Abstract
Circulating levels of endogenous ouabain (EO), a vasopressor hormone of adrenocortical origin, are increased by sodium depletion. Further, lanosterol synthase (LSS), an enzyme involved in cholesterol biosynthesis, has a missense polymorphism (rs2254524 V642L) that affects EO biosynthesis in adrenocortical cells. Here we investigated the hypothesis that LSS rs2254524 alleles in vivo impact the BP and EO responses evoked by a low dietary Na intake (<100 mEq/day, 2 weeks) among patients with mild essential hypertension. During the low salt diet, the declines in both systolic (−8.7±1.7 vs −3.0±1.5 p= 0.013), and diastolic (−5.1±0.98 vs −1.4±0,.94 mmHg, p<0.05) BP and the slope of the long-term pressure-natriuresis relationship were affected significantly by the presence of the LSS rs2254524 A variant (AA: 0.71±0,22, AC 0.09±0.13, CC 0.04±0.11 mEq/mmHg/24h, p=0.028). In addition, BP rose in ~25% of the patients in response to the low salt diet and this was associated with increased circulating EO. LSS gene polymorphisms influence both the salt-sensitivity of BP and changes in circulating EO in response to a low salt diet. The response of BP and EO to the low salt diet is markedly heterogeneous. Approximately 25% of patients experienced adverse effects i.e., increased BP and EO when salt intake was reduced and may be at increased long-term risk. The augmented response of EO to the low salt diet further supports the view that adrenocortical function is abnormal in some essential hypertensives.
Keywords: Na-K ATPase, digitalis like factor, diet, hypertension, genetic, polymorphisms
INTRODUCTION
High blood pressure (BP) is the most common modifiable risk factor for cardiovascular disease and death. Dietary salt reduction is now a global priority for the prevention of premature morbidity and mortality from cardiovascular disease, as recommended by the World Health Organization (WHO)1,2. However, recent studies have raised questions about the potential for adverse effects associated with low sodium intake, including cardiovascular disease and death3–5 in some individuals.
Endogenous Ouabain (EO) is an adrenocortical cardiac glycoside whose circulating level is influenced by body Na balance. Na+ depletion raises circulating EO6,7 whereas long-term high salt diets are thought to suppress circulating EO levels in normal individuals much as they do for plasma aldosterone. However, relative to Na+ intake, plasma EO8 is inappropriately elevated in about 45% of patients with essential hypertension and correlates with blood pressure9. Further, the elevated levels of EO are related to heart10 and kidney11 damage.
The recent ongoing controversy concerning the paradoxical and potentially hazardous effects of low sodium intake in some hypertensive patients3–5 and the adverse effect of high circulating EO mentioned above, led us to consider that EO might be (over) activated by low salt in some patients but not others. EO is a vasopressor stress hormone stimulated by the decline in blood pressure that occurs, for example, during cardio-pulmonary by-pass12. EO has a sustained vasopressor action and, in response to the stress of a low salt intake, the increase in EO is believed to help minimize an excessive decline in BP.
Further, recent work has indicated that the lanosterol synthase gene (LSS) is transcribed in discrete nephron sites13 and that LSS is involved in EO synthesis14. Here, using never-treated patients with mild to moderate essential hypertension, we test the hypothesis that the stress of a low salt diet raises circulating EO, that this response is modulated by the LSS genotype, and explore the proposal that changes in EO may help identify those patients that may experience adverse effects of low salt diets.
MATERIALS AND METHODS
Inclusion criteria
The present study was conducted according to the principles outlined in the Helsinki declaration. The Committee for Research on Human Subjects of the San Raffaele Scientific Institute, Milan, approved the protocol. Participants gave informed, written consent. In this protocol, according to the “Guideline for management of arterial hypertension”15 criteria we enrolled 394 never treated, recently discovered, essential hypertensive patients with high normal blood pressure level or grade 1 or 2 hypertension, hereinafter termed naïve essential hypertensive (NEH) patients. Of these participants, 340 had 24-hour ambulatory BP monitoring that met with the European Society of Hypertension guidelines (>14 and 7 readings for the computation of day and night means, respectively). Inclusion criteria were:
Essential hypertension: exclusion of secondary causes of hypertension (renal arterial stenosis, hyperaldosteronism, pheocromocytoma, thyroid disorders);
Age between 18 and 65 years old;
BMI < 30 kg/m2;
-
Absence of:
Chronic and acute concomitant diseases (cardiocerebrovascular diseases, diabetes, hepatic and renal diseases);
Gestational hypertension or pregnancy;
Drug and alcohol abuse
Use of pharmacological therapy, included estroprogestinic therapy and hormone replacement therapy.
To exclude secondary hypertension, renal artery ultrasound scan, electrolytes and hormonal dosage (PRA, aldosterone) were performed (urinary catecholamines were measured when pheaochromocytoma was suspected). Echocardiography was performed to assess the presence or absence of cardiac organ damage (left ventricular hypertrophy). The procedures followed were in accordance with institutional guidelines.
Low sodium diet
Patients were instructed to follow a low sodium dietary protocol for a month with three office visits: at enrolment, after 15 days and at the end of the month. During the visits, office blood pressure was measured three times after at least five minutes in the sitting position; blood and 24-hour urine samples were collected to measure renal function, electrolytes and hormones (PRA, aldosterone, and EO). At the first visit, patients received a list of suggested foods with a low content of sodium and a list of forbidden foods with high content of sodium. To assess compliance with the low sodium diet we measured 24-hour urinary sodium excretion at each visit: we considered compliant (C) only those patients who reduced their urinary sodium by at least 40% of their basal value or achieved a urinary sodium less than 100 mmol/day. Patients who did not reach this target were termed non-compliant.
Pressure-Natriuresis relationship
The slope of the relationship between BP and urinary Na+ excretion (PNat, pressure-natriuresis relationship, mEq/mmHg per minute) was calculated by plotting the Na+ excretion on the y axis as a function of mean blood pressure (MBP) on the x axis measured both under basal conditions, day 0 and after 15 days of the low salt diet16 in compliant patients.
Biochemical and renal parameters
Serum and urinary sodium (PNa and UNa) and potassium (PK and UK) were measured by flame photometry, serum and urinary creatinine (Pcrea and Ucrea) by an automated enzymatic method. Plasma renin activity and plasma aldosterone were measured by radioimmunoassay (RIA; Medical System, Genova, Italy). Plasma EO was determined by C-18-extracted samples using a specific antiserum as previously described17. Intraindividual variability was <10%, as previously discussed18.
Genotyping
In the LSS gene (40,684 base-pairs; chromosome location 21q22.3) we selected the missense rs2254524 polymorphism because it was previously associated with ouabain synthesis; i.e., reduced mRNA expression in cells transfected with the A LSS variant but higher LSS activity and ouabain levels, normalized for LSS protein expression, in the A variant vs the C variant transfected cells14.
After DNA extraction from peripheral blood, the LSS SNP was genotyped using the TaqMan® OpenArray™ Genotyping System (Life Technologies, Foster City, CA). All DNA samples were loaded at 50 ng per microliter and amplified on customized arrays following the manufacturer’s instructions. For analysis of the genotypes, we used autocalling methods as implemented in the TaqMan® Genotyper software version 1.3 (Life Technologies). Next, genotype clusters were evaluated manually. Twenty duplicate samples gave 100% reproducibility.
Statistical Analysis
Plasma EO, PRA and aldosterone were not normally distributed (Kolmogorov–Smirnov test). Accordingly, we normalized the distributions by logarithmic transformation. For database management and statistical analysis, we used the SPSS software package (SPSS Inc., Chicago, Illinois, USA), version 21. Power calculation. Sample size calculation was done with the statistical program G*Power. Assuming significant a difference between genotypic groups in SBP variation equal or greater to 5 mmHg (SD 11 mmHg in preliminary data) with α err prob = 0,05 and Power (1-β err prob) = 0,90 we need a total sample size of 168 compliant patients.
Quartile values corresponding to the 25th, 50th, and 75th percentiles were then calculated for BP changes from baseline. For the study NHE cohort, 48 naïve hypertensive patients, all of whom had undergone the pressure natriuresis study, were included in each quartile. We performed analysis of variance to compare the means between groups. ANOVA for repeated measures was used to test the effect of the Na load within and between patients. Our statistical methods also included trend analyses, single and multiple linear regressions. We included in our models co-variables with known physiological relevance for arterial BP and renal phenotypes such as age, sex, body mass index (BMI), baseline PRA and aldosterone, and urinary Na excretion.
RESULTS
Three-hundred-sixty-five naïve hypertensive patients (NEH) underwent the low salt intake protocol for four weeks. The clinical characteristics of these patients are reported in Table 1.
Table 1.
Clinical characteristics of the hypertensive population studied (n=365)
| Parameter | Mean | SD |
|---|---|---|
| Age (years) | 44.9 | 8.9 |
| BMI (kg/m2) | 25.7 | 2.8 |
| Gender (female/male) | 80/314 | --- |
| SBP (mmHg) | 142.0 | 12.3 |
| DBP (mmHg) | 89.3 | 8.5 |
| UNa (mEq/day) | 170.6 | 68.9 |
| UK (mEq/day) | 66.4 | 23.3 |
| PRA (ng/ml/hr) | 1.02 | (0.4–2.65) |
| Aldosterone (ng/dl) | 112.0 | (51–225) |
| EO (pmol/L) | 178.2 | (92–313) |
| Creat Cl (ml/m) | 122.5 | 36.2 |
| Creat. (mg/L) | 0.86 | 0.14 |
All data are means, and standard deviations (SD). PRA, aldosterone and EO are geometric means (IQR).
The number of NEH patients complaint with the low sodium diet and that completed the 4 week dietary period were 139 (35%) of the 365 NEH enrolled. For analysis, we chose only 192 compliant patients that successfully reached 2 weeks of the low salt diet because of the larger number (52.6%). Clinical characteristics of the compliant and non-compliant groups are reported in Table 2. Compliant patients showed a greater reduction in both SBP and DBP (−5.17 and −2.63 mmHg, respectively) compared with their non-compliant counterparts. The decline in urinary sodium excretion was associated with increases in PRA and aldosterone in the compliant but not the non-compliant group (Table 2). The reduction in UNa excretion was associated with a linear rise in PRA (β= −0.187, p<0.010) and aldosterone (β= −0.375, p<0.001), as expected. No changes in PRA and plasma aldosterone were observed among the non-compliant patients, consistent with the urinary measurements that their adherence to the diet was inconsistent (Table 2). Furthermore, the changes in UNa excretion were not associated with detectable changes in circulating EO in either group (C: β=0.049, p=0.585, and NC: β= −0.034, p=0.753).
Table 2.
Impact of low salt intake among Compliant and Non-Compliant Patients
| Parameter | COMPLIANT (n = 192) | NON-COMPLIANT (n = 173) | P |
|---|---|---|---|
| SBP baseline (mmHg) | 141.0 ± 0.9 | 142.3 ± 0.9 | ns |
| DBP baseline(mmHg) | 88.4 ± 0.6 | 90.1 ± 0.6 | 0.046 |
| ΔSBP (mmHg) | −5.17 ± 0.9 | −2.23 ± 0.9 | 0.018 |
| ΔDBP (mmHg) | −2.63 ± 0.9 | −0.14 ± 0.9 | 0.003 |
| UNa baseline (mEq/24 h) | 164.2 ± 4.4 | 176 ± 5.2 | ns |
| UK baseline (mEq/24 h) | 47.1 ± 1.3 | 41.7 ± 1.3 | 0.004 |
| UNa day 15 (mEq/24 h) | 81.6 ± 2.5 | 188.4 ± 5.3 | <0.001 |
| UK day 15 (mEq/24 h) | 47.7 ± 1.6 | 46.6 ± 1.5 | ns |
| Δ PRA (ng/ml/min) | +0.56 ± 0.14 | −0.35 ± 0.13 | <0.001 |
| Δ Aldo (ng/dL) | +24 ± 7.05 | −15.7 ± 4.46 | <0.001 |
| Δ EO (pmol/L) | +11.3± 4.3 | −3.24 ± 3.8 | Ns |
| Creatinine Cl. baseline (ml/m/1.73m2) | 121.4 ± 2.7 | 123.7 ± 2.5 | Ns |
| Creatinine Cl. day 15 (ml/m/1.73m2) | 111.8 ± 2.2 | 131.8 ± 3.0 | <0.0001 |
All data are mean ± SEM.
Creatinine Cl = creatinine clearance
Δ = changes from baseline
Among the complaint group, creatinine clearance declined ~10% during the low salt dietary period whereas clearance increased among non-compliant patients (Table 2).
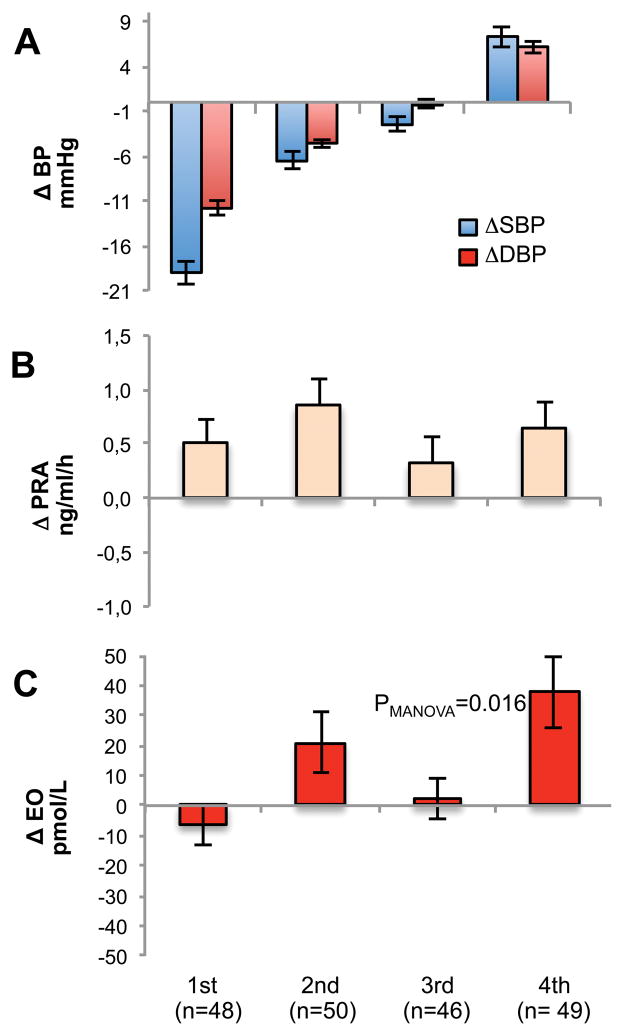
To explore the responses of the measured parameters at the extremes of blood pressure variability, we divided our NEH patients into quartiles according to their BP changes. By definition, the decline in systolic and diastolic BP were most pronounced (−7.5 and −10.8 mmHg, respectively p<0.0001) in the 1st quartile after the low salt dietary period among the compliant patients (Figure 1, A panel). On the other hand, patients in the 4th quartile displayed a significant increase in both systolic and diastolic BP (+9.6 and +7. 4 mmHg, respectively, p<0.0001) indicating “reverse salt-sensitivity” (RSS). Among compliant patients, and regardless of normal or reverse salt-sensitivity, the urinary Na excretion reached target values with the average being <100 mEq/24 h, with no significant difference among quartile groups. PRA (Figure 1, panel B) and plasma aldosterone (not shown) increased in all quartiles. Circulating EO was significantly elevated (p=0.009) only in the 4th quartile of BP responses, and was unchanged (Figure 1, C panel) in the first three quartiles.
Figure 1.
Panel A, changes in systolic (blue bar) and diastolic (red bar) BP among the quartile groups. The 1st quartile group exhibited the greatest fall in BP in response to low salt intake. The 4th quartile group showed a “parodoxical” increase in BP. Panel B. Changes (Δ) in PRA in the 4 groups from baseline. Panel C. Changes (Δ) in plasma EO from baseline.
In contrast with the compliant group, all members of the non-compliant group displayed UNa excretions (Table 2) above the target value (>100 mEq/24 h). In addition, PRA, plasma aldosterone and EO were unchanged in all BP quartiles.
When the dietary induced decline in BP (DBP) and the changes in plasma EO (ΔEO) were analyzed in the compliant patients, a borderline significant (p=0.056) and direct (β=0.16) relationship was present; no relationship was found among the non-compliant patients (data not shown).
Genetic analysis
Clinical characteristics of compliant patients genotyped for LSS rs2224524 are reported in the Supplementary Table S1. Despite clear trends in the changes in BP and EO across the LSS genotypes, no significant differences were found.
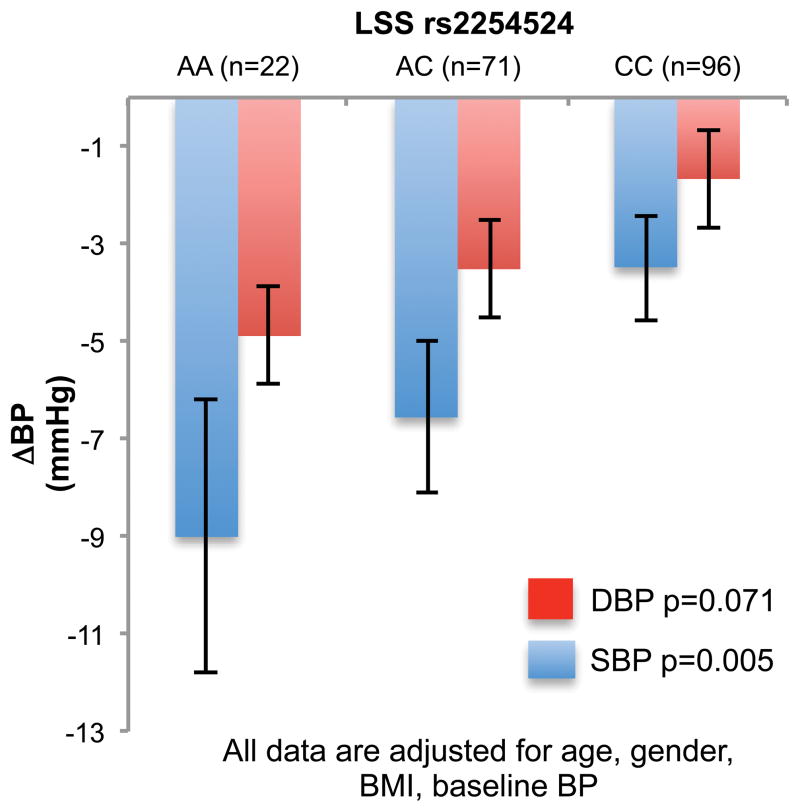
Compliant patients carrying the AA genotype of the LSS rs2254524 polymorphism, showed greater declines in systolic and diastolic BP, than in AC and CC carriers (Figure 2), with an additive effect of the A allele. However, among non-compliant patients, no significant BP or EO differences were present (Supplementary Figures S1 and S2), and thus highly significant (p<0.001) differences in the BP responses among the genotypes were observed between the compliant and non-compliant groups.
Figure 2.
Decline in systolic and diastolic BP during the low salt diet in the LSS rs2254524 AA + AC carriers (red bar systolic BP, orange diastolic BP) compared to LSS (blue bar systolic BP, light blue diastolic BP) CC among compliant patients.
Among the compliant patients, contingency crosstab analysis (Table 3) showed significant (χ2=8,88, p=0.011) differences in the genotype distribution among the BP quartiles. Much smaller numbers (n=3, 1.6%) of AA carriers were represented in the 4th quartile relative to their CC wild type counterparts (n=26, 27%).
Table 3.
Lanosterol synthase genotype frequency distribution among compliant patients grouped according to their BP quartiles
| LSS rs2254524
|
|||||
|---|---|---|---|---|---|
| AA | AC | CC | |||
|
|
|||||
| Quartile Δ PAM15 | 1st | no. | 8 | 20 | 20 |
| % | 4.2% | 28.6% | 20.6% | ||
| 2nd | no. | 7 | 21 | 21 | |
| % | 3.7% | 30.0% | 21.6% | ||
| 3rd | no. | 4 | 15 | 30 | |
| % | 2.1% | 17.1% | 30.9% | ||
| 4th | no. | 3 | 17 | 26 | |
| % | 1.6% | 24.3% | 26.8% | ||
|
| |||||
| Total | no. | 22 | 73 | 97 | |
| % | 11.6% | 37.0% | 51.3% | ||
X2 = 8.846 p = 0.011
ΔPAM15 = MBP difference between day 15 – day 0
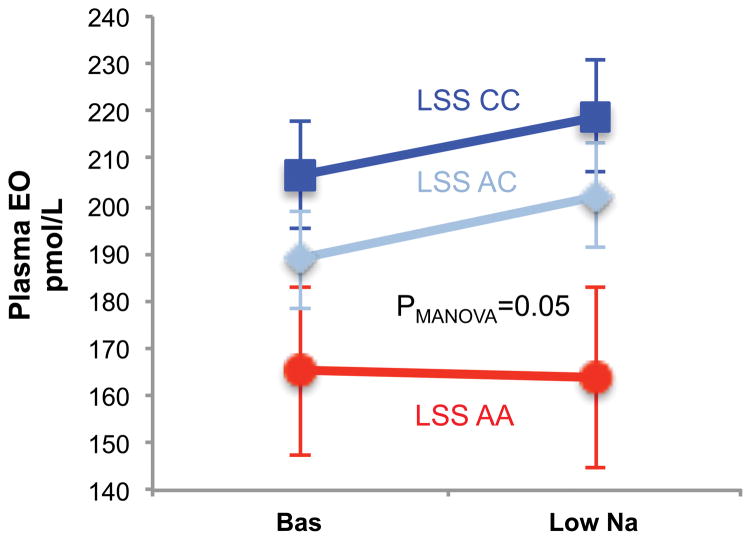
Furthermore, among the AA patients, plasma EO was unchanged by the low Na diet (165.1± 17.6.1 vs 163.8 ± 19.2 pM/L), while a significant (p=0.05) increase in EO was present in those carrying the AC or CC variants (from 206.2 ± 11.2 to 218.9 ± 11.84 pM/L, and from 188.6 ± 10.2 to 202.2 ± 10.9 pM/L, respectively) as shown in Figure 3.
Figure 3.
Circulating EO levels according to the LSS rs2254524 in compliant patients. In LSS AA genotype (red line) no significant changes in circulating EO is present, while increase are observed in AC (light blue) and CC (bluu line) genotypes.
Pressure-natriuresis relationship
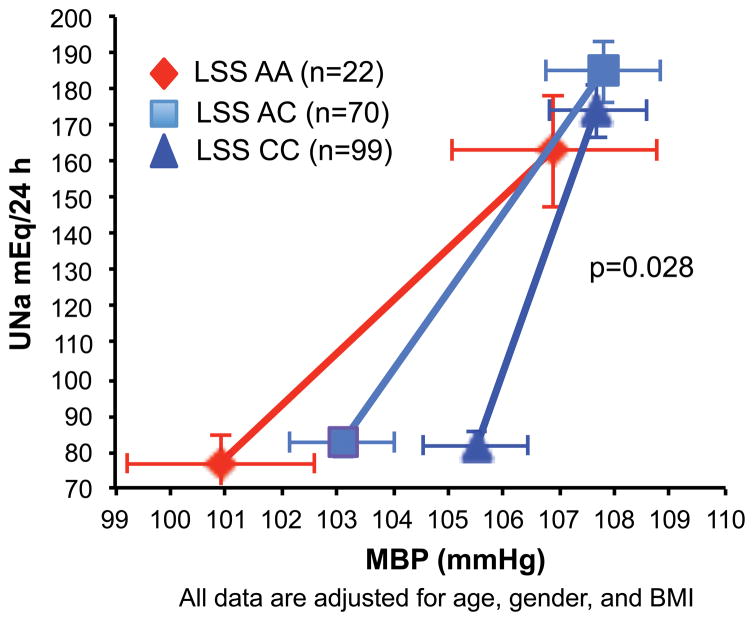
Figure 4 presents the PNat relationship according to the LSS genotype among compliant patients. The slope among AA carriers was significantly (0.696±0.22 mEq/L/mmHg, p=0.028 covariate for age, sex, BMI) different from that of AC heterozygotes (0.100±0.13 mEq/L/mmHg) and the CC wild type (0.046±0.18 mEq/L/mmHg). The pressure-natriuresis relationship was notably less steep among AA carriers than among C allele carriers, indicating increased salt-sensitivity of BP to changes in dietary salt in the former. Among non-compliant patients, no such association between the slope of the PNat and genotype was detected (data not shown).
Figure 4.
Renal pressure-natriuresis relationship during low salt diet grouped according to the LSS rs2254524 genotypes. Shown is the urinary sodium excretion (UNa) as a function of the systemic mean blood pressure (MBP). The slope of the AA carriers (red diamonds 0.696±0.25 mEq/L/mmHg) was significantly different than the AC heterozygotes (light blue squares, 0.100±0.13 mEq/L/mmHg) and the CC wild type (blue triangle, 0.046±0.18 mEq/L/mmHg).
DISCUSSION
The main findings of this study are as follows: First, among patients with newly diagnosed untreated essential hypertension, and in response to the lowered sodium intake, circulating EO increased significantly among those patients whose BP rose (reverse salt-sensitivity). Second, the LSS rs2254524 AA genotype was strongly associated with greatest declines in BP in response to the low salt diet. Third, among patients with the LSS rs2254524 A variant, circulating EO was unchanged by the low Na diet. Our results show that the increase in plasma EO during the low salt diet was restricted to compliant patients that also carried the LSS AC and CC alleles. Circulating EO was unchanged among compliant patients that carried the LSS AA genotype. Further, our results raise the possibility that the adverse effects associated with moving to a low salt intake recently reported may be linked with elevated circulating EO.
Sodium pump inhibitors like EO were suggested as salt-sensitive and rapidly acting natriuretic hormones19. However, various studies6–8,20,21 show that changes in circulating EO are confined to long-term variations in electrolyte balance. Further, in the Belgian general population, the behaviour of circulating EO indicated that it was acting to minimize the depressor action of lower sodium intakes22. The present study in a group of patients with naïve mild hypertension in which a deliberate and specific restrictive urinary Na limit (< 100 mEq/day) was applied, confirms and extends the impression from the Belgian study. We show that among ~25% of patients with mild hypertension, the imposition of a low salt intake leads to significant increases in circulating EO and systolic and diastolic BPs.
Reverse salt-sensitivity, i.e., the seemingly paradoxical increase in BP observed in some patients during reduced salt intake is an underreported phenomenon whose mechanism has remained unexplained. In prior studies, patients with both normal and RSS were not distinguished by changes in classical biochemical markers such as PRA and aldosterone23 even following very marked reductions of Na intake (20 mEq/day)24,25. Our results agree in this regard. More importantly, we observe that the RSS group is characterized by increased circulating EO. This is especially intriguing because EO is a key component of a recently recognized CNS-humoral-vascular axis26. The proximal components of this axis are central neuronal pathways that are activated by elevated peripheral and brain Angiotensin II. Activation of this axis by low salt leads to increased sympathetic drive, elevated plasma EO and increased arterial myogenic- and evoked-tone that together help to sustain or even raise BP26. Recent evidence12, confirms27 the notion that circulating EO behaves as a vasopressor stress hormone that changes during volume expansion and also in circumstances where blood pressure falls.
In contrast to the RSS group, the large majority (75%) of the patients showed no change in EO in response to the low salt regimen; we suggest this may be important for the manifestation of the depressor effect of a low salt intake. The response of circulating EO and BP, and the link to the LSS AC and CC genotypic variants, indicate that the RSS subgroup is a distinct phenotype; it is also notable that the AA genotype is less represented in the RSS group. Another issue concerns the role of the LSS which mediates the biosynthesis of cholesterol precursors. Why are LSS polymorphisms (a VAL A→LEU C missense mutation) associated with profound differences in the pressure-natriuresis relationship when plasma renin and aldosterone are not linked with the LSS genotypes? The renal tubules express LSS at multiple discrete sites13 and the transporters Slco1b2 and MDR (Abcb4) are co-expressed with LSS in some nephron sites. Both Slco1b2 and MDR mediate transmembrane steroid movements, including those for digoxin, ouabain27 and bile acids and by affecting the reabsorption of filtered steroids such as EO28 may influence its circulating levels at baseline and the response to the low salt diet.
The AA LSS genotype was strongly associated with the greatest drop in BP after 2 weeks of the low salt intake. Furthermore, the pressure-natriuresis relationship in AA carriers versus their AC and CC counterparts indicates that the former hypertensive patients are especially salt-sensitive. Thus, LSS polymorphisms identify those patients most likely to benefit from a low salt diet as a lifestyle modification. At the moment, LSS animal models that may help dissect the underlying mechanisms involved in the LSS-EO-low salt pathway are not available, and further studies will need to be specifically designed to address this point.
In our original study,14 a direct association between the LSS polymorphism and ouabain production was demonstrated, the LSS A variant showed reduced mRNA expression in transfected cells, whereas LSS activity and ouabain concentrations were higher in cells transfected with mutated A versus wild type C alleles. Furthermore, in two different studies14, the antihypertensive effect of rostafuroxin, an anti-EO antagonist,29 reduced BP in an allele-dependent manner. Those observations, when taken together with the data presented in this study, suggest that the decline in BP is especially associated with the A variant of the LSS gene. The significant increases in circulating EO we observed were restricted to C allele carriers, and likely reflect increased secretion by the adrenal gland or reduced renal excretion. Conversely, beneficial effects of the AA genotype on plasma EO that are mediated by reduced renal excretion per se are unlikely because of the reduced creatinine clearance among the compliant patients.
Finally, there is on-going debate about the potential for increased risk associated with low salt intakes3–5,23,30. Our studies indicate that among patients with EH, there are three distinct BP phenotypes; a large group (~50%) of the patients whose BP falls, another group (~25%) in whom BP is largely unchanged and a third group (~25%) in which EO and BP both rise with low salt intake. We noted in other studies that co-elevated EO and BP have been repeatedly associated with increased morbidity and mortality3–5. Our results therefore suggest that circulating EO is a functional marker that predicts the beneficial and adverse effects of low salt diets.
Limitations
One limitation in this outpatient study was the inability to lower salt intake below 100 mmol/day. Reducing urinary sodium excretion to 50 mmol Na/day provokes PRA, aldosterone and BP responses24 beyond those we observed. More dramatic reduction in urinary sodium is feasible with an inpatient design that was not practical in our setting31. Nevertheless, the observation of elevated EO inappropriate to sodium excretion in the RSS group leads us to share the impression32–34 that there may be a subtle adrenocortical dysfunction in some patients with EH irrespective of whether the primary focus of the measurement is aldosterone, cortisol, or EO. Second, our study was of a relatively short duration; several weeks to months may be required to uncover the full depressor or pressor effect of a dietary manoeuvre involving dietary salt or diuretics35,36. Third, single 24 hour urine sodium measurements, even when available, may not fully capture changes in dietary salt intake due to infradian variations in salt balance35. Fourth, we did not include a normal dietary control group in the study design. The non-compliant patients are not valid placebo controls37; they complied unpredictably and intermittently with the low salt regimen.
Supplementary Material
PERSPECTIVES.
Reverse salt-sensitivity, i.e., the paradoxical increase in BP observed in some patients during reduced salt intake is an oft underreported if not ignored phenomenon in studies that investigate the impact of salt on BP. In this study, we demonstrate that phenomenon is present among ≈ 25 % of newly diagnosed hypertensive patients and is related to increased circulating EO. Long-term studies are clearly needed to probe the potential relationship of elevated circulating EO with increased morbidity and mortality among susceptible patients with low Na excretion. Nevertheless, the apparent protective effects and blood pressure decline associated with the LSS AA genotype may already identify those patients most likely to benefit from a low salt diet as a lifestyle modification.
NOVELTY AND SIGNIFICANCE.
What is new?
Polymorphisms in the Lanosterol synthase gene have a strong influence on the salt-sensitivity of BP and changes in circulating EO in response to a low salt diet.
Circulating EO increased significantly among the 25% of the patients studied whose BP rose (reverse salt sensitivity) in response to the lowered sodium intake.
What is relevant?
Dietary compliance may account for genetic difference between different studies and should always considered.
Among patients with the LSS rs2254524 AA genotype, circulating EO was unchanged during the low Na diet while resulted significantly increased in those carrying the wild type C allele.
Summary
Circulating EO is increased by sodium depletion and influences BP and renal Na excretion via the transport and/or signaling actions of the renotubular and vascular myocyte Na–K pump. Lanosterol synthase (LSS) mediates an early step in the biosynthesis of cholesterol. A missense polymorphism in this gene affects its expression, enzymatic activity and, unexpectedly, impacts the synthesis of Endogenous Ouabain (EO) when transfected into adrenocortical cells. Thus in this study we investigate the hypothesis that LSS rs2254524 alleles might impact BP and EO responses evoked by a low dietary Na intake (<100 mEq/day, 2 weeks) among dietary compliant patients with mild essential hypertension. Among the study participants, the decline in both systolic BP and the slope of the long-term pressure-natriuresis relationship were affected significantly by the presence of the LSS rs2254524 A variant Further, in ~25% of the patients studied, both EO and BP rose significantly during the low salt diet.
Acknowledgments
We thank Prof Giuseppe Bianchi for its suggestions, and Cinzia Scotti for the technical assistance preparing the manuscript.
SOURCES OF FUNDING
This study was supported in part by the Italian Ministry of Health RF-FSR-2008-1141719 (PM) and CVie grant (PM), supported in part by USPHS grants HL107555 and HL45215 (JH).
Footnotes
DISCLOSURES
None.
References
- 1.Campbell NRC, Correa-Rotter R, Cappuccio FP, Webster J, Lackland DT, Neal B, MacGregor GA. Proposed nomenclature for salt intake and for reductions in dietary salt. J Clin Hypertens (Greenwich) 2015;17:247–251. doi: 10.1111/jch.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Fernandez R, Siopa M, Simpson SJ, Amiya RM, Breda J, Cappuccio FP. Current salt reduction policies across gradients of inequality-adjusted human development in the WHO European region: minding the gaps. Public Health Nutr. 2014;17:1894–1904. doi: 10.1017/S136898001300195X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graudal NA, Hubeck-Graudal T, Jürgens G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review) Am J Hypertens. 2012;25:1–15. doi: 10.1038/ajh.2011.210. [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Eng J Med. 2014;371:612–623. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 5.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovsky J, Kawecka-Jaszcz K, Nikitin Y, Staessen JA European Project on Genes in Hypertension (EPOGH) Investigators. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–1785. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 6.Manunta P, Messaggio E, Ballabeni C, Sciarrone MT, Lanzani C, Ferrandi M, Hamlyn JM, Cusi D, Galletti F, Bianchi G Salt Sensitivity Study Group of the Italian Society of Hypertension. Plasma ouabain-like factor during acute and chronic changes in sodium balance in essential hypertension. Hypertension. 2001;38:198–203. doi: 10.1161/01.hyp.38.2.198. [DOI] [PubMed] [Google Scholar]
- 7.Hamlyn JM, Manunta P. Endogenous ouabain: a link between sodium intake and hypertension. Curr Hypertens Rep. 2010;13:14–20. doi: 10.1007/s11906-010-0161-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manunta P, Stella P, Rivera R, Ciurlino D, Cusi D, Ferrandi M, Hamlyn JM, Bianchi G. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension. 1999;34:450–456. doi: 10.1161/01.hyp.34.3.450. [DOI] [PubMed] [Google Scholar]
- 9.Manunta P, Hamlyn JM, Simonini M, Messaggio E, Lanzani C, Bracale M, Argiolas G, Casamassima N, Brioni E, Glorioso N, Bianchi G. Endogenous ouabain and the renin–angiotensin–aldosterone system: distinct effects on Na handling and blood pressure in human hypertension. J Hypertens. 2011;29:349–356. doi: 10.1097/HJH.0b013e32833ea821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuznetsova T, Manunta P, Casamassima N, Messaggio E, Jin Y, Thijs L, Richart T, Fagard RH, Bianchi G, Staessen JA. Left ventricular geometry and endogenous ouabain in a Flemish population. J Hypertens. 2009;27:1884–1891. doi: 10.1097/HJH.0b013e32832e49a8. [DOI] [PubMed] [Google Scholar]
- 11.Bignami E, Casamassima N, Frati E, et al. Preoperative endogenous ouabain predicts acute kidney injury in cardiac surgery patients. Crit Care Med. 2013;41:744–755. doi: 10.1097/CCM.0b013e3182741599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bignami E, Casamassima N, Frati E, Messaggio E, Corno L, Zangrillo A, Manunta P. Endogenous Ouabain changes rapidly during cardiac pulmonary by pass. J Steroids Horm Sci. 2011:1–6. [Google Scholar]
- 13.Lee JW, Chou C-L, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014111067. ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanzani C, Citterio L, Glorioso N, et al. Adducin- and Ouabain-related gene variants predict the antihypertensive activity of Rostafuroxin, Part 2: clinical studies. Sci Transl Med. 2010;2:59ra87–59ra87. doi: 10.1126/scitranslmed.3001814. [DOI] [PubMed] [Google Scholar]
- 15.Mancia G, Fagard R, Narkiewicz K, et al. Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 16.Manunta P, Cusi D, Barlassina C, Righetti M, Lanzani C, D’Amico M, Buzzi L, Citterio L, Stella P, Rivera R, Bianchi G. Alpha-adducin polymorphisms and renal sodium handling in essential hypertensive patients. Kidney Int. 1998;53:1471–1478. doi: 10.1046/j.1523-1755.1998.00931.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferrandi M, Manunta P, Balzan S, Hamlyn JM, Bianchi G, Ferrari P. Ouabain-like factor quantification in mammalian tissues and plasma: comparison of two independent assays. Hypertension. 1997;30:886–896. doi: 10.1161/01.hyp.30.4.886. [DOI] [PubMed] [Google Scholar]
- 18.Manunta P, Ferrandi M, Bianchi G, Hamlyn JM. Endogenous ouabain in cardiovascular function and disease. J Hypertens. 2009;27:9–18. doi: 10.1097/HJH.0b013e32831cf2c6. [DOI] [PubMed] [Google Scholar]
- 19.Gault MH, Vasdev SC, Longerich LL, Fernandez P, Prabhakaran V, Dawe M, Maillet C. Plasma digitalis-like factor(s) increase with salt loading. N Eng J Med. 1983;309:1459–1459. doi: 10.1056/NEJM198312083092316. [DOI] [PubMed] [Google Scholar]
- 20.Manunta P. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men. Am J Physiol Regul Integr Comp Physiol. 2005;290:R553–R559. doi: 10.1152/ajpregu.00648.2005. [DOI] [PubMed] [Google Scholar]
- 21.Manunta P, Maillard M, Tantardini C, Simonini M, Lanzani C, Citterio L, Stella P, Casamassima N, Burnier M, Hamlyn JM, Bianchi G. Relationships among endogenous ouabain, alpha-adducin polymorphisms and renal sodium handling in primary hypertension. J Hypertens. 2008;26:914–920. doi: 10.1097/HJH.0b013e3282f5315f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J-G, Staessen JA, Messaggio E, Nawrot T, Fagard R, Hamlyn JM, Bianchi G, Manunta P. Salt, endogenous ouabain and blood pressure interactions in the general population. J Hypertens. 2003;21:1475–1481. doi: 10.1097/00004872-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Alderman MH, Madhavan S, Cohen H, Sealey JE, Laragh JH. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension. 1995;25:1144–1152. doi: 10.1161/01.hyp.25.6.1144. [DOI] [PubMed] [Google Scholar]
- 24.MacGregor GA, Markandu ND, Sagnella GA, Singer DR, Cappuccio FP. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet. 1989;2:1244–1247. doi: 10.1016/s0140-6736(89)91852-7. [DOI] [PubMed] [Google Scholar]
- 25.Coffman TM, Crowley SD. Kidney in Hypertension: Guyton Redux. Hypertension. 2008;51:811–816. doi: 10.1161/HYPERTENSIONAHA.105.063636. [DOI] [PubMed] [Google Scholar]
- 26.Hamlyn JM, Linde CI, Gao J, Huang BS, Golovina VA, Blaustein MP, Leenen FHH. Neuroendocrine humoral and vascular components in the pressor pathway for brain Angiotensin II: A new axis in long term blood pressure control. Plos One. 2014;9:e108916. doi: 10.1371/journal.pone.0108916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulanger BR, Lilly MP, Hamlyn JM, Laredo J, Shurtleff D, Gann DS. Ouabain is secreted by the adrenal gland in awake dogs. Am J Physiol. 1993;264:E413–419. doi: 10.1152/ajpendo.1993.264.3.E413. [DOI] [PubMed] [Google Scholar]
- 28.Tripodi G, Citterio L, Kouznetsova T, Lanzani C, Florio M, Modica R, Messaggio E, Hamlyn JM, Zagato L, Bianchi G, Staessen JA, Manunta P. Steroid biosynthesis and renal excretion in human essential hypertension: association with blood pressure and Endogenous Ouabain. Am J Hypertens. 2009;22:357–363. doi: 10.1038/ajh.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrari P. Rostafuroxin: An ouabain-inhibitor counteracting specific forms of hypertension. Biochim Biophys Acta - Molecular Basis of Disease. 2010;1802:1254–1258. doi: 10.1016/j.bbadis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 30.McCarron DA, Kazaks AG, Geerling JC, Stern JS, Graudal NA. Response to “Salt: the dying echoes of the food industry”. Am J Hypertens. 2014;27:282–284. doi: 10.1093/ajh/hpt230. [DOI] [PubMed] [Google Scholar]
- 31.Chamarthi B, Williams JS, Williams GH. A mechanism for salt-sensitive hypertension: abnormal dietary sodium-mediated vascular response to angiotensin-II. J Hypertens. 2010;28:1020–1026. doi: 10.1097/HJH.0b013e3283375974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollenberg NK, Williams GH. Non modulation and essential hypertension. Curr Hypertens Rep. 2006;8:127–131. doi: 10.1007/s11906-006-0008-9. [DOI] [PubMed] [Google Scholar]
- 33.Connell JMC, MacKenzie SM, Freel EM, Fraser R, Davies E. A lifetime of aldosterone excess: long-term consequences of altered regulation of aldosterone production for cardiovascular function. Endocr Rev. 2008;29:133–154. doi: 10.1210/er.2007-0030. [DOI] [PubMed] [Google Scholar]
- 34.Watt GC, Harrap SB, Foy CJ, Holton DW, Edwards HV, Davidson HR, Connor JM, Lever AF, Fraser R. Abnormalities of glucocorticoid metabolism and the renin-angiotensin system: a four-corners approach to the identification of genetic determinants of blood pressure. J Hypertens. 1992;10:473–482. doi: 10.1097/00004872-199205000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Rakova N, Jüttner K, Dahlmann A, et al. Long-term space flight simulation reveals infradian rhythmicity in human Na+ balance. Cell Metab. 2013;17:125–131. doi: 10.1016/j.cmet.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Roos JC, Boer P, Koomans HA, Geyskes GG, Dorhout Mees EJ. Haemodynamic and hormonal changes during acute and chronic diuretic treatment in essential hypertension. Eur J Clin Pharmacol. 1981;19:107–112. doi: 10.1007/BF00568396. [DOI] [PubMed] [Google Scholar]
- 37.Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu Rev Pharmacol Toxicol. 2008;48:33–60. doi: 10.1146/annurev.pharmtox.48.113006.094711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.