Abstract
Becoming a skilled reader requires building a functional neurocircuitry for printed-language processing that integrates with spoken-language-processing networks. In this longitudinal study, functional MRI (fMRI) was used to examine convergent activation for printed and spoken language (print-speech coactivation) in selected regions implicated in printed-language processing (the reading network). We found that print-speech coactivation across the left-hemisphere reading network in beginning readers predicted reading achievement 2 years later beyond the effects of brain activity for either modality alone; moreover, coactivation effects accounted for variance in later reading after controlling for initial reading performance. Within the reading network, effects of coactivation were significant in bilateral inferior frontal gyrus (IFG) and left inferior parietal cortex and fusiform gyrus. The contribution of left and right IFG differed, with more coactivation in left IFG predicting better achievement but more coactivation in right IFG predicting poorer achievement. Findings point to the centrality of print-speech convergence in building an efficient reading circuitry in children.
Keywords: reading, cognitive neuroscience, neuroimaging, language, literacy
The human ability to process spoken language provides a biological foundation through which printed language may be learned (Liberman, 1992). Oral language skills that require explicit phonological processing (the ability to detect, categorize, retrieve, and manipulate speech segments) are causally linked to the ability to rapidly and accurately map letters to speech sounds for successful reading (Bradley & Bryant, 1983; Wagner & Torgesen, 1987). For example, skilled readers perform better than poorer readers on tasks that require access to phonological information and metaphonological skills (National Reading Panel, 2000; Shankweiler et al., 1995). Skilled readers may have detailed internal representations for speech, which allows them to learn to map printed language quickly and accurately onto existing phonological representations (Elbro, 1996; Wagner & Torgesen, 1987). Additionally, skilled readers are more successful than less-skilled readers at learning to pair novel visual and verbal information (Hulme, Goetz, Gooch, Adams, & Snowling, 2007; Vellutino, Scanlon, & Spearing, 1995).
Learning to connect printed letters or words to their spoken forms is an example of a task requiring integration across modalities. Warmington and Hulme (2012) reported that children’s performance on paired verbal-visual learning tasks was a strong indicator of their ability to read both real words and nonwords. The presumption is that poor integration across modalities may contribute to poor reading outcomes. At the level of the brain, this implies a neurolinguistic system that is suitably adept at processing information through both spoken and printed modalities, and one that might depend on functional convergence across modalities to achieve proficient reading (Braze et al., 2011; Kovelman et al., 2015; Liberman, 1992; Shankweiler et al., 2008). Functional convergence is the cooperative relationship between visual and auditory language processing. How it develops within early readers’ neurobiological networks and how reorganization of language systems for reading supports the development of fluent and automated reading over time are key questions with implications for both theory and practice (Dehaene, Cohen, Morais, & Kolinsky, 2015; Shankweiler et al., 2008).
Surprisingly, the contribution of cross-modal neural convergence to reading ability has been explored only recently. In the present research, we explored such convergence by measuring individual differences in coactivation to printed and spoken stimuli (henceforth referred to as print-speech coactivation). Studies in our lab have found a robust relationship between print-speech coactivation in left-hemisphere language regions and reading-related skills in both children (Frost et al., 2009) and adults (Constable et al., 2004; Shankweiler et al., 2008). Frost et al. (2009) found that young readers’ behavioral performance on phonological awareness tasks is associated with variability in print-speech convergence in left-hemisphere language-related regions—the better the child’s phonological awareness, the smaller the difference between activation levels for printed and spoken stimuli in left-hemisphere networks, especially the superior temporal gyrus (STG). A study with young adults showed a similar brain-behavior pattern for reading-comprehension skill (Shankweiler et al., 2008). In left inferior frontal gyrus (IFG), greater convergence for spoken and printed sentences was associated with higher reading-comprehension scores. Blau et al. (2010) reported compatible findings that 9-year-olds’ activations in response to letters and speech sounds in planum temporale and Heschl’s gyrus correlated with word and pseudoword reading skills. McNorgan, Randazzo-Wagner, and Booth (2013) observed that typically developing readers between the ages of 8 and 13 showed significant correlations between phonological awareness (elision) and their functional activation in a cross-modal (auditory-visual) rhyme-judgment task in fusiform gyrus, posterior superior temporal sulcus, and planum temporale. Poor readers did not show this cross-modal integration, which suggests that they may fail to engage the same system when processing phonological information in different modalities. In these studies, individual differences in reading skills correlated with the degree to which print and speech materials engaged overlapping neural networks.
The neural circuitry for reading has been extensively studied in children and adults, and there is strong consensus on the topology (see Pugh et al., 2010, for a review). Skilled readers show robust activation and functional connectivity across left-hemisphere dorsal (temporoparietal), ventral (occipitotemporal), and inferior frontal networks along with subcortical networks in print-processing tasks (Pugh et al., 2010; Pugh et al., 2013; B. A. Shaywitz et al., 2002). These functional differences for print are evident early on; we recently reported that greater activation in reading-related left-hemisphere cortical regions (IFG, temporoparietal, and occipitotemporal) and subcortical networks were associated with better concurrent decoding skills in children learning to read (Pugh et al., 2013). For both children and adults with poor reading achievement, there are marked functional differences in activity generated in these systems during reading. Specifically, left-hemisphere networks used by strong readers tend to be underactivated in poor readers (Brunswick, McCrory, Price, Frith, & Frith, 1999). Additionally, poor readers often show evidence of two, apparently compensatory, patterns associated with their left-hemisphere dysfunction: an increased functional role for right-hemisphere posterior sites (S. E. Shaywitz et al., 1998) and increased bihemispheric IFG activation (Brunswick et al., 1999; B. A. Shaywitz et al., 2002; S. E. Shaywitz et al., 1998). In the current study, we targeted bilateral IFG, temporoparietal regions, and occipitotemporal regions; we extended research beyond cross-sectional correlations toward developing a neurobiological account of how individual differences in print-speech convergence at the initial stages of learning to read actually predict later reading outcomes.
A few longitudinal studies of neural activation patterns support the expectation that functional activation for print in critical regions can predict future reading performance. For example, in children as young as 8 years, future improvement in decoding has been found to positively correlate with earlier functional activation in left IFG and left basal ganglia (McNorgan, Alvarez, Bhullar, Gayda, & Booth, 2011) as well as right occipitotemporal and bilateral middle temporal gyrus (Hoeft et al., 2007). No studies to date have examined print-speech convergence as an indicator of later reading skill. In the current investigation, we explored for the first time the hypothesis that print-speech convergence, as measured by coactivation in left- and right-hemisphere networks (previously implicated in skilled and less-skilled reading), predicts reading 2 years later as children transition from learning to read to fluent and automatic decoding. We asked whether convergence differences account for outcomes above and beyond behavioral measures or general activation for printed and spoken stimuli.
Method
Participants
We aimed to recruit children with varying reading ability, from skilled to poor. Children entered the study during a 3-year recruiting wave and were followed for 2 years. Data collection ended when the 2-year follow-up visits were completed on all participants who remained available for the study. Sample size was determined via power analyses informed by brain-behavior correlational studies of reading in young adults (Shankweiler et al., 2008) and by prior longitudinal studies that identified brain regions that were significant predictors in multiple regression models (Hoeft et al., 2007). From the full sample of 128 children who provided longitudinal behavioral data, the current study included all 68 who met the following criteria, regardless of their reading ability. First, they completed the functional MRI (fMRI) task and their fMRI data met our quality standards (see the section on fMRI acquisition and processing). In addition, they were between the ages of 6 and 10 years at entry into the study (Time 1), were native English speakers, and had no history of hearing or vision impairment, intellectual disability, or developmental disability. Only children who provided behavioral data on our outcome reading measure at the 2-year follow-up (Time 2) were included in the analysis. Demographic data and performance on behavioral tasks is reported in Table 1.
Table 1.
Demographic and Descriptive Data for the 68 Children in the Study
| Variable | Time 1 | Time 2 |
|---|---|---|
| Age (years) | 8.5 (1.2) | 10.5 (1.3) |
| WJ-III Broad Reading scorea | 110 (19) | 108 (17) |
| WJ-III Basic Reading score | 111 (17) | 106 (14) |
| TOWRE Sight Word Efficiency score | 102 (16) | 102 (13) |
| TOWRE Phonemic Decoding score | 103 (17) | 103 (18) |
| WASI Verbal IQ | 111 (15) | 116 (14) |
| WASI Performance IQ | 111 (17) | 111 (16) |
| PPVT-III score | 113 (13) | 115 (14) |
| CTOPP Phonological Awareness score | 108 (17) | 104 (15) |
Note: The table shows means, with standard deviations in parentheses. WJ-III = Woodcock-Johnson Test of Achievement, third edition (Woodcock, McGrew, & Mather, 2001); TOWRE = Test of Word Reading Efficiency (Torgesen, Wagner, & Rashotte, 1999); WASI = Wechsler Abbreviated Scales of Intelligence (Wechsler, 1999); PPVT-III = Peabody Picture Vocabulary Test, third edition (Dunn & Dunn, 1997); CTOPP = Comprehensive Test of Phonological Processing (Wagner, Torgesen, & Rashotte, 1999).
WJ-III Broad Reading score was the outcome variable at Time 2.
Behavioral measures
To evaluate reading skills, we administered the third edition of the Woodcock-Johnson Test of Achievement (WJ-III; Woodcock, McGrew, & Mather, 2001) at both Time 1 and Time 2. The primary outcome variable of the study was the WJ-III Broad Reading Composite (WJBR) score, which was a composite of scores on the following subtests: Letter-Word Identification (recognizing letters and reading real words of increasing difficulty), Reading Fluency (speeded reading of sentences), and Passage Comprehension (reading and understanding short passages). Additional reading measures consisted of accurately identifying single words and pseudowords, which was assessed by the WJ-III Basic Reading subtest (a combination of the Letter-Word Identification and Word Attack measures) and the Phonemic Decoding and Sight Word Efficiency subtests of the Test of Word Reading Efficiency (TOWRE; Torgesen, Wagner, & Rashotte, 1999).
IQ was assessed for descriptive purposes using the Wechsler Abbreviated Scales of Intelligence (Wechsler, 1999), and spoken language skills were evaluated using the third edition of the Peabody Picture Vocabulary Test (Dunn & Dunn, 1997) and the oral language subtests of the WJ-III (Woodcock et al., 2001). In addition, the Elision and Blending Words subtests of the Comprehensive Test of Phonological Processing (Wagner, Torgesen, & Rashotte, 1999) were administered to evaluate phonological awareness.
fMRI task
To assess functional activation in response to print and to speech, we asked children to complete a picture-identification task (see Frost et al., 2009). An event-related design was used in which a picture remained on screen while a series of comparison items appeared one at a time. Comparison items were words or pseudowords that were either printed or spoken. There was a jittered interstimulus interval of 4 to 7 s between each item. There were four sets of pictures, each with seven to eight comparison items, per functional imaging run. Words and pseudowords either matched the picture on screen (20% of trials) or did not match the picture on screen (80% of trials). Participants indicated a match or mismatch by pressing a button. To average over similar response types, we restricted our analysis to functional activation for processing of printed and spoken tokens for the mismatch trials (collapsing responses to words and pseudowords). Mean accuracy for the task was 85% (SD = 12%).
fMRI acquisition and processing
We acquired fMRI data on a Siemens 1.5T (Sonata) scanner at Time 1 on a separate day from the behavioral testing. Participants’ heads were enclosed in a standard head coil with padding to ensure there was no movement throughout the scan. Twenty axial-oblique slices were collected using a single-shot, gradient-echo, echo-planar imaging sequence—flip angle = 80°, echo time (TE) = 50 ms, repetition time (TR) = 2,000 ms, field of view (FOV) = 200 × 200 mm; slice thickness was set to 6 mm without gaps. All children completed between 6 and 10 functional runs. Additionally, a high-resolution anatomic image was acquired using the magnetization-prepared rapid gradient-echo pulse sequence (flip angle = 8°, TE = 3.65 ms, TR = 2,000 ms, FOV = 256 × 256 mm, voxel resolution = 1 × 1 × 1 mm).
Functional data were analyzed in AFNI (Cox, 1996) after first adjusting for differences in slice-acquisition times and motion, coregistered to the individual’s high-resolution anatomical data and linearly normalized to a standard template (Colin 27) using a single concatenated transform. Data were smoothed with an 8-mm full-width half-maximum kernel and submitted to a standard general linear model with gamma-based hemodynamic response function, baseline drift terms, and six motion parameters (three translation, three rotation). Images that were more than 2 mm displaced or 2° rotated from the first image in the entire functional series were discarded, as were images more than 1 mm displaced or 1° rotated from the previous image.
Identifying coactivation in regions of interest (ROIs)
We examined a reading network consisting of ROIs selected using a model of the canonical reading circuit developed from our previous work (Pugh et al., 2010; Pugh et al., 2013). Four primary ROIs in each hemisphere were anatomically identified using atlas-defined regions (SPM Anatomy Toolbox Version 1.8; Eickhoff et al., 2005). These regions consisted of bilateral IFG (pars opercularis and pars triangularis), temporoparietal regions including STG (anterior and posterior), inferior parietal cortex (IPC; inferior parietal lobule and supramarginal gyrus), and the fusiform gyrus, which contains the occipitotemporal region. Within each anatomical region, we created a metric of print-speech convergence based on coactivation, defined as the total number of voxels for each participant that were significantly activated (p < .01) for both speech and print stimuli (conjoint probability p < .0001). This threshold was used in our previous investigation (Frost et al., 2009) and was chosen here because it revealed reliable individual differences in coactivation that were predictive of relevant behavioral differences. Coactivation in the reading network was the sum of the coactive voxels in these four regions within the respective hemispheres. In addition, the number of voxels activated at p < .01 across the whole brain for spoken and printed stimuli was computed to control for the relative degree of brain activation for each participant. It is important to point out that although we assume that coactivation as measured here reflects neuronal populations that are responding to both modalities, we are limited by the spatial resolution of fMRI technology, and results can be interpreted only within this resolution.
Data analysis
The outcome variable was WJBR score at Time 2. The primary analysis involved predicting Time 2 WJBR scores from Time 1 coactivation to printed and spoken stimuli summed across the reading network in both the left and right hemispheres, while controlling for age at Time 1 and whole-brain activation for both printed and spoken stimuli. We also conducted analyses of the individual ROIs of the reading network to gain insight into the relative contribution of each region. Although the main focus was on establishing whether coactivation within the reading circuit predicts future reading performance, our previous work has indicated that coactivation is correlated with concurrent (Time 1) reading skill, and we therefore conducted an analysis to rule out the possibility that any observed predictive relationship between convergence and Time 2 reading could be attributed solely to a third-order correlation with Time 1 reading performance (i.e., autoregressive effects).
Results
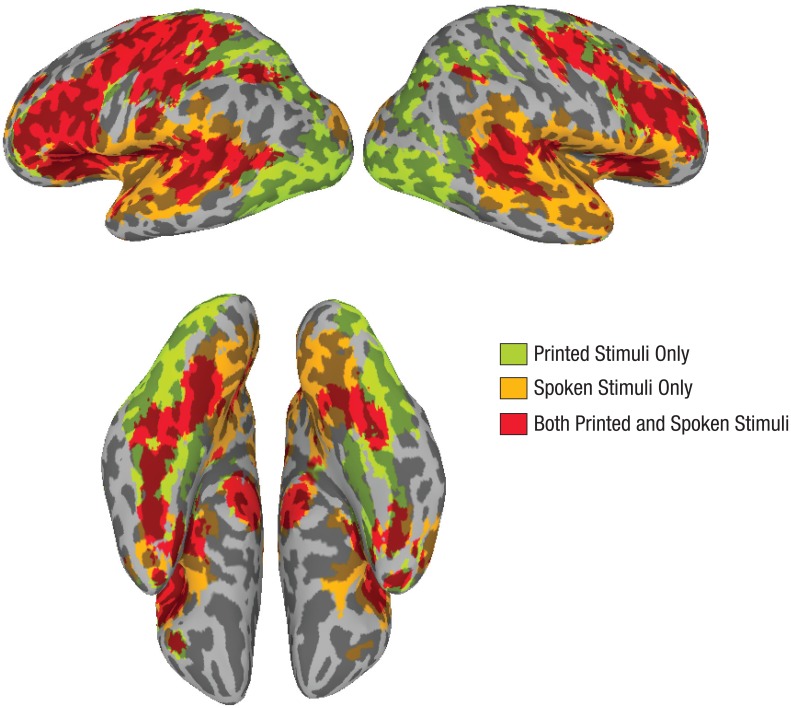
The brain maps in Figure 1 show regions in the reading network associated with significant (p < .01) activation to printed stimuli, spoken stimuli, and both printed and spoken stimuli across the entire cohort. The figure shows that readers strongly engaged the canonical reading network for printed stimuli, and much of this network was also engaged for spoken stimuli.
Fig. 1.
Brain regions that showed significant (p < .01) activation across the sample in response to printed stimuli only, spoken stimuli only, and both printed and spoken stimuli. The top images are lateral views, and the bottom images are inferior views. For both views, the left hemisphere is shown on the left and the right hemisphere on the right.
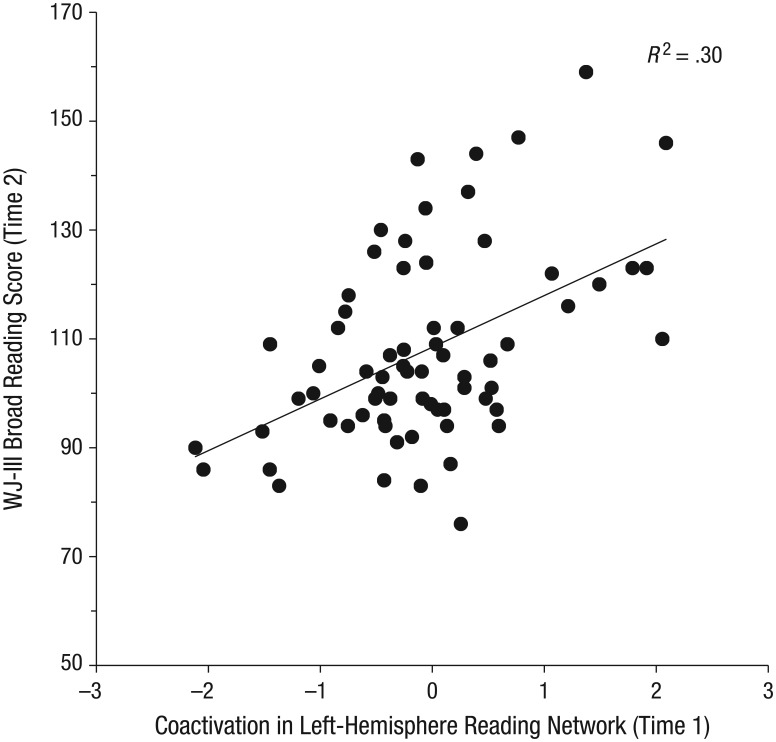
The raw correlation between Time 2 WJBR scores and coactivation was significant for left-hemisphere reading-network ROIs (r = .374, p = .002) but not for right-hemisphere reading-network ROIs (r = .197, p = .107). Results from the regression predicting Time 2 WJBR scores from Time 1 coactivation within the reading network (controlling for Time 1 age and whole-brain activation for printed and spoken stimuli) suggest that the model accounted for significant variance in Time 2 reading (see Table 2). Specifically, Time 1 coactivation in the left-hemisphere reading network was the only significant predictor of Time 2 reading scores. Figure 2 displays a scatterplot of this relationship.
Table 2.
Results From the Multiple Regression Predicting Time 2 Reading Score From Time 1 Print-Speech Coactivation in the Reading Network
| Predictor | β | t(62) | p |
|---|---|---|---|
| Control variables | |||
| Age at Time 1 | −0.16 | −1.41 | .163 |
| Whole-brain activation to printed stimuli | −0.60 | −1.65 | .103 |
| Whole-brain activation to spoken stimuli | −0.12 | −1.77 | .083 |
| Coactivation in regions of interest | |||
| Left-hemisphere reading network | 1.59 | 4.32 | < .001 |
| Right-hemisphere reading network | −0.28 | −1.00 | .323 |
Note: The model accounted for a significant amount of variance, R2 = .30, F(5, 62) = 5.4, p < .001. Reading scores were obtained from the Woodcock-Johnson Test of Achievement (WJ-III; Woodcock, McGrew, & Mather, 2001) Broad Reading composite measure. Print-speech coactivation refers to the number of voxels significantly active in the region for both printed and spoken stimuli.
Fig. 2.
Scatterplot (with best-fitting regression line) showing the association between Time 2 reading scores and standardized residuals for Time 1 coactivation in the left-hemisphere reading network in response to printed and spoken stimuli. For Time 1 coactivation, we controlled for age, whole-brain activation in response to the two types of stimuli, and right-hemisphere coactivation. Reading scores were obtained from the Woodcock-Johnson Test of Achievement (WJ-III; Woodcock, McGrew, & Mather, 2001) Broad Reading composite measure.
Next, we tested a stepwise regression model to explore how coactivation within specific regions accounted for variance in Time 2 reading. Each of the eight regions that comprised the reading network (left and right IFG, IPC, STG, and fusiform gyrus) were entered in a stepwise regression model (p < .05 to enter, p > .10 to remove). Results are shown in Table 3. The control variables were again force-entered first but were not significant. However, the stepwise regression model resulted in the entry of four ROIs: left IPC (Step 2), left IFG (Step 3), left fusiform gyrus (Step 4), and right IFG (Step 5). This suggests independent contributions of coactivation in each of these regions in predicting later reading. Note that coactivation in left IFG, IPC, and fusiform gyrus were positively associated with reading outcomes, but right IFG was negatively associated with later reading achievement.
Table 3.
Results From the Stepwise Multiple Regression Predicting Time 2 Reading Score From Time 1 Print-Speech Coactivation in Selected Regions of Interest
| Predictor | Step 1:
R2 = .09, F(3,
64) = 1.98, p < .126 |
Step 2:
R2 = .20, F(4,
63) = 4.00, p < .006 |
Step 3:
R2 = .26, F(5,
62) = 4.42, p < .002 |
Step 4:
R2 = .32, F(6,
61) = 4.78, p < .001 |
Step 5:
R2 = .37, F(7,
60) = 4.97, p < .001 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | t(64) | p | β | t(64) | p | β | t(64) | p | β | t(64) | p | β | t(64) | p | |
| Age at Time 1 | −0.13 | −1.09 | .280 | −0.14 | −1.18 | .243 | −0.18 | −1.55 | .125 | −0.09 | −0.81 | .423 | −0.08 | −0.68 | .500 |
| Whole-brain activation to printed stimuli | 0.04 | 0.201 | .841 | −0.20 | −0.90 | .369 | −0.44 | −1.82 | .073 | −0.82 | −2.86 | .006 | −0.73 | −2.52 | .014 |
| Whole-brain activation to spoken stimuli | 0.25 | 1.14 | .257 | −0.13 | −0.55 | .582 | −0.37 | −1.46 | .150 | −0.62 | −2.30 | .025 | −0.32 | −1.05 | .296 |
| Left IPC | — | — | — | 0.69 | 3.05 | .003 | 0.53 | 2.31 | .024 | 0.55 | 2.45 | .017 | 0.47 | 2.16 | .035 |
| Left IFG | — | — | — | — | — | — | 0.65 | 2.25 | .028 | 0.64 | 2.29 | .026 | 0.68 | 2.49 | .016 |
| Left fusiform gyrus | — | — | — | — | — | — | — | — | — | 0.64 | 2.26 | .027 | 0.63 | 2.28 | .026 |
| Right IFG | — | — | — | — | — | — | — | — | — | — | — | — | −0.42 | −2.11 | .039 |
Note: Age at Time 1, whole-brain activation in response to printed stimuli, and whole-brain activation in response to spoken stimuli were force-entered in Step 1. For the remaining steps, the number of voxels included in the anatomically defined regions of interest for both printed and spoken stimuli (print-speech coactivation) were selected among left and right inferior parietal cortex (IPC), inferior frontal gyrus (IFG), fusiform gyrus, and superior temporal gyrus (STG) until no additional regions of interest resulted in significant improvement in model fit. Reading scores were obtained from the Woodcock-Johnson Test of Achievement (WJ-III; Woodcock, McGrew, & Mather, 2001) Broad Reading composite measure. Print-speech coactivation refers to the number of voxels significantly active in the region for both printed and spoken stimuli.
Finally, given our previous findings (Frost et al., 2009; Shankweiler et al., 2008), we questioned whether print-speech convergence effects might be attributable to correlations with Time 1 reading scores (r = .267, p = .033). To assess whether our findings might be due to a third-order correlation with Time 1 reading, we ran an additional regression (see Table 4) in which we entered coactivation in the left-hemisphere reading network and Time 1 reading scores as predictors of Time 2 reading performance. Four participants were missing Time 1 reading scores, and thus the analysis was conducted on 64 children. The results demonstrate that the effects of left-hemisphere coactivation cannot be accounted for by a spurious third-order relation to Time 1 reading scores. Also note that a stepwise regression assessing the variance added beyond Time 1 reading autoregressive effects showed that coactivation in the left-hemisphere reading network independently contributed variance in addition to the variance in reading outcome accounted for by Time 1 reading (when the control variables and coactivation in the right-hemisphere reading network were also entered in stepwise regression or backward regression, only the left-hemisphere coactivation and Time 1 reading scores significantly contributed to Time 2 reading).
Table 4.
Results from the Multiple Regression Predicting Time 2 Reading Score From Time 1 Reading Score and Print-Speech Coactivation in the Left Hemisphere
| Variable | β | t(61) | p |
|---|---|---|---|
| Time 1 reading score (control) | 0.85 | 13.78 | < .001 |
| Left-hemisphere coactivation in the reading network | 0.124 | 2.03 | .047 |
Note: The model accounted for a significant amount of variance, R2 = .79, F(1, 61) = 112, p < .001. Reading scores were obtained from the Woodcock-Johnson Test of Achievement (WJ-III; Woodcock, McGrew, & Mather, 2001) Broad Reading composite measure. Print-speech coactivation refers to the number of voxels significantly active in the reading network for both printed and spoken stimuli.
Discussion
This study examined the neurobiological underpinnings of reading achievement in young readers as a function of print-speech convergence (as measured by coactivation) in key networks 2 years earlier. Results reinforce the notion, which has been emphasized in behavioral research, that learning to read is better understood as an achievement involving synthesis of speech and print than as simply a visual-orthographic learning challenge (cf. Hulme et al., 2007; Warmington & Hulme, 2012). This longitudinal study extends our previous findings of concurrent relationships in children and adults (Frost et al., 2009; Shankweiler et al., 2008). Empirically, cross-modal brain activity in the reading network accounted for significant variance in reading achievement 2 years later after we controlled for general activation for print or speech alone (which by chance could have produced more overlap) and for initial scores on the same reading tests (ruling out third-order correlations with Time 1 reading ability). Thus, children whose early language experiences reinforce connections between speech and print are developing an overlapping organization of language cortex that supports reading (Dehaene et al., 2015). This is the predicted consequence of a biological system built for language used to support both speech and reading (Joanisse & McClelland, 2015). Whereas previous studies have shown that functional response to print in children is associated with concurrent reading (Pugh et al., 2013) and reading development (Hoeft et al., 2007; McNorgan et al., 2011; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003), the present study shows unequivocally that print-speech convergence is the operative construct in developing a fluent reading system.
The loci of these convergence effects is unsurprising, as IFG and IPC have been previously shown to generally overlap in adults while processing language (Constable et al., 2004). Furthermore, the inverse relationship of left and right IFG in predicting future reading achievement is in agreement with models indicating that right-hemisphere compensation is a feature of disrupted left-hemisphere circuits in individuals with reading disability (Pugh et al., 2010) and data showing rightward shifts for print processing in poor readers (Pugh et al., 2008). The critical element added here is that children with poorer reading trajectories over a 2-year period not only utilized left IFG less and right IFG more for printed stimuli, but they show this pattern for both printed and spoken stimuli (thus, convergence). This implies a shift in core language functions in these children. Left IFG has been known to be active for phonological and articulatory coding of both spoken and printed language (Price, 2012; Pugh et al., 2010), which is foundational for early reading. The increased right-hemisphere engagement for processing phonological information observed in adolescents who are poor readers (Pugh et al., 2008) may be evident in younger children whose trajectories for reading development are poorer. Finally, whether this rightward integration reflects compensation or failure to make age-appropriate shifts to the left hemisphere (B. A. Shaywitz et al., 2004; Turkeltaub et al., 2003), these data reinforce the increased right-hemisphere profile of poorer readers (S. E. Shaywitz et al., 1998).
The significant effects of convergence on later reading arose across the reading network. Bilateral IFG and left IPC are believed to be tuned to phonological coding for speech and print (Binder et al., 1997; Constable et al., 2004; Pugh et al., 2008; Shankweiler et al., 2008; B. A. Shaywitz et al., 2002), and the findings here may be biological indicators of the ability to integrate orthographic and phonological representations. At later stages, fusiform gyrus also plays a role in skilled reading (Pugh et al., 2010; B. A. Shaywitz et al., 2004), and convergence in this region also contributed to later reading. Coactivation in phonologically and visually tuned regions were important predictors of later reading outcomes on tasks that tap reading speed and comprehension (i.e., WJBR), which suggests that early attunement of these regions enables the circuit to build a mechanism that can support reading efficiency. Effectively utilizing these systems during reading may aid with efficient transfer of letters into sound-based representations (Wagner & Torgesen, 1987).
With respect to the current understanding of the topology of print and speech organization, research on spoken-word processing implicates a bilateral circuitry with key functional divisions between dorsal and frontal networks and ventral networks for different aspects of spoken-word processing (Price, 2012). Reading networks for skilled adult readers (Price, 2012; Schlaggar & McCandliss, 2007) show substantial overlap with these spoken-language networks, especially in left temporoparietal and inferior frontal networks (Constable et al., 2004; Shankweiler et al., 2008). Interestingly, studies that have contrasted performance of literate and illiterate adults on spoken-language tasks have shown that literacy modulates organization for speech with increased involvement of temporoparietal and inferior frontal regions in simple speech tasks (Castro-Caldas, Petersson, Reis, Stone-Elander, & Ingvar, 1998; Kovelman et al., 2015; Rogalsky et al., 2015). Moreover, these changes in language cortex with literate language experience also have direct consequences on quality of speech processing, such that literates (with greater engagement of distributed left parietal and inferior frontal networks for speech tasks) actually perform certain speech tasks with greater proficiency than illiterates (Rogalsky et al., 2015). Thus, the impact of literacy on speech and of speech on literacy is bidirectional in that not only does learning to read affect speech processing (Dehaene et al., 2015), but also it very much depends on convergence of these networks (Frost et al., 2009; Shankweiler et al., 2008). These findings support our assertion that early convergence is critical in developing efficient reading skills.
The longitudinal design in the present study enabled us to move closer to achieving causal models of the neural bases of early reading success and failure. The results are consistent with behavioral studies showing associations between reading ability and verbal-visual learning (cf. Hulme et al., 2007; Vellutino et al., 1995; Warmington & Hulme, 2012). However, questions remain about how the speech and reading circuits influence one another in development and how integrated processing might be mutually facilitative for both modalities (Monzalvo & Dehaene-Lambertz, 2013). By utilizing theoretically motivated neurobiological indicators of future reading achievement, the present study provides a necessary foundation for studies of the brain basis of poor reading outcomes that may be explored across languages. Children whose brains begin to leverage the reading network for cross-modal processing are likely to have better reading achievement in the future. These results reinforce the idea that reading development emerges as a connection between spoken and printed linguistic representations.
Footnotes
Declaration of Conflicting Interests: The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Funding: This research was supported by National Institutes of Health Grant Nos. P01HD001994 and R01HD048830.
References
- Binder J. R., Frost J. A., Hammeke T. A., Cox R. W., Rao S. M., Prieto T. (1997). Human brain language areas identified by functional magnetic resonance imaging. The Journal of Neuroscience, 17, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau V., Reithler J., van Atteveldt N., Seitz J., Gerretsen P., Goebel R., Blomert L. (2010). Deviant processing of letters and speech sounds as proximate cause of reading failure: A functional magnetic resonance imaging study of dyslexic children. Brain, 133, 868–879. doi: 10.1093/brain/awp308 [DOI] [PubMed] [Google Scholar]
- Bradley L., Bryant P. E. (1983). Categorizing sounds and learning to read—a causal connection. Nature, 301, 419–421. [Google Scholar]
- Braze D., Mencl W. E., Tabor W., Pugh K. R., Constable R. T., Fulbright R. K., . . . Shankweiler D. P. (2011). Unification of sentence processing via ear and eye: An fMRI study. Cortex, 47, 416–431. doi: 10.1016/j.cortex.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick N., McCrory E., Price C., Frith C., Frith U. (1999). Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke’s Wortschatz? Brain, 122, 1901–1917. [DOI] [PubMed] [Google Scholar]
- Castro-Caldas A., Petersson K. M., Reis A., Stone-Elander S., Ingvar M. (1998). The illiterate brain: Learning to read and write during childhood influences the functional organization of the adult brain. Brain, 121, 1053–1063. [DOI] [PubMed] [Google Scholar]
- Constable R. T., Pugh K. R., Berroya E., Mencl W. E., Westerveld M., Ni W., Shankweiler D. (2004). Sentence complexity and input modality effects in sentence comprehension: An fMRI study. NeuroImage, 22, 11–21. [DOI] [PubMed] [Google Scholar]
- Cox R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L., Morais J., Kolinsky R. (2015). Illiterate to literate: Behavioural and cerebral changes induced by reading acquisition. Nature Reviews Neuroscience, 16, 234–244. [DOI] [PubMed] [Google Scholar]
- Dunn L. M., Dunn L. M. (1997). Peabody Picture Vocabulary Test (3rd ed.). Circle Pines, MN: AGS. [Google Scholar]
- Eickhoff S., Stephan K. E., Mohlberg H., Grefkes C., Fink G. R., Amunts K., Zilles K. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25, 1325–1335. [DOI] [PubMed] [Google Scholar]
- Elbro C. (1996). Early linguistic abilities and reading development: A review and a hypothesis. Reading and Writing: An Interdisciplinary Journal, 8, 453–485. [Google Scholar]
- Frost S. J., Landi N., Mencl W. E., Sandak R., Fulbright R. K., Tejada E. T., . . . Pugh K. R. (2009). Phonological awareness predicts activation patterns for print and speech. Annals of Dyslexia, 59, 78–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F., Ueno T., Reiss A. L., Meyler A., Whitfield-Gabrieli S., Glover G. H., . . . Gabrieli J. D. E. (2007). Prediction of children’s reading skills using behavioral, functional, and structural neuroimaging measures. Behavioral Neuroscience, 121, 602–613. [DOI] [PubMed] [Google Scholar]
- Hulme C., Goetz K., Gooch D., Adams J., Snowling M. J. (2007). Paired-associate learning, phoneme awareness, and learning to read. Journal of Experimental Child Psychology, 96, 150–166. [DOI] [PubMed] [Google Scholar]
- Joanisse M. F., McClelland J. L. (2015). Connectionist perspectives on language learning, representation and processing. Wiley Interdisciplinary Reviews: Cognitive Science, 6, 235–247. doi: 10.1002/wcs.1340 [DOI] [PubMed] [Google Scholar]
- Kovelman I., Wagley N., Hay J. S. F., Ugolini M., Bowyer S. M., Lajiness-O’Neill R., Brennan J. (2015). Multimodal imaging of temporal processing in typical and atypical language development. Annals of the New York Academy of Sciences, 1337, 7–15. doi: 10.1111/nyas.12688 [DOI] [PubMed] [Google Scholar]
- Liberman A. M. (1992). The relation of speech to reading and writing. Advances in Psychology, 94, 167–178. [Google Scholar]
- McNorgan C., Alvarez A., Bhullar A., Gayda J., Booth J. R. (2011). Prediction of reading skill several years later depends on age and brain region: Implications for developmental models of reading. The Journal of Neuroscience, 31, 9641–9648. doi: 10.1523/jneurosci.0334-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNorgan C., Randazzo-Wagner M., Booth J. R. (2013). Cross-modal integration in the brain is related to phonological awareness only in typical readers, not in those with reading difficulty. Frontiers in Human Neuroscience, 7, Article 388. doi: 10.3389/fnhum.2013.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzalvo K., Dehaene-Lambertz G. (2013). How reading acquisition changes children’s spoken language network. Brain & Language, 127, 356–365. doi: 10.1016/j.bandl.2013.10.009 [DOI] [PubMed] [Google Scholar]
- National Reading Panel. (2000). Teaching children to read: An evidence-based assessment of the scientific research literature on reading and its implications for reading instruction. Washington, DC: National Institutes of Health. [Google Scholar]
- Price C. J. (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62, 816–847. doi: 10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K. R., Frost S. J., Sandak R., Landi N., Moore D., Della Porta G., Rueckl J. G., Mencl W. E. (2010). Mapping the word reading circuitry in skilled and disabled readers. In Cornelissen P. L., Hansen P. C., Kringelback M. L., Pugh K. R. (Eds.), The neural basis of reading (pp. 281–305). New York, NY: Oxford University Press. [Google Scholar]
- Pugh K. R., Frost S. J., Sandak R., Landi N., Rueckl J. G., Constable R. T., . . . Mencl W. E. (2008). Effects of stimulus difficulty and repetition on printed word identification: An fMRI comparison of nonimpaired and reading-disabled adolescent cohorts. Journal of Cognitive Neuroscience, 20, 1146–1160. doi: 10.1162/jocn.2008.20079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K. R., Landi N., Preston J. L., Mencl W. E., Austin A. C., Sibley D., . . . Frost S. J. (2013). The relationship between phonological and auditory processing and brain organization in beginning readers. Brain & Language, 125, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C., Poppa T., Chen K.-H., Anderson S. W., Damasio H., Love T., Hickok G. (2015). Speech repetition as a window on the neurobiology of auditory–motor integration for speech: A voxel-based lesion symptom mapping study. Neuropsychologia, 71, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B. L., McCandliss B. D. (2007). Development of neural systems for reading. Annual Reviews of Neuroscience, 30, 475–503. [DOI] [PubMed] [Google Scholar]
- Shankweiler D., Crain S., Katz L., Fowler A. E., Liberman A. M., Brady S. A., . . . Shaywitz B. A. (1995). Cognitive profiles of reading-disabled children: Comparison of language skills in phonology, morphology, and syntax. Psychological Science, 6, 149–156. [Google Scholar]
- Shankweiler D., Mencl W. E., Braze D., Tabor W., Pugh K. R., Fulbright R. K. (2008). Reading differences and brain: Cortical integration of speech and print in sentence processing varies with reader skill. Developmental Neuropsychology, 33, 745–775. [DOI] [PubMed] [Google Scholar]
- Shaywitz B. A., Shaywitz S. E., Blachman B. A., Pugh K. R., Fulbright R. K., Skudlarski P., . . . Gore J. C. (2004). Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biological Psychiatry, 55, 926–933. [DOI] [PubMed] [Google Scholar]
- Shaywitz B. A., Shaywitz S. E., Pugh K. R., Mencl W. E., Fulbright R. K., Skudlarski P., . . . Gore J. C. (2002). Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry, 52, 101–110. [DOI] [PubMed] [Google Scholar]
- Shaywitz S. E., Shaywitz B. A., Pugh K. R., Fulbright R. K., Constable R. T., Mencl W. E., . . . Gore J. C. (1998). Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Sciences, USA, 95, 2636–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen J. K., Wagner R. K., Rashotte C. A. (1999). Test of Word Reading Efficiency. Austin, TX: Pro-Ed. [Google Scholar]
- Turkeltaub P. E., Gareau L., Flowers D. L., Zeffiro T. A., Eden G. F. (2003). Development of neural mechanisms for reading. Nature Neuroscience, 6, 767–773. doi: 10.1038/nn1065 [DOI] [PubMed] [Google Scholar]
- Vellutino F. R., Scanlon D. M., Spearing D. (1995). Semantic and phonological coding in poor and normal readers. Journal of Experimental Child Psychology, 59, 76–123. [DOI] [PubMed] [Google Scholar]
- Wagner R. K., Torgesen J. K. (1987). The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin, 101, 192–212. [Google Scholar]
- Wagner R. K., Torgesen J. K., Rashotte C. A. (1999). Comprehensive Test of Phonological Processing. Austin, TX: Pro-Ed. [Google Scholar]
- Warmington M., Hulme C. (2012). Phoneme awareness, visual-verbal paired-associate learning, and rapid automatized naming as predictors of individual differences in reading ability. Scientific Studies of Reading, 16, 45–62. [Google Scholar]
- Wechsler D. (1999). Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Woodcock R. W., McGrew K. S., Mather N. (2001). Woodcock-Johnson Test of Achievement (3rd ed.). Itasca, IL: Riverside Publishing. [Google Scholar]