Abstract
Aims
Chronic kidney disease (CKD) is associated with worse outcomes in heart failure with preserved ejection fraction (HFpEF). Whether this association is due the effect of CKD on intrinsic abnormalities in cardiac function is unknown. We hypothesized that CKD is independently associated with worse cardiac mechanics in HFpEF.
Methods and Results
We prospectively studied 299 patients enrolled in the Northwestern University HFpEF Program. Using the creatinine-based CKD-Epi equation to calculate estimated glomerular filtration rate (eGFR), study participants were analyzed by CKD status (using eGFR <60 ml/min/1.73 m2 to denote CKD). Indices of cardiac mechanics (longitudinal strain parameters) were measured using speckle-tracking echocardiography. Using multivariable-adjusted linear and Cox regression analyses, we determined the association between CKD and echocardiographic parameters and clinical outcomes (cardiovascular hospitalization or death). Of 299 study participants, 48% had CKD. CKD (dichotomous variable) and reduced eGFR (continuous variable) were both associated with worse cardiac mechanics indices including LA reservoir strain, left ventricular longitudinal strain, and right ventricular free wall strain, even after adjusting for potential confounders, including comorbidities, EF and volume status. For example, for each 1-SD decrease in eGFR, LA reservoir strain was 3.52%-units lower (P<0.0001) after multivariable adjustment. Reduced eGFR was also associated with worse outcomes (adjusted hazard ratio [HR] 1.28 [95% CI 1.01–1.61] per 1-SD decrease in eGFR; P=0.039). The association was attenuated after adjustment for indices of cardiac mechanics (P=0.064).
Conclusion
In HFpEF, CKD is independently associated with worse cardiac mechanics, which may explain why HFpEF patients with CKD have worse outcomes.
Keywords: diastolic heart failure, chronic kidney disease, cardiac mechanics, outcomes
INTRODUCTION
Due to a variety of factors, chronic kidney disease (CKD) and heart failure with preserved ejection fraction (HFpEF) are becoming more prevalent.1, 2 Whether due to a common etiology or arising independently, CKD and HFpEF are often coincident in patients. Furthermore, the patient population with both problems is expanding. Importantly, studies published ~10 years ago found that renal dysfunction is associated with worse outcomes and higher mortality in HFpEF patients,3–6 and the trajectory of renal dysfunction may contribute to outcome differences.7 Despite the association between CKD and adverse outcomes, the interplay between CKD, clinical characteristics, and cardiac structural and functional abnormalities in HFpEF, particularly indices of cardiac mechanics, have not been well described.
Recent literature suggests that indices of cardiac mechanics (e.g., speckle tracking echocardiography strain parameters) are superior to conventional echocardiographic measures for evaluation of subclinical cardiac disease and abnormalities in cardiomyocyte calcium homeostasis.8, 9 Furthermore, abnormalities in left ventricular (LV) longitudinal systolic strain occur in HFpEF despite a normal ejection fraction.10 HFpEF has also been shown to be associated with abnormalities in left atrial (LA) mechanics,11 and there is a growing appreciation of the clinical importance of contractile dysfunction of the LV, right ventricle (RV), and LA in HFpEF.12–14 However, whether CKD is associated with these abnormalities in myocardial function in HFpEF is unknown.
Despite the growing body of literature evaluating speckle tracking in HFpEF patients, there has been limited evaluation of speckle tracking parameters in patients with concurrent CKD. Therefore, we aimed to better characterize the association between CKD and abnormalities in cardiac structure and function in HFpEF. We hypothesized that presence of CKD is associated with worse longitudinal strain parameters in HFpEF independent of other comorbidities, cardiac structural parameters, and indices of volume overload.
METHODS
Study population
As part of the Northwestern University HFpEF Program, a systematic observational study of HFpEF (ClinicalTrials.gov identified #NCT01030991) was conducted enrolling consecutive patients between March 2008 and May 2011. The Northwestern University institutional review board approved this study and all participants gave written, informed consent. Further details of the study design have been described previously.15, 16 The investigation conforms with the principles outlined in the Declaration of Helsinki (Br Med J 1964; ii: 177). In brief, participants were recruited after hospitalization for heart failure as identified by an automated daily query of the inpatient electronic medical record at Northwestern Memorial Hospital. The following search criteria were used: diagnosis of HF or the words ‘heart failure’ in the hospital notes OR B-type natriuretic peptide (BNP) >100 pg/ml OR administration of 2 or more doses of intravenous diuretics. Patients were offered post-discharge follow-up in a specialized HFpEF program if they met the following 3 inclusion criteria: age >21 years, LV ejection fraction (EF) >50% and presence of heart failure as defined by Framingham criteria.
Post-hospitalization, HF was confirmed in the clinic, and all patients were found to have at least 1 of the following diagnostic hallmarks: grade 2+ LV diastolic dysfunction on echocardiography; elevated LV filling pressures on invasive hemodynamic test; or elevated BNP (>100 pg/mL). All study patients met European Society of Cardiology consensus statement for the diagnosis of HFpEF.17 Patients were excluded from participation if there was a prior history of LVEF <40% that had since recovered, LV end-diastolic volume (EDV) >97 ml/m2, they had constrictive pericarditis or greater than moderate valvular disease. Of the 419 consecutive patients who were enrolled, 56 were excluded we excluded because echocardiographic images could not be retrieved; 55 were excluded due to poor image quality (in whom speckle tracking-strain analysis was not feasible); and 9 were excluded due to lack of data on renal function (i.e., estimated glomerular filtration rate [GFR]) at time of study enrollment. Thus, 299 patients were available for the final analysis.
Clinical characteristics
Demographics, race/ethnicity, NYHA functional class, comorbidities, medications, vitals, body mass index and labs including serum sodium, blood urea nitrogen, hemoglobin, and BNP were recorded or measured in the study participants. Hypertension was defined by systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg, physician documented history of hypertension (HTN) or current use of antihypertensive medications. Coronary artery disease (CAD) was defined by physician-documented history of CAD, known coronary stenosis >50%, history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft or abnormal stress test results consistent with myocardial ischemia. Diabetes mellitus was defined by physician-documented history, use of oral hypoglycemic or insulin for the treatment of hyperglycemia. Obesity was defined by body mass index >30kg/m2.
Exposure variable: renal function
Serum creatinine was measured in all patients. Estimated glomerular filtration rate was calculated using the creatinine based CKD-Epi equation.18 CKD was defined as eGFR < 60 ml/min/1.73m2 (which corresponds to Stage 3 or worse CKD). Participants were classified in to 5 groups based on the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) 2000 guidelines.19 Stage 0/1 CKD was defined as eGFR >90 ml/min/1.73m2; Stage 2 CKD as eGFR 60–89 ml/min/1.73m2; Stage 3 CKD as eGFR 30–59 ml/min/1.73m2; Stage 4 CKD as eGFR 15–29ml/min/1.73m2; and Stage 5 CKD as eGFR <15ml/min/1.73m2 or if the participant was on dialysis. Stage 1 CKD represents a unique population with renal vulnerability (e.g., microalbuminuria or renal structural abnormality) in the presence of eGFR > 90 ml/min/1.73 m2. For the purposes of this study, patients with normal renal function and those with Stage 1 CKD were combined.
Echocardiographic characteristics
All study participants underwent comprehensive 2-dimensional echocardiography with Doppler and tissue Doppler imaging (TDI) using commercially available ultrasound systems with harmonic imaging (Philips iE33 or 7500; Philips Medical Systems, Andover, MA; or Vivid 7, GE Healthcare, General Electric Corp, Waukesha, WI). The test was performed with the patient in the left lateral decubitus position. Blood pressure was recorded at the time of echocardiography using a digital blood pressure monitor (Omron HEM-907XL; Omron Healthcare Inc, Vernon Hills, IL). Cardiac structure and function were quantified as recommended by the American Society of Echocardiography,20–22 including the use of the biplane method of discs for the calculation of LV volumes. LA volume was measured by the biplane area-length method using apical 4- and 2-chamber views at the end-systolic frame preceding mitral valve opening. Dedicated acquisitions of the LA were performed in order to avoid foreshortening. LA volumes were then indexed to body surface area. TDI peak LV longitudinal systolic velocity (s′) and early (e′) and late (a′) LV diastolic velocities were obtained in the apical 4-chamber view at the lateral mitral annulus. Right atrial pressure was estimated based on the size of the inferior vena cava and percent collapse during sniff maneuver.
All measurements were performed by an experienced research sonographer (blinded to all clinical data [including CKD status, creatinine, and eGFR] and outcomes) using ProSolv 4.0 echocardiographic analysis software (ProSolv CardioVascular; FujiFilm, Indianapolis, IN). All measurements were verified by an experienced investigator with expertise in echocardiography.
Speckle-tracking echocardiography
All images used for speckle-tracking echocardiographic analysis were obtained at a frame rate of 50–70 fps. Strain was analyzed by a single investigator using a customized software package (2D Cardiac Performance Analysis, TomTec v4.5, Munich, Germany). Three consecutive cardiac cycles were recorded and averaged. Speckle-tracking analysis was not performed in patients with unacceptable image quality, defined as >1 segment dropout, missing view, or significant foreshortening of the LV, RV, or LA.
The QRS was used as the reference point (i.e. zero baseline) to calculate all strain parameters. Peak LV longitudinal systolic strain, peak longitudinal RV systolic free wall strain, and left atrial reservoir, conduit, and booster strains were measured. The LV endocardial border was manually traced in the apical 4-, 3-, and 2- chamber views, and the RV endocardial border traced in the apical 4-chamber RV-focused view. LV longitudinal strain was calculated by averaging the 6 segments in each view, and RV free wall strain was calculated by averaging the 3 RV free wall segments in the apical 4-chamber view. Intraobserver variability of ventricular strain parameters was low (intraclass correlations 0.88–0.92; coefficient of variation 7.2–11.3%).
Left atrial strain was measured in the apical 4- and 2-chamber views. We defined the following components of LA strain: LA reservoir strain = peak (maximal) longitudinal LA strain; LA booster strain = longitudinal LA strain measured between onset of the P wave and onset of the QRS complex; and LA conduit strain = LA reservoir strain – LA booster strain. In patients who were in atrial fibrillation at the time of echocardiography, there is no LA booster function because of the loss of coordinated LA contraction; in these cases, LA conduit strain = LA reservoir strain. Intraobserver variability of LA strain parameters was low (intraclass correlations 0.84–0.94, coefficient of variation 2.4–6.0%).
Outcomes
While enrolled in the study, participants were evaluated at the Northwestern HFpEF Program at least every 6 months or more frequently if clinically indicated. At each visit, antecedent hospitalizations were reviewed, documented and categorized. For cardiovascular hospitalizations, the specific cause was identified (e.g. HF, acute coronary syndrome, arrhythmia, etc.). At the same intervals, participants or their proxies were contacted to determine vital status. Deaths were verified through query of the Social Security Death Index. Enrollment date for participants was defined as the first visit to the outpatient clinic. Last follow-up date was defined as date of death or date of last HFpEF clinic visit.
Statistical analysis
Study participants were stratified into 2 groups based on the presence of Stage 3 CKD using a cut-off of eGFR < 60 ml/min/1.73 m2. Clinical characteristics, laboratory data, and echocardiographic parameters were compared between groups. Categorical variables were compared between groups using Chi-squared tests or Fisher exact tests, as appropriate. Continuous variables were compared between groups using t-tests (or Wilcoxon rank-sum test, when appropriate). For the echocardiographic data stratified by CKD, we additionally used linear (or logistic regression, where appropriate) to show the p-value for differences between CKD vs. no CKD adjusted for age, hypertension, diabetes, and beta-blocker use as these factors could potentially confound the association between CKD and echocardiographic parameters.
We used linear regression models to determine the independent association between CKD and indices of cardiac mechanics. We also used linear regression to test the trend in indices of cardiac mechanics across CKD stages. We used Cox proportional-hazard analyses and the log-rank statistic to evaluate the relationship between CKD and outcomes, which included cardiovascular hospitalization, HF hospitalization, and death. Covariates in multivariable-adjusted models included were based on clinical relevance and included age, sex, race, smoking status, BMI, hypertension, hyperlipidemia, diabetes mellitus, chronic obstructive pulmonary disease, obstructive sleep apnea, coronary artery disease, and atrial fibrillation. All models were further adjusted for ejection fraction and a marker of volume overload (echocardiographic estimation of right atrial pressure).
A two-sided p-value <0.05 was considered significant. All analyses were performed using Stata 12 (StataCorp, College Station, TX).
RESULTS
Baseline clinical characteristics of the study patients
We prospectively enrolled 299 outpatients with HFpEF after hospitalization for HF as described above. Mean age was 65±12 years, 64% were female, and 38% were African American. The mean eGFR was 62±28 ml/min/1.73 m2 (25th–75th percentile 40–82 ml/min/1.73 m2). CKD (Stage 3 or worse) was present in 145 patients (48%).
Table 1 shows the clinical and demographic characteristics by presence of CKD (eGFR < 60 ml/min/1.73 m2). Those with CKD were older, had worse NYHA functional class, and were more likely to have a history of systemic hypertension and diabetes mellitus. Additionally, those with CKD had higher BNP (and were more likely to be on vasodilators, loop diuretics, and aspirin than the study participants without CKD).
Table 1.
Clinical Characteristics Stratified by Presence or Absence of CKD
| Clinical characteristic | No CKD (n = 154) | CKD (n = 145) | p-value |
|---|---|---|---|
| Age (years) | 60.9±12.3 | 69.3±12.1 | <0.001 |
| Female, n(%) | 96(62) | 95(66) | 0.57 |
| Race, n(%) | 0.58 | ||
| White | 78 (51) | 77(53) | |
| African American | 63(41) | 52(37) | |
| Other | 13(8) | 16(11) | |
| NYHA functional class, n(%) | 0.004 | ||
| I | 26(17) | 16(11) | |
| II | 68(44) | 48(33) | |
| III | 56(37) | 81(56) | |
| IV | 3(2) | 0(0) | |
| Coronary artery disease, n(%) | 71(46) | 75(52) | 0.33 |
| Hypertension, n(%) | 104(68) | 121(83) | 0.001 |
| Hyperlipidemia, n(%) | 76(49) | 83(57) | 0.17 |
| Diabetes mellitus, n(%) | 33(21) | 57(39) | <0.001 |
| Current or former smoker, n(%) | 62(40) | 60(41) | 0.84 |
| Atrial fibrillation, n(%) | 34(22) | 44(30) | 0.1 |
| Obesity, n(%) | 77(50) | 73(50) | 0.95 |
| COPD, n(%) | 48(31) | 56(39) | 0.18 |
| Obstructive sleep apnea, n(%) | 47(31) | 57(39) | 0.11 |
| Systolic blood pressure (mmHg) | 123.2±19.8 | 126.9±20.7 | 0.12 |
| Diastolic blood pressure (mmHg) | 71.4±11.4 | 68.5±12.5 | 0.035 |
| Body mass index (kg/m2) | 31.8±8.7 | 31.5±8.4 | 0.7 |
| Serum creatinine (mg/dl) | 0.9±0.2 | 2.3±2.1 | <0.001 |
| Estimated GFR (ml/min/1.73m2) | 83.5±16.5 | 37.2±15.7 | <0.001 |
| Hemoglobin (g/dl) | 12.1±1.9 | 11.8±1.7 | 0.12 |
| B-type natriuretic peptide (pg/ml)* | 113 (37–290) | 338 (187–640) | <0.001 |
| Medications, n(%) | |||
| ACE-I/ARB | 77(50) | 84(58) | 0.17 |
| β-blocker | 90(58) | 110(76) | 0.001 |
| Calcium channel blocker | 45(29) | 54(37) | 0.14 |
| Nitrate | 8(5) | 32(22) | <0.001 |
| Loop diuretic | 69(45) | 96(66) | <0.001 |
| Thiazide diuretic | 34(22) | 30(21) | 0.77 |
| Aldosterone blocker | 20(13) | 17(12) | 0.74 |
| Statin | 65(42) | 81(56) | 0.018 |
| Aspirin | 52(34) | 79(54) | <0.001 |
| Warfarin | 32(21) | 38(26) | 0.27 |
Continuous variables expressed as mean ± standard deviation unless otherwise specified
Values represent median (25th–75th percentile)
CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; GFR = glomerular filtration rate; ACE-I = angiotensin converting enzyme-inhibitor; ARB = angiotensin receptor blocker
Association of kidney function with echocardiographic parameters and cardiac mechanics
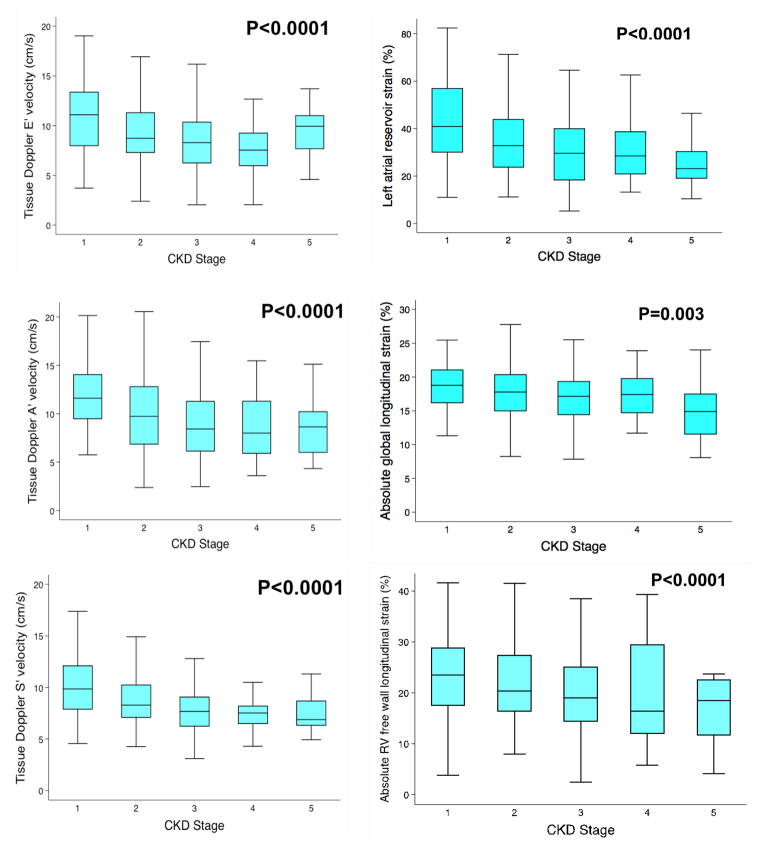
On echocardiographic analysis, as displayed in Table 2, patients with CKD had significantly higher LV mass index. Additionally, diastolic relaxation as measured by e′ velocity was more impaired in patients with CKD than in those without CKD. Longitudinal systolic function, as estimated by LV systolic tissue velocities on tissue Doppler imaging, and late diastolic velocity (a′) were also more impaired in patients with CKD compared to those without CKD. Figure 1 (left panel) demonstrates the differences in tissue Doppler (s′, e′ and a′) indices by CKD stage revealing a relatively linear association between declining renal function and worse longitudinal function of the LV (p<0.0001 for all). Pulmonary artery systolic pressure was higher, and indices of RV systolic function (e.g., RV fractional area change) were lower, in patients with CKD. Adjustment for age, hypertension, diabetes, and beta-blocker did not eliminate the associations between CKD and the majority of echocardiographic parameters (Table 2).
Table 2.
Echocardiographic Characteristics Stratified by Presence or Absence of CKD
| Echocardiographic parameter | No CKD (n=154) | CKD (n=145) | P-value | Adjusted P-value* |
|---|---|---|---|---|
| LV ejection fraction, % | 61.5±6.3 | 60.9±6.6 | 0.44 | — |
| LV end-systolic volume index, ml/m2 | 16.6±6.6 | 16.7±8.6 | 0.92 | — |
| LV end-diastolic volume index, ml/m2 | 42.1±12.1 | 40.9±12.9 | 0.41 | — |
| LV mass index, g/m2 | 96.4±27.9 | 113.7±48.2 | <0.001 | <0.001 |
| Stroke volume, ml | 86.7±30.0 | 85.1±30.7 | 0.66 | — |
| Left atrial volume index, ml/m2 | 32.5±12.0 | 36.5±15.4 | 0.012 | 0.055 |
| E/A ratio | 1.3±0.7 | 1.4±0.7 | 0.67 | — |
| Tissue Doppler e′ velocity, cm/s | 10.1±4.1 | 8.5±3.4 | <0.001 | 0.056 |
| Tissue Doppler s′ velocity, cm/s | 9.5±2.9 | 7.9±2.5 | <0.001 | <0.001 |
| Tissue Doppler a′ velocity, cm/s | 10.9±3.9 | 8.8±3.8 | <0.001 | <0.001 |
| E/e′ ratio | 11.8±7.7 | 14.8±7.6 | <0.001 | 0.072 |
| PA systolic pressure, mmHg (n=222) | 40±15 | 47±16 | 0.001 | 0.014 |
| PA systolic pressure > 40 mmHg (n=222), n(%) | 51(44) | 67(63) | 0.004 | 0.026 |
| RA pressure, mmHg | 6.6±3.4 | 7.6±4.1 | 0.022 | 0.095 |
| RV basal diameter, cm | 3.8±0.6 | 3.9±0.6 | 0.50 | — |
| RV basal diameter > 4.2 cm, n(%) | 35(23) | 40(28) | 0.33 | — |
| RV end-diastolic area, cm2 | 27±7 | 27±7 | 0.66 | — |
| RV end-systolic area, cm2 | 15±5 | 16±5 | 0.13 | — |
| RV fractional area change, % | 44±7 | 42±7 | 0.009 | 0.007 |
| TAPSE, cm | 2.2±0.6 | 1.9±0.6 | 0.003 | 0.12 |
| LV longitudinal strain, %** | 18.2±4.0 | 16.8±4.1 | 0.005 | 0.030 |
| RV free wall strain, %** | 22.7±8.1 | 19.6±7.7 | <0.001 | 0.014 |
| Left atrial reservoir strain, % | 36.7±16.3 | 28.8±14.9 | <0.001 | 0.007 |
| Left atrial conduit strain, % | 19.6±8.9 | 15.4±7.2 | <0.001 | 0.013 |
| Left atrial booster strain, % | 19.1±8.3 | 15.9±7.9 | <0.001 | 0.008 |
Continuous variables expressed as mean ± standard deviation
LV = left ventricular; RV = right ventricular; PA = pulmonary arterial; TAPSE = tricuspid annular plane systolic excursion
P-value after adjustment for age, hypertension, diabetes, and beta-blocker use
LV and RV strains are presented as absolute values
Figure 1.
Relationship between Chronic Kidney Disease Stages and Left Ventricular Longitudinal Tissue Velocities Measured by Tissue Doppler Imaging (left panel) and Longitudinal Strain Indices Measured by Speckle-Tracking Echocardiography (right panel) in Patients with Heart Failure and Preserved Ejection Fraction
LA strain parameters (booster, conduit, and reservoir strains) were also significantly worse in the CKD compared to those without CKD. Furthermore, LA strain showed a stepwise decline in function with progressive CKD stage as demonstrated in Figure 1 (right panel; p<0.001). Absolute longitudinal strain and RV free wall strain were also significantly worse in patients with CKD. Again, these findings persisted when participants were evaluated based on CKD stage (Figure 1, right panel; p = 0.003 for LV longitudinal strain and p = 0.001 for RV free wall strain).
All speckle-tracking echocardiography strain parameters remained significant after sequential adjustments: model 1) age, sex, race, smoking status and BMI; 2) model 1 plus hypertension, hyperlipidemia, diabetes mellitus, chronic obstructive pulmonary disease, coronary artery disease, atrial fibrillation and obstructive sleep apnea; and 3) model 2 plus ejection fraction and right atrial pressure (Table 3). This was true for both the dichotomous CKD variable and for the linear eGFR variable. For example, in the fully adjusted model, for every 1-SD decrease in GFR, absolute LV longitudinal strain decreased by 0.53%-units (p=0.037) and LA reservoir strain decreased by 3.52 %-units (p<0.0001). Substituting LV end-diastolic volume for right atrial pressure as a marker of volume status did not change the results (Table 3). Similarly, substituting LV mass for hypertension also did not change the results (Table 3).
Table 3.
Association of Glomerular Filtration Rate and Chronic Kidney Disease with Indices of Cardiac Mechanics on Multivariable-Adjusted Linear Regression Analyses
| Model 1 | β-Coefficient for 1-SD decrease in GFR | p-value | β-Coefficient for CKD | p-value |
|---|---|---|---|---|
| LA reservoir strain | −3.59 (−5.42, −1.76) | <0.0001 | −5.60 (−9.27, −1.92) | 0.003 |
| LA conduit strain | −1.93 (−2.88, −0.99) | <0.0001 | −2.72 (−4.60, −0.84) | 0.005 |
| LA booster strain | −1.54 (−2.60, −0.47) | 0.005 | −2.92 (−5.04, −0.80) | 0.007 |
| LV longitudinal strain | −0.71 (−1.21, −0.22) | 0.005 | −1.36 (−2.33, −0.39) | 0.006 |
| RV free wall strain | −1.10 (−2.06, −0.13) | 0.026 | −2.66 (−4.55, −0.78) | 0.006 |
|
| ||||
| Model 2 | ||||
|
| ||||
| LA reservoir strain | −3.01 (−4.84, −1.18) | 0.001 | −4.59 (−8.22, −0.96) | 0.013 |
| LA conduit strain | −1.60 (−2.54, −0.66) | 0.001 | −2.18 (−4.02, −0.33) | 0.021 |
| LA booster strain | −1.38 (−2.44, −0.33) | 0.011 | −2.64 (−4.72, −0.56) | 0.013 |
| LV longitudinal strain | −0.59 (−1.10, −0.08) | 0.023 | −1.20 (−2.20, −0.21) | 0.018 |
| RV free wall strain | −1.20 (−2.20, −0.21) | 0.018 | −2.79 (−4.71, −0.86) | 0.005 |
|
| ||||
| Model 3 | ||||
|
| ||||
| LA reservoir strain | −2.52 (−4.40, −0.63) | 0.009 | −3.63 (−7.34, 0.09) | 0.055 |
| LA conduit strain | −1.33 (−2.29, −0.38) | 0.006 | −1.69 (−3.56, 0.17) | 0.074 |
| LA booster strain | −1.12 (−2.2, −0.03) | 0.044 | −2.21 (−4.35, −0.06) | 0.044 |
| LV longitudinal strain | −0.42 (−0.94, 0.10) | 0.11 | −0.92 (−1.91, 0.08) | 0.072 |
| RV free wall strain | −1.23 (−2.25, −0.21) | 0.018 | −2.82 (−4.79, −0.86) | 0.005 |
|
| ||||
| Model 4 | ||||
|
| ||||
| LA reservoir strain | −3.52 (−5.23, −1.81) | <0.0001 | −4.63 (−8.01, −1.26) | 0.007 |
| LA conduit strain | −1.97 (−2.90, −1.05) | <0.0001 | −2.27 (−4.10, −0.44) | 0.015 |
| LA booster strain | −1.64 (−2.77, −0.52) | 0.004 | −2.69 (−4.91, −0.48) | 0.017 |
| LV longitudinal strain | −0.53 (−1.03, −0.03) | 0.037 | −1.08 (−2.05, −0.12) | 0.028 |
| RV free wall strain | −1.19 (−2.22, −0.16) | 0.024 | −2.68 (−4.67, −0.69) | 0.008 |
|
| ||||
| Model 5 | ||||
|
| ||||
| LA reservoir strain | −3.07 (−4.85, −1.28) | 0.001 | −4.72 (−8.27, −1.17) | 0.009 |
| LA conduit strain | −1.57 (−2.47, −0.66) | 0.001 | −1.99 (−3.77, −0.20) | 0.029 |
| LA booster strain | −1.44 (−2.48, −0.39) | 0.007 | −2.77 (−4.83, −0.71) | 0.009 |
| LV longitudinal strain | −0.56 (−1.03, −0.08) | 0.022 | −1.01 (−1.94, −0.08) | 0.033 |
| RV free wall strain | −1.19 (−2.16, −0.21) | 0.017 | −2.68 (−4.57, 0.80) | 0.005 |
LA = left atrial; LV = left ventricular; RV = right ventricular
• Model 1: adjusted for age, sex, race, smoking status, and body mass index
• Model 2: adjusted for model 1 covariates plus hypertension, hyperlipidemia, diabetes mellitus, chronic obstructive pulmonary disease, coronary artery disease, atrial fibrillation, and obstructive sleep apnea
• Model 3: adjusted for model 1 covariates plus hyperlipidemia, diabetes mellitus, chronic obstructive pulmonary disease, coronary artery disease, atrial fibrillation, obstructive sleep apnea, and LV mass (identical to model 2 except LV mass replaces hypertension)
• Model 4: adjusted for model 2 covariates plus ejection fraction and right atrial pressure
• Model 5: adjusted for model 2 covariates plus ejection fraction and LV end-diastolic volume
Association of kidney function with adverse outcomes
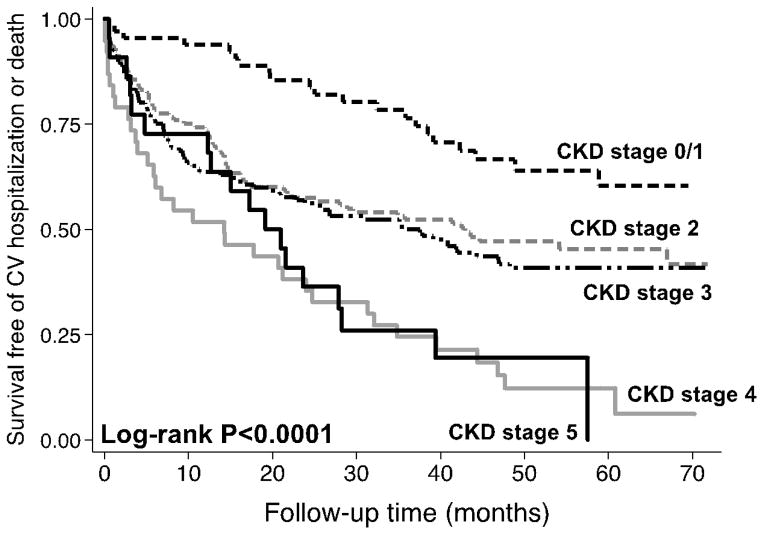
The median follow-up time was 14 months (25th–75th percentile 5–24 months). Of the 299 study patients, 65 (22%) had a HF hospitalization, 94 (31%) had a cardiovascular hospitalization, 36 (12%) died, and 113 (38%) experienced the combined endpoint of cardiovascular hospitalization or death. As shown in Table 4, CKD, when analyzed as a dichotomous variable with a cut-off value of eGFR 60 ml/min/1.73 m2, was not associated with adverse outcomes. However, when examining outcomes for patients with HFpEF by eGFR, there are significantly worse outcomes for each 1-SD decrease in eGFR. To further illustrate this relationship, Figure 2 displays a Kaplan-Meier curve by CKD stage, which shows a decline in survival free of the combined endpoint of cardiovascular hospitalization (including HF hospitalization) or death by CKD stage (log-rank p<0.0001). Based on the survival curves, patients with CKD stage 2 and 3 had similar (and worse) outcomes than CKD stage 1, and patients with CKD stage 4 and 5 were similar and had the worst outcomes.
Table 4.
Association of Glomerular Filtration Rate and Chronic Kidney Disease with Adverse Outcomes on Multivariable-Adjusted Cox-Proportional Hazard Analyses
| Hazard Ratio for 1-SD decrease in GFR | p-value | Hazard Ratio for CKD | p-value | |
|---|---|---|---|---|
| Model 1 | ||||
|
| ||||
| HF hospitalization | 1.36 (1.03, 1.79) | 0.031 | 1.47 (0.85, 2.55) | 0.17 |
| Cardiovascular hospitalization | 1.39 (1.10, 1.75) | 0.006 | 1.16 (0.73, 1.84) | 0.52 |
| Death | 1.21 (0.84, 1.73) | 0.31 | 0.84 (0.41, 1.73) | 0.63 |
| Combined endpoint | 1.37 (1.11, 1.69) | 0.003 | 1.13 (0.74, 1.71) | 0.57 |
|
| ||||
| Model 2 | ||||
|
| ||||
| HF hospitalization | 1.20 (0.88, 1.62) | 0.24 | 1.09 (0.61, 1.93) | 0.77 |
| Cardiovascular hospitalization | 1.29 (1.01, 1.65) | 0.04 | 0.97 (0.60, 1.56) | 0.89 |
| Death | 1.20 (0.81, 1.79) | 0.37 | 0.67 (0.30, 1.49) | 0.32 |
| Combined endpoint | 1.32 (1.06, 1.64) | 0.013 | 1.00 (0.66, 1.53) | 0.99 |
|
| ||||
| Model 3 | ||||
|
| ||||
| HF hospitalization | 1.19 (0.86, 1.65) | 0.29 | 1.10 (0.59, 2.04) | 0.76 |
| Cardiovascular hospitalization | 1.25 (0.96, 1.63) | 0.10 | 0.97 (0.58, 1.61) | 0.89 |
| Death | 1.17 (0.76, 1.81) | 0.47 | 0.69 (0.29, 1.65) | 0.40 |
| Combined endpoint | 1.28 (1.01, 1.61) | 0.039 | 1.02 (0.65, 1.60) | 0.93 |
|
| ||||
| Model 4 | ||||
|
| ||||
| HF hospitalization | 1.17 (0.82, 1.69) | 0.39 | 1.21 (0.60, 2.47) | 0.59 |
| Cardiovascular hospitalization | 1.25 (0.93, 1.68) | 0.13 | 1.04 (0.59, 1.84) | 0.90 |
| Death | 1.22 (0.75, 1.99) | 0.42 | 0.80 (0.30, 2.14) | 0.64 |
| Combined endpoint | 1.23 (0.99, 1.53) | 0.064 | 1.08 (0.65, 1.80) | 0.77 |
HF = heart failure; SD = standard deviation; GFR = glomerular filtration rate; CKD = chronic kidney disease
• Model 1: adjusted for age, sex, race, smoking status, and body mass index
• Model 2: adjusted for model 1 covariates plus hypertension, hyperlipidemia, diabetes mellitus, chronic obstructive pulmonary disease, coronary artery disease, atrial fibrillation, and obstructive sleep apnea
• Model 3: adjusted for model 2 covariates plus ejection fraction and right atrial pressure
• Model 4: adjusted for model 3 covariates plus indices of cardiac mechanics (left atrial reservoir strain, left ventricular longitudinal strain, and right ventricular free wall strain)
Figure 2.
Kaplan-Meier Curves Demonstrating the Relationship between Chronic Kidney Disease Stages and the Combined Outcome of Cardiovascular Hospitalization or Death in Patients with Heart Failure with Preserved Ejection Fraction
Table 4 demonstrates that eGFR was associated with the combined outcome of cardiovascular hospitalization (which included HF hospitalization) or death, even after adjustment for age, sex, race, comorbidities, ejection fraction, and right atrial pressure (HR 1.28, 95% CI 1.01, 1.61; P=0.039). However, the association between reduced eGFR and adverse outcomes was somewhat attenuated after further adjustment for LA reservoir strain, absolute longitudinal strain, and RV free wall strain (HR 1.23, 95% CI 0.99–1.53; P=0.064).
DISCUSSION
In a prospective analysis of a cohort of 299 patients with HFpEF, we found that CKD was associated with LV longitudinal strain, RV free wall strain, and LA reservoir, conduit, and booster strain parameters. These differences remained significant even after multivariable adjustment controlling for common comorbidities, cardiac structural abnormalities, and markers of volume status. To our knowledge, this is the first study to evaluate the association between CKD and cardiac mechanics in HFpEF. While previous studies have demonstrated similar abnormalities in strain parameters in end-stage renal disease patients, a thorough examination of all stages of CKD in HFpEF patients has not yet been performed.23
Conventional echocardiography has historically been used to evaluate cardiac structure and function in CKD patients. Prior studies have shown that CKD is associated with lower mitral annular e′ velocity and higher E/e′ ratio, markers of LV diastolic dysfunction.24 In attempts to diagnose cardiac dysfunction earlier in the clinical course, Edwards et al. showed that patients with CKD without history of cardiovascular disease have abnormal strain parameters, including reduced longitudinal strain and strain rate in the setting of normal LV ejection fraction and regional systolic tissue velocities, thereby demonstrating the increased sensitivity of strain parameters above and beyond tissue velocities and global LV ejection fraction in patients with CKD.25 Yan et al. expanded this analysis by looking at speckle tracking in ESRD patients on hemodialysis without HF. These investigators also determined that LV myocardial function is impaired in these patients prior to the onset of clinical manifestations.26 Our study expands on these earlier studies by demonstrating the linear relationship between worsening renal function (i.e., increasing CKD stage and worsening eGFR) and abnormalities in indices of cardiac mechanics.
Our results confirm prior studies showing that CKD is associated with worse prognosis in patients with HFpEF. We augment prior research by showing that this association is independent of important comorbidities, ejection fraction, and estimates of volume overload (right atrial pressure). Taken together with our novel cross-sectional findings that lower eGFR is associated with worse cardiac mechanics, these results suggest a potential role for CKD in driving adverse outcomes in HFpEF. The complete mechanism by which renal dysfunction could lead to poor outcomes in these patients is likely complex and multifaceted. Mechanistically, Edwards et al. showed that CKD stage 2 and 3 is associated with reduced aortic distensibility, increased arterial elastance, increased end-systolic elastance, reduced early diastolic (e′) tissue velocities and increased LV diastolic stiffness. Thus, patients with CKD stage 2 and 3 resemble early HFpEF even before the onset of overt HF.27
Although we demonstrated an association between CKD and adverse cardiac mechanics, the direction of this association cannot be ascertained given the cross-sectional design of our study. CKD may be a pathogenic factor that results in abnormal cardiac mechanics. However, it is also quite possible that underlying abnormalities in cardiac structure/function in HFpEF contribute to the development of CKD. Worse diastolic function and abnormal cardiac mechanics could lead to reduced cardiac output and/or increased renal venous congestion, thereby leading to worse renal function in HFpEF. In reality, it is likely a two-way association that leads to the detrimental cardiorenal interactions in HFpEF patients.
Multiple prior studies have shown that renal insufficiency remains an independent predictor of mortality in HFpEF patients.5 Furthermore, CKD-associated mortality is worse in patients with HFpEF compared to patients with HF with reduced ejection fraction even after controlling for covariates.28 Go and colleagues demonstrated a step-wise increase in mortality with decreasing eGFR in HFpEF.4 Newer literature led by Kramann et al. suggests that speckle tracking and strain analysis can detect uremic cardiomyopathy and therefore predict cardiovascular mortality.23 However, this analysis was limited to patients with ESRD whereas our study evaluated a broader population of all stages of CKD allowing for a more robust investigation of the relationship between eGFR and outcomes in HFpEF. In rat models, CKD was significantly correlated with interstitial myocardial fibrosis suggesting a mechanism of action for abnormal cardiac mechanics in these patients. Other potential etiologies of abnormal cardiac mechanics in CKD include abnormalities in cardiomyocyte calcium hemostasis, which we have previously demonstrated in the spontaneously hypertensive rat,29 and/or generalized endothelial dysfunction, which may result in abnormal cardiac mechanics. Indeed, in a prior epidemiologic study, we demonstrated an association between low-grade albuminuria (a marker of generalized endothelial dysfunction) and adverse cardiac mechanics.30
Strengths of the study include the standardized recruitment of high-risk patients, the comprehensive nature of echocardiographic evaluation, and inclusion of novel speckle-tracking strain parameters for the evaluation of cardiac mechanics. However, there are several limitations of the present study that require acknowledgement. First, participants were enrolled from a single academic center potentially limiting generalizability. However, Northwestern Memorial Hospital serves a large, diverse, urban population, and the clinical characteristics of the study participants were similar to those of HFpEF epidemiologic studies and observational registries. Second, recruitment occurred exclusively among patients with a prior history of HF hospitalization with all study-related evaluation and testing done in the outpatient setting. Nevertheless, the study participants likely represent a higher risk population than those that would be seen exclusively in the outpatient setting, though the study population did represent those HFpEF patients most likely to be enrolled in clinical trials. Furthermore, we only measured renal function at baseline; thus we cannot evaluate the effect of trajectories of change in renal function over time with cardiac mechanics and outcomes in HFpEF. We also did not measure plasma or blood volume, which could provide important insight in future studies of CKD in HFpEF. Finally, we lack the ability to make causal inferences due to the cross-sectional nature of our study of the association of CKD and echocardiographic findings in HFpEF.
Conclusions
In the setting of HFpEF, CKD is associated with increased cardiac remodeling, significantly worse cardiac mechanics, and worse outcomes. Further studies of cardiac mechanics in relation to CKD in HFpEF could provide insight into mechanisms underlying these associations, which could lead to novel therapeutic paradigms for these high-risk patients.
Acknowledgments
FUNDING:
This work was supported by grants from the American Heart Assocation (#0835488N) and National Institutes of Health (R01 HL107557), both to S.J.S.
Footnotes
CONFLICT OF INTEREST:
No relationships with industry related to this manuscript.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149(2):209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113(23):2713–23. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 5.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113(5):671–8. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 6.Tribouilloy C, Rusinaru D, Mahjoub H, Souliere V, Levy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29(3):339–47. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 7.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43(1):61–7. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Kimura K, Takenaka K, Ebihara A, Okano T, Uno K, Fukuda N, Ando J, Fujita H, Morita H, Yatomi Y, Nagai R. Speckle tracking global strain rate E/E′ predicts LV filling pressure more accurately than traditional tissue Doppler E/E′. Echocardiography. 2012;29(4):404–10. doi: 10.1111/j.1540-8175.2011.01587.x. [DOI] [PubMed] [Google Scholar]
- 9.Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Stoylen A, Ihlen H, Lima JA, Smiseth OA, Slordahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47(4):789–93. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(5):447–56. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyoshi H, Oishi Y, Mizuguchi Y, Iuchi A, Nagase N, Ara N, Oki T. Association of left atrial reservoir function with left atrial structural remodeling related to left ventricular dysfunction in asymptomatic patients with hypertension: evaluation by two-dimensional speckle-tracking echocardiography. Clin Exp Hypertens. 2015;37(2):155–65. doi: 10.3109/10641963.2014.933962. [DOI] [PubMed] [Google Scholar]
- 12.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic Importance of Impaired Systolic Function in Heart Failure With Preserved Ejection Fraction and the Impact of Spironolactone. Circulation. 2015;132(5):402–14. doi: 10.1161/CIRCULATIONAHA.115.015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8(2):295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 14.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35(48):3452–62. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110(6):870–6. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz DH, Beussink L, Sauer AJ, Freed BH, Burke MA, Shah SJ. Prevalence, clinical characteristics, and outcomes associated with eccentric versus concentric left ventricular hypertrophy in heart failure with preserved ejection fraction. Am J Cardiol. 2013;112(8):1158–64. doi: 10.1016/j.amjcard.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28(20):2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Kramann R, Erpenbeck J, Schneider RK, Rohl AB, Hein M, Brandenburg VM, van Diepen M, Dekker F, Marx N, Floege J, Becker M, Schlieper G. Speckle Tracking Echocardiography Detects Uremic Cardiomyopathy Early and Predicts Cardiovascular Mortality in ESRD. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruch C, Rothenburger M, Gotzmann M, Wichter T, Scheld HH, Breithardt G, Gradaus R. Chronic kidney disease in patients with chronic heart failure--impact on intracardiac conduction, diastolic function and prognosis. Int J Cardiol. 2007;118(3):375–80. doi: 10.1016/j.ijcard.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 25.Edwards NC, Hirth A, Ferro CJ, Townend JN, Steeds RP. Subclinical abnormalities of left ventricular myocardial deformation in early-stage chronic kidney disease: the precursor of uremic cardiomyopathy? J Am Soc Echocardiogr. 2008;21(12):1293–8. doi: 10.1016/j.echo.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Yan P, Li H, Hao C, Shi H, Gu Y, Huang G, Chen J. 2D-speckle tracking echocardiography contributes to early identification of impaired left ventricular myocardial function in patients with chronic kidney disease. Nephron Clin Pract. 2011;118(3):c232–40. doi: 10.1159/000321383. [DOI] [PubMed] [Google Scholar]
- 27.Edwards NC, Ferro CJ, Townend JN, Steeds RP. Aortic distensibility and arterial-ventricular coupling in early chronic kidney disease: a pattern resembling heart failure with preserved ejection fraction. Heart. 2008;94(8):1038–43. doi: 10.1136/hrt.2007.137539. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99(3):393–8. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah SJ, Aistrup GL, Gupta DK, O’Toole MJ, Nahhas AF, Schuster D, Chirayil N, Bassi N, Ramakrishna S, Beussink L, Misener S, Kane B, Wang D, Randolph B, Ito A, Wu M, Akintilo L, Mongkolrattanothai T, Reddy M, Kumar M, Arora R, Ng J, Wasserstrom JA. Ultrastructural and cellular basis for the development of abnormal myocardial mechanics during the transition from hypertension to heart failure. Am J Physiol Heart Circ Physiol. 2014;306(1):H88–100. doi: 10.1152/ajpheart.00642.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz DH, Selvaraj S, Aguilar FG, Martinez EE, Beussink L, Kim KY, Peng J, Sha J, Irvin MR, Eckfeldt JH, Turner ST, Freedman BI, Arnett DK, Shah SJ. Association of low-grade albuminuria with adverse cardiac mechanics: findings from the hypertension genetic epidemiology network (HyperGEN) study. Circulation. 2014;129(1):42–50. doi: 10.1161/CIRCULATIONAHA.113.003429. [DOI] [PMC free article] [PubMed] [Google Scholar]