Abstract
The developing brain undergoes substantial maturation into adulthood and the development of specific neural structures occurs on differing timelines. Transient imbalances between developmental trajectories of corticolimbic structures, which are known to contribute to regulation over fear learning and anxiety, can leave an individual susceptible to mental illness, particularly anxiety disorders. There is a substantial body of literature indicating that the endocannabinoid system critically regulates stress responsivity and emotional behavior throughout the life span, making this system a novel therapeutic target for stress- and anxiety-related disorders. During early life and adolescence, corticolimbic endocannabinoid signaling changes dynamically and coincides with different sensitive periods of fear learning, suggesting that endocannabinoid signaling underlies age-specific fear learning responses. Moreover, perturbations to these normative fluctuations in corticolimbic endocannabinoid signaling, such as stress or cannabinoid exposure, could serve as a neural substrate contributing to alterations to the normative developmental trajectory of neural structures governing emotional behavior and fear learning. In this review, we first introduce the components of the endocannabinoid system and discuss clinical and rodent models demonstrating endocannabinoid regulation of fear learning and anxiety in adulthood. Next, we highlight distinct fear learning and regulation profiles throughout development and discuss the ontogeny of the endocannabinoid system in the central nervous system, and models of pharmacological augmentation of endocannabinoid signaling during development in the context of fear learning and anxiety.
Keywords: adolescence, neonatal, early life, juvenile, stress, anandamide, CB1 receptor, cannabinoid, development, emotional behaviour, 2-AG, fatty acid amide hydrolase, FAAH
In search of more effective therapeutic strategies for mood disorders, a substantial body of work has been devoted to understanding exaggerated or inappropriate fear responses within neurocircuitry implicated in stress and anxiety-related disorders in children and adolescents (e.g. Glenn et al., 2012, Lau et al., 2008, Swartz et al., 2015). The shift towards identifying the relationship between development and anxiety and stress regulation is largely based on observations that there is a heightened incidence of mental illnesses, particularly for mood disorders, that emerges during the adolescent period. In fact, estimates indicate that one in five adolescents have a mental illness that will persist into adulthood (Paus et al., 2008), and depression and anxiety disorders occur in as many as one in ten adolescents (Costello et al., 2005, Kessler et al., 2005). Anxiety disorders, specifically, are considered among the most prevalent psychopathologies affecting between 15-20% of youth (Kessler et al., 2007, Kessler et al., 2005, Merikangas et al., 2010). Moreover, at least 75% of adults with a fear/anxiety-related disorder are reported to have met diagnostic criteria as children or adolescents (Kim-Cohen et al., 2003). These statistics highlight the importance of characterizing physiological and neural mechanisms underlying anxiety and stress regulation throughout development (Pattwell et al., 2013), and emphasize the need to enhance our understanding of how clinical treatments may be more or less effective based on age.
There is an extensive history of medicinal and recreational cannabis use in humans and in many cases, cannabis is utilized for its mood-enhancing, anxiolytic and stress-relieving properties (Cheung et al., 2010, Green et al., 2003, Hunault et al., 2014, Temple et al., 2014). This led to the subsequent identification of the major psychoactive constituents of cannabis known as Δ-9-tetrahydrocannabinol (THC; Gaoni & Mechoulam, 1964) and the eventual characterization of the cannabinoid type 1 receptor (CB1R) with which it interacts (Devane et al., 1988). Although cannabis use has been reported to produce enhanced anxiety and paranoia in specific contexts and predisposed individuals (e.g. Moreira & Wotjak, 2010), studies indicate that THC and other natural and synthetic cannabinoids generally alleviate anxiety symptoms in recreational users (Green et al., 2003, Hunault et al., 2014, Temple et al., 2014) and in patients with an anxiety disorder (Crippa et al., 2011, Fabre & McLendon, 1981, Fraser, 2009, Jetly et al., 2015, Nakano et al., 1978, Roitman et al., 2014). Similarly, the anxiolytic properties of THC and other cannabinoids in humans is consistent with the results from preclinical rodent studies (Gunduz-Cinar et al., 2013a, Moreira & Wotjak, 2010).
Aside from being the biological target of THC from cannabis, the endocannabinoid (eCB) system has been widely studied for its ability to maintain homeostasis by exerting regulation over cognitive, behavioral, emotional, developmental and physiological processes (see review, Mechoulam & Parker, 2012). As natural and synthetic cannabinoids are reported to produce both anxiolytic, mood-enhancing effects as well as dysphoric, panic-like responses (Akirav, 2011), these biphasic effects of cannabinoids on anxiety suggest that the eCB system is an important regulator of emotional homeostasis, in particular. Moreover, the eCB system is likely a major contributor to individual variation in anxiety levels, while dysregulated eCB signaling could serve as a risk for developing an anxiety disorder (Gunduz-Cinar et al., 2013a). Moreover, there is preclinical evidence that eCB signaling in corticolimbic structures such as the prefrontal cortex, amygdala and hippocampus play a critical role in regulating adult stress responses by the hypothalamic-pituitary-adrenal (HPA) axis and emotional behavior such as anxiety (Campolongo et al., 2011, Lee & Gorzalka, 2012, Morena et al., in press, Rubino & Parolaro, 2008). Consequently, the eCB system has become a therapeutic target for a number of clinical conditions, including depression and anxiety-related disorders (Hill & Patel, 2013, Mangieri & Piomelli, 2007).
There is strong evidence indicating that the eCB system regulates both neurodevelopmental processes and adult conditioned and unconditioned anxiety behaviors: the eCB system modulates neuronal development and circuit connectivity (Harkany et al., 2008, Maccarrone et al., 2014), corticolimbic eCB signaling changes dynamically during development (Ellgren et al., 2008, Heng et al., 2011, Lee & Gorzalka, 2012, Rubino et al., 2015, Wenger et al., 2002) and deficiencies in central eCB ligand, N-arachidonoylethanolamine (anandamide; AEA), are associated with greater anxiety behavior (Bluett et al., 2014, Gray et al., 2015). Moreover, there are preliminary indications reviewed here suggesting that corticolimbic eCB signaling is an important underlying mechanism mediating interactions between maturational stage (e.g., adolescence) and fear learning/anxiety behavior. Early life and adolescence are characterized by distinct maturational alterations in the eCB system that occurs on a similar timeline as sensitive periods in fear learning. Furthermore, studies employing cannabinoid agonists indicate that the eCB system regulates emotional behavior and HPA axis stress responses from early stages of development and also suggest that developmental cannabinoid exposure can contribute to long-term dysregulation of the brain and anxiety behavior, which is reminiscent of the results of extended glucocorticoid exposure (Campolongo et al., 2007, D'asti et al., 2010, Fride et al., 2005). In this article, we first introduce the components of the eCB system and fear learning neurocircuitry, and then discuss clinical and rodent models demonstrating eCB regulation of fear learning and anxiety in adulthood. Next, we highlight distinct fear learning and regulation profiles throughout development and describe the ontogeny of the eCB system in the central nervous system. Lastly, we discuss findings from pharmacological studies of perinatal and periadolescent eCB signaling disruption and discuss their relevance in relation to our current understanding of anxiety and fear learning regulation by eCBs.
The endocannabinoid system
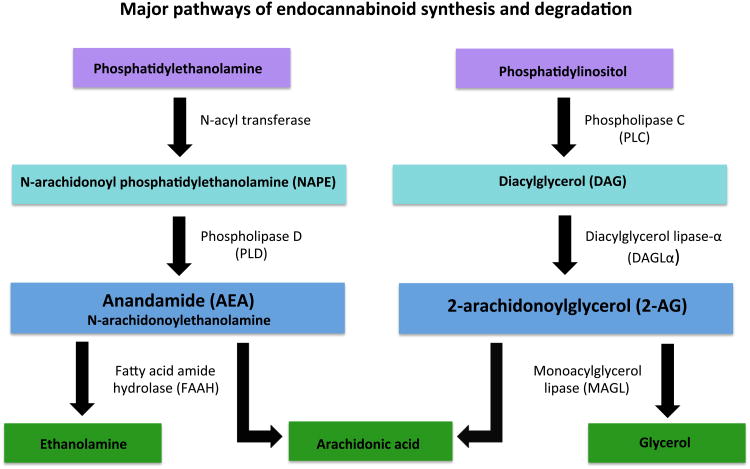
The eCB system includes two inhibitory G-protein coupled receptors, the CB1 and CB2 receptors. CB1Rs are widely expressed in the brain on multiple neuronal populations such as GABA and glutamate, while CB2 receptors are predominantly found in peripheral tissues. Although, CB2 receptors have been detected in the central nervous system relatively recently (Onaivi et al., 2006, Van Sickle et al., 2005), the majority of these reports localize their presence in microglia rather than neurons (e.g. Cabral et al., 2008). The eCB system also possesses two major endogenous ligands, N-arachidonylethanolamine (anandamide; AEA) and 2-arachidonoylglycerol (2-AG) which are synthesized “on demand” and act as retrograde messengers to regulate the release of other neurotransmitters (see reviews, Jutras-Aswad et al., 2009, Katona & Freund, 2012) and contribute to both short- and long-term synaptic plasticity (Mackie, 2006). Both AEA and 2-AG are synthesized post-synaptically by activity-dependent cleavage of phospholipid head groups. The synthesis of AEA most commonly occurs through hydrolysis of N-arachidonoyl phosphatidylethanolamine (NAPE) by phospholipase D (NAPE-PLD), although it should be noted there are at least 3 other biosynthetic pathways by which AEA can be synthesized (Figure 1; Di Marzo, 2011, Liu et al., 2008). There are two proposed pathways of 2-AG synthesis; however, the hydrolysis of inositol phospholipids containing arachidonic acid at the sn-2 position by phospholipase C into diacylglycerol, which is then further hydrolyzed to 2-AG by diacylglycerol lipase-α (DAGLα), is likely the most important (Figure 1; Hillard, 2000, Sugiura et al., 2002, Ueda et al., 2011). AEA is subject to rapid intracellular degradation primarily by hydrolytic enzyme, fatty acid amide hydrolase (FAAH; Di Marzo, 2011), resulting in arachidonic acid and ethanolamine (Ahn et al., 2008). The pathways leading to breakdown of 2-AG are less clear, with at least 8 participating enzymes; however, 2-AG hydrolysis by monoacylglycerol lipase (MAGL) to arachidonic acid and glycerol is considered to be its major degradative pathway accounting for approximately 85% of 2-AG hydrolysis in the brain (Ueda et al., 2011).
Figure 1. Major pathways of anandamide and 2-arachidonoylglycerol (2-AG) synthesis and degradation.
It is not entirely clear why there are two ligands for CB1Rs; however, distinct biosynthetic and degradative pathways as well as pharmacokinetic differences between AEA and 2-AG facilitate differential patterns of signaling that could be contributing to the intricacies of regulation over a variety of complex processes with multiple targets, such as stress responsivity and emotionality (Hill & Tasker, 2012). AEA has a relatively high binding affinity for the CB1R, yet induces somewhat poor intracellular signal transduction (i.e. partial agonist properties); in contrast, 2-AG has a relatively low binding affinity to the CB1R, but produces a robust intracellular response (Hillard et al., 1995). To add further complexity to this picture, AEA also binds to transient receptor potential vanilloid type (TRPV1) channels (Smart et al., 2000, Zygmunt et al., 1999), which is also known to modulate fear and anxiety responses (Moreira et al., 2012, Moreira & Wotjak, 2010). Moreover, FAAH is found post-synaptically, whereas MAGL is mostly colocalized with CB1Rs pre-synaptically (Haring et al., 2012). It is currently believed that these differences in signaling properties implicates 2-AG as representing a phasic signal (stimulus-induced) in response to sustained depolarization and is involved in several forms of activity-dependent synaptic plasticity, particularly two models of retrograde neurotransmission known as depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE; Blankman & Cravatt, 2013). In contrast, AEA appears to represent a tonic signal that gates and maintains steady state conditions (Hill & Tasker, 2012). In support of this, blockade of neuronal firing in hippocampal slice preparations is found to facilitate AEA uptake and degradation, thereby reducing AEA tone, and resulting in a reduction of CB1R-mediated suppression of GABA release (Kim & Alger, 2010). Moreover, in vivo studies of FAAH disruption suggest AEA pathways control select behavioral processes such as anxiety behavior (Bluett et al., 2014); although, simultaneous increases in both AEA and 2-AG via inhibition of MAGL and FAAH are known to produce some synergistic CB1R-mediated effects, indicating cross-talk between the two pathways to regulate behavior (Blankman & Cravatt, 2013).
Neurocircuitry of fear learning and anxiety
Although the terms, “stress” and “anxiety” are often used together and interchangeably, they are distinct, yet related concepts. Stress is considered a state of strain elicited by a real or perceived threat to homeostatic functioning and initiates multiple mechanisms that facilitate adaptation and an organism's ability to deal with the threat at hand (e.g. McEwen, 2007). Fear or anxiety is often a component of this stress response, which includes anticipatory feelings of worry and unease to a stimulus or event (usually negative) that may or may not occur. Given the similarity between these concepts, it is not surprising that stress responsivity and anxiety are regulated by common corticolimbic structures including the amygdala, hippocampus and prefrontal cortex (PFC; Romeo & McEwen, 2006). Commonly used rodent tests of unconditioned anxiety include the elevated plus maze, open field, light/dark box, social interaction and novelty-induced hypophagia. These behavioral assessments are generally based on the rodent's innate conflict between fearfulness of being left vulnerable to prey and the exploratory drive to visit open, unprotected spaces or unfamiliar/familiar conspecifics (Lafenetre et al., 2007).
In situations where fear responses are disproportionate to the risk and intensity of the real or perceived situation, this can lead to a maladaptive consequences such as developing an anxiety disorder (Akirav, 2011). Furthermore, a key maladaptive characteristic of anxiety disorders is the inability or difficulty in learning appropriate cues and contexts that signify safety and those that convey a threat (e.g. Akirav, 2011, Pine, 2007, Shin & Liberzon, 2010). Thus, experimental studies have also focused on understanding neural mechanisms underlying the human and rodent's ability to learn associations between previously experienced negative events and the cues and contexts that might predict their re-occurrence (i.e., fear learning) using a Pavlovian conditioning paradigm (Casey et al., in press). This typically involves pairing a neutral cue (e.g. tone) with an innately aversive stimulus (unconditioned stimulus; US; e.g. a brief electric shock). Once an association between the neutral cue and aversive stimulus is established, the presentation of the neutral cue, now considered a conditioned stimulus (CS), produces similar physiological and behavioral responses (conditioned responses; CR) to the anticipated threat as presentation of the US itself. In rodents, freezing behavior is the most commonly assessed CR, while in humans, changes in skin conductance, startle responses and pupil dilation are measured. Despite the persistence of the learned fear memory, extinction learning can occur in which CRs (e.g. freezing behavior) to the CS (e.g. tone) can be inhibited by repeatedly presenting the CS in the absence of the US. In this modified paradigm, the organism learns the CS is now safe. One limitation of the extinction training paradigm is that the memory of the extinguished fear can re-emerge with the passage of time (i.e., spontaneous recovery; Bouton, 2004).
The amygdala is implicated in a variety of emotional functions including mood regulation, mediating fear and anxiety behavior, reward processing to reinforce or motivate behavior, and plays a fundamental role in fear learning acquisition, expression and extinction (Ledoux, 2007). The amygdala has been shown to be responsive to multiple types of fear-related stimuli including pharmacological induction of fear, emotional stimuli, facial expressions and fear conditioning (Akirav, 2011). Furthermore, the amygdala can be divided into several nuclei that have divergent inputs and outputs and thus, functional contributions to fear learning and anxiety (see reviews, Ledoux, 2007, and Orsini & Maren, 2012). In particular, the basolateral complex of the amygdala (BLA) consists of the lateral nucleus, basolateral nucleus, and basomedial nucleus, which are collectively responsible for establishing a CS-US association (Orsini & Maren, 2012).
Specifically, the lateral nucleus of the amygdala receives inputs from sensory systems and in conjunction with the basolateral nucleus of the amygdala, serves to integrate relevant sensory information and relay it to the central nucleus of the amygdala. The central nucleus of the amygdala is a key output region for the expression of fear responses via downstream projections to hypothalamic and brain stem nuclei to produce autonomic responses (e.g. increased heart rate, freezing, stress response; Maren, 2001, Orsini & Maren, 2012).
The ventromedial PFC is important for an individual's ability to shift from fear expression to fear suppression (Milad & Quirk, 2002, Milad et al., 2007, Santini et al., 2004). Subregions within the ventromedial PFC are reported to differentially regulate the expression and extinction of conditioned fear responses; the prelimbic cortex is necessary for the expression of conditioned fear responses (Corcoran & Quirk, 2007) whereas the infralimbic region is likely involved in the suppression of these responses (Burgos-Robles et al., 2009, Hefner et al., 2008). It has been proposed that the infralimbic cortex is able to achieve this via projections to a cluster of GABAergic intercalated cells in the amygdala that suppress central nucleus output and thus, reduce the expression of the conditioned fear response (Likhtik et al., 2008). However, more recent work indicates that the infralimbic cortex lacks direct functional innervation to intercalated neurons (Strobel et al., 2015). Thus, it has been suggested that the infralimbic cortex guides amygdalar output by modulating BLA-related plasticity which could then recruit intercalated cells to suppress fear responses (see review, Maren & Holmes, 2015, Strobel et al., 2015). This is consistent with findings of a recent optogenetic study indicating that the infralimbic cortex and its connections to the BLA are specifically necessary for facilitating the storage of extinction memory during extinction training, rather than the retrieval of the extinction memory (Do-Monte et al., 2015). In line with this preclinical literature, patients diagnosed with post-traumatic stress disorder (PTSD) have reduced activity in the PFC coupled to high activity in the amygdala, when exposed to reminders of a traumatic event (Shin et al., 2001). Taken together, it has been proposed that a deficiency in ventromedial PFC input to the amygdala is likely to result in a reduction of inhibitory tone within this structure, which could ultimately lead to the overexpression of fear responses and development of an anxiety disorder.
The hippocampus and amygdala are strongly interconnected, with projections from the ventral CA1 region to the basal nucleus of the amygdala and projections from the ventral subiculum to lateral nucleus, basal nucleus, basomedial nucleus, and central nucleus of the amygdala (Orsini & Maren, 2012). Reciprocal projections from the amygdala stem from the basal nucleus of the amygdala and terminate in the CA1, CA2, CA3 and ventral subiculum of the hippocampus (Orsini & Maren, 2012). The hippocampus plays a role in the acquisition and storage of contextual fear memory; however, the exact role of this neural structure remains unclear (Orsini & Maren, 2012). From what is known, the high level of interconnectivity between the hippocampus and amygdala is important for fear extinction as the hippocampus provides information about the safety or threat of an environment based on contextual representations formed by previous experience (Fanselow & Dong, 2010, Orsini & Maren, 2012). Furthermore, the ventral subiculum and CA1 region have projections that terminate in the prelimbic and infralimbic cortex of the ventromedial PFC. Communication between the hippocampus and PFC is also known to be of high importance for fear extinction, which is supported by findings that a low frequency stimulation of the dorsal hippocampus impairs extinction recall and disrupts extinction-related long term potentiation (LTP) in the PFC whereas high frequency stimulation in the dorsal hippocampus facilitates extinction recall and LTP in the PFC (Farinelli et al., 2006, Orsini & Maren, 2012).
Adult corticolimbic endocannabinoid signaling regulates adult unconditioned anxiety behavior
Cannabinoids are often reported to have both anxiolytic and anxiogenic properties (Campolongo et al., 2011, Haring et al., 2012, Hill & Gorzalka, 2009, Marco & Laviola, 2012). While cannabis use is generally associated with stress-relief and mood elevation, in some cases, it produces dysphoric responses that include panic or heightened anxiety (Hall & Solowij, 1998, Lafenetre et al., 2007, Mechoulam & Parker, 2012, Micale et al., 2013). These biphasic effects are consistent with rodent studies demonstrating dose-dependent effects of CB1R agonists, such as THC, on anxiety behavior; moderate to high doses of CB1R agonists enhance anxiety- and stress-responses, while low to moderate doses of CB1R agonists exhibit anxiolytic effects in male and female rodents (Berrendero & Maldonado, 2002, Lutz, 2009, Patel & Hillard, 2006, Rey et al., 2012, Rodríguez de Fonseca et al., 1996). In contrast, pharmacological and genetic deletion of CB1Rs in rodents are generally known to have anxiogenic effects (Blasio et al., 2013, Patel & Hillard, 2006) and produce a behavioral and neural phenotype indicative of chronic stress exposure, including heightened emotional behavior (Haller et al., 2002, Maccarrone et al., 2002, Martin et al., 2002, Urigüen et al., 2004), hypothalamic-pituitary-adrenal (HPA) axis dysregulation (Barna et al., 2004, Cota et al., 2007), and shorter, less complex pyramidal neurons in the medial PFC (Hill et al., 2011, Lee et al., 2014c). Correspondingly, CB1R agonists directly infused into the hippocampus and medial PFC are known to exert anxiolytic effects (Lisboa et al., 2015). However, there are known inconsistent results reported in the literature (e.g. no or opposing effects) which are at least partly due to differences between anxiety test used, strain, sex, drug, dose, age of testing and aversiveness of the environment (e.g. dim versus bright illumination in the testing room; Lafenetre et al., 2007).
Adult corticolimbic endocannabinoid signaling regulates adult conditioned anxiety behavior
Pharmacological and genetic deletion of the CB1R is generally found to have no effect on the acquisition of cued and contextual fear learning (Marsicano et al., 2002, Suzuki et al., 2004). Although, systemic injections of the CB1R antagonist, AM-251, increases the acquisition and expression of freezing behavior in a trace (hippocampal-dependent) and delay (amygdala-dependent) fear conditioning paradigm (Reich et al., 2008). In contrast, low to moderate doses of CB1R agonist, WIN55212-2, and FAAH inhibitor, URB597, injected directly into the medial PFC decreases conditioned fear as measured by cue-fear-potentiated startle reflexes (Lin et al., 2008, Lin et al., 2009). Local infusion of AEA or AEA transport inhibitor, AM-404, into the ventromedial PFC or dorsolateral column of the periaqueductal grey attenuated conditioned freezing behavior, which was blocked by pretreatment with a CB1R antagonist (Lisboa et al., 2010, Resstel et al., 2008). Thus, the overall body of literature suggests that similar to unconditioned fear, moderate enhancement of eCB signaling reduces conditioned fear responses while inhibition of eCB signaling increases these fear responses (Akirav, 2011).
There is a wealth of evidence demonstrating that eCB signaling modulates extinction of conditioned fear. Inhibition of eCB signaling is found to attenuate or prolong fear extinction (Akirav, 2011, Chhatwal et al., 2005, Lafenetre et al., 2007, Lutz, 2007, Marsicano et al., 2002, Pamplona et al., 2006, Reich et al., 2008, Simone et al., 2015, Suzuki et al., 2004), while an enhancement of eCB signaling is known to facilitate fear extinction (Abush & Akirav, 2010, Chhatwal et al., 2005, Gunduz-Cinar et al., 2013b, Pamplona et al., 2008, Pamplona et al., 2006, Rabinak et al., 2013, Simone et al., 2015, Suzuki et al., 2004). Foundational work by Marsicano and colleagues (2002) demonstrated that CB1R knockout mice exhibit no anxiety behavior differences during the acquisition and consolidation of conditioned fear, however CB1R deficient mice display impaired fear extinction. Moreover, pharmacological blockade of CB1Rs in rodents is reported to similarly impair fear extinction without affecting the acquisition or consolidation of the fear memory (Bitencourt et al., 2008, Chhatwal et al., 2005, Lafenetre et al., 2007, Lin et al., 2009, Lutz, 2007, Pamplona et al., 2008). However, activation of the eCB system with CB1R agonists, such as THC, during extinction learning is known to facilitate extinction recall by preventing the recovery of extinguished fear memories in rodents (Chhatwal et al., 2005, Pamplona et al., 2008, Pamplona et al., 2006) and humans (Rabinak et al., 2013).
CB1R signaling in the BLA appears to serve as a neural locus for gating behavioral and neuroendocrine responses to stress and fear responses (Bluett et al., 2014, Gray et al., 2015, Gunduz-Cinar et al., 2013a, Hill et al., 2009a). In support of this, CB1R antagonism is found to increase neuronal activation within the BLA (Newsom et al., 2012, Patel et al., 2005), while also increasing HPA axis activity and anxiety behavior (Dono & Currie, 2012, Ganon-Elazar & Akirav, 2009, Hill et al., 2009a). In mouse fear conditioning studies, extinction training produces an increase in BLA AEA, but not 2-AG levels (Marsicano et al., 2002), and local administration of a FAAH inhibitor directly into the BLA enhances fear extinction (Gunduz-Cinar et al., 2013b). Consistent with this, local administration of a CB1R agonist into the BLA prevents the effects of stress on fear extinction (Ganon-Elazar & Akirav, 2009, Ganon-Elazar & Akirav, 2012), acoustic startle response (Ganon-Elazar & Akirav, 2012), and activation of CB1R in the BLA after fear memory reactivation blocks fear memory reconsolidation (Lin et al., 2006). Collectively, these data indicate that activation of eCB signaling within the BLA can reduce neurobehavioral indices of stress, anxiety and fear.
The role of endocannabinoid signaling in human fear learning
Human studies are consistent with preclinical work implicating a regulatory role for the eCB system in modulating emotional processing and fear learning. Acute THC administration reduces amygdalar reactivity to social signs of threat, without affecting activity in primary visual cortex and motor cortex (Phan et al., 2008) and impairs recognition of facial fear and anger, but not sadness or happiness (Ballard et al., 2012). Indeed, presentation of negative stimuli (pictures of fearful faces) reduces activity in neural structures such as the amygdala, orbital frontal gyrus, hippocampus, parietal gyrus, PFC, and regions in the occipital cortex following acute THC exposure, whereas presentation of positive stimuli (pictures of happy faces) increases activity within that network (Bossong et al., 2013). Furthermore, THC facilitates extinction of learned fear in humans and increases activation in the ventromedial PFC and hippocampus upon presentation of the CS relative to subjects that received a placebo (Rabinak et al., 2014, Rabinak et al., 2013). Cannabidiol (CBD), a non-psychoactive cannabinoid found in cannabis, has also been found to enhance consolidation of fear extinction (Das et al., 2013). In a study of genetic variability in human eCB signaling, fear learning was assessed in individuals that were genotyped for two single nucleotide polymorphisms (SNP) within the promotor region of the human CB1R gene (known as CNR1). Carriers of either the rs2180619 or rs1049353 SNP demonstrated comparable acquisition and expression of fear potentiation of the eyeblink startle reflex; however, only homozygote (A/A) A-allele carriers of rs2180619 exhibited an absence of fear extinction in contrast to robust extinction of the fear potentiated eyeblink startle response in homozygote (G/G) and heterzygote (A/G) G-allele carriers (Heitland et al., 2012). Through interaction with the 5HTTLPR SNP in the serotonin transporter gene, the rs2180619 SNP is also associated with individual differences in trait anxiety (Lazary et al., 2009), however, the molecular mechanisms affecting CB1R functionality and expression by this specific SNP remain to be determined (Heitland et al., 2012).
In addition to playing a role in emotional learning in humans, there is also evidence that aberrant eCB signaling could contribute to mood and anxiety disorders in humans. Specifically, the results of clinical studies indicate that compromised eCB signaling could serve as a molecular underpinning for neuropsychiatric conditions such as depression and anxiety disorders. Basal serum concentrations of AEA and 2-AG are lower in female patients with major depression (Hill et al., 2009b), suggesting that hypoactive eCB signaling could be involved in the etiology of depression (Hill & Patel, 2013). Similarly, lower circulating eCB concentrations are reported in human PTSD patients concomitant with an upregulation of CB1Rs (Hill et al., 2013, Neumeister et al., 2013). Consistent with these findings, clinical trials using CB1R antagonists (e.g. Rimonabant (Sanofi-Avantis) and Taranabant (Merck)) for the treatment of metabolic and cardiac conditions also yielded an increase in the incidence of depressed mood, anxiety and risk of suicide (Christensen et al., 2007, Lutz, 2009). Collectively, the emerging picture indicates that the eCB system functions to reduce stress and anxiety responses primarily through activation of the CB1R.
Therapeutic potential of FAAH and MAGL inhibition on fear learning and anxiety
Emerging research indicates that genetic and pharmacological enhancement of eCB signaling by augmentation of central AEA and 2-AG levels could ameliorate some of the neural and behavioral sequelae (e.g. HPA axis dysregulation and increased anxiety behavior) associated with depression and anxiety disorders. Preclinical work indicates that pharmacological inhibition of FAAH (e.g. URB-597, PF-3845; Hill et al., 2007, Kathuria et al., 2003) and AEA transport (AM-404; Bortolato et al., 2006) has anxiolytic properties in several tests of anxiety, including: the elevated zero maze, elevated plus maze, defensive withdrawal and social isolation-induced ultrasonic vocalization tests. Moreover, transgenic mice with a FAAH deficiency exhibit increased central AEA levels and a less anxious phenotype in the elevated plus maze and light dark box relative to wild type controls, which is prevented by systemic injection of a CB1R antagonist, Rimonabant (Moreira et al., 2008).
In light of the preclinical literature, researchers have undertaken investigations of genetic models of natural variation in circulating AEA levels and identified a common SNP (C385; rs324420) in the human FAAH gene that results in elevated AEA levels due to substitution of an evolutionarily conserved proline at amino-acid position 129 with a threonine residue that renders the FAAH protein more vulnerable to proteolytic degradation (Chiang et al., 2004, Dincheva et al., 2015, Sipe et al., 2002, Sipe et al., 2010). Adults carrying this variant exhibit reduced amygdalar responses to threat stimuli of fearful faces and trait anxiety levels (Hariri et al., 2009). Extending these findings, carriers of this variant are also found to exhibit accelerated habituation of amygdalar activity to repeated presentation of threat images and lower scores on the personality trait of stress-reactivity (Gunduz-Cinar et al., 2013b). A parallel mouse study using FAAH inhibitors further demonstrate that this habituation is associated with enhanced extinction-related plasticity in the amygdala (Gunduz-Cinar et al., 2013b). Lastly, we recently developed a knock-in mouse that biologically recapitulates the C385A SNP of the human FAAH gene. Adult humans and mice carrying the allele variant exhibit paralleled reductions in FAAH expression, elevated central AEA (Boileau et al., in press, Dincheva et al., 2015), enhanced fronto-amygdala connectivity and fear extinction learning as well as reduced anxiety behavior (Dincheva et al., 2015). Collectively, these pharmacological, genetic and behavioral findings provide preliminary evidence that FAAH inhibition could be an effective treatment option specifically for stress-related neuropsychiatric disorders (Bluett et al., 2014, Gunduz-Cinar et al., 2013a).
Acute pharmacological MAGL inhibition (resulting in elevated 2-AG levels) has also been reported to generally reduce rodent anxiety behavior in the elevated plus maze (Aliczki et al., 2012, Aliczki et al., 2013, Almeida-Santos et al., 2013, Busquets-Garcia et al., 2011, Sciolino et al., 2011), elevated zero maze (Busquets-Garcia et al., 2011) and marble burying tasks (Kinsey et al., 2011). Consistent with these findings, genetic knockout studies indicate that deficient 2-AG levels result in enhanced anxiety behavior. In one model, DAGLα knock out mice were generated in which, central, but not circulating 2-AG levels are reduced and associated with impaired eCB-mediated retrograde synaptic signaling at excitatory synapses in the BLA as well as greater anxiety behavior and anhedonia than wild-type mice (Shonesy et al., 2014). Interestingly, this behavioral and neural phenotype is reversed by pharmacological normalization of 2-AG levels by MAGL inhibitor, JZL-184 (Shonesy et al., 2014). In similar fashion, another DAGLα knock out mouse model indicates mice had an 80% reduction of central 2-AG levels and reductions in cortical and amygdalar AEA that corresponded with a behavioral and neural phenotype reminiscent of pharmacological and genetic deletion of CB1Rs: enhanced anxiety behavior in the open field and light/dark box, impaired fear extinction, maternal neglect behavior, reduced hippocampal neurogenesis, and reduced stress coping behavior in the forced swim test (Jenniches et al., in press). Adeno-associated virus-mediated overexpression of MAGL in hippocampal glutamatergic neurons also results in a 50% reduction in 2-AG levels, impairs DSE (but not DSI) and enhanced anxiety behavior in the elevated plus maze and open field tests (Guggenhuber et al., in press). In contrast, genetic deletion of MAGL (resulting in elevated 2-AG levels) was found to enhance anxiety behavior in the marble burying test, accompanied with impaired CB1R signaling and enhanced excitatory drive in mPFC/BLA circuitry (Imperatore et al., in press).
In summary, the evidence indicates that elevated levels of AEA and 2-AG by FAAH and MAGL generally have an anxiolytic effect while reductions in these eCB ligands are associated with enhanced anxiety. Further research determining the exact role of the endogenous ligands of the eCB system in fear learning and anxiety behavior is clearly warranted.
Fear learning and regulation across development
The neurocircuitry underlying fear learning and extinction in adulthood has been relatively well characterized. However, far less is known about the developmental processes shaping maturation of this neurocircuitry and how it may modulate fear learning and extinction by age (Casey et al., in press). Indeed, prefrontal and sub-cortical circuitry that is implicated in the regulation of fear learning and extinction is known to undergo substantial change from childhood to adulthood (Gogtay et al., 2004).
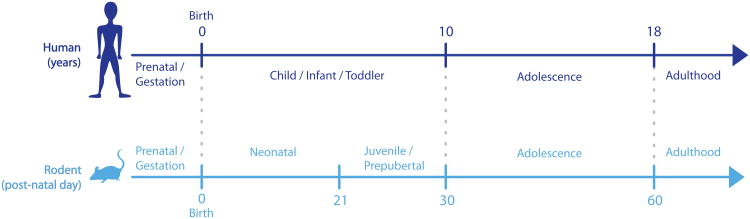
Rodent studies indicate that fear learning is linked to the maturation of the amygdala, which begins early in life and is tightly linked to maternal presence (Figure 2; Landers & Sullivan, 2012). Early postnatal life is considered a sensitive period for attachment learning in which rodent pups generally exhibit a profile characterized by suppressed fear responding, presumably to promote infant-caregiver attachment, even in the face of diminished quality of care (Landers & Sullivan, 2012, Raineki et al., 2010, Roth & Sullivan, 2005). During this period, odor-shock conditioning produces a paradoxical odor preference despite being paired with a shock that produces a pain response (Landers & Sullivan, 2012, Moriceau & Sullivan, 2006, Rudy & Cheatle, 1977). Post natal day (PND) 10 marks the end of this sensitive period in that amygdalar GABAergic-related synaptic plasticity begins to emerge (Thompson et al., 2008) and rodent pups can demonstrate adult-like cued fear learning to odor-shock conditioning (Moriceau & Sullivan, 2006); however, maternal presence, which maintains relatively low levels of circulating corticosterone in the pup (Sullivan & Holman, 2011), can provide social buffering and modulate the pup's learning to reinstate odor preference instead of aversion (Landers & Sullivan, 2012). Although able to learn fear associations, infant rodents (Figure 2) demonstrate a functional “infantile amnesia” in which fear memories are not as persistent as in adulthood (Akers et al., 2014, Campbell & Spear, 1972, Kim & Richardson, 2007). In support of this, animals conditioned on PND 18 appear to forget the fear memory within 10 days (Kim & Richardson, 2007). This may be related to the infant's lack of ability to associate environmental cues with the conditioned fear memory. In contrast to cued fear learning, contextual fear conditioning is thought to emerge by PND 24, coinciding with maturation of amygdala-hippocampus connectivity (Raineki et al., 2010) and developmental-related increases in hippocampal neurogenesis that have been shown to interfere with infant contextual fear memory persistence (Akers et al., 2014).
Figure 2. Human and rodent development timelines with commonly used terms for referring to those stages of development (adapted from Lee & Gorzalka, 2012).
However, by PND 23, a new sensitive period for extinction learning emerges (Pattwell et al., 2013). As juveniles, fear memories are persistently attenuated following extinction training, an effect not observed in adult rodents (Pattwell et al., 2012). That is, unlike adults, juvenile rodents do not display spontaneous recovery of the fear memory that typically follows extinction training (Kim & Richardson, 2007, Pattwell et al., 2012, Yap & Richardson, 2007). Consistent with this behavioral pattern, extinction training in the juvenile rodent is found to be amygdala-dependent and does not require infralimbic cortical engagement at PND 18 (Kim et al., 2009, Kim & Richardson, 2008). In contrast, we have previously found that by PND 23, the juvenile rodent exhibits persistent attenuation of fear memory; however, the fear memory appears more susceptible to interference by paralleled potentiation of the infralimbic cortex during extinction training, rather than degrading on its own (Pattwell et al., 2012).
In non-linear fashion, both fear extinction learning and retention are attenuated during adolescence relative to younger and older mice and humans (Casey et al., in press, Kim et al., 2011, McCallum et al., 2010, Pattwell et al., 2012, Pattwell et al., 2013). Adolescent mice display blunted fear extinction learning that is paralleled by an absence of infralimbic extinction learning-induced plasticity (Kim et al., 2011, Pattwell et al., 2012). Reminiscent of risky behavior in adolescent humans, a second behavioral pattern that emerges in the adolescent rodent is diminished hippocampal-dependent contextual fear expression (Pattwell et al., 2011) or “fearlessness” in contexts that have been previously paired with negative consequences. Unlike juveniles and adults, adolescent mice do not display a fear response when returned to the context in which they experienced the aversive event (shock). In this case, the suppression of contextual fear is a result of contextual fear retrieval failure, rather than acquisition, given that the same animals that exhibited diminished contextual fear as adolescents, demonstrate a fear response to the context when tested as young adults (Pattwell et al., 2011).
The adolescent period is characterized by increased exploration and a transition to independence, making specific danger cues particularly relevant in this novelty-seeking period. Thus, diminished fear extinction and contextual fear during this period does hold some benefits in this context. Furthermore, the differential rates of development in adolescent subcortical-prefrontal connections (e.g. myelination, synaptic pruning) are also likely to contribute to these shifts in fear regulation (Somerville & Casey, 2010). For example, in a human fMRI study examining developmental changes in connectivity between the medial PFC and the amygdala, blood oxygen level-dependent (BOLD) activity within the ventromedial PFC and the amygdala shifts from a positive to negative correlation from childhood to adolescence and is then stabilized during adulthood (Gee et al., 2013). Thus, it is possible that changes in connectivity between the amygdala, hippocampus and ventromedial PFC during the adolescent period could mediate a shift from restricted subcortical circuitry governing fear learning in juvenile stages, towards a more flexible and expansive circuit for fear regulation that is more evident in adulthood.
It is clear there are multiple sensitive periods for fear learning and its underlying neurocircuitry during normative development from early life to adulthood. Furthermore, the developmental course of fear learning and its associated neural correlates occurs in a highly ordered pattern. While these sensitive periods appear to facilitate greater flexibility in some situations, they may also confer greater susceptibility to external events (e.g. stress exposure) that could initiate a cascade of negative consequences, possibly leading to risk for mental illness. Much of our understanding regarding fear learning and regulation during development is based on descriptive work, leaving the exact molecular mechanisms of action and conditions by which risk for mental illness is conferred largely unexplored.
Dynamic changes in corticolimbic endocannabinoid signaling during different developmental periods
Numerous human and rodent studies indicate that the eCB system is significantly involved in the regulation of adult fear learning and unconditioned anxiety behavior (Riebe et al., 2012). Together with in vitro and in vivo studies that demonstrate regulation of neurodevelopmental processes by eCBs, it is reasonable to suggest that corticolimbic eCB signaling is involved in the developmental course of fear learning and anxiety behavior described above. Previous work has established that eCB signaling plays a multifaceted role in structural and functional neurodevelopment (see review Harkany et al., 2008, Maccarrone et al., 2014), regulating proliferation of neural progenitors and cell lineage commitment (Mulder et al., 2008), immature neuronal migration and axonal path finding (Berghuis et al., 2005, Berghuis et al., 2007, Harkany et al., 2008, Mulder et al., 2008), as well as initiation of synaptic communication of neural networks in perinatal rat tissue (Berghuis et al., 2007, Bernard et al., 2005). Moreover, studies investigating the ontogeny of eCB signaling indicate that this system changes dynamically and in a temporal-specific fashion throughout development, particularly in adolescence (Figure 3). Distinct patterns of eCB activity during development coincide with distinct fear learning sensitive periods, suggesting that corticolimbic eCB signaling could serve as an underlying mechanism mediating age-specific sensitive periods in fear learning and regulation outlined above. The presence of the eCB system has been detected during early embryonic stages in chicken and mice (Psychoyos et al., 2012). Furthermore, the eCB system is found to be functional in the rat central nervous system as early as gestational days 11-14 (Berrendero et al., 1999, Harkany et al., 2008, Rodriguez de Fonseca et al., 1993). Rodent central 2-AG levels peak around birth then decrease to PND 5 concentrations, which are comparable to those found in adults (Berrendero et al., 1999, Fernandez-Ruiz et al., 2000). Conversely, AEA is reported to steadily increase from early life to adulthood (Berrendero et al., 1999, Fernandez-Ruiz et al., 2000). However, no late neonatal, juvenile, or adolescent time points were included in these studies (i.e., no sampling between PND 5 – 56), leaving an existing gap in our understanding of the ontogeny of central AEA and 2-AG during early life to juvenile ages and highlighting the need for a comprehensive determination of the trajectory of AEA and 2-AG signaling throughout this period.
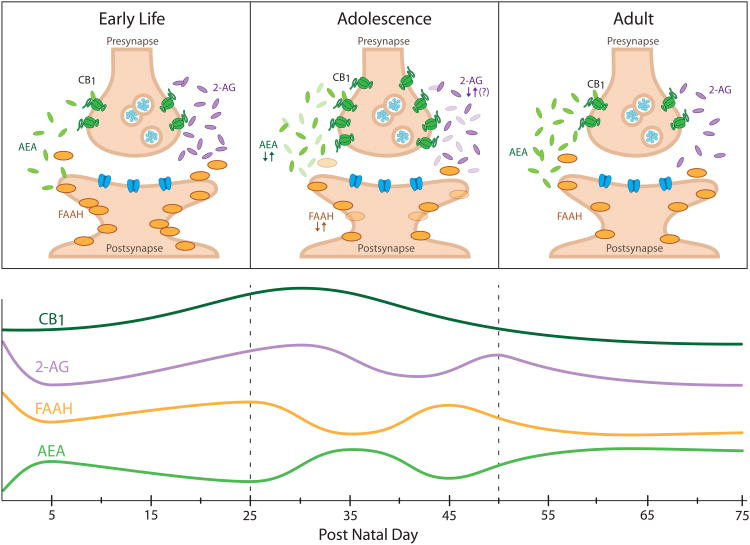
Figure 3. Corticolimbic endocannabinoid signaling changes dynamically across rodent development.
(A) Distinct endocannabinoid signaling profiles in early life, adolescence and adulthood. Components of the endocannabinoid system are represented schematically within a synapse (adapted from(Long et al., 2012). (B) Developmental trajectories of the components of the endocannabinoid system. CB1 receptor (CB1) expression peaks with the onset of adolescence. 2-arachidonoylglycerol (2-AG) is highest around birth and may fluctuate throughout adolescence. N-arachidonoylethanolamine (anandamide; AEA) gradually increases during early life and fluctuates during adolescence. Fatty acid amide hydrolase (FAAH) activity fluctuates in reciprocal fashion to AEA during adolescence. Based on data from (Berrendero et al., 1999, Ellgren et al., 2008, Fernandez-Ruiz et al., 2000, Heng et al., 2011, Lee et al., 2013, Rodriguez de Fonseca et al., 1993, Rubino et al., 2015, Wenger et al., 2002).
Accumulating evidence suggests there is considerable variation in the developmental course of adolescent corticolimbic AEA and 2-AG levels within neural structures regulating anxiety and fear learning. A comparison of cortical and hippocampal AEA and 2-AG levels in wild type and CB1R knockout male mice at PND 28 and 120 revealed no significant differences between wild type groups; however, elevated hippocampal FAAH activity and reduced AEA levels were observed only in adult knockout mice (Maccarrone et al., 2002). Behaviorally, young (PND 28) CB1R knockout mice exhibited moderately elevated anxiety behavior relative to older knockout mice, yet no age-specific differences in anxiety behavior in the open field or light-dark box were detected in the wild type mice (Maccarrone et al., 2002). In the male rat PFC, AEA was found to exhibit a gradual and progressive increase (i.e., PND 29-50) to adult levels while 2-AG levels were highest in early adolescence (PND 29), decreasing in mid-adolescence (PND 38) before increasing again in late adolescence (PND 50; Ellgren et al., 2008). Conversely, in the female rat PFC, AEA concentration increases from PND 46-60, but decreases from PND 60-75, with no differences in 2-AG concentrations as well as FAAH and MAGL activity (Rubino et al., 2015). Furthermore, hypothalamic AEA content is observed to increase immediately preceding vaginal opening (as a physical marker of pubertal onset; Wenger et al., 2002). We have also previously reported that corticolimbic AEA concentrations consistently fluctuate in the same pattern across corticolimbic structures: amygdala, PFC, hippocampus and hypothalamus, throughout the male rodent adolescent period (PND 25-70; Lee et al., 2013). These fluctuations in AEA were found to be at least partially due to corresponding changes in FAAH activity (Lee et al., 2013).
CB1Rs are detected and functional in human fetal tissue as early as gestational week 9 (Zurolo et al., 2010) and gestational days 11-14 in rats (Berrendero et al., 1999, Fernandez-Ruiz et al., 2000), with high CB1R expression limited to the amygdala and hippocampus and low distributions in striatum, thalamus and cerebral cortex during early to mid-gestation in the human fetus (gestational weeks 17-22) and rat neonate (Rodriguez de Fonseca et al., 1993). In contrast, higher and wider density of these ubiquitous receptors is reported in the adult rat (Herkenham et al., 1991) and human brain (Jutras-Aswad et al., 2009). Furthermore, from gestation into the first few days after birth, CB1R expression is localized to white matter areas of the midbrain, commissural tracts and brainstem; however, by adulthood, this phenomenon is no longer observable and CB1Rs are primarily expressed within grey matter (Berrendero et al., 1998, Berrendero et al., 1999, Fernandez-Ruiz et al., 2000, Romero et al., 1997). By PND 10, limbic, striatal and midbrain structures are reported to exhibit low concentrations of CB1R expression (Rodriguez de Fonseca et al., 1993) and the ability of eCBs to regulate synaptic transmission begins to emerge and increases throughout development to adulthood (Liang et al., 2014, Zhu & Lovinger, 2010).
The onset of adolescence at PND 30-40 marks a peak in limbic, striatal and midbrain CB1R expression, which then decreases to reach adult levels of expression by PND 70 in male and female rats (Rodriguez de Fonseca et al., 1993). In contrast, CB1R binding from birth to PND 60 is also reported to increase gradually to adult levels in male and female whole rat brains, although there are no adolescent time points included in this study (Belue et al., 1995). Nonetheless, CB1R expression in the rat is most consistently reported to be highest with the onset of adolescence (PND 25-30), followed by a general linear decline to adult levels within the PFC (Ellgren et al., 2008, Heng et al., 2011), limbic, striatal and cortical structures (Rodriguez de Fonseca et al., 1993). Furthermore, more recent work has demonstrated differential rates at which these CB1R declines occur, with declines in limbic/associative regions happening gradually and throughout adolescence whereas major changes in sensorimotor regions are not exhibited until mid- to late- adolescence (Heng et al., 2011). Functionality of these receptors, as measured by DSE, also follows the same developmental pattern (Heng et al., 2011). In summary, despite somewhat inconsistent findings that are at least partially due to strain and sex differences between studies, the rodent literature indicates that corticolimbic eCB ligand concentrations, particularly with respect to AEA, fluctuate throughout adolescence while CB1R expression peaks at the onset of adolescence before declining to adult levels (Ellgren et al., 2008, Lee & Gorzalka, in press, Lee et al., 2013, Rubino et al., 2015, Wenger et al., 2002).
In humans, CB1R expression is reported to gradually increase to adult levels in post-mortem brain tissue (Mato et al., 2003). However, a more recent study reports that CB1R expression peaks during infancy (<1 year old) to toddler-age (1.5 – 4.5 years old), then gradually decreases to adult levels in human PFC tissue samples (Choi et al., 2012, Long et al., 2012). Moreover, expression of enzymes that contribute to AEA synthesis (i.e., NAPE-PLD) and degradation (i.e., FAAH) both increase from infancy to adulthood, suggesting greater AEA regulation in adulthood than earlier in life (Long et al., 2012). In terms of 2-AG, synthetic enzyme, DAGLα, peaks between school age and young adulthood, while MAGL follows a similar pattern as CB1R expression (i.e., expression peaks around toddler age; Long et al., 2012). However, there is a critical gap in our current understanding of eCB signaling development in humans; to date, there is no information regarding the ontogeny of AEA or 2-AG concentration in humans, even in the periphery. Interestingly, although a similar general pattern of CB1R expression occurs in humans and rodents, the maturational trajectories of these receptors exhibit a peak around toddler-age in humans whereas this peak occurs closer to pubertal onset/early adolescence in the rodent. While the functional consequences of these developmental differences in eCB signaling require further investigation, it is possible that the dynamic changes in eCB signaling reflect a general structural and functional instability compared to that of adults. For example, adolescent AEA fluctuations and declining CB1R expression could underlie adolescent behavioral profiles that are regulated by corticolimbic circuits, such as relatively low anxiety, high responsivity to rewards and reduced inhibitory control (Casey & Jones, 2010). Given that this behavioral profile is maximal during adolescence and coincides with changes in AEA/CB1R expression and activity, corticolimbic eCB signaling may represent a neural substrate of the adolescent phenotype.
Long-term consequences of developmental exposure to cannabinoids
To date, there are no studies investigating the role of eCB signaling on fear learning during development. Despite this fundamental gap in our understanding of eCB regulation over fear learning during development, there are several studies that have investigated the impact of developmental cannabinoid exposure on unconditioned anxiety behavior. The majority of these studies indicate that cannabinoid exposure during development, whether by maternal use or during adolescence, can contribute to long-term dysregulation of the brain, the HPA axis and emotionality, which is reminiscent of the results of extended glucocorticoid exposure (Lee & Gorzalka, in press, Lee & Gorzalka, 2012). Early life and adolescence also appear to be periods of susceptibility to the effects of chronic cannabinoid exposure as adult rats exposed to the same treatment do not appear to be as greatly affected as adolescents (e.g. Bambico et al., 2010, Zamberletti et al., 2014).
There is clear evidence that the eCB system is involved in the regulation of emotional behavior from early developmental stages (Trezza et al., 2008) and perinatal cannabinoid exposure is found to induce a variety of long-term neural, cognitive and emotional alterations in adulthood (see review, Higuera-Matas et al., 2015). This is demonstrated in a longitudinal rodent study that found perinatal THC exposure increases ultrasonic vocalizations following removal from the nest during early life, reduces social interactions and play during adolescence, and increases anxiety-like behavior in the elevated plus maze with no impairment to general locomotor activity in adulthood (Trezza et al., 2008, Trezza & Vanderschuren, 2008). This treatment regimen was also found to impair inhibitory avoidance task (as a measure of long-term aversive memory) performance in adulthood, paralleled with reductions in extracellular norepinephrine and glutamate concentrations in the adult male rat PFC (Campolongo et al., 2007). Similarly, perinatal THC and synthetic cannabinoid agonist, CP-55,940, treatment increases adult anxiety behavior in the open field (Newsom & Kelly, 2008), social interaction and emergence tests (O'Shea et al., 2006). However, perinatal exposure to FAAH inhibitior, URB-597, moderately reduces stress-coping behavior in the forced swim test without affecting anxiety behavior in the elevated plus maze in adulthood (Wu et al., 2014).
Adolescent CB1R agonist exposure generally produces enduring increases in anxiety behavior in a number of rodent behavioral tests (Rubino & Parolaro, 2008, Schneider et al., 2005) and results in a multitude of other long-term, sex-dependent neural and behavioral consequences associated with stress-induced HPA axis dyregulation (Rubino et al., 2009a, Rubino et al., 2009b, Rubino et al., 2008). On a behavioral level, adolescent THC exposure reduces stress coping behavior in the forced swim test in adult female rats, impairs spatial working memory in adult males, and leads to anhedonia in both adult males and females as measured by the sucrose preference test (Rubino et al., 2008). Neural consequences of adolescent cannabinoid exposure include differential reductions in amygdalar and hippocampal CB1R binding and signaling capacity in adult males and females (Rubino et al., 2008), decreases in markers of neuroplasticity such as synaptophysin and PSD 95 in the PFC of females (Rubino et al., 2009a), dendritic atrophy and reduced number of spines in hippocampal neurons in males (Rubino et al., 2009b), and reductions in cell proliferation (Realini et al., 2011) and survival (Lee et al., 2014b) in the dentate gyrus of the hippocampus in adult female and male rats. Chronic administration of the FAAH inhibitor URB-597 in adulthood reverses effects induced by adolescent THC administration such as reductions in stress-coping and increases in anxiety behaviors in female rats (Realini et al., 2011) and decreases hippocampal CB1R expression in male rats (Marco et al., 2009). These findings suggest that a persistent deficit in AEA signaling in adulthood from adolescent cannabinoid exposure may mediate many of the sustained detrimental effects of this treatment. Adolescent exposure to CB1R agonist, WIN55,212-2, produces similar results as adolescent THC exposure (reduced stress coping and increased anhedonia) as well as increased anxiety responses in the novelty-suppressed feeding test coupled to attenuated serotonergic and heightened noradrenergic activity (Bambico et al., 2010). Consistent with these studies, sustained CB1R blockade by CB1R antagonist/inverse agonist, AM-251, treatment during adolescence increases stress-coping behavior in the forced swim test and moderately increases risk assessment behavior in the elevated plus maze in adult male rats (Lee et al., in press).
Recent work has demonstrated that the detrimental impact of adolescent THC treatment is due, at least in part, to disruption of normative adolescent eCB signaling that regulates PFC maturation (Rubino et al., 2015). Moreover, adolescent THC exposure results in impaired eCB-mediated signaling and plasticity in glutamatergic synapses in the PFC of adult female mice, which is reversible with MAGL inhibitor, JZL184 (Lovelace et al., in press). In line with this, administration of CB1R agonist, CP 55,940, during PND 28-43 increases CB1R activity in the PFC of adult male but not female rats (Mateos et al., 2011). Moreover, multiple studies (Rubino et al., 2015, Rubino et al., 2009a, Rubino et al., 2009b, Rubino et al., 2008, Zamberletti et al., 2014) have demonstrated that chronic adolescent THC exposure results in long term modulation of corticolimbic CB1R density and binding as well as other long term neural and behavioral changes similar to those produced by chronic stress (via glucocorticoid hypersecretion; e.g. McEwen, 2005) and prolonged glucocorticoid exposure (Hill et al., 2008). Together, these studies suggest that eCB signaling during adolescence impacts the normative development of circuits that regulate stress and emotionality in adulthood. As such, perturbations of the eCB system during adolescence, due to either stress or exogenous cannabinoid exposure, may have long-lasting effects on emotional stability and flexibility in adulthood. However, there are some contrasting reports that chronic adolescent exposure to the CB1R agonist, CP 55,940, either decreases (Wegener & Koch, 2009) or has no effect on anxiety behavior as measured in the open field test, elevated plus maze (Biscaia et al., 2003) and social interaction test (O'Shea et al., 2006). Similar to the adult literature, the results of these developmental cannabinoid exposure studies likely vary based on sex, strain/species, drug, dose and age of exposure (Rubino & Parolaro, 2008, Schneider, 2008).
Concluding Remarks
eCB signaling has been established to be involved in the regulation of unconditioned and conditioned anxiety behavior in adulthood, making it a current therapeutic target for the treatment of stress- and anxiety-related disorders. However, with the emergence of mental illness peaking in adolescence (Paus et al., 2008), it is critical that we gain a more comprehensive understanding of normative developmental processes that confer both plasticity and vulnerability to pathogenic experiences. Previous work has established there are distinct sensitive periods for fear learning during normative development. At the same time, there are multiple temporal-specific alterations in corticolimbic eCB signaling (Figure 3) that may facilitate the fear responses characterizing each of these sensitive periods. During the infantile sensitive period (suppressed fear responding), 2-AG levels are high while AEA and CB1Rs gradually increase (Berrendero et al., 1999, Fernandez-Ruiz et al., 2000). Moreover, during the juvenile sensitive period for fear extinction (absence of spontaneous recovery or reinstatement of fear memory), AEA (Lee et al., 2013, Wenger et al., 2002) and CB1R expression (Heng et al., 2011, Rodriguez de Fonseca et al., 1993) peak between the end of the juvenile period and onset of adolescence. Lastly, the adolescent sensitive period for fear extinction and contextual fear conditioning (blunted fear extinction and contextual fear memory) coincides with fluctuating corticolimbic AEA levels (Ellgren et al., 2008, Lee et al., 2013, Rubino et al., 2015) and declining CB1R expression (Rodriguez de Fonseca et al., 1993). Research has also revealed that developmental exposure to cannabinoid agonists is associated with a multitude of long-term behavioral and neural consequences on unconditioned anxiety behavior (Campolongo et al., 2007, Rubino et al., 2015). Given that each sensitive period of fear learning also coincides with a distinct eCB signaling profile, it is tempting to speculate that the eCB system is a neural correlate for fear learning throughout the life span and accordingly, can facilitate the plasticity associated with each sensitive period as well as serve as a neural substrate by which external factors can lead to the development of an anxiety disorder. However, there is a significant gap in the literature investigating the role of normative eCB signaling in the corticolimbic circuit and regulation of fear learning during different developmental ages. Future research determining the exact role of eCB signaling in fear learning during development will contribute to our understanding of vulnerability to mental illness and may inform which treatments are more effective based on age.
Acknowledgments
The authors wish to thank Caitlin Riebe for her technical assistance generating figures for this article. This work was supported in part by the Canadian Institutes of Health Research (TTYL, MNH), Natural Sciences and Engineering Research Council of Canada (MNH) and the National Institute of Mental Health Grant P50 MH079513 (FSL).
Footnotes
The authors have no conflict of interest to declare.
References
- Abush H, Akirav I. Cannabinoids modulate hippocampal memory and plasticity. Hippocampus. 2010;20:1126–1138. doi: 10.1002/hipo.20711. [DOI] [PubMed] [Google Scholar]
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endo-cannabinoid signaling in the nervous system. Chemical Reviews. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Akirav I. Role of the Endocannabinoid System in Anxiety and Stress-Related Disorders. In: Kalinin V, editor. Anxiety Disorders. 2011. InTech. [Google Scholar]
- Aliczki M, Balogh Z, Tulogdi A, Haller J. The temporal dynamics of the effects of monoacylglycerol lipase blockade on locomotion, anxiety, and body temperature. Behavioural Pharmacology. 2012;23 doi: 10.1097/FBP.0b013e3283564dfa. [DOI] [PubMed] [Google Scholar]
- Aliczki M, Zelena D, Mikics E, Varga ZK, Pinter O, Bakos NV, Varga J, Haller J. Monoacylglycerol lipase inhibition-induced changes in plasma corticosterone levels, anxiety and locomotor activity in male CD1 mice. Hormones and Behavior. 2013;63:752–758. doi: 10.1016/j.yhbeh.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Almeida-Santos A, Gobira P, Rosa L, Guimaraes F, Moreira F, Aguiar D. Modulation of anxiety-like behavior by the endocannabinoid 2-arachidonoylglycerol (2-AG) in the dorsolateral periaqueductal gray. Behavioral Brain Research. 2013;252:10–17. doi: 10.1016/j.bbr.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Ballard ME, Bedi G, de Wit H. Effects of delta-9-tetrahydrocannabinol on evaluation of emotional images. Journal of Psychopharmacology. 2012;26:1289–1298. doi: 10.1177/0269881112446530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico F, Nguyen N, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiology of Disease. 2010;37:641–655. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Barna I, Zelena D, Arszovszki AC, Ledent C. The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: in vivo and in vitro studies in CB1 receptor knockout mice. Life Sciences. 2004;75:2959–2970. doi: 10.1016/j.lfs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicology and Teratology. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, Schulte G, Ernfors P, Mackie K, Paratcha G, Hurd YL, Harkany T. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proceedings of the National Academy of Sciences. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Bernard C, Milh M, Morozov YM, Ben-Ari Y, Freund TF, Gozlan H. Altering cannabinoid signaling during development disrupts neuronal activity. Proceedings of the National Academy of Sciences. 2005;102:9388–9393. doi: 10.1073/pnas.0409641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, García-Gil L, Hernández ML, Romero J, Cebeira M, de Miguel R, Ramos JA, Fernández-Ruiz JJ. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–3188. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Maldonado R. Involvement of the opioid system in the anxiolytic-like effects induced by delta(9)-tetrahydrocannabinol. Psychopharmacology. 2002;163:111–117. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernández-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Biscaia M, Marín S, Fernández B, Marco EM, Rubio M, Guaza C, Ambrosio E, Viveros MP. Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology. 2003;170:301–308. doi: 10.1007/s00213-003-1550-7. [DOI] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. European Neuropsychopharmacology. 2008;18:849–859. doi: 10.1016/j.euroneuro.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacological Reviews. 2013;65:849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasio A, Iemolo A, Sabino V, Petrosino S, Steardo L, Rice KC, Orlando P, Iannotti FA, Di Marzo V, Zorrilla EP, Cottone P. Rimonabant precipitates anxiety in rats withdrawn from palatable food: role of the central amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:2498–2507. doi: 10.1038/npp.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluett R, Gamble-George J, Hermanson D, Hartley N, Marnett L, Patel S. Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Translational Psychiatry. 2014;4:e408. doi: 10.1038/tp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Tyndale RF, Williams B, Mansouri E, Westwood DJ, Foll BL, Rusjan PM, Mizrahi R, De Luca V, Zhou Q, Wilson AA, Houle S, Kish SJ, Tong J. The fatty acid amide hydrolase C385A variant affects brain binding of the positron emission tomography tracer [11C]CURB. Journal of Cerebral Blood Flow and Metabolism. doi: 10.1038/jcbfm.2015.119. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, La Rana G, Russo R, Calignano A, Gessa GL, Cuomo V, Piomelli D. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- Bossong MG, van Hell HH, Jager G, Kahn RS, Ramsey NF, Jansma JM. The endocannabinoid system and emotional processing: a pharmacological fMRI study with ∆9-tetrahydrocannabinol. European Neuropsychopharmacology. 2013;23:1687–1697. doi: 10.1016/j.euroneuro.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. Journal of Neuroscience. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biological psychiatry. 2011;70:479–486. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F. CB2 receptors in the brain: role in central immune function. British Journal of Pharmacology. 2008;153:240–251. doi: 10.1038/sj.bjp.0707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BA, Spear NE. Ontogeny of memory. Psychological Review. 1972;79:215–236. doi: 10.1037/h0032690. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Trezza V, Cassano T, Gaetani S, Morgese MG, Ubaldi M, Soverchia L, Antonelli T, Ferraro L, Massi M, Ciccocioppo R, Cuomo V. Perinatal exposure to delta-9-tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addiction Biology. 2007;12:485–495. doi: 10.1111/j.1369-1600.2007.00074.x. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Trezza V, Ratano P, Palmery M, Cuomo V. Developmental consequences of perinatal cannabis exposure: behavioral and neuroendocrine effects in adult rodents. Psychopharmacology. 2011;214:5–15. doi: 10.1007/s00213-010-1892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE, Lee FS. Treating the developing versus the developed brain: Translating mouse and human preclinical studies to practice. Neuron. doi: 10.1016/j.neuron.2015.05.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1189–1285. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JT, Mann RE, Ialomiteanu A, Stoduto G, Chan V, Ala-Leppilampi K, Rehm J. Anxiety and mood disorders and cannabis use. American Journal of Drug and Alcohol Abuse. 2010;36:118–122. doi: 10.3109/00952991003713784. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Human Molecular Genetics. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Choi K, Le T, McGuire J, Xing G, Zhang L, Li H, Parker CC, Johnson LR, Ursano RJ. Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. Journal of Psychiatric Research. 2012;46:882–889. doi: 10.1016/j.jpsychires.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. The Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. Journal of Neuroscience. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Egger H, Angold A. 10-year research update review: the epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:972–986. doi: 10.1097/01.chi.0000172552.41596.6f. [DOI] [PubMed] [Google Scholar]
- Cota D, Steiner MA, Marsicano G, Cervino C, Herman JP, Grübler Y, Stalla J, Pasquali R, B L, GK S, Pagotto U. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology. 2007;148:1574–1581. doi: 10.1210/en.2005-1649. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, Simões MV, Bhattacharyya S, Fusar-Poli P, Atakan Z, Santos Filho A, Freitas-Ferrari MC, McGuire PK, Zuardi AW, Busatto GF, Hallak JE. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. Journal of Psychopharmacology. 2011;25:121–130. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- D'asti E, Long H, Tremblay-Mercier J, Grajzer M, Cunnane S, Di Marzo V, Walker C. Maternal dietary fat determines metabolic profile and themagnitude of endocannabinoid inhibition of the stress response in neonatal rat offspring. Endocrinology. 2010;151:1685–1694. doi: 10.1210/en.2009-1092. [DOI] [PubMed] [Google Scholar]
- Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E, Curran HV, Morgan CJ. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology. 2013;226:781–792. doi: 10.1007/s00213-012-2955-y. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FAr, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmacology. 1988;34:605–613. [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nature Neuroscience. 2011;14:9–15. doi: 10.1038/nn.2720. [DOI] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, Huang C, Cravatt BF, Glatt CE, Hill MN, Casey BJ, Lee FS. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nature Communications. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte FH, Manzano-Nievesy G, Quiñones-Laracuente K, Ramos-Medina L, Quirk GJ. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. Journal of Neuroscience. 2015;35:3607–3615. doi: 10.1523/JNEUROSCI.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dono LM, Currie PJ. The cannabinoid receptor CB₁ inverse agonist AM251 potentiates the anxiogenic activity of urocortin I in the basolateral amygdala. Neuropharmacology. 2012;62:192–199. doi: 10.1016/j.neuropharm.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, Devi LA, Hurd YL. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. European Neuropsychopharmacology. 2008;18:826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre LF, McLendon D. The efficacy and safety of nabilone (a synthetic cannabinoid) in the treatment of anxiety. Journal of Clinical Pharmacology. 1981;21:377S–382S. doi: 10.1002/j.1552-4604.1981.tb02617.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learning & Memory. 2006;13 doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Berrendero F, Hernandez ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends in Neurosciences. 2000;23:14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD) CNS Neuroscience & Therapeutics. 2009;15:84–88. doi: 10.1111/j.1755-5949.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E, Suris R, Weidenfeld J, Mechoulam R. Differential response to acute and repeated stress in cannabinoid CB1 receptor knockout newborn and adult mice. Behavioral Pharmacology. 2005;16:431–440. doi: 10.1097/00008877-200509000-00016. [DOI] [PubMed] [Google Scholar]
- Ganon-Elazar E, Akirav I. Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. Journal of Neuroscience. 2009;29:11078–11088. doi: 10.1523/JNEUROSCI.1223-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganon-Elazar E, Akirav I. Cannabinoids prevent the development of behavioral and endocrine alterations in a rat model of intense stress. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:456–466. doi: 10.1038/npp.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. Journal of the American Chemical Society. 1964;86:1646–1647. [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, Hajcak G. The development of fear learning and generalization in 8-13 year-olds. Developmental Psychobiology. 2012;54:675–684. doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Vecchiarelli HA, Morena M, Lee TTY, Hermanson DJ, Kim AB, Mclaughlin RJ, Hassan KI, Kuhne C, Wotjak CT, Deussing JM, Patel S, Hill MN. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. Journal of Neuroscience. 2015;35:3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B, Kavanagh D, Young R. Being stoned: a review of self-reported cannabis effects. Drug and Alcohol Review. 2003;22:453–460. doi: 10.1080/09595230310001613976. [DOI] [PubMed] [Google Scholar]
- Guggenhuber S, Romo-Parra H, Bindila L, Leschik J, Lomazzo E, Remmers F, Zimmermann T, Lerner R, Klugmann M, Pape HC, Lutz B. Impaired 2-AG signaling in hippocampal glutamatergic neurons: aggravation of anxiety-like behavior and unaltered seizure susceptibility. International Journal of Neuropsychopharmacology. doi: 10.1093/ijnp/pyv091. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Hill M, McEwen B, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends in Pharmacological Sciences. 2013a;34:637–644. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, MacPherson K, Cinar R, Gamble-George J, Sugden K, Williams B, Godlewski G, Ramikie T, Gorka A, Alapafuja S, Nikas S, Makriyannis A, Poulton R, Patel S, Hariri A, Caspi A, Moffitt T, Kunos G, Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Molecular Psychiatry. 2013b;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Solowij N. Adverse effects of cannabis. The Lancet. 1998;352:1611–1616. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. European Journal of Neuroscience. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Haring M, Guggenhuber S, Lutz B. Neuronal populations mediating the effects of endocannabinoids on stress and emotionality. Neuroscience. 2012;204:145–158. doi: 10.1016/j.neuroscience.2011.12.035. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, B MS. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biological psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T, Keimpema E, Barabás K, Mulder J. Endocannabinoid functions controlling neuronal specification during brain development. Molecular and Cellular Endocrinology. 2008;286:S84–90. doi: 10.1016/j.mce.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. Journal of Neuroscience. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitland I, Klumpers F, Oosting R, Evers D, Leon Kenemans J, Baas J. Failure to extinguish fear and genetic variability in the human cannabinoid receptor 1. Translational Psychiatry. 2012;2:e162. doi: 10.1038/tp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65:278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. Journal of Neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuera-Matas A, Ucha M, Ambrosio E. Long-term consequences of perinatal and adolescent cannabinoid exposure on neural and psychological processes. Neuroscience and Biobehavioral Reviews. 2015;55:119–146. doi: 10.1016/j.neubiorev.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, Hillard CJ, Yehuda R. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. 2013;38:2952–2961. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, Ho WS, Shi L, Patel S, Gorzalka BB, Hillard CJ. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS and Neurological disorders - Drug Targets. 2009;8:451–458. doi: 10.2174/187152709789824624. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, McEwen BS. Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cerebral Cortex. 2011;21:2056–2064. doi: 10.1093/cercor/bhq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32:350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009a;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009b;34:1257–1262. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S. Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biology of Mood & Anxiety Disorders. 2013;3:19. doi: 10.1186/2045-5380-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ. Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins and Other Lipid Mediators. 2000;61:3–18. doi: 10.1016/s0090-6980(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Wilkison DM, Edgemond WS, Campbell WB. Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochimica et Biophysica Acta. 1995;1257:249–256. doi: 10.1016/0005-2760(95)00087-s. [DOI] [PubMed] [Google Scholar]
- Hunault CC, Böcker KB, Stellato RK, Kenemans JL, de Vries I, Meulenbelt J. Acute subjective effects after smoking joints containing up to 69 mg Δ9-tetrahydrocannabinol in recreational users: a randomized, crossover clinical trial. Psychopharmacology. 2014;231:4723–4733. doi: 10.1007/s00213-014-3630-2. [DOI] [PubMed] [Google Scholar]
- Imperatore R, Morello G, Luongo L, Taschler U, Romano R, De Gregorio D, Belardo C, Maione S, Di Marzo V, Cristino L. Genetic deletion of monoacylglycerol lipase (MAGL) leads to impaired cannabinoid receptor CB1R signaling and anxiety-like behavior. Journal of Neurochemistry. doi: 10.1111/jnc.13267. in press. [DOI] [PubMed] [Google Scholar]
- Jenniches I, Ternes S, Albayram O, Otte DM, Bach K, Bindila L, Michel K, Lutz B, Bilkei-Gorzo A, Zimmer A. Anxiety, stress, and fear response in mice with reduced endocannabinoid levels. Biological psychiatry. doi: 10.1016/j.biopsych.2015.03.033. in press. [DOI] [PubMed] [Google Scholar]
- Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–588. doi: 10.1016/j.psyneuen.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Jutras-Aswad D, DiNieri JA, Harkany T, Hurd YL. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:395–412. doi: 10.1007/s00406-009-0027-z. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nature Medicine. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annual Review of Neuroscience. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Current Opinion in Psychiatry. 2007;20:359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. New England Journal of Medicine. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses. Nature Neuroscience. 2010;13:592–600. doi: 10.1038/nn.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. Journal of Neuroscience. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Li S, Richardson R. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cerebral Cortex. 2011;21:530–538. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behavioral Neuroscience. 2007;121:131–139. doi: 10.1037/0735-7044.121.1.131. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. Journal of Neuroscience. 2008;28:1282–1290. doi: 10.1523/JNEUROSCI.4736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Archives of General Psychiatry. 2003;60:709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, O'Neal ST, Long JZ, Cravatt BF, Lichtman AH. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacology, Biochemistry and Behavior. 2011;98:21–27. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafenetre P, Chaouloff F, Marsicano G. The endocannabinoid system in anxiety, fear memory and habituation. Journal of Psychopharmacology. 2007;26:23–39. doi: 10.1177/0269881111408958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Developmental Neuroscience. 2012;34:101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, Jenness J, Ernst M, Grillon C, Pine DS. Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Hunyady L, Juhasz G, Bagdy G. Promoter variants of the cannabinoid receptor 1 gene (CNR1) in interaction with 5-HTTLPR affect the anxious phenotype. American Journal of Medical Genetics Part B Neuropsychiatric genetics. 2009;150B:1118–1127. doi: 10.1002/ajmg.b.31024. [DOI] [PubMed] [Google Scholar]
- Ledoux JE. The amygdala. Current Biology. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, Šestan N, Weinberger DR, Casey BJ. Mental health. Adolescent mental health--opportunity and obligation. Science. 2014a;346:547–549. doi: 10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Wainwright S, Hill M, Galea L, Gorzalka B. Sex, drugs, and adult neurogenesis: sex-dependent effects of escalating adolescent cannabinoid exposure on adult hippocampal neurogenesis, stress reactivity, and amphetamine sensitization. Hippocampus. 2014b;24:280–292. doi: 10.1002/hipo.22221. [DOI] [PubMed] [Google Scholar]
- Lee TT, Filipski SB, Hill MN, McEwen BS. Morphological and behavioral evidence for impaired prefrontal cortical function in female CB1 receptor deficient mice. Behavioral Brain Research. 2014c;271:106–110. doi: 10.1016/j.bbr.2014.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TT, Gorzalka BB. Evidence for a role of adolescent endocannabinoid signaling in regulating HPA axis stress responsivity and emotional behaviour development. International Review of Neurobiology. doi: 10.1016/bs.irn.2015.09.002. in press. [DOI] [PubMed] [Google Scholar]
- Lee TT, Hill MN, Hillard CJ, Gorzalka BB. Disruption of peri-adolescent endocannabinoid signaling modulates neuroendocrine and behavioral responses to stress in adulthood. Neuropharmacology. doi: 10.1016/j.neuropharm.2015.07.021. in press. [DOI] [PubMed] [Google Scholar]
- Lee TTY, Gorzalka BB. Timing is everything: evidence for a role of corticolimbic endocannabinoids in modulating hypothalamic-pituitary-adrenal axis activity across developmental periods. Neuroscience. 2012;204:17–30. doi: 10.1016/j.neuroscience.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Lee TTY, Hill MN, Hillard CJ, Gorzalka BB. Temporal changes in N-acylethanolamine content and metabolism throughout the peri-adolescent period. Synapse. 2013;67:4–10. doi: 10.1002/syn.21609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SL, Alger BE, McCarthy MM. Developmental increase in hippocampal endocannabinoid mobilization: role of metabotropic glutamate receptor subtype 5 and phospholipase C. Journal of Neurophysiology. 2014;112:2605–1615. doi: 10.1152/jn.00111.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Chen PS, Gean PW. Chronic cannabinoid administration in vivo compromises extinction of fear memory. Learning & Memory. 2008;15:876–884. doi: 10.1101/lm.1081908. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learning & Memory. 2006;13:316–321. doi: 10.1101/lm.217006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cerebral Cortex. 2009;19:165–175. doi: 10.1093/cercor/bhn075. [DOI] [PubMed] [Google Scholar]
- Lisboa SF, Borges AA, Nejo P, Fassini A, Guimarães FS, Resstel LB. Cannabinoid CB1 receptors in the dorsal hippocampus and prelimbic medial prefrontal cortex modulate anxiety-like behavior in rats: additional evidence. Progress in Neuropsychopharmacology & Biological Psychiatry. 2015;59:76–83. doi: 10.1016/j.pnpbp.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Lisboa SF, Reis DG, da Silva AL, Corrêa FM, Guimarães FS, Resstel LB. Cannabinoid CB1 receptors in the medial prefrontal cortex modulate the expression of contextual fear conditioning. International Journal of Neuropsychopharmacology. 2010;13:1163–1173. doi: 10.1017/S1461145710000684. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, Palmiter RD, Krystal G, Rai R, Mahadevan A, Razdan RK, Kunos G. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54:1–7. doi: 10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LE, Lind J, Webster M, Weickert CS. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neuroscience. 2012;13:87. doi: 10.1186/1471-2202-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace JW, Corches A, Vieira PA, Mackie K, Korzus E. An animal model of female adolescent cannabinoid exposure elicits a long-lasting deficit in presynaptic long-term plasticity. Neuropharmacology. doi: 10.1016/j.neuropharm.2015.04.034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. The endocannabinoid system and extinction learning. Molecular Neurobiology. 2007;36:92–101. doi: 10.1007/s12035-007-8004-x. [DOI] [PubMed] [Google Scholar]
- Lutz B. Endocannabinoid signals in the control of emotion. Current Opinion in Pharmacology. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Guzmán M, Mackie K, Doherty P, Harkany T. Programming of neural cells by (endo) cannabinoids: From physiological rules to emerging therapies. Nature Reviews Neuroscience. 2014;15:786–801. doi: 10.1038/nrn3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Valverde O, Barbaccia M, Castañé A, Maldonado R, Ledent C, Parmentier M, Finazzi-Agrò A. Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. European Journal of Neuroscience. 2002;15:1178–1186. doi: 10.1046/j.1460-9568.2002.01957.x. [DOI] [PubMed] [Google Scholar]
- Mackie K. Mechanisms of CB1 receptor signaling: endocannabinoid modulation of synaptic strength. International Journal of Obesity. 2006;30:S19–23. doi: 10.1038/sj.ijo.0803273. [DOI] [PubMed] [Google Scholar]
- Mangieri RA, Piomelli D. Enhancement of endocannabinoid signaling and the pharmacotherapy of depression. Pharmacological Research. 2007;56:360–366. doi: 10.1016/j.phrs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Laviola G. The endocannabinoid system in the regulation of emotions throughout lifespan: a discussion on therapeutic perspectives. Journal of Psychopharmacology. 2012;26:150–163. doi: 10.1177/0269881111408459. [DOI] [PubMed] [Google Scholar]
- Marco EM, Rubino T, Adriani W, Viveros MP, Parolaro D, Laviola G. Long-term consequences of URB597 administration during adolescence on cannabinoid CB1 receptor binding in brain areas. Brain Research. 2009;1257:25–31. doi: 10.1016/j.brainres.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Holmes A. Stress and fear extinction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015:1–22. doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak C, Azad S, Bisogno T, Rammes G, Cascio M, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Castelli M, Viveros MP. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB1 cannabinoid receptors. Journal of Psychopharmacology. 2011;25:1676–1690. doi: 10.1177/0269881110370503. [DOI] [PubMed] [Google Scholar]
- Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. European Journal of Neuroscience. 2003;17:1747–1754. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression and mood disorders: Structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker L. The endocannabinoid system and the brain. Annual Review of Psychology. 2012;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burnstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micale V, Di Marzo V, Sulcova A, Wotjak CT, Drago F. Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacology & Therapeutics. 2013;138:18–37. doi: 10.1016/j.pharmthera.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Terzian AL, Guimarães FS, Wotjak CT. Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin? Neuroscience. 2012;204:186–192. doi: 10.1016/j.neuroscience.2011.08.046. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Wotjak CT. Cannabinoids and anxiety. Current Topics in Behavioral Neuroscience. 2010;2:429–450. doi: 10.1007/7854_2009_16. [DOI] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, Hill MN. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. doi: 10.1038/npp.2015.166. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabas K, Ballester Rosado CJ, Nguyen L, Monory K, Marsicano G, Di Marzo V, Hurd YL, Guillemot F, Mackie K, Lutz B, Guzman M, Lu HC, Galve-Roperh I, Harkany T. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proceedings of the National Academy of Sciences. 2008;105:8760–8765. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S, Gillespie HK, Hollister LE. A model for evaluation of antianxiety drugs with the use of experimentally induced stress: Comparison of nabilone and diazepam. Clinical Pharmacology and Therapeutics. 1978;23:54–62. doi: 10.1002/cpt197823154. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, Potenza MN, Bailey CR, Lin SF, Najafzadeh S, Ropchan J, Henry S, Corsi-Travali S, Carson RE, Huang Y. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Molecular Psychiatry. 2013;18:1034–1040. doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom RJ, Kelly SJ. Perinatal delta-9-tetrahydrocannabinol exposure disrupts social and open field behavior in adult male rats. Neurotoxicology and Teratology. 2008;30:213–219. doi: 10.1016/j.ntt.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom RJ, Osterlund C, Masini CV, Day HE, Spencer RL, Campeau S. Cannabinoid receptor type 1 antagonism significantly modulates basal and loud noise induced neural and hypothalamic-pituitary-adrenal axis responses in male Sprague-Dawley rats. Neuroscience. 2012;204:64–73. doi: 10.1016/j.neuroscience.2011.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar long lasting deficits in object recognition and reduced social interaction in rats. Journal of Psychopharmacology. 2006;20:611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- Onaivi E, Ishiguro H, Gong J, Patel S, Perchuk A, Meozzi P, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola B, Liu Q, Hope B, Iwasaki S, Arinami T, Uhl G. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Annals of the New York Academy of Sciences. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neuroscience and Biobehavioral Reviews. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Bitencourt RM, Takahashi RN. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiology of Learning and Memory. 2008;90:290–293. doi: 10.1016/j.nlm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology. 2006;188:641–649. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- Patel S, Cravatt BF, Hillard CJ. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:497–507. doi: 10.1038/sj.npp.1300535. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. Journal of Pharmacology and Experimental Therapeutics. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proceedings of the National Academy of Sciences. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, Lee FS. Altered fear learning across development in both mouse and human. Proceedings of the National Academy of Sciences. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Lee FS, Casey BJ. Fear learning and memory across adolescent development: Hormones and Behavior Special Issue: Puberty and Adolescence. Hormones and Behavior. 2013;64:380–389. doi: 10.1016/j.yhbeh.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. Journal of Neuroscience. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Psychoyos D, Vinod KY, Cao J, Xie S, Hyson RL, Wlodarczyk B, He W, Cooper TB, Hungund BL, Finnell RH. Cannabinoid receptor 1 signaling in embryo neurodevelopment. Birth Defects Research Part B, Developmental and Reproductive Toxicology. 2012;95:137–150. doi: 10.1002/bdrb.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Lyons M, Mori S, Milad MR, Liberzon I, Phan KL. Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiology of Learning and Memory. 2014;113:125–134. doi: 10.1016/j.nlm.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, Phan KL. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64:396–402. doi: 10.1016/j.neuropharm.2012.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological psychiatry. 2010;67:1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini N, Vigano' D, Guidali C, Zamberletti E, Rubino T, Parolaro D. Chronic URB597 treatment at adulthood reverted most depressive-like symptoms induced by adolescent exposure to THC in female rats. Neuropharmacology. 2011;60:235–243. doi: 10.1016/j.neuropharm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Reich CG, Mohammadi MH, Alger BE. Endocannabinoid modulation of fear responses: learning and state-dependent performance effects. Journal of Psychopharmacology. 2008;11:769–777. doi: 10.1177/0269881107083999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Lisboa SF, Aguiar DC, Corrêa FM, Guimarães FS. Activation of CB1 cannabinoid receptors in the dorsolateral periaqueductal gray reduces the expression of contextual fear conditioning in rats. Psychopharmacology. 2008;198:405–411. doi: 10.1007/s00213-008-1156-1. [DOI] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros MP, Lutz B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2624–2634. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe CJ, Pamplona FA, Kamprath K, Wotjak CT. Fear relief-toward a new conceptual frame work and what endocannabinoids gotta do with it. Neuroscience. 2012;204:159–185. doi: 10.1016/j.neuroscience.2011.11.057. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernández-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal stages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, Navarro M. Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. Journal of Pharmacology and Experimental Therapeutics. 1996;276:56–64. [PubMed] [Google Scholar]
- Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, open-label, pilot study of add-on oral Δ9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clinical Drug Investigation. 2014;34:587–591. doi: 10.1007/s40261-014-0212-3. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Annals of the New York Academy of Sciences. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Romero J, Garcia-Palomero E, Berrendero F, Garcia-Gil L, Hernandez ML, Ramos JA, Fernández-Ruiz JJ. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26:317–323. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Molecular and Cellular Endocrinology. 2008;286:S108–113. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, Sagheddu C, Ligresti A, Tonini R, Di Marzo V, Parolaro D. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiology of Disease. 2015;73:60–69. doi: 10.1016/j.nbd.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Alberio T, Capurro V, Viganò D, Guidali C, Sala M, Fasano M, Parolaro D. The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotoxic Research. 2009a;15:567–607. doi: 10.1007/s12640-009-9031-3. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, Guidali C, Pinter M, Sala M, Bartesaghi R, Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009b;19:363–372. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1760–1769. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Cheatle MD. Odor-aversion learning in neonatal rats. Science. 1977;198:845–846. doi: 10.1126/science.918668. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. Journal of Neuroscience. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addiction Biology. 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- Schneider M, Drews E, Koch M. Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212-2. Behavioural Pharmacology. 2005;16:447–454. doi: 10.1097/00008877-200509000-00018. [DOI] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacological Research. 2011;64:226–234. doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shonesy BC, Bluett RJ, Ramikie TS, Báldi R, Hermanson DJ, Kingsley PJ, Marnett LJ, Winder DG, Colbran RJ, Patel S. Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Reports. 2014;9:1644–1653. doi: 10.1016/j.celrep.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone JJ, Green MR, Hodges TE, McCormick CM. Differential effects of CB1 receptor agonism in behavioural tests of unconditioned and conditioned fear in adult male rats. Behavioral Brain Research. 2015;279:9–16. doi: 10.1016/j.bbr.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Sipe JC, K C, L GA, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proceedings of the National Academy of Sciences. 2002;99:8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Scott TM, Murray S, Harismendy O, Simon GM, Cravatt BF, Waalen J. Biomarkers of endocannabinoid system activation in severe obesity. PLoS One. 2010;5:e8792. doi: 10.1371/journal.pone.0008792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) British Journal of Pharmacology. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel C, Marek R, Gooch HM, Sullivan RK, Sah P. Prefrontal and auditory input to intercalated neurons of the amygdala. Cell Reports. 2015;10:1435–1442. doi: 10.1016/j.celrep.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Holman P. Transitions in sensitive period attachment learning in infancy: The role of corticosterone. Neuroscience and Biobehavioral Reviews. 2011;34:835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. Journal of Neuroscience. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, Hariri AR. Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. American Journal of Psychiatry. 2015;172:276–283. doi: 10.1176/appi.ajp.2014.14020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple EC, Driver M, Brown RF. Cannabis use and anxiety: is stress the missing piece of the puzzle? Frontiers in Psychiatry. 2014;5:168. doi: 10.3389/fpsyt.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JV, Sullivan RM, Wilson DA. Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Research. 2008;1200:58–65. doi: 10.1016/j.brainres.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Cassano T, Macheda T, Dipasquale P, Carratù MR, Gaetani S, Cuomo V. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology. 2008;198:529–537. doi: 10.1007/s00213-008-1162-3. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology. 2008;197:217–227. doi: 10.1007/s00213-007-1025-3. [DOI] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T, Ohnishi T. Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. Biofactors. 2011;37:1–7. doi: 10.1002/biof.131. [DOI] [PubMed] [Google Scholar]
- Urigüen L, Pérez-Rial S, Ledent C, Palomo T, Manzanares J. Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology. 2004;46:966–973. doi: 10.1016/j.neuropharm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Van Sickle M, Duncan M, Kingsley P, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison J, Marnett L, Di Marzo V, Pittman Q, Patel K, Sharkey K. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Wegener N, Koch M. Behavioural disturbances and altered Fos protein expression in adult rats after chronic pubertal cannabinoid treatment. Brain Research. 2009;1253:81–91. doi: 10.1016/j.brainres.2008.11.081. [DOI] [PubMed] [Google Scholar]
- Wenger T, Gerendai I, Fezza F, González S, Bisogno T, Fernandez-Ruiz J, Di Marzo V. The hypothalamic levels of the endocannabinoid, anandamide, peak immediately before the onset of puberty in female rats. Life Sciences. 2002;70:1407–1414. doi: 10.1016/s0024-3205(01)01516-8. [DOI] [PubMed] [Google Scholar]
- Wu CS, Morgan D, Jew CP, Haskins C, Andrews MJ, Leishman E, Spencer CM, Czyzyk T, Bradshaw H, Mackie K, Lu HC. Long-term consequences of perinatal fatty acid amino hydrolase inhibition. British Journal of Pharmacology. 2014;171:1420–1434. doi: 10.1111/bph.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CS, Richardson R. Extinction in the developing rat: an examination of renewal effects. Developmental Psychobiology. 2007;49:565–575. doi: 10.1002/dev.20244. [DOI] [PubMed] [Google Scholar]
- Zamberletti E, Beggiato S, Steardo LJ, Prini P, Antonelli T, Ferraro L, Rubino T, Parolaro D. Alterations of prefrontal cortex GABAergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiology of Disease. 2014;63:35–47. doi: 10.1016/j.nbd.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Zhu P, Lovinger D. Developmental alteration of endocannabinoid retrograde signaling in the hippocampus. Journal of Neurophysiology. 2010;103:1123–1129. doi: 10.1152/jn.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurolo E, Iyer AM, Spliet WGM, Van Rijen PC, Troost D, Gorter JA, Aronica E. CB1 and CB2 cannabinoid receptor expression during development and in epileptogenic developmental pathologies. Neuroscience. 2010;170:28–41. doi: 10.1016/j.neuroscience.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]