Abstract
Introduction
The optimal methodology for assessing comorbidity to predict various surgical outcomes such as mortality, readmissions, complications and failure to rescue (FTR) using claims data has not been established.
Objective
Compare diagnosis- and prescription-based comorbidity scores for predicting surgical outcomes.
Methods
We used 100% Texas Medicare data (2006–2011) and included patients undergoing coronary artery bypass grafting (CABG), pulmonary lobectomy, endovascular repair of abdominal aortic aneurysm, open repair of abdominal aortic aneurysm, colectomy, and hip replacement (N=39,616). The ability of diagnosis-based (Charlson comorbidity score, Elixhauser comorbidity score, Combined Comorbidity Score, Centers for Medicare & Medicaid Services-Hierarchical Condition Categories [CMS-HCC]) vs. prescription-based chronic disease (CDS) score in predicting 30-day mortality, 1-year mortality, 30-day readmission, complications, and FTR were compared using c-statistics (c) and integrated discrimination improvement (IDI).
Results
The overall 30-day mortality was 5.8%, 1-year mortality was 17.7%, 30-day readmission was 14.1%, complication rate was 39.7%, and FTR was 14.5%. CMS-HCC performed the best in predicting surgical outcomes (30-day mortality, c=0.791, IDI=4.59%; 1-year mortality, c=0.798, IDI=9.60%; 30-day readmission, c=0.630, IDI=1.27%; complications, c=0.766, IDI=9.37%; FTR, c=0.811, IDI=5.24%) followed by Elixhauser comorbidity index/disease categories (30-day mortality, c=0.750, IDI=2.37%; 1-year mortality, c=0.755, IDI=5.82%; 30-day readmission, c=0.629, IDI=1.43%; complications, c=0.730, IDI=3.99%; FTR, c=0.749, IDI=2.17%). Addition of prescription-based scores to diagnosis-based scores did not improve performance.
Conclusions
The CMS-HCC had superior performance in predicting surgical outcomes. Prescription-based scores, alone or in addition to diagnosis-based scores, were not better than any diagnosis-based scoring system.
Keywords: Surgery, Charlson comorbidity score, Elixhauser comorbidity score, chronic disease score, CMS-HCC, surgical outcomes
INTRODUCTION
In the hierarchy of study designs, randomized controlled trials (RCTs) are considered the gold standard because they can establish causal effect between the treatment and the outcome. However, surgical treatment decisions are half as likely to be based on RCTs compared to medical treatment decisions due to several unique aspects of surgery. (1) It is often challenging, or sometimes unethical, to randomize patients to surgical treatment or no treatment. Likewise, it is difficult to blind patients to receipt of surgery. In addition, the surgical learning curve and the complex process of coordinating care across disciplines and individuals make RCTs in surgery challenging. (2–4) As such, the use of observational studies to evaluate surgical outcomes research is increasing. (5)
Observational studies include the challenge of controlling for patient risk factors. (6) Patient selection based on comorbidity can significantly bias estimates of the treatment effect. As such, it is critical to control for comorbidity. The most commonly used diagnosis-based comorbidity scores include the Charlson comorbidity score (CCS) and the Elixhauser comorbidity score. (7, 8) The Combined Comorbidity Score, developed by combining disease conditions from Charlson and Elixhauser, was superior to either score alone in predicting mortality in Medicare patients. (9) The Centers for Medicare and Medicaid Services (CMS) have also developed a risk adjustment model, the CMS Hierarchical Condition Categories (CMS-HCC), to adjust capitation payments to health plans. (10) Prescription-based comorbidity scores include the chronic disease score (CDS). (11)
Previous studies have compared the ability of different claim-based methodologies to evaluate patient comorbidities. (12) However, no study has established the optimal methodology using claims data for assessing comorbidity in order to predict various surgical outcomes such as mortality, readmissions, complications and failure to rescue (FTR). The goal of our study was twofold: (i) to conduct a review of studies in surgery to describe the use of comorbidity sores, and (ii) to compare the performance of commonly used comorbidity scores in predicting mortality, readmission rates, complications and FTR in surgical patients.
METHODS
Use of Comorbidity Scores in Surgical Studies Using Medicare Claims Data
We performed a review of surgical studies to describe how comorbidity scores were used to control for confounding in Medicare claims data. In order to discuss results of our study in the context of Medicare data, we limited the literature review to Medicare data only. We selected articles from PubMed using following search criteria: “surgery” OR “surgical” AND “SEER” OR “Medicare”. All articles from 2012 onwards published in English were kept for further review (N=1,723). We included articles in the final review that met the following criteria: (i) the study was an original research article using Medicare data, (ii) the study included surgical patients or compared surgical interventions and (iii) the study controlled for comorbidities in multivariable models. Full text articles were reviewed and summarized for the use of comorbidity scores.
Assessment of the Performance of Comorbidity Scores
Data Source
In this retrospective study, we used 100% Texas Medicare claims data from 2006 to 2011. Medicare claims data collects patients’ demographic and enrollment information, outpatient visits, and inpatient visits. Specifically, we used the Medicare Provider Analysis and Review (MEDPAR) file, the outpatient file, the carrier file and the denominator file.
Study sample
The study sample included patients from 2007 to 2010 who underwent a primary surgical procedure for one of the following: coronary-artery bypass grafting (CABG), pulmonary lobectomy, endovascular repair of abdominal aortic aneurysm, open repair of abdominal aortic aneurysm, colectomy or hip replacement. (Appendix 1) We selected these procedures because they are common for Medicare beneficiaries and they reflect several surgical subspecialties (cardiac, thoracic, vascular, colorectal and orthopedic), enhancing the generalizability of results across the spectrum of surgical procedures. (13) Patients over 65 years of age who were continuously enrolled in Medicare part A, B and D without health maintenance organization (HMO) enrollment in the year prior to the surgery were included in the cohort.
Outcome
We included five commonly-used surgical outcomes:(i) 30-day mortality, defined as death within 30 days from the procedure date (in-hospital or after discharge); (ii) 1-year mortality, defined as mortality within 1 year of the procedure date; (iii) 30-day readmission, defined as readmission within 30 days from the index hospital discharge date; (iv) complications occurring in-hospital or within 30 days of the surgery date, defined using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes previously validated by chart review in The Complication Program; and (v) FTR, defined as the number deaths in patients who developed a postoperative complication (numerator) relative to the total number of patients who developed a postoperative complication (denominator). (13–16) In evaluating readmissions, we excluded patients who died during the index hospitalization or after admission to a non-prospective payment system hospital from both index and readmission causes. We considered eight major complications: pulmonary failure, pneumonia, myocardial infarction, deep venous thrombosis/pulmonary embolism, acute renal failure, hemorrhage, surgical site infection and gastrointestinal bleeding. (Appendix 1)
Covariates
Age, gender, race/ethnicity, type of surgical procedure, Medicare and Medicaid dual eligibility status and original reason for entitlement were included as covariates in this study.
Comorbidity scores
Diagnosis-based scores
Medicare inpatient and outpatient claims data were used to identify comorbidities. All claims in the year prior to the index surgery date were queried for the presence of comorbidities. We did not include comorbidities found only at the index hospitalization, to ensure that we captured comorbidities only, and not complications. Patients were considered as having a given comorbidity if they had (i) at least one diagnosis from the inpatient file or (ii) at least two distinct diagnoses recorded more than 30 days apart from the outpatient file. (17)
The Charlson comorbidity score (CCS) adapted for the use with claims data includes 17 disease conditions. (7, 18, 19) Weights are assigned to each disease condition and summed for each patient to obtain a single summary score. (20) Four different versions of CCS weights are available for prediction of 1-year mortality. (Appendix 2) The original weights for CCS were derived by Charlson et al. using all inpatient records from New York Hospital Cornell Medical Center and validated in breast cancer patients. (7) Schneeweiss et al. derived CCS weights using New Jersey Medicare data and validated their model using Pennsylvania Medicare data. (21) Quan et al. updated the CCS weights using hospital data from Canada and validated their model with hospital data from six different countries. (22, 23) The Royal College of Surgeons Charlson score included 14 disease conditions (peptic ulcer disease was omitted; mild and severe liver disease were combined; and diabetes with and without complications were combined) and assigned an equal weight to all conditions to develop a summary score. (24) We also constructed CCS by summing up the 17 disease conditions for each patient without applying any type of weights. Therefore, five different versions of summary CCS were constructed.
The Elixhauser comorbidity score includes 30 disease conditions. The original comorbidity index was not developed as a summary score, and it included 30 indicator variables for disease conditions. (8, 25) Walraven et al. used inpatient records from the Ottawa Hospital, Canada and derived weights for the Elixhauser comorbidity score to make it as a summary score. (26) We also constructed an Elixhauser score by summing the number of the 30 disease conditions for each patient.
The Combined Comorbidity Score was developed by including disease conditions from the Charlson and the Elixhauser comorbidity scores. The Combined Comorbidity Score includes 20 unique disease conditions from the possible 37 disease conditions obtained from Charlson and Elixhauser. Weights were derived for these disease conditions using Pennsylvania Medicare data and validated in New Jersey Medicare data to predict one year mortality. (9) We also constructed the Combined Comorbidity number of conditions for each patient by summing up the 20 disease conditions.
The CMS-HCC risk index was developed by the CMS to calculate the risk of adjustment to capitation payments to Medicare Advantage health plans. The CMS-HCC uses 87 HCCs (of a total of 189 HCCs) to predict Part A and Part B medical expenditures. In addition to 87 HCCs, it also uses certain HCC interactions, age-gender interactions, an indicator for at least one month of Medicaid enrollment in the base year and an indicator for original disability status. The CMS-HCC assigns expenditure-related weights to 87 HCC and other demographic variables to create a summary core. In this study, we used CMS-HCC version 21 using a publicly available SAS code. (10) We did not use the 87 indicator variables as this method would have limited application to smaller datasets or for patients with rare outcomes; data over fitting and lack of convergence can also be issues. CMS-HCC cannot be included as a count variable because it uses interactions of HCCs and demographic variables.
Prescription medication-based score
Medicare part D prescription claims data were used to define disease categories. The Red Book and American Hospital Formulary System (AHFS) were used to classify drugs into appropriate categories, each of which represents a disease condition.
The Chronic disease score (CDS) includes 29 disease conditions identified using prescription drug classes. The original CDS derived weights for total cost, outpatient cost and primary care visit weights. In this study, we used primary care visit weights to make a summary comorbidity score. Prior studies have used a physician-visit weight-based CDS to predict 1-year mortality. (11) We constructed the CDS number of disease conditions for each patient by summing up the 29 disease conditions. The CDS has been used in Medicare patients to predict different outcomes. (27, 28) However, no prior study has determined the performance of CDS in surgical patients for surgical outcomes.
Statistical analysis
Descriptive statistics were used to describe the study cohort and the distribution of summary comorbidity scores. Spearman correlation coefficients were used to describe the correlation between comorbidity scores.
Logistic regression models were constructed for five surgical outcomes. The baseline model included age, gender, race/ethnicity, type of surgical procedure, Medicare and Medicaid dual eligibility and original reason for entitlement. For each comorbidity score model, we included the baseline model covariates plus a particular comorbidity score. In addition to summary comorbidity score models, we also considered models in which comorbidities were entered as indicator variables, except for CMS-HCC. A total of 30 logistic regression models were constructed for each outcome (1-baseline model, 13-diagnosis-based score models, 3-prescription-based score model and 13-diagnosis plus prescription-based score models). (Appendix 3)
The performance of comorbidity scores were evaluated with c-statistics and integrated discrimination improvement (IDI). (29) The discrimination ability of all logistic models was also compared using c-statistics with asymptotic 95% confidence intervals. A general guideline to evaluate different models based on the c-statistics is as follows: 0.5=chance prediction; 0.7–0.8=acceptable; 0.8–0.9=excellent; 1.0=perfect prediction. (30) The IDI is the difference in discrimination slopes between two models, where the discrimination slope for a model is the mean difference in predicted probability of mortality between cases and controls. (31, 32) A higher IDI value indicates that the new model performs better than the referent model.
All statistical analyses were performed using SAS 9.4. This study was considered non-human subjects research and was deemed exempt by the Institutional Review Board at the University of Texas Medical Branch, Galveston.
RESULTS
Use of comorbidity scores in surgical studies
A total of 1,723 studies were identified using the initial search strategy; of these, 245 studies met the inclusion criteria. Fully 147 (60%) studies used Charlson comorbidity scores, 47 (19.2%) used Elixhauser comorbidity scores, 46 (18.8%) studies used some other method such as controlling clinically relevant comorbidities and 5 (2.0%) studies used the CMS-HCC score. None of the studies used a Combined Comorbidity Score or a prescription-based comorbidity score. Of the studies that used the Charlson comorbidity score, 8 (5.4%) used 17 disease conditions as separate covariates, 133 (90.5%) used the summary score and 6 (4.1%) used the total number of conditions. For studies using the Elixhauser comorbidity score, 34 (72.3%) used distinct disease conditions, 10 (21.3%) used the total number of conditions and 3 (6.4%) used a summary score. (Appendix 4)
Comparison of comorbidity scores
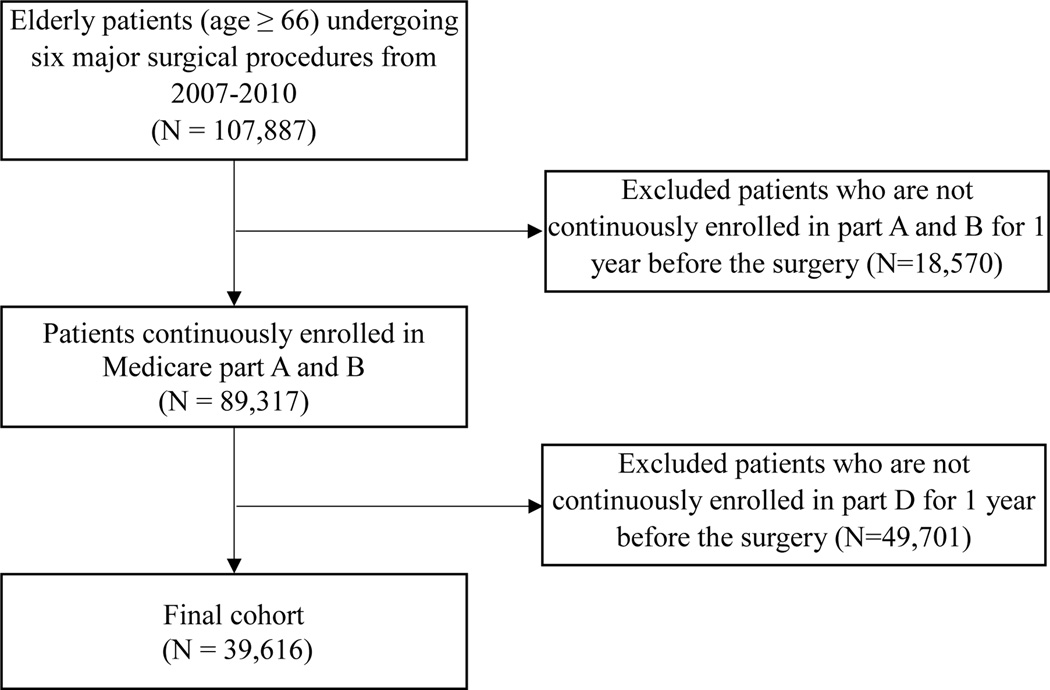
The study included 39,616 patients (Figure 1). The mean age of the cohort was 77.3 ± 7.5 years and 60.7% were females. The cohort predominantly consisted of non-Hispanic whites (86.3%), non-Hispanic Blacks (5.8%) and Hispanics (5.8%). Hip replacement was the most common procedure (44.4%), followed by colectomy (24.6%), CABG (21.8%), endovascular repair of abdominal aortic aneurysm (4.8%), lobectomy (2.9%) and open repair of abdominal aortic aneurysm (1.5%). The overall 30-day mortality was 5.8%, 1-year mortality was 17.7%, 30-day readmission was 14.1%, the complication rate was 39.7% and FTR was 14.5%. (Table 1)
Figure 1.
Cohort Selection
Table 1.
Descriptive Statistics of the Cohort
| Characteristic | Number (%) |
|---|---|
| N | 39,616 |
| Age, mean (SD) | 77.3 (7.5) |
| Race/ethnicity | |
| Non-Hispanic whites | 34,189 (86.3) |
| Non-Hispanic blacks | 2,312 (5.8) |
| Hispanics | 2,282 (5.8) |
| Others | 833 (2.1) |
| Female | 24,036 (60.7) |
| Surgery type | |
| Coronary-artery bypass grafting | 8,643 (21.8) |
| Lobectomy | 1,164 (2.9) |
| Endovascular repair of abdominal aortic aneurysm | 1,895 (4.8) |
| Open repair of abdominal aortic aneurysm | 575 (1.5) |
| Colectomy | 9,744 (24.6) |
| Hip replacement | 17,595 (44.4) |
| Medicare and Medicaid dual eligible patients | 10,944 (27.6) |
| Original reason for entitlement | |
| Old age | 34,812 (90.4) |
| Disability or end stage renal disease | 3,804 (9.6) |
| Outcomes | |
| 30-day mortality | 2,282 (5.8) |
| 1-year mortality | 6,991 (17.7) |
| 30-day readmissions† | 4,433 (14.1) |
| Complications | 15,718 (39.7) |
| Failure to rescue‡ | 2,282 (14.5) |
Denominator is 30,848. Patient died in the index hospitalization or admissions to non-prospective payment system hospital from both index and readmission were excluded (n=8,768).
Denominator is 15,718.
Table 2 reports the distribution of summary comorbidity scores. The mean score was highest for the Elixhauser summary score (4.7 ± 6.8) and lowest for the Royal College of Surgeon’s CCS (1.2 ± 1.1). More than 30% of patients had zero score for the CCS, Elixhauser comorbidity score or the Combined Comorbidity Score. In contrast, less than 10% of patients had a zero score for the CDS and no patient had a zero score for the CMS-HCC. Diagnosis-based comorbidity scores were highly correlated (ranging from 0.57 to 0.97), whereas the correlation between diagnosis- and prescription-based comorbidity scores was low to moderate (ranging from 0.14 to 0.32). (Appendix 5)
Table 2.
Distribution of Summary Comorbidity Scores in the Cohort
| Comorbidity Score | Mean (SD) | % with 0 | 25th Percentile |
Median | 75th Percentile |
Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| CCS original | 1.7 (2.0) | 34.9 | 0 | 1 | 3 | 0 | 17 |
| CCS/Schneeweiss | 2.3 (2.7) | 36.3 | 0 | 1 | 4 | 0 | 20 |
| CCS/Quan | 1.4 (1.9) | 51.6 | 0 | 0 | 2 | 0 | 15 |
| Royal College of Surgeons CCS | 1.6 (1.1) | 35.1 | 0 | 1 | 2 | 0 | 3 |
| Elixhauser summary score | 4.7 (6.8) | 35.3 | 0 | 2 | 7 | −11 | 49 |
| Combined Comorbidity Score | 1.2 (2.3) | 30.4 | 0 | 0 | 2 | −2 | 17 |
| CMS-HCC | 2.7 (1.7) | 0 | 1.4 | 2.2 | 3.5 | 0.3 | 15.4 |
| CDS | 2.4 (1.5) | 3.7 | 1.3 | 2.2 | 3.2 | −1.2 | 10.7 |
Abbreviations: CCS, Charlson comorbidity score; CMS-HCC, Center for Medicare & Medicaid – Hierarchical Conditions Categories; CDS, chronic disease score
Table 3 reports the c-statistics for different comorbidity scores. The baseline model which included age, gender, race/ethnicity, type of surgery, dual eligibility status and original reason for entitlement performed poorly in predicting 30-day mortality (c=0.698), 1-year mortality (0.689), 30-day readmission (c=0.591), complications (c=0.697) and FTR (c=0.701). The addition of any comorbidity score to the baseline model improved the model performance significantly. Among models in which individual disease conditions were included as indicator variables, Elixhauser consistently performed the best in predicting the five surgical outcomes studied, followed by the Combined Comorbidity Score. Among diagnosis- and prescription-based summary comorbidity measures, the CMS-HCC performed the best, followed by the Combined Comorbidity Score for all five surgical outcomes. Overall, the ranking of the top four comorbidity scores were as follows: CMS-HCC > Elixhauser disease categories > Combined Comorbidity Score disease categories > Combined Comorbidity Score. When comorbidities were controlled for (as the number of disease conditions in the Elixhauser or the Combined Comorbidity Score), the instruments performed poorly compared to their respective weighted comorbidity score; the CCS/number of disease conditions performed poorer than the CCS/Schneeweiss summary score. The performance of prescription-based summary comorbidity scores was no better than any diagnosis-based summary comorbidity scores. The c-statistics results showed that addition of CDS to diagnosis-based scores improved c-statistics by less than 3%. (Appendix 6) A large proportion of patients were excluded because of Part D eligibility criteria. In order to address concerns regarding generalizability, we tested the performance of diagnosis-based scores in 89,317 patients who met Part A and B criteria. The relative performance of diagnosis-based comorbidity scores remained the same in the 89,317 patients as it was in the 39,616 patients. (Appendixes 7 and 8)
Table 3.
Comparative Performance of Comorbidity Scores in Predicting Surgical Outcomes
| Comorbidity Score C-Statistics (95% CI) |
30-Day Mortality |
1-Year Mortality |
30-Day Readmission |
Complications | Failure To Rescue |
|---|---|---|---|---|---|
| Baseline model (age, gender, race/ethnicity, procedure type, dual eligibility status, original reason for entitlement) | 0.698 (0.687, 0.709) |
0.689 (0.682, 0.696) |
0.591 (0.582, 0.600) |
0.697 (0.692, 0.703) |
0.701 (0.689, 0.713) |
| Comorbidity scores with disease categories † | |||||
| Charlson disease categories | 0.742 (0.732, 0.752) |
0.746 (0.740, 0.753) |
0.624 (0.615, 0.633) |
0.726 (0.721, 0.731) |
0.741 (0.730, 0.752) |
| Elixhauser disease categories | 0.750 (0.740, 0.760) |
0.755 (0.749, 0.761) |
0.629 (0.620, 0.637) |
0.730 (0.725, 0.735) |
0.749 (0.738, 0.760) |
| Combined disease categories | 0.749 (0.739, 0.759) |
0.753 (0.747, 0.759) |
0.627 (0.618, 0.636) |
0.729 (0.724, 0.734) |
0.747 (0.736, 0.759) |
| CDS disease categories | 0.725 (0.715, 0.736) |
0.727 (0.721, 0.733) |
0.617 (0.609, 0.627) |
0.714 (0.709, 0.719) |
0.726 (0.715, 0.737) |
| Diagnosis-based summary comorbidity score ‡ | |||||
| CCS original | 0.729 (0.719, 0.739) |
0.737 (0.731, 0.743) |
0.615 (0.606, 0.624) |
0.715 (0.709, 0.720) |
0.729 (0.717, 0.740) |
| CCS/Schneeweiss | 0.735 (0.725, 0.746) |
0.742 (0.736, 0.749) |
0.620 (0.611, 0.629) |
0.719 (0.714, 0.724) |
0.735 (0.724, 0.746) |
| CCS/Quan | 0.730 (0.719, 0.741) |
0.737 (0.730, 0.744) |
0.614 (0.605, 0.623) |
0.713 (0.708, 0.718) |
0.730 (0.718, 0.741) |
| Royal College of Surgeons CCS§ | 0.731 (0.721, 0.741) |
0.734 (0.727, 0.740) |
0.616 (0.607, 0.625) |
0.717 (0.712, 0.722) |
0.731 (0.720, 0.742) |
| CCS/number of disease | 0.731 (0.721, 0.742) |
0.734 (0.728, 0.740) |
0.617 (0.608, 0.626) |
0.717 (0.712, 0.722) |
0.731 (0.720, 0.742) |
| Elixhauser summary score | 0.741 (0.731, 0.752) |
0.745 (0.739, 0.751) |
0.618 (0.609, 0.626) |
0.716 (0.711, 0.721) |
0.740 (0.729, 0.751) |
| Elixhauser/number of disease | 0.720 (0.710, 0.730) |
0.718 (0.711, 0.724) |
0.615 (0.607, 0.624) |
0.713 (0.708, 0.718) |
0.722 (0.711, 0.734) |
| Combined Comorbidity Score | 0.745 (0.735, 0.756) |
0.751 (0.745, 0.757) |
0.619 (0.610, 0.628) |
0.721 (0.716, 0.726) |
0.743 (0.732, 0.754) |
| Combined Comorbidity/number of disease | 0.728 (0.718, 0.738) |
0.728 (0.721, 0.734) |
0.616 (0.608, 0.625) |
0.716 (0.711, 0.721) |
0.730 (0.719, 0.741) |
| CMS-HCC | 0.797 (0.789, 0.806) |
0.798 (0.793, 0.803) |
0.630 (0.622, 0.639) |
0.766 (0.762, 0.771) |
0.811 (0.802, 0.820) |
| Prescription-based summary comorbidity score | |||||
| CDS | 0.700 (0.690, 0.710) |
0.696 (0.689, 0.702) |
0.603 (0.594, 0.612) |
0.703 (0.698, 0.708) |
0.704 (0.692, 0.716) |
| CDS/number of disease | 0.700 (0.690, 0.710) |
0.691 (0.684, 0.698) |
0.595 (0.586, 0.604) |
0.700 (0.695, 0.705) |
0.704 (0.692, 0.717) |
Abbreviations: CCS, Charlson comorbidity score; CMS-HCC, Center for Medicare & Medicaid – Hierarchical Conditions Categories; CDS, chronic disease score
Top 2 comorbidity scores from disease categories are highlighted in light gray color.
Top 2 comorbidity scores from diagnosis-based summary score are highlighted in light gray color.
Table 4 reports the IDI for comorbidities scores. All comorbidity scores were compared against the baseline model. The results of IDI were in accordance with the c-statistics results. The relative ranking of the top four comorbidity scores were as follows: CMS-HCC > Elixhauser disease categories > Combined Comorbidity Score disease categories > Combined Comorbidity Score.
Table 4.
Integrated Discrimination Improvement (IDI)of Comorbidity Scores in Predicting Surgical Outcomes
| Comorbidity Score IDI (%) |
30-Day Mortality |
1-Year Mortality |
30-Day Readmission |
Complications | Failure To Rescue |
|---|---|---|---|---|---|
| Baseline model (age, gender, race/ethnicity, procedure type, dual eligibility status, original reason for entitlement) | Ref | Ref | Ref | Ref | Ref |
| Comorbidity scores with disease categories † | |||||
| Charlson disease categories | 1.96 | 4.96 | 1.18 | 3.36 | 1.74 |
| Elixhauser disease categories | 2.37 | 5.82 | 1.43 | 3.99 | 2.17 |
| Combined disease categories | 2.31 | 5.66 | 1.36 | 3.85 | 2.07 |
| CDS disease categories | 1.24 | 3.17 | 0.96 | 1.91 | 1.05 |
| Diagnosis-based summary comorbidity score ‡ | |||||
| CCS original | 1.03 | 3.79 | 0.73 | 1.96 | 0.85 |
| CCS/Schneeweiss | 1.42 | 4.33 | 0.97 | 2.63 | 1.23 |
| CCS/Quan | 1.07 | 3.82 | 0.67 | 1.75 | 0.87 |
| Royal College of Surgeons CCS | 1.29 | 3.47 | 0.77 | 2.33 | 1.07 |
| CCS/number of disease | 1.30 | 3.47 | 0.79 | 2.36 | 1.08 |
| Elixhauser summary score | 1.71 | 4.74 | 0.90 | 2.27 | 1.51 |
| Elixhauser/number of disease | 0.71 | 1.96 | 0.78 | 1.84 | 0.63 |
| Combined Comorbidity Score | 1.88 | 5.45 | 1.00 | 2.82 | 1.60 |
| Combined Comorbidity/number of disease | 1.01 | 2.75 | 0.82 | 2.20 | 0.91 |
| CMS-HCC | 4.59 | 9.60 | 1.27 | 9.37 | 5.24 |
| Prescription-based summary comorbidity score | |||||
| CDS | 0.10 | 0.46 | 0.35 | 0.63 | 0.11 |
| CDS/number of disease | 0.06 | 0.11 | 0.10 | 0.23 | 0.07 |
Abbreviations: CCS, Charlson comorbidity score; CMS-HCC, Center for Medicare & Medicaid – Hierarchical Conditions Categories; CDS, chronic disease score
Top 2 comorbidity scores from disease categories are highlighted in light gray color.
Top 2 comorbidity scores from diagnosis-based summary score are highlighted in light gray color.
DISCUSSION
We compared the performance of commonly-used diagnosis- and prescription-based comorbidity scores in predicting 30-day mortality, 1-year mortality, 30-day readmission, complications and FTR in patients undergoing high-risk surgery. Our review of the use of comorbidity scores in surgical studies found that the majority of studies used Charlson comorbidity score; only five studies used CMS-HCC. Our study results showed that CMS-HCC performed the best in predicting all surgical outcomes, followed by Elixhauser disease categories, Combined Comorbidity Score disease categories and Combined Comorbidity Score.
A recent systematic review described the development and validation of comorbidity scores. The authors reviewed 76 articles; similar to our study, diagnosis-based measures performed better in predicting mortality, but prescription-based measures demonstrated better performance in predicting health care utilization. (33) Prior studies have shown that use of CCS+CDS, compared to CCS alone, improved c-statistics for mortality prediction by 1.7% and adjusted R2 in explaining healthcare expenditure variation by 25%. (27, 34) We found that, in surgical patients, diagnosis-based comorbidity scores performed better than prescription-based scores and the use of both did not offer any advantage in predicting surgical outcomes compared to diagnosis-based score alone.
Only three studies have compared the performance of comorbidity scores in surgical patients. Atherly et al. compared National Surgical Quality Improvement Program (NSQIP), DxCG and CCS in predicting mortality in surgery and concluded that NSQIP performed better (c=0.94) than DxCG (c=0.59) or CCS (c=0.52). (35) However, this is a case of comparing “apples to oranges”. NSQIP includes data on preoperative comorbidities, lab values and intraoperative events (45 preoperative and 17 intraoperative factors), whereas CCS only includes 17 comorbidities. (36) Two other studies compared Charlson and Elixhauser comorbidity scores in patients having orthopedic surgery and concluded that Elixhauser outperformed Charlson. (28, 37) In our study, Elixhauser had better performance compared to the Charlson comorbidity score.
Our results are consistent with findings evaluating comorbidity scores in patients with cardiovascular diseases, cancer, renal disease, respiratory disease and patients undergoing orthopedic surgery. (12) This meta-analysis (54 articles) compared the performance of diagnosis-based comorbidity indices using administrative data and the authors concluded that the Elixhauser comorbidity index performed better than CCS in predicting long-term mortality. In our study of surgical patients, when disease conditions were entered as indicator variables, Elixhauser performed better than the Combined Comorbidity Score and Charlson comorbidity score. Of disease conditions, the Elixhauser includes 30, the Combined Comorbidity Score includes 20 and the Charlson includes 17. This greater detail likely explains why the Elixhauser comorbidity measure performed the best. Ours is the first study that compared the ability of CMS-HCC to predict surgical outcomes. We found that the CMS-HCC score performed the best in predicting all surgical outcomes. Future studies evaluating surgical outcome using Medicare data can use the CMS-HCC to control for confounding due to comorbidity.
In the best case scenario, a summary score can perform only as well as individual disease categories. (38) A few studies have utilized CCS and Elixhauser by summing up the number of disease conditions without applying any kind of weights; such a method ignores the complexity of the various disease conditions. (39, 40) For example, patients with metastatic cancer and those with ulcer disease receive equal weight, a process which is intuitively incorrect. Therefore, it would appear to be better to use weights to construct a summary comorbidity score rather than using the number of disease conditions. Our study results support this argument, with the worst performance coming from scores summing unweighted comorbidities.
Weighted CCS summary score was the most popular measure for comorbidity control in surgical studies, with most studies using original CCS weights derived in 1987. (7) Four different versions of weights are available to construct the summary CCS. (Appendix 2) In our study, summary comorbidity scores performed slightly worse than comorbidity models that included indicator variables for individual disease conditions. Furthermore, the weights for comorbidity scores were not derived for surgical patients, which may explain the lower performance of summary scores in this population. We found that weights derived by Schneeweiss et al. using Medicare data performed better than other weights. (21) Prior studies have shown that a comorbidity score developed for specific a population and outcome of interest can perform better than generic scores. (12, 21, 30, 41)
Summary comorbidity scores can be very useful as they offer several advantages over other systems of measure. Summary comorbidity scores can be used to control for several comorbidities in smaller datasets without loss of statistical power. (26) The overall comorbidity burden of the cohort can be easily summarized using a single summary score, and summary scores allow investigators to model interactions between other variables and comorbidity as well as to construct time-dependent models of the comorbidity score. (42, 43) Furthermore, a summary comorbidity score can be easily incorporated when developing a prognostic nomogram. (44)
The study had the following limitations. We selected older adults and six major surgical procedures to represent the spectrum of surgical specialties. Therefore, results should be generalizable to older adults with similar surgical complexity. The study used the Medicare data from Texas and we recognize the differences in demographic differences between Texas and national population. However, the rates of outcomes and distribution of comorbidity scores are similar. (13) The CMS-HCC can be applied only to the Medicare data. The performance of comorbidity scores in other healthcare claims and electronic medical records datasets is yet to be tested. We did not include comorbidities from the index hospitalization to ensure that we do not inadvertently include complications. While including disease conditions from the index hospitalization may increase the overall predictive power of comorbidity scores, it runs the risk of misclassifying complications as comorbidities. Identifying complications from Medicare data without “present on admission” codes can be problematic. However, we chose to include a subset of complications that have high sensitivity and specificity. (14, 15, 45)
Conclusion
In conclusion, all diagnosis-based comorbidity scores performed better than prescription-based scores in predicting surgical outcomes, and combining both types of scores did not improve the performance. Based on the comparative performance of comorbidity scores, we recommend the use of CMS-HCC to control for confounding in surgical studies that use Medicare data.
Supplementary Material
Acknowledgments
We thank Sarah Toombs Smith, PhD, Science Editor and Assistant Professor in the Sealy Center on Aging, University of Texas Medical Branch at Galveston, for her editorial assistance.
Funding: Supported by grants from the UTMB Clinical and Translational Science Award #UL1TR000071, NIH T-32 Grant # 5T32DK007639, and AHRQ Grant # 1R24HS022134.
Footnotes
Author Disclosures: None
REFERENCES
- 1.Farrokhyar F, Karanicolas PJ, Thoma A, et al. Randomized controlled trials of surgical interventions. Ann Surg. 2010;251:409–416. doi: 10.1097/SLA.0b013e3181cf863d. [DOI] [PubMed] [Google Scholar]
- 2.McCulloch P, Taylor I, Sasako M, et al. Randomised trials in surgery: problems and possible solutions. BMJ. 2002;324:1448–1451. doi: 10.1136/bmj.324.7351.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macefield RC, Boulind CE, Blazeby JM. Selecting and measuring optimal outcomes for randomised controlled trials in surgery. Langenbecks Arch Surg. 2014;399:263–272. doi: 10.1007/s00423-013-1136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karanicolas PJ, Farrokhyar F, Bhandari M. Practical tips for surgical research: blinding: who, what, when, why, how? Can J Surg. 2010;53:345–348. [PMC free article] [PubMed] [Google Scholar]
- 5.Merkow RP, Ko CY. Evidence-based medicine in surgery: the importance of both experimental and observational study designs. JAMA. 2011;306:436–437. doi: 10.1001/jama.2011.1059. [DOI] [PubMed] [Google Scholar]
- 6.Brookhart MA, Sturmer T, Glynn RJ, et al. Confounding control in healthcare database research: challenges and potential approaches. Med Care. 2010;48:S114–S120. doi: 10.1097/MLR.0b013e3181dbebe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 8.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Gagne JJ, Glynn RJ, Avorn J, et al. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.2013 Model Software/ICD-9-CM Mappings. [Accessed August 1, 2015]; Available at: https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Risk-Adjustors-Items/Risk2013.html.
- 11.Clark DO, Von Korff M, Saunders K, et al. A chronic disease score with empirically derived weights. Med Care. 1995;33:783–795. doi: 10.1097/00005650-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50:1109–1118. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 13.Tsai TC, Joynt KE, Orav EJ, et al. Variation in surgical-readmission rates and quality of hospital care. N Engl J Med. 2013;369:1134–1142. doi: 10.1056/NEJMsa1303118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250:1029–1034. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 15.Sheetz KH, Dimick JB, Ghaferi AA. The association between hospital care intensity and surgical outcomes in medicare patients. JAMA surgery. 2014;149:1254–1259. doi: 10.1001/jamasurg.2014.552. [DOI] [PubMed] [Google Scholar]
- 16.Tamirisa NP, Parmar AD, Vargas GM, et al. Relative Contributions of Complications and Failure to Rescue on Mortality in Older Patients Undergoing Pancreatectomy. Ann Surg. 2015 doi: 10.1097/SLA.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 18.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute. SEER-Medicare: Calculation of comorbidity weights. [Accessed August 1, 2015];2014 Available at: http://appliedresearch.cancer.gov/seermedicare/program/comorbidity.html.
- 21.Schneeweiss S, Wang PS, Avorn J, et al. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38:1103–1120. doi: 10.1111/1475-6773.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 24.Armitage JN, van der Meulen JH, Royal College of Surgeons Co-morbidity Consensus G. Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. The British journal of surgery. 2010;97:772–781. doi: 10.1002/bjs.6930. [DOI] [PubMed] [Google Scholar]
- 25.Healthcare Cost and Utilization Project (HCUP) HCUP Comorbidity Software. [Accessed August 1, 2015];2014 Available at: http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp.
- 26.van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 27.Schneeweiss S, Seeger JD, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154:854–864. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 28.Gordon M, Stark A, Skoldenberg OG, et al. The influence of comorbidity scores on re-operations following primary total hip replacement: comparison and validation of three comorbidity measures. The bone & joint journal. 2013;95-B:1184–1191. doi: 10.1302/0301-620X.95B9.31006. [DOI] [PubMed] [Google Scholar]
- 29.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iezzoni LI. Risk adjustment for measuring health care outcomes. Chicago, Ill: Health Administration Press; 2013. [Google Scholar]
- 31.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 32.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yurkovich M, Avina-Zubieta JA, Thomas J, et al. A systematic review identifies valid comorbidity indices derived from administrative health data. J Clin Epidemiol. 2014 doi: 10.1016/j.jclinepi.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Baser O, Palmer L, Stephenson J. The estimation power of alternative comorbidity indices. Value Health. 2008;11:946–955. doi: 10.1111/j.1524-4733.2008.00343.x. [DOI] [PubMed] [Google Scholar]
- 35.Atherly A, Fink AS, Campbell DC, et al. Evaluating alternative risk-adjustment strategies for surgery. Am J Surg. 2004;188:566–570. doi: 10.1016/j.amjsurg.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 36.ACS NSQIP. [Accessed August 1, 2015];2014 Available at: http://site.acsnsqip.org/. [Google Scholar]
- 37.Menendez ME, Neuhaus V, van Dijk CN, et al. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472:2878–2886. doi: 10.1007/s11999-014-3686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin SR, Wong YN, Uzzo RG, et al. Why Summary Comorbidity Measures Such As the Charlson Comorbidity Index and Elixhauser Score Work. Med Care. 2013 doi: 10.1097/MLR.0b013e318297429c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evan Pollack C, Wang H, Bekelman JE, et al. Physician social networks and variation in rates of complications after radical prostatectomy. Value Health. 2014;17:611–618. doi: 10.1016/j.jval.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schootman M, Lian M, Pruitt SL, et al. Hospital and geographic variability in thirty-day all-cause mortality following colorectal cancer surgery. Health Serv Res. 2014;49:1145–1164. doi: 10.1111/1475-6773.12171a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng C, Ellis JL, Steiner JF, et al. Assessment of morbidity over time in predicting health outcomes. Med Care. 2014;52(Suppl 3):S52–S59. doi: 10.1097/MLR.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang CY, Baldwin LM, Saver BG, et al. The contribution of longitudinal comorbidity measurements to survival analysis. Med Care. 2009;47:813–821. doi: 10.1097/MLR.0b013e318197929c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parmar AD, Sheffield KM, Adhikari D, et al. PREOP-Gallstones: A Prognostic Nomogram for the Management of Symptomatic Cholelithiasis in Older Patients. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994;32:700–715. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.