | Multivariable | |||
|---|---|---|---|---|---|---|
|
| ||||||
| OR (95% CI) | P | aOR (95% CI) | P | |||
|
| ||||||
| Age, median (IQR) | 1.00 (0.99 to 1.00) | 0.10 | 1.00 (0.99 to .100) | 0.17 | ||
|
| ||||||
| College graduate or higher | 2,212 (68.8%) | 948 (64.5%) | 0.69 (0.59 to 0.80) | <0.001 | 0.68 (0.57 to 0.80) | <0.001 |
|
| ||||||
| Annual income | ||||||
| <$17,999 | 585 (18.5%) | 253 (17.4%) | Ref | ** | Ref | ** |
| $18,000 to $29,999 | 414 (13.1%) | 217 (14.9%) | 1.45 (1.12 to 1.86) | 0.004 | 1.52 (1.16 to 2.00) | 0.003 |
| $30,000 to $59,999 | 948 (29.9%) | 444 (30.6%) | 1.16 (0.94 to 1.42) | 0.17 | 1.36 (1.07 to 1.71) | 0.01 |
| ≥$60,000 | 1,222 (38.6%) | 538 (37.1%) | 1.03 (0.85 to 1.26) | 0.76 | 1.33 (1.04 to 1.70) | 0.02 |
|
| ||||||
| Sexual orientation | ||||||
| Homosexual/Gay | 2,668 (83.0%) | 1,250 (85.1%) | Ref | ** | Ref | ** |
| Bisexual | 504 (15.7%) | 205 (14.0%) | 0.78 (0.64 to 0.94) | 0.01 | 0.80 (0.65 to 0.98) | 0.03 |
| Other | 43 (1.3%) | 14 (0.95%) | 0.55 (0.29 to 1.04) | 0.07 | 0.57 (0.29 to 1.12) | 0.10 |
|
| ||||||
| In a monogamous relationship | 384 (11.9%) | 192 (13.1%) | 1.22 (0.98 to 1.50) | 0.07 | 1.68 (1.33 to 2.12) | <0.001 |
|
| ||||||
| Self-perceived HIV risk, per 1-unit increase in perceived risk (10-point scale), median (IQR) | 3 (2 to 5) | 3 (2 to 6) | 1.28 (1.24 to 1.3) | <0.001 | 1.26 (1.22 to 1.31) | <0.001 |
|
| ||||||
| Depression | 806 (25.2%) | 375 (25.6%) | 1.04 (0.89 to 1.22) | 0.64 | 0.99 (0.83 to 1.18) | 0.91 |
|
| ||||||
| Problematic alcohol use | 538 (16.8%) | 251 (17.1%) | 1.05 (0.87 to 1.26) | 0.64 | 1.04 (0.85 to 1.27) | 0.71 |
|
| ||||||
| CAI more than 5 times in last 3 months | 389 (12.1%) | 251 (17.1%) | 2.40 (1.92 to 2.99) | <0.001 | 1.70 (1.34 to 2.17) | <0.001 |
|
| ||||||
| Visited provider in past 12 months | 2,888 (89.8%) | 1,302 (88.5%) | 0.78 (0.62 to 0.98) | 0.03 | 0.76 (0.60 to 0.98) | 0.03 |
Descriptive Analysis of Sexual Frequency and Planning
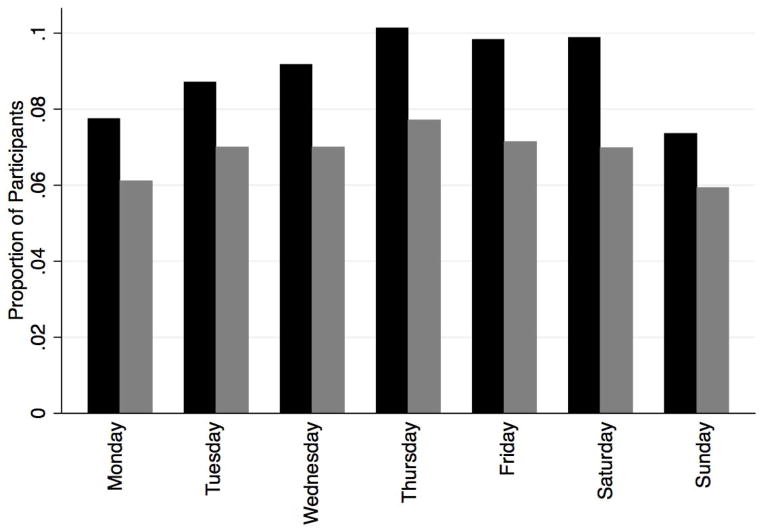
Figure 1 demonstrates the days in the past week participants reported having CAI, as well as whether CAI was planned. A majority of CAI over the course of the week was planned in advance, as up to 80% of the respondents who reported CAI on any particular day indicated that their first sex act of the day was planned. Few (14%) of respondents reported having sex only on the weekends (Friday–Sunday) over the last 3 months. Overall, 8% of participants reported engaging in CAI more than once per week on average. Nearly half (46%) of participants reported engaging in unplanned CAI at least once in the last 3 months.
Figure 1.
Condomless anal intercourse by day of week, total and planned
Black bars = percentage of respondents reporting CAI; Gray bars = percentage of respondents reporting planned CAI
Bivariate and Multivariable analysis of factors associated with frequent CAI and planning
Bivariate and multivariable analyses of factors associated with engaging in unplanned CAI at least once in the last 3 months are reported in Table 1. Multivariable analysis indicated that respondents who reported unplanned CAI in the last 3 months were significantly less likely to have a college degree or higher (aOR 0.68, 95% CI 0.57–0.80). They were also less likely to report being bisexual as compared to homosexual/gay (aOR 0.80, 95% CI 0.65–0.98) and less likely to have visited a provider in the last 12 months (aOR 0.76, 95% CI 0.60–0.98). Factors associated with unplanned CAI included higher self-perceived HIV risk (aOR 1.26, 95%CI 1.22–1.31 for every 1 unit increase in perceived risk on a 10 point scale), being in a monogamous relationship (aOR 1.68, 95% CI 0.1.33–2.12), engaging in CAI more than 5 times in the last 3 months (aOR 1.70, 95% CI 1.34–2.17), as well as having a higher annual income (as compared to <$17,999, $18,000–29,999: aOR 1.52, 95% CI 1.16–2.0; $30,000–59,999: aOR 1.36, 95% CI 1.07–1.71; ≥$60,000: aOR 1.33, 95% CI 1.04–1.70).
Table 2 demonstrates bivariate and multivariable analysis of factors associated with frequent CAI (>1 times per week). Respondents who reported frequent CAI tended to be younger (median age 37 versus 40, aOR 0.98, 95% CI 0.97–0.99), in a monogamous relationship (aOR 4.23, 95% CI 3.05–5.86), and had a higher self-perceived risk of HIV acquisition (OR 1.17, 95% CI 1.11–1.23 per 1-unit increase in perceived risk on a 10 point scale). Identifying as bisexual as compared to homosexual/gay was associated with a decreased likelihood of reporting frequent CAI (aOR 0.50, 95% CI 0.33–0.77).
Table 2.
Factors associated with engaging in frequent condomless anal intercourse (>1 time per week) (N=3,217)
| Engaged in Frequent CAI (N=264) | Bivariate | Multivariable | |||
|---|---|---|---|---|---|
|
| |||||
| OR (95% CI) | P | aOR (95% CI) | P | ||
|
| |||||
| Age, median (IQR) | 37 (27 to 46) | 0.98 (0.97 to 0.99) | 0.002 | 0.98 (0.97 to 0.99) | 0.001 |
|
| |||||
| College graduate or higher | 173 (65.5%) | 0.86 (0.66 to 1.12) | 0.26 | 0.91 (0.67 to 1.23) | 0.55 |
|
| |||||
| Annual income | |||||
| <$17,999 | 47 (17.9%) | Ref | ** | Ref | ** |
| $18,000 to $29,999 | 41 (15.7%) | 1.25 (0.80 to 1.93) | 0.32 | 1.33 (0.84 to 2.12) | 0.22 |
| $30,000 to $59,999 | 80 (30.5%) | 1.07 (0.74 to 1.56) | 0.72 | 1.28 (0.84 to 1.93) | 0.25 |
| ≥$60,000 | 94 (35.9%) | 0.96 (0.67 to 1.39) | 0.85 | 1.16 (0.74 to 1.81) | 0.52 |
|
| |||||
| Sexual orientation | |||||
| Homosexual/Gay | 233 (88.3%) | Ref | ** | Ref | ** |
| Bisexual | 27 (10.2%) | 0.59 (0.39 to 0.89) | 0.01 | 0.50 (0.33 to 0.77) | 0.002 |
| Other | 4 (1.5%) | 1.09 (0.38 to 3.07) | 0.88 | 0.88 (0.30 to 2.56) | 0.82 |
|
| |||||
| In a monogamous relationship | 70 (26.5%) | 3.00 (2.23 to 4.04) | <0.001 | 4.23 (3.05 to 5.86) | <0.001 |
|
| |||||
| Self-perceived HIV risk, per 1-unit increase in perceived risk (10-point scale), median (IQR) | 3 (2 to 6) | 1.12 (1.07 to 1.18) | <0.001 | 1.17 (1.11 to 1.23) | <0.001 |
|
| |||||
| Depression | 61 (23.4%) | 9.90 (0.67 to 1.21) | 0.49 | 0.89 (0.65 to 1.22) | 0.48 |
|
| |||||
| Problematic alcohol use | 36 (13.7%) | 0.78 (0.54 to 1.12) | 0.18 | 0.81 (0.56 to 1.18) | 0.27 |
|
| |||||
| Visited provider in past 12 months | 238 (90.2%) | 1.07 (0.70 to 1.63) | 0.75 | 1.29 (0.82 to 2.04) | 0.28 |
Associations between sex frequency/planning behaviors and interest in different PrEP regimens
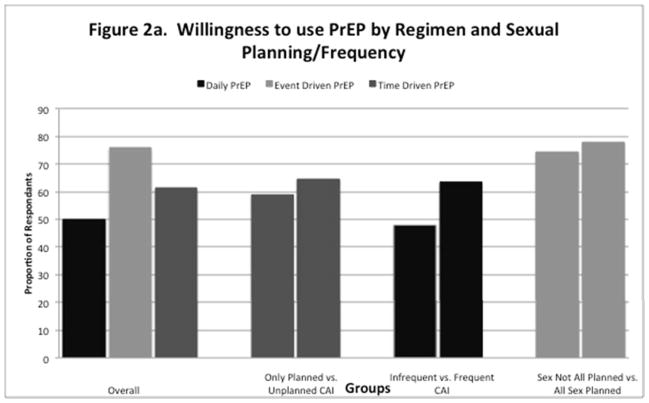
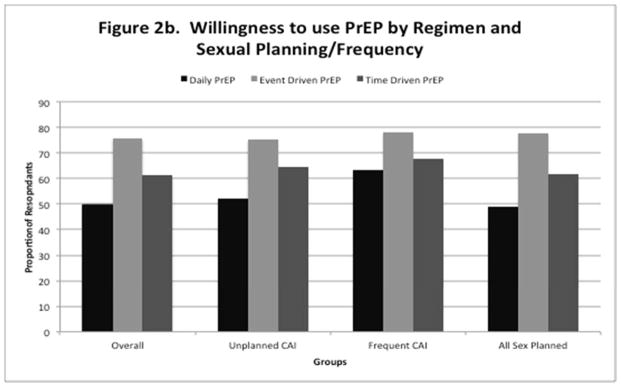
Table 3 demonstrates associations between reporting unplanned CAI in the past 3 months and interest in using different PrEP regimens. In multivariable analysis, having unplanned CAI in the past 3 months was significantly associated with interest in using time driven (fixed interval dosing) PrEP (aOR 1.24, 95% CI 1.07–1.43). Although not significant, there was a trend toward willingness to use daily PrEP (aOR 1.15, 95% CI 1.00–1.33) as well. Table 4 demonstrates associations between frequent CAI and interest in using different PrEP regimens. In multivariable analysis, engaging in frequent CAI was significantly associated with interest in using daily PrEP (aOR 1.49, 95% CI 1.13–1.91). Table 5 demonstrates associations between only engaging in planned sex over the past 3 months and preferences for different PrEP regimens. In multivariable analysis, only having planned sex was associated with interest in using event-driven PrEP (aOR 1.21, 95% CI 1.02–1.43). Across the entire sample, respondants were most willing to use event driven PrEP (75.8%), followed by time driven PrEP (61.2%), and daily PrEP (49.8%). Overall willingness to use these regimens remained high across all subgroups analyzed by sexual frequency and planning behaviors (supplemental figure 2a–b).
Table 3.
Associations between unplanned CAI in the past 3 months and preferences for PrEP dosing
| PrEP dosing | Bivariate Models | Multivariable Models1 | ||
|---|---|---|---|---|
| OR (95% CI) | P | aOR (95% CI) | P | |
| Daily PrEP | 1.18 (1.03 to 1.36) | 0.02 | 1.15 (1.00 to 1.33) | 0.052 |
| Event-driven PrEP2 | 0.94 (0.80 to 1.10) | 0.43 | 0.93 (0.79 to 1.10) | 0.42 |
| Time-driven PrEP3 | 1.27 (1.10 to 1.46) | 0.001 | 1.24 (1.07 to 1.43) | 0.004 |
Adjusted for age, education (college grad or above versus less than), income, sexual orientation, in a monogamous relationship, depression, problematic alcohol use, having visited a provider in the past 12 months, and nation of residence (United States or Canada);
Defined as having to take a pill right before sex and soon after for it to work;
Defined as having to take a pill every Monday and Friday plus another pill soon after sex for it to work
Table 4.
Associations between frequent CAI (> 1 time/week) and preferences for PrEP dosing
| PrEP dosing | Bivariate Models | Multivariable Models1 | ||
|---|---|---|---|---|
| OR (95% CI) | P | aOR (95% CI) | P | |
| Daily PrEP | 1.39 (1.08 to 1.79) | 0.01 | 1.49 (1.13 to 1.91) | 0.004 |
| Event-driven PrEP2 | 0.71 (0.54 to 0.94) | 0.02 | 0.76 (0.57 to 1.02) | 0.065 |
| Time-driven PrEP3 | 0.94 (0.73 to 1.22) | 0.65 | 0.98 (0.75 to 1.27) | 0.87 |
Adjusted for age, education (college grad or above versus less than), income, sexual orientation, in a monogamous relationship, depression, problematic alcohol use, having visited a provider in the past 12 months, and nation of residence (United States or Canada);
Defined as having to take a pill right before sex and soon after for it to work;
Defined as having to take a pill every Monday and Friday plus another pill soon after sex for it to work
Table 5.
Association between only having “planned” sex over the past 3 months and preferences for PrEP dosing
| PrEP dosing | Bivariate Models | Multivariable Models1 | ||
|---|---|---|---|---|
| OR (95% CI) | P | aOR (95% CI) | P | |
| Daily PrEP | 0.95 (0.84 to 1.09) | 0.49 | 0.96 (0.83 to 1.11) | 0.57 |
| Event-driven PrEP2 | 1.22 (1.03 to 1.44) | 0.02 | 1.21 (1.02 to 1.43) | 0.03 |
| Time-driven PrEP3 | 1.02 (0.89 to 1.18) | 0.75 | 1.03 (0.89 to 1.20) | 0.68 |
Adjusted for age, education (college grad or above versus less than), income, sexual orientation, in a monogamous relationship, depression, problematic alcohol use, having visited a provider in the past 12 months, and nation of residence (United States or Canada);
Defined as having to take a pill right before sex and soon after for it to work;
Defined as having to take a pill every Monday and Friday plus another pill soon after sex for it to work
DISCUSSION
These results provide insight into temporal patterns of sexual activity as well as planning behaviors in a large sample of North American MSM, extending observations from a previous online study of North American MSM (35). In our study population, most episodes of condomless anal intercourse appear to be planned in advance, which could suggest that event driven PrEP would be feasible. A prior on-line study of North American MSM found that most (85%) reported sex that was infrequent enough (sex on two days per week or less) to justify intermittent PrEP, though 58% of the cohort reported essentially no planning prior to their most recent sexual encounter (35). A study of sexual frequency and planning behavior of an MSM population in Thailand showed that most (86%) had sex that was infrequent enough to justify consideration of an intermittent PrEP regimen (34). A significant portion (65%) of this cohort also reported anticipation of their most recent sex act. A sizable group of MSM in our study reported only having sex only on weekends. As their risk for HIV acquisition is theoretically confined to 3 days out of the week, this group of MSM may be best suited for less than daily PrEP regimens such as time-driven or event-driven PrEP.
In the current study, having unplanned CAI was associated with lower educational level. This finding corresponds with prior studies that have shown that lower educational achievement (35) is associated with lack of sexual planning in MSM. Our study also demonstrated that having unplanned CAI was associated with frequent CAI as well as having a higher self-perceived risk of HIV. Although no study that we are aware of has investigated an association between self-perceived risk of HIV and sexual planning behaviors, van Griensven et al. have shown that risk factors for HIV acquisition such as younger age and engaging in receptive anal intercourse are associated with less sexual planning (34). These results suggest that those individuals who tend to be at higher risk for HIV acquisition (younger age, lower socioeconomic status) are also those who are unable to anticipate their next sex act. Providers should consider these factors when considering who may not be candidates for event based PrEP, as event based PrEP would require anticipation of sex acts in advance. These patients may be better suited for a time driven or daily PrEP regimen. In our study, being in a monogamous relationship and not having visited a provider in the last 12 months was also associated with less sexual planning. These results are similar to those reported by Volk et al., which showed that having a committed partner and undergoing less frequent HIV testing was associated with having unplanned sex (35). This suggests that persons in HIV serodiscordant relationships or in stable partnerships where one or both partners have additional sexual contacts, such as part of “open relationships”, may benefit from counseling about the availability of daily PrEP or time driven PrEP.
In our survey, engaging in frequent CAI was associated with younger age, which has been shown in MSM previously (35). Having a higher self-perceived risk of HIV acquisition was also associated with frequent CAI. Factors associated with a high risk of HIV acquisition such as use of alcohol, engaging in group sex, and engaging in sex in exchange for money have been associated with having more frequent sex in prior studies. Being in a monogamous relationship was also associated with frequent CAI, which correlates with previous data indicating having a committed partner is associated with having more frequent sex (35).
Few studies have attempted to characterize attitudes regarding intermittent versus daily dosing regimens (36–40) with results indicating either no difference in acceptability (37,40) or a preference for intermittent dosing (38). Our results show that those who engaged in frequent CAI were more interested in taking daily PrEP than those who engaged in infrequent CAI. Indeed, those MSM who are having frequent sex may benefit most from daily PrEP regimen, as some of the advantages of intermittent PrEP compared to daily PrEP, such as decreased pill burden and cost, are diminished by frequent PrEP use. Our results indicate that those who engaged in unplanned CAI were more willing to use time driven PrEP as compared to those who did not engage in unplanned CAI. It makes sense that those respondents who do not plan sexual activity in advance would find a regimen that does not require anticipation of sex acts more appealing. We also found that those participants who reported engaging only in planned sex preferred an event driven regimen. These results correspond to recently published survey results of HIV serodiscordant couples in Kenya, which showed that participants tended to prefer less than daily dosing if sex was usually anticipated in advance (40). These results together indicate that preferences for different PrEP regimens seem to correlate with and may be influenced by the sexual frequency and planning behaviors of potential PrEP users.
There are several limitations to this study. First, the survey was conducted through a popular online sexual networking site for MSM in North America, potentially limiting the generalizability of the results. As the survey population was >80% White with a median age of 40, these results may not apply to other high risk MSM groups such as young minority populations, though almost 40% of new infections among U.S. MSM occur among those who identify as white (25). Furthermore, although the overall number of respondents who completed surveys was high, the overall response rate was low. Other studies of MSM using large online sampling data have experienced similar response rates (41). We are unable to determine whether responders differed from non-responders in a significant way, thus introducing a potential selection bias. Also, we were unable to assess whether the monogamous respondents in our analysis were in a seroconcordant relationship, in which case their risk of HIV acquisition would theoretically be low. An additional limitation is the cross-sectional design, which allows for analysis of participants’ behaviors at one point in time as opposed to longitudinally. Furthermore, while the survey was conducted online and therefore may allow for more candid responses by reducing social desirability bias (42,43), the data gathering relied on self-report, which is subject to recall bias.
To our knowledge, this is the largest study to report on sexual frequency and planning behaviors and their associations with preferences for PrEP dosing schedules in a population of North American MSM. Our results indicate that preferences for PrEP dosing regimens seem to be informed by sexual frequency and planning behaviors. As new strategies for HIV prevention continue to develop, more studies will be needed to evaluate the acceptability of intermittent PrEP strategies among potential consumers and to help define which dosing strategies will be optimal for which individuals.
Supplementary Material
Figure 2.
Figure 2a–b. “Unplanned CAI” defined as respondents who reported any unplanned CAI in last 3 months. “Frequent CAI” defined as respondents who reported CAI more than 1×/week. “All Sex Planned” defined as respondents who only had “planned” sex over the previous 3 months.
Acknowledgments
CO was supported by NIH grants (T32DA013911, R25MH083620). DK was supported by a Career Development Award from the National Institutes of Health (K23 MH098795). SS was supported by NIH (K24MH094214). KM was supported by NIH grant (P30 AI06354).
Contributor Information
Conor Stack, Department of Medicine Beth Israel Deaconess Medical Center.
Catherine E Oldenburg, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA Fenway Health, The Fenway institute, Boston, United States.
Matthew Mimiaga, Fenway Health, The Fenway institute, Boston, United States Harvard T.H. Chan School of Public Health, Epidemiology, Boston, United States, Massachusetts General Hospital, Psychiatry, Boston, United States.
Steven Alan Elsesser, The Fenway Institute, Fenway Health, Boston, MA.
Douglas Krakower, Division of Infectious Diseases, Beth Israel Deaconess Medical Center, Boston, MA.
David S. Novak, On-Line Buddies, Cambridge, United States.
James E. Egan, Department of Behavioral and Community Health Sciences, Graduate School of Public Health, University of Pittsburgh.
Ron Stall, Center for LGBT Health Research, Graduate School of Public Health, University of Pittsburgh
Steve Safren, Harvard Medical School, Medicine, Boston, United States, Massachusetts General Hospital, Psychiatry, Boston, United States.
Kenneth H. Mayer, Fenway Health, The Fenway institute, Boston, United States, Harvard Medical School, Medicine, Boston, United States, Harvard T.H. Chan School of Public HealthGlobal Health and Population, Boston, United States.
References
- 1.Beyrer C, Baral SD, van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012 Jul 28;380(9839):367–77. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013 Nov 13;27(17):2665–78. doi: 10.1097/01.aids.0000432449.30239.fe. [DOI] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012 Aug 2;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 6.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013 Jun 15;381(9883):2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 7.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012 Aug 2;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015 Feb 5;372(6):509–18. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeten J, Celum C. Systemic and topical drugs for the prevention of HIV infection: antiretroviral pre-exposure prophylaxis. Annu Rev Med. 2013;64:219–32. doi: 10.1146/annurev-med-050911-163701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012 Sep 12;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011 Jan 28;60(3):65–8. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6003a1.htm. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012 Aug 10;61(31):586–9. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6131a2.htm. [PubMed] [Google Scholar]
- 13.Krakower D, Mayer KH. Engaging healthcare providers to implement HIV pre-exposure prophylaxis. Curr Opin HIV AIDS. 2012 Nov;7(6):593–9. doi: 10.1097/COH.0b013e3283590446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks RA, Kaplan RL, Lieber E, et al. Motivators, concerns, and barriers to adoption of preexposure prophylaxis for HIV prevention among gay and bisexual men in HIV-serodiscordant male relationships. AIDS Care. 2011 Sep;23(9):1136–45. doi: 10.1080/09540121.2011.554528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krakower D, Ware N, Mitty JA, et al. HIV providers’ perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: a qualitative study. AIDS Behav. 2014 Sep;18(9):1712–21. doi: 10.1007/s10461-014-0839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina JM, Pintado C, Gatey C, et al. Challenges and opportunities for oral pre-exposure prophylaxis in the prevention of HIV infection: where are we in Europe? BMC Med. 2013 Aug 23;11:186. doi: 10.1186/1741-7015-11-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. [Accessed 18 June 2015];FACTS 001 Trial: Questions and Answers. Available at: http://www.usaid.gov/sites/default/files/documents/1864/FACTS-001.pdf.
- 19.ANRS (Agence nationale de recherches sur le sida et les hépatites virales) [Accessed 12 June, 2014]; Available: http://www.ipergay.fr/
- 20. [Accessed: 01/05/2015];Press Release: “A significant breakthrough in the fight agains HIV/AIDS”. Available: www.avac.org/sites/default/files/u44/ipergayPR.pdf.
- 21.Molina JM, CC, Charreau I, et al. On Demand PrEP With Oral TDF-FTC in MSM: Results of the ANRS Ipergay Trial [abstract 23LB]. Conference on Retroviruses and Opportunistic Infections 2015; Seattle, Washington. February 23–26, 2015; CROI; 2015. Available: http://www.croiconference.org/sessions/demand-prep-oral-tdf-ftc-msm-results-anrs-ipergay-trial. [Google Scholar]
- 22.HIV Prevention Trials Network. [Accessed 12 June 2014]; Available: http://www.hptn.org/
- 23.HPTN 067/ADAPT Study. [Accessed: 03 March 2015];Results Brief on Daily vs. Non-daily PrEP Dosing Regimens for South African Women. Available: http://www.hptn.org/web%20documents/HPTN067/HPTN067_SAresults_Fact%20Sheet_V1.0.pdf.
- 24.HPTN 067 – The Adapt Study. [Accessed 03 March 2015];A Phase II, Randomized, Open-Label, Pharmacokinetic and Behavioral Study of the Use of Intermittent Oral Emtricitabine/Tenofovir Disoproxil Fumarate Pre-Exposure Prophylaxis (PrEP) Available: http://www.hptn.org/research_studies/hptn067.asp.
- 25.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFarlane M, Bull SS, Rietmeijer CA. The Internet as a newly emerging risk environment for sexually transmitted diseases. JAMA. 2000 Jul 26;284(4):443–6. doi: 10.1001/jama.284.4.443. [DOI] [PubMed] [Google Scholar]
- 27.Tashima KT, Alt EN, Harwell JI, et al. Internet sex-seeking leads to acute HIV infection: a report of two cases. Int J STD AIDS. 2003 Apr;14(4):285–6. doi: 10.1258/095646203321264926. [DOI] [PubMed] [Google Scholar]
- 28.Rosser BR, Oakes JM, Horvath KJ, et al. HIV sexual risk behavior by men who use the Internet to seek sex with men: results of the Men’s INTernet Sex Study-II (MINTS-II) AIDS Behav. 2009;13:488–498. doi: 10.1007/s10461-009-9524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krakower DS, Mimiaga MJ, Rosenberger JG, et al. Limited Awareness and Low Immediate Uptake of Pre-Exposure Prophylaxis among Men Who Have Sex with Men Using an Internet Social Networking Site. PLoS One. 2012;7(3):e33119. doi: 10.1371/journal.pone.0033119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 31.Knowlton R, McCusker J, Stoddard A, et al. The use of the CAGE questionnaire in a cohort of homosexually active men. J Stud Alcohol. 1994;55:692–694. doi: 10.15288/jsa.1994.55.692. [DOI] [PubMed] [Google Scholar]
- 32.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 33.Mimiaga MJ, Goldhammer H, Belanoff C, et al. Men who have sex with men: perceptions about sexual risk, HIV and sexually transmitted disease testing, and provider communication. Sex Transm Dis. 2007;34:113–119. doi: 10.1097/01.olq.0000225327.13214.bf. [DOI] [PubMed] [Google Scholar]
- 34.van Griensven F, Thienkrua W, Sukwicha W, et al. Sex frequency and sex planning among men who have sex with men in Bangkok, Thailand: implications for pre- and post-exposure prophylaxis against HIV infection. J Int AIDS Soc. 2010 Apr 14;13:13. doi: 10.1186/1758-2652-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volk JE, Liu A, Vittinghoff E, et al. Sexual frequency and planning among at-risk men who have sex with men in the United States: implications for event-based intermittent pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2012 Sep 1;61(1):112–5. doi: 10.1097/QAI.0b013e31825bd87d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Elst EM, Mbogua J, Operario D, et al. High acceptability of HIV pre-exposure prophylaxis but challenges in adherence and use: qualitative insights from a phase I trial of intermittent and daily PrEP in at-risk populations in Kenya. AIDS Behav. 2013 Jul;17(6):2162–72. doi: 10.1007/s10461-012-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kibengo FM, Ruzagira E, Katende D, et al. Safety, adherence and acceptability of intermittent tenofovir/emtricitabine as HIV pre-exposure prophylaxis (PrEP) among HIV-uninfected Ugandan volunteers living in HIV-serodiscordant relationships: a randomized, clinical trial. PLoS One. 2013 Sep 26;8(9):e74314. doi: 10.1371/journal.pone.0074314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisingerich AB, Wheelock A, Gomez GB, et al. Attitudes and acceptance of oral and parenteral HIV preexposure prophylaxis among potential user groups: a multinational study. PLoS One. 2012;7(1):e28238. doi: 10.1371/journal.pone.0028238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutua G, Sanders E, Mugo P, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7(4):e33103. doi: 10.1371/journal.pone.0033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts ST, Heffron R, Ngure K, et al. Preferences for daily or intermittent pre-exposure prophylaxis regimens and ability to anticipate sex among HIV uninfected members of Kenyan HIV serodiscordant couples. AIDS Behav. 2014 Sep;18(9):1701–11. doi: 10.1007/s10461-014-0804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weatherburn P, Schmidt A, Hickson F, et al. The European Men-Who-Have-Sex-WithMen Internet Survey (EMIS): Design and Methods. Sexuality Research and Social Policy. 2013 Dec;10(4):243–257. [Google Scholar]
- 42.Metzger DS, Koblin B, Turner C, et al. Randomized controlled trial of audio computer-assisted self-interviewing: utility and acceptability in longitudinal studies. HIVNET Vaccine Preparedness Study Protocol Team. Am J Epidemiol. 2000;152:99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- 43.Ellen JM, Gurvey JE, Pasch L, et al. A randomized comparison of A-CASI and phone interviews to assess STD/HIV-related risk behaviors in teens. J Adolesc Health. 2002;31:26–30. doi: 10.1016/s1054-139x(01)00404-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.