Abstract
Background
Esophageal and gastric cancers differ in their epidemiology but have several risk factors in common. The aim of this study was to assess age and sex differences in the burden of esophageal and gastric cancers in the context of the global obesity epidemic.
Methods
Data from 50 countries were obtained from Cancer Incidence in Five Continents Volume X and GLOBOCAN 2012. Age-specific and -standardized incidence rates of esophageal adenocarcinoma (EAC) and squamous cell carcinoma (ESCC), as well as cardia (CGC) and non-cardia (NCGC) gastric cancer, were estimated. Countries were grouped and analyzed according to their obesity prevalence.
Results
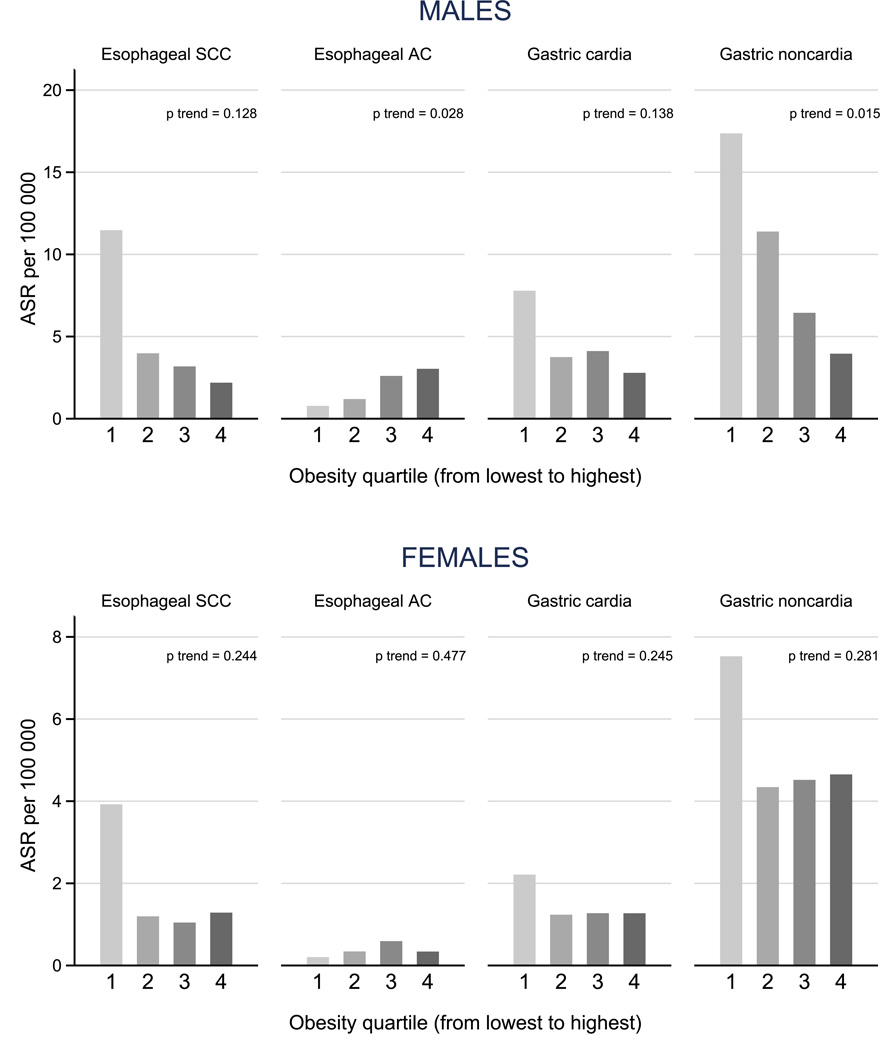
A gradient across quartiles of obesity prevalence was found for EAC, with the highest incidence rates in high prevalence countries (ASR 3.0 vs. 0.8 per 100,000 in highest vs. lowest obesity quartiles, males). In contrast, for ESCC as well as for CGC and NCGC the reverse was true, with the highest rates observed in countries with the lowest obesity prevalence (ESCC: 2.2 vs. 11.5, CGC: 2.8 vs. 7.8, NCGC: 3.9 vs. 17.4 in highest vs. lowest obesity quartiles, males). While for EAC, sex and age differences in incidence were most pronounced in countries with a high prevalence of obesity, these differences were much smaller for the other cancer sites assessed.
Conclusions
Variation in obesity prevalence may partly explain age and sex differences in the incidence of EAC.
Impact
Ecological studies can help assess relationships between risk factors and cancer, and generate new hypotheses that may be pursued through more directed research.
Keywords: esophageal neoplasms, gastric neoplasms, histology, epidemiology, obesity, smoking
INTRODUCTION
In 2012, worldwide almost 1.5 million people developed esophageal or gastric cancer (1). Although closely allied in terms of their anatomical location and shared exposures, incidence patterns and trends of the two cancers are very different. This observation has led to an increasing interest in the epidemiology of different subtypes or subsites of both malignancies in order to disentangle the possible contribution of risk factors and to plan appropriate cancer control strategies. Most esophageal cancers can be subdivided into two main histological subtypes: esophageal adenocarcinomas (EAC) and esophageal squamous cell carcinomas (ESCC). Whereas EACs usually develop in the lower third of the esophagus, ESCC occurs mostly in the upper two-thirds of the esophagus (2). Gastric cancers can generally be classified into two topographical categories: cardia gastric cancers (CGC) arising from the area of the stomach adjoining the esophageal-gastric junction, and non-cardia gastric cancers (NCGC) developing in more distal regions of the stomach.
Recent studies have highlighted the different epidemiologic profiles and incidence trends of esophageal and gastric cancer, respectively by subtype and subsite. While the incidence of EAC is highest and has been increasing in Caucasian populations of high-income countries, ESCC is most common in Asian populations (3, 4). In contrast, the incidence of both gastric cancer subsites is highest in Eastern and South-Eastern Asia, whilst rates of CGC exceeded those of NCGC in North America and several European countries (3, 4). The underlying reasons for these patterns remain partly unexplained. Differences in the prevalence of risk factors have been suggested to contribute to this pattern. While tobacco smoking is a stronger risk factor for ESCC (5) and both types of gastric cancer (6), it has also been shown to be associated with an increased risk of EAC (7). Obesity, conversely, has been estimated to be responsible for a third of all global EAC cases in males in 2012 (8), but has also been linked to CGC (9) – not only as an independent risk factor but also through its involvement in the development of gastro-esophageal reflux disease (GERD) and the metaplastic precursor of EAC, Barrett’s esophagus (10, 11). Moreover, while NCGC is strongly linked to Helicobacter pylori (H. pylori) infection (12, 13), inverse associations have been found for CGC and EAC (14, 15). A similar relationship has also been observed for socioeconomic status, with clear gradients and the highest risks for NCGC and ESCC found with increasing deprivation but no clear associations for CGC and EAC (16).
Parallels in the epidemiology of esophageal and gastric cancers are also more evident when looking at incidence patterns across age and sex. While males are at higher risk for all included cancers, this is particularly true for EAC (male-to-female sex ratio: 4.4 : 1) and CGC (3.2 : 1) (3, 4). These two cancer types are also referred to as cancers of the gastro-esophageal junction and are frequently considered one clinical entity (15). Males developing these junctional cancers are also more likely to present at younger ages than females (17). Understanding how risk factors are influencing these age and sex differences will facilitate the unravelling of underlying mechanisms in the etiology of these tumors.
In view of the global increase in obesity and its potential impact on the incidence of esophageal and gastric malignancies, we assessed this relationship with the aim to identify potential synergies and to increase the understanding of existing age and sex differences. Using an ecological approach, we provide a wider view on obesity as a linking risk factor and the findings may generate new hypotheses in cancer epidemiology.
MATERIAL AND METHODS
For all analyses, data from Cancer Incidence in Five Continents (CI5) Vol. X (18) and GLOBOCAN 2012 (1) were used. Both databases are governed by the International Agency for Research on Cancer but differ in their underlying data and purpose. CI5 provides high quality data on the incidence of cancer from cancer registries around the world and its Vol. X contains information from 290 cancer registries in 68 countries about cancers diagnosed from 2003 to 2007. The GLOBOCAN project provides estimates of the incidence of, mortality and prevalence from major cancer types at the national level, for 184 countries of the world, for the year 2012. These estimates are based on most recent data available and further details are provided elsewhere (19).
Using both data sources, age-specific and age-standardized (world population, 1960) incidence rates were estimated for esophageal cancer by histological subtype (EAC/ESCC) and gastric cancer by topographical subsite (CGC/NCGC) in 2012. The exact method and the resulting incidence rates have been described in more detail in Arnold et al (3) and Colquhoun et al (4). In brief, sex- and age-specific (<65; ≥65 years) proportions of EAC/ESCC were computed for all countries included in CI5X except for those with no cases of EAC/ESCC in one of four substrata (male, female; <65, ≥65 years). Similarly, the proportions of CGC (C16.0) and NCGC (C16.1–6) cases out of all gastric cancers with known topography (C16.0–6) were calculated for each country included in CI5X and stratified by sex and the same broad age group, provided there were two or more cases of CGC and NCGC within each sex and age group stratification. Where data were available from multiple regional registries within a country, cases and populations were pooled to obtain national proportions. The histological types of esophageal cancer and the topographical classification of gastric cancers were defined according to the third edition of the International Classification of Diseases for Oncology (ICD-O-3) as presented in Cancer Incidence in Five Continents Vol. IX (CI5IX) (20, 21). For the current study, the age- and sex-specific proportions of CGC/NCGC and EAC/ESCC determined in the previous steps were then applied to the 2012 gastric and esophageal cancer incidence estimates in GLOBOCAN 2012 for 50 countries
The prevalence of obesity, defined as a body mass index (BMI) ≥30 kg/m2, by country and sex for the year 2008 was obtained from the Global Health Observatory (22). For this analysis, countries were grouped into quartiles of their obesity prevalence by sex. The quartiles of obesity prevalence were <16.5%, 16.5–<21.4%, 21.4–<24.6% and ≥24.6% in males and <16.9%, 16.9–<23.1%, 23.1–<26.8% and ≥26.8% in females. All included countries are listed by obesity quartiles in Supplementary Table 1. Age-specific and age-standardized incidence rates were then calculated for each quartile and compared across cancer sites. Tests for linear trend were done by performing a linear regression treating obesity quartiles as a continuous variable. P-values were considered statistically significant at the 0.05 level.
Of the countries included in this study, China, Japan and Republic of Korea have the highest burden of gastric cancer (1, 4). Diets high in salt as well as a high prevalence of H. pylori infection in those areas have been proposed to be the main drivers of the increased risk of NCGC (23, 24). In the past decades, there have been programs to increase awareness and implement early detection in these countries. This has led to increased cancer diagnosis, especially of small lesions, and hence increased cancer incidence (25). Such activities may obscure the apparent relationship with other risk factors and we therefore conducted a sensitivity analysis, excluding China, Japan and Republic of Korea.
Given that tobacco smoking is another important risk factor for both esophageal and gastric cancer, the interaction between obesity prevalence and smoking was assessed for each cancer by reclassifying the included countries according to tertiles of obesity prevalence combined with tertiles of lung cancer mortality in 2012 obtained from the GLOBOCAN database (1), as a proxy for past smoking patterns (Supplementary Tables 2a and 2b). Age-standardized incidence rates were calculated for each new exposure category by sex and cancer. Incidence rate ratios for each combined exposure category were then computed relative to the reference category (lowest obesity tertile and lowest lung cancer mortality tertile).
Stata 12 was used for data processing and analysis.
RESULTS
Clear gradients in age-standardized incidence rates (ASR) were found across obesity quartiles in males (Figure 1). While the incidence of gastric cancer (both CGC and NCGC) and ESCC was highest in countries in the lowest quartile of obesity prevalence, the reverse was true for EAC. For NCGC and ESCC, the incidence rate in the lowest obesity quartile was five-fold the rate in the highest obesity quartile (NCGC: ASR 17.4 and 3.9 per 100,000; ESCC: 11.5 and 2.2, respectively). The rates for EAC between the highest and the lowest obesity quartiles differed by four-fold (ASR: 3.0 and 0.8). These patterns were less pronounced in females, where incidence rates were, in general, lower than males for all four cancers.
Figure 1.
Age-standardized incidence rates (ASR) of esophageal and gastric cancers by sex and obesity quartile (proportion of body mass index≥30kg/m2)
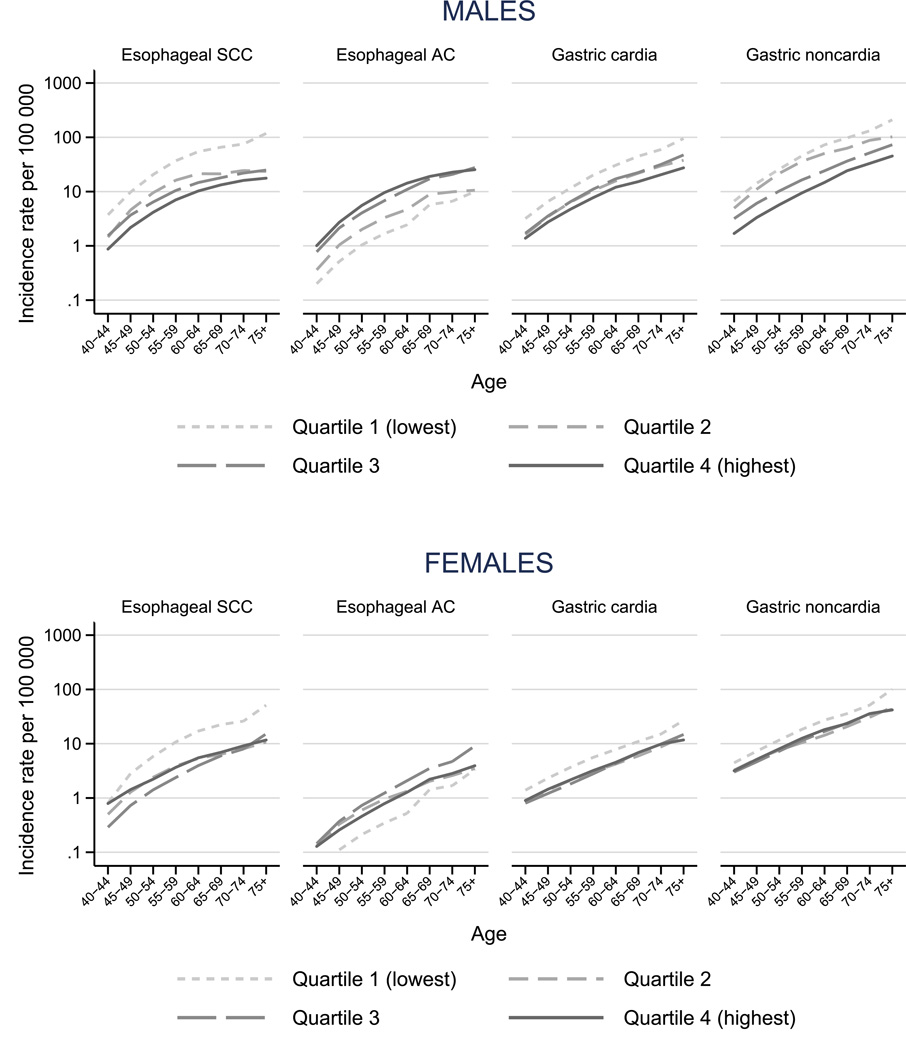
Age-specific incidence rates largely reflected similar patterns, increasing with age across cancers (Figure 2). For ESCC, differences in incidence between lower and higher obesity quartiles increased with age: whereas the incidence rate of ESCC among 40–44 years old males was four times higher in countries in the lowest obesity quartile relative to countries in the highest quartile (ASR 3.7 vs. 0.9, respectively), among the 75+ years old it was seven-fold higher (ASR 117.8 vs. 17.7). In females, the differences in incidence between obesity groups across ages were smaller than in males, with the exception of the lowest quartile of obesity, which stood out for all cancer sites considered.
Figure 2.
Age-specific incidence rates of esophageal and gastric cancers by sex and obesity quartile (proportion of body mass index≥30kg/m2)
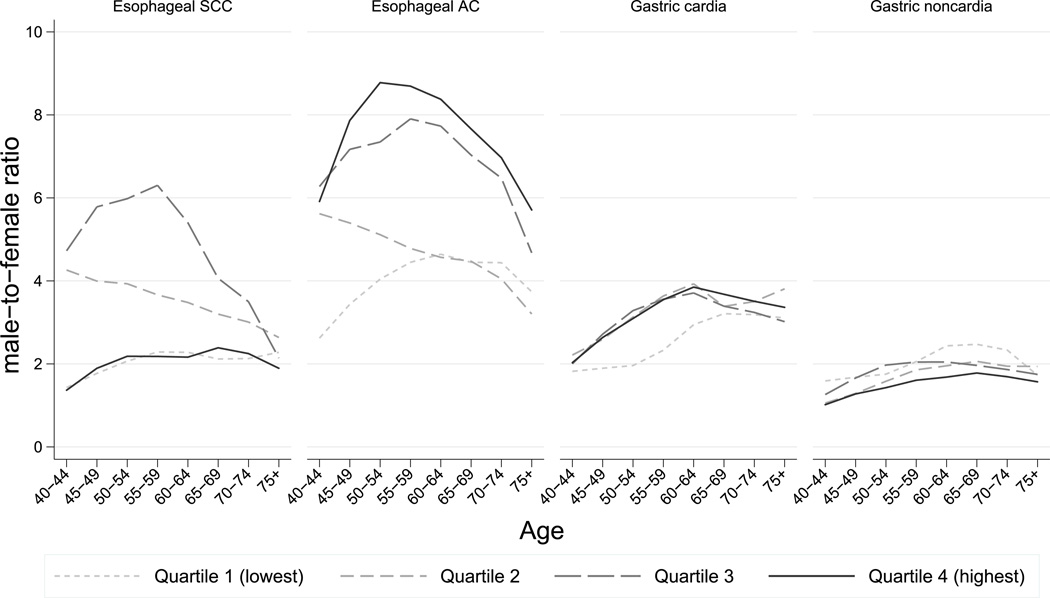
Male-female ratios in age-standardized incidence rates were greatest for EAC, ranging from a ratio of 9 in the highest obesity quartile to 3.8 in the lowest quartile. In contrast, the differences between male and female rates were smallest in the highest obesity quartile for all other sites (male-to-female ratio range for ESCC: 1.7–3.3; CGC: 2.2–3.5; NCGC: 0.9–2.6). Sex differences in incidence rates increased with age before levelling off after age 60 years for CGC, with no appreciable differences between obesity quartiles (Figure 3). Large differences across sex and age were found for esophageal cancer with contrasting patterns for the two subtypes. Whereas male-female differences declined with increasing age for both sites, this was most pronounced in the two highest obesity quartiles in the case of EAC, and in the second quartile for ESCC. For the latter site, the smallest differences between sexes were observed in the highest obesity quartile with an opposing pattern across ages.
Figure 3.
Male-to-female ratios of esophageal and gastric cancers by age and obesity quartile (proportion of body mass index≥30kg/m2)
Excluding China, Japan and Republic of Korea from the analyses resulted in slightly different patterns from those described above (Supplementary Figures 1–3). This was especially evident in females, where gradients in rates across obesity quartiles almost disappeared. In males, while patterns changed for the two gastric cancer subsites, gradients for both subtypes of esophageal cancer remained. In a second sensitivity analysis, we assessed the possible interaction between obesity and tobacco smoking for each cancer site and sex (Supplementary Table 3). An independent relation with obesity was observed for EAC in males, where we found a gradually increasing risk with increasing obesity level at each population-level exposure of tobacco smoking. As for tobacco smoking, an independent effect was seen for ESCC where incidence rose by increasing smoking subgroup in all levels of obesity prevalence. Yet, while the risk of ESCC decreased with increasing obesity prevalence, this decrease was largest where tobacco smoking was highly prevalent.
DISCUSSION
In this study, and for the first time, we assessed age and sex disparities in gastric and esophageal cancer incidence, respectively by subsite and subtype, in the context of the global obesity epidemic. We showed that taking into account the major subsites and -types of both esophageal and gastric cancer is essential when trying to disentangle the potential impact of risk factors on their respective epidemiology. A gradient across obesity quartiles was found for EAC, with the highest incidence rates occurring in countries with the highest prevalence of obesity, particularly for men. In contrast, for ESCC as well as for CGC and NCGC the gradient was reversed, with the highest rates observed in countries with the lowest obesity prevalence. While for ESCC, differences in incidence between lower and higher obesity quartiles increased with age, differences in the incidence of EAC across ages were most pronounced in countries with a high prevalence of obesity. This only partly applied to CGC and NCGC, where differences were greatest in low obesity countries. Sex differences were greatest for EAC and most pronounced in the highest obesity quartile as well as before age 60. For all other sites, sex differences were smallest in the highest obesity quartile.
Obesity is a known risk factor for various morbidities and mortality. Although wide variations exist in its prevalence, overweight (BMI ≥25kg/m2) and obesity (BMI ≥30 kg/m2) have been increasing substantially on a global scale. Recent global statistics indicated that 35% of the adult population is overweight and 12% obese (22). While increases in prevalence have affected both sexes, they have been more pronounced in females. Yet, in recent years, mean BMI of males exceeded that of females in several high-income countries (22, 26). Overweight and obesity prevalence commonly increases with age in females, while males are most likely to be overweight between ages 40 and 60 (27). Also, the prevalence of abdominal or android obesity, most commonly measured by waist circumference, is higher among males than among females (28). Obesity, especially abdominal fat, has been found to promote the development of gastro-esophageal reflux and the metaplastic precursor of EAC—Barrett’s esophagus (10, 11). In addition, it also acts as an independent risk factor for EAC (29, 30). While high BMI has been estimated to increase the risk of EAC by 52% (per 5kg/m2 increase in BMI) in males and 51% in females (31), studies looking at waist circumference have shown even stronger effects (32). In contrast, inverse associations for both high BMI and waist circumference have been described for ESCC (Supplementary Figure 4), independent of smoking status (31, 33). Obesity has also been linked to gastric cancer, with different effects for the two subsites: while being overweight or obese was associated with a 55% increase in risk of CGC, no statistically significant association was observed for NCGC (9). This is consistent with what we found in this study, with the highest incidence rates of CGC, NCGC and ESCC in the lowest obesity quartile and of EAC in the highest obesity quartile. Yet, gradients were less clear for females, where differences in incidence rates were similar across obesity quartiles 2–4 for CGC, NCGC and ESCC. This might be due to substantially lower rates of all four cancers in females and as to EAC, possibly related to a lower waist circumference in females relative to males.
The two other much discussed risk factors linked to gastric and esophageal cancer—and often mentioned in the context of obesity—are smoking and H. pylori infection. Recent evidence has suggested an inverse association of CGC and EAC with H. pylori infection, which is most likely due to its potential to lower gastric acid secretion and reduce GERD (14, 15). A similar association has been found between H. pylori infection and the prevalence of overweight, where eradication of the bacterium was followed by a significant increase in body weight (34, 35). Gradual decreases in H. pylori prevalence, especially in younger birth cohorts, could thus be one of the reasons for the rising incidence of EAC and CGC. Similarly, tobacco smoking is inversely associated with obesity (32), but increases the risk of ESCC (5) and both types of gastric cancer (6). Recently, it has also been shown to be associated with an increased risk of EAC (7). The combined effect of ongoing decreases in the prevalence of tobacco smoking and H. pylori infection has been found to be responsible for 47% of the observed decline in NCGC incidence in the US (36). In our study, we assessed the interaction between smoking and obesity and found an interaction between the two factors for all four cancer sites studied: smoking and obesity clearly had independent effects on incidence of ESCC and EAC, respectively. We also observed a seemingly protective effect of higher BMI on ESCC which is consistent with previous reports and is thought to be due to reverse causation (33).
Strong male predominance is a well-known and often debated phenomenon in the epidemiology of both gastric and esophageal cancer (37, 38). Yet the reasons underlying this pattern are still largely unknown. While smoking didn’t offer an explanation for sex disparities in EAC incidence (39), higher levels of adipokines such as leptin produced by visceral adipose tissue, which is more often observed in males, have been suggested to contribute to the marked sex differences (40). Patients with Barrett’s esophagus were previously found to have a greater waist circumference than population controls, even within strata of BMI (41). This observation supports the hypothesis that central adiposity, rather than BMI, is a better predictor of EAC risk. In general, males are more likely to have a larger waist circumference and develop Barrett’s esophagus at younger ages than females (42, 43). This might explain some of our results showing larger sex disparities in the incidence of EAC and CGC in younger age groups (17, 44).
The large sex disparities in EAC incidence, which were most pronounced in countries with higher obesity levels, furthermore suggest that sex steroid hormones may influence this association. Hypotheses include a protective effect of estrogens, responsible for lower risks observed in females, and a positive association with free testosterone, elevating the risk in males. Increased levels of estrogen have been shown to be involved in the suppression of inflammatory responses and cytokine production in certain tissues (45). They could thus have a similar effect on reflux-induced inflammation of the gastro-esophageal junction. Obesity, in turn, increases the circulating levels of estrogen and may therefore protect females against some malignancies such as EAC. Furthermore, breastfeeding has been found to decrease the risk for esophageal and gastric junction adenocarcinoma (46). In males, levels of free testosterone and dihydrotestosterone have been positively associated with an increased risk of Barrett’s esophagus (47). The fact that total testosterone levels decrease with age and have been shown to be negatively related with obesity (48) is in line with our observation that sex disparities for both types of esophageal cancer were most pronounced at younger ages and progressively decreased after age 65 years - as opposed to sex differences in gastric cancer incidence, which increased until age 60 years before levelling off. In brief, high levels of testosterone in males, concurring with the protective effect of estrogen in females, could thus partly explain the large difference in incidence of EAC between sexes at younger ages.
Strengths & Limitations
To ensure the validity of our approach, we used only high quality data (including only registries that have been selected for CI5) to estimate incidence rates of esophageal and gastric cancers. This was done by applying country-, age- and sex-specific proportions of esophageal and gastric cancers by subtype/-site obtained from CI5X to GLOBOCAN 2012 estimates. Hence, these rates represent estimates of the true incidence within each country and should be interpreted with caution.
We only included countries represented in CI5X in our analyses, resulting in a selection of 50 mostly high-income countries, which may not be representative on the global level. As a consequence, the obesity groupings and the respective incidence rates depend on the countries included and can potentially change if other countries were included. It is also possible that misclassification of the different cancers has affected our results. This is rather unlikely in the case of ESCC because its histological appearance is quite distinct from that of EAC. However, misclassification of cancers of the gastro-esophageal junction could have led to an under- or over-estimation of both EAC and CGC incidences, with potentially varying effects across countries (15, 49). This might have influenced our results in a way that true patterns for EAC and CGC might be even more distinct than what we found. In our study, we have seen some similarities in the epidemiology of EAC and CGC, which have been argued to represent one clinical entity (15). Adenocarcinomas of the gastro-esophageal junction are thus thought to be a mixed group composed of tumors originating either from the stomach or the lower esophagus. This is why tumors of the gastric cardia are assumed to share risk factors with both NCGC and EAC. The similar sex disparities of EAC incidence and CGC incidence have been suggested to be related to the intestinal histological subtype rather than tumor location (17).
Data on obesity prevalence was used for the year 2008 and thus preceded the cancer data, which is biologically plausible and in line with evidence from other studies, even though the precise lag-time between the exposure to obesity and the occurrence of cancer is not well-established (50). We used an ecological study design and the results can serve to generate new hypotheses surrounding the relationship between obesity and the different esophageal and gastric cancers. Using this approach, we compared groups of persons and countries with different levels of obesity, including persons both with and without cancer, in order to make inferences about the relationship between obesity and cancer on the individual level. Given the evidence that already exists on the individual level, e.g. between EAC and obesity, we do not believe that our results are due to an ecological fallacy. Furthermore, in this type of study design it is difficult to control for confounding variables, but we did attempt to assess the role of an important confounder such as smoking by including a proxy country-level smoking variable (lung cancer mortality rates) in our sensitivity analyses. The temporal sequence between outcome and exposure is another critical point often ascribed to ecological studies. In view of large increases in the incidence of EAC among white Caucasian populations in high-income countries, the rising prevalence of obesity has been discussed as the central driver of this development, although some controversy exists around this hypothesis with regard to the temporality of both epidemics (51, 52). Assessing trends in incidence and obesity prevalence, as well as differences across age, cohorts, sex and subsite/-type could provide further insights into the etiology of those tumors and should be pursued in the future.
Conclusion
In this study, and for the first time, we have assessed age and sex disparities in esophageal and gastric cancer incidence, respectively by subtype and subsite, in the context of the global obesity epidemic. Obesity had been associated with rises in the incidence of both EAC and CGC and might act in conjunction with sex hormones and potential effect modification of smoking as well as declines in the prevalence of H. pylori infection. Higher rates for EAC and CGC in males could be explained by a higher prevalence of abdominal obesity, which is the most metabolically active, and increases the risk of reflux. Considering the different subtypes and subsites of both esophageal and gastric cancer is vital when trying to disentangle the potential impact of risk factors. Ecological studies as this one can help generate new hypotheses in this field; yet potentially causal associations need to be confirmed using other study designs.
Supplementary Material
Acknowledgments
Grant support
A. Colquhoun is supported by a Vanier Canada Graduate Scholarship and a Michael Smith Foreign Study Supplement.
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 4.Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015 doi: 10.1136/gutjnl-2014-308915. [DOI] [PubMed] [Google Scholar]
- 5.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. Br J Cancer. 2009;101:855–859. doi: 10.1038/sj.bjc.6605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 7.Cook MB, Kamangar F, Whiteman DC, Freedman ND, Gammon MD, Bernstein L, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst. 2010;102:1344–1353. doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867–2873. doi: 10.1016/j.ejca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 11.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 12.An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group. Lancet. 1993;341:1359–1362. [PubMed] [Google Scholar]
- 13.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 14.Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445–1452. doi: 10.1093/jnci/djj393. [DOI] [PubMed] [Google Scholar]
- 15.McColl KE, Going JJ. Aetiology and classification of adenocarcinoma of the gastro-oesophageal junction/cardia. Gut. 2010;59:282–284. doi: 10.1136/gut.2009.186825. [DOI] [PubMed] [Google Scholar]
- 16.Brewster DH, Fraser LA, McKinney PA, Black RJ. Socioeconomic status and risk of adenocarcinoma of the oesophagus and cancer of the gastric cardia in Scotland. Br J Cancer. 2000;83:387–390. doi: 10.1054/bjoc.2000.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derakhshan MH, Liptrot S, Paul J, Brown IL, Morrison D, McColl KE. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58:16–23. doi: 10.1136/gut.2008.161331. [DOI] [PubMed] [Google Scholar]
- 18.Forman D, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, et al. Cancer Incidence in Five Continents, Vol. X (electronic version) Lyon: IARC; 2013. [DOI] [PubMed] [Google Scholar]
- 19.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 20.Geneva, Switzerland: World Health Organization; 2013. International Classification of Diseases for Oncology, Third Edition, First Revision. [Google Scholar]
- 21.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Lyon, France: International Agency for Research on Cancer; 2007. Cancer Incidence in Five Continents, Vol. IX. [Google Scholar]
- 22.Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10:22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, et al. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer. 2004;7:46–53. doi: 10.1007/s10120-004-0268-5. [DOI] [PubMed] [Google Scholar]
- 24.Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196–201. doi: 10.1002/ijc.21822. [DOI] [PubMed] [Google Scholar]
- 25.Mackenbach JP, Bakker M. Reducing inequalities in health : a European perspective. London; New York: Routledge; 2002. [Google Scholar]
- 26.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 28.Ford ES, Li C, Zhao G, Tsai J. Trends in obesity and abdominal obesity among adults in the United States from 1999–2008. International journal of obesity. 2011;35:736–743. doi: 10.1038/ijo.2010.186. [DOI] [PubMed] [Google Scholar]
- 29.Lagergren J. Influence of obesity on the risk of esophageal disorders. Nature reviews Gastroenterology & hepatology. 2011;8:340–347. doi: 10.1038/nrgastro.2011.73. [DOI] [PubMed] [Google Scholar]
- 30.Hoyo C, Cook MB, Kamangar F, Freedman ND, Whiteman DC, Bernstein L, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol. 2012;41:1706–1718. doi: 10.1093/ije/dys176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 32.Steffen A, Schulze MB, Pischon T, Dietrich T, Molina E, Chirlaque MD, et al. Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18:2079–2089. doi: 10.1158/1055-9965.EPI-09-0265. [DOI] [PubMed] [Google Scholar]
- 33.Lahmann PH, Pandeya N, Webb PM, Green AC, Whiteman DC, Australian Cancer S. Body mass index, long-term weight change, and esophageal squamous cell carcinoma: is the inverse association modified by smoking status? Cancer. 2012;118:1901–1909. doi: 10.1002/cncr.26455. [DOI] [PubMed] [Google Scholar]
- 34.Lender N, Talley NJ, Enck P, Haag S, Zipfel S, Morrison M, et al. Review article: Associations between Helicobacter pylori and obesity--an ecological study. Alimentary pharmacology & therapeutics. 2014;40:24–31. doi: 10.1111/apt.12790. [DOI] [PubMed] [Google Scholar]
- 35.Lane JA, Murray LJ, Harvey IM, Donovan JL, Nair P, Harvey RF. Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased body mass index in a placebo-controlled study. Alimentary pharmacology & therapeutics. 2011;33:922–929. doi: 10.1111/j.1365-2036.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- 36.Yeh JM, Hur C, Schrag D, Kuntz KM, Ezzati M, Stout N, et al. Contribution of H. pylori and smoking trends to US incidence of intestinal-type noncardia gastric adenocarcinoma: a microsimulation model. PLoS medicine. 2013;10:e1001451. doi: 10.1371/journal.pmed.1001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edgren G, Liang L, Adami HO, Chang ET. Enigmatic sex disparities in cancer incidence. Eur J Epidemiol. 2012;27:187–196. doi: 10.1007/s10654-011-9647-5. [DOI] [PubMed] [Google Scholar]
- 39.Freedman ND, Derakhshan MH, Abnet CC, Schatzkin A, Hollenbeck AR, McColl KE. Male predominance of upper gastrointestinal adenocarcinoma cannot be explained by differences in tobacco smoking in men versus women. Eur J Cancer. 2010;46:2473–2478. doi: 10.1016/j.ejca.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Q, Zhuang H, Liu Y. The association between obesity factor and esophageal caner. Journal of gastrointestinal oncology. 2012;3:226–231. doi: 10.3978/j.issn.2078-6891.2012.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kubo A, Cook MB, Shaheen NJ, Vaughan TL, Whiteman DC, Murray L, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett's oesophagus: a pooled analysis from the international BEACON consortium. Gut. 2013;62:1684–1691. doi: 10.1136/gutjnl-2012-303753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu X, Chen VW, Ruiz B, Andrews P, Su LJ, Correa P. Incidence of esophageal and gastric carcinomas among American Asians/Pacific Islanders, whites, and blacks: subsite and histology differences. Cancer. 2006;106:683–692. doi: 10.1002/cncr.21542. [DOI] [PubMed] [Google Scholar]
- 43.van Blankenstein M, Looman CW, Johnston BJ, Caygill CP. Age and sex distribution of the prevalence of Barrett's esophagus found in a primary referral endoscopy center. Am J Gastroenterol. 2005;100:568–576. doi: 10.1111/j.1572-0241.2005.40187.x. [DOI] [PubMed] [Google Scholar]
- 44.Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213–219. doi: 10.1007/s101200200037. [DOI] [PubMed] [Google Scholar]
- 45.Kane SV, Reddy D. Hormonal replacement therapy after menopause is protective of disease activity in women with inflammatory bowel disease. Am J Gastroenterol. 2008;103:1193–1196. doi: 10.1111/j.1572-0241.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- 46.Cronin-Fenton DP, Murray LJ, Whiteman DC, Cardwell C, Webb PM, Jordan SJ, et al. Reproductive and sex hormonal factors and oesophageal and gastric junction adenocarcinoma: a pooled analysis. Eur J Cancer. 2010;46:2067–2076. doi: 10.1016/j.ejca.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook MB, Wood SN, Cash BD, Young P, Acosta RD, Falk RT, et al. Association between circulating levels of sex steroid hormones and Barrett's esophagus in men: a case-control analysis. Clin Gastroenterol Hepatol. 2015;13:673–682. doi: 10.1016/j.cgh.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, Chaudhuri A, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes care. 2010;33:1186–1192. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindblad M, Ye W, Lindgren A, Lagergren J. Disparities in the classification of esophageal and cardia adenocarcinomas and their influence on reported incidence rates. Annals of surgery. 2006;243:479–485. doi: 10.1097/01.sla.0000205825.34452.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renehan AG, Soerjomataram I, Tyson M, Egger M, Zwahlen M, Coebergh JW, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126:692–702. doi: 10.1002/ijc.24803. [DOI] [PubMed] [Google Scholar]
- 51.Edgren G, Adami HO, Weiderpass E, Nyren O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62:1406–1414. doi: 10.1136/gutjnl-2012-302412. [DOI] [PubMed] [Google Scholar]
- 52.Kroep S, Lansdorp-Vogelaar I, Rubenstein JH, Lemmens VE, van Heijningen EB, Aragones N, et al. Comparing trends in esophageal adenocarcinoma incidence and lifestyle factors between the United States, Spain, and the Netherlands. Am J Gastroenterol. 2014;109:336–343. doi: 10.1038/ajg.2013.420. quiz 5, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.