Abstract
This study reports the consequences of knocking out NADPH oxidase 4 (Nox4) upon the development of hypertension and kidney injury in the Dahl salt-sensitive (SS) rat. Zinc finger nuclease injection of single cell SS embryos was used to create an 8 base-pair frame-shift deletion of Nox4 resulting in a loss of the ~68 kD band in Western blot analysis of renal cortical tissue of the SSNox4−/− rats. SSNox4−/− rats exhibited a significant reduction of salt-induced hypertension compared to SS rats after 21 days of 4.0% NaCl diet (134±5 vs 151±3 mmHg in SS) and a significant reduction of albuminuria, tubular casts, and glomerular injury. Optical fluorescence 3D cryoimaging revealed significantly higher redox ratios (NADH/FAD) in the kidneys of SSNox4−/− rats even when fed the 0.4% NaCl diet indicating greater levels of mitochondrial electron transport chain metabolic activity and reduced oxidative stress compared to SS rats. Prior to the development of hypertension, RNA expression levels of NADPH oxidase subunits Nox2, p67phox, and p22phox were found to be significantly lower (p<0.05) in SSNox4−/− compared to SS rats in the renal cortex. Thus the mutation of Nox4 appears to modify transcription of a number of genes in ways that contribute to the protective effects observed in the SSNox4−/− rats. We conclude that the reduced renal injury and attenuated blood pressure response to high salt in the SSNox4−/− rat could be the result of multiple pathways including gene transcription, mitochondrial energetics, oxidative stress, and protein matrix production impacted by the knock out of Nox4.
Keywords: Nox4, Dahl salt-sensitive rat, hypertension, oxidative stress, renal injury
Introduction
Kidney function, which plays a key role in hypertension, can be significantly compromised by pathways of oxidative stress, particularly if an imbalance between production of nitric oxide (NO) and reactive oxygen species (ROS) develops in the kidney1,2,3,4,5,6,7. NADPH oxidases (Noxs) are a major source of ROS within all regions of the kidney6,8,9,10,11,12,13,14,15,16,17,18,19 with the renal outer medulla exhibiting the greatest levels of Nox enzyme activity based on the rate of O•2− production per gram tissue12. Elevations of O•2− levels, specifically in the renal medulla of normal Sprague Dawley rats, have been shown to result in sodium retention and reductions of medullary blood flow leading to hypertension13,20,21. Conversely, local reduction of NADPH oxidase by chronic medullary interstitial infusions of apocynin resulted in a reduced hypertension in Dahl salt-sensitive (SS) rats which express elevated levels of medullary Nox2 and tissue ROS7. The renal medulla of SS rats naturally exhibits excess medullary ROS production with tissue O•2− and H2O2 concentrations nearly twice that of salt-resistant consomic SS.13BN control rats6,7, even in the pre-hypertensive, low salt-fed state5.
Yet, the relative abundance, localization, regulation and functional roles within the kidney of the various Nox isoforms have only begun to be elucidated. Nox1 and Nox 5 (not expressed in rodents9) are expressed at very low levels within the kidney and no specific functions have been yet ascribed to them19,22, despite clear implications that they play an important role in vascular pathology of a variety of disease states9,23. Nox2, the prototypical Nox isoform, has been the most widely studied and is found in the vasculature, heart, brain and kidneys. Nox4 has been found to be the most abundant Nox isoform in the kidney18,19 although its distribution and functional relevance to kidney function and in hypertension has remained poorly understood. The present study examined the contribution of the Nox4 isoform of NADPH-oxidase in salt-induced hypertension and renal injury of the SS rat, a model that recapitulates many aspects of hypertension in African Americans24. To define the role of Nox4 in SS rat, we have produced a ubiquitous knock out of the Nox4 in the SS rat utilizing zinc finger nuclease (ZFN) technology25,26. Additionally, given recent evidence that chemiluminescence signals in tissue/cell homogenates in the presence of enhancers such as lucigenin may not accurately reflect important sources of cellular ROS production27, the redox state of the kidneys in the present study was assessed by optical fluorescence three dimensional (3D) cryoimaging, a novel technique we have recently described28. Together, the results of our studies demonstrate that Nox4 contributes importantly to the development of salt-induced hypertension in the SS rat via alterations of mitochondrial electron transport chain and redox state, and through transcription effects on several NADPH oxidase subunits and the intrarenal production of collagens.
Methods
Experimental animals
Male rats were obtained at weaning from colonies developed and maintained at the Medical College of Wisconsin under controlled environmental conditions with parents and offspring fed a purified AIN-76A rodent food (Dyets, Bethlehem, PA) containing 0.4% NaCl with water provided ad libitum until the experimental period of 4.0% NaCl diet (high salt; Dyets, Bethlehem, PA). All experimental protocols were approved by the MCW Institutional Animal Care and Use Committee.
Development of SS rat with Nox4 (SSNox4−/−) knocked out
A knock out (KO) of Nox4 in the SS rat (SSNox4−/−) was developed using ZFNs25,26 designed by Sigma to target the Nox4 exon 7 sequence GGTTACAGCTTCTACctatgcAATAAGGTAAGGGTC, where ZFN binds to each underlined sequence on complementary strands. A similar approach was used successfully to study the function of p67phox17, renin29 and Rag130 in SS rats and some details of the breeding strategy are included in the Online Supplement. To confirm the mutation in homozygous animals, Nox4 cDNA of SS and SSNox4−/− products were amplified from kidney cortex samples and sequenced. The Nox4 gene in the KO rat was found to be missing 8 nt (CCTATGCA) at site +535 in exon 7. The frame shift caused by this deletion introduces a 4 amino acid and an early stop codon. The SS Nox4 protein has 578 aa while the deleted form in the KO rat maintains Nox4 N-terminal 178 aa containing the first 4 transmembrane domains but missing most of the C-terminal including D, E loop and FAD, NADPH binding domains (see Online Supplement for details).
Western blot analysis, using an antibody provided by Dr. Ajay Shah and techniques previously described by his group31 was performed on renal cortex and outer medulla tissue collected from SS and SSNox4−/− rats to confirm the absence of the Nox4 protein. Renal cortex and outer medulla tissue snap frozen at time of collection were homogenized, protein quantified and the lysates prepared for loading of 30 ug as we have previously reported7,17. Proteins were then separated on a 4–20% SDS-PAGE gel (BioRad) and transferred to a PVDF membrane prepared and probed as we have described in detail7. Additional membranes were prepared from cortex and medullary tissues from rats fed 4.0% NaCl diet for 21 days and were probed with the antibody for the Nox2 (Becton Dickinson). A ChemiDox XRS+ imaging system (BioRad) was used for chemiluminescence detection of bands and Image Lab software, version 5.1 used for quantification by densitometry. The membrane was stained with Coomassie blue and the total Coomassie blue intensity in each lane was used for normalization. As the sequencing of the homozygous Nox4 knockout cDNA fragment indicated deletion of 8 nucleotides there was a resulting shorter piece of Nox4 with N-terminal 178 aa plus 4 extra aa and molecular weight of approximately 25kD comparing to the full length of 594 aa and molecular weight around 68kD. Western blot with a Nox4 antibody from Dr.A.M. Shah15 confirmed the loss of the specific Nox4 band about 68 kD as well as 25 kD. The short isoform of Nox4 (band at 25 kD) was not observed since the antibody recognized only the C –terminal part of Nox4 protein15. We also performed western blots to determine the total abundance of S-nitrosylated products in the renal cortex and outer medulla of rats of the two strains. We used a NO2Tyr antibody (Abcam) to determine the nitrosylation of proteins in the samples and quantified as described above.
Chronic measurement of arterial blood pressure and heart rate, and collection and analysis of urine
Mean arterial pressure (MAP) and heart rate (HR) were measured by radiotelemetry in adult male rats. At six weeks of age on the day prior to surgery, rats were placed in metabolic cages for an overnight urine collection (18 h) for the measurement of urine albumin. The following day, as we have described17,32, rats were surgically prepared then given a 5–7 day recovery period before recording of blood pressure (see Online Supplement for more details). Baseline MAP was recorded 24 hrs/day for three days while animals were maintained on the 0.4% salt diet. The diet was then switched from 0.4% to 4.0% NaCl diet. MAP and HR were recorded for 24 hrs/day for 21 days. Following the last recording of MAP and HR on day 21, the rats were again placed in a metabolic cage for a second overnight urine collection. Albuminuria was quantified using an Albumin Blue 580 (Molecular Probes) fluorescence assay.
Histology
Following the last day of 4.0% NaCl diet and measurement of blood pressure and collection of urine, rats were anesthetized; the left kidney was flushed with saline and fixed with a 10% buffered formalin solution. Kidneys were paraffin embedded and 3 µm sections mounted and stained with Gomori’s trichrome and immunostained for α-smooth muscle actin (SMA; Dako Cytomation) for quantification of renal injury and fibrosis as we have described in detail33,34. One stained section from the left kidney of each rat studied was scored by a trained observer blinded to the strain/group. Tubular necrosis was determined by quantifying the percentage of the outer medullary tissue containing blocked tubules filled with protein using MetaMorph Image Analysis software (version 7.6; Universal Imaging System). For the glomerular scoring, 60 superficial glomeruli and 30 juxtamedullary glomeruli were selected and scored for each kidney section and the percent of glomeruli with a score of ≥2 calculated. The scoring of each glomerulus was a modification of the injury score described by Raij et al.35 Each glomerulus was examined and a score of 0 to 4 given according to the severity of the lesion (mesangial expansion and glomerulosclerosis) with a score of 1 represented an involvement of 25% of the glomerulus up to a score of 4 indicating 100% of the glomerulus was involved.
Quantitative PCR
Tissue was collected from a separate group of rats of both strains maintained on 0.4% NaCl diet from birth. The outer medulla was dissected free from the cortex and samples of both regions snap frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted from both the renal cortex and the outer medulla tissue using TrizolR reagent. The quality of each sample was assessed using an Agilent 2100 BioAnalzyer36 and quantity determined by spectrophotometry (NanodropR). Two ug of total RNA was reverse transcribed by random hexamer primers into cDNA (Thermo Scientific RevertAid First Strand cDNA synthesis kit) and real time PCR analysis performed using 8–10 ng total RNA as we have described using SYBR green chemistry37 on a ABI Prism 7900HT (Applied Biosystems). All primer sequences are given in the Online Supplement. Similarly, tissue was collected from the rats used for blood pressure recording at the end of the study following 21 days of 4.0% NaCl diet and renal cortex and outer medulla tissue for determination of gene expression.
Optical fluorescence 3D cryoimaging
In separate groups of SS (n=6–8/salt diet) and SSNox4−/− (n=5–9/salt diet) rats, kidneys were collected for the 3D imaging protocol. We have recently reported this technique for the determination of the oxidative state of the kidney in SS rats28. The frozen kidneys, stored at −80°C until the day of study, were mounted in the cryoimager, a custom designed and fabricated optical fluorescence imaging system from the Biophotonics Lab at the University of Wisconsin-Milwaukee previously described in detail28,38,39.
Separate images for NADH and FAD autofluorescence were obtained to probe the oxidative state of the metabolism in the tissue. These images were obtained from each of the sequentially sliced kidneys and these images from each group of kidneys were analyzed using MATLAB (The Mathworks Inc). Representations of the kidneys were assembled into 3D images using z-stacks of all the image slices for both NADH and FAD signals and the NADH to FAD redox ratio of each kidney calculated as we have recently described28. An intensity histogram distribution of NADH/FAD ratio for each kidney was calculated and the mean value determined for the histogram of each kidney28. A more detailed description of the calculation of the mean values of the histogram is given in the Online Supplement along with other additional methodological descriptions.
Statistical Methods
Data are presented as mean values ± standard error. A two-way analysis of variance (ANOVA) for repeated measures (repeated measurements in the same subjects) followed by a Holm-Sidak post hoc test were used to assess differences within and between the strains for the hemodynamic data and for the urinary excretion of albumin. For the comparison of the tissue redox ratio between the separate groups of collected kidneys from both strains on both salt diets, a two-way ANOVA followed by a Holm-Sidak post hoc test was used. Students’ t-test was used to compare between the two strains for the histological analysis of glomerular injury and tubular necrosis and for the mRNA expression analysis and for the quantification of the nitrosylated proteins. A p<0.05 was considered significant with a power of >0.69–1.0 and a β<0.31-0.
Results
Validation of the null mutation of Nox4 in the SS rat and impact on gene expression
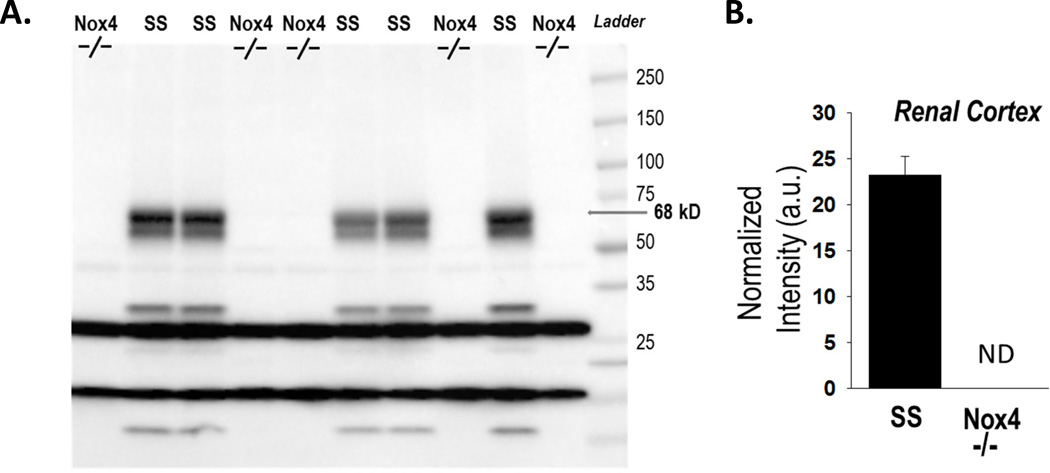
cDNA sequencing was performed on RT-PCR products amplified from the mRNA obtained from the renal cortex from SSNox4−/− rats and SS rats. Each of the SSNox4−/− rats (n=8) exhibited an 8 base-pair frame-shift deletion within exon 7 which resulted in a truncation of the 578 amino acid Nox4 protein with 178 amino acids remaining at the N-terminal (see Online Supplement for details of sequence). Figure 1 (left panel) shows the Western blot membrane with no detectable Nox4 protein band at 68 kD in the SSNox4−/− (n=5) renal cortical tissue collected after 21 days of 4.0% NaCl diet compared to tissue from the SS rat (n=5) on the same diet as quantified by densitometry (right panel). The molecular weight bands seen on our Western blot were previously observed by Anilkumar et al.15, the group from whom we obtained the antibody used in this study. Importantly, the ~68 kD band was absent in the renal cortical tissue from SSNox4−/−. Other bands which were also observed by Anikumar et al.15 using this same antibody were absent in the SSNox4−/− rats, including those bands at 62, 28, 22 and 10 kD. It has been proposed that the 28 kD band represents a splice variant of Nox4 and localized to the nucleus in vascular cells15. Another group of bands in our blot was present in both strains and appears to be the result of non-specific binding. In our preliminary Western blot, there was no signal for Nox4 detected in homogenized outer medullary tissue in the SS rat so the data is not shown.
Figure 1.
Western blot analysis of Nox4 protein expression in renal cortex tissue homogenates prepared from SSNox4−/− (n=5) and SS (n=5) rats maintained on 4.0% NaCl diet. Panel A. No expression of the full length Nox4 isoform was detected at the 68 kD in the mutant rats SSNox4−/− (Nox4−/−) compared to the SS in renal cortex tissue. No expression was noted in either strain in the outer medulla. Panel B. In the renal cortical tissue, densitometry was used to quantify the band at 68 kD in each lane normalized to the protein loaded as determined by Coomassie staining of the membrane. The Nox4 protein was not detectable (ND) in the renal cortex from the SSNox4−/− rats.
Arterial pressure responses to the 4.0% NaCl diet
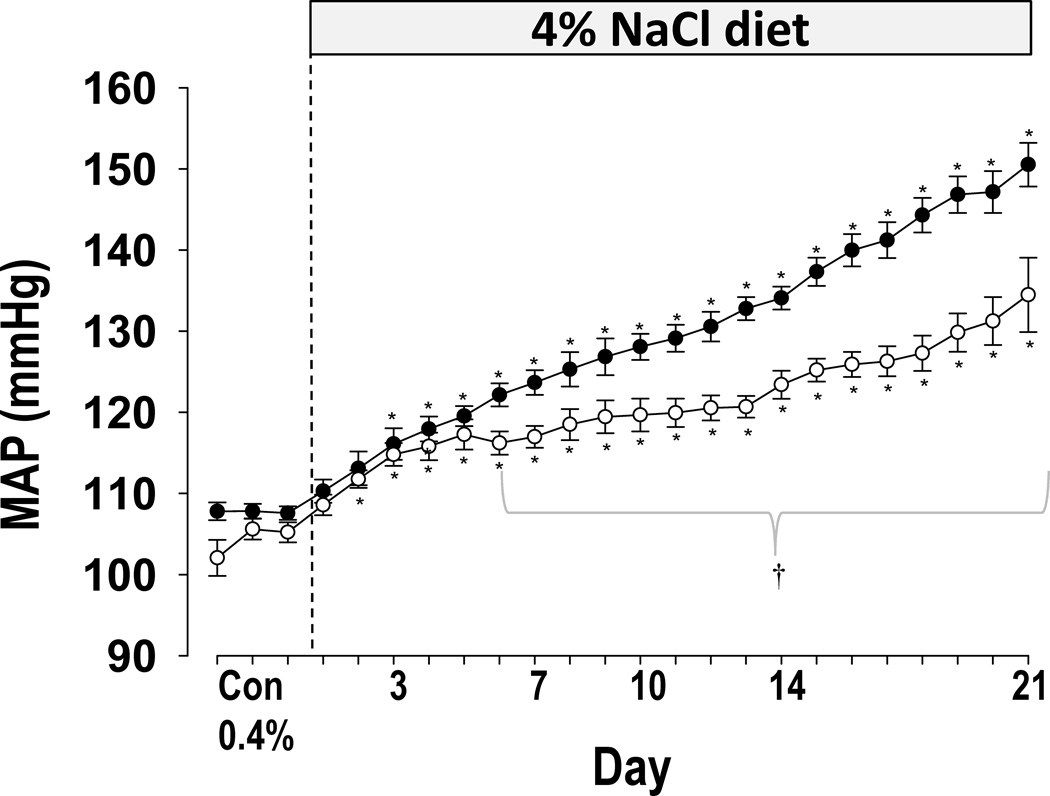
As summarized in Figure 2, SSNox4−/− rats exhibited a marked reduction of salt-induced hypertension compared to SS rats. Mean arterial pressure (MAP) 24 hr averages did not differ significantly (p<0.05) between SS (n=8; 108±1 mmHg) and SSNox4−/− rats (n=9; 105±1) on the last day of 0.4% salt diet. However, after 21 days of the high salt (4.0% NaCl) diet, the average 24 hr MAP of SSNox4−/− rats was significantly lower (134±5 mmHg; n=9) relative to SS rats (151±3 mmHg; n=8; p<0.05). The continuous 24 hr pulsatile pressure data were analyzed to determine if the circadian rhythm was modified in SSNox4−/− compared to SS rats. MAP was averaged in 12 hr bins to correspond to the day (6am–6pm) and night (6pm–6am) cycles. No significant differences in rhythmicity were observed between strains although the amplitudes of cycles were greater in SS rats fed the 4.0% NaCl diet (p<0.01; Online Supplement Figure S1). To control for the effect of pressure itself on the amplitude of the cycling, the amplitude data were normalized to the MAP. Analysis of these normalized data showed no significant differences between the strains during the control 0.4% NaCl diet or after the transition to the high salt diet.
Figure 2.
Mean arterial pressure (MAP) was measured on 0.4% NaCl diet for three days and for 21 days after the diet was switched to 4.0% NaCl diet in SS (closed circles; n=8) and SSNox4−/− (open circles; Nox4−/−; n=9). † indicates significant difference between SS and SSNox4−/− (p<0.05; β<0.2). * indicates significant difference of a 4.0% NaCl diet day from the three control days within a strain (p<0.05; β<0.2). (Two-way ANOVA for repeated measures; Holm-Sidak post hoc)
The 24 hr heart rate for SS rats maintained on the 0.4% NaCl diet averaged 446±5 and 455±3 beats per minute (bpm) in SSNox4−/− rats. After transition to high salt diet, heart rate fell significantly in both strains by day 8 and remained reduced through day 21 of 4.0% NaCl diet (405±2 bpm in SS; 409±4 in SSNox4−/−) and was not significantly different between strains.
Indexes of renal injury
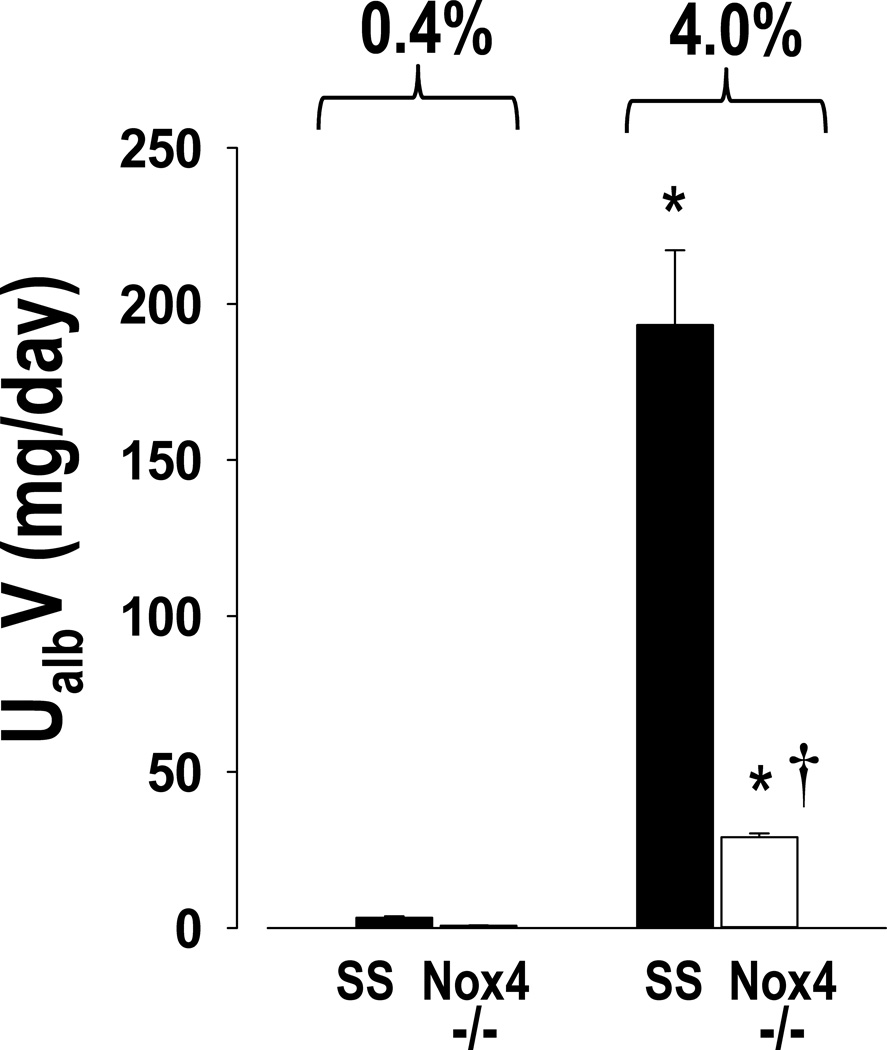
The daily excretion of albumin determined on the last day of 0.4% NaCl and after 21 days of 4.0% NaCl diet is summarized in Figure 3. Significantly lower levels of albumin excretion were observed in the SSNox4−/− rats even when receiving the 0.4% NaCl diet when compared to the SS strain. Most evident, however, was the marked protection from severe albuminuria observed after 21 days of 4.0% NaCl diet at which time SSNox4−/− rats excreted only about 1/7 as much albumin as SS rats.
Figure 3.
Albumin was measured in urine samples collected overnight on the last day of 0.4% NaCl diet and on day 21 of 4.0% NaCl diet for the determination of urinary excretion of albumin (UalbV) in SS (n=8) and SSNox4−/− (n=9) rats. * indicates significant difference between strains at each salt level (p<0.05; β<0.1). (Two-way ANOVA for repeated measures; Holm-Sidak post hoc)
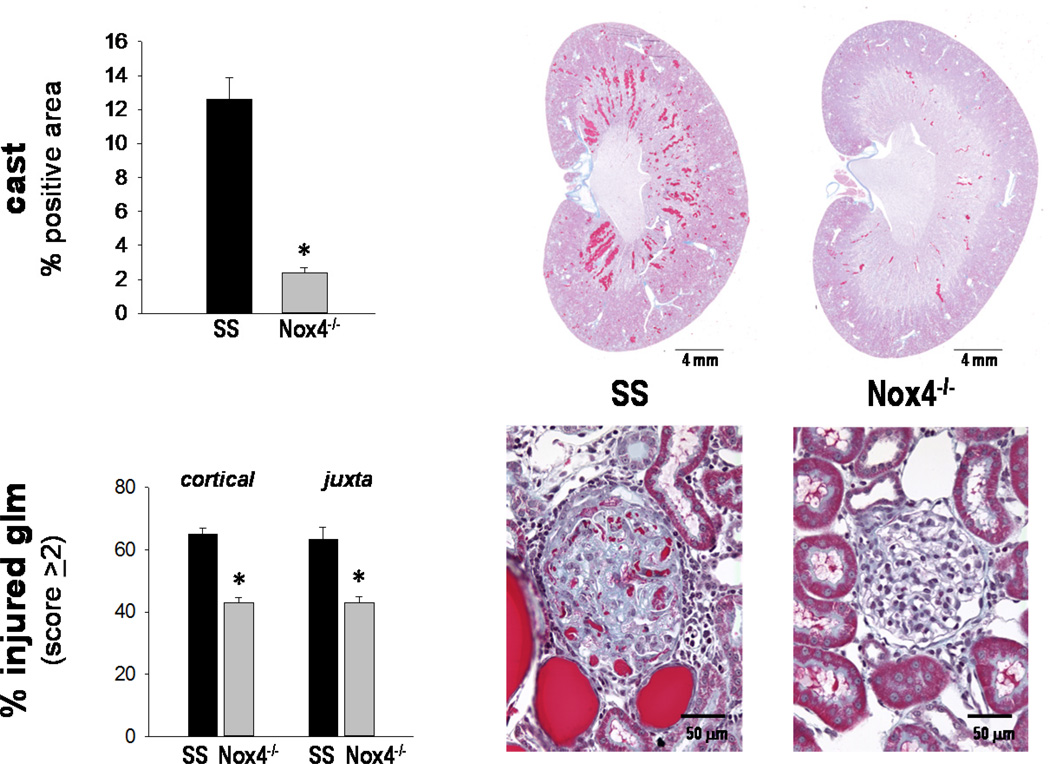
Histological analyses of renal injury in the kidneys of both strains after 21 days of high salt (n=9–10/strain) included determination of tubular necrosis and glomerular injury. As summarized in Figure 4, SSNox4−/− rats exhibited markedly less tubular necrosis as evidenced by a large reduction of tubular cast staining (Gomori’s trichrome; 2.4±0.3% positive strained region) compared to SS rats (12.6±1.3%). Glomerular injury was also significantly less (p<0.05) in SSNox4−/− than in SS rats when comparing the percentage of glomeruli with an injury score of ≥ 2 (scale of 0 for no injury to 4 with marked glomerulosclerosis and mesangial expansion). Kidneys of SSNox4−/− rats averaged 43±1.5% of the glomeruli compared to the SS kidneys with 65±3.9% of the glomeruli with an injury score of ≥ 2 in both the cortical and juxtamedullary areas. α-SMA immunostaining for renal fibrosis in the renal outer medulla was not significantly different between SSNox4−/− (7.3±0.5%) and SS (7.9±1.0%) rats (p<0.05).
Figure 4.
Panel A: Summary of the outer medullary tubular injury (percentage of tubular cast positive region) and the percentage of injured glomeruli with a score ≥2 determined in the cortical and juxtamedullary (juxta) regions from both SS (n=9) and SSNox4−/− (n=10) rats. * indicates significant difference when SSNox4−/− was compared to SS (p<0.05; β<0.2). Panel B: A representative section of trichrome stained kidney from each strain is shown to demonstrate the overview of the tubular injury. Higher magnification at 40x is also shown to demonstrate the considerable injury in the SS compared to the SSNox4−/− with a representative cortical glomerulus from each strain shown.
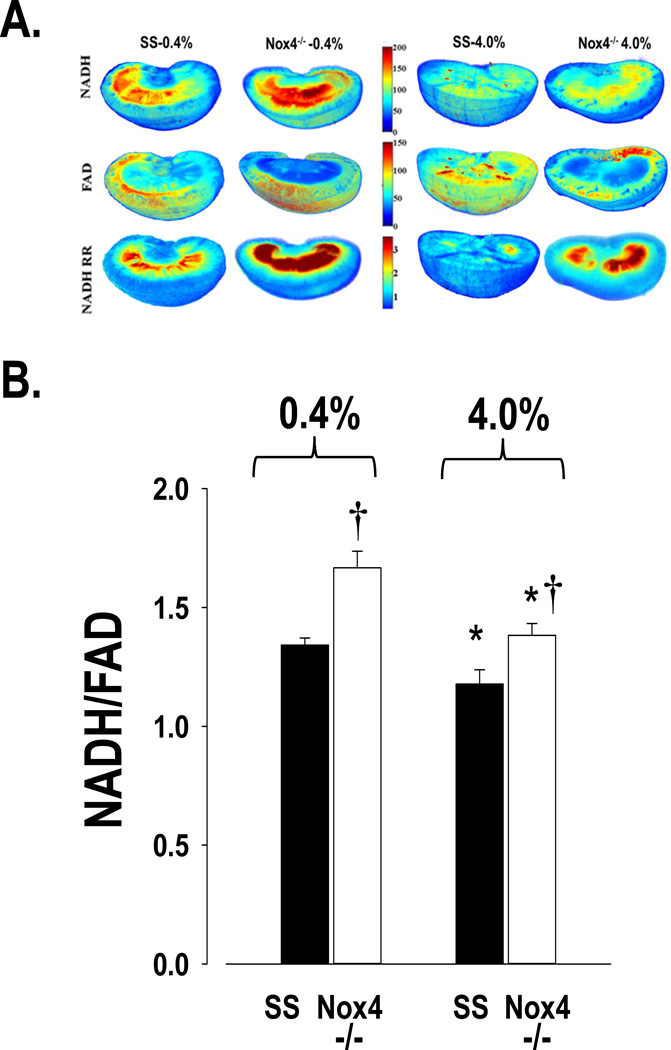
NADH/FAD redox ratios and determination of oxidative stress levels
Figure 5A presents representative 3D images showing the NADH and FAD fluorescence signals and the tissue redox ratios (NADH/FAD) obtained from a SS and a SSNox4−/− rat fed either the 0.4% NaCl diet or the 4.0% NaCl diet for 21 days. Higher NADH and lower FAD fluorescence signals (i.e. higher redox ratios) are evident in the kidneys of SSNox4−/− rats compared to SS rats fed either salt diet. Lower tissue redox ratios represent higher levels of oxidative stress and the tissue redox ratio shows an inverse relationship with the oxidative stress level of the tissue. In both strains, the 4.0% NaCl diet resulted in lower redox ratios throughout the kidney, especially in the mitochondria-rich renal medulla as apparent by the pseudo-red color intensity. However, redox ratios remained comparably greater in the SSNox4−/− rats. Images of each of the kidneys analyzed for these studies are presented in the Online Supplement (Figure S2).
Figure 5.
Panel A shows a composite of representative three-dimensional rendered images of kidneys from each strain on both salt diets. The distribution of fluorescence signal for NADH and FAD and the tissue redox ratio (NADH/FAD) indicate those regions highest in red (see intensity scale) that are protected from the oxidative stress as demonstrated by the higher NADH/FAD ratio in the SSNox4−/− strain. Panel B summarizes the mean values of the tissue redox ratio of NADH/FAD for the separate groups of SS and SSNox4−/− (Nox4−/−) rats on 0.4% (SS n=6; Nox4−/− n=5) and 4.0% NaCl diets (SS n=8; Nox4−/− n=9). * indicates significant difference within the strain between the two diets (p<0.05; β<0.1). † indicates a significant difference between strains on the same salt diet (p<0.05; β<0.1). (Two-way ANOVA; Holm-Sidak post hoc)
Figure 5B summarizes the group data for the kidney tissue redox ratios of SS and SSNox4−/− rats on the two salt diets. Kidneys of SSNox4−/− rats exhibited significantly higher tissue redox ratios than kidneys of SS rats whether fed a 0.4% or the 4.0 NaCl diet indicating reduced oxidative stress. The redox ratio of SS kidneys of rats fed the 4.0% NaCl diet (n=8) was significantly lower than those fed the 0.4% NaCl diet (n=6), 1.18±0.06 versus 1.34±0.03 respectively as was the case in SSNox4−/− rats (n=9) which averaged 1.38±0.05 when fed the 4.0% NaCl diet compared to SS Nox4−/− rats (n=5) which averaged 1.67±0.07 (p<0.001) when fed 0.4% NaCl diet. Based on a two-way ANOVA analysis, there was a statistically significant difference due to strain (p<0.001) and salt diet (p<0.001) and a significant interaction of the two (p= 0.046). These data indicate that although oxidative stress, as assessed from the redox ratios, was increased with the high salt diet in both strains, it was buffered in the SSNox4−/− rats.
These results were further supported by the determination of nitrosylated protein (NO2Tyr) by western blots of the renal cortex and outer medulla from the SS and SSNox4−/− rats on 0.4% and 21 days of 4.0% NaCl diet. In these studies, there was no significant difference between the abundance of NO2Tyr in the renal cortex or outer medulla of the SS (n=6) or SSNox4−/− (n=5) rats on 0.4% NaCl diet. However, the outer medulla of the SS rats fed 4.0% NaCl for 21 days, had significantly higher abundance of NO2Tyr (1.3 fold higher; p<0.005) than the SSNox4−/− rats. The renal cortex of the 4.0% NaCl fed SS rats was slightly but significantly less than the SSNox4−/− rats (p<0.053).
Gene expression profiles of SSNox4−/− rats
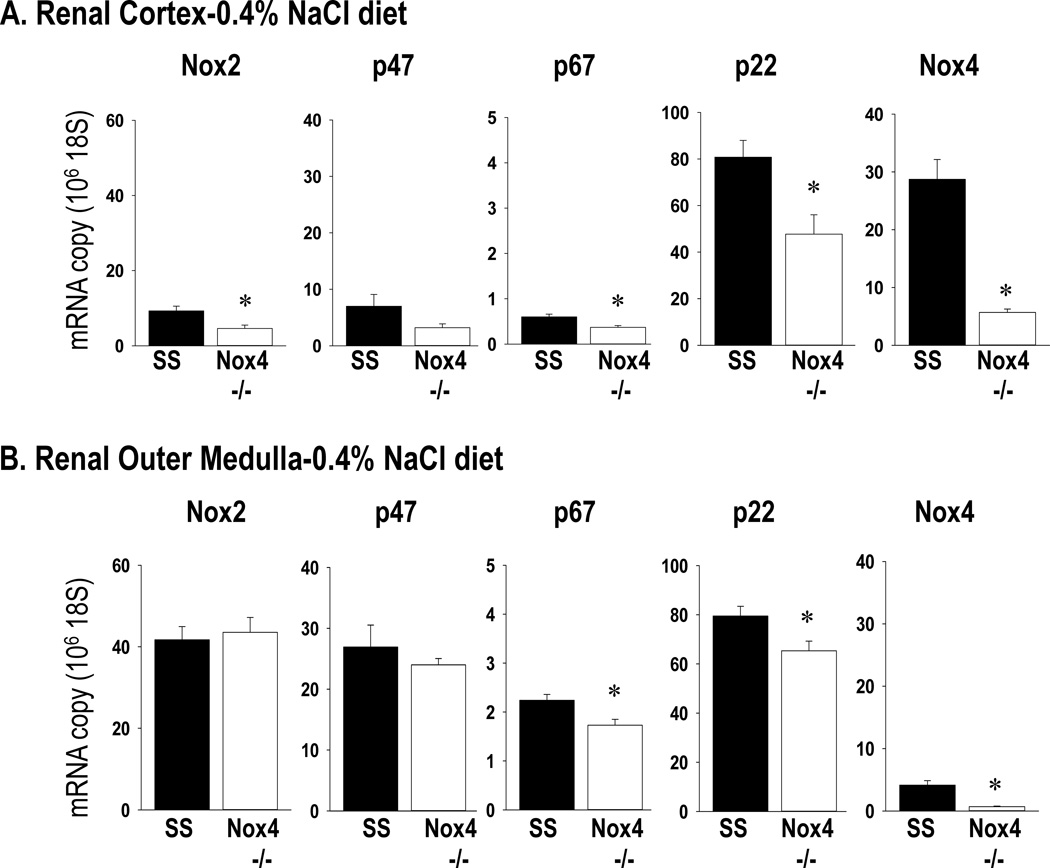
Figure 6 summarizes the gene expression of the NADPH subunits and Nox4 in the renal cortex and renal outer medulla tissue collected in the pre-hypertensive state from rats maintained on the 0.4% NaCl diet. Expression of p67phox, p22phox, and Nox4 were significantly reduced (p<0.05) in the renal cortex and outer medulla of SSNox4−/− rats compared to SS rats. Nox2 was significantly less in the SSNox4−/− compared to the SS (p<0.05) but only in the renal cortex. Nox1 mRNA was detected in the renal tissue by RT-PCR but the expression was too low for quantitative RT-PCR to be run.
Figure 6.
mRNA expression was determined by qPCR from RNA extracted from homogenates of renal cortical (Panel A) and outer medullary (Panel B) tissue collected from SS rats (n=6) and SSNox4−/− (n=6) rats maintained on 0.4% NaCl diet. The RNA expression levels of the NADPH subunits gp91phox (Nox2), p47phox (p47), p67phox (p67) and p22phox (p22) and of Nox4. Tissues from both strains were run for any given gene on the same plate thus expression levels can be compared between the two renal regions. * indicates significant difference between strains (p<0.05; β<0.3). (Students’ t-test)
Shown in Figure S3 (Online Supplement) are the results of the Western blot analysis for Nox2 protein in homogenates prepared from the renal cortex and outer medulla tissue also collected in the pre-hypertensive state from rats maintained on the 0.4% NaCl diet. Nox2 protein expression was significantly reduced in the renal outer medulla of the SSNox4−/− rats compared to the SS rats.
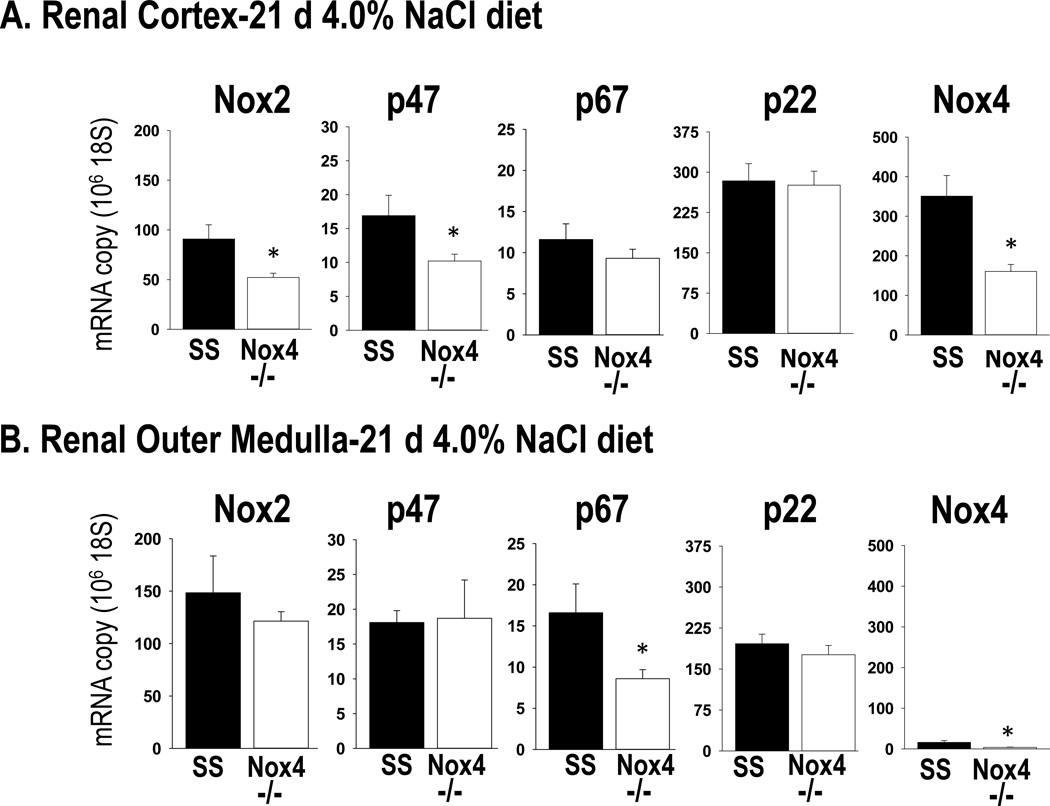
Results obtained when the expression of these genes was analyzed in renal cortex and outer medulla tissue collected at the end of the study after 21 days of 4.0% NaCl diet also indicated that Nox2 was reduced in the SSNox4−/− rats. As shown in Figure 7, analysis of mRNA expression of the NADPH subunits Nox2 and p47phox from renal cortex found that SSNox4−/− rats (n=4) exhibited a nearly 50% reduction (p< 0.05) of these isoforms in the renal cortical tissue while no significant differences were observed in outer medullary tissue as compared to SS rats (n=4). In contrast to the rats fed 0.4% NaCl diet, no significant differences were observed in expression of p67phox or p22 in the cortex in SS (N=4) versus SSNox4−/− (N=4) rats but there was a significant reduction of the p67phox isoform in the outer medulla.
Figure 7.
mRNA expression was determined by qPCR from RNA extracted from homogenates of renal cortical (Panel A) and outer medullary (Panel B) tissue collected from SS rats (n=6) and SSNox4−/− (n=6) rats after 21 days of 4.0% NaCl diet. The RNA expression levels of the NADPH subunits gp91phox (Nox2), p47phox (p47), p67phox (p67) and p22phox (p22) and of Nox4. Tissues from both strains were run for any given gene on the same plate thus expression levels can be compared between the two renal regions. * indicates significant difference between strains (p<0.05; β<0.3). (Students’ t-test)
Figure S4 (Online Supplement) summarizes the results of the Western blots analysis for Nox2 protein in homogenates prepared from renal cortex and outer medulla tissue collected in those rats of both strains at the end of study after 21 days of 4.0% NaCl. Nox 2 protein expression significantly reduced in the renal cortex of SSNox4−/− rats (n=9) compared to SS (n=6). Consistent with the mRNA expression data, these results indicate that the reduction of Nox2 in SSNox4−/− rats was confined largely to the renal cortex after 21 days of 4.0% NaCl diet.
Since we did not detect a difference in α-SMA staining for renal fibrosis between strains, we tested expression levels of collagen. RNA expression levels of Collagen 1a1, 1a2, and 4a2 were also significantly lower in SSNox4−/− compared to SS rats (p<0.05) in both the renal cortex and renal outer medulla in rats maintained on 0.4% NaCl diet and in rats after 21 days of 4.0% NaCl diet (see Figure S5 and S6 in the Online Supplement).
Together these results indicate that knock out of the Nox4 gene resulted in mRNA expression changes related to proteins critically important to the activity of Nox2 and matrix proteins of the cortex and medulla of the kidney. Remarkably, rather than observing compensatory increases of Nox2 related subunits in the kidney tissue from SSNox4−/− rats, the changes measured moved in parallel with the reduction of Nox4 expression in this mutated SS rat. Since these transcriptional changes were observed in the pre-hypertensive period when the blood pressure was the same in both rat strains prior to switching to the high salt diet, it is evident that these changes were a consequence of the reduction of Nox4 rather than changes secondary to the high salt diet and/or hypertension. The reduction of Nox2 expression and of the collagen matrix proteins would be expected to further enhance the protection of the kidneys from fibrotic injury and contribute to the blunting of salt-induced hypertension beyond that achieved by reduction of Nox4 alone.
Discussion
The role of Nox4 in hypertension and associated renal injury has remained largely unexplored. Nox4 is known to be abundantly expressed in the kidney18,19,40,41 as confirmed by the present study. We found that tissue levels of Nox4 mRNA in the kidney were expressed at levels nearly seven fold higher in the renal cortex than medulla of pre-hypertensive SS rats fed a 0.4% NaCl diet. Western blotting further revealed that Nox4 is highly expressed in the cortex versus outer medulla in rats fed 4.0% NaCl diet. Most importantly, however, the knock out of the Nox4 gene provided the SS rat significant protection from renal oxidative stress, renal injury and hypertension. Moreover, the results revealed mechanistic insights whereby Nox4 exerted these protective effects including alterations of cellular mitochondrial bioenergetics, alterations of the transcriptional regulation of NADPH oxidase subunits, and by transcriptional regulation of collagen synthesis within the kidney.
Nox4 effects on renal mitochondrial bioenergetics and oxidative stress
The 3D cryofluorescence images show that the tissue redox ratio (NADH/FAD) was significantly higher in the kidneys of SSNox4−/− rats compared to SS rats whether fed the 0.4% or 4.0% NaCl diet, similar to the differences we recently observed between the SS and SSp67phox− null mutant rats28. Lower tissue redox ratios represent higher levels of oxidative stress and the tissue redox ratio shows an inverse relationship with the oxidative stress level of the tissue. The higher NADH signal and lower FAD signal observed in the mitochondria-rich renal medulla of the SSNox4−/− rats indicates the greater presence of NAD in the reduced form (NADH) in the mitochondria and less FADH2 present in its oxidized form (FAD) compared to SS rats. Enhanced pairing of electrons with hydrogen and less leakage of electrons in the mitochondrial electron transport chain (ETC) to available oxygen would result in less production of ROS42. Importantly, since NADH and FAD signals originate exclusively from the mitochondria42,43, the present results indicate greater mitochondrial ETC metabolic activity and decreased oxidative stress in the mitochondria-rich renal medulla of the SSNox4−/− rats. It is recognized that in the presence of oxidative stress, the ETC does not function efficiently due to oxidative damage of ETC complexes. This leads to excess electron leak and ROS production which leads to the accumulation of the mitochondrial coenzymes NADH and FADH2 in their oxidized forms NAD and FAD. Hence, a decrease in NADH fluorescence and an increase in FAD fluorescence results in a decreased redox ratio (NADH/FAD). Therefore the inverse relationship between the redox ratio and oxidative stress shows that the lower redox ratios observed in the SS rats represent higher levels of renal oxidative stress. The presence of greater abundance of nitrosylated protein products (NO2Tyr) in the outer medulla of the SS rat fed 4.0% NaCl diet further supports the greater levels of oxidative stress in this strain than the SSNox4−/−.
It is interesting that although we were able to detect greater Nox4 protein expression in cortical tissue homogenates than in medullary tissue of SS rats, alterations in the redox state by the mutation of the Nox4 were more apparent in the renal medulla. This appears to be explained by the insensitivity of the Nox4 antibody since preliminary functional studies in our lab have shown that Nox4 plays an important role in ROS production in the freshly isolated perfused mTAL of SS rat44.
We have reported in previous studies that kidneys of SS rats exhibit a fumarase insufficiency45 and the mTAL of SS rats exhibit a reduced efficiency of O2 utilization46. Fumarase is an enzyme in the TCA cycle which facilitates a transition step in the production of energy in the form of NADH and chronic infusion of a fumarate precursor into the medullary interstitium of a salt-resistant control strain (SS.BN13) restored blood pressure salt-sensitivity45. It has recently been found that Nox4 can potently inhibit the enzyme fumarate hydratase (FH) leading to accumulation of fumarate47. The kidney tissue of diabetic mice and human subjects exhibited reduced expression of FH which could be restored in vitro by the administration of Nox1/Nox4 inhibitor GKT13783147. Transgenic overexpression of Nox4 in mice resulted in overexpression of fumarate as found in diabetic mice and this was associated with endoplasmic reticulum stress, matrix gene expression, expression of hypoxia-inducible factor-1a (HIF-1a) and TGF-β, events attenuated by NOX1/NOX4 inhibition in diabetic mice47. These data suggest that the knock out of Nox4 in SS rats may result in restoration of fumarase which could account for the enhanced energy production and reduced mitochondrial ROS production observed in the SSNox4−/−.
Evidence of Nox4 regulation on NADPH oxidase gene transcription
It was found that SSNox4−/− rat kidneys expressed reduced levels of Nox2, p67phox, and p22phox mRNAs in the renal cortical tissue of rats fed the 0.4% NaCl diet and prior to the development of hypertension. As these subunits are critical to the activity of Nox2, the reduction of renal ROS in SSNox4−/− rat kidneys could be attributed to these observed changes although Nox2 activity levels were not specifically quantified in the present study. The parallel reductions of the Nox2 subunits in the SSNox4−/− rat kidneys indicates that Nox4 is participating in the transcriptional regulation of these genes whereby Nox4 and Nox2 may act in concert in determining renal function in the SS rat. It should be noted that Nox1 mRNA is expressed at nearly undetectable and unquantifiable levels in rodent kidneys22, as we have also observed (data not shown).
These findings are of particular importance since, as the field moves forward and relies increasingly on the application of gene editing model systems, it is important to appreciate that the resulting phenotypes may not represent changes that can be directly attributable to the gene that was targeted. The present data are a good example of a response that was not a predictable compensatory response, which would offset the loss of function of the Nox4. Rather, the KO resulted in responses which would appear to amplify the effect of simply removing Nox4. It will not be easy to quantify the relative contribution of these interacting systems but important to recognize the complexity of these systems as we move forward.
Although Nox4 is the most abundantly expressed Nox isoform in the kidney, we have shown that Nox2 also contributes importantly to renal dysfunction and hypertension in the SS rats. The null mutation of the p67phox gene in SS rats, a critical subunit of Nox217, also resulted in reductions of salt-induced hypertension and renal injury very similar to those presently observed with the knock out of Nox4. SSp67phox−/− null rats also exhibit a significant reduction of ROS production in the renal interstitium as determined by microdialysis17 and by optical fluorescence 3D cryoimaging28. As p67phox is important for the activation only of Nox2 (and not Nox4), it is evident that Nox2 contributes to hypertension in SS rats. Given the data from the present study showing that knock out of Nox4 results in reductions of several of the key Nox2 subunits, it appears that the protective effects of Nox4 KO could be attributed to the parallel reduction of Nox2 activity.
Nox4 effects on transcriptional regulation of collagen production
Knock out of Nox4 in SS rats on the 0.4% NaCl diet prior to hypertension in response to high salt, resulted in reduced mRNA expression levels of Collagens 1a1, 1a2, 4a2 in both the cortex and medulla. The reduced expression of these collagens indicate that Nox4 was indeed having a protective effect from fibrosis in these rats even though the immunostaining and quantification of kidney α-SMA did not demonstrate reduced levels of fibrosis in the SSNox4−/− rats following 4.0% NaCl diet for 21 days. We have previously reported that expression of Col1a1 was upregulated in SS rats as compared to the congenic salt-resistant SS.13BN control strain48. Col1a2 and Col3a1 have been shown to be elevated in response to MMP-7 and also in aging kidneys and in diabetes49 where these proteins are thought to contribute to the development of fibrosis leading to chronic kidney disease50. TGFβ1, which is elevated in kidneys of SS rats51 and increases the progression of renal interstitial fibrosis independent of blood pressure, is associated with increases of fibronectin-1 and collagen 1a152. Based on these observations, the reduced expression of collagens 1a1, 1a2 and 4a2 would be expected to provide added protection from the salt-induced hypertension and renal injury in the SSNox4−/− rats.
Other possible contributors to renal protection in SSNox4−/− rats
Given the global nature of the Nox4 knock out, we also recognize that other sites of action of Nox4 (e.g. vascular or CNS) could have contributed to the observed protection from hypertension and renal injury in SSNox4−/− rats. However, given the abundance of Nox4 in the kidney18,19, it is likely that the reduced intrarenal actions of Nox4 contributed in large measure to the reduced oxidative stress and injury of the SSNox4−/− rats. Finally, it should be recognized that reduced renal injury in SSNox4−/− rats could also be a consequence of the blunting of hypertension since it has been shown using servo-control techniques, that renal perfusion pressure contributes importantly to the renal injury in SS rats34.
Nox4 – a unique Nox isoform
The general biochemical features of the Nox family of enzymes have been well characterized and described by others14,15,16. Nox4 is unique in that it does not require the binding of cytosolic p67phox, p47phox or Rac1 or 2 proteins to produce ROS which is produced constitutively and mostly as H2O29,53,54. In addition to the cell membrane, Nox4 may also be localized to intracellular locations such as the mitochondria, ER, the nucleus and focal adhesions55 but observations are quite variable and controversial given the lack of antibody selectivity, variations in staining approaches, splice variants56 and possible transitioning between intracellular compartments57,58.
The role of Nox4 in hypertension has not been previously studied in rats. Most studies related to the functional role of Nox4 have focused on the systemic and pulmonary vasculature, and largely in cultured cells, where this isoform has been shown to be constitutively active, regulated at the gene level, and influenced by angiotensin II, shear stress/flow, hypoxia and microRNAs57,59,60,61. Nox4 was found to be a major source of oxidative stress in rat NRK-52E cells62 (proximal tubule-like) when stimulated with angiotensin II. In freshly isolated mTAL of Sprague Dawley rats, Nox4 siRNA was found to reduce O•2− responses to acute stimulation with either angiotensin II19 or increased luminal flow22. In other studies, Nox4 has been importantly implicated in glomerular podocyte injury and apoptosis in a rat podocyte injury model induced by puromycin aminonucleoside treatment63. Results of this study indicated that TGF-β1 induced mitochondrial Nox4 activation and ROS production through the TGF-β receptor-Smad2/3 signaling pathway. This conforms to the recent studies in Nox4 transgenic mice with specific expression in glomerular podocytes which resulted in accumulation of fumarate which is a key regulator of HIF-1α and TGF-β. In mice, knock out of Nox4 has been found to provide protection from oxidative stress and injury of the systemic vasculature during ischemic or inflammatory stress induced by angiotensin II infusion64. Deletion of Nox4 in streptozotocin-induced diabetic ApoE(−/−) mice also was found to confer renal protection from glomerular injury64. Together, these observations are consistent with the present observations indicating that the knock out of Nox4 in SS rats results in protection from renal glomerular, tubular and interstitial injury.
Perspectives
Nox4 is the most highly expressed NADPH-oxidase isoform in the kidney18,19 and appears to be involved in a variety of diseases such as idiopathic pulmonary fibrosis, pulmonary arterial hypertension, diabetic nephropathy, complications of diabetic cardiomyopathy and neuropathy and retinopathy, and metastatic carcinoma65. Little is known about the role of Nox4 in normal renal function and sodium homeostasis, or in the pathophysiology of hypertension15,18,66. The broad significance of the present study is: First, an SS rat with a null nutation in Nox4 was produced enabling assessment of this NADPH oxidase isoform in an hereditary model of salt-sensitive hypertension. Second, data is presented showing that Nox4 contributes importantly to the hypertension, renal oxidative stress, and the renal injury observed in SS rats fed a high salt diet. Third, mechanistic insights were obtained indicating that Nox4 exerts its effects through multiple pathways, most notably by transcriptional regulation of Nox2 enzyme subunits, transcriptional regulation of collagen synthesis, and via its effects on mitochondrial energetics.
Given that prior studies have utilized various cell types in culture (human and mouse), a variety of methods for inhibiting Nox4 (nonselective inhibitors, siRNA, knockout, etc.), and different disease models, it is not surprising that observations and conclusions regarding the role of Nox4 in several pathological states have been inconsistent. The results of the present study provide strong evidence that Nox4 contributes significantly to the development of salt-induced hypertension in the SS rat. Studies will now be required to reveal in greater detail the underlying pathways and mechanisms involved such that targeted therapeutics can be developed and the relevance to human hypertension can be determined.
Supplementary Material
Novelty and Significance.
What is new?
The SSNox4−/− first rat model with a deletion of the Nox4 gene.
The study represents the first to evaluate the specific contribution of Nox4 in a naturally occurring form of hypertension in the rat, Dahl salt-sensitive (SS) rats.
Mechanistic insights were obtained indicating that Nox4 exerts its effects through multiple pathways, most notably by transcriptional regulation of Nox2 enzyme subunits, transcriptional regulation of collagen synthesis, and via its effects on mitochondrial energetics.
What is relevant
Nox4 was found to play an important role in salt-sensitive hypertension which is relevant to human disease.
Deletion of Nox4 in the salt-sensitive Dahl (SS) rat resulted in a significant reduction of salt-induced hypertension.
Nox4 is the most highly expressed NADPH-oxidase isoform in the kidney and the results of this study indicate that this largely unexplored Nox isoform plays an important role in fluid-electrolyte homeostasis and in hypertension.
Summary
The knockout of the Nox4 isoform of NADPH oxidase in the Dahl salt-sensitive rat revealed that Nox4 plays a very important role in the development of this form of hypertension and the associated renal injury. It will now be important to determine the sites and mechanisms whereby Nox4 exerts this potent effect on blood pressure regulation.
Acknowledgments
A. Cowley designed the study, participated in data analysis, and drafted the manuscript. C. Yang and K. Sadovnikov performed the Western blot analysis and the RNA expression experiments. T. Kurth performed the blood pressure studies. C. Yang, N. Zheleznova, F. Salehpour, and M. Ranji performed the tissue collection and 3D optical imaging studies. R. Ryan performed the histological analysis for the quantification of renal injury. L. Rein provided statistical consultation and analyses. A. Dayton and M. Hoffman performed the analysis of the circadian patterns. A. Staruschenko participated in the study design and data interpretation. M. Skelton performed data analysis and manuscript preparation. A. Geurts produced the mutant rat strain and participated in the study design and data interpretation.
Sources of Funding
This work was supported by National Institutes of Health, National Institute of Heart, Lung and Blood grants HL-116264 (A. Cowley, A. Geurts) and HL-122662 (A. Cowley, A. Staruschenko).
Footnotes
Conflicts(s) of Interest
Medical College of Wisconsin may one day receive royalties on the sales of genetically modified rats.
References
- 1.Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension. 2008;52:777–786. doi: 10.1161/HYPERTENSIONAHA.107.092858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowley AW, Jr, Abe M, Mori T, O’Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol. 2015;308:F179–F197. doi: 10.1152/ajprenal.00455.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manning RD, Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol. 2005;25:311–317. doi: 10.1159/000086411. [DOI] [PubMed] [Google Scholar]
- 4.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Ryan RP, Cowley AW, Jr, Staruschenko A. Real-time electrochemical detection of ATP and H2O2 release in freshly isolated kidneys. Am J Physiol. 2013;305:F134–F141. doi: 10.1152/ajprenal.00129.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor NE, Cowley AW., Jr Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol. 2005;289:R1573–R1579. doi: 10.1152/ajpregu.00525.2005. [DOI] [PubMed] [Google Scholar]
- 6.Taylor NE, Maier KG, Roman RJ, Cowley AW., Jr NO synthase uncoupling in the kidney of Dahl S rats: role of dihydrobiopterin. Hypertension. 2006;48:1066–1071. doi: 10.1161/01.HYP.0000248751.11383.7c. [DOI] [PubMed] [Google Scholar]
- 7.Taylor NE, Glocka P, Liang M, Cowley AW., Jr NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47:692–698. doi: 10.1161/01.HYP.0000203161.02046.8d. [DOI] [PubMed] [Google Scholar]
- 8.Cifuentes-Pagano E, Csanyi G, Pagano PJ. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell Mol Life Sci. 2012;69:2315–2325. doi: 10.1007/s00018-012-1009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirker A, Zhang M, Shah AM. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takac I, Schroder K, Brandes RP. The Nox family of NADPH oxidases: friend or foe of the vascular system? Curr Hypertens Rep. 2012;14:70–78. doi: 10.1007/s11906-011-0238-3. [DOI] [PubMed] [Google Scholar]
- 12.Zou AP, Li N, Cowley AW., Jr Production and actions of superoxide in the renal medulla. Hypertension. 2001;37:547–553. doi: 10.1161/01.hyp.37.2.547. [DOI] [PubMed] [Google Scholar]
- 13.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW., Jr Increased renal medullary oxidative stress produces hypertension. Hypertension. 2002;39:667–672. doi: 10.1161/hy0202.103469. [DOI] [PubMed] [Google Scholar]
- 14.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31:S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 15.Anilkumar N, San Jose G, Sawyer I, Santos CX, Sand C, Brewer AC, Warren D, Shah AM. A 28-kDa splice variant of NADPH oxidase-4 is nuclear-localized and involved in redox signaling in vascular cells. Arterioscler Thromb Vasc Biol. 2013;33:e104–e112. doi: 10.1161/ATVBAHA.112.300956. [DOI] [PubMed] [Google Scholar]
- 16.Brandes RP, Schroder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends Cardiovascu Med. 2008;18:15–19. doi: 10.1016/j.tcm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Feng D, Yang C, Geurts A, Kurth T, Liang M, Lazar J, Mattson D, O’Connor P, Cowley AW., Jr Increased expression of NAD(P)H oxidase subunit p67phox in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metabolism. 2012;15:201–208. doi: 10.1016/j.cmet.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 19.Massey KJ, Hong NJ, Garvin JL. Angiotensin II stimulates superoxide production in the thick ascending limb by activating NOX4. Am J Physiol. 2012;303:C781–C789. doi: 10.1152/ajpcell.00457.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YF, Cowley AW, Jr, Zou AP. Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol. 2003;285:R827–R833. doi: 10.1152/ajpregu.00636.2002. [DOI] [PubMed] [Google Scholar]
- 21.Makino A, Skelton MM, Zou AP, Cowley AW., Jr Increased renal medullary H2O2 leads to hypertension. Hypertension. 2003;42:25–30. doi: 10.1161/01.HYP.0000074903.96928.91. [DOI] [PubMed] [Google Scholar]
- 22.Hong NJ, Garvin JL. NADPH oxidase 4 mediates flow-induced superoxide production in thick ascending limbs. Am J Physiol. 2012;303:F1151–F1156. doi: 10.1152/ajprenal.00181.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 24.Cowley AW, JR, Roman RJ. The role of the kidney in hypertension. JAMA. 1996;275:1581–1589. [PubMed] [Google Scholar]
- 25.Geurts AM, Cost GJ, Freyvert Y, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geurts AM, Cost GJ, Remy S, Cui X, Tesson L, Usal C, Menoret S, Jacob HJ, Anegon I, Buelow R. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol Biol. 2010;597:211–225. doi: 10.1007/978-1-60327-389-3_15. [DOI] [PubMed] [Google Scholar]
- 27.Rezende F, Loewe O, Helfinger V, Prior KK, Walter M, Zukunft S, Fleming I, Weissmann N, Brandes RP, Fleming I, Weissmann N, Brandes RP, Schroder K. Unchanged NADPH oxidase activity in Nox1-Nox2-Nox4 triple knockout mice-What do NADPH-stimulated chemiluminescence assays really detect? Antioxid Redox Signal. 2015 Jun 30; doi: 10.1089/ars.2015.6314. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Salehpour F, Ghanian Z, Yang C, Zhelezenova N, Kurth T, Dash RK, Cowley AW, Jr, Ranji M. Effects of p67phox on the mitochondrial oxidative state in the kidney of Dahl salt-sensitive rats: optical fluorescence 3D cryoimaging. Am J Physiol. 2015;309:F377–F382. doi: 10.1152/ajprenal.00098.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno C, Hoffman M, Stodola TJ, Didier DN, Lazar J, Geurts AM, North PE, Jacob HJ, Greene AS. Creation and characterization of a renin knockout rat. Hypertension. 2011;57:614–619. doi: 10.1161/HYPERTENSIONAHA.110.163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol. 2013;304:R407–R414. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and Nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 32.De Miguel C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and lead to hypertension and renal disease. Am J Physiol. 2011;300:F734–F742. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori T, Cowley AW., Jr Role of pressure in angiotensin II-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension. 2004;43:752–759. doi: 10.1161/01.HYP.0000120971.49659.6a. [DOI] [PubMed] [Google Scholar]
- 34.Mori T, Cowley AW., Jr High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol. 2008;19:1472–1482. doi: 10.1681/ASN.2007121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984;26:137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 36.Lu L, Li P, Yang C, Kurth T, Misale M, Skelton M, Moreno C, Roman RJ, Greene AS, Jacob HJ, Lazar J, Liang M, Cowley AW., Jr Dynamic convergence and divergence of renal genomic and biological pathways in protection from Dahl salt-sensitive hypertension. Physiol. Genomics. 2010;41:63–70. doi: 10.1152/physiolgenomics.00170.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison J, Knoll K, Hessner MJ, Liang M. Effect of high glucose on gene expression in mesangial cells: upregulation of the thiol pathway is an adaptational response. Physiol Genomics. 2004;17:271–282. doi: 10.1152/physiolgenomics.00031.2004. [DOI] [PubMed] [Google Scholar]
- 38.Sepehr R, Staniszewski K, Maleki S, Jacobs ER, Audi S, Ranji M. Optical imaging of tissue mitochondrial redox state in intact rat lungs in two models of pulmonary oxidative stress. J Biomed Opt. 2012;17:046010. doi: 10.1117/1.JBO.17.4.046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staniszewski K, Audi SH, Sepehr R, Jacobs ER, Ranji M. Surface fluorescence studies of tissue mitochondrial redox state in isolated perfused rat lungs. Ann Biomed Eng. 2013;41:827–836. doi: 10.1007/s10439-012-0716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–1888. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 41.Gorin Y, Block K, Hernandez J, Bhandari b, Wagner B, Barnes JL, Abboud HE. Nox4 NADPH oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 42.Chance B, Baltscheffsky H. Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide. J Biol Chem. 1958;233:736–739. [PubMed] [Google Scholar]
- 43.Chance B, Schoener B, Oshino R, Itshak F, Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J Biol Chem. 1979;254:4764–4771. [PubMed] [Google Scholar]
- 44.Zheleznova NN, Geurts AM, Yang C, Cowley AW., Jr Contribution of Nox4 to luminal flow induced H2O2 production in medullary thick ascending limbs of Dahl salt-sensitive rats. Hypertension. 2013;62:A83. [Google Scholar]
- 45.Tian Z, Liu Y, Usa K, Mladinov D, Fang Y, Ding X, Greene AS, Cowley AW, Jr, Liang M. Novel role of fumarate metabolism in Dahl salt-sensitive hypertension. Hypertension. 2009;54:255–260. doi: 10.1161/HYPERTENSIONAHA.109.129528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheleznova NN, Yang C, Ryan RP, Halligan BD, Liang M, Greene AS, Cowley AW., Jr Mitochondrial proteomic analysis reveals deficiencies in oxygen utilization in medullary thick ascending limb of Henle in the Dahl salt-sensitive rat. Physiol Genomics. 2012;44:829–842. doi: 10.1152/physiolgenomics.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.You YH, Quach T, Saito R, Pham J, Sharma K. Metabolomics reveal a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J Am Soc Nephrol. 2015;27 doi: 10.1681/ASN.2015030302. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Taylor NE, Lu L, Usa K, Cowley AW, Jr, Ferreri NR, Yeo NC, Liang M. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension. 2010;55:974–982. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korrapati MC, Howell LA, Shaner BE, Megyesi JK, Siskind LJ, Schnellmann RG. Suramin: a potential therapy for diabetic nephropathy. PLoS One. 2013;8:e73655. doi: 10.1371/journal.pone.0073655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oelusarz A, Nichols LA, Grunz-Borgmann EA, Chen G, Akintola AD, Catania JM, Burghardt RC, Trzeciakowski JP, Parrish AR. Overexpression of MMP-7 increases collagen 1A2 in the aging kidney. Physiol Rep. 2013;1:e00090. doi: 10.1002/phy2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CC, Geurts AM, Jacob HJ, Fan F, Roman RJ. Heterozygous knockout of transforming growth factor-b1 protects Dahl S rats against high salt-induced renal injury. Physiol Genomics. 2013;45:110–118. doi: 10.1152/physiolgenomics.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryoo IG, Ha H, Kwak MK. Inhibitory role of the KEAP1-NRF2 pathway in TGFB1-stimulated renal epithelial transition to fibroblastic cells: a modulatory effect on SMAD signaling. PLoS One. 2014;9:e93265. doi: 10.1371/journal.pone.0093265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause K-H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen F, Haigh S, Barman S, Fulton DJR. From form to function: the role of Nox4 in the cardiovascular system. Frontiers in Physiol. 2012;3 doi: 10.3389/fphys.2012.00412. article 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goyal P, Weissmann N, Rose F, Grimminger F, Schafers HJ, Seeger W, Hanze J. Identification of novel Nox4 splice variants with impact on ROS levels in A549 cells. Biochem Biophys Res Commun. 2005;329:32–39. doi: 10.1016/j.bbrc.2005.01.089. [DOI] [PubMed] [Google Scholar]
- 57.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Paunkova L, Du P, Papaharalambus C, Lasseque B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Von Lohneysen K, Noach D, Wood MR, Friedman JS, Knaus UG. Structural insights into Nox4 and Nox2, motifs involved in function and cellular localization. Mol Cell Biol. 2010;30:961–975. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 60.Fu Y, Zhang Y, Wang Z, Wang L, Wei X, Zhang B, Wen Z, Fang H, Pang Q, Yi F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am J Nephrol. 2010;32:581–589. doi: 10.1159/000322105. [DOI] [PubMed] [Google Scholar]
- 61.Montezano AC, Burger D, Ceravolo GS, Yusug H, Montero M, Touyz RM. Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond) 2011;120:131–141. doi: 10.1042/CS20100384. [DOI] [PubMed] [Google Scholar]
- 62.Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW, Ihm CG, Moon JY. Angiotensin II-induced mitochondrial Nox4 is a major endogenous sources of oxidative stress in kidney tubular cells. PLoS One. 2012;7:e39739. doi: 10.1371/journal.pone.0039739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu L, Liu Y, Wu Y, Liu Q, Geng J, Gu X, Xiong Y, Fan Q, Ye J. Smad3/Nox4-mediated mitochondrial dysfunction plays a crucial role in puromycin aminonucleoside-induced podocyte damage. Cell Signal. 2014;26:2979–2991. doi: 10.1016/j.cellsig.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 64.Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Manikoshi T, Thallas-Bonke V, Wingler K, Szyndralewiez C, Heitz F, Touyz RM, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol. 2104;25:1237–1254. doi: 10.1681/ASN.2013070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laleu B, Gaggini F, Orchard M, Gioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem. 2010;53:7715–7730. doi: 10.1021/jm100773e. [DOI] [PubMed] [Google Scholar]
- 66.Nistala R, Whaley-Connell A, Sowers JR. Redox control of renal function and hypertension. Antioxid Redox Signal. 2008;10:2047–2089. doi: 10.1089/ars.2008.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.