Abstract
Background
Body mass index (BMI) has been associated with breast cancer (BC) outcomes. However, few studies used clinical trial settings where treatments and outcomes are consistently evaluated and documented. There are also limited data assessing how patient/disease characteristics and treatment may alter the BMI/BC association.
Methods
We evaluated 15,538 BC participants from four NSABP protocols. B-34 studied early-stage BC patients (N=3,311); B-30 and B-38 included node-positive BC patients (N=5,265 and 4,860); B-31 studied node-positive and HER2-positive BC patients (N=2,102). We used Cox proportional hazards regression to calculate adjusted hazards ratios (HRs) for risk of death and recurrence, and conducted separate analyses by ER-status and treatment group.
Results
In B-30, increased BMI was significantly related to survival. Compared with BMI<25, HRs were 1.04 for BMI 25–29.9 and 1.18 for BMI≥30 (p=0.02). Separate analyses indicated the significant relationship was only in ER-positive disease (p=0.002) and the sub-group treated with doxorubicin/cyclophosphamide (p=0.005). There were no significant trends across BMI for the other three trials. Similar results were found for recurrence. Increased BMI was significantly related to recurrence in B-30 (p=0.03); and the significant relationship was only in ER-positive BCs (p=0.001). Recurrence was also significant among ER-positive disease in B-38 (p=0.03).
Conclusions
In our investigation, we did not find a consistent relationship between BMI at diagnosis and BC recurrence or death.
Impact
This work demonstrates that the heterogeneity of BC between different BC populations and the different therapies used to treat them may modify any association that exists between BMI and BC outcome.
Keywords: breast cancer, recurrence, survival, body mass index, estrogen receptor status
INTRODUCTION
In the United States, a woman has a 1 in 8 chance of being diagnosed with breast cancer. Early detection combined with treatment advancements has greatly improved prognosis and survival. As of January 2014 there were more than 2.8 million American women living with a history of breast cancer including both current patients and survivors (1). Potentially modifiable lifestyle factors, such as overweight and obesity, have gained popularity as indicators for breast cancer prognosis and tools for risk reduction among these women.
Excess weight remains a large public health problem with two-thirds of Americans being overweight and obese. Body mass index (BMI) is often the standardized method used for measuring and classifying excess weight. Existing literature suggests a relationship between increased BMI and an increased risk for developing breast cancer (2–6). However, associations by menopausal status have not been consistent, especially among premenopausal women (3, 5–12). Despite the inconsistencies by menopausal status, results have shown that the relationship between BMI and breast cancer risk appears to be limited to estrogen receptor (ER)-positive cancers.
In addition to breast cancer incidence, high BMI has also been associated with more aggressive or advanced tumors (13, 14) and with poorer outcomes (15–19). Earlier investigations using data from the National Surgical Adjuvant Breast and Bowel Project (NSABP) treatment trials of node-negative patients who were ER-positive (Protocol B-14) and ER-negative (Protocols B-13, B-19, and B-23) found that obesity was related to an increased risk of overall mortality, other primary cancers, and contralateral breast cancers but not recurrence (20, 21). More recently, a meta-analysis of 43 studies showed that survival among obese women with breast cancer was worse than that among non-obese women (16). However, the majority of the trials included were observational studies and included very few results from clinical trials with consistently evaluated and well documented treatment regimens and outcome measurements. Additionally, a 2014 review summarized the findings of 11 studies (22). It included four retrospective investigations (23–26), four population-based epidemiological studies (19, 27–29), and three secondary analyses from treatment efficacy clinical trials (17, 30, 31). In the review, the authors concluded that obesity is associated with negative clinical outcomes in breast cancer patients and is evident in several ethnic populations. They also observed a stronger relationship among ER-positive cancers regardless of menopausal status and no evidence of association in women with triple-negative disease. Furthermore, researchers from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) recently found obesity to be strongly related to breast cancer recurrence and mortality only in pre/peri-menopausal women with ER-positive disease (32).
Despite the abundance of literature regarding obesity, BMI, and breast cancer, there are limited data evaluating how patient characteristics, disease characteristics, and treatment may alter the association of BMI with breast cancer risk and outcomes. These are important aspects to consider, because we know there is substantial heterogeneity in breast cancer outcome by stage, type (i.e., node status, ER-status, and HER2-status), and the treatments used for these types of disease. Furthermore, it remains unclear whether the differences in findings throughout the literature have biological basis, or are due to varied methods of the investigating studies. The purpose of this study was to investigate the consistency of the relationship between BMI at diagnosis and overall survival (OS) as well as that of BMI and breast cancer recurrence in well-documented and controlled clinical trial populations with various types of breast cancer and different treatments for the disease. The populations consisted of participants from four more recently reported breast cancer treatment trials conducted by the NSABP: B-30, B-31, B-34, and B-38.
MATERIALS AND METHODS
Description of Trials
NSABP protocols B-30, B-31, B-34, and B-38 were all multicenter, randomized, clinical trials. For each trial, OS and breast cancer recurrence were either primary or secondary endpoints. Generally, participants included women diagnosed with invasive breast cancer (IBC) who underwent either total mastectomy or lumpectomy with either an axillary dissection or sentinel node biopsy. Patients could not have had a prior history of IBC and were required to be free from other non-breast malignancies for at least five years before entry. Other inclusion and exclusion criteria, along with further details of study designs have been previously published (33–36). All clinical centers obtained approval from institutional review boards, and all participants provided written informed consent. All significant values reported are statistically significant.
Protocol B-30 was conducted among women with operable, node-positive breast cancer. Between 1999 and 2004, 5,351 women were randomly assigned to one of three adjuvant treatment regimens: doxorubicin (A) and cyclophosphamide (C) followed by docetaxel (T) (AC→T), AT, or a concurrent regimen of TAC. Results were published in 2010 indicating a significant 17% reduction in mortality in AC→T versus AT, and a non-significant 14% reduction versus TAC. There was no difference in OS between AT and TAC (33).
Protocol B-31 comprised women with operable, node-positive breast tumors who overexpressed the HER2 protein. B-31 opened in February 2000 and began to randomly assign participants to a treatment regimen of either A and C followed by paclitaxel (P) (AC→P) or A and C followed by P plus trastuzumab (H) (AC→PH). Prior to the first interim analysis, the trial statistical plan was modified to be reported in a joint analysis with North Central Cancer Treatment Group (NCCTG) N9831. In response to the first joint interim analyses, enrollment to B-31 was stopped on April 26, 2005, at which time 2,119 patients had been randomly assigned. The joint analysis of B-31 and N9831 showed that the addition of trastuzumab reduced the rates of recurrence by half and the mortality rate by one third (34).
Protocol B-34 was a double-blind, placebo-controlled trial in a general population of early-stage breast cancer patients. A total of 3,323 women with operable breast cancer and no evidence of metastases were enrolled between 2001 and 2004. Most of these women were node-negative and had small tumors. Women were randomly assigned to receive either adjuvant oral clodronate for three years or a placebo. Local and systemic therapies were administered at the treating physician’s discretion. Results were reported in 2012 and indicated no difference between treatment groups for disease-free survival (DFS), OS, recurrence-free interval (RFI), or bone metastasis-free interval (35).
Similar to B-30, protocol B-38 was limited to patients with node-positive breast cancer. Between 2004 and 2007, 4,894 women were randomly assigned to one of three treatment regimens: TAC, AC→P, and AC followed by P plus gemcitabine (G) (AC→PG). Results for B-38 were reported in 2013 (36). Adding G to AC→P improved neither DFS nor OS. There were also no significant differences in efficacy between AC→P and TAC, although toxicity profiles differed.
Collection of follow-up data for the trials was similar. Clinical, hematological, and biochemical assessments were required at the beginning of each treatment cycle, every six months for the first five years, and annually thereafter. Patients received a physical breast examination at each follow-up visit and annual bilateral mammograms. Staff members from each clinical center performed follow-up and were required to submit documentation for each event reported. To confirm the diagnosis of each event, all documentation was centrally reviewed by medical professionals at the NSABP.
Study Design
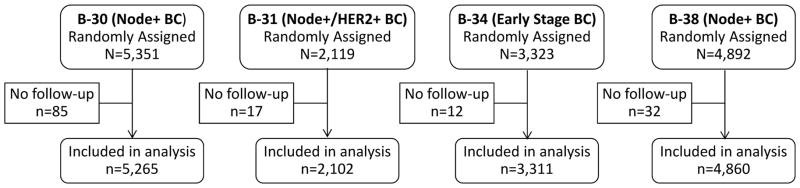
We assessed data from 15,538 breast cancer patients who participated in the four NSABP protocols. All participants with follow-up and supporting documentation were included (Figure 1). Follow-up information for B-30, B-31, and B-34 included data as of December 31, 2012, representing an average of 9.0, 8.3, and 8.4 years, respectively. For B-38, a data cutoff of March 31, 2013 was used, representing an average follow-up of 5.9 years.
Figure 1.
CONSORT diagram: NSABP Protocols B-30, B-31, B-34, and B-38. BC=Breast Cancer
In all of the trials, the height and weight of each participant upon entry were measured by staff at each contributing center. Individual BMIs were calculated as weight in kilograms divided by height in meters squared. BMI is usually grouped into four categories of weight classification for adults: underweight (<18.5), normal (18.5–24.9), overweight (25.0–29.9), and obese (≥30.0). Approximately 1% of participants were underweight in these populations; therefore, they were combined with the normal group for analysis.
Information about important explanatory variables was also assessed upon entry. For all four studies, these included age, ER-status, number of positive nodes, and tumor size. Tumor grade was collected in all except B-38. Menopausal status was also collected; however, the defining criteria were different for each trial. In B-31, it was only collected for those who were ER-positive and thus was unknown for almost 80% of participants.
Statistical Analysis
Because we know there is heterogeneity in breast cancer outcome by disease characteristics and treatment, we decided a priori to analyze each trial individually to explore the relationship of BMI with OS and recurrence among these four diverse populations. We used Cox proportional hazards regression to calculate adjusted hazards ratios (HRs) for risk of recurrence and death. BMI was explored as a continuous variable and a categorical variable. However, because BMI categories are based on established cut points for overweight and obesity, and are both recognizable and clinically meaningful markers for excess weight, we only present results for the categorical classification. Breast cancer recurrence was defined as any local, regional, or distant recurrence. OS was defined as death from any cause. Time-to-event was measured from the date of assignment to the date of diagnosis or death, and all participants with follow-up were included in the analysis using the intent-to-treat method. P-values for tests for trend across BMI categories were obtained by including BMI as a single ordinal term (with values 0, 1, and 2) in Cox regression models and evaluating the global p-value for the term. Adjustment variables were treatment, age, menopausal status, ER-status, number of positive nodes, tumor size, and tumor grade, when available. We conducted separate analyses among ER-positive and ER-negative breast cancers because we had noted differences in the BMI/breast cancer risk association in previous studies (12) and it was of particular interest to investigate. We also conducted separate analyses among the different treatment groups to determine whether the effect of BMI was altered by different regimens. Forest plots were used to display results for which a HR>1 indicates poorer survival or greater risk for recurrence when compared to participants with BMI<25.0. Assessments of the statistical significance of interactions were based on the multivariable model for the respective study populations. P-values used to assess statistical significance of parameters in all modeling were determined using the likelihood ratio test. All tests were evaluated using a 2-sided p-value of 0.05. Analyses were performed using SAS version 9.2 software (SAS Institute, Inc).
RESULTS
The majority of women participating in each of the four trials were white (range 83–86%). The mean ages for participants of B-30, B-31, B-34, and B-38 were 51.1 (SD, 9.7), 49.6 (SD, 10.1), 54.1 (SD, 10.4), and 51.2 (SD, 9.5) years, respectively. Patient and tumor characteristics for this analysis by protocol and BMI group are presented in Table 1. Generally, obese women were older and postmenopausal with slightly larger tumors.
Table 1.
Percent of women by protocol, body mass index, demographic characteritcs and tumor characteristics: NSABP B-30, B-31, B-34, and B-38
| Demographic and tumor characteristics |
B-30 Node-positive BC (N=5,265) |
B-31 Node and HER2-positive BC (N=2,102) |
B-34 Early Stage BC (N=3,311) |
B-38 Node-positive BC (N=4,860) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Body Mass Index
| ||||||||||||
| < 25.0 (n=1807) |
25–29.9 (n=1664) |
≥ 30.0 (n=1794) |
< 25.0 (n=792) |
25–29.9 (n=646) |
≥ 30.0 (n=664) |
< 25.0 (n=1065) |
25–29.9 (n=1060) |
≥ 30.0 (n=1186) |
< 25.0 (n=1481) |
25–29.9 (n=1462) |
≥ 30.0 (n=1917) |
|
| Age | ||||||||||||
| <50 years | 52.6 | 44.6 | 38.8 | 57.4 | 46.3 | 46.2 | 47.2 | 30.9 | 29.2 | 52.9 | 42.3 | 37.3 |
| ≥50 years | 47.4 | 55.4 | 61.2 | 42.6 | 53.7 | 53.8 | 52.8 | 69.1 | 70.8 | 47.1 | 57.7 | 62.7 |
| Menopausal Status | ||||||||||||
| Premenopausal | 54.0 | 44..8 | 39.9 | 11.6 | 10.1 | 9.3 | 50.6 | 35.6 | 31.5 | 58.1 | 46.3 | 40.2 |
| Postmenopausal | 46.0 | 55.2 | 60.1 | 6.8 | 10.2 | 12.8 | 49.3 | 64.2 | 68.5 | 40.7 | 52.9 | 59.3 |
| Unknown | 0 | 0 | 0 | 81.6 | 79.7 | 77.9 | 0.1 | 0.2 | 0 | 1.1 | 0.8 | 0.5 |
| Estrogen Receptor Status | ||||||||||||
| Negative | 24.2 | 25.7 | 24.1 | 45.3 | 50.3 | 46.5 | 23.0 | 24.2 | 23.3 | 20.0 | 20.7 | 21.7 |
| Positive | 75.8 | 74.3 | 75.9 | 54.4 | 49.5 | 53.5 | 76.8 | 75.5 | 76.6 | 78.9 | 78.4 | 77.8 |
| Unknown | 0 | 0 | 0 | 0.3 | 0.2 | 0 | 0.2 | 0.4 | 0.1 | 1.1 | 0.9 | 0.5 |
| Number of Positive Nodes | ||||||||||||
| None | --- | --- | --- | --- | --- | --- | 74.7 | 76.0 | 76.7 | --- | --- | --- |
| 1–3 | 68.1 | 63.6 | 64.8 | 59.1 | 56.7 | 56.8 | 19.3 | 17.4 | 16.8 | 64.2 | 62.8 | 65.9 |
| 4–9 | 23.3 | 26.4 | 25.7 | 28.0 | 28.8 | 30.1 | 4.7 | 4.7 | 5.2 | 25.9 | 25.5 | 24.3 |
| ≥10 | 7.9 | 8.5 | 8.2 | 12.9 | 14.6 | 13.1 | 1.2 | 1.9 | 1.3 | 8.8 | 10.9 | 9.2 |
| Unknown | 0.8 | 1.5 | 1.3 | 0 | 0 | 0 | 0 | 0 | 0 | 1.1 | 0.8 | 0.6 |
| Tumor Size | ||||||||||||
| ≤ 2.0 cm | 48.5 | 40.8 | 39.0 | 43.8 | 37.0 | 37.7 | 72.0 | 67.2 | 63.9 | 45.3 | 38.8 | 40.6 |
| 2–4.0 cm | 36.7 | 41.8 | 44.4 | 41.8 | 46.7 | 45.0 | 23.8 | 28.5 | 30.9 | 40.5 | 45.3 | 43.8 |
| > 4.0 cm | 13.8 | 15.6 | 15.3 | 14.4 | 15.5 | 17.0 | 3.8 | 4.1 | 5.1 | 13.0 | 15.1 | 15.1 |
| Unknown | 0.9 | 1.8 | 1.3 | 0 | 0.8 | 0.3 | 0.4 | 0.3 | 0.2 | 1.2 | 0.8 | 0.5 |
| Tumor Grade | ||||||||||||
| Good/Intermediate | 54.0 | 52.0 | 52.8 | 32.1 | 31.1 | 30.9 | 64.3 | 61.4 | 62.5 | --- | --- | --- |
| Poor | 42.1 | 44.0 | 43.7 | 67.7 | 67.6 | 67.8 | 33.1 | 36.2 | 35.8 | --- | --- | --- |
| Unknown | 3.9 | 4.0 | 3.5 | 0.3 | 1.2 | 1.4 | 2.6 | 2.4 | 1.8 | --- | --- | --- |
BC=Breast Cancer
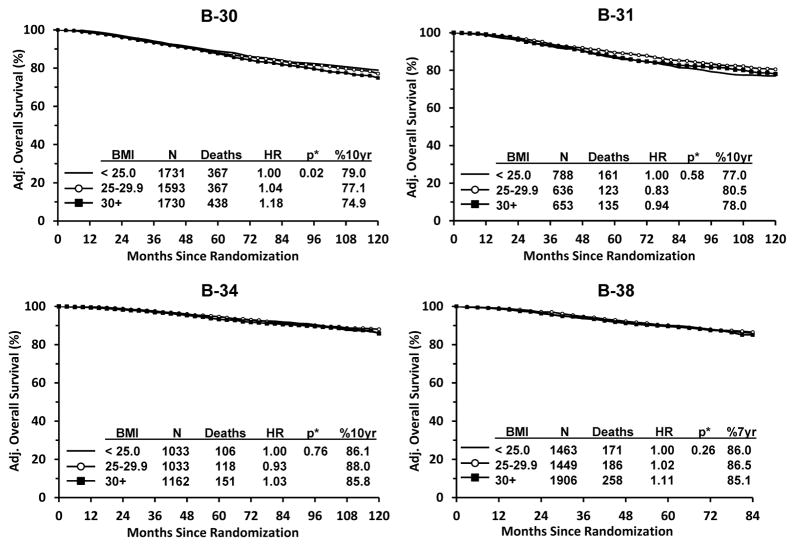
Results for OS are presented in Figure 2. Among participants in B-30 (node-positive breast cancer patients), we found a statistically significant relationship between increased BMI and poorer survival. Compared with BMI <25, adjusted HRs were 1.04 for BMI 25–29.9 and 1.18 for BMI ≥30 (p=0.02). Ten-year survival estimates were 79.0% for BMI<25, 77.1% for BMI 25–29.9, and 74.9% for BMI≥30. For the other trials there were no statistically significant trends in OS across BMI categories. The adjusted HRs for overweight and obese women were: B-31: 0.83 and 0.94 (p=0.58); B-34: 0.93 and 1.03 (p=0.76); and B-38: 1.02 and 1.11 (p=0.26). Absolute differences in ten-year (B-31 and B-34) and seven-year (B-38) survival estimates between BMI<25 and the other two BMI categories were small ranging from 0.3%–3.5% (Figure 2).
Figure 2.
Overall Survival by Protocol: NSABP Protocols B-30, B-31, B-34, and B-38. * Adjusted for treatment, age, menopausal status, ER status, number of positive nodes, tumor size and tumor grade, when available.
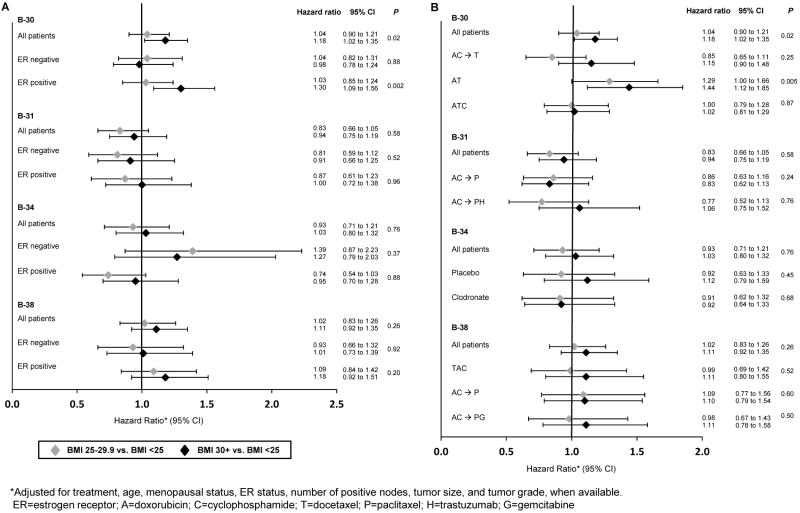
Separate analyses by ER-status (Figure 3A) showed that the statistically significant relationship in B-30 between increased BMI and OS was present in those with ER-positive breast cancer (p=0.002) but not ER-negative (p=0.88). The adjusted HRs for overweight and obese women were 1.03 and 1.30 for ER-positive breast cancer and 1.04 and 0.98 for ER-negative. No statistically significant results were found for either ER-positive or ER-negative disease for the other trials.
Figure 3.
Overall Survival by Protocol, ER-Status (A) and Treatment Group (B): NSABP Protocols B-30, B-31, B-34, and B-38
Separate analyses by treatment group (Figure 3B) showed that the statistically significant relationship in B-30 between increased BMI and OS was present only in the AT group. The adjusted HRs for overweight and obese women in this group were 1.29 and 1.44 (p=0.005). There was not a statistically significant trend among those in the AC→T group (HRs: 0.85 and 1.15; p=0.25) or the TAC group (HRs: 1.00 and 1.02; p=0.87). There were also no statistically significant results among any of the treatment groups in the remaining trials.
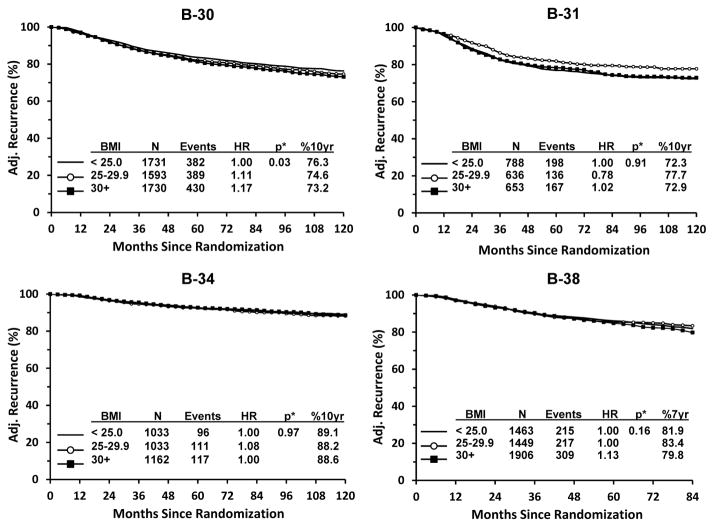
Similarly to the results for OS, we found a statistically significant relationship between increased BMI and an increased risk of recurrence among B-30 participants only (Figure 4). Compared with BMI <25, adjusted HRs were 1.11 for BMI 25–29.9 and 1.17 for BMI ≥30 (p=0.03). Ten-year recurrence-free estimates were 76.3% for BMI<25, 74.6% for BMI 25–29.9, and 73.2% for BMI ≥30. For the remaining trials, there was no statistically significant trend across BMI categories. The adjusted HRs for overweight and obese women were: B-31: 0.78 and 1.02 (p=0.91), B-34: 1.08 and 1.00 (p=0.97), and B-38: 1.00 and 1.13 (p=0.16). Absolute differences in ten-year (B-31 and B-34) and seven-year (B-38) recurrence-free estimates between BMI <25 and the other two BMI categories ranged from 0.5%–5.4% (Figure 4).
Figure 4.
Breast Cancer Recurrence by Protocol: NSABP Protocols B-30, B-31, B-34, and B-38. * Adjusted for treatment, age, menopausal status, ER status, number of positive nodes, tumor size and tumor grade, when available.
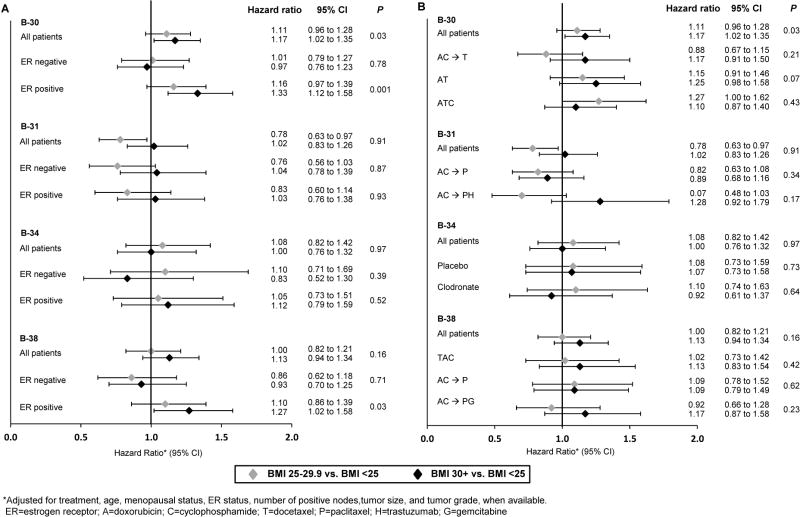
Separate analyses by ER-status (Figure 5A) again showed that the statistically significant relationship between increased BMI and recurrence in B-30 was present in those with ER-positive (HRs: 1.16 and 1.33; p=0.001) but not ER-negative disease (HRs: 1.01 and 0.97; p=0.78). There was also a statistically significant relationship in B-38 among ER-positive breast cancer (HRs: 1.10 and 1.27; p=0.03) but not ER-negative breast cancer (HRs: 0.86 and 0.93; p=0.71). No statistically significant results were found for either ER-positive or ER-negative disease for the remaining trials.
Figure 5.
Breast Cancer Recurrence by Protocol, ER-Status (A) and Treatment Group (B): NSABP Protocols B-30, B-31, B-34, and B-38
Separate analyses of the association between BMI and breast cancer recurrence by treatment group (Figure 5B) showed no statistically significant results in any of the protocols. In each of the trials we tested for interactions between BMI and ER-status, between BMI and treatment group, and between BMI and menopausal status when available. There were no statistically significant interactions found in any of the populations (data not shown).
Protocols B-30 and B-38 studied similar populations of node-positive breast cancer patients; therefore similar findings were expected for these two trials. A possible explanation for why B-30 reached statistical significance and B-38 did not may be the amount of follow-up information that was available. B-38 is a newer trial and thus did not have as much follow-up data as B-30. We analyzed B-30 data that were truncated at seven years to determine whether the association between BMI and OS would have remained significant with less follow-up. We found that although a similar pattern in HRs remained, the p-value for trend in the assessment of all B-30 patients was no longer significant (p=0.07). However, the significant effect found among ER-positive patients remained (p=0.01, data not shown).
DISCUSSION
In our investigation, we did not find a consistent relationship between BMI at diagnosis and OS or breast cancer recurrence. For OS, an association was evident in only one of the four trial populations. Furthermore, the significant relationship in B-30 (node-positive breast cancer patients) was evident only in those with ER-positive breast cancer, and those in the AT treatment group. For breast cancer recurrence, an association was again only evident in the B-30 population and only in those with ER-positive disease. However, there was also a significant relationship among ER-positive breast cancer participants in B-38. This finding was expected given that B-30 and B-38 studied similar populations of node-positive breast cancer patients, but included a longer follow-up period in B-30.
Breast cancer is known to be a heterogeneous disease. Therefore, it is not surprising that its relationship with behavioral risk factors such as BMI may not be consistent when considering populations with various disease characteristics and different treatment regimens. Comparable to our results in B-30, a study by Sparano et al. found an association between higher BMI at diagnosis and higher risk of recurrence and death, specifically in HR-positive and HER2-negative disease (30). Another study among patients with triple-negative disease reported no significant association between obesity and OS or DFS (25). Recently, researchers from the EBCTCG provided results from 80,000 participants of 70 trials. They again found obesity to be strongly related to breast cancer mortality only in women with ER-positive disease, and furthermore only among pre/peri-menopausal women (32). Different populations with different disease characteristics and treatments likely all contribute to the difference in findings among multiple studies. It is unclear exactly why, for example, we found evidence of a positive relationship between increased BMI and breast cancer outcomes among patients with ER-positive disease in B-30 and B-38, but not in B-31 and B-34. However, both B-31 and B-34 had different disease characteristics (B-31 patients were HER2-positive, and B-34 patients were mostly node-negative with small tumors) that likely contributed to the difference in findings. Furthermore, administration of adjuvant systemic chemotherapy was at the discretion of the investigator in B-34, which may also have affected the results.
Not only did we not find a consistent relationship between BMI and breast cancer outcomes among the four trials included in this study, but we also found HRs consistently smaller than other reports. One explanation may be that we included more recently conducted trials than other investigations. In a 2010 review by Goodwin (37), she speculated that lower HRs may reflect real differences in the prognostic effects of obesity in women receiving contemporary treatments. In addition, participants in clinical trial populations are more strictly monitored than in observational studies. Therefore comorbidities associated with obesity such as diabetes and cardiovascular disease may be less likely to impact breast cancer outcomes. In all four of the trials the majority of deaths were due to breast cancer (74% in B-30, 77% in both B-31 and B-38, and 52% in B-34).
In our findings and throughout the literature, increased BMI has been repeatedly associated with ER-positive breast cancer only. This is believed to be related to excess estradiol production in adipose tissue of obese participants. However, this process is only relevant in postmenopausal women because estrogen levels are controlled by the ovaries in premenopausal women. Other potential biologic factors that have been associated with obesity and could potentially affect the obesity and breast cancer relationship include insulin-like growth factors, adipocytokines, and inflammatory cytokines (37). Future studies investigating these potential mediators should also look for any interactions with host or tumor characteristics and treatment effects that could help to explain why BMI seems to only be an important factor for certain subgroups of breast cancer patients.
In addition to disease characteristics, various patient characteristics may also alter the effect of BMI on breast cancer outcomes. As mentioned above, the EBCTCG found that menopausal status affects the relationship between obesity and breast cancer mortality. We found no evidence of a difference by menopausal status in the trials in which this information was available, nor was there a difference by age group when using age 55 as a proxy for menopausal status (data not shown). Another example that could affect the association of BMI with outcome is cigarette smoking. Kroenke et al. published results in 2005 using data from the Nurses’ Health Study. They found that weight before breast cancer diagnosis was related to worse prognosis among never smokers but not ever smokers (15). Based on a prior study by Manson et al. (38) they theorized that by mixing smokers and nonsmokers in analyses, studies may underestimate the impact of weight. We did not collect smoking status in these trials and so were unable to account for such findings.
The current study has other limitations. First, BMI may not be the best marker of overweight and obesity because it does not consider body size or distinguish between the amount of fat and lean tissue (39). Furthermore, a one-time measure of BMI may not capture a woman’s lifetime exposure to excess weight. Perhaps a combination of multiple assessments from adolescence through adulthood would be a more reliable marker. We were also unable to assess change in BMI after breast cancer diagnosis. It may be that this is a more clinically meaningful and relevant marker than BMI at diagnosis only. Concern may also arise from reports that the relationship between body size and breast outcomes may be J- or U-shaped curves (37). This would mean that combining underweight participants with those of normal weight could underestimate obesity effects. However, in our populations the number of participants who were underweight was very small (1%) and therefore unlikely to affect the results. Another limitation was that not all explanatory variables of interest were available in each of the trials. Tumor grade was not collected in B-38 and menopausal status was unknown for the majority of B-31 participants. Additionally, the criteria used to define menopausal status were not the same in all trials.
Despite these limitations, the current study has much strength. It was conducted among participants in clinical trials, therefore having consistently defined and measured outcomes and uniform treatment delivery. Furthermore, defined protocol eligibility requirements of clinical trials yield participants with similar disease stages at diagnosis as well as limited comorbidities at study entry. BMI was known for all participants and the measurements of height and weight used to calculate BMI were collected by trained medical staff, which is more accurate than relying on self-reported information (40). Finally, this was one the few analyses able to explore multiple populations individually, thereby representing the heterogeneity of breast cancer.
Using existing data from four separate breast cancer treatment clinical trials, we were able to conduct an exploratory analysis of the consistency of the association between increased BMI and breast cancer outcomes among various breast cancer populations receiving different types of treatment. Based on our findings, BMI does not appear to be a consistent factor for breast cancer prognosis among all breast cancer populations. However, increased BMI does appear to be related to poorer survival and an increased risk for recurrence among the specific population of ER-positive breast cancer patients with node-positive disease. Further studies are needed to confirm this finding and to better identify and target specific populations for which weight loss may be used as a treatment option to improve breast cancer outcome.
Acknowledgments
Support: All authors received support from the following: NCI U10-CA-180868, U10-CA-180822, and UG1-CA-189867; Aventis Pharmaceuticals; Genentech, Inc; Bayer Oy; Amgen Inc; and Eli Lilly & Company.
This manuscript is funded by the NIH; please archive accordingly.
Footnotes
The authors declare the following potential conflicts of interest:
Dr. Tang: Consultant/advisory board: Incyte. All other authors report no other potential conflicts of interest.
ClinicalTrials.gov:
NSABP B-30: NCT00003782
NSABP B-31: NCT00004067
NSABP B-34: NCT00009945
NSABP B-38: NCT00093795
The authors retain the right to provide a copy of the final manuscript to the NIH upon acceptance for journal publication for public archiving in PubMed Central as soon as possible but no later than 12 months after publication by the journal.
References
- 1.American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014. [Accessed 5-5-2015]. http://www.cancer.org/acs/groups/cid/documents/webcontent/003090-pdf.pdf. [Google Scholar]
- 2.Trentham-Dietz A, Newcomb PA, Storer BE, et al. Body size and risk of breast cancer. Am J Epidemiol. 1997;145:1011–19. doi: 10.1093/oxfordjournals.aje.a009057. [DOI] [PubMed] [Google Scholar]
- 3.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–27. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 4.Morimoto LM, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: The Women’s Health Initiative (United States) Cancer Causes Control. 2002;13:741–51. doi: 10.1023/a:1020239211145. [DOI] [PubMed] [Google Scholar]
- 5.Lahmann PH, Hoffmann K, Allen N, et al. Body size and breast cancer risk: Findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2004;111:762–71. doi: 10.1002/ijc.20315. [DOI] [PubMed] [Google Scholar]
- 6.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–36. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vatten LJ, Kvinnsland S. Prospective study of height, body mass index and risk of breast cancer. Acta Oncol. 1992;31:195–200. doi: 10.3109/02841869209088902. [DOI] [PubMed] [Google Scholar]
- 8.Ursin G, Longnecker MP, Haile RW, Greenland S. A meta-analysis of body mass index and risk of premenopausal breast cancer. Epidemiology. 1995;6:137–41. doi: 10.1097/00001648-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006;166:2395–2402. doi: 10.1001/archinte.166.21.2395. [DOI] [PubMed] [Google Scholar]
- 10.Pichard C, Plu-Bureau G, Neves-E Castro M, Gompel A. Insulin resistance, obesity and breast cancer risk. Maturitas. 2008;60:19–30. doi: 10.1016/j.maturitas.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Kaaks R, Van Noord PA, Den Tonkelaar I, Peeters PH, Riboli E, Grobbee DE. Breast-cancer incidence in relation to height, weight and body-fat distribution in the dutch “DOM” cohort. Int J Cancer. 1998;76:647–51. doi: 10.1002/(sici)1097-0215(19980529)76:5<647::aid-ijc6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Cecchini RS, Costantino JP, Cauley JA, et al. Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev Res. 2012;5:583–92. doi: 10.1158/1940-6207.CAPR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biglia N, Peano E, Sgandurra P, et al. Body mass index (BMI) and breast cancer: Impact on tumor histopatologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecol Endocrinol. 2013;29:263–67. doi: 10.3109/09513590.2012.736559. [DOI] [PubMed] [Google Scholar]
- 14.Haakinson DJ, Leeds SG, Dueck AC, et al. The impact of obesity on breast cancer: A retrospective review. Ann Surg Oncol. 2012;19:3012–18. doi: 10.1245/s10434-012-2320-8. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–78. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 16.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 17.de Azambuja E, McCaskill-Stevens W, Francis P, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: The experience of the BIG 02-98 trial. Breast Cancer Res Treat. 2010;119:145–53. doi: 10.1007/s10549-009-0512-0. [DOI] [PubMed] [Google Scholar]
- 18.Imkampe AK, Bates T. Impact of a raised body mass index on breast cancer survival in relation to age and disease extent at diagnosis. Breast J. 2010;16:156–61. doi: 10.1111/j.1524-4741.2009.00872.x. [DOI] [PubMed] [Google Scholar]
- 19.Conroy SM, Maskarinec G, Wilkens LR, White KK, Henderson BE, Kolonel LN. Obesity and breast cancer survival in ethnically diverse postmenopausal women: The Multiethnic Cohort Study. Breast Cancer Res Treat. 2011;129:565–74. doi: 10.1007/s10549-011-1468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dignam JJ, Wieand K, Johnson KA, Fisher B, Xu L, Mamounas EP. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J Natl Cancer Inst. 2003;95:1467–76. doi: 10.1093/jnci/djg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dignam JJ, Wieand K, Johnson KA, et al. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2006;97:245–54. doi: 10.1007/s10549-005-9118-3. [DOI] [PubMed] [Google Scholar]
- 22.Azrad M, Denmark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: A review of the recent literature. Curr Nutr Rep. 2014;3:9–15. doi: 10.1007/s13668-013-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24:2506–14. doi: 10.1093/annonc/mdt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamineni A, Anderson ML, White E, et al. Body mass index, tumor characteristics, and prognosis following diagnosis of early-stage breast cancer in a mammographically screened population. Cancer Causes Control. 2013;24:305–12. doi: 10.1007/s10552-012-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ademuyiwa FO, Groman A, O’Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer. 2011;117:4132–40. doi: 10.1002/cncr.26019. [DOI] [PubMed] [Google Scholar]
- 26.Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Impact of body mass index on survival outcome among women with early stage triple-negative breast cancer. Clin Breast Cancer. 2012;12:364–72. doi: 10.1016/j.clbc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Ewertz MR, Jensen M, Gunnarsdottir KA, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 28.Kwan ML, Chen WY, Kroenke CH, et al. Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat. 2012;132:729–39. doi: 10.1007/s10549-011-1914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Lu W, Zheng W, et al. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat. 2010;122:823–33. doi: 10.1007/s10549-009-0708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118:5937–46. doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crozier JA, Moreno-Aspitia A, Ballman KV, Dueck AC, Pockaj BA, Perez EA. Effect of body mass index on tumor characteristics and disease-free survival in patients from the HER2-positive adjuvant trastuzumab trial N9831. Cancer. 2013;119:2447–54. doi: 10.1002/cncr.28051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan H, Gray RG. Effect of obesity in premenopausal ER+ early breast cancer: EBCTCG data on 80,000 patients in 70 trials. J Clin Oncol. 2014;32:15s. Abstract 503. [Google Scholar]
- 33.Swain SM, Jeong JH, Geyer CE, Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–65. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 35.Paterson AH, Anderson SJ, Lembersky BC, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): A multicentre, placebo-controlled, randomized trial. Lancet Oncol. 2012;13:734–42. doi: 10.1016/S1470-2045(12)70226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swain SM, Tang G, Geyer CE, Jr, et al. Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: The NSABP B-38 trial. J Clin Oncol. 2013;31:3197–3204. doi: 10.1200/JCO.2012.48.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodwin PJ. Commentary on: “Effect of obesity on survival in women with breast cancer: systematic review and meta-analysis” (Melinda Protani, Michael Coory, Jennifer H. Martin) Breast Cancer Res Treat. 2010;123:637–640. doi: 10.1007/s10549-010-1101-y. [DOI] [PubMed] [Google Scholar]
- 38.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–85. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 39.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 40.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]