Abstract
Increased neuroinflammation and oxidative stress resulting from heightened microglial activation is associated with age-related cognitive impairment. The objectives of this study were to examine the effects of the bioactive sulforaphane (SFN) on the nuclear factor E2-related factor 2 (Nrf2) pathway in BV2 microglia and primary microglia, and to evaluate proinflammatory cytokine expression in lipopolysaccharide (LPS)-stimulated primary microglia from adult and aged mice. BV2 microglia and primary microglia isolated from young adult and aged mice were treated with SFN and LPS. Changes in Nrf2 activity, expression of Nrf2 target genes, and levels of proinflammatory markers were assessed by quantitative PCR and immunoassay. SFN increased Nrf2 DNA-binding activity and upregulated Nrf2 target genes in BV2 microglia, while reducing LPS-induced interleukin (IL-)1β, IL-6, and inducible nitric oxide synthase (iNOS). In primary microglia from adult and aged mice, SFN increased expression of Nrf2 target genes and attenuated IL-1β, IL-6, and iNOS induced by LPS. These data indicate that SFN is a potential beneficial supplement that may be useful for reducing microglial mediated neuroinflammation and oxidative stress associated with aging.
Keywords: Aging, BV2 microglia, primary microglia, neuroinflammation, Nrf2, sulforaphane
1. Introduction
Microglia are activated in response to inflammatory stimuli and stress. Activated microglia produce proinflammatory cytokines and reactive oxygen and nitrogen species, and express genes associated with the newly defined microglial sensome (Eggen et al., 2013; Hickman et al., 2013). Another factor that results in microglial activation is aging. Microglia from old but otherwise healthy mice tend to transition from the quiescent M0 phenotype to the inflammatory M1 phenotype (Crain et al., 2013). This shift in microglial phenotype results in chronic, low grade neuroinflammation which is considered a contributing factor to some aspects of cognitive aging and other aging-related diseases. Furthermore, microglia from aged mice are hypersensitive to stress and peripheral immune stimuli and produce excessive levels of inflammatory mediators when further provoked. Microglial hypersensitivity leads to prolonged behavioral deficits (i.e. sickness behavior) following peripheral infection (Godbout et al., 2005; Henry et al., 2009). This suggests that it is necessary to regulate the proinflammatory status of microglia in the aged brain to promote successful aging.
Nuclear factor E2-related factor 2 (Nrf2) provides a critical compensatory mechanism to counteract oxidative stress through its ability to upregulate genes containing the antioxidant response element (ARE) promoter sequence (Itoh et al., 1997). Involvement of Nrf2 in regulation of inflammation and microglial activation has also been reported (Innamorato 2008; Rojo et al., 2010). Because the Nrf2 pathway can be activated by pharmacological and dietary sources, it has become a potential therapeutic target for reducing oxidative stress associated with chronic, age-related neuroinflammation (Barger et al., 2007; de Vries et al., 2008; Innamorato 2008).
Sulforaphane (SFN) is a small-molecule Nrf2 activator that can be obtained naturally from cruciferous vegetables, with especially high concentrations derived from broccoli and broccoli sprouts (Fahey et al., 1997; Talalay et al., 1995). Clinical and pre-clinical studies have established that SFN from broccoli is readily absorbed (Vermeulen et al., 2008) and has a low toxicity profile, making it suitable to be provided in supplement form (Shapiro et al., 2006; Ye et al., 2002). However, in a recent study where a 10% broccoli diet was fed to aged mice, several markers indicative of reduced glial cell activity were altered, but other markers of inflammation were not (Townsend et al., 2014). Other studies suggest that Nrf2 signaling might be disrupted during aging, leading to decreased endogenous antioxidant response (Duan et al., 2009; Suh et al., 2004), but this has not been examined in microglia. Therefore, the aim of this study was to determine if SFN mitigates markers of neuroinflammation in primary microglia from young adult and aged mice. We hypothesized that SFN would activate Nrf2 target genes and reduce production of inflammatory mediators in microglia from adult and aged mice.
2. Materials and Methods
2.1 BV2 microglia culture and treatment
The immortalized murine microglia cell line, BV2, has been used as a model to investigate the neuroimmune system (Bocchini et al., 1992; Jang et al., 2008). Cells were maintained at 37°C under 5% CO2 in 75-cm2 flasks in Dulbecco modified Eagle medium (Lonza, Allendale, NJ) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 200 mM glutamine, and streptomycin/penicillin (Invitrogen, Carlsbad, CA). In order to determine time-dependent change in ARE gene expression, cells were plated in 6-well culture dishes, then treated with vehicle (complete medium) or 2.5 μM SFN (LKT Laboratories, St. Paul, MN). Cells were harvested after 3, 6, 9, and 24 h. In subsequent experiments, cells were treated for 1 h with SFN or vehicle, then with vehicle ± lipopolysaccharide (LPS, 100 ng/mL) (Sigma, St. Louis, MO; 0127:B8) for 8 h. Each treatment was replicated 3 times in separate but identical trials.
2.2 CD11b+ primary microglia cell isolation and treatment
Adult (4–5 month-old) and aged (18–20 month-old) male BALB/c mice from our in-house colony were individually housed in polypropylene cages in a temperature controlled environment (21°C) with a reversed phase 12 h light:dark cycle (lights out at 09:00 h). Mice were given ad libitum access to rodent chow and water. Mice were euthanized using CO2 asphyxiation and brains rapidly removed for microglia isolation. All studies were carried out in accordance with United States National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the University of Illinois Institutional Animal Care and Use Committee.
To obtain primary microglia, we used an isolation method slightly modified from a protocol previously described that yields an enriched population of CD11b+/CD45low microglia that retain phenotypic integrity and inflammatory cytokine production in response to LPS (Nikodemova and Watters 2012). Cells that were positive for CD11b were isolated from brains of young adult (n = 16) and aged (n = 16) BALB/c mice. Whole brains were enzymatically digested using a Neural Tissue Dissociation Kit (Miltenyi Biotec, San Diego CA) for 35 min at 37°C. Digested tissue was then passed through a 40 μm strainer to further separate cells and remove debris and then pelleted by centrifugation at 300 × g for 15 min. Myelin removal was facilitated by suspending the pelleted cells in 30% Percoll-Plus (GE Healthcare, Princeton, NJ) and centrifuging for 10 min at 1000 × g. After centrifugation, myelin and percoll were aspirated and remaining cells were washed with PEB solution consisting of sterile phosphate-buffered saline (PBS), 0.2 mM EDTA, and 0.5% BSA. Cells were then pelleted by centrifugation, PEB solution was removed, and cells were incubated with anti-CD11b magnetic microbeads (10 μL beads 90 μL PEB; Miltenyi Biotec, San Diego CA) for 15 min. MS columns were used to magnetically separate CD11b+ cells (Miltenyl Biotec, San Diego CA). Cells were collected and suspended in medium (DMEM, 10% FBS) containing 10 ng/mL granulocyte-macrophage colony stimulated factor and plated in 12-well culture plates pre-coated with poly-L-ornithine (Sigma, St. Louis, MO). After 7–8 days in culture, primary cells were treated and harvested. All primary cells were treated with vehicle (medium) ± SFN (2.5 μM) for 1 h followed by vehicle ± LPS (10 ng/mL) for 8 h.
2.3 Nrf2 DNA-binding assay
The TransAm Nrf2 kit was used to measure Nrf2 nuclear protein binding to the ARE promoter sequence (Active Motif, Carlsbad, CA). BV2 cells were treated with SFN ± LPS as described above, then harvested with 0.25% Trypsin-EDTA and washed once with cold PBS. Cells were pelleted by centrifugation for 5 min at 500 × g. Nuclear proteins were extracted using NE-PER reagent (Pierce, Rockford, IL). Nuclear protein was quantified using the 660 nm Protein Assay Reagent from Pierce (Rockford IL) and 4.5 μg of nuclear protein per sample was used for the assay.
2.4 Markers of inflammation and oxidative stress
Total RNA was isolated from BV2 cells using E.Z.N.A. total RNA kits (Omega Biotek, Norcross, GA). RNA from primary microglia was isolated using the Tri Reagent protocol (Sigma, St. Louis, MO). Synthesis of cDNA was carried out using a high capacity RT kit (Applied Biosystems, Grand Island, NY) according to the manufacturer’s instructions. Quantitative real-time RT-PCR (qPCR) was used to detect changes in mRNA expression of ARE genes NAD(P)H quinone oxidoreductase 1 (NQO1, Mm.PT.56a.9609207), heme oxygenase 1 (HMOX1, Mm.PT.56a.9675808), and glutamate-cysteine ligase, modifier subunit (GCLM, Mm.PT.56a.11654780), and proinflammatory markers interleukin (IL)-1β (Mm.PT.56a.41616450), IL-6 (Mm.PT.56a.13354106), and inducible nitric oxide synthase (iNOS, Mm.PT.56a.43705194) using PrimeTime qPCR Assays (Integrated DNA Technologies, Coralville, IA). All mRNA expression changes were compared to the housekeeping control gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Mm.PT.39.a.1) and the 2−ΔΔCt calculation method as previously described (Jurgens et al., 2012). Data are expressed as fold change relative to the adult vehicle control.
To determine whether SFN attenuated secretion of proinflammatory cytokines, media from BV2 cells and primary microglia were collected 9 h after SFN. IL-6 protein expression was quantified using a commercially available OptEIA ELISA kit (BD Biosciences, San Jose, CA) according to manufacturer’s instructions.
Nitrite production in medium from treated BV2 cells was measured using the Promega Griess Reagent System (Madison, WI). The assay was conducted according to the manufacturer’s instructions. Absorbance was read at 532 nm.
2.5 Statistical analysis
All data were analyzed using Statistical Analysis System (Cary, NC). Data from BV2 cells were subjected to analysis of variance (ANOVA) to assess main effects of SFN at each time point, or effects of SFN, LPS and the SFN × LPS interaction. Similarly, data from primary microglia were subjected to ANOVA to assess main effects of Age, SFN, LPS, and all interactions. Where ANOVA revealed a significant interaction, post hoc Student’s t test using Fisher’s least significant differences was used to determine means separation. All data are expressed as means ± SEM.
3. Results
3.1 SFN increased expression of ARE genes in BV2 cells
Because upregulation of antioxidants may contribute to SFN’s anti-inflammatory potential, we measured the transcriptional ARE response to SFN in BV2 cells. SFN upregulates ARE genes including NQO1, HMOX1, and GCLM (Thimmulappa et al., 2002). We assessed NQO1, HMOX1, and GCLM mRNA at four time points to determine the optimal time of gene induction. As shown in Figure 1, NQO1 was increased at 6, 9, and 24 h after SFN (P<0.0001, for each) compared to vehicle controls. HMOX1 was increased at 3, 6, and 9 h after SFN (P<0.0001, for each) while GCLM was increased at 3, 6, 9, and 24 h after SFN (P<0.0001, for each). These data indicate that the BV2 microglia cell line is highly responsive to SFN.
Figure 1. SFN increased expression of ARE genes in BV2 cells.
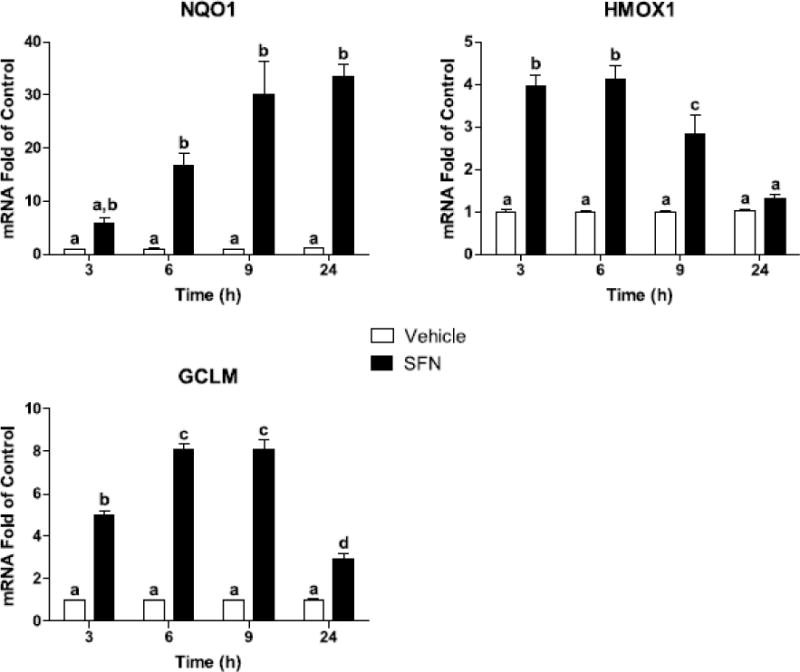
SFN increased NQO1, HMOX1, and GCLM. Bars represent means ± SEM (n=3). Means with different letters are significantly different from each other (P<0.05).
3.2 SFN increased Nrf2 DNA-binding activity and decreased proinflammatory markers in BV2 cells
Activation of Nrf2 in response to oxidative stress or chemical inducers results in its translocation to the nucleus where it regulates antioxidant genes by binding to the ARE promoter region (Itoh et al., 1997). Both SFN (P<0.05) and LPS (P<0.05) increased Nrf2 activity in BV2 cells (Figure 2) but the SFN × LPS interaction was not significant (P=0.74).
Figure 2. SFN increased Nrf2 DNA binding activity in BV2 cells.
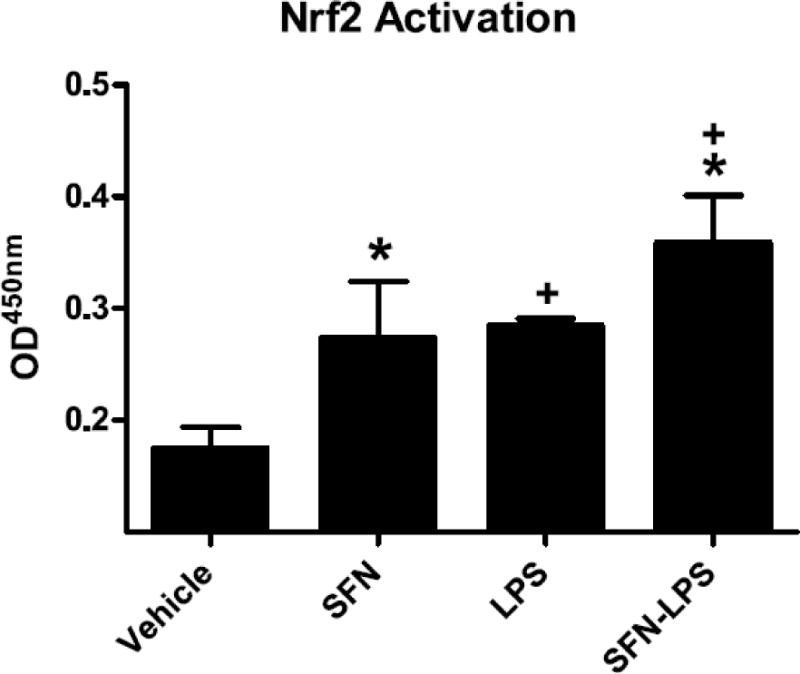
Bars represent means ± SEM (n=3). * indicates main effect of SFN and + a main effect of LPS (P<0.05).
In order to examine inhibitory effects of SFN on LPS-induced inflammation, the expression of several proinflammatory markers was assessed. As anticipated, LPS increased IL-1β, IL-6, and iNOS mRNA (P<0.01, for each) (Figure 3). The SFN × LPS interaction indicated that SFN decreased LPS-induced IL-1β (P<0.05), IL-6 (P<0.01), and iNOS (P<0.0001).
Figure 3. SFN decreased proinflammatory markers in BV2 cells.
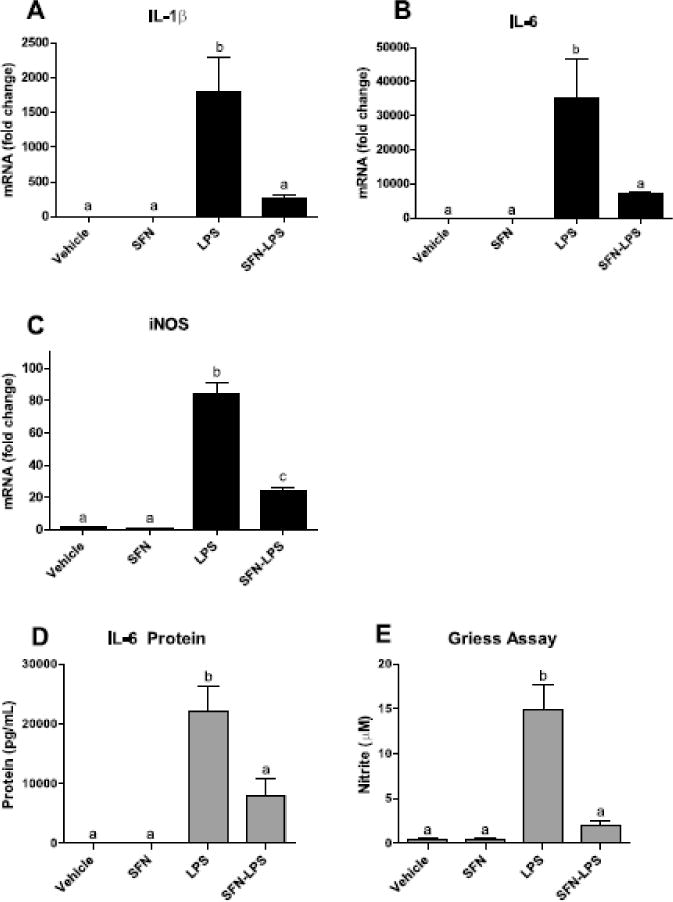
(A–C) SFN decreased LPS-induced IL-1β, IL-6, and iNOS mRNA. (D–E) SFN decreased LPS-induced IL-6 protein and nitrite equivalents. Bars represent means ± SEM (n=3). Means with different letters are significantly different from each other (P<0.05).
To determine if the change in mRNA expression was reflected by a change in protein, IL-6 protein was measured in supernatants. Consistent with its effects on IL-6 mRNA, SFN reduced IL-6 protein secretion by LPS-treated cells (P<0.05) (Figure 3). IL-1β protein is not secreted by BV2 cells and was therefore not assayed. Finally, SFN was found to inhibit nitric oxide production by LPS-stimulated BV2 cells, as measured by the Griess assay (P<0.0001).
3.3 SFN increased expression of ARE genes in primary microglia from adult and aged mice
Although SFN-induced ARE gene expression has been previously reported in microglia (Brandenburg et al., 2010; Konwinski et al., 2004), it has not been determined whether aging microglia are sensitive to the antioxidant-inducing effects of SFN. This is important because dysfunctional Nrf2 signaling may underlie age-related changes reported for microglia. To address this question, microglia isolated from adult and aged mouse brain were treated with SFN for 9 h. Exposure to SFN increased NQO1, HMOX1, and GCLM mRNA in adult and aged microglia (P<0.0001, for each) (Figure 4), and LPS further stimulated HMOX1 expression in SFN-treated microglia (SFN × LPS, P<0.01). Additionally, SFN increased GCLM in aged microglia more than adult microglia (Age × SFN, P<0.05). However, no three-way interactions were evident for NQO1, HMOX1, or GCLM. These data confirm that primary adult and aged microglia had increased ARE gene expression following exposure to SFN similar to that observed in BV2 microglia. Moreover, microglia from aged mice responded to SFN equal to or better than microglia from adult mice.
Figure 4. SFN increased expression of ARE genes in adult and aged primary brain microglia.
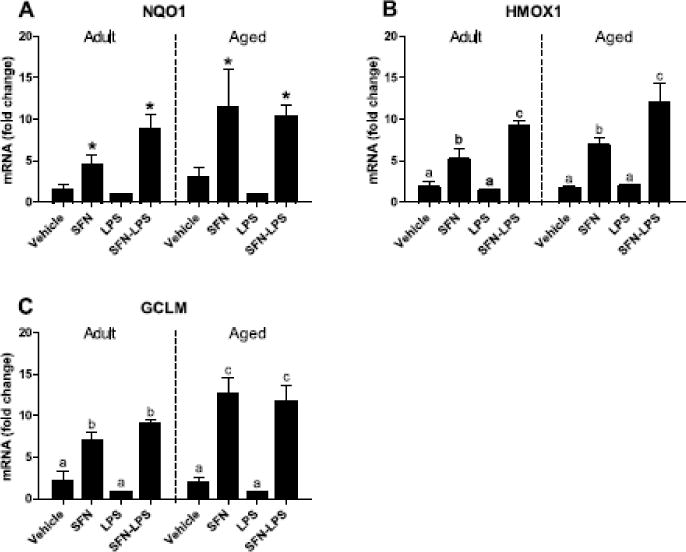
SFN increased NQO1, HMOX1, and GCLM mRNA. Bars represent means ± SEM (n=4). Means with an asterisk indicate main effect of SFN (P<0.0001). Means with different letters are significantly different from each other (P<0.05).
3.4 SFN decreased LPS-induced proinflammatory markers in primary microglia from adult and aged mice
The aging brain displays persistent microglial activity which is thought to contribute to chronic, low-grade neuroinflammation that negatively impacts long-term brain health (Godbout and Johnson 2009). Because the previous study demonstrated that microglia from aged mice were responsive to SFN, we next examined if exposure to SFN would reduce inflammation in LPS-treated primary microglia from adult and aged mice. As shown in Figure 5, LPS increased proinflammatory cytokines IL-1β and IL-6 (P<0.0001, for each) in all primary microglia. Interactions between SFN and LPS (P<0.0001) were evident for IL-1β and IL-6, providing evidence that SFN reduces LPS-induced proinflammatory markers in both adult and aged microglia. Although IL-1β was not significantly affected by age, a three-way interaction for IL-6 indicated that SFN had a greater inhibitory effect on LPS-induced IL-6 mRNA in aged microglia compared to adult microglia (Age × SFN × LPS, P<0.05). Consistent with the changes observed in IL-6 mRNA, SFN also inhibited LPS-induced secretion of IL-6 (SFN × LPS interaction, P<0.0001) in primary microglia. However, IL-6 protein levels did not differ between age groups.
Figure 5. SFN reduced proinflammatory markers in primary microglia from adult and aged mice.
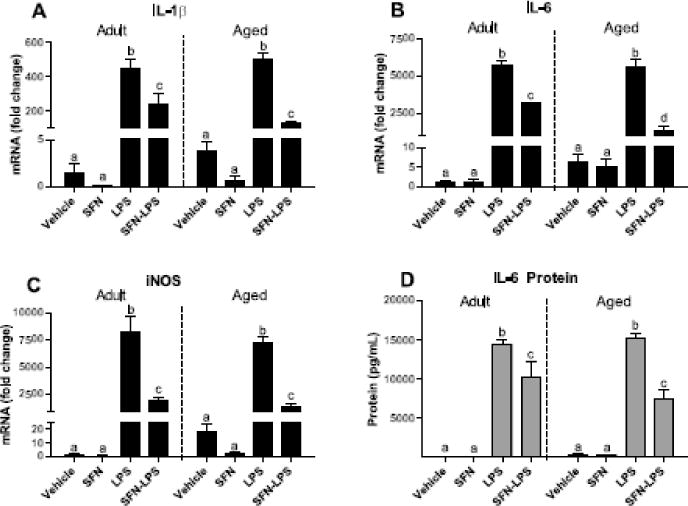
SFN decreased LPS-induced IL-1β, IL-6, and iNOS mRNA. Bars represent means ± SEM (n=4). Means with different letters are significantly different from each other (SFN × LPS, P<0.0001).
Because iNOS is induced by proinflammatory cytokines such as IL-1β, we examined the possibility that SFN could indirectly reduce oxidative stress by inhibiting iNOS expression (Liu et al., 1996). In agreement with the reduction in LPS-induced IL-1β expression in SFN-treated adult and aged microglia, SFN attenuated LPS-induced increase in iNOS mRNA (SFN × LPS interaction, P<0.0001). Age did not significantly impact iNOS expression.
4. Discussion
Heightened microglial activation contributes to the chronic low-grade neuroinflammation that is evident in the elderly (Fagiolo et al., 1993; Streit et al., 2004). Due to the rapidly expanding aging population, coupled with the numerous detrimental effects of chronic neuroinflammation such as decreased cognition, research investigating nutritional and pharmacological means to reduce neuroinflammation has garnered increasing interest. The data presented here demonstrated that microglia from aged mice respond to SFN with induction of ARE genes, and that SFN is a potent inhibitor of LPS-induced inflammation and oxidative stress. Therefore, the present study suggests that SFN supplementation may be a viable strategy to reduce age-related neuroinflammation.
Microglia are major producers of inflammatory mediators that exacerbate the production of reactive oxygen and nitrogen species in the brain (Lucin and Wyss-Coray 2009). To counter oxidative stress, microglia, along with other cells of the central nervous system, produce endogenous antioxidants that are regulated predominately through the Nrf2/ARE pathway. Our initial experiments utilizing BV2 microglia sought to determine if SFN would increase expression of ARE genes. The data obtained using BV2 microglia confirmed that ARE gene expression is increased in the presence of SFN and this is consistent with a previous study (Konwinski et al., 2004) that showed increased Nrf2 protein expression in BV2 cells after 9 h of exposure to SFN. Interestingly, our study revealed increased Nrf2 activity and induction of Nrf2 target genes using a much lower dose of SFN (2.5 μM compared to 50 μM) than used previously (Konwinski et al., 2004)
The current study was to determine if SFN could upregulate ARE genes in primary microglia from aged mice. Here, we provide evidence that SFN increased NQO1, HMOX1, and GCLM transcription in aged mouse microglia. These data indicate that the mechanisms responsible for ARE gene induction are responsive to SFN, a potent Nrf2 pathway inducer, in aging microglia. This finding is particularly important because the progression of age-related cognitive decline and neurodegenerative pathologies is closely associated with increased oxidative stress and microglial activation (Barnham et al., 2004; Dröge and Schipper 2007; Liu and Hong 2003). Additionally, some evidence points to loss of Nrf2 activity during aging, which leads to reduced expression of endogenous antioxidants (Duan et al., 2009; Suh et al., 2004). A dysregulated Nr2 pathway could render aged microglia more prone to take on an inflammatory state. However, studies reporting age-related loss of Nrf2 activity have focused on astrocytes and liver tissue (Duan et al., 2009; L’Episcopo et al., 2013; Shih and Yen 2007; Suh et al., 2004), suggesting that there may be regional or cell-specific differences in the aging Nrf2 pathway. The present study found no evidence that the Nrf2 pathway was affected in microglia from aged mice. While it is possible that the aged mice were not old enough for loss of Nrf2 activity to be detected, this is unlikely as age-related phenotypic changes have been previously demonstrated in microglia isolated from mice at the same age as those used in this study (18–20 months) (Henry et al., 2009). Nonetheless, an important finding is that the Nrf2/ARE pathway was intact in aged microglia, meaning that SFN may be useful for decreasing microglial-mediated neuroinflammation and inducing endogenous antioxidant expression through the Nrf2 pathway.
An additional benefit of SFN supplementation may be derived from reduction in proinflammatory mediators following activation of the Nrf2/ARE pathway, as SFN has been reported to reduce inflammation in a Nrf2-dependent manner. Sulforaphane inhibits elevated inflammatory cytokines in wild-type macrophages, but has no known anti-inflammatory effect on cells lacking Nrf2 (Lin et al., 2008). Upregulation of the Nrf2 pathway in endothelial cells prevented hydrogen peroxide induced oxidative toxicity and reduced inflammation by suppressing activation of the MAPK pathway (Chen et al., 2006). Further, Nrf2-mediated inhibition of P38 phosphorylation reduced inflammatory cytokines in a BV2 model of neuroinflammation (Koh et al., 2009). Here, we demonstrate that SFN attenuated the LPS-induced expression of cytokines IL-1β and IL-6 in primary microglia from aged mice. These proinflammatory markers are linked to sickness behavior and increased frailty during aging. Additionally, oxidative stress contributes to the neuroinflammatory milieu, both as a result of decreased antioxidant capacity, and increased oxidative damage. This increase in oxidative stress is associated with cognitive decline (Dröge and Schipper 2007; Floyd and Hensley 2002). Within the central nervous system, upregulation of iNOS is an important oxidative component of the microglial inflammatory response to toxins and pathogenic stimuli, but excessive production of nitric oxide can damage surrounding cells (Dawson et al., 1994; Wong et al., 1996). We observed that SFN attenuated upregulation of iNOS in microglia from aged mice, supporting our hypothesis that the antioxidant-enhancing effect of SFN attenuates increased oxidative stress in stimulated aging microglia. Due to the limited microglia that can be isolated from a single mouse brain, we were unable to detect secreted nitrate as an indication of nitric oxide production. However, in BV2 cells, LPS induced secretion of nitrate was substantially reduced by SFN, supporting the observed reduction of iNOS mRNA.
Aged microglia are characteristically less responsive to anti-inflammatory signals compared to adult microglia, thus activation of alternative signaling pathways and transcriptional regulation using SFN may offer a unique approach for reducing neuroinflammation during aging (Fenn et al., 2012). Although our studies used ex vivo SFN treatment, peripherally administered SFN has been shown to cross the blood brain barrier and reduce microglial-mediated inflammation and oxidative stress in adult mice (Innamorato 2008). Our current data further demonstrate SFN’s anti-inflammatory properties, specifically with regard to microglia isolated from aged mice. Previous studies have demonstrated that elevated neuroinflammatory mediators are found in brain tissue of otherwise healthy aged animals, implying a dysregulated neuroimmune status independent of disease (Godbout et al., 2005). Although our data did not reveal a main effect due to age, basal expression of IL-1β, IL-6, and iNOS were several-fold higher in aged control microglia compared to adult controls, in agreement with previous reports of heightened basal inflammatory markers in microglia (Frank et al., 2010). These results indicate that proinflammatory markers in aging microglia are reduced by SFN, highlighting the need for future in vivo studies examining SFN induced attenuation of microglial-mediated inflammation.
5. Conclusions
In summary, the data presented here indicate that SFN attenuates expression of IL-1β, a key proinflammatory cytokine involved in neuroinflammatory signaling. SFN also reduced proinflammatory cytokine IL-6 and oxidative stress marker iNOS. These data support the hypothesis that SFN can be used to reduce potentially adverse properties of activated aging microglia. Furthermore, because neuroinflammation is closely associated with deficits in cognitive abilities and behavioral function (Abraham et al., 2008; Abraham and Johnson 2009; Berg et al., 2005), an intervention such as SFN that reduces microglial-mediated inflammation may be advantageous to preventing age-induced decline in brain health.
Highlights.
Sulforaphane (SFN) activated Nrf2 in BV2 cells and increased antioxidant genes
SFN reduced proinflammatory cytokines in LPS-stimulated BV2 microglia
SFN increased expression of antioxidant genes in adult and aged microglia
SFN reduced LPS-induced proinflammatory cytokines in adult and aged microglia
SFN is a potential therapeutic supplement for aging-related neuroinflammation
Acknowledgments
We would like to acknowledge Dr. Marcus Lawson and Jennifer Rytych for assistance with primary microglia isolation techniques. This work was supported by NIH R01AG16710 to R.W.J.
Abbreviations
- ARE
antioxidant response element
- GCLM
glutatmate-cysteine ligand modifier subunit
- HMOX1
heme oxygenase I
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- NQO1
NAD(P)H quinone oxidoreductase
- Nrf2
nuclear factor erythroid 2-related factor
- qPCR
real-time quantitative RT-PCR
- SFN
sulforaphane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None reported
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuv Res. 2009;12:445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger SW, Goodwin ME, Porter MM, Beggs ML. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem. 2007;101:1205–1213. doi: 10.1111/j.1471-4159.2007.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Berg BM, Godbout JP, Chen J, Kelley KW, Johnson RW. α-Tocopherol and selenium facilitate recovery from lipopolysaccharide-induced sickness in aged mice. J Nutr. 2005;135:1157–1163. doi: 10.1093/jn/135.5.1157. [DOI] [PubMed] [Google Scholar]
- Bocchini V, Mazzolla R, Barluzzi R, Blasi E, Sick P, Kettenmann H. An immortalized cell line expresses properties of activated microglial cells. J Neurosci Res. 1992;31:616–621. doi: 10.1002/jnr.490310405. [DOI] [PubMed] [Google Scholar]
- Brandenburg LO, Kipp M, Lucius R, Pufe T, Wruck C. Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflamm Res. 2010;59:443–450. doi: 10.1007/s00011-009-0116-5. [DOI] [PubMed] [Google Scholar]
- Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, Kunsch C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290:H1862–H1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J Neurosci Res. 2013;91:1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson VL, Brahmbhatt HP, Mong JA, Dawson TM. Expression of inducible nitric oxide synthase causes delayed neurotoxicity in primary mixed neuronal-glial cortical cultures. Neuropharmacology. 1994;33:1425–1430. doi: 10.1016/0028-3908(94)90045-0. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Witte M, Hondius D, Rozemuller AJM, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45:1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Dröge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Zhang R, Guo Y, Jiang Y, Huang Y, Jiang H, Li C. Nrf2 activity is lost in the spinal cord and its astrocytes of aged mice. In vitro Cell Dev-AN. 2009;45:388–397. doi: 10.1007/s11626-009-9194-5. [DOI] [PubMed] [Google Scholar]
- Eggen BJ, Raj D, Hanisch UK, Boddeke HW. Microglial phenotype and adaptation. J Neuroimmune Pharmacol. 2013;8:807–823. doi: 10.1007/s11481-013-9490-4. [DOI] [PubMed] [Google Scholar]
- Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, Monti D, Franceschi C, Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RA, Hensley K. Oxidative stress in brain aging: Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol. 2010;226:181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunol Allergy Clin. 2009;29:321–337. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang L-c, Means TK, El Khour J. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innamorato N, Rojo AI, Garcia-Yague AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci. 2008;105:7534–7539. doi: 10.1073/pnas.0802865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci. 2012;32:3958–3968. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K, Cha Y, Kim S, Kim J. tBHQ inhibits LPS-induced microglial activation via Nrf2-mediated suppression of p38 phosphorylation. Biochem Bioph Res Co. 2009;380:449–453. doi: 10.1016/j.bbrc.2009.01.082. [DOI] [PubMed] [Google Scholar]
- Konwinski RR, Haddad R, Chun JA, Klenow S, Larson SC, Haab BB, Furge LL. Oltipraz, 3H-1,2-dithiole-3-thione, and sulforaphane induce overlapping and protective antioxidant responses in murine microglial cells. Toxicol Lett. 2004;153:343–355. doi: 10.1016/j.toxlet.2004.06.006. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Impagnatiello F, Pluchino S, Marchetti B. Aging-induced Nrf2-ARE pathway disruption in the subventricular zone drives neurogenic impairment in Parkinsonian mice via PI3K-Wnt/β-catenin dysregulation. J Neurosci. 2013;33:1462–1485. doi: 10.1523/JNEUROSCI.3206-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Wu RT, Wu T, Khor TO, Wang H, Kong AN. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem Pharmacol. 2008;76:967–973. doi: 10.1016/j.bcp.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhao ML, Brosnan CF, Lee SC. Expression of type II nitric oxide synthase in primary human astrocytes and microglia: role of IL-1beta and IL-1 receptor antagonist. J Immunol. 1996;157:3569–3576. [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Watters J. Efficient isolation of live microglia with preserved phenotypes from adult mouse brain. J Neuroinflam. 2012;9:147. doi: 10.1186/1742-2094-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo AI, Innamorato NG, Martín-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia. 2010;58:588–598. doi: 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- Shih PH, Yen GC. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007;8:71–80. doi: 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflamm. 2004;1:14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y. Chemoprotection against cancer by Phase 2 enzyme induction. Toxicol Lett. 1995;82–83:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- Townsend BE, Chen YJ, Jeffery EH, Johnson RW. Dietary broccoli mildly improves neuroinflammation in aged mice but does not reduce lipopolysaccharide-induced sickness behavior. Nutr Res. 2014;34:990–999. doi: 10.1016/j.nutres.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen M, Klöpping-Ketelaars IWAA, van den Berg R, Vaes WHJ. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J Agr Food Chem. 2008;56:10505–10509. doi: 10.1021/jf801989e. [DOI] [PubMed] [Google Scholar]
- Wong ML, Rettori V, Al-Shekhlee A, Bongiorno PB, Canteros G, McCann SM, Gold PW, Licinio J. Inducible nitric oxide synthase gene expression in the brain during systemic inflammation. Nat Med. 1996;2:581–584. doi: 10.1038/nm0596-581. [DOI] [PubMed] [Google Scholar]
- Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]


