Summary
The filamentous meshwork formed by the lamin nucleoskeleton provides a scaffold for the anchoring of highly condensed heterochromatic DNA to the nuclear envelope, thereby establishing the three-dimensional architecture of the genome [1]. Insight into the importance of lamins to cellular viability can be gleaned from the laminopathies, severe disorders caused by mutations in genes encoding lamins. A cellular consequence of lamin dysfunction in laminopathies is relaxation of heterochromatic DNA [1]. Similarly, we have recently reported the widespread relaxation of heterochromatin in tauopathies [1]: age-related progressive neurodegenerative disorders, including Alzheimer’s disease, which are pathologically characterized by aggregates of phosphorylated tau protein in the brain [2, 3]. Here we demonstrate that acquired lamin misregulation though aberrant cytoskeletal-nucleoskeletal coupling promotes relaxation of heterochromatin and neuronal death in an in vivo model of neurodegenerative tauopathy. Genetic manipulation of lamin function significantly modifies neurodegeneration in vivo, demonstrating that lamin pathology plays a causal role in tau-mediated neurotoxicity. We show that lamin dysfunction is conserved in human tauopathy, as super-resolution microscopy reveals a significantly disrupted nuclear lamina in postmortem tissue from human Alzheimer’s disease brain. Our study provides strong evidence that tauopathies are neurodegenerative laminopathies, and identifies a new pathway mediating neuronal death in currently untreatable human neurodegenerative disorders, including Alzheimer’s disease.
Results
The model organism Drosophila provides a genetically tractable platform that can be used to identify and validate molecular mechanisms. In addition, panneuronal expression of transgenic human tau in Drosophila (Figure S1A) recapitulates aspects of human tauopathies such as heterochromatin relaxation [4], DNA damage [5], activation of the cell cycle in postmitotic neurons [6], synapse loss [7], and progressive neurodegeneration [8]. Since pathogenic tau induces heterochromatin relaxation [4], and the lamin nucleoskeleton regulates chromatin dynamics [1], we investigated a potential role for lamin dysfunction in tauopathy. We first determined if lamin levels are altered in the brains of adult Drosophila panneuronally expressing a disease-associated mutant form of human tau [9], tauR406W. Like vertebrate B-type lamin, Drosophila “Lamin” is expressed in most cells and developmental stages, and has a CaaX box that targets it to the nuclear membrane [10]. In 10-day-old tauR406W transgenic Drosophila heads, Lamin protein levels were significantly decreased (Figure 1A), whereas protein levels of Drosophila A-type lamin, termed “Lamin C,” were not affected (Figure S1B). Since Lamin C was unchanged in tauR406W transgenic Drosophila, we focused subsequent studies on the Drosophila B-type lamin.
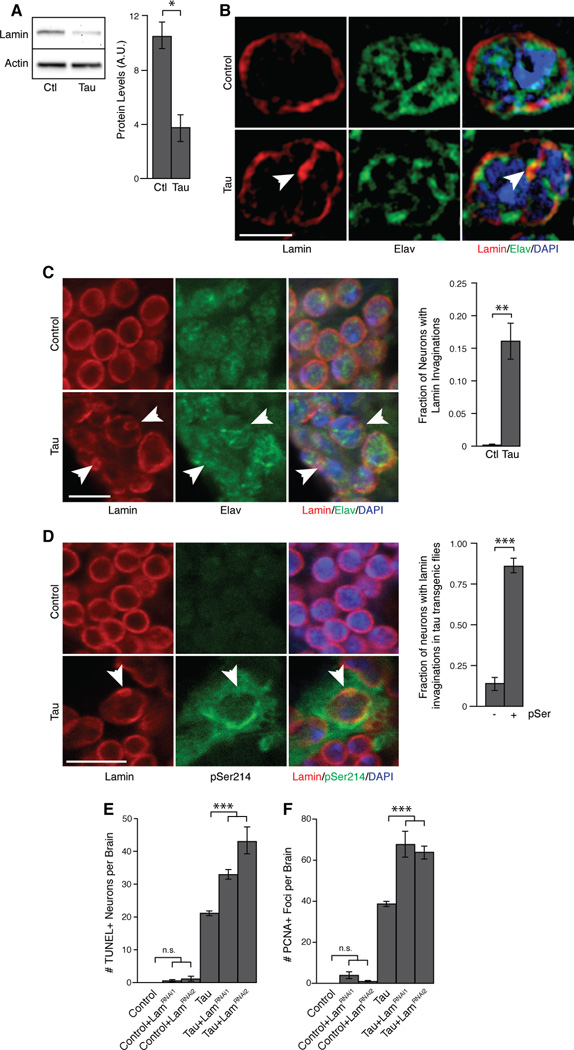
Figure 1. Lamin pathology in tau transgenic Drosophila.
(A) Western blot for Lamin in homogenates from control and tau transgenic Drosophila heads, n=3.
(B) Super-resolution microscopy of Lamin and elav immunostaining in control and tau transgenic Drosophila brains. Arrowhead indicates Lamin invagination.
(C) Immunostaining of Lamin and elav in control and tau transgenic Drosophila brains. Arrowheads indicate neurons with Lamin invaginations, n=3.
(D) Immunostaining of Lamin and tau phosphorylated at serine 214 in control and tau transgenic Drosophila brains. Arrowhead indicates Lamin invagination in a pSer214-positive neuron, n=3.
(E) Neuronal degeneration assayed by TUNEL staining in brains of control and tau transgenic Drosophila harboring RNAi transgenes targeted to Lamin, n=6.
(F) Cell cycle activation assayed by PCNA staining in brains of control and tau transgenic Drosophila harboring RNAi transgenes targeted to Lamin, n=6.
All flies are 10 days old. Controls are elav-GAL4/+. Scale bars are 1 µm in (B) and 5 µm in (C) and (D). Data are presented as mean ± SEM, unpaired t-test or ANOVA, **p<0.01, ***p<0.001. See also Figure S1.
Abnormal nuclear morphology, including invagination of the nuclear envelope, is common in cells from laminopathy patients [11]. Similarly, we observed invaginations of the nuclear envelope in tauR406W transgenic Drosophila. Costaining with elav, a neuron-specific protein, indicated that nuclei harboring invaginations were neuronal (Figure 1B–C). In addition to decreased Lamin protein levels in tau transgenic Drosophila brains, morphological changes of the lamin nucleoskeleton suggest that tau causes dysfunction of neuronal Lamin. We next determined if Lamin invaginations coincide with pathological tau. Tau phosphorylation is a well-characterized pathogenic event in Alzheimer’s disease and related tauopathies [12]. 86% of nuclei containing Lamin invaginations in tauR406W transgenic Drosophila were positive for tau phosphorylated at serine 214, a disease-associated tau phosphopepitope (Figure 1D). Taken together, these data suggest that pathological tau reduces Lamin protein levels and causes lamin dysfunction by altering the three-dimensional morphology of the lamin nucleoskeleton.
To determine whether Lamin reduction and disorganization are specific to tauR406W versus a general feature of tau pathology, we utilized Drosophila transgenic for human wild-type tau (tauWT) [8] or a pseudohyperphosphorylated form of human tau (tauE14) [13]. Panneuronal expression of tauWT or tauE14 significantly reduced Lamin levels in adult Drosophila brains (Figure S1C–D), and caused Lamin to invaginate (Figure S1E–F). The extent of Lamin reduction and invagination caused by expression of tauWT or tauE14 correlated with their toxicities, which cause substantially less and more, respectively, neuronal toxicity than tauR406W [8, 13]. These data suggest that Lamin pathology is a general feature of tau-induced toxicity and is downstream of aberrant tau phosphorylation. Since expression of tauR406W provides a level of toxicity that is well suited for genetic manipulation and biochemical analysis [8], 10-day-old tauR406W transgenic flies were used in our subsequent experiments, and are referred to as tau hereafter for simplicity.
We next determined if Lamin dysfunction plays a causal role in promoting cell death. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) detects DNA fragmentation, and has been used to quantify neuronal death in tau transgenic Drosophila [4, 6, 14]. Further depletion of Lamin levels in neurons of tau transgenic Drosophila by RNAi-mediated reduction of Lamin significantly increased neuronal death compared to tau expressed alone (Figure 1E). As an additional control, we measured TUNEL levels in control and tau transgenic Drosophila with RNAi targeting luciferase (Figure S1G). We next determined if Lamin depletion activates the cell cycle in postmitotic neurons of tau transgenic flies. Ectopic expression of cell cycle markers in postmitotic neurons and their coincidence with tau pathology is a well-described feature of tauopathies [15, 16], and abnormal activation of the cell cycle in neurons can drive apoptosis in tau transgenic Drosophila [6]. Based on staining with proliferating cell nuclear antigen (PCNA), we found a significant increase in ectopic cell cycle activation in tau transgenic Drosophila brains when Lamin was panneuronally depleted via RNAi (Figure 1F). RNAi-mediated Lamin reduction enhanced locomotor defects [4] and shortened median lifespan [8] of tau transgenic Drosophila (Figure S1H–I), suggesting that Lamin depletion affects the overall health of tau transgenic Drosophila. We validated Lamin mRNA knockdown (Figure S1J), and Lamin protein knockdown in control and tau transgenic Drosophila heads (Figure S1K–S1L). RNAi targeted to Lamin did not change total levels of transgenic tau protein (Figure S1M). Together, these genetic manipulations are consistent with a causal role for tau-induced lamin dysfunction in driving cell cycle activation and subsequent apoptotic cell death of postmitotic neurons.
To determine if Lamin reduction and disorganization are specific to tauopathy versus a general consequence of neurodegeneration, we utilized a Drosophila model of spinocerebellar ataxia type 3 (SCA3; Machado-Joseph disease) [17]. We did not detect reduced Lamin levels or a disrupted Lamin nucleoskeleton in brains of adult SCA3 model Drosophila (Figure S1N–O). As previously reported, we observed loss of Kenyon cells, the projection neurons of the mushroom bodies, in brains of SCA3 model Drosophila [18]; however, genetic reduction of Lamin did not exacerbate Kenyon cell loss (Figure S1P). These data suggest that Lamin reduction and disorganization are not a result of general neurodegeneration.
While knockout of B-type lamins in the mouse forebrain reduces neuronal number [19], the effects of lamin dysfunction in the adult brain are not well characterized. We thus determined whether Lamin pathology can drive heterochromatin relaxation, DNA damage, cell cycle activation, and apoptosis in neurons. Since modest reduction of Lamin levels mediated by transgenic RNAi was not toxic in the absence of transgenic human tau expression, while null or strong Lamin mutations are reported to be lethal or semilethal [20, 21], we used a homozygous partial loss of function allele, LamA25, to genetically induce Lamin dysfunction in Drosophila. LamA25 removes sequences encoding the CaaX domain responsible for localizing Lamin to the inner nuclear membrane [22] (Figure S2A). Like pathological tau [4], Lamin dysfunction significantly reduced total levels of dimethylated lysine 9 of histone 3 (H3K9me2) and Heterochromatin Protein 1α (HP1α), markers of heterochromatic DNA, in adult Drosophila brains (Figure 2A). Like many other cell types, Drosophila neurons possess cytologically distinct regions of DAPI-dense heterochromatic DNA termed “chromocenters.” We observed loss of chromocenter staining in neurons of LamA25 mutant Drosophila (Figure 2B–C, Figure S2B–C), suggesting that, like pathological tau [4], Lamin dysfunction disrupts genomic architecture in neurons. We have previously reported that tau-induced relaxation of heterochromatin causes increased expression of genes in the brain that are normally silenced by heterochromatin [4]. Three of the six genes that we previously reported as most significantly affected by heterochromatin relaxation in tau transgenic fly heads [4] were also expressed at higher levels in LamA25 mutant fly heads (Figure S2D). Since low baseline gene expression causes variability in RT-PCR, it is possible that modest increases in gene expression were undetected. DNA damage is a cellular hallmark of tauopathies in both Drosophila and humans [5, 16], and is a known consequence of heterochromatin relaxation [23]. Significantly more neurons of flies expressing transgenic tau or harboring the LamA25 mutation had DNA damage compared to control (Figure 2D). In tau transgenic flies, all nuclei harboring DNA damage also had a lamin invagination (Figure S2E), providing further evidence of the strong connection between lamin pathology and DNA damage in adult neurons.
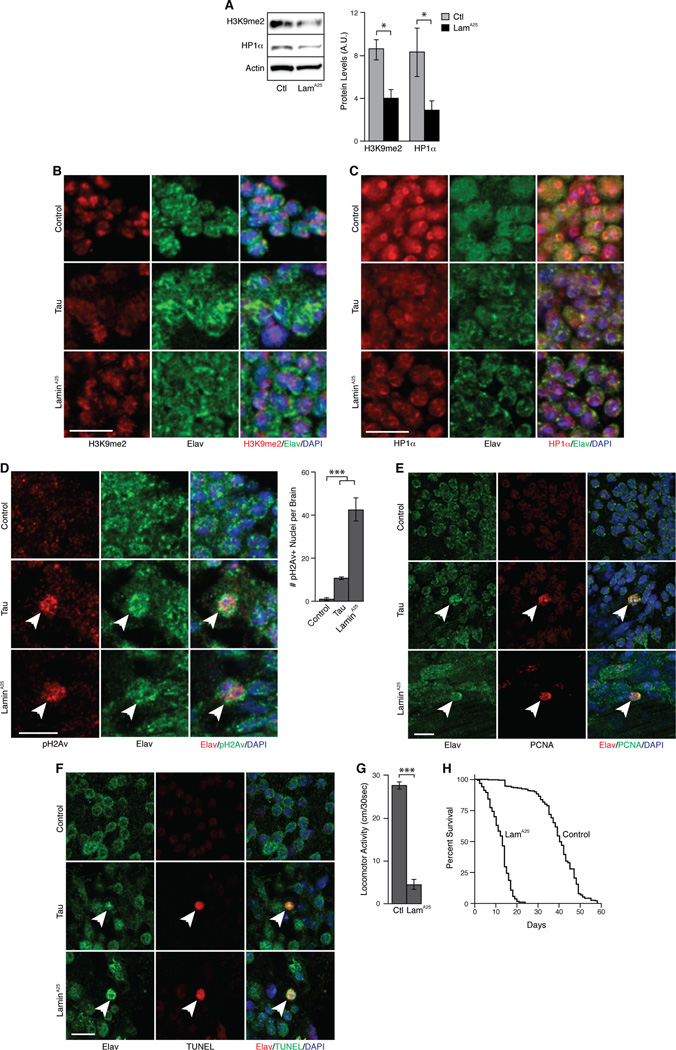
Figure 2. Lamin dysfunction causes neurodegeneration, heterochromatin relaxation, and DNA damage in the adult Drosophila brain.
(A) Western blot for markers of heterochromatin in homogenates from control and LamA25 mutant Drosophila heads, n=3.
(B) Neuronal heterochromatin assayed by immunostaining of H3K9me2 and elav in control, tau transgenic, and LamA25 mutant fly brains.
(C) Neuronal heterochromatin assayed by immunostaining of HP1α and elav in control, tau transgenic, and LamA25 mutant fly brains.
(D) Neuronal DNA damage assayed by immunostaining of pH2Av and elav in control, tau transgenic, and LamA25 mutant fly brains. Arrows indicate DNA damage in elav-positive neurons, n=6.
(E) Neuronal cell cycle activation assayed by immunostaining of control, tau transgenic, and LamA25 mutant fly brains with elav and PCNA. Arrows indicate cell cycle activation in Elav-positive neurons. n=6.
(F) Neuronal degeneration assayed by immunostaining of control, tau transgenic, and LamA25 mutant fly brains with elav and TUNEL. Arrows indicates TUNEL staining in elav-positive neurons. n=6.
(G) Locomotor activity of control and LamA25 mutant Drosophila. n=18.
(H) Lifespan of control and LamA25 mutant Drosophila. n=300, p<0.0001, log-rank test. Flies are 10 days old except in (H). Control is w1118 in (A, G, and H) and elav-GAL4/+ in (B–F). LamA25 mutants are homozygous. Scale bars are 5 µm. Data are presented as mean ± SEM, t-test or ANOVA unless otherwise stated, *p<0.05, ***p<0.001. See also Figure S2.
Having established that Lamin dysfunction is sufficient to cause both heterochromatin relaxation and DNA damage in neurons, we next asked if Lamin pathology induces neuronal cell cycle activation and apoptosis. Both PCNA (Figure 2E, average of 23.5 in six LamA25 mutant brains, SEM=4.91, control=0) and phosphorylated histone 3 (Figure S2F) staining of adult fly brains indicated that Lamin misregulation causes ectopic activation of the cell cycle in postmitotic neurons. Similarly, TUNEL staining revealed neuronal apoptosis in LamA25-mutant brains (Figure 2F, average of 87.5 in six LamA25 mutant brains, SEM=10.46, control=0), demonstrating that blocking incorporation of Lamin into the nuclear envelope triggers cell death in postmitotic neurons. LamA25 mutant animals also had significantly reduced locomotor activity (Figure 2G) and lifespan (Figure 2H), both of which are features of tauopathy [4, 8, 24].
As additional evidence that lamin pathology lies in the pathway of tau-induced neurotoxicity, heterozygous LamA25 mutation synergizes with transgenic tau in the brain to induce significantly more neurodegeneration (Figure S2G) and neuronal cell cycle activation (Figure S2H) than either does alone. Similarly, in the background of heterozygous LamA25 mutation, transgenic tau causes further heterochromatin relaxation (Figure S2I). Together, these experiments provide strong evidence that Lamin dysfunction is upstream of heterochromatin relaxation, DNA damage, neuronal cell cycle activation, and apoptosis in the pathway of tau-induced neurotoxicity.
We next investigated the mechanism by which pathological tau reduces Lamin protein levels and alters its nuclear distribution. In addition to its role as a microtubule-binding protein, tau also binds actin and induces overstabilization and bundling of filamentous actin (F-actin) in tauopathy [25, 26]. Genetically reversing actin overstabilization significantly suppresses tau neurotoxicity [14, 26], demonstrating that overstabilization of F-actin is a causal event in tau-induced neurodegeneration. Since the actin cytoskeleton has intimate connections to the lamin nucleoskeleton through the nuclear envelope-spanning linker of nucleoskeleton and cytoskeleton (LINC) complex [27], we determined if manipulating levels of F-actin affects the nuclear lamina. In tau transgenic Drosophila brains, reversing F-actin stabilization by overexpressing Gelsolin [14], an actin-severing protein, significantly increased Lamin protein levels (Figure 3A–B), strongly supporting the hypothesis that tau-induced actin stabilization drives neurotoxicity by disrupting the nuclear lamina. Gelsolin overexpression significantly decreased the number of neurons harboring disruptions in the nuclear lamina in tau transgenic Drosophila (Figure 3C). In addition, Gelsolin overexpression significantly improved locomotion and prolonged the median lifespan of tau transgenic Drosophila (Figure S3A–B), consistent with our previous finding that Gelsolin overexpression reduces tau-induced apoptosis in Drosophila brains [14]. To further test if stabilization of F-actin disrupts Lamin levels and localization, we promoted formation of Factin in neurons by overexpressing WASp (Figure S3C) or the RD domain of spire (Figure S3D) [28, 29] in the absence of transgenic tau. Like transgenic expression of human tau, ectopic stabilization of F-actin in neurons significantly decreased Lamin protein levels in the brain (Figures 3D–E). In addition, genetically promoting actin polymerization induced invagination of the nuclear lamina (Figure 3F). We observed concentrations of F-actin [30, 31] in close proximity to Lamin invaginations in tau transgenic flies (Figures 3G–H), supporting the hypothesis that tau-induced stabilization of actin filaments causes local disruptions of the nuclear lamina. In addition to regulating actin dynamics in the cytoplasm, nuclear Gelsolin, WASp, or spire [32–34] could potentially affect Lamin levels and/or morphology.
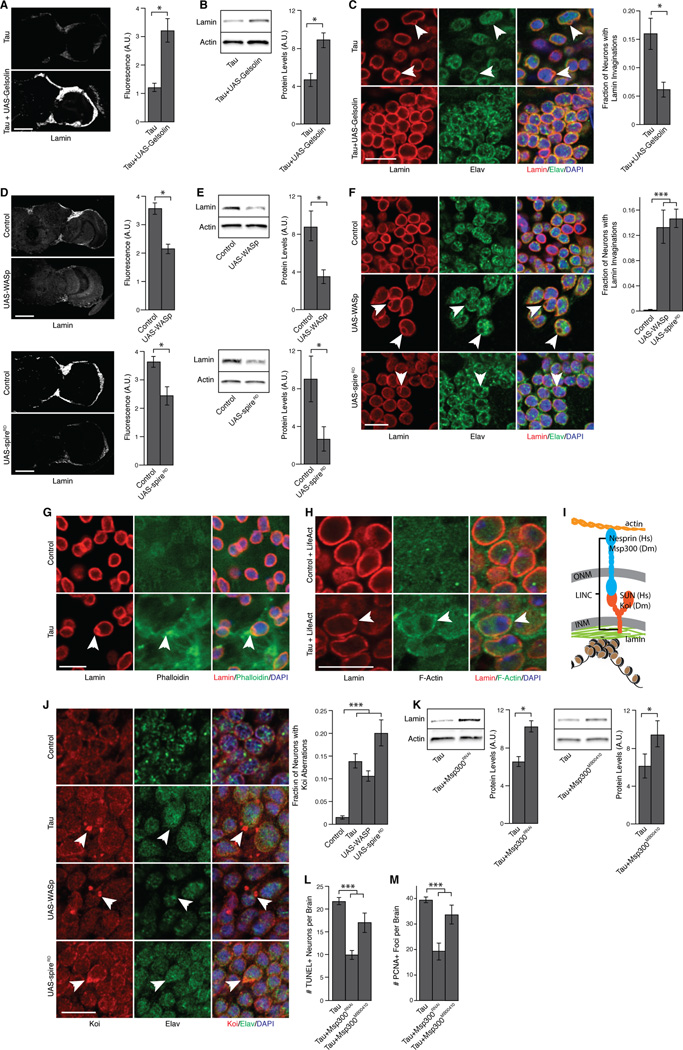
Figure 3. Tau disrupts lamin via stabilization of F-actin and LINC complex dysfunction in Drosophila.
(A) Lamin immunofluorescence in cortical neurons of tau transgenic flies with and without overexpression of Gelsolin, n=3.
(B) Lamin protein levels in brains of tau transgenic flies with and without overexpression of Gelsolin, n=3.
(C) Immunofluorescence of Lamin in elav-positive neurons of tau transgenic flies with and without overexpression of Gelsolin, n=3, arrows indicate Lamin invaginations, n=3.
(D) Lamin immunofluorescence in cortical neurons of Drosophila overexpressing WASp or spireRD, n=3.
(E) Lamin protein levels in brains of Drosophila overexpressing WASp or spireRD, n=3.
(F) Immunofluorescence of Lamin in elav-positive neurons of Drosophila overexpressiong WASp or spireRD, n=3, arrows indicate Lamin invaginations.
(G) Immunostaining of Lamin and phalloidin in control and tau transgenic fly brains, arrow indicates Lamin disruption adjacent to F-actin.
(H) Immunostaining of Lamin and GFP in control and tau model Drosophila transgenic for a GFP-based reporter of F-actin, arrow indicates Lamin disruption adjacent to F-actin.
(I) Schematic diagram of the LINC complex.
(J) Immunostaining of koi in control, tau transgenic, and WASp- and spireRD-overexpressing fly brains, n=3, arrows indicate altered koi distribution in elav-positive neurons, n=3.
(K) Lamin protein levels in brains of tau transgenic Drosophila versus tau transgenic Drosophila with Msp300MB00410 or RNAi targeted to Msp300, n=3.
(L) Neuronal apoptosis assayed by TUNEL staining in tau transgenic Drosophila versus tau transgenic Drosophila with Msp300MB00410 or RNAi targeted to Msp300, n=6.
(M) Cell cycle activation assayed by PCNA staining in tau transgenic Drosophila versus tau transgenic Drosophila with Msp300MB00410 or RNAi targeted to Msp300, n=6.
Control is elav-GAL4/+. All flies are 10 days old. Scale bars are 30 µm in (A) and (D), and 5 µm in (C), (F–H), and (J). Data are presented as mean ± SEM, unpaired t-test or ANOVA, *p<0.05, **p<0.01, ***p<0.001. See also Figure S3.
Since the LINC complex acts as a physical bridge between the actin cytoskeleton and the lamin nucleoskeleton, we determined if the LINC complex facilitates F-actin-induced disruption of the lamin nucleoskeleton in tauopathy. In Drosophila, Lamin binds directly to LINC complex component koi, the Drosophila homolog of human SUN1 [35]. Koi traverses the inner nuclear envelope and binds Msp300, the Drosophila homolog of human nesprin, in the perinuclear space. Together, koi and Msp300 span the nuclear envelope, and Msp300 binds F-actin in the cytoplasm [36] (Figure 3I). Overstabilizing F-actin via transgenic tau or overexpression of WASp or spireRD altered the distribution of neuronal koi. While koi lined the nuclear envelope in neurons of control brains, we observed a significant increase in koi-positive blebs and focal concentrations of koi along the nuclear envelope in tau transgenic and WASp or spireRD-overexpressing neurons (Figure 3J). Reducing the interaction between F-actin and the LINC complex by RNAi-mediated reduction of Msp300 (Figure S3E) or a transposable element insertion into Msp300, Msp300MB00410 [37], significantly increased Lamin levels in tau transgenic Drosophila brains (Figure 3K) and reduced neuronal apoptosis and aberrant cell cycle activation (Figure 3L–M). Neither of these suppressors of tau toxicity altered levels of total transgenic tau protein (Figure S3F). Collectively, these experiments provide strong support for a model in which tau-induced overstabilization of F-actin disrupts LINC complex organization and reduces lamin levels and localization, which promotes heterochromatin relaxation, subsequent aberrant cell cycle activation and neurodegeneration in tauopathy.
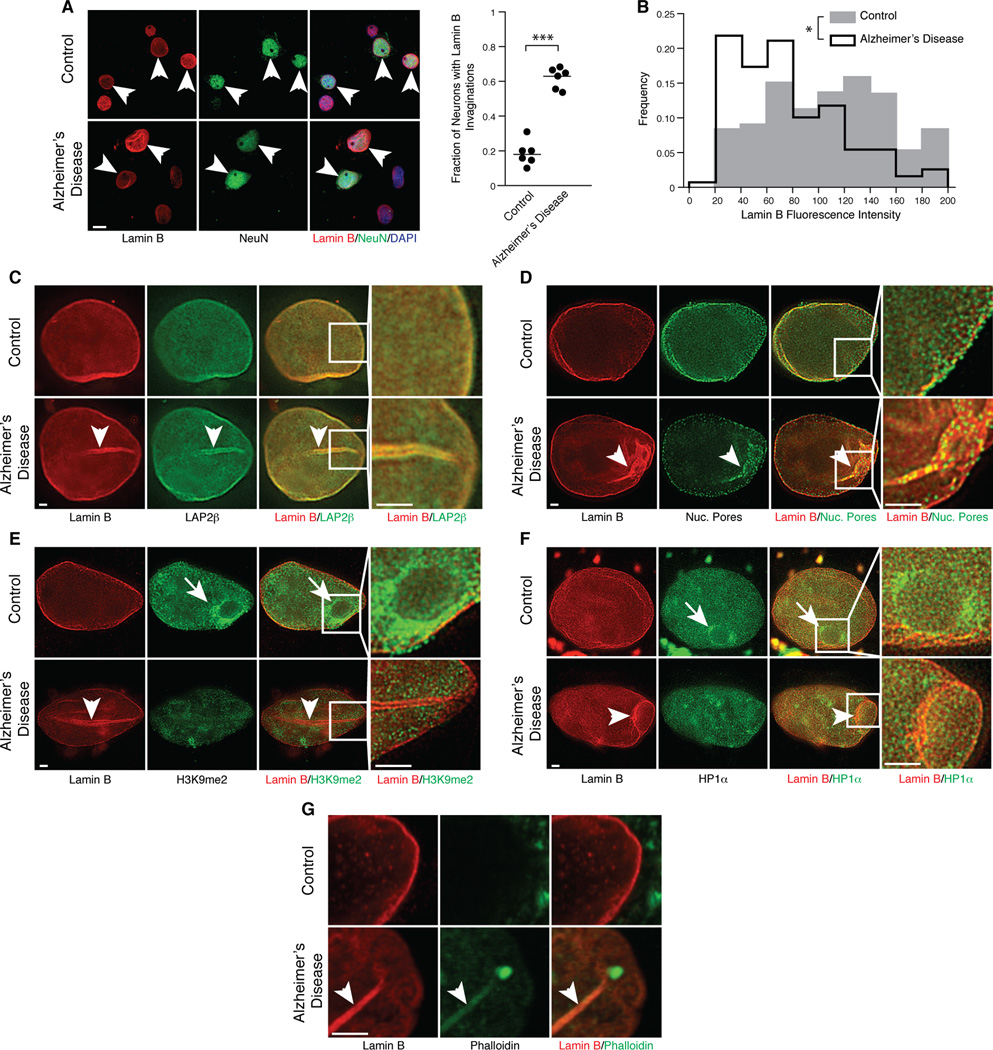
We next investigated lamin in human Alzheimer’s disease, the most common tauopathy. As predicted, we detected a disruption of the nuclear lamina in neurons from Alzheimer’s disease brains, where a significant number of neurons harbored invaginations of the nuclear lamina into the deep nuclear interior (Figure 4A, Movie S1). Quantitative analysis of lamin B revealed a significant reduction in lamin B protein levels in neurons from brains of patients with Alzheimer’s disease versus controls (Figure 4B). Lamina-associated protein 2β LAP2β) also lined the nuclear invaginations, suggesting that invaginations are not restricted to the lamin protein itself (Figure 4C). In addition, membrane invaginations were lined with nuclear pores (Figure 4D), suggesting that inner and outer membranes of the nuclear envelope invaginate, and that invaginations are likely filled with cytoplasm. To visualize the interaction between heterochromatin and lamin, we costained neuronal nuclei with lamin and H3K9me2 or HP1α, both of which are altered in Alzheimer’s disease brains [4]. Unlike neuronal nuclei from controls, in which we observed formation of typical perinucleolar chromocenters, nuclei from Alzheimer’s disease brains did not exhibit chromocenter staining in neurons harboring lamin invaginations (Figures 4E and 4F). Finally, we observed that F-actin (Figure 4G) and disease-associated phosphotau (Movie S2) lined the nuclear envelope invaginations in neurons from Alzheimer’s disease brains, supporting our hypothesis that tau-induced stabilization of F-actin disrupts the lamin nucleoskeleton.
Figure 4. Lamin pathology in human Alzheimer’s disease.
(A) Lamin B and NeuN immunostaining in nuclei from postmortem control and Alzheimer’s disease frontal cortex. Arrows indicate NeuN-positive neurons. n=6, ***p<0.001, t-test.
(B) Lamin B levels in NeuN-positive neurons from control and Alzheimer’s disease frontal cortex. n=6, *p=0.02, Mixed Effect Model.
(C) Super-resolution microscopy of Lamin B and LAP2β in neurons from control and Alzheimer’s disease frontal cortex.
(D) Super-resolution microscopy of Lamin B and nuclear pores in neurons from control and Alzheimer’s disease frontal cortex.
(E) Super-resolution microscopy of Lamin B and H3K9me2 in neurons from control and Alzheimer’s disease frontal cortex.
(F) Super-resolution microscopy of Lamin B and HP1α in neurons from control and Alzheimer’s disease frontal cortex.
(G) Super-resolution microscopy of Lamin B and F-actin visualized via phalloidin staining in neurons from control and Alzheimer’s disease frontal cortex.
Arrowheads indicate lamin invaginations and arrows indicate perinucleolar staining of heterochromatin in (E–F). Scale bars are 5 µm in (A) and 1 µm in (C–G). See also Movies S1 and S2.
Discussion
Our data suggest that tauopathies are neurodegenerative laminopathies, and establish that B-type lamins are required for maintaining genomic architecture, genomic integrity, function and survival of adult neurons. We find that lamin abnormalities are present in both Drosophila and human tauopathy, emphasizing the clinical relevance of these data. While irregularities in nuclear shape in Alzheimer’s disease have been previously reported [38], our genetic data from tau transgenic Drosophila suggest that lamin pathology and nuclear envelope invagination are early events that precede heterochromatin relaxation, neuronal cell cycle activation, and apoptosis. Mechanistically, we show that tau-induced stabilization of F-actin [26] causes LINC complex dysfunction and reduction and disorganization of lamin in neurons, allowing untethering of heterochromatin from the nuclear periphery, subsequent heterochromatin relaxation, DNA damage, cell cycle activation and apoptosis. Tau-induced overstabilization of F-actin is also known to disrupt mitochondrial dynamics, causing increased oxidative stress [14]. Since oxidative stress is known to cause DNA damage in the central nervous system [39], it is possible that lamin pathology and oxidative stress both contribute to some of the observed phenotypes. Our studies identify cellular mechanisms that are shared between tauopathies and laminopathies, and lay the groundwork for exploration of lamin and the LINC complex as novel therapeutic targets for the treatment of tauopathies.
Supplementary Material
Acknowledgements
Human brains were obtained from the Brigham and Women’s Hospital and the Massachusetts Alzheimer’s Disease Research Center. Super-resolution and confocal imaging were performed at the Harvard NeuroDiscovery Center Enhanced Neuroimaging Core Facility and the Harvard Center for Biological Imaging. Transgenic RNAi fly stocks were provided by the TRiP at Harvard Medical School (NIH/HIGMS R01-GM084947) and the Vienna Drosophila RNAi Center. Dr. Rebecca Betensky from the Data Management and Statistics Core of the Massachusetts Alzheimer’s Disease Research Center provided statistical assistance. Antibodies obtained from the Developmental Studies Hybridoma Bank (DSHB) were developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242. Antibodies obtained from the UC Davis/NIH NeuroMab facility are supported by NIH grant U24NS050606 and maintained by the Department of Neurobiology, Physiology and Behavior, College of Biological Sciences, University of California, Davis, CA 95616.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
B.F. and M.B.F. conceptualized the study, participated in interpreting data and wrote the manuscript. B.F. and F.H.B. performed experiments. M.B.F. supervised the research.
The authors declare no conflicts of interest.
References
- 1.Camozzi D, Capanni C, Cenni V, Mattioli E, Columbaro M, Squarzoni S, Lattanzi G. Diverse lamin-dependent mechanisms interact to control chromatin dynamics. Focus on laminopathies. Nucleus (Austin, Tex.) 2014;5:427–440. doi: 10.4161/nucl.36289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 4.Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014;17:357–366. doi: 10.1038/nn.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khurana V, Merlo P, DuBoff B, Fulga TA, Sharp KA, Campbell SD, Gotz J, Feany MB. A neuroprotective role for the DNA damage checkpoint in tauopathy. Aging Cell. 2012;11:360–362. doi: 10.1111/j.1474-9726.2011.00778.x. [DOI] [PubMed] [Google Scholar]
- 6.Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Current biology : CB. 2006;16:230–241. doi: 10.1016/j.cub.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Merlo P, Frost B, Peng S, Yang YJ, Park PJ, Feany M. p53 prevents neurodegeneration by regulating synaptic genes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:18055–18060. doi: 10.1073/pnas.1419083111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science (New York, N.Y.) 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 9.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 10.Riemer D, Stuurman N, Berrios M, Hunter C, Fisher PA, Weber K. Expression of Drosophila lamin C is developmentally regulated: analogies with vertebrate A-type lamins. Journal of cell science. 1995;108(Pt 10):3189–3198. doi: 10.1242/jcs.108.10.3189. [DOI] [PubMed] [Google Scholar]
- 11.Worman HJ. Nuclear lamins and laminopathies. J Pathol. 2012;226:316–325. doi: 10.1002/path.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medina M, Avila J. Further understanding of tau phosphorylation: implications for therapy. Expert review of neurotherapeutics. 2015;15:115–122. doi: 10.1586/14737175.2015.1000864. [DOI] [PubMed] [Google Scholar]
- 13.Steinhilb ML, Dias-Santagata D, Fulga TA, Felch DL, Feany MB. Tau phosphorylation sites work in concert to promote neurotoxicity in vivo. Molecular biology of the cell. 2007;18:5060–5068. doi: 10.1091/mbc.E07-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DuBoff B, Gotz J, Feany MB. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron. 2012;75:618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25:5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost B, Gotz J, Feany MB. Connecting the dots between tau dysfunction and neurodegeneration. Trends in cell biology. 2015;25:46–53. doi: 10.1016/j.tcb.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warrick JM, Paulson HL, Gray-Board GL, Bui QT, Fischbeck KH, Pittman RN, Bonini NM. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell. 1998;93:939–949. doi: 10.1016/s0092-8674(00)81200-3. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, Feany MB. Comparison of pathways controlling toxicity in the eye and brain in Drosophila models of human neurodegenerative diseases. Human molecular genetics. 2004;13:2011–2018. doi: 10.1093/hmg/ddh214. [DOI] [PubMed] [Google Scholar]
- 19.Coffinier C, Jung HJ, Nobumori C, Chang S, Tu Y, Barnes RH, 2nd, Yoshinaga Y, de Jong PJ, Vergnes L, Reue K, et al. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Molecular biology of the cell. 2011;22:4683–4693. doi: 10.1091/mbc.E11-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenz-Bohme B, Wismar J, Fuchs S, Reifegerste R, Buchner E, Betz H, Schmitt B. Insertional mutation of the Drosophila nuclear lamin Dm0 gene results in defective nuclear envelopes, clustering of nuclear pore complexes, and accumulation of annulate lamellae. The Journal of cell biology. 1997;137:1001–1016. doi: 10.1083/jcb.137.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osouda S, Nakamura Y, de Saint Phalle B, McConnell M, Horigome T, Sugiyama S, Fisher PA, Furukawa K. Null mutants of Drosophila B-type lamin Dm(0) show aberrant tissue differentiation rather than obvious nuclear shape distortion or specific defects during cell proliferation. Developmental biology. 2005;284:219–232. doi: 10.1016/j.ydbio.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Patterson K, Molofsky AB, Robinson C, Acosta S, Cater C, Fischer JA. The functions of Klarsicht and nuclear lamin in developmentally regulated nuclear migrations of photoreceptor cells in the Drosophila eye. Molecular biology of the cell. 2004;15:600–610. doi: 10.1091/mbc.E03-06-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luijsterburg MS, Dinant C, Lans H, Stap J, Wiernasz E, Lagerwerf S, Warmerdam DO, Lindh M, Brink MC, Dobrucki JW, et al. Heterochromatin protein 1 is recruited to various types of DNA damage. The Journal of cell biology. 2009;185:577–586. doi: 10.1083/jcb.200810035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leffelaar D, Grigliatti T. Age-dependent behavior loss in adult Drosophila melanogaster. Developmental Genetics. 1983;4:211–227. [Google Scholar]
- 25.Moraga DM, Nunez P, Garrido J, Maccioni RB. A tau fragment containing a repetitive sequence induces bundling of actin filaments. J Neurochem. 1993;61:979–986. doi: 10.1111/j.1471-4159.1993.tb03611.x. [DOI] [PubMed] [Google Scholar]
- 26.Fulga TA, Elson-Schwab I, Khurana V, Steinhilb ML, Spires TL, Hyman BT, Feany MB. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nature cell biology. 2007;9:139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- 27.Mellad JA, Warren DT, Shanahan CM. Nesprins LINC the nucleus and cytoskeleton. Current opinion in cell biology. 2011;23:47–54. doi: 10.1016/j.ceb.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Berger S, Schafer G, Kesper DA, Holz A, Eriksson T, Palmer RH, Beck L, Klambt C, Renkawitz-Pohl R, Onel SF. WASP and SCAR have distinct roles in activating the Arp2/3 complex during myoblast fusion. Journal of cell science. 2008;121:1303–1313. doi: 10.1242/jcs.022269. [DOI] [PubMed] [Google Scholar]
- 29.Quinlan ME, Heuser JE, Kerkhoff E, Mullins RD. Drosophila Spire is an actin nucleation factor. Nature. 2005;433:382–388. doi: 10.1038/nature03241. [DOI] [PubMed] [Google Scholar]
- 30.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, et al. Lifeact: a versatile marker to visualize F-actin. Nature methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatan M, Shinder V, Israeli D, Schnorrer F, Volk T. The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures. The Journal of cell biology. 2011;192:307–319. doi: 10.1083/jcb.201007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li GH, Shi Y, Chen Y, Sun M, Sader S, Maekawa Y, Arab S, Dawood F, Chen M, De Couto G, et al. Gelsolin regulates cardiac remodeling after myocardial infarction through DNase I-mediated apoptosis. Circulation research. 2009;104:896–904. doi: 10.1161/CIRCRESAHA.108.172882. [DOI] [PubMed] [Google Scholar]
- 33.Sadhukhan S, Sarkar K, Taylor M, Candotti F, Vyas YM. Nuclear role of WASp in gene transcription is uncoupled from its ARP2/3-dependent cytoplasmic role in actin polymerization. Journal of immunology (Baltimore, Md. : 1950) 2014;193:150–160. doi: 10.4049/jimmunol.1302923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belin BJ, Lee T, Mullins RD. DNA damage induces nuclear actin filament assembly by Formin-2 and Spire-(1/2) that promotes efficient DNA repair. eLife. 2015;4 doi: 10.7554/eLife.07735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kracklauer MP, Banks SM, Xie X, Wu Y, Fischer JA. Drosophila klaroid encodes a SUN domain protein required for Klarsicht localization to the nuclear envelope and nuclear migration in the eye. Fly. 2007;1:75–85. doi: 10.4161/fly.4254. [DOI] [PubMed] [Google Scholar]
- 36.Xie X, Fischer JA. On the roles of the Drosophila KASH domain proteins Msp-300 and Klarsicht. Fly. 2008;2:74–81. doi: 10.4161/fly.6108. [DOI] [PubMed] [Google Scholar]
- 37.Metaxakis A, Oehler S, Klinakis A, Savakis C. Minos as a genetic and genomic tool in Drosophila melanogaster. Genetics. 2005;171:571–581. doi: 10.1534/genetics.105.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheffield LG, Miskiewicz HB, Tannenbaum LB, Mirra SS. Nuclear pore complex proteins in Alzheimer disease. Journal of neuropathology and experimental neurology. 2006;65:45–54. doi: 10.1097/01.jnen.0000195939.40410.08. [DOI] [PubMed] [Google Scholar]
- 39.McKinnon PJ. Maintaining genome stability in the nervous system. Nat Neurosci. 2013;16:1523–1529. doi: 10.1038/nn.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.