Abstract
Background
Motor retraining for non-specific chronic low back pain (LBP) often focuses on voluntary postural tasks. This training, however, may not transfer to other known postural impairments, such as automatic postural responses to external perturbations.
Objectives
To evaluate the extent current treatments of motor retraining ameliorate impaired postural coordination when responding to a perturbation of standing balance.
Design
Planned secondary analysis of a prospectively registered (NCT01362049), randomized controlled trial with a blinded assessor.
Method
Sixty-eight subjects with chronic, recurrent, non-specific LBP were allocated to perform a postural response task as a secondary assessment one week before and one week after receiving either stabilization or Movement System Impairment (MSI)-directed treatment over 6 weekly 1-h sessions plus home exercises. For assessment, subjects completed the Oswestry disability and numeric pain rating questionnaires and then performed a postural response task of maintaining standing balance in response to 3 trials in each of 4 randomly presented directions of linear surface translations of the platform under the subjects' feet. Integrated amplitudes of surface electromyography (EMG) were recorded bilaterally from the rectus abdominis (RA), internal oblique (IO), and external oblique (EO) muscles during the postural response task.
Results
No significant effects of treatment on EMG responses were evident. Oswestry and numeric pain ratings decreased similarly following both treatments.
Conclusions
Stabilization and MSI-directed treatments do not affect trunk EMG responses to perturbations of standing balance in people with LBP, suggesting current methods of motor retraining do not sufficiently transfer to tasks of reactive postural control.
Keywords: Low back pain, Movement system impairment, Stabilization, Posture, Balance, Treatment
1. Introduction
Chronic low back pain (LBP) represents a common, disabling, and costly health condition with a rising prevalence (Andersson, 1999; Katz, 2006; Friedly et al., 2010). This rise in prevalence and inability to prevent chronic or recurrent episodes of pain suggest a lack of long-term treatment efficacy and demonstrates a significant need to optimize treatment.
Although the causes of chronic LBP are likely multi-factorial – including altered psychological, motor, mechanical, and sensory factors (Langevin and Sherman, 2007) – people with chronic LBP exhibit many impairments of postural control across several contexts of motor behavior. These impairments include altered sway during quiet stance (Mazaheri et al., 2013), altered anticipatory postural adjustments preceding voluntary movements (Hodges and Richardson, 1996; Sihvonen et al., 1997; Danneels et al., 2002; Jacobs et al., 2009, 2010; Macdonald et al., 2011), as well as diminished stability and altered patterns of muscle activation in response to an externally induced postural perturbation (Radebold et al., 2000; Newcomer et al., 2002; Cholewicki et al., 2005; Henry et al., 2006; Stokes et al., 2006; MacDonald et al., 2010; Jacobs et al., 2011; Mok et al., 2011). It should be noted, however, that impairments during quiet stance or of anticipatory postural adjustments are not consistently identified across studies or are not ubiquitously evident across all subjects with LBP (Silfies et al., 2009; Gubler et al., 2010; Jacobs et al., 2010; Mazaheri et al., 2013). Likewise, during responses to external postural perturbations, reports vary with regard to whether persons with LBP exhibit delayed muscle responses (Radebold et al., 2000; Cholewicki et al., 2005; Reeves et al., 2005), decreased incidence or altered amplitudes of muscle activation (Radebold et al., 2000; MacDonald et al., 2010; Jacobs et al., 2011; Jones et al., 2012a,b), co-contracted muscle activation patterns (Radebold et al., 2000), and/or increased baseline activation (Stokes et al., 2006; Jacobs et al., 2011; Jones et al., 2012a). This evidence of impaired postural control with LBP suggests a need for physical intervention (e.g., movement exercises) for people with LBP and, indeed, current practice guidelines recommend that clinicians refer their patients to physical therapy because early use of physical therapy associates with decreased subsequent use of medical services (Gellhorn et al., 2012).
In addition to heterogenous laboratory measures of postural impairment, patients with LBP exhibit a heterogenous clinical presentation. Thus, physical therapy interventions were developed to classify patients into homogenous groups and provide patient-specific treatments in order to ameliorate LBP (Karayannis et al., 2015). Although short-term indicators suggest patient-specific motor rehabilitation could be beneficial, superior outcomes in the long term are not often evident (Ferreira et al., 2007; Macedo et al., 2008; Unsgaard-Tondel et al., 2010; George et al., 2011; Surkitt et al., 2012; Henry et al., 2014; Saner et al., 2015). Motor retraining has been found to successfully modify some postural impairments (Tsao and Hodges, 2008; Hoffman et al., 2011), however the tasks used for assessment often exhibit strong similarities to the exercises practiced in treatment. It, therefore, becomes imperative to understand whether such treatments are effective in addressing known motor impairments associated with LBP during transfer tasks that are not specifically involved in the treatment paradigm. If the motor retraining treatments are deemed successful when a transfer task is assessed, then past reports that demonstrate a lack of superior clinical outcomes could reflect that ameliorating motor impairments provides no added benefit to treatment outcomes. In contrast, if these motor retraining treatments do not modify transfer tasks of postural coordination, then such treatments may still have potential for superior outcomes if the training strategies were improved to better address the general array of postural impairments associated with LBP.
The objective of this study, which represents a planned secondary analysis to the original clinical trial (Henry et al., 2014), was to determine whether two examples of motor retraining treatment – stabilization and Movement System Impairment (MSI)-directed exercises (McGill, 1998; Richardson et al., 1999; Sahrmann, 2002; Van Dillen et al., 2003) – are effective at ameliorating impairments in postural responses to perturbations of standing balance. We hypothesized that stabilization and MSI-directed treatments (which do not treat postural responses to perturbations of standing balance) would not ameliorate these impairments during early, automatic response phases, but would modify late-phase responses that have potential for voluntary influence (Jacobs and Horak, 2007; Jacobs et al., 2011; Tokuno et al., 2013).
2. Methods
2.1. Design overview
This study was a prospectively registered (NCT01362049), 2-arm randomized controlled trial with a blinded assessor. The primary objective of the trial was to examine the relative efficacy of trunk stabilization versus MSI-directed treatments for improving short- (6 weeks) and long-term (12 months) clinical outcomes in people with chronic LBP (Henry et al., 2014). Primary outcome measures were Modified Oswestry Disability scores and Numeric Pain Ratings (Fritz and Irrgang, 2001; Childs et al., 2005). Subjects were randomly assigned to receive either stabilization or MSI-directed treatment. This study reports on a planned secondary objective to determine the efficacy of stabilization and MSI-directed treatments to modify known impairments of postural responses associated with LBP. In addition to the baseline assessment prior to treatment onset, responses to externally induced perturbations of standing balance were assessed within one week after treatment and were not assessed at the 12-month time point. The study began March 2010 and short-term (6-week) follow-up was completed September 2011.
In order to confirm the nature of the subjects' postural-response impairments, the data from the subjects with LBP in this trial were compared to the data of 27 subjects without LBP. This cross-sectional analysis provided the basis for determining whether the MSI and stabilization treatments are effective at ameliorating differences in postural responses between those with and without LBP.
2.2. Setting and participants
Assessments, before and after treatment, were conducted at a university motion analysis laboratory, whereas treatment was conducted at one of six outpatient physical therapy clinics. Subjects were assigned to a care provider according to the location that was most convenient for them in order to optimize adherence. Care providers had an average of 14.2 (range 1–27) years of experience in orthopedic physical therapy, and case volumes for the diagnosis of interest at each site ranged from 130 to 950 per year. Adherence to the protocol by the care providers was evaluated by chart audits of 46% of the subjects and in-person observation audits of 36% of the subjects – on average during the third treatment visit – by trained physical therapists who did not provide treatment. Chart audits for data accuracy and thoroughness were also performed following treatment on all subjects.
Subjects with LBP were assessed for study inclusion through phone and email contact. Subjects with LBP admitted to the study (1) were between 21 and 55 years old, (2) had a history of chronic LBP (≥12 months) with or without recurrences, (3) could stand and walk independently, (4) had a Modified Oswestry Disability score of ≥19%, and/or a score less than 8 on at least one activity from the Patient Specific Functional Scale (Stratford et al., 1995), (5) could understand English, and (6) were currently employed or actively engaged in daily activities. The referring physician and the treating physical therapist screened for exclusion criteria through patient report with corroboration by the subject's medical record. Exclusion criteria included evidence or report of: a structural spinal deformity, spinal fracture, osteoporosis, systemic disease processes, disc herniation with corroborating clinical signs and symptoms, previous spinal surgery, pregnancy or less than 6 months post-partum or post-weaning, magnified symptom behavior, a body-mass index of greater than 30, receiving worker's compensation, or in litigation for the LBP. The subjects without LBP were recruited using the following inclusion criteria: an absence of neurological, psychiatric, cardiovascular or musculoskeletal disorders, as well as no uncorrected vision problems or severe musculoskeletal injuries.
2.3. Randomization and interventions
2.3.1. Assessment protocol
Prior to randomization, subjects visited the motion analysis laboratory for initial assessments. Subjects first completed a battery of questionnaires (including the Modified Oswestry Disability Index and Numeric Pain Rating Scale) and underwent a standardized clinical exam. Regarding the secondary objective to determine the effects of treatment on impaired postural coordination, 68 subjects were allocated for assessment on postural responses to support surface translations (movements of a platform under the subjects' feet) that perturb standing balance, and 56 were allocated to a protocol of voluntary postural coordination that is reported separately (Lomond et al., 2015). Following the clinical assessments, subjects allocated to this protocol with support surface translations were prepared for surface electromyography (EMG) recordings. Electrodes (Norotrodes with fixed 2-cm inter-electrode distance; Myotronics, Kent, WA, USA) were placed over the bilateral external oblique (EO), internal oblique (IO), and rectus abdominis (RA) muscles. Electrode placement and EMG recording parameters were as previously reported (Jacobs et al., 2011).
During the experimental task, subjects were instructed to stand looking forward on a moveable platform at their self-selected stance width and with their arms hanging comfortably at their sides. The subjects were then instructed to maintain their standing balance in response to the 9-cm platform movements. Subjects were given practice trials in each of two perturbation directions (leftward and forward translations) in order to familiarize them with the task. Following these practice trials, three trials in each of 4 directions of linear surface translations (forward, backward, left, and right) were presented in random order and at unpredictable intervals.
2.3.2. Randomization
Following laboratory testing for initial assessments, subjects with LBP were randomized to receive one of two treatments: stabilization or MSI-directed exercise treatment. The study's statistician used a covariate adaptive randomization schema. The treatment assignment was transmitted to the study coordinator and the treating physical therapist. All other personnel were unaware of the treatment assignment for the duration of the study. Subjects were instructed not to provide information regarding their treatment during laboratory assessments; laboratory personnel were unable to guess treatment assignment better than chance.
2.3.3. Treatment protocols
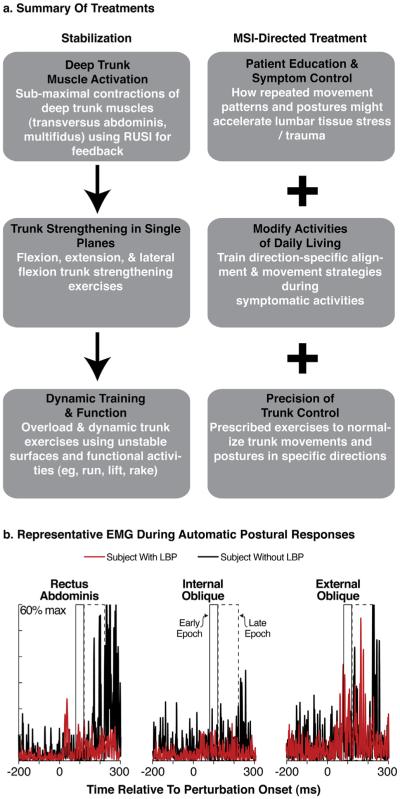
Both interventions were comprised of six weekly 1-h sessions plus prescribed home exercises (Fig. 1a). The stabilization protocol focused on 3 components of spinal stability: (1) motor control of the deep trunk muscles (Richardson et al., 1999; Hicks et al., 2005); (2) coordination and strengthening of the flexor, extensor, and oblique trunk muscles (Hicks et al., 2005) by focusing on repeated submaximal efforts that progressed to maximal efforts (Richardson and Jull, 1995; McGill, 1998), and (3) an education booklet (Melnick et al., 1998) that describes how to use proper body mechanics in order to protect the spine during activities of daily living.
Fig. 1.
Illustrations of (a) treatment protocols during intervention, and (b) EMG measures taken during assessment of postural responses. In (b), exemplar EMG traces from a subject with (red traces) and without (black traces) LBP illustrate the early (box with solid line; 80–120 ms) and late (box with dashed outline; 120–220 ms) EMG epochs from which integrated EMG amplitudes were calculated. These illustrated traces were derived from responses to rightward translations of muscles from the right side of the body. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
For subjects assigned to the MSI-directed treatment protocol (Sahrmann, 2002; Harris-Hayes et al., 2005; Van Dillen et al., 2005; Hoffman et al., 2011; Henry et al., 2014; Azevedo et al., 2015), the physical therapist tailored treatment to focus on: (1) education regarding how the subject's specific habitual lumbopelvic movement patterns and postures might accelerate lumbar-tissue stress as well as education about positions or postures to control symptoms; (2) exercises to modify the subject's specific trunk movements and postures; and (3) functional-activity modifications to change the subject's trunk movement and alignment patterns during activities identified as troublesome on the Patient Specific Functional Scale.
2.4. Outcome measures and follow-up
Outcome measures were assessed a week prior to the start of treatment and one week after completing the 6-week intervention. Integrated EMG amplitudes were generated over three epochs as outcome measures for the postural-response task: (1) a baseline 75-ms epoch immediately preceding the perturbation, (2) an early-phase epoch from 80 to 120 ms after perturbation onset, and (3) a late-phase epoch from 120 to 220 ms after perturbation onset (Fig. 1b). Responses were differentiated by epoch given our hypothesis that the early- and late-phase epochs represent response phases with distinct underlying neural control. Specifically, the early phase represents automatic, sub-cortical response generation, whereas the late phase has greater potential for cortical (and perhaps voluntary) influence (Jacobs and Horak, 2007; Jacobs et al., 2011). We predicted that our treatment protocols had potential to increase integrated EMG amplitudes in only the late-phase epoch because both protocols treat voluntary postural coordination and the extent of training is not likely to elicit transfer to the more automated, sub-cortical processes of postural control in the early-phase epochs (Tokuno et al., 2013).
Integrated EMG amplitudes were generated using Matlab software (Matlab, Natick, MA, USA). The EMG signals were band-pass filtered at 35–200 Hz, baseline corrected by subtracting the mean of the signal, and full-wave rectified. The high-pass limit was set to minimize cardiac artifact (Drake and Callaghan, 2006). Each subject's mean integrated EMG amplitude was then generated across the three trials of each direction of perturbation. Rather than the typical maximum voluntary contraction, non-normalized EMG amplitudes were analyzed because people with LBP may be unwilling to generate a voluntary contraction to their maximum capability (Lariviére et al., 2003).
Oswestry disability percentage scores were generated by summing the scores of each response item, then dividing by the total possible 50 points and multiplying by 100 to express the ratio as a percentage; higher percentages reflect higher levels of reported disability. Numeric pain ratings were recorded as the reported score out of 10, zero representing no pain and 10 representing maximal pain.
2.5. Sample size estimation
The clinical trial's sample size was powered based on the primary outcome measures of Oswestry disability scores and numeric pain ratings. However, for the secondary objective of this study, we estimated the minimal detectable differences between groups with and without LBP (assuming this study's sample size, 80% power, and a significance level of 0.05) in EMG response amplitudes based on previously published findings, which demonstrated significantly smaller IO and RA muscle response amplitudes as well as significantly larger EO muscle response amplitudes with LBP (Jones et al., 2012b). For the IO, EO, and RA muscles, respectively, we estimated minimal detectable differences from before to after treatment across both treatment groups to be 4.0%, 3.2% and 3.3%, with within group standard deviations of 11.8%, 9.1% and 9.4%.
2.6. Statistical analysis
Integrated EMG amplitudes of each epoch were analyzed separately due to their unique functional implications. Responses to lateral (combined left and right), forward, and backward perturbations were also analyzed separately because LBP, and treatment for LBP, may differentially affect responses across these conditions based on different mechanical constraints (Jacobs et al., 2011; Jones et al., 2012a,b). Neural mechanisms controlling individual abdominal and oblique muscles are also task-specific to different directions of surface translations (Carpenter et al., 2008). The effects of each treatment on integrated EMG amplitudes were assessed by mixed-model analyses of variance (ANOVA) to evaluate differences between treatment groups (stabilization versus MSI) and visits (pre- versus post-treatment). When analyzing responses to lateral perturbations, the ANOVA included a third factor for direction in order to compare responses between leftward and rightward surface translations. Because each specific variable was compared three times by separate ANOVA based on perturbation direction, significance was assigned a Bonferroni-corrected P-value of 0.0167. Although visit main effects represent the primary comparison of interest based on the power analysis of combined treatment groups, we also included the treatment-by-visit interactions to examine the potential differential effects of treatment groups on the outcomes of interest. Because no statistically significant interactions were found, they will not be discussed further. Statistical analyses were performed using SAS 9.2 Software (SAS Institute Inc., USA).
Measures of participant characteristics (i.e., age, height, weight, body-mass index as well as pre-treatment Oswestry scores and Numeric Pain Ratings) were compared using independent samples t-tests, comparing subjects with LBP treated with stabilization exercises versus those treated with MSI-directed exercises. Differences in gender between treatment groups were assessed by Chi-square.
In order to confirm specific impairments evident with this trial's cohort of subjects with LBP, similar ANOVA, t-test, and chi-square analyses were generated to compare this trial's subjects with LBP to the group of subjects without LBP. The ANOVA analyses controlled for the effects of age and gender as covariates due to significant differences between the groups with and without LBP on these variables.
3. Results
3.1. Recruitment and clinical outcomes of the clinical trial
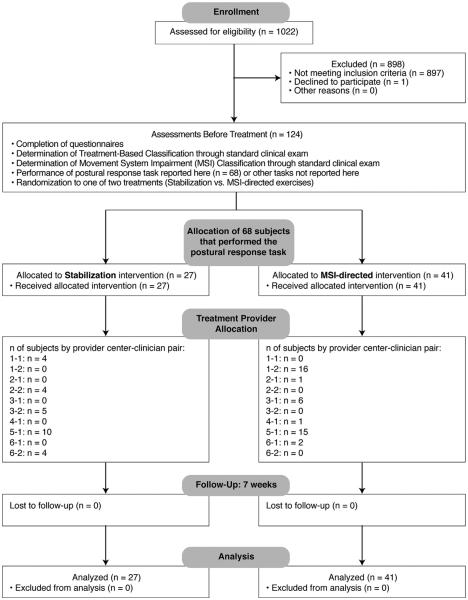
Recruitment and selection (Fig. 2) yielded a group of 124 subjects with LBP, of whom 68 were selected and participated in this protocol on postural responses to perturbed standing balance. Group characteristics are summarized in Table 1. No adverse events were reported. The groups with LBP randomized to stabilization versus MSI-directed treatments were not significantly different with regard to physical characteristics, numeric pain ratings, or Oswestry disability scores (Table 1). Oswestry disability scores and numeric pain ratings significantly decreased for both groups from before to after treatment, with no significant differences between groups (Table 1).
Fig. 2.
Flow diagram of participants in the clinical trial.
Table 1.
Frequency or mean (95% confidence interval) values of subject characteristics.
| Measure | Visit | Treatment groups |
Between-groups P-value |
Treatment main effect P-valuea |
|
|---|---|---|---|---|---|
| MSI | Stabilization | ||||
| Number | Pre & post | 41 | 27 | – | – |
| % Male | Pre | 63 | 44 | 0.123 | – |
| Age | Pre | 42.6 (39.4–45.8) yr | 39.6 (35.2–43.9) yr | 0.268 | – |
| Height | Pre | 174 (172–176) cm | 172 (169–175) cm | 0.308 | – |
| Weight | Pre | 72.8 (69.0–76.6) kg | 68.2 (63.5–72.9) kg | 0.142 | – |
| Body-mass index | Pre | 24 (23–25) kg/m2 | 23 (22–24) kg/m2 | 0.248 | – |
| Stance width | Pre & post | 21.2 (19.7–22.7) cm | 22.0 (20.0–24.0) cm | 0.519 | – |
| Oswestry Disability Index | Pre | 19.4 (16.7–22.0) % | 18.6 (14.9–22.3) % | 0.731 | <0.0001 |
| Post | 12.1 (9.5–14.7) % | 11.7 (8.7–14.6) % | 0.836 | ||
| Numeric Pain Rating | Pre | 2.68 (2.10–3.26) | 2.37 (1.63–3.11) | 0.517 | 0.00015 |
| Post | 1.58 (1.11–2.04) | 1.78 (1.20–2.35) | 0.598 | ||
Treatment main effects are reported across both treatment groups; group or interaction effects were not significant (P-values > 0.21).
3.2. Differences between subjects with and without LBP in EMG responses to perturbations
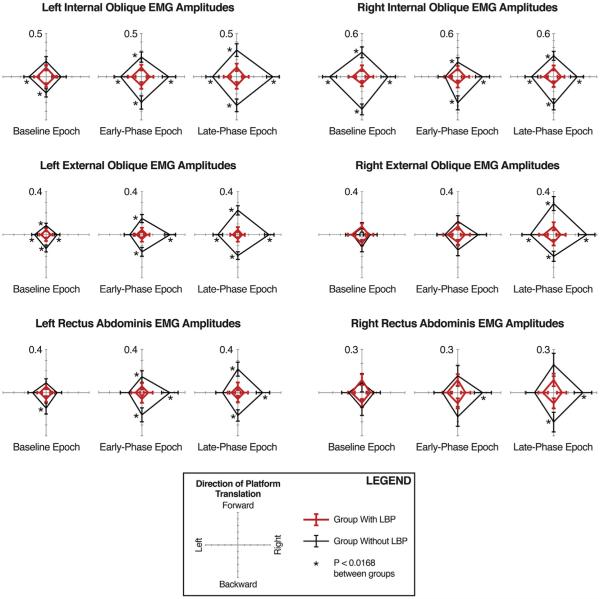
When comparing subjects with and without LBP, the groups were significantly different with regard to numeric pain ratings, age, and the distribution of males and females (Table 2); thus, age and gender were added as covariates to the analyses of EMG responses. For each recorded muscle, the group with LBP exhibited significantly lower integrated EMG amplitudes than the group without LBP in response to at least one direction of platform translation (Fig. 3; Table 3).
Table 2.
Frequency or mean (95% confidence interval) values of subject characteristics of groups with versus without LBP.
| Measure | With LBP (pre-treatment) | Without LBP | Between-groups P-value |
|---|---|---|---|
| Number | 68 | 27 | – |
| % Male | 56 | 33 | 0.047 |
| Age | 41.4 (38.8–44.0) yr | 32.5 (28.8–36.2) yr | 0.0004 |
| Height | 173 (171–175) cm | 170 (167–173) cm | 0.103 |
| Weight | 70.9 (68.0–73.9) kg | 66.8 (62.371.2) kg | 0.141 |
| Body-mass index | 24 (23–25) kg/m2 | 23 (22–24) kg/m2 | 0.287 |
| Stance width | 21.5 (20.3–22.7) cm | 20.7 (18.3–23.0) cm | 0.500 |
| Oswestry Disability Index | 19.1 (16.9–21.2) % | – | – |
| Numeric Pain Rating | 2.56 (2.17–2.95) | 0.22 (0.06–0.38) | 0.0001 |
Fig. 3.
Integrated EMG amplitudes between groups with and without LBP. Polar plot triplets are grouped by muscle across the baseline (−75 to 0 ms), early-phase (80–120 ms), and late-phase (120–220 ms) recording epochs. Polar plot directions indicate directions of surface translation, which induce body sway in the opposite direction. Red lines indicate mean (95% confidence interval) values for the group with LBP; black lines, the group without LBP. Asterisks (*) denote significant (P < 0.0168 after Bonferroni correction) differences between groups. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
Table 3.
Main group effects between groups with and without LBP.
| Muscle | Epoch | Perturbation direction | Group effects F-statistics, P-values |
|---|---|---|---|
| Left IO | Baseline | Forward, backward Lateral |
F = 5.40, 10.63; P = 0.023, 0.0016 F = 6.98, P = 0.0098 |
| Early-phase | Forward, backward Lateral |
F = 11.19, 18.83; P = 0.0013, <0.0001 F = 19.13, P < 0.0001 |
|
| Late-phase | Forward, backward Lateral |
F = 20.21, 28.36; P < 0.0001, <0.0001 F = 37.01, P < 0.0001 |
|
| Right IO | Baseline | Forward, backward Lateral |
F = 25.76, 67.58; P < 0.0001, <0.0001 F = 30.98, P < 0.0001 |
| Early-phase | Forward, backward Lateral |
F = 6.20, 20.92; P = 0.015, <0.0001 F = 8.87, P = 0.0038 |
|
| Late-phase | Forward, backward Lateral |
F = 20.30, 42.57; P < 0.0001, <0.0001 F = 17.92, P < 0.0001 |
|
| Left EO | Baseline | Forward, backward Lateral |
F = 10.39, 21.43; P = 0.0018, <0.0001 F = 14.55, P= 0.0003 |
| Early-phase | Forward, backward Lateral |
F = 23.72, 24.76; P < 0.0001, <0.0001 F = 31.73, P < 0.0001 |
|
| Late-phase | Forward, backward Lateral |
F = 52.77, 49.17; P < 0.0001, <0.0001 F = 70.32, P < 0.0001 |
|
| Right EO | Baseline | Forward, backward Lateral |
F = 0.12, 1.62; P = 0.73, 0.21 F = 0.00, P = 0.98 |
| Early-phase | Forward, backward Lateral |
F = 2.10, 5.29; P = 0.15, 0.024 F = 3.71, P = 0.058 |
|
| Late-phase | Forward, backward Lateral |
F = 30.79, 20.58; P < 0.0001, <0.0001 F = 21.30, P < 0.0001 |
|
| Left RA | Baseline | Forward, backward Lateral |
F = 1.88, 8.92; P = 0.17, 0.0037 F = 5.31, P = 0.024 |
| Early-phase | Forward, backward Lateral |
F = 8.21, 14.52; P = 0.0053, 0.0003 F = 11.57, P = 0.0010 |
|
| Late-phase | Forward, backward Lateral |
F = 17.67, 23.27; P < 0.0001, <0.0001 F = 14.86, P = 0.0002 |
|
| Right RA | Baseline | Forward, backward Lateral |
F = 0.29, 1.06; P = 0.59, 0.31 F = 1.03, P = 0.31 |
| Early-phase | Forward, backward Lateral |
F = 0.43, 5.31; P = 0.51, 0.024 F = 5.95, P = 0.0168 |
|
| Late-phase | Forward, backward Lateral |
F = 5.20, 10.79; P = 0.025, 0.0015 F = 10.26, P = 0.0019 |
3.3. Effects of treatment on EMG responses to perturbations in subjects with LBP
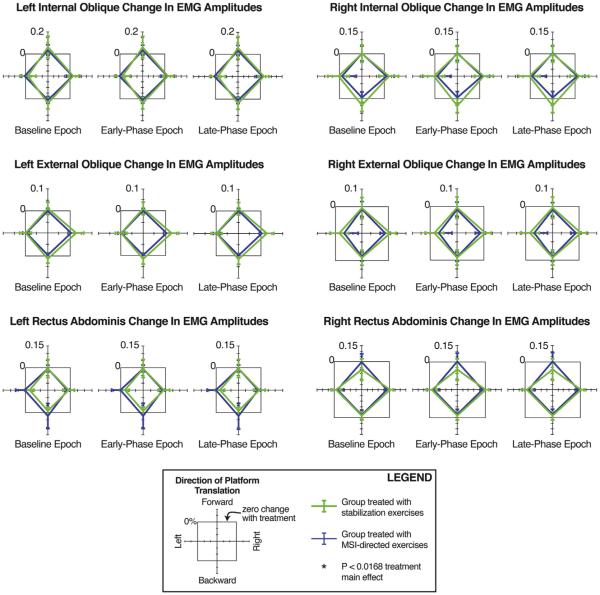
Fig. 4 presents changes in mean integrated EMG amplitudes following treatment (post-treatment minus pre-treatment). Neither treatment significantly altered integrated EMG amplitudes (range of visit main effects: F = 0.00–3.68; P = 0.06–0.95 for all epochs and muscles). Although not reaching the corrected level of significance (P = 0.0167), visit-by-direction effects were evident below a P-value of 0.05 at the left EO muscle because increases in EMG amplitudes were larger in response to rightward translations than to leftward translations (F = 4.32, P = 0.042 for early-phase responses; F = 4.50, P = 0.038 for late-phase responses).
Fig. 4.
Changes in integrated EMG amplitudes after treatment with either the stabilization or MSI-directed exercise protocol. Green lines indicate mean (95% confidence interval) values of change (post- minus pre-treatment) for the group receiving stabilization exercises; blue lines, the group receiving MSI-directed treatment. Gray square boxes indicate zero change with treatment. No significant main effects of session or group-by-session interactions were evident. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
4. Discussion
Before treatment, the subjects with LBP exhibited smaller muscle activation amplitudes than the subjects without LBP at all recorded muscles, and these effects were often evident across multiple directions of perturbations as well as across the baseline, early, and late phases of the response. Although both treatments significantly decreased subject-reported levels of pain and disability, these treatments did not significantly modify muscle response amplitudes to postural perturbations of standing balance.
Both interventions decreased short-term pain and disability, the primary outcome measures of this clinical trial. The improvement in pain and disability could represent benefits conferred by successful modification of untested motor impairments other than postural responses to perturbations, or the improvement could represent general benefits of exercise that are not dependent on retraining new patterns of motor coordination. Although not specifically tested by this study, we speculate that the improved pain and disability likely represent non-specific effects of exercise because other reports on this clinical trial demonstrate (1) no superior benefit on pain and disability between groups assigned to patient-matched retraining versus those receiving unmatched treatment assignment, and (2) no significant amelioration of another transfer task targeting anticipatory postural adjustments during voluntary leg movements (Henry et al., 2014; Lomond et al., 2015). Other clinical trials also demonstrate that patient-matched motor control exercises do not provide superior outcomes on pain and disability levels compared to unmatched motor control treatment or general strengthening exercises (Ferreira et al., 2007; Macedo et al., 2008; Unsgaard-Tondel et al., 2010; George et al., 2011; Surkitt et al., 2012; Lomond et al., 2014). Thus, based on this previous literature and on this study's findings that motor retraining did not elicit modified EMG responses to perturbations of standing balance, we interpret the improved pain and disability scores in this study as a general benefit of physical exercise rather than a demonstration of successful motor retraining.
To our knowledge, this study represents the first blinded randomized trial to evaluate the effects of motor retraining treatments on responses to an induced perturbation of standing balance. A strength of the study is the evaluation of automatic postural responses as a transfer task that is not specifically trained by the treatment protocols. This choice in task enables an assessment of the generalizability of motor retraining treatments for ameliorating automatic postural impairments in a different context of motor behavior. This study also benefits from blinded assessments and processing of outcome measures in order to minimize the potential for bias.
We are aware of only one other study to evaluate changes with motor retraining in automatic postural responses to an externally induced perturbation. Navalgund et al. (2013) recently reported that subjects with subacute LBP exhibit delayed trunk muscle responses compared to subjects without LBP, and that 10 weeks of stabilization treatment did not ameliorate these delays, but muscle response amplitudes were increased following treatment. Navalgund's study and ours, therefore, similarly demonstrated that stabilization does not effectively ameliorate identified differences between people with and without LBP. Their study's results diverge from ours, however, in that muscle response amplitudes increased following treatment. Differences between the studies' results might reflect differences in perturbation characteristics, EMG normalization methods, treatment duration, or blinded assessment and data processing.
Our study does carry some notable methodological considerations. First, EMG outcome measures for the automatic postural response protocol were only assessed immediately prior to and after treatment. Second, although six 1-h weekly sessions of treatment reflect local clinical practice, this dosage may not be sufficient to progress patients through stages of motor learning that enable consolidated, automated, and transferable motor skills. Third, the inter-trial and inter-session reliability of the EMG responses to perturbations measured in this study have yet to be confirmed, although significant differences between subjects with and without LBP were demonstrated in this study and in others that used the same protocol (Henry et al., 2006; Jacobs et al., 2011; Jones et al., 2008; Jones et al., 2012a). We can, however, conclude that the stabilization and MSI treatments of this study did not significantly modify muscle response amplitudes in a manner that ameliorated differences between subjects with and without LBP that were identified using the same protocol and methods of recording.
Although not supported given their current practice, patient-specific motor retraining protocols, if improved, may still have potential to provide superior outcomes compared to unmatched or non-specific physical exercises. The caveat to this statement, however, is the causative relationships among specific motor impairments with pain severity or disability remain speculative. Thus, the value of intervening on any specific motor impairment remains uncertain. Supposing, then, that treating postural impairments associated with LBP is of value, current motor retraining protocols appear to require improvement in order to better address the spectrum of known motor impairments associated with LBP. Clinical outcomes from patient-specific motor training might benefit from training a larger repertoire of behaviors – perhaps including postural responses to external perturbations (Jacobs et al., 2011; Tokuno et al., 2013) – in order to ensure that training comprehensively addresses the motor impairments of people with LBP. Motor retraining must also adhere to principles of motor learning in order to effectively engender new motor skills and transfer those motor skills across activities that are not specifically trained during treatment. Thus, further study must evaluate the adequacy of different treatment modalities, durations, and dosages to accomplish advanced stages of motor learning in patients with LBP.
Acknowledgments
This study (NIH2R01HD040909) was funded by the National Institutes of Health, USA, and awarded to SM Henry as Principal Investigator. The authors would like to acknowledge the consultation services of Dr. Julie Fritz on the Treatment Based Classification schema for the stabilization exercise protocol and Dr. Linda Van Dillen on the Movement System Impairment exercise protocol and the related standardized clinical exam items. The authors would also like to acknowledge the Vermont physical therapists that participated in this study: Justine Dee, MS PT, Matthew Odachowski, MPT and Jeffrey Albertson, MPT of Dee Physical Therapy, South Burlington; Janet Carscadden, PT and Andrea Trombley, MPT of Evolution Physical Therapy and Yoga, Burlington; Rose Bernier, PT, Traci Glanz, PT, Lucia Ryan, PT, Diane Stevens, PT and Sonya Worth, PT, PGDipHSc of Fletcher Allen Health Care, Burlington; Candice Brueck, MPT of Timberlane Physical Therapy, South Burlington; Karen Westervelt, PT, PGDipHSc and Jane Eliasson, PT of Copley Hospital, Morrisville, Vermont. We further acknowledge Rebecca Ouellette-Morton, MS, MPT for assistance with data collection.
Footnotes
Ethical approval statement
The university's Institutional Review Board approved the protocol. All subjects provided written informed consent, and the rights of each subject were protected. There were no changes to methods after the trial began. The trial ended once recruitment goals were met and the data were collected.
References
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–5. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- Azevedo DC, Van Dillen LR, Santos HO, Oliveira DR, Ferreira PH, Costa LO. Movement system impairment-based classification versus general exercise for chronic low back pain: protocol of a randomized controlled trial. Phys Ther. 2015 doi: 10.2522/ptj.20140555. http://dx.doi.org/10.2522/ptj.20140555. [DOI] [PubMed]
- Carpenter MG, Tokuno CD, Thorstensson A, Cresswell AG. Differential control of abdominal muscles during multi-directional support-surface translations in man. Exp Brain Res. 2008;188:445–55. doi: 10.1007/s00221-008-1377-x. [DOI] [PubMed] [Google Scholar]
- Childs J, Piva S, Fritz J. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30:1331–4. doi: 10.1097/01.brs.0000164099.92112.29. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Silfies SP, Shah RA, Greene HS, Reeves NP, Alvi K, et al. Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine. 2005;30:2614–20. doi: 10.1097/01.brs.0000188273.27463.bc. [DOI] [PubMed] [Google Scholar]
- Danneels LA, Coorevits PL, Cools AM, Vanderstraeten GG, Cambier DC, Witvrouw EE, et al. Differences in electromyographic activity in the multifidus muscle and the iliocostalis lumborum between healthy subjects and patients with sub-acute and chronic low back pain. Eur Spine J. 2002;11:13–9. doi: 10.1007/s005860100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JD, Callaghan JP. Elimination of electrocardiogram contamination from electromyogram signals: an evaluation of currently used removal techniques. J Electromyogr Kinesiol. 2006;16:175–87. doi: 10.1016/j.jelekin.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Ferreira ML, Ferreira PH, Latimer J, Herbert RD, Hodges PW, Jennings MD, et al. Comparison of general exercise, motor control exercise and spinal manipulative therapy for chronic low back pain: a randomized trial. Pain. 2007;131:31–7. doi: 10.1016/j.pain.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Friedly J, Standaert C, Chan L. Epidemiology of spine care: the back pain dilemma. Phys Med Rehabil Clin N Am. 2010;21:659–77. doi: 10.1016/j.pmr.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JM, Irrgang JJ. A comparison of a modified Oswestry low back pain disability questionnaire and the Quebec back pain disability scale. Phys Ther. 2001;81:776–88. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]
- Gellhorn AC, Chan L, Martin B, Friedly J. Management patterns in acute low back pain: the role of physical therapy. Spine. 2012;37:775–82. doi: 10.1097/BRS.0b013e3181d79a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SZ, Childs JD, Teyhen DS, Wu SS, Wright AC, Dugan JL, et al. Brief psychosocial education, not core stabilization, reduced incidence of low back pain: results from the prevention of low back pain in the military (POLM) cluster randomized trial. BMC Med. 2011;9:128. doi: 10.1186/1741-7015-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D, Mannion AF, Schenk P, Gorelick M, Helbling D, Gerber H, et al. Ultrasound tissue Doppler imaging reveals no delay in abdominal muscle feed-forward activity during rapid arm movements in patients with chronic low back pain. Spine. 2010;35:1506–13. doi: 10.1097/BRS.0b013e3181c3ed41. [DOI] [PubMed] [Google Scholar]
- Harris-Hayes M, Van Dillen LR, Sahrmann SA. Classification, treatment and outcomes of a patient with lumbar extension syndrome. Physiother Theory Pract. 2005;21:181–96. doi: 10.1080/09593980500212987. [DOI] [PubMed] [Google Scholar]
- Henry SM, Hitt JR, Jones SL, Bunn JY. Decreased limits of stability in response to postural perturbations in subjects with low back pain. Clin Biomech. 2006;21:881–92. doi: 10.1016/j.clinbiomech.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Henry SM, Van Dillen LR, Ouellette-Morton RH, Hitt JR, Lomond KV, DeSarno MJ, et al. Outcomes are not different for patient-matched versus nonmatched treatment in subjects with chronic recurrent low back pain: a randomized clinical trial. Spine J. 2014;14:2799–810. doi: 10.1016/j.spinee.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GE, Fritz JM, Delitto A, McGill SM. Preliminary development of a clinical prediction rule for determining which patients with low back pain will respond to a stabilization exercise program. Arch Phys Med Rehabil. 2005;86:1753–62. doi: 10.1016/j.apmr.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine. 1996;21:2640–50. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- Hoffman SL, Johnson MB, Zou D, Harris-Hayes M, Van Dillen LR. Effect of classification-specific treatment on lumbopelvic motion during hip rotation in people with low back pain. Man Ther. 2011;16:344–50. doi: 10.1016/j.math.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114:1339–48. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Henry SM, Nagle KJ. People with chronic low back pain exhibit decreased variability in the timing of their anticipatory postural adjustments. Behav Neurosci. 2009;123:455–8. doi: 10.1037/a0014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Henry SM, Nagle KJ. Low back pain associates with altered activity of the cerebral cortex prior to arm movements that require postural adjustment. Clin Neurophysiol. 2010;121:431–40. doi: 10.1016/j.clinph.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Henry SM, Jones SL, Hitt JR, Bunn JY. A history of low back pain associates with altered electromyographic activation patterns in response to perturbations of standing balance. J Neurophysiol. 2011;106:2506–14. doi: 10.1152/jn.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Henry SM, Raasch CC, Hitt JR, Bunn JY. Responses to multi-directional surface translations involve redistribution of proximal versus distal strategies to maintain upright posture. Exp Brain Res. 2008;187:407–17. doi: 10.1007/s00221-008-1312-1. [DOI] [PubMed] [Google Scholar]
- Jones SL, Henry SM, Raasch CC, Hitt JR, Bunn JY. Individuals with non-specific low back pain use a trunk stiffening strategy to maintain upright posture. J Electromyogr Kinesiol. 2012a;22:13–20. doi: 10.1016/j.jelekin.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Hitt JR, DeSarno MJ, Henry SM. Individuals with non-specific low back pain in an active episode demonstrate temporally altered torque responses and direction-specific enhanced muscle activity following unexpected balance perturbations. Exp Brain Res. 2012b;221:413–26. doi: 10.1007/s00221-012-3183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayannis NV, Jull GA, Hodges PW. Movement-based subgrouping in low back pain: synergy and divergence in approaches. Physiotherapy. 2015 doi: 10.1016/j.physio.2015.04.005. http:// dx.doi.org/10.1016/j.physio.2015.04.005 [DOI] [PubMed]
- Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Jt Surg Am. 2006;88(Suppl. 2):21–4. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Sherman KJ. Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med Hypotheses. 2007;68:74–80. doi: 10.1016/j.mehy.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Lariviére C, Arsenault AB, Gravel D, Gagnon D, Loisel P. Surface electromyography assessment of back muscle intrinsic properties. J Electromyogr Kinesiol. 2003;13:305–18. doi: 10.1016/s1050-6411(03)00039-7. [DOI] [PubMed] [Google Scholar]
- Lomond KV, Henry SM, Hitt JR, DeSarno MJ, Bunn JY. Altered postural responses persist following physical therapy of general versus specific trunk exercises in people with low back pain. Man Ther. 2014;19:425–32. doi: 10.1016/j.math.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomond KV, Jacobs JV, Hitt JR, DeSarno MJ, Bunn JY, Henry SM. Effects of low back pain stabilization or movement system impairment treatments on voluntary postural adjustments: a randomized controlled trial. Spine J. 2015;15:596–606. doi: 10.1016/j.spinee.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D, Moseley GL, Hodges PW. People with recurrent low back pain respond differently to trunk loading despite remission from symptoms. Spine. 2010;35:818–24. doi: 10.1097/BRS.0b013e3181bc98f1. [DOI] [PubMed] [Google Scholar]
- Macdonald DA, Dawson AP, Hodges PW. Behavior of the lumbar multifidus during lower extremity movements in people with recurrent low back pain during symptom remission. J Orthop Sports Phys Ther. 2011;41:155–64. doi: 10.2519/jospt.2011.3410. [DOI] [PubMed] [Google Scholar]
- Macedo LG, Latimer J, Maher CG, Hodges PW, Nicholas M, Tonkin L, et al. Motor control or graded activity exercises for chronic low back pain? A randomised controlled trial. BMC Musculoskelet Disord. 2008;9:65. doi: 10.1186/1471-2474-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri M, Coenen P, Parnianpour M, Kiers H, van Dieen JH. Low back pain and postural sway during quiet standing with and without sensory manipulation: a systematic review. Gait Posture. 2013;37:12–22. doi: 10.1016/j.gaitpost.2012.06.013. [DOI] [PubMed] [Google Scholar]
- McGill SM. Low back exercises: evidence for improving exercise regimens. Phys Ther. 1998;78:754–65. doi: 10.1093/ptj/78.7.754. [DOI] [PubMed] [Google Scholar]
- Melnick MS, Saunders HD, Saunders R. Self help manual: managing back pain; daily activities guide for back pain patients. The Saunders Group; St. Paul, MN: 1998. [Google Scholar]
- Mok NW, Brauer SG, Hodges PW. Changes in lumbar movement in people with low back pain are related to compromised balance. Spine. 2011;36:E45–52. doi: 10.1097/BRS.0b013e3181dfce83. [DOI] [PubMed] [Google Scholar]
- Navalgund A, Buford JA, Briggs MS, Givens DL. Trunk muscle reflex amplitudes increased in patients with subacute, recurrent LBP treated with a 10-week stabilization exercise program. Mot Control. 2013;17:1–17. doi: 10.1123/mcj.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer KL, Jacobson TD, Gabriel DA, Larson DR, Brey RH, An KN. Muscle activation patterns in subjects with and without low back pain. Arch Phys Med Rehabil. 2002;83:816–21. doi: 10.1053/apmr.2002.32826. [DOI] [PubMed] [Google Scholar]
- Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25:947–54. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- Reeves NP, Cholewicki J, Milner TE. Muscle reflex classification of low-back pain. J Electromyogr Kinesiol. 2005;15:53–60. doi: 10.1016/j.jelekin.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Richardson CA, Jull GA. An historical perspective on the development of clinical techniques to evaluate and treat the active stabilising system of the lumbar spine. Aust J Physiother. 1995;1:5–13. [Google Scholar]
- Richardson CA, Jull GA, Hodges PW, Hides JA. Therapeutic exercise for spinal segmental stabilization in low back pain: scientific basis and clinical approach. Churchill Livingstone; Edinburgh: 1999. [Google Scholar]
- Sahrmann S. Diagnosis and treatment of movement impairment syndromes. Mosby; St. Louis: 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saner J, Kool J, Sieben JM, Luomajoki H, Bastiaenen CH, de Bie RA. A tailored exercise program versus general exercise for a subgroup of patients with low back pain and movement control impairment: a randomised controlled trial with one- year follow-up. Man Ther. 2015 doi: 10.1016/j.math.2015.02.005. http://dx.doi.org/10.1016/j.math.2015.02.005. [DOI] [PubMed]
- Sihvonen T, Lindgren KA, Airaksinen O, Manninen H. Movement disturbances of the lumbar spine and abnormal back muscle electromyographic findings in recurrent low back pain. Spine. 1997;22:289–95. doi: 10.1097/00007632-199702010-00012. [DOI] [PubMed] [Google Scholar]
- Silfies SP, Mehta R, Smith SS, Karduna AR. Differences in feedforward trunk muscle activity in subgroups of patients with mechanical low back pain. Arch Phys Med Rehabil. 2009;90:1159–69. doi: 10.1016/j.apmr.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Stokes IA, Fox JR, Henry SM. Trunk muscular activation patterns and responses to transient force perturbation in persons with self-reported low back pain. Eur Spine J. 2006;15:658–67. doi: 10.1007/s00586-005-0893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford P, Gill C, Westaway M, Binkley J. Assessing disability and change on individual patients: a report of a patient specific measure. Physiother Can. 1995;47:258–63. [Google Scholar]
- Surkitt LD, Ford JJ, Hahne AJ, Pizzari T, McMeeken JM. Efficacy of directional preference management for low back pain: a systematic review. Phys Ther. 2012;92:652–65. doi: 10.2522/ptj.20100251. [DOI] [PubMed] [Google Scholar]
- Tokuno CD, Cresswell AG, Thorstensson A, Carpenter MG. Recruitment order of the abdominal muscles varies with postural task. Scand J Med Sci Sports. 2013;23:349–54. doi: 10.1111/j.1600-0838.2011.01394.x. [DOI] [PubMed] [Google Scholar]
- Tsao H, Hodges PW. Persistence of improvements in postural strategies following motor control training in people with recurrent low back pain. J Electromyogr Kinesiol. 2008;18:559–67. doi: 10.1016/j.jelekin.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Unsgaard-Tondel M, Fladmark AM, Salvesen O, Vasseljen O. Motor control exercises, sling exercises, and general exercises for patients with chronic low back pain: a randomized controlled trial with 1-year follow-up. Phys Ther. 2010;90:1426–40. doi: 10.2522/ptj.20090421. [DOI] [PubMed] [Google Scholar]
- Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, McDonnell MK, Bloom NJ. Movement system impairment-based categories for low back pain: stage 1 validation. J Orthop Sports Phys Ther. 2003;33:126–42. doi: 10.2519/jospt.2003.33.3.126. [DOI] [PubMed] [Google Scholar]
- Van Dillen LR, Sahrmann SA, Wagner JM. Classification, intervention, and outcomes for a person with lumbar rotation with flexion syndrome. Phys Ther. 2005;85:336–51. [PubMed] [Google Scholar]