Abstract
Natural killer (NK) cells can provide effective immunotherapy for ovarian cancer. Here, we evaluated the ability of NK cells isolated from peripheral blood (PB-) and NK cells derived from induced pluripotent stem cell (iPSC-) to mediate killing of ovarian cancer cells in a mouse xenograft model. A mouse xenograft model was used to evaluate the intraperitoneal delivery of three different NK cell populations: iPSC-derived NK cells, PB-NK cells that had been activated and expanded in long-term culture, and overnight activated PB-NK cells that were isolated through CD3/CD19 depletion of peripheral blood B and T cells. Bioluminescent imaging was used to monitor tumor burden of luciferase expressing tumor lines. Tumors were allowed to establish prior to administering NK cells via intraperitoneal injection. These studies demonstrate a single dose of any of the three NK cell populations significantly reduced tumor burden. When mice were given 3 doses of either iPSC-NK cells or expanded PB-NK cells, the median survival improved from 73 days in mice untreated to 98 and 97 days for treated mice, respectively. From these studies, we conclude iPSC-derived NK cells mediate anti-ovarian cancer killing at least as well as PB-NK cells, making these cells a viable resource for immunotherapy for ovarian cancer. Due to their ability to be easily differentiated into NK cells and their long-term expansion potential, iPSCs can be used to produce large numbers of well-defined NK cells that can be banked and used to treat a large number of patients including treatment with multiple doses if necessary.
Keywords: induced pluripotent stem cells, natural killer cells, ovarian cancer, immunotherapy
Introduction
Patients with recurrent ovarian cancer face a poor prognosis due to the limited efficacy of standard therapies [1]. Recently, there has been rapid advancement in the production of novel immunotherapies for treatment of refractory malignancies. Natural killer (NK) cells are lymphocytes with anti-tumor properties that represent a potent cytotoxic population for allogeneic adoptive cell transfer. Use of haplo-identical NK cells has shown tremendous promise for the treatment of acute myeloid leukemia (AML), and a Phase II clinical trial at our institution has utilized NK cells intravenously for the treatment of ovarian cancer [2, 3]. While this approach is promising, limitations of the therapy still exist. Recently we have demonstrated NK cells to be more effective in mediating anti-ovarian cancer activity when delivered via intraperitoneal (IP) injection rather than intravenously [4]. These studies facilitated the opening of an ongoing clinical trial to assess IP delivery of NK cells in patients with refractory ovarian cancer (clinicaltrials.gov, NCT02118285).
One of the limitations to these approaches has been the source NK cells. Currently NK cells are typically isolated from the peripheral blood (PB) of haplo-identical donors through CD3 (T cells) and CD19 (B cells) depletion followed by overnight stimulation with IL-2. However, this cellular product is a heterogeneous mixture of cells, with typically only about 30% of infused cells being NK cells [5]. While devoid of T cells and B cells, this cell product still contains monocytes and other blood cells in addition to the NK cells. Furthermore, this approach yields only enough cells for a single dose, must be performed separately for each patient, and is time consuming and costly.
To produce a homogeneous and well-defined NK cell product, we have developed a clinically translatable method for the development and expansion of NK cells derived from human induced pluripotent stem cells (iPSCs) [6]. With the ability to produce large quantities, iPSC-NK cells are now becoming a viable cell population for use in immunotherapy [7]. We have previously demonstrated that iPSC-NK cells are effective against leukemia and HIV infection [8, 9]. Since NK cells are not HLA restricted, NK cells derived from iPSCs can be utilized as an allogeneic “off-the-shelf” immunotherapy for the treatment of cancer. Also, repeated dosing of NK cells becomes feasible as many cell “doses” can be banked and stored. These studies now evaluate the use of iPSC-derived NK cells and peripheral blood NK cells (PB-NK cells) that have been expanded using artificial antigen presenting cells (aAPCs) compared to the current clinical product, overnight activated PB-NK cells. We find that aAPC expanded PB-NK and iPSC-NK cells provide an improved anti-tumor effect in vivo when compared to overnight-activated PB-NK cells.
Materials and Methods
Cell Lines
iPSCs (UCBiPS7, derived from umbilical cord blood CD34+ cells) were produced and maintained on as described previously [10]. The serous epithelial ovarian tumor cell lines MA-148 and A1847 were kindly provided by Sundaram Ramakrishnan (University of Minnesota) and Reuben Harris (University of Minnesota), respectively. Luciferase expressing MA-148 and A1847 cells were created as previously described [6]. Briefly, 500,000 cells were nucleofected with 1 µg of pKT2 plasmid containing a GFP:zeocin fusion protein and firefly luciferase as well as 1 µg of SB100X transposase using the 4D-NucleofectorTM system (Lonza). Cells were then selected with zeocin and flow cytometry was used to confirm all cells were GFP positive.
Isolation of Peripheral Blood NK cells
Overnight-activated PB-NK cells were collected as previously described [4]. In short, mononuclear cells were isolated through density gradient centrifugation from an apheresis product (Memorial Blood Center), and NK cells were enriched by depleting CD3+ and CD19+ cells using magnetic beads (Miltenyi Biotec, Auburn, CA). Use of peripheral blood mononuclear cells from donors was approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota. Following CD3/CD19 depletion, cells were activated overnight with 100 Units/ml interleukin 2 (IL-2, Promethus).
Flow Cytometry
The following antibodies were used: CD56-APC, CD16-PercpCy5.5, NKp44-PE, NKp46-PE, NKG2D-PE, and NKG2A all from Becton Dickson. CD158a/h-PE, CD158j-PE, CD158i-PE, CD158e1/e2, CD159a-PE were obtained from Beckman Coulter and used as a cocktail for overall killer cell immunoglobulin (Ig)-like receptors (KIR) expression. Flow cytometry was done on a BD FACS Calibur and data analyzed using FlowJo (Treestar).
Derivation of iPSC-NK cells
The derivation of NK cells from iPSCs has been previously described [6, 11]. Briefly, 3,000 TrypLE adapted iPSCs were seeded in the middle 60 wells of 96-well round-bottom plates in BPEL (bovine serum albumin polyvinyl alcohol essential lipids) [11] containing stem cell factor (SCF) (40 ng/ml, R&D Systems, Minneapolis, MN), vascular endothelial growth factor (20 ng/ml, R&D Systems, Minneapolis, MN), and bone morphogenic protein 4 (20 ng/ml, R&D Systems, Minneapolis, MN). At day 11 of hematopoietic differentiation to produce CD34+/CD43+ cells, EBs were directly transferred into each well of uncoated 24-well plates. Cells were then further differentiated into NK cells as previously reported [9] using IL-3 (first week only, 5 ng/mL), IL-15 (10 ng/mL), IL-7 (20 ng/mL), SCF (20 ng/mL), and flt3 ligand (10 ng/mL) (R&D Systems, Minneapolis, MN). Half-media changes were performed weekly and NK cell were harvested after 28–32 days for aAPC expansion.
aAPC Expansion of PB-NK and iPSC-NK Cells
NK cells were expanded using clone 9.mbIL-21 artificial antigen-presenting cells (aAPCs) (kindly provided by Dr. Dean Lee, MD Anderson Cancer Center), as previously described [6, 12]. Briefly, NK cells were maintained in RPMI-1640 (Life Technologies, Carlsbad, CA) containing 10% FBS (Life Technologies, Carlsbad, CA), 1% penicillin/streptomycin (Life Technologies, Carlsbad, CA), and 50 units/mL of interleukin-2. Cells were stimulated 2:1 with irradiated aAPCs (10,000 cGy) upon initiation of cultures. Subsequently, media was changed twice weekly and cells were re-stimulated with aAPCs every 7 days.
51-Chromium Release Assay
Tumor targets (K562, MA-148, and A1847 cells) were loaded with 51-Chromium by incubating target cells for 1 hour at 37°C. Target cells were then washed three times and co-cultured with NK cells at the indicated effector to target ratios. Total lysis was achieved using 5% Triton-X 100. After 4 hour incubation with NK cells, the tumor cells were harvested and analyzed. Specific 51-Cr lysis was calculated using the equation: % specific lysis = 100 × (release - spontaneous release) / (maximal release - spontaneous release).
Animal Models
NOD/SCID/γc−/− (NSG) mice (Jackson Laboratories, Bar Harbor, ME) were used for all in vivo experiments. Mice were given 2×105 luciferase expressing tumor cells via IP injection four days prior to NK cell injection (D-4). On D-1 mice were sub-lethally irradiated (225 cGy), and bioluminescent imaging (BLI) was used to ensure tumor engraftment. Mice were then grouped based on BLI to ensure each group started with same average tumor burden. NK cells (20×106 cells/mouse) were then given IP to mice on D0. BLI was used to track tumor growth weekly using the Xenogen IVIS Imaging system (Caliper Life Science, Hopkinton, MA). Mice received IP injections of IL-2 (5 µg/mouse/day) for the first 7 days, followed by injections every Monday, Wednesday, and Friday for 3 additional weeks to help stimulate NK cell survival and expansion in vivo.
Statistical Analysis
Tumor volume, measured by bioluminescence, was log10 transformed to ensure normality. Data were compared for each experiment separately. Tumor volume was compared at each time point by treatment group (excluding negative control) using analysis of variance (ANOVA) and pairwise comparisons were conducted using t-tests. P-values for pairwise comparisons at each time were conservatively adjusted for multiple comparisons using a Bonferroni correction. Tumor volume over time was compared by treatment group (excluding negative control) using repeated measures mixed models, including treatment, day and treatment by day interaction as fixed effects and a random effect for mouse. Overall survival (OS) of the mice was calculated from treatment initiation to death or censored at the end of the experiment (112 days). OS was summarized using Kaplan-Meier methods and compared by treatment group using log rank tests; median survival and 95% confidence intervals (CI) are presented. P-values <0.05 were considered statistically significant.
Results
Expansion of NK cells with artificial antigen presenting cells (aAPCs) yields a pure population of NK cells
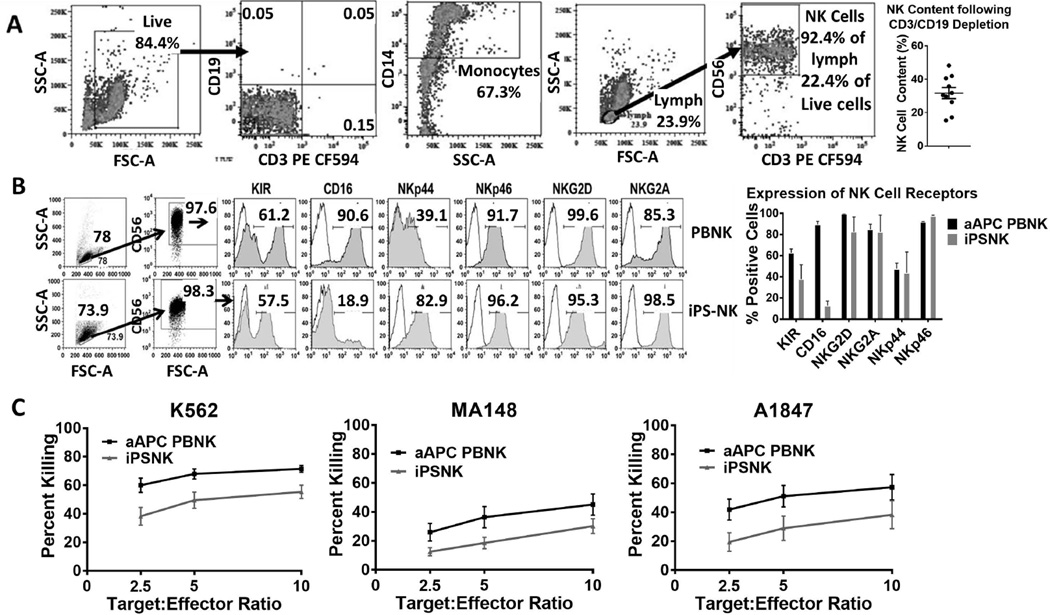
Previous studies demonstrate that PB-NK cells can be adoptively transferred from haplo-identical allogeneic donors for the treatment of AML and ovarian cancer [2, 3]. In addition, we have recently reported the efficacy of PB-NK cells administered IP for the treatment of ovarian cancer in a pre-clinical xenograft model [4]. PB-NK cells used for these studies are enriched from peripheral blood using CD3/CD19 magnetic beads to deplete T and B cells, respectively. This method results in a cell product containing approximately 30–40% NK cells, while the rest of the cell product contains mostly monocytes (CD14+) (Figure 1A) [5]. We hypothesized that a more pure population of NK cells would result in better anti-tumor response. Previously, we have demonstrated efficient derivation of NK cells from iPSCs in quantities suitable for clinical translation [6]. NK cells differentiated from induced pluripotent stem cells (iPSCs) are >97% pure (Figure 1B) [6], defined by CD56+/CD3− cells, following expansion via artificial aAPCs containing membrane bound (mb-) IL-21 in conjunction with 50 U/mL IL-2. In order to compare iPSC-NK cells with PB-NK of equal purity, aAPC expansion was also performed on the CD3/CD19 depleted cell product. During culture with aAPCs, NK cells are preferentially expanded leading to an APC-expanded PB-NK cell product that is also >97% pure. Both the PB-NK cells and iPSC-NK cells underwent a several log-fold increase upon expansion. Flow cytometric analysis demonstrated that both the PB-NK cells and iPSC-NK cells were phenotypically mature and expressed typical NK cell activating receptors (Figure 1). The initial in vitro killing ability of aAPC expanded PB-NK cells and iPSC-NK cells was then evaluated using a standard 51-Chromium release assay (Figure 1C). The standard K562 cell line was used in addition to two ovarian cell lines, MA148 and A1847 cells. The aAPC expanded PB-NK cells had slightly better in vitro killing ability compared to the iPSC-NK cells across all of the targets tested.
Figure 1.
Flow cytometric analysis of NK cell products. A) Phenotype of CD3/CD19 depleted PB-NK cell product with no aAPC expansion. Cells were gated based on live gate for CD3/CD19 and monocyte percentages. A lymphocyte gate based off the live gate was used to determine percent CD56+ NK cells. Final percentage of NK cells is percentage of NK cells in live gate. Dot plot represents the NK cell content of 10 CD3/CD19 depletions with SEM displayed. B) Phenotypic analysis of aAPC expanded PB-NK and iPSC-derived NK cells. A lymphocyte gate and a CD56+ gate were applied for individual NK cell receptors. Flow plots are representative of at least 3 independent experiments. The bar graph is the average + SEM for n=3. C) In vitro killing ability of NK cells was assessed using a standard 4-hour 51-Cr release assay. Lines shown are the average tumor lysis at the indicated effector:target ratios for at least 3 independent experiments.
NK cells have anti-tumor activity
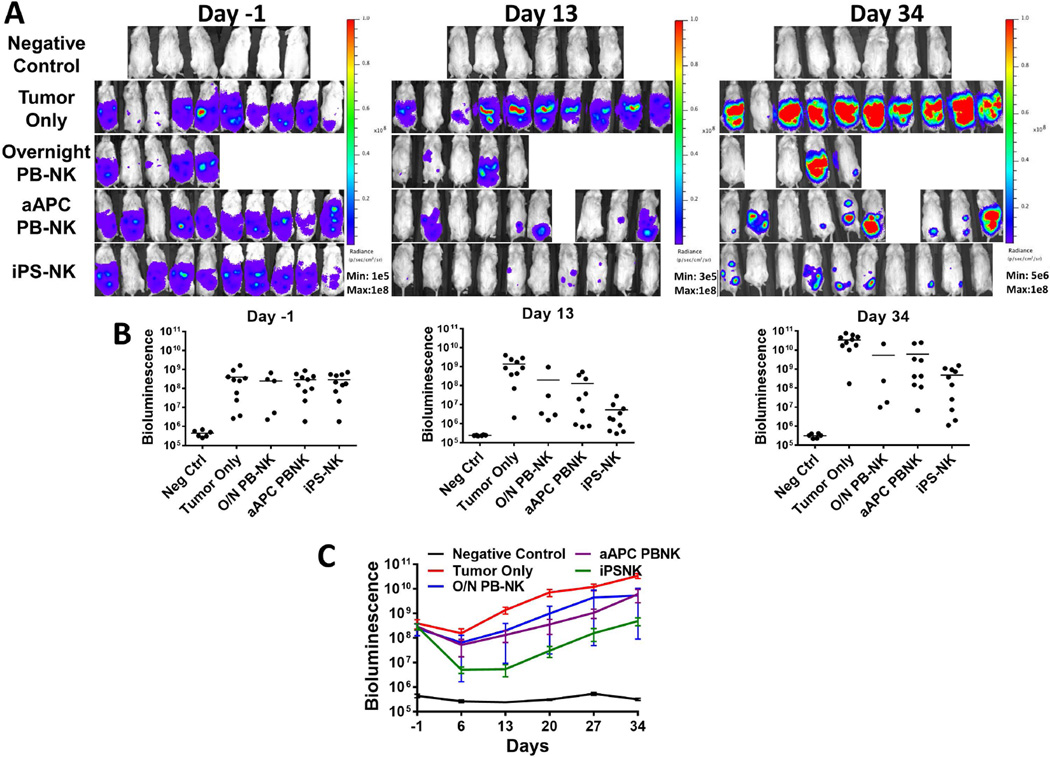
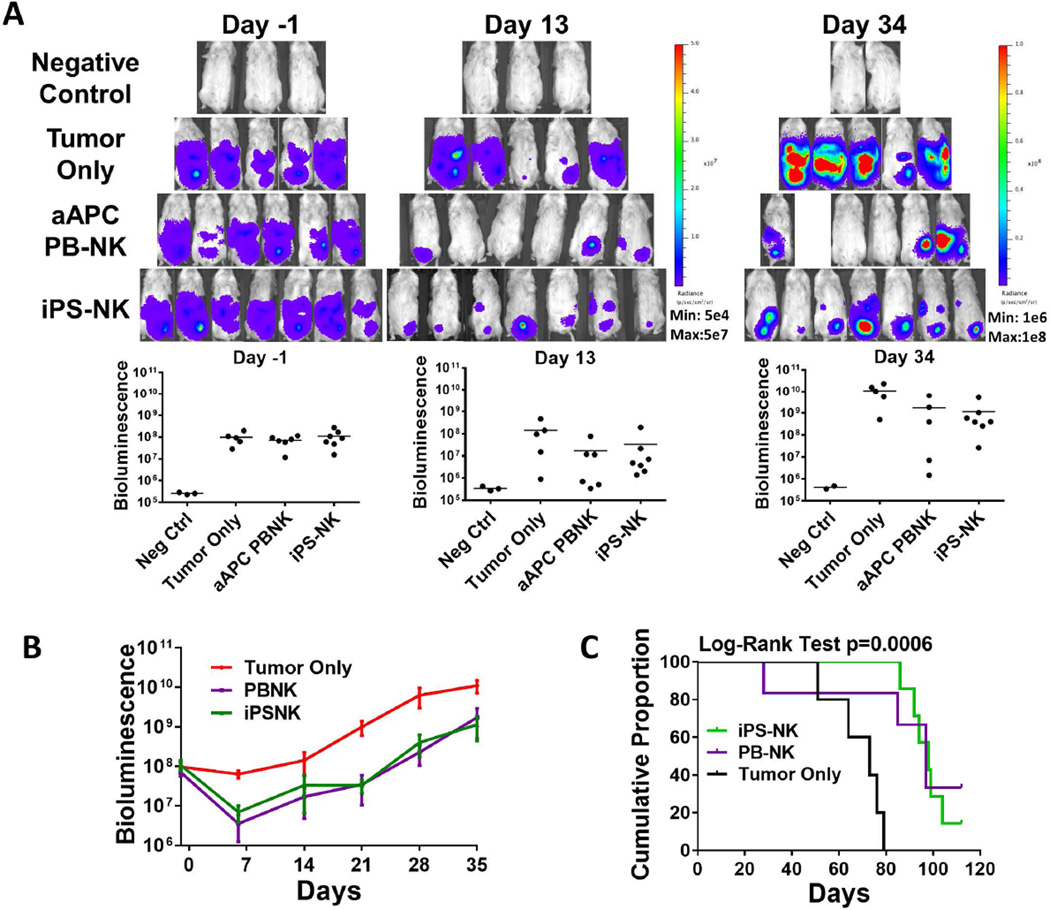
We used a mouse xenograft model to test the hypothesis that the highly enriched populations of NK cells produced by aAPC expansion would have better anti-tumor activity compared to overnight IL-2 activated PB-NK cells without aAPC culture (the current NK cell clinical product). Four days after inoculation with luciferase-expressing MA-148 ovarian tumor cells, mice were treated with a single dose of 20 × 106 NK cells. As in previous NK cell xenograft studies, IL-2 was administered to promote the growth and in vivo survival of NK cells [4]. BLI was used to monitor tumor growth in the mice (Figure 2). When compared to untreated tumor bearing mice, all three NK cell sources produced a significant reduction in tumor burden over the treatment period (Figure 2C; p<0.0001). There was no statistical difference between the overnight activated PB-NK cells and either of the aAPC activated NK cell populations due to the variation in tumor response observed in the overnight activated PB-NK cell treatment group. However, statistical analysis did reveal that the aAPC-expanded iPSC-NK cell treatment was better than the aAPC expanded PB-NK cells over the treatment period (p=0.014).
Figure 2.
PB-NK cells and iPSC-derived NK cells mediate in vivo killing of ovarian cancer cells in a MA-148 tumor model. Mice were inoculated with 2×10<sup>5</sup> MA-148 GFP:Luc tumor cells 4 days prior to NK cell injection. Bioluminescent imaging was used to monitor tumor establishment and growth. Day -1 images were used to group mice prior to NK cell injection. A) Bioluminescent imaging of mice treated with overnight PB-NK, aAPC PB-NK, or iPS-NK cells as indicated in figures. B) Dot plots representing each group at shown time points. Bars are plotted at the mean. C) Means + SEM plotted for each group as shown. All treatment groups were statistically significantly different over the treatment period (p<0.0001) compared to tumor only, and iPSC-NK were significantly better than aAPC PB-NK (p=0.014).
NK cells in blood of treated mice
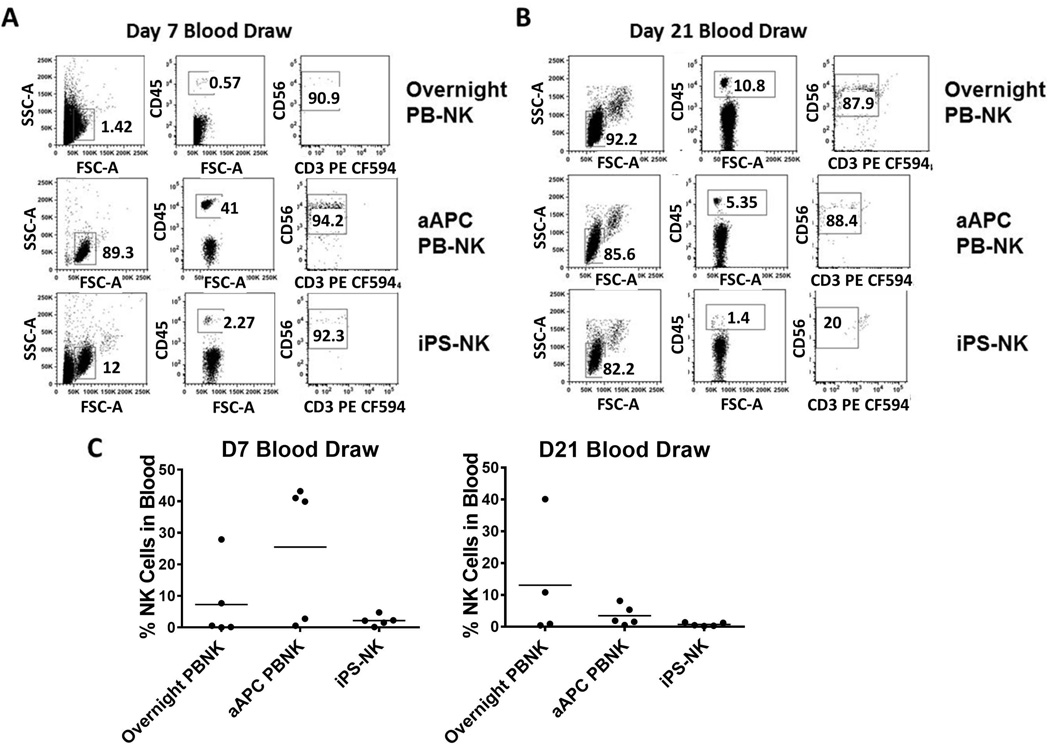
In order to evaluate NK cell engraftment, in vivo persistence and homing, peripheral blood was analyzed by flow cytometry to test for the presence of human NK cells. At day 7 after NK cell administration, mice treated with the aAPC expanded PB-NK cells had the highest amount of NK cells (mean 25%) in circulation (Figure 3). In contrast, the overnight activated PB-NK cell group (mean 7%) and iPSC-derived NK cell group (mean 2%) had relatively low numbers of circulating NK cells (Figure 3). By day 21, the overnight activated PB-NK cell treated group continued to show an increased expansion of circulating NK cells (mean 13%), whereas mice treated with aAPC expanded PB-NK cells exhibited a decrease in circulating NK cells (mean 3%). iPSC-derived NK cells remained low in the circulation at both early and late time points (mean 1%).
Figure 3.
Analysis of NK cells in blood of mice treated with NK cells. A representative mouse blood draw for either overnight activated PB-NK, aAPC PB-NK, or iPS-NK cells at (A) Day 7 or (B) Day 21. Cells were first gated based on FSC/SSC, then by human CD45+ cells, and finally CD56+ cells. C) Dot plots demonstrating the percentage of human NK cells in mice.
NK cells work against multiple ovarian tumor lines
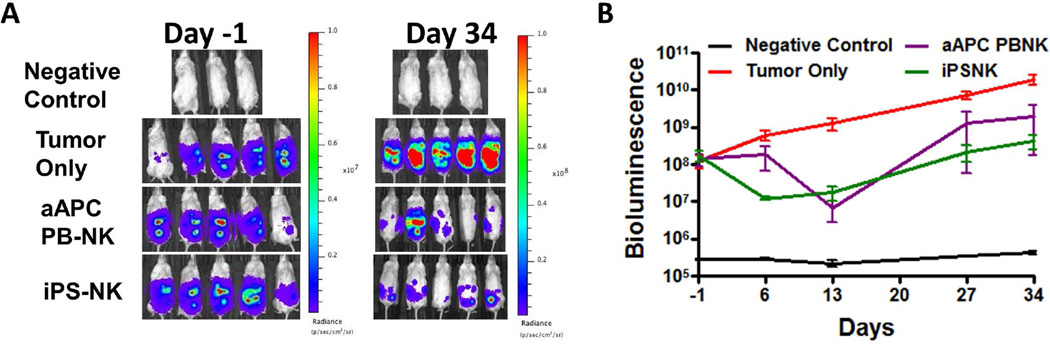
Next, aAPC expanded PB-NK and iPSC-NK cells were tested against a second ovarian tumor line, A1847. Mice were treated similar to the protocol with the MA-148 cells. Once again, both expanded PB-NK and iPSC-NK cells markedly inhibited tumor growth (Figure 4). BLI data revealed both treatment groups caused a significant decrease in tumor burden over the treatment period (p<0.0001 for both aAPC expanded PB-NK and iPSC-NK cells when compared to the tumor only group). As opposed to the MA-148 tumor line, there was no difference between the aAPC expanded PB-NK and iPSC-NK cells against the A1847 tumor line (p-value=1.00).
Figure 4.
PB-NK cells and iPSC-derived NK cells mediate in vivo killing of ovarian cancer cells in a A1847 tumor model. Mice were inoculated with 2×10<sup>5</sup> A1847 GFP:Luc tumor cells 4 days prior to NK cell injection. Bioluminescent imagining was used to monitor tumor establishment and growth. Day -1 images were used to group mice prior to NK cell injection. A&B) Bioluminescent imaging data for mice treated with aAPC PB-NK, or iPS-NK cells as indicated in figures. Line graph represents mean + SEM. Both treatment groups were statistically significantly better than tumor alone over the treatment period (both p<0.0001).
Multiple dosing of NK cells improves anti-ovarian cancer activity
While the results for a single dose of NK cells were promising in both tumor models studied, we were interested to evaluate if multiple doses of NK cells could further improve tumor response. Again, aAPC-expanded cells afford the opportunity to treat with multiple cell doses; whereas the overnight IL-2 treated PB-NK cells (the current clinical product) can only be used as a one-time treatment. Here, MA148 were again used after tumor had established for 4 days, 3 doses of NK cells were given on day 0, 7, and 14. Once again mice were monitored by BLI. Here, mice were also monitored for their overall survival. Imaging data once again revealed that both aAPC PB-NK and iPSC-NK cells had significant anti-ovarian cancer activity and markedly reduced tumor burden in mice (Figure 5). By day 20, there were 2 mice in the aAPC PB-NK cell treated group and 1 mouse in the iPSC-NK cell treated group that had bioluminescent values equivalent to the negative control group, demonstrating this anti-tumor activity leads to elimination of tumor cells below the limits of detection in this sensitive BLI system. Both the aAPC expanded PB-NK cells and iPSC-derived NK cells produced a significant tumor response (Figure 5B; both p-values <0.0001). Furthermore, the iPSC-derived NK group again had a trend toward overall better activity (p-value 0.109). Overall survival (OS) was significantly improved in mice treated with either PB-NK or iPSC-derived NK compared to those not treated (Figure 5C; overall Log-Rank Test p=0.0006 5C). Specifically, the median OS for the PB-NK cell treated group was 97 days (95% CI: 28-not estimable days) and for the iPS-NK cell treated group was 98 days (95% CI: 86–104 days), both of which were statistically significantly longer than the median OS in the control group, which was 73 days (95% CI: 51–79 days; p=0.048 and p=0.0008, respectively).
Figure 5.
Anti-ovarian cancer activity using multiple doses of NK cells. Mice were inoculated with 2×10<sup>5</sup> MA-148 GFP:Luc tumor cells 4 days prior to NK cell injection. Bioluminescent imagining was used to monitor tumor establishment and growth. NK cells were administered on D0, D7, and D14. A&B) Bioluminescent imaging data for mice treated with aAPC PB-NK, or iPS-NK cells as indicated in figures. Line graph represents mean + SEM. Both treatment groups were significantly better than tumor alone over the treatment period (both p<0.0001) C) Kaplan-Meier curve for overall survival of mice. Tumor only n=5 (5 failed), aAPC PB-NK n=6 (4 failed), and iPS-NK n=7 (6 failed). Treatment with iPS-NK and aAPC PB-NK statistically significantly improved overall survival compared to tumor only (p=0.008 and p=0.048, respectively).
Discussion
NK cells are gaining increasing interest for adoptive immunotherapy to treat multiple cancer types [13–15]. While both pre-clinical models and clinical trials demonstrate varying degrees of effectiveness against AML and ovarian cancer, there are still several limitations regarding the NK cell source. The process of isolating PB-NK cells prior to treatment can be costly and requires time, as well as a suitable donor. More importantly, the typical cell product of PB-NK cells is actually a heterogeneous mixture of NK cells and monocytes that varies markedly from donor to donor and the technical quality of the apheresis procedure. Finally, it is becoming more evident that mismatching KIRs, a family of NK cell inhibitory receptors, may improve patient outcome [16–20]. If this becomes standard in NK cell-based immunotherapy, it would require that each donor is KIR haplotyped prior to NK cell isolation. These obstacles could result in treatment delay, in addition to introducing variation in treatment because the cell product is not standardized. To overcome these limitations, we have evaluated the use of aAPC expanded PB-NK cells and iPSC-NK cells in a mouse xenograft model for ovarian cancer. Both of these cell populations can be easily standardized so that patients receive a pure population of NK cells with known KIR haplotyping and can be ready to be used as an “off-the-shelf” therapy. With the expansion capability of PB-NK cells in aAPCs, isolation of NK cells from a single donor can produce enough cells to treat multiple patients. In the case of iPSC-NK cells, reports have shown that enough NK cells to treat a patient can be produced from as little as 1×106 undifferentiated iPSCs [6]. This method is free of both serum and xenobiotics, and it is suitable for clinical translation. Since iPSCs can be maintained indefinitely, once a single line is developed the number of patients that can be treated with NK cells derived from iPSCs is nearly limitless.
As noted, the freshly isolated PB-NK cells were found to contain only about 30% NK cells (Figure 1), which is comparable to previous studies [6], whereas aAPC expanded NK cells were almost entirely pure (>97%) NK cells. Consistent with previous reports, the PB-NK and iPSC-NK cells had a similar NK cell phenotype for CD56, NKG2D, NKp44, and NKp46. NKG2D is a C-type lectin protein and NKp44 and NKp46 are natural cytotoxicity receptors that are all important for NK cell activation (Figure 1). One difference between PB-NK cells and iPSC-NK cells is the level of CD16, a membrane receptor involved in antibody-dependent cell-mediated cytotoxicity (ADCC). Typically, iPSC-NK cells have slightly lower levels of CD16 (Figure 1). Overall given their respective phenotypes, we hypothesized that both aAPC PB-NKs and iPSC-NK cells would function similarly. Both cell types expanded extremely well in the aAPC culture conditions to generate an ample supply of cells.
After confirming the cell types had similar phenotypes, we compared the various cell populations in the ovarian xenograft model using MA-148 cells. While all three treatment groups demonstrated statistically significant reduction in tumor burden compared to the tumor only group, both the aAPC expanded PB-NK and iPSC-NK cells induced a more consistent response in the mice compared to freshly isolated PB-NK cells. As we have observed previously, approximately 20–40% of mice treated with freshly isolated PB-NK cells do not show any tumor response [4]. Conversely, all the mice treated with iPSC-derived NK cells and the aAPC expanded PB-NK cells showed tumor response. Due to the heterogeneity of the NK cell product when CD3/CD19 depletion was performed and the variable response observed, we focused on using the more standardized iPSC-NK cells and aAPC expanded PB-NK cells in further experiments. The iPSC-NK cells performed significantly better than the PB-NK group against the MA-148 tumor line (Figure 2), suggesting that iPSC-NK cells may provide a better source of NK cells for immunotherapy. Interestingly, when the blood was examined for the presence of NK cells, it was found that both freshly isolated PB-NK cells and aAPC expanded PB-NK cells were circulating at D7 and D21 whereas iPSC- derived NK cells had a relatively low amount of cells in the circulating blood (Figure 2). Of note, both iPSC-derived NK and aAPC expanded PB-NK cells could be found in the peritoneal cavity of mice for at least 21 days (data not shown). These data suggest that iPSC-NK cells do not transit into the circulation as well as PB-NK cells; however, they do remain within the peritoneum and it did not impair their ability to reduce tumor burden. Previous studies by our group demonstrate that compared to PB-NK cells, it is know that iPSC- derived NK cells typically express lower levels of CD62L, a receptor important in the migration of NK cells into the peripheral blood [8]. This may be one reason why iPSC-NK cells were not found to be circulating in the blood.
In order to further assess aAPC expanded PB-NK and iPSC-NK cells in vivo, we wanted to test both cell products against a second tumor line. The ovarian cancer cell line A1847 was used to carry out another round of mouse xenograft studies (Figure 4). Mice were treated the same way as with the MA-148 tumor cells and were again dosed with 20 × 106 NK cells. Similar to the MA-148 tumor line, both aAPC expanded PB-NK and iPSC-NK cells significantly reduced the tumor burden in mice.
Based on the promising results seen when mice were given a single dose of NK cells, we hypothesized that giving mice multiple doses would further enhance the anti-tumor effect. Given the large expansion potential of either PB-NK or iPSC- derived NK cells using aAPCs, generating sufficient cell numbers to carry out multiple doses is very feasible. Dosing mice 3 times with NK cells dramatically increased their overall survival compared to non-treated control mice with BLI measurements for several mice reduced to negative control levels. Some treated mice survived for >125 days while mice left untreated survived no longer than 80 days. These results clearly demonstrate that aAPC PB-NK cells and iPSC-NK cells are effective in vivo at reducing established ovarian cancer.
While these studies represent an important step forward for use of NK cell-based immunotherapy to treat ovarian cancer, the cell sources currently evaluated were not able to completely eliminate all tumor in the mice, and eventually mice succumbed to the disease. In order to continue to improve NK cell-based immunotherapy, iPSC-NK cells represent an ideal population to build upon this promising direction. With the ability to produce iPSC-NK cells in clinically suitable amounts, many opportunities exist to improve upon current NK cell based therapies. For example, iPSCs can be genetically engineered to express chimeric antigen receptors (CARs) or similar constructs prior to differentiation into NK cells, as we have shown in vivo using iPSC-NK cells to treat HIV infection [6, 8]. By engineering the iPSCs, an NK cell population can be created with 100% CAR expression. Indeed, CARs targeting mesothelin, an antigen commonly overexpressed in ovarian cancer [21, 22], have already been previously developed for T cells and tested for patients with refractory pancreatic cancer [23, 24].
Conclusion
In conclusion, we have found that using aAPC expanded PB-NK cells or iPSC- derived NK cells is superior to the use of CD3/CD19 depleted apheresis cell product. Most importantly, the pure (>97%) NK cell populations provided a better overall tumor reduction in vivo. In addition, higher doses of NK cells can be achieved, administration of multiple doses is possible, and the cells could be banked to provide more immediate treatment options. While both the aAPC PB-NK and iPSC-NK cells function similarly, the ability to provide a standardized cell source for “off-the-shelf” adoptive immunotherapies makes iPSC- derived NK cells represent an attractive cell population for future therapies [7].
Acknowledgements
This research was supported by funding from the Minnesota Ovarian Cancer Alliance (MOCA) (Geller, M) “NK Cell Immunotherapy for Ovarian Cancer”, Mayo Clinic Ovarian Cancer SPORE (P50 CA136393), and NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. Melissa Geller, MD and Dan Kaufman, MD, PhD are supported by a Research Scholar Grant, RSG-14-151-01-CCE from the American Cancer Society. Dan Kaufman, MD, PhD has support from the Minnesota Ovarian Cancer Alliance and a Minnesota Partnership Grant for Biotechnology. David Hermanson, PhD has been supported by an NIH Hematology Research Training Grant (2T32HL007062) and an Irvington Postdoctoral Fellowship from the Cancer Research Institute.
Footnotes
Author Contributions: David Hermanson conception and design, collection of data, data analysis and interpretation, manuscript writing; Laura Bendzick conception and design and collection of data; Lee Pribyl conception and design and collection of data; Valarie McCullar collection of data and data analysis; Rachel Isaksson Vogel analysis of data, Jeff S. Miller conception and design, Melissa A. Geller conception and design, financial support, final approval of manuscript and Dan S. Kaufman conception and design, financial support, final approval of manuscript
Conflict of Interest: The authors declare no conflict of interest.
Disclosure of Potential Conflicts of Interest:
Dr. Kaufman consults for Fate Therapeutics. This relationship has been reviewed and managed by the University of Minnesota in accordance with its conflicts of interest policies.
REFERENCES
- 1.Armstrong DK. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist. 2002;7(Suppl 5):20–28. doi: 10.1634/theoncologist.7-suppl_5-20. [DOI] [PubMed] [Google Scholar]
- 2.Geller MA, Cooley S, Judson PL, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 4.Geller MA, Knorr DA, Hermanson DA, et al. Intraperitoneal delivery of human natural killer cells for treatment of ovarian cancer in a mouse xenograft model. Cytotherapy. 2013;15:1297–1306. doi: 10.1016/j.jcyt.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koepsell SA, Miller JS, McKenna DH. Natural killer cells: a review of manufacturing and clinical utility. Transfusion. 2013;53:404–410. doi: 10.1111/j.1537-2995.2012.03724.x. [DOI] [PubMed] [Google Scholar]
- 6.Knorr DA, Ni Z, Hermanson D, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2:274–283. doi: 10.5966/sctm.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eguizabal C, Zenarruzabeitia O, Monge J, et al. Natural killer cells for cancer immunotherapy: pluripotent stem cells-derived NK cells as an immunotherapeutic perspective. Front Immunol. 2014;5:439. doi: 10.3389/fimmu.2014.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni Z, Knorr DA, Clouser CL, et al. Human pluripotent stem cells produce natural killer cells that mediate anti-HIV-1 activity by utilizing diverse cellular mechanisms. J Virol. 2011;85:43–50. doi: 10.1128/JVI.01774-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woll PS, Grzywacz B, Tian X, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng ES, Davis R, Stanley EG, et al. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat Protoc. 2008;3:768–776. doi: 10.1038/nprot.2008.42. [DOI] [PubMed] [Google Scholar]
- 12.Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 14.Yang XY, Zeng H, Chen FP. Cytokine-induced killer cells: A novel immunotherapy strategy for leukemia. Oncol Lett. 2015;9:535–541. doi: 10.3892/ol.2014.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodduluru LN, Kasala ER, Madhana RM, et al. Natural killer cells: The journey from puzzles in biology to treatment of cancer. Cancer Lett. 2015;357:454–467. doi: 10.1016/j.canlet.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia. J Immunol. 2014;192:4592–4600. doi: 10.4049/jimmunol.1302517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Impola U, Turpeinen H, Alakulppi N, et al. Donor Haplotype B of NK KIR Receptor Reduces the Relapse Risk in HLA-Identical Sibling Hematopoietic Stem Cell Transplantation of AML Patients. Front Immunol. 2014;5:405. doi: 10.3389/fimmu.2014.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaelis SU, Mezger M, Bornhäuser M, et al. KIR haplotype B donors but not KIR-ligand mismatch result in a reduced incidence of relapse after haploidentical transplantation using reduced intensity conditioning and CD3/CD19-depleted grafts. Ann Hematol. 2014;93:1579–1586. doi: 10.1007/s00277-014-2084-2. [DOI] [PubMed] [Google Scholar]
- 20.Oevermann L, Michaelis SU, Mezger M, et al. KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood. 2014;124:2744–2747. doi: 10.1182/blood-2014-03-565069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scales SJ, Gupta N, Pacheco G, et al. An antimesothelin-monomethyl auristatin e conjugate with potent antitumor activity in ovarian, pancreatic, and mesothelioma models. Mol Cancer Ther. 2014;13:2630–2640. doi: 10.1158/1535-7163.MCT-14-0487-T. [DOI] [PubMed] [Google Scholar]
- 22.Schwab CL, English DP, Roque DM, et al. Past, present and future targets for immunotherapy in ovarian cancer. Immunotherapy. 2014;6:1279–1293. doi: 10.2217/imt.14.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]